Abstract
Background: Notoriety bias is defined as “a selection bias in which a case has a greater chance of being reported if the subject is exposed to the studied factor known to cause, thought to cause, or likely to cause the event of interest.” This study aimed to determine the existence of notoriety bias in the FDA Adverse Event Reporting System (FAERS) database and estimate the impact of potential notoriety bias induced by safety alerts on signal estimation using disproportionality analysis. Methods: Publicly available FAERS data were downloaded and used for analysis. Thirty-one drugs which had label change/safety alert issued by FDA from 2009 to 2013 were considered. These drugs were reviewed 4 quarters before and after the safety alert notification for the existence of notoriety bias. The impact of notoriety bias induced by safety alerts was analyzed by comparing the signal strength using reporting odds ratio (ROR) and proportional reporting ratio (PRR), 2 years before and after the safety alert. Wilcoxon signed rank test was used to determine whether there were a statistically significant difference before and after the safety alert. Results: There was increased reporting for 11 drugs after the safety alert/label change by the FDA. The reporting of 20 drugs decreased or remained unchanged after the safety alert/label change by the FDA. Wilcoxon signed rank test showed that there is no statistically significant difference with respect to the number of reports before and after the safety alert (P = .330, Z = −0.974). Fourteen (45.16%) drugs had an increase in ROR, while 17 (54.83%) drugs had a decrease in ROR after safety alert issued by FDA (P = .953, Z = −0.059). Fourteen (45.16%) drugs had an increase in PRR, while 17 (54.83%) drugs had a decrease in PRR after safety alert issued by the FDA (P = .914, Z = −0.108). Conclusion: Although few FDA safety alert/warnings had a strong and immediate impact, many had no impact on reporting of AE and signal strength. This study found that overreporting due to notoriety bias does not exist in the FAERS database and the overall disproportionality in signal estimates is not altered by the safety alert.
Keywords: drug information, medication safety, medication errors, adverse drug reactions reporting/monitoring
Introduction
Pharmacovigilance systems worldwide rely on spontaneous reporting for the identification of suspected adverse drug reactions (ADRs) and risk assessment because of the limitations of clinical trials (restricted sample size, reduced follow-up, appraisal of surrogate markers) in establishing the safety profile of a drug in the premarketing setting.1
The unfolding of adverse event (AE) profiles of numerous drugs (e.g., cerivastatin, sparfloxacin, thalidomide, sibutramine, tolcapone)2-4 after they were approved by the US Food and Drug Administration (FDA) led to the blooming of postmarketing surveillance. The analysis of spontaneous reports reflects the real world scenario, in which patients experienced the primary outcome in a complex pharmacological context.5 However, there is a delay in broadcasting the relevant postmarketing AE information which is of significant concern. Such delays, combined with the aforesaid limitations of premarketing clinical trial, emphasize the need for meticulous postmarketing vigilance.
The FDA Adverse Event Reporting System (FAERS) database is designed to support the FDA’s postmarketing surveillance of all approved drugs and therapeutic biologic products. The FAERS encompasses voluntary AE reports from health care professionals and consumers, which are reported either through manufacturers or directly to the FDA.6 The database encounters many challenges including underreporting, Weber effect,7 and notoriety bias.8
With respect to underreporting, it reduces the sensitivity because it underestimates the frequency and thereby impact of the problem. In addition, it makes the system more vulnerable to selective reporting, which may introduce a serious bias.9 Various initiatives have been introduced in recent years by FDA, global regulatory agencies, and the healthcare industry to encourage and facilitate the reporting of AEs, such as greater accessibility to the spontaneous reporting database through electronic and online reporting. With regard to Weber effect, it is a substantial increase in the spontaneous reporting of ADRs, particularly during the first 2 years after the initial approval of the drug, which then plateaus and eventually drops.10 However, the recent study suggests that it may not be a significant factor in the analysis of postmarketing AE data.7
Healthcare providers depend heavily on safety information from drug label “inserts” which mainly contain premarketing clinical trial results as FAERS data are not readily accessible.11 To keep healthcare professionals abreast with evolving postmarketing safety risks and help guide prescribing decisions, the FDA issues warnings and safety alerts.12 The dissemination of an alert can alter the reporting of an event. This phenomenon is called notoriety bias and has been defined as a “selection bias in which a case has a greater chance of being reported if the subject is exposed to the studied factor known to cause, thought to cause, or likely to cause the event of interest.”8
Many studies have previously evaluated the impact of FDA alerts on drug use,13-17 but very few have studied the impact of FDA alerts on AE reporting. The abovementioned studies have either considered a single drug or single class of drug for analysis. This study aimed to determine the existence of notoriety bias in FAERS database and estimate the impact of potential notoriety bias induced by safety alerts on signal estimates using disproportionality analysis.
