Abstract
Objective:
We examined the association between neighborhood disadvantages and percent person-time spent with increased transmission risk (VL>1500 copies/ml) for people living with HIV (PLWH) in South Carolina (SC).
Design:
Observational cohort in SC.
Methods:
Study population included PLWH between 1/1/2014 – 12/31/2017, with two or more VL tests 6 months apart (n=2,076). Proportion of time living with VL>1500 copies/ml after linkage to care was determined. Neighborhood disadvantage was assessed using area deprivation index (ADI). A generalized linear model was fit to generate parameter estimates for time spent with detectable VL.
Results:
Almost half of PLWH (49.5%) lived with VL>1500 copies/ml for some time (median days =46). Young adults and PLWH who injected drugs experienced the highest proportion for time living with detectable VL.
Conclusions:
Targeted programs are needed to improve VL suppression, reduce new transmissions and decrease disparities in HIV outcomes in all neighborhoods.
Keywords: HIV, viral suppression, detectable, care continuum, VIH, supresión viral, cuidado continuo
Resumen
Resumen Objetivo:
Examinamos la asociación entre las desventajas del vecindario y el porcentaje de tiempo que la persona pasa con un mayor riesgo de transmisión (VL> 1500 copias / ml) para las personas que viven con el VIH (PLWH) en Carolina del Sur (SC).
Diseño:
cohorte observacional en SC.
Métodos:
la población de estudio incluyó PVVS entre el 1/1/2014 y el 31/12/2017, con dos o más pruebas de LV con 6 meses de diferencia (n = 2,076). Se determinó la proporción de tiempo viviendo con VL> 1500 copias / ml después de la vinculación a la atención La desventaja del vecindario se evaluó mediante el índice de privación de área (IDA). Se ajustó un modelo lineal generalizado para generar estimaciones de parámetros para el tiempo dedicado con VL detectable.
Resultados:
Casi la mitad de las PLWH (49.5%) vivió con VL> 1500 copias / ml por algún tiempo (días promedio = 46). Los adultos jóvenes y las PVVS que se inyectaron drogas experimentaron la mayor proporción de tiempo viviendo con LV detectable
Conclusiones:
Se necesitan programas específicos para mejorar la supresión de VL, reducir las nuevas transmisiones y disminuir las disparidades en los resultados del VIH en todos los vecindarios.
Introduction
Ending the human immunodeficiency virus (HIV) epidemic in the United States and South Carolina remains unattainable if persons living with HIV (PLWH) remain unaware of their infection, and/or PLWH are unable to sustain HIV viral suppression (1). Policy and programmatic efforts remain focused on closing gaps in the HIV treatment continuum, from early linkage to care, to achieving viral suppression (defined as viral load <= 200 RNA copies/ml). Timely linkage to care and commencement of antiretroviral therapy (ART) remain vital to attaining viral suppression (2). Unfortunately, nearly 25–40% of the PLWHs in the United States do not maintain viral suppression with implications for HIV transmission (3). Studies show about 25–51% of diagnosed PLWH account for about 50% of new HIV transmissions (4–6). PLWH with HIV viral load (VL) above 1500 copies/ml have an increased risk of transmitting HIV to others (7–10,12). Evidence of HIV transmission at lower VL levels is rare, making retention in care and continuous VL suppression crucial to ending the HIV epidemic. Thus, it is important to examine factors associated with PLWH having detectable VL. Considerable interest remains in understanding how social determinants of health affect the HIV treatment continuum. The neighborhood where an individual lives, is a social determinant of health, which profoundly impact the outcomes of the HIV continuum of care (13–15). Higher rates of HIV risk behaviors are seen in populations residing in the most disadvantaged neighborhoods (16, 17), and individuals residing in the most disadvantaged neighborhoods are more likely to experience delayed linkage into HIV care (6). Racial and sexual minorities already disproportionately affected by HIV, may face additional risks based on the neighborhood in which they live (18, 19). The recently released 2013 area deprivation index (ADI) is a geographic area-based measure of socioeconomic deprivation experienced by residents in different neighborhoods. The ranking for the ADI exists in two forms, one assessing the deprivation of a neighborhood relative to the rest of the state (rank 0–10) and one assessing neighborhood deprivation relative to the entire country (percentile score out of 100). The higher the score or percentile, the more disadvantaged the neighborhood is deemed to be (20–23). Recent population health and disease specific studies show the ADI’s value and broad applicability to accounting for neighborhoods as a social determinant of health. The goal of this study was to examine the effects of neighborhood and individual-level characteristics on time spent living with detectable VL (>1500 copies/ml). We hypothesized that individuals living in neighborhoods with higher ADI scores spend a higher proportion of time spent living with detectable VL.
Methods
Study Population
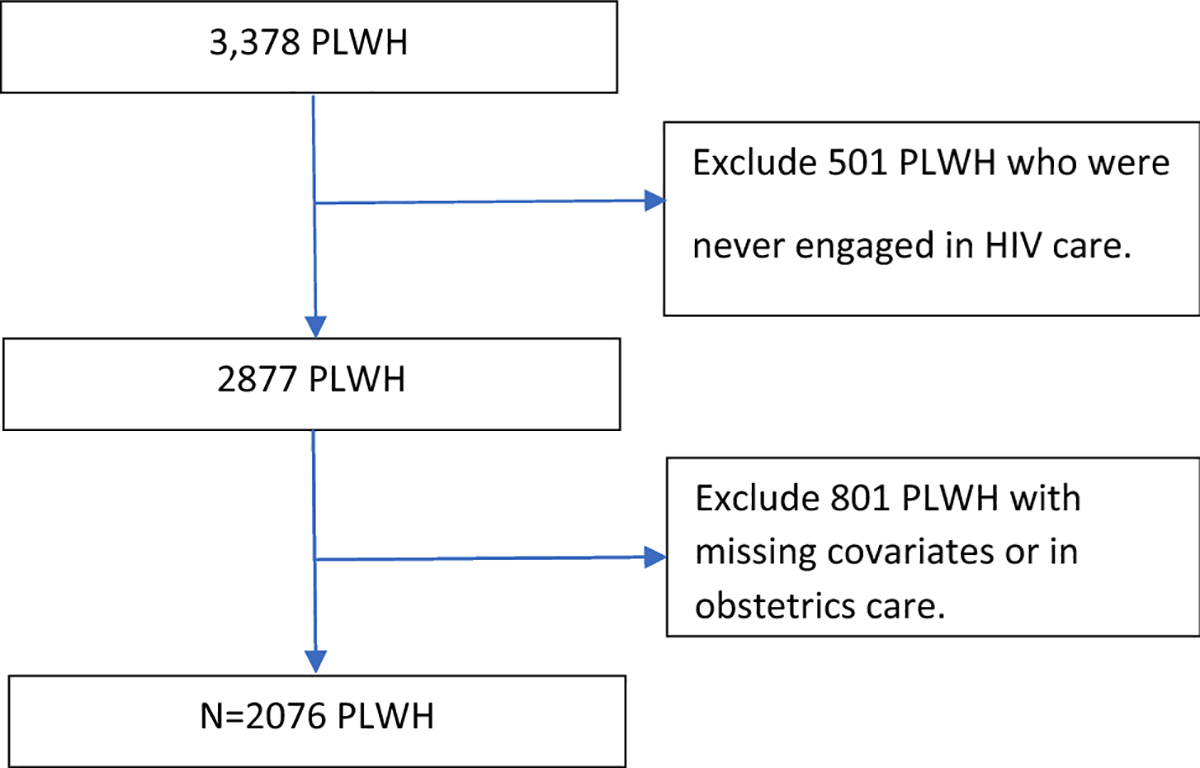
The South Carolina (SC) enhanced HIV/AIDS electronic reporting system (e-HARS database), collected and maintained by the SC Department of Health and Environmental Control (DHEC), was used to examine the effects of neighborhood characteristics and individual-level social determinants on time spent living with VL>1500 copies/ml. There were a total of 3378 newly diagnosed adults (15 years of age or older) in South Carolina between 1/1/2014 and 12/31/2017. The eligible population was restricted to those newly diagnosed with HIV and remained alive between 1 January 2014 and 1 January 2018. Excluded from the analysis were individuals who never engaged in HIV care and women who were pregnant at any time between 1 January 2014 and 31 December 2017. Pregnant women were excluded to avoid obstetric care being a confounder. Additional information about which data were missing and excluded are presented in Figure 1. We analyzed all VL tests up through January 1, 2018. The study population after applying the exclusion criteria was 2,076 (Figure 1).
Figure I: Exclusion criteria flow chart.