Methodology
Database
The FAERS is a database that comprises AE reports, medication error reports, and product quality complaints resulting in AEs that are submitted to FDA. The database is primarily designed to assist the FDA’s postmarketing safety surveillance program for drug and therapeutic biologic products. It is in compliance with the International Conference on Harmonization (ICH) issued safety reporting guidance (ICH E2B). The reports in FAERS are evaluated by clinical reviewers, in the Center for Drug Evaluation and Research and the Center for Biologics Evaluation and Research. Reports are submitted to the FAERS database from healthcare professionals, consumers, and manufacturers. The database consists of the following components: Demo file (patient demographics and administrative information), Drug file (details about reported drugs), Reaction file (reactions coded in MedDRA terminology), Outcome file (patient outcomes for the event), Therapy file (drug therapy start dates and end dates), and Indication file (coded in MedDRA for indication of reported drug).
Study Procedure
Publicly available FAERS data were downloaded from FAERS website (https://open.fda.gov/data/faers/). Text file was extracted into excel worksheet for further analysis. “New safety information” which is given by USFDA was considered as the source of safety signal. Thirty-one drugs which had label change/safety alert issued by FDA from 2009 to 2013 were considered in this study. These drugs were reviewed 4 quarters before and after the safety alert notification for the existence of notoriety bias. Normalization of reports was done for ease of graphical representation. The highest count of the report of any of the 8 quarters of the drug was considered as 100 and remaining count of reports of that particular drug was normalized accordingly. The safety alert for biologicals and for drugs which had unspecific safety alert (eg, psychiatric events, skin reactions) were eliminated from the study.
Disproportionality Analysis
The impact of notoriety bias induced by safety alerts was analyzed using reporting odds ratio (ROR) and proportional reporting ratio (PRR).5 The ROR is the ratio of the odds of reporting of one specific event versus all other events for a given drug compared with the reporting odds for all other drugs present in the database. The higher the value, the stronger the disproportion appears to be. The PRR involves the calculation of the rate of reporting of one specific event among all events for a given drug, the comparator being this reporting rate for all drugs present in the database (including the drug of interest). The computational formula for calculating ROR and PRR18,19 is given in Tables 1 and 2. ROR and PRR for the drugs were calculated 2 years before and after the safety alert were issued. Wilcoxon signed rank test was used to determine whether there were statistical differences in signal strength before and after the safety alert.
Table 1.
Method of Calculation of the Signals.
| Drug of interest | Other drugs | |
|---|---|---|
| ADR of interest | A | B |
| Other ADR | C | D |
Note. ADR = adverse drug reaction; A = the number of reports containing both suspected drug and suspected ADRs; B = the number of reports containing drug of choice but with other ADRs; C = the number of reports containing the event of interest but with other medications; D = the number of reports concerning other medications and other ADRs.
Table 2.
Formula for Computation of Signals.
| Measure | Computation |
|---|---|
| ROR | ROR = (A/B)/(C/D) SE = |
| PRR | PRR = A(A+C)/B(B+D) SE = |
Note. ROR = reporting odds ratio; PRR = proportional reporting ratio.