Calculation of person-time with viral load (VL) exceeding 1500 copies/ml
We adopted a slightly modified version from Marks et al. to determine the proportion of time in which an individual lived with a VL >1500 copies/ml (6). We chose the 1500 copies/ml as the cut-off value because in several studies it was reported that PLWH with VL>1500 copies/ml had increased risk of transmitting HIV (6–8). Total person time consisted of total observation time in days between first and last viral load test dates. We reviewed all dates and values for all VL tests. If VL test results met or exceeded the threshold of 1500 copies/ml, the person-time in which PLWH had a VL exceeding 1500 copies/ml is the time range between the median of current examination date and previous examination date and median time between current examination date to the next examination date. For example, if there are four test dates as T1, T2, T3, T4, and only T3 has VL test that met or exceeded the threshold of 1500 copies/ml, the person-time in which PLWH had a VL exceeding 1500 copies/ml is (T3-T2)*0.5+(T4-T3)*0.5. Total person time is T4-T1, then the proportion of person-time in which PLWH had a VL exceeding 1500 copies/ml is ((T3-T2)*0.5+(T4-T3)*0.5)/(T4-T1)). If VL test met or exceeded the threshold of 1500 copies/ml at both T2 and T3, the person-time in which PLWH had a VL exceeding 1500 copies/ml is (T2-T1)*0.5+(T3-T2)+(T4-T3)*0.5 (Figure 2). We present findings on person-time (days), and proportion of person-time in which PLWH had a VL exceeding 1500 copies/ml.
Figure II: Schematic for calculating person-time with viral load (VL) exceeding 1500 copies/ml*.
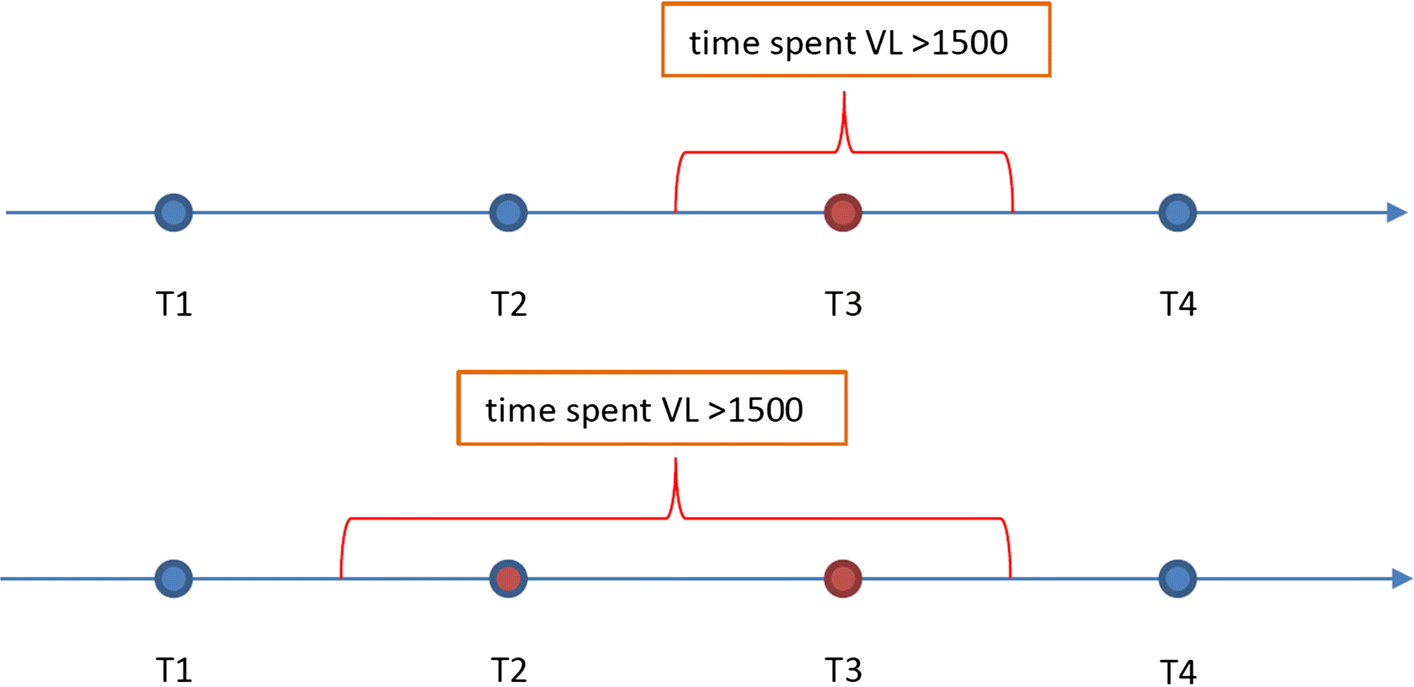
*Red circles represent time period spent living with increased transmission risk (detectable viral load).