Results
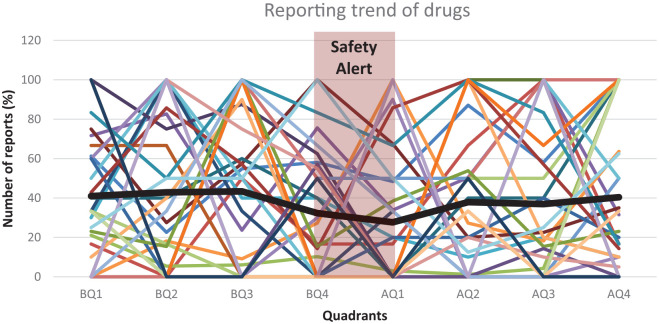
The analysis was performed for 31 drugs which had safety alert/label change notification by FDA between 2009 and 2013 (Table 3). The reporting trend between 4 quarters before and after the safety alert, along with the moving average is shown in Figure 1. The moving average was not significantly altered before and after the alert which indicates that the drugs are not affected by the safety alert notification.
Table 3.
Drugs Which Had Safety Alert/Label Change Notification by FDA Between 2009 and 2013 and Difference in Number of Reports, ROR and PRR Before and After Safety Alert.
| Drug | Safety alert | Date of alert | Number of reports before | Number of reports after | ROR before | ROR after | PRR before | PRR after |
|---|---|---|---|---|---|---|---|---|
| Zoledronic acid | Renal impairment | March 13, 2009 | 61 | 91 | 0.44 | 0.49 | 0.44 | 0.49 |
| Ceftriaxone | Hemolytic anemia | June 7, 2009 | 5 | 12 | 6.45 | 12.46 | 6.48 | 12.74 |
| Varenicline | Visual impairment | July 01, 2009 | 6 | 11 | 1.08 | 1.12 | 1.09 | 1.13 |
| Montelukast | Suicidal behavior | August 19, 2009 | 322 | 274 | 8.77 | 3.35 | 9.60 | 3.41 |
| Didanosine | Portal hypertension | January 25, 2010 | 21 | 6 | 341.80 | 525.86 | 470.53 | 607.94 |
| Trazodone | Prolongation of the electrocardiogram QT interval | February 02, 2010 | 6 | 23 | 4.08 | 4.32 | 4.06 | 4.31 |
| Propylthiouracil | Hepatotoxicity | April 1, 2010 | 5 | 1 | 13.23 | 38.41 | 13.04 | 37.14 |
| Orlistat | Hepatotoxicity | May 25, 2010 | 104 | 58 | 0.50 | 1.23 | 0.50 | 1.22 |
| Solifenacin | Angioedema | July 12, 2010 | 0 | 2 | 0.77 | 0.71 | 0.77 | 0.71 |
| Minocycline | DRESS | September 14, 2010 | 26 | 0 | 45.45 | 6.96 | 43.59 | 6.92 |
| Tapentadol hydrochloride | Serotonin syndrome | November 1, 2010 | 9 | 9 | 19.70 | 40.31 | 19.33 | 39.47 |
| Oxybutynin chloride | Angioedema | January 31, 2011 | 4 | 3 | 1.28 | 1.70 | 1.28 | 1.70 |
| Doxycycline hyclate | Stevens Johnson Syndrome | March 21, 2011 | 3 | 3 | 2.72 | 6.45 | 2.73 | 6.49 |
| Finasteride | Male breast cancer | April 13, 2011 | 3 | 5 | 77.45 | 37.60 | 93.67 | 41.80 |
| Lanthanum carbonate | Intestinal obstruction | April 27, 2011 | 3 | 12 | 0.983 | 0.52 | 0.98 | 0.53 |
| Simvastatin | Muscle injury | June 8, 2011 | 19 | 10 | 9.87 | 9.01 | 13.15 | 10.90 |
| Dutasteride | Male breast cancer | June 9, 2011 | 4 | 3 | 70.33 | 146.97 | 83.19 | 192.56 |
| Leuprolide acetate | Osteopenia | June 17, 2011 | 14 | 13 | 0.59 | 0.21 | 0.59 | 0.21 |
| Dronedarone hydrochloride | Pulmonary toxicity | June 21, 2011 | 8 | 5 | 10.89 | 29.86 | 11.17 | 30.80 |
| Bevacizumab | Osteonecrosis of jaw | September 30, 2011 | 14 | 18 | 4.43 | 2.52 | 4.51 | 2.56 |
| Infliximab | Sarcoidosis | October 26, 2011 | 20 | 17 | 5.67 | 2.04 | 6.12 | 2.09 |
| Voriconazole | Periostitis | November 16, 2011 | 11 | 1 | 224.86 | 195.93 | 362.26 | 301.37 |
| Adalimumab | Optic neuritis | December 26, 2011 | 19 | 16 | 1.72 | 0.74 | 1.77 | 0.74 |
| Solifenacin succinate | Somnolence | January 17, 2012 | 4 | 8 | 0.19 | 0.28 | 0.19 | 0.28 |
| Drospirenone | Pancreatitis | February 13, 2012 | 6 | 1 | 3.73 | 3.36 | 3.86 | 3.44 |
| Pegloticase | Anaphylaxis | April 16, 2012 | 46 | 7 | 42.80 | 74.69 | 38.92 | 65.03 |
| Trospium chloride | Somnolence | July 23, 2012 | 1 | 4 | 0.03 | 0.01 | 0.04 | 0.01 |
| Tolterodine tartrate | Somnolence | August 1, 2012 | 2 | 4 | 1.48 | 1.18 | 1.48 | 1.18 |
| Fesoterodine fumarate | Somnolence | August 1, 2012 | 18 | 12 | 2.55 | 1.78 | 2.52 | 1.77 |
| Dalfampridine | Anaphylaxis | January 22, 2013 | 3 | 2 | 0.55 | 0.27 | 0.56 | 0.27 |
| Lacosamide | Neutropenia | April 17, 2013 | 3 | 1 | 0.79 | 0.69 | 0.79 | 0.69 |
Note. FDA = US Food and Drug Administration; ROR = reporting odds ratio; PRR = proportional reporting ratio; DRESS = drug reaction with eosinophilia and systemic symptoms.
Figure 1.
Reporting trend of 31 drugs, 4 quarters before and after the safety alert.
Note. Each color represents the reporting trend of individual drug. Pink zone indicates the period during which the safety alert was issued. Thick black line indicates the moving average of reporting trends of all drugs. Q = quadrant; moving average of 31 drugs AE reports; BQ = before the safety alert; AQ = after the safety alert.
Drugs Whose Reporting Increased After Safety Alert
There was increased reporting for 11 drugs after the safety alert/label change by FDA. Drugs included Zoledronic acid (number of reports before: 61; number of reports after: 91), Ceftriaxone (5; 12), Varenicline (6; 11), Trazodone (6; 23), Solifenacin (0; 2), Finasteride (3; 5), Lanthanum carbonate (3; 12), Bevacizumab (14; 18), Solifenacin succinate (4; 8), Trospium chloride (1; 4), and Tolterodine tartrate (2; 4) (Table 3). An average increase of 17.18 ± 31.63 was observed for the abovementioned drugs.
Drugs Whose Reporting Decreased or Remained Unchanged After Safety Alert
The reporting of 20 drugs decreased or remained unchanged after the safety alert/label change by FDA. Drugs included Montelukast (number of reports before: 322; number of reports after: 274), Didanosine (21; 6), Propylthiouracil (5; 1), Orlistat (104; 58), Minocycline (26; 0), Tapentadol hydrochloride (9; 9), Oxybutynin chloride (4; 3), Doxycycline hyclate (3; 3), Simvastatin (19; 10), Dutasteride (4; 3), Leuprolide acetate (14; 13), Dronedarone HCl (8; 5), Infliximab (20; 17), Voriconazole (11; 1), Adalimumab (19; 16), Drospirenone (6; 1), Pegloticase (46; 7), Fesoterodine fumarate (18; 12), Dalfampridine (3; 2), and Lacosamide (3; 1) (Table 3).