Exposure and Outcome Variable
The baseline period for the study is defined as the time of first VL for the PLWH starting on or after 1 January 2014. The outcome variable is the proportion of person-time (days) spent living with VL>1500 copies/ml. It reflects the proportion of time the PLWH spent living with VL >1500 copies/ml relative to the total number of person-days days observed.
Covariates
Individual demographic covariates, as reported in eHARS, included in this analysis were race, [Black, non-Black (White, Hispanic, other race)], age at diagnosis (18–24, 25–50 years and >50 years), gender (male, female), urban or rural residence at last known residence, HIV transmission risk group [heterosexual, men who have sex with men (MSM), injection drug use (IDU), or non-identified risk (NIR)], initial CD4 count (first CD4 in eHARS), time to linkage to care (date of diagnosis to date of first VL or CD4 count) and proportion of time from diagnosis spent living with VL>1500 copies/ml. Proportion of time in-care was defined based on receipt of a CD4 count/VL every three months, accounting for number of years from first HIV medical care visit to the last visit. Rural/urban residence was determined using the Rural Urban Commuting Area (RUCA) codes crosswalk with the zip codes of the PLWH residence (21). Small rural, large rural, and isolated areas were aggregated into rural areas. Neighborhood deprivation scores were determined using the 2013 Area Deprivation Index [ADI] state ranks. The 2013 ADI is a validated index-based measure which uses U.S. Census poverty, education, housing and employment indicators to characterize census-based regions by zip code (22). Several studies have correlated the ADI with health outcomes like Medicare 30-day rehospitalizations, cardiovascular diseases, cancer prevalence and childhood mortality (25–28). Its construction based on the U.S. census block allows for ordered ranking of neighborhoods based on socioeconomic status. The ADI incorporates important census block domain factors such as income, education, employment, and housing quality. For this study, SC ADI state ranking deciles were grouped into three groups: 1 ( ADI ranks 1–3) for least disadvantaged, 2 (ADI ranks 4–7) moderate and 3 (ADI ranks 8–10) most disadvantaged neighborhoods. We used the mode for assigning individual zip codes ranks using the ADI. The combination of the ADI with individual level factors allowed us to measure the impact of social determinants of health on time spent with detectable VL for newly diagnosed adult PLWH in South Carolina (SC). Timely linkage to care was defined as linkage to care within 30 days of HIV diagnosis.
Statistical Analysis
Univariate analyses were performed to compare characteristics of those who spent time with VL>1500 copies/ml and those who did not. Median proportion of time in days spent living with VL>1500 copies/ml were assessed. Medians were preferred to mean values for more precision and to account for outliers. We used the Mann Whitney U nonparametric test to compare medians. We fitted a generalized linear model to identify significant factors association with time spent living with VL >1500 copies/ml. Factors analyzed included demographic factors (age, race, gender HIV risk group, rural or urban residence), neighborhood ADI, and retention in care measures such viral factors such as initial CD4 counts. The proportion of time spent living with VL>1500 copies/ml was modeled as a continuous predictor variable. Generalized linear models were used, controlling for individual VLs, to generate point estimates and 95% confidence intervals (CI) for the proportion of time spent with VL>1500 copies/ml in both univariate and multivariate models. We estimated marginal means using least-square means (LSMEANS). Model selection was based on a combination of p-value less 0.10 (from the univariate model), use of “a priori” characteristics based on literature and model fit statistics. The final multivariable model was adjusted for the main effects of age, gender, race, transmission risk, and neighborhood lived (ADI measure). We conducted a sensitivity analyses and found no effects using deciles for ADI rankings in the adjusted model. We assessed interaction effects between selected variables based on literature and found no significant interactions. All statistical analyses were carried out using SAS version 9.4 (29).