Wilcoxon signed rank test was performed to identify the impact of safety alert on reporting trend. It was observed that there is no statistically significant difference with respect to the number of reports before and after the safety alert (P = .330, Z = −0.974) (Tables 4 and 5).
Table 4.
Descriptive Statistics.
| n | Mean | Std. Deviation | |
|---|---|---|---|
| Number of reports | |||
| Before | 31 | 26.9355 | 59.53371 |
| After | 25.8387 | 57.98684 | |
| Signal strength using ROR | |||
| Before | 31 | 29.2003 | 72.29670 |
| After | 37.1373 | 100.85342 | |
| Signal strength using PRR | |||
| Before | 31 | 38.6842 | 104.31360 |
| After | 44.5129 | 121.97943 | |
Note. ROR = reporting odds ratio; PRR = proportional reporting ratio.
Table 5.
Wilcoxon Signed Rank Analysis.
| n | Mean rank | Sum of ranks | Z value | P value | |
|---|---|---|---|---|---|
| Number of reports | |||||
| Negative ranks | 18a | 14.58 | 262.50 | −0.974b | .330 |
| Positive ranks | 11c | 15.68 | 172.50 | ||
| Ties | 2d | ||||
| Signal strength using ROR | |||||
| Negative ranks | 17a | 14.76 | 251.00 | −0.059b | .953 |
| Positive ranks | 14c | 17.50 | 245.00 | ||
| Ties | 0d | ||||
| Signal strength using PRR | |||||
| Negative ranks | 17a | 14.91 | 253.50 | −0.108b | .914 |
| Positive ranks | 14c | 17.32 | 242.50 | ||
| Ties | 0d | ||||
Note. ROR = reporting odds ratio; PRR = proportional reporting ratio.
After < before.
Based on positive ranks.
After > before.
After = before.
Impact of Safety Alert on Signal Strength (ROR)
Fourteen (45.16%) drugs had increase in ROR after safety alert, while 17 (54.83%) drugs had decrease in ROR after safety alert issued by FDA. Zoledronic acid (ROR before: 0.446; ROR after: 0.499), Ceftriaxone (6.45; 12.64), Varenicline (1.08; 1.12), Montelukast (8.77; 3.35), Didanosine (341.8; 525.86); Trazodone (4.08; 4.32), Propylthiouracil (13.23; 38.41), Orlistat (0.5; 1.23), Solifenacin (0.77; 0.71), Minocycline (45.45; 6.96), Tapentadol hydrochloride (19.7; 40.31), Oxybutynin chloride (1.28; 1.70), Doxycycline hyclate (2.72; 6.45), Finasteride (77.45; 37.6), Lanthanum carbonate (0.983; 0.52), Simvastatin (9.87; 9.01), Dutasteride (70.33; 146.97), Leuprolide acetate (0.59; 0.21), Dronedarone HCl (10.89; 29.86), Bevacizumab (4.43; 2.52), Infliximab (5.67; 2.04), Voriconazole (224.86; 195.93), Adalimumab (1.72; 0.74), Solifenacin succinate (0.19; 0.28), Drospirenone (3.73; 3.36), Pegloticase (42.8; 74.69), Trospium chloride (0.03; 0.01), Tolterodine tartrate (1.48; 1.18), Fesoterodine fumarate (2.55; 1.78), Dalfampridine (0.55; 0.27), Lacosamide (0.79; 0.69) (Table 3). There was no significant difference in the ROR before and after safety alert (P = .953, Z = −0.059) (Tables 4 and 5).
Impact of Safety Alert on Signal Strength (PRR)
Fourteen (45.16%) drugs had increase in PRR after safety alert, while 17 (54.83%) drugs had decrease in PRR after safety alert issued by FDA. Zoledronic acid (PRR before: 0.44; PRR after: 0.49), Ceftriaxone (6.48; 12.74), Varenicline (1.09; 1.13), Montelukast (9.6; 3.41), Didanosine (470.53; 607.94), Trazodone (4.06; 4.31), Propylthiouracil (13.04; 37.14), Orlistat (0.5; 1.22), Solifenacin (0.77; 0.71), Minocycline (43.59; 6.92), Tapentadol hydrochloride (19.33; 39.47), Oxybutynin chloride (1.28; 1.70), Doxycycline hyclate (2.73; 6.49), Finasteride (93.67; 41.8), Lanthanum carbonate (0.983; 0.53), Simvastatin (13.15; 10.9), Dutasteride (83.19; 192.56), Leuprolide acetate (0.59; 0.21), Dronedarone HCl (11.17; 30.8); Bevacizumab (4.51; 2.56), Infliximab (6.12; 2.09), Voriconazole (362.26; 301.37), Adalimumab (1.77; 0.74), Solifenacin succinate (0.19; 0.28), Drospirenone (3.86; 3.44), Pegloticase (38.92; 65.03), Trospium chloride (0.04; 0.01), Tolterodine tartrate (1.48; 1.18), Fesoterodine fumarate (2.52; 1.77), Dalfampridine (0.56; 0.27), Lacosamide (0.79; 0.69) (Table 3). Significant difference was not observed in the PRR before and after safety alert (P = .914, Z = −0.108) (Tables 4 and 5).
Discussion
Our study aimed to determine the existence of notoriety bias in FAERS database and estimate the impact of potential notoriety bias induced by safety alerts on signal estimates using disproportionality analysis. We found that overreporting due to notoriety bias does not exist in the FAERS database and the overall disproportionality in signal estimates is not altered by safety alert. Although few drugs demonstrated increased AE reporting following safety alert, there was no significant increase in the reports except for visual impairment due to Varenicline which had more than 50% increase in the number of reports after the FDA alert. Nevertheless, the overall reporting in the database was not significantly affected.
When we compared the number of reports 4 quarters before and after the safety alert, there was an increase in reporting for few and the others remained unaffected by the FDA alert. Wilcoxon signed rank analysis showed no significant difference between the number of reports before and after the safety alert. This was consistent with the study conducted by Hoffman KB et al, which suggests that modern day AE reporting trends do not appear to be substantially affected by FDA alerts.20 The decreased reporting of AE after FDA alert can be attributed to substantial decline in the use or prescribing of the drugs or increase in the use of other drugs in the same class after the release of information by FDA on the dangers of that particular drug.
Disproportionality in signal strength was assessed using ROR and PRR 2 years before and after the alert. Few drugs showed increase in ROR and PRR after the alert, while it decreased for others. Statistical analysis showed no significant difference in the signal strength before and after the safety alert. This was contradictory to the study conducted by Pariente et al, which concludes that disproportionality in spontaneous reporting databases increases after safety alert.21 However, the above study analyzed the consequences of safety alert only on 4 drugs.
Notoriety bias/stimulated reporting of a drug is dependent on numerous factors such as specificity of the warning, seriousness of the alert, traditional media coverage, acceptance of warning/alert/label change, if the guidance increases the work load for the physicians and whether the alert comes with guidance for selection of safer alternatives.
The increasing use of social media sites to disseminate the emerging safety information of licensed medicinal products can have a significant impact on FDA warnings. A study conducted by Faasse et al reported that the traditional news coverage has the potential to considerably increase the overall AE reporting as it increase the anxiety in viewers.22 All the newspaper articles mentioned at least one benefit of the drug; however, only 32% mentioned one harmful effect. None listed harm without mentioning a benefit.23 Public is much more in-tune with social media and direct-to-consumer advertising since 2009-2013 and extrapolating these results to present day is a question. Further analysis should be done using more recent data for better understanding on the impact of social media on safety alerts.
Another factor which can have a significant impact is the physician’s acceptance and willingness to follow the FDA guidance. Morrato et al compared the frequency of provider contact after FDA advisory of “close supervision” for risk of suicide and found that it was not increased after FDA advisory was issued. This might have occurred as FDA advisory was issued without lack of supporting evidence that close supervision may lead to improved outcomes and physicians believed that their current level of monitoring was sufficient and advisory was overlooked.24 A study found that physicians are unlikely to abide to FDA guidance that requires increased patient contact.17
The effectiveness of risk communication is under question. A study conducted previously concluded that safety alert can be effective, but not always sufficiently.25 Safety alert can result in both intended and unintended effects. There was decreased number of prescriptions for selective serotonin receptor inhibitors (SSRIs) after safety warning of increased risk of suicidality and suicide thoughts in children and adolescents.26 The unintended effect was decrease in prescription of SSRIs in adults as well.27 This requires adoption of clear and effective risk communication strategies.
In most of the cases, warnings are vague and difficult to interpret. FDA warnings/alerts/label changes are more successful if they are repeated, precise, brief, offer alternatives, communicated through media and newsletters, and come with decision support.28
Limitations of this study were that the impact of confounding factors such as media coverage on FDA warning could not be assessed, drug selection was biased as we did not include all the alerts between 2009 to 2013, and generalizing this result to the entire database might not be appropriate. In the present day scenario, social media and advertising have more influence over public which could have shown an increase in the reporting ratio; however, due to the unavailability of “new safety information” our study includes only data from 2009 to 2013. The reasons for reporting and signal strength being unaffected by the FDA alert could not be evaluated.
Conclusion
Although few FDA safety alert/warnings had strong and immediate impact, many had no impact on reporting of AE and signal strength. This study found that overreporting due to notoriety bias does not exist in the FAERS database and the overall disproportionality in signal estimates is not altered by safety alert. Even though few drugs exhibited increase in reporting, it was not found to be significant. Future studies are required to assess the impact of confounding factors on notoriety bias.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Viswam Subeesh  https://orcid.org/0000-0002-1721-4553
https://orcid.org/0000-0002-1721-4553
References
- 1. Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med. 2003;18(1):57-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Charatan F. Bayer decides to withdraw cholesterol lowering drug. BMJ. 2001;323(7309):359. [PMC free article] [PubMed] [Google Scholar]
- 3. Qureshi ZP, Seoane-Vazquez E, Rodriguez-Monguio R, Stevenson KB, Szeinbach SL. Market withdrawal of new molecular entities approved in the United States from 1980 to 2009. Pharmacoepidemiol Drug Saf. 2011;20(7):772-777. [DOI] [PubMed] [Google Scholar]
- 4. McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;278(7216):1358. [Google Scholar]
- 5. Poluzzi E, Raschi E, Piccinni C, De Ponti F. Data mining techniques in pharmacovigilance: analysis of the publicly accessible FDA Adverse Event Reporting System (AERS). In: Karahoca A, ed. Data Mining Applications in Engineering and Medicine. Rijeka, Croatia: InTech; 2012:265-302. [Google Scholar]
- 6. US Food and Drug Administration. FDA Adverse Event Reporting System (FAERS) public dashboard. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance/adversedrugeffects/ucm070093.htm. Accessed January 12, 2019.
- 7. Arora A, Jalali RK, Vohora D. Relevance of the Weber effect in contemporary pharmacovigilance of oncology drugs. Ther Clin Risk Manag. 2017;13:1195-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bégaud B, Dangoumau J. Pharmacoepidemiology: definitions, problems, methodology. Therapie. 2000;55(1):113-117. [PubMed] [Google Scholar]
- 9. Alvarez-Requejo A, Carvajal A, Begaud B, Moride Y, Vega T, Arias LM. Under-reporting of adverse drug reactions. Estimate based on a spontaneous reporting scheme and a sentinel system. Eur J Clin Pharmacol. 1998;54(6):483-488. [DOI] [PubMed] [Google Scholar]
- 10. Beulah E, Reddy N, Subeesh V, Maheswari E, Pudi C. Weber effect: an extended analysis for ten years of reporting trends in FDA Adverse Event Reporting System (FAERS). Value Health. 2018;21:S369. [Google Scholar]
- 11. Harrington CA, Garcia AS, Sircar-Ramsewak F. Evaluation of adverse drug event information in US manufacturer labels. Curr Drug Saf. 2011;6(1):30-35. [DOI] [PubMed] [Google Scholar]
- 12. Piening S, Haaijer-Ruskamp FM, de Vries JT, et al. Impact of safety-related regulatory action on clinical practice: a systematic review. Drug Saf. 2012;35(5):373-385. [DOI] [PubMed] [Google Scholar]
- 13. Busch SH, Frank RG, Leslie DL, et al. Antidepressants and suicide risk: how did specific information in FDA safety warnings affect treatment patterns? Psychiatr Serv. 2010;61(1):11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cluxton RJ, Li Z, Heaton PC, et al. Impact of regulatory labeling for troglitazone and rosiglitazone on hepatic enzyme monitoring compliance: findings from the state of Ohio Medicaid program. Pharmacoepidemiol Drug Saf. 2005;14(1):1-9. [DOI] [PubMed] [Google Scholar]
- 15. Shrank WH, Choudhry NK, Tong A, et al. Warnings without guidance: patient responses to an FDA warning about ezetimibe. Med Care. 2012;50:479-484. [DOI] [PubMed] [Google Scholar]
- 16. Valluri S, Zito JM, Safer DJ, Zuckerman IH, Mullins CD, Korelitz JJ. Impact of the 2004 Food and Drug Administration pediatric suicidality warning on antidepressant and psychotherapy treatment for new-onset depression. Med Care. 2010;48:947-954. [DOI] [PubMed] [Google Scholar]
- 17. Olfson M, Marcus SC, Druss BG. Effects of Food and Drug Administration warnings on antidepressant use in a national sample. Arch Gen Psychiatry. 2008;65(1):94-101. [DOI] [PubMed] [Google Scholar]
- 18. Subeesh V, Singh H, Maheswari E, Beulah E. Novel adverse events of vortioxetine: a disproportionality analysis in USFDA Adverse Event Reporting System database. Asian Journal of Psychiatry. 2017;30:152-156. [DOI] [PubMed] [Google Scholar]
- 19. Subeesh V, Maheswari E, Singh H, Beulah TE, Swaroop AM. Novel adverse events of iloperidone: a disproportionality analysis in US Food and Drug Administration Adverse Event Reporting System (FAERS) database. Curr Drug Saf. 2019;14(1):21-26. [DOI] [PubMed] [Google Scholar]
- 20. Hoffman KB, Demakas AR, Dimbil M, Tatonetti NP, Erdman CB. Stimulated reporting: the impact of US Food and Drug Administration-issued alerts on the adverse event reporting system (FAERS). Drug Saf. 2014;37(11):971-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N. Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf. 2007;30(10):891-898. [DOI] [PubMed] [Google Scholar]
- 22. Faasse K, Gamble G, Cundy T, Petrie KJ. Impact of television coverage on the number and type of symptoms reported during a health scare: a retrospective pre-post observational study. BMJ Open. 2012;2(4):e001607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cassels A, Hughes MA, Cole C, Mintzes B, Lexchin J, McCormack JP. Drugs in the news: an analysis of Canadian newspaper coverage of new prescription drugs. CMAJ. 2003;168(9):1133-1137. [PMC free article] [PubMed] [Google Scholar]
- 24. Morrato EH, Libby AM, Orton HD, et al. Frequency of provider contact after FDA advisory on risk of pediatric suicidality with SSRIs. Am J Psychiatry. 2008;165(1):42-50. [DOI] [PubMed] [Google Scholar]
- 25. Goldman SA. Communication of medical product risk. Drug Saf. 2004;27(8):519-534. [DOI] [PubMed] [Google Scholar]
- 26. Isacsson G, Rich CL. Antidepressant drugs and the risk of suicide in children and adolescents. Pediatr Drugs. 2014;16(2):115-122. [DOI] [PubMed] [Google Scholar]
- 27. Gibbons RD, Brown CH, Hur K, et al. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356-1363. [DOI] [PubMed] [Google Scholar]
- 28. Lasser KE, Seger DL, Yu DT, et al. Adherence to black box warnings for prescription medications in outpatients. Arch Intern Med. 2006;166(3):338-344. [DOI] [PubMed] [Google Scholar]