Results
Our study population incudes 2,076 individuals who were newly diagnosed with HIV in SC between January 2014 and December 2017. The mean age of the study population is 34.8 years. Most of the individuals were Black (69.4%), male (79.7%), MSM (57.8%), urban residents (75.1%) and living in the most disadvantaged neighborhoods (37.9%). Table 1 summarizes the study population’s characteristics and compares those who spent any time living with detectable VL and those who did not. Almost half (49.5%), spent time living with detectable VL after linkage to care. On average, individuals spent 18% of their total time after initial linkage to HIV care living with detectable VL. Young age, being Black, timely linkage to care and proportion of time spent in care after diagnosis were significantly associated with time spent living with detectable VL.
Table I:
Characteristics of Newly Diagnosed Adult PLWHs in South Carolina (2014–2017)
| Characteristics | Total N(%) | Detectable N(%) | Undetectable1 N(%) | P-Value |
|---|---|---|---|---|
| N (%) | 2,076 (100) | 1,028 (49.5) | 1,048 (50.5) | |
| Proportion of total time spent with VL>1500 copies/ml Mean [SD] | ||||
| 0.09 [0.15] | 0.18 [0.17] | NA | ||
| Age Group | 0.0032 | |||
| 18–25 years | 652 (31.4) | 349 (34.0) | 303 (28.9) | |
| 26–50 years | 1,107(53.3) | 546 (53.1) | 561 (53.5) | |
| >50 years | 317 (15.3) | 133 (12.9) | 184 (17.6) | |
| Sex | 0.5830 | |||
| Male | 1,654 (79.7) | 814 (79.2) | 840 (80.2) | |
| Female | 422 (16.5) | 214 (20.8) | 208 (19.8) | |
| Race | 0.0199 | |||
| Black | 1,441 (69.4) | 738 (71.8) | 703 (67.1) | |
| Non-Black | 635 (30.6) | 290 (28.2) | 345 (32.9) | |
| State Neighborhood ADI Rank2 | 0.5854 | |||
| Least Disadvantaged Neighborhood | 546 (26.3) | 264 (25.7) | 282 (26.9) | |
| Moderate Disadvantaged Neighborhood | 743(35.8) | 379 (36.9) | 364 (34.7) | |
| Most Disadvantaged Neighborhood | 787 (37.9) | 385 (37.4) | 402 (38.4) | |
| Residence | 0.8801 | |||
| Rural | 516 (24.9) | 257 (25.0) | 259 (24.7) | |
| Urban | 1,560 (75.1) | 771 (75.0) | 789 (75.3) | |
| Transmission Risk Group | 0.1013 | |||
| Non-identified risk (NIR) | 544 (26.20) | 264 (25.7) | 280 (26.7) | |
| Heterosexual | 273 (13.2) | 131 (12.7) | 142 (13.6) | |
| Men who have sex with men (MSM) | 1,199 (57.8) | 594 (57.8) | 605 (57.7) | |
| Injection drug user (IDU) | 60 (2.9) | 39 (3.8) | 20 (2.00) | |
| Proportion of Time In-Care3 | <0.0001 | |||
| 0–25% | 375 (18.1) | 98 (9.5) | 277 (26.4) | |
| 26–75% | 188 (9.1) | 133 (12.9) | 55 (5.3) | |
| 100% | 1,513 (72.8) | 797 (77.5) | 716 (68.3) | |
| Initial CD4+ Count | 0.2956 | |||
| ≤ 200 cells/mm3 | 605 (29.1) | 289 (28.1) | 316 (30.1) | |
| 200–500 cells/mm3 | 930 (44.8) | 464 (45.1) | 466 (44.5) | |
| > 500 cells/mm3 | 541 (26.1) | 275 (26.7) | 266 (25.4) | |
| Timely Linkage to care | 0.0335 | |||
| No | 494 (23.8) | 224 (21.8) | 270 (25.8) | |
| Yes | 1,582 (76.2) | 804 (78.2) | 778 (74.2) |
Undetectable People Living with HIV (PLWHs) were defined as those who had every viral load test not detectable.
The ADI neighborhood index pertains to zip codes and is a geographic area-based measure of socioeconomic deprivation experienced by residents in different neighborhoods.
Proportion of time spent in-care represents duration in-care relative to time since first diagnosis.
Profile of days spent living with increased transmission risk (detectable viral loads)
Among the 1,028 PLWH with time spent with VL >1500 copies/ml, the median days spent living with VL > 1500 copies/ml was 46 days. Median days living with VL > 1500 copies/ml was greatest among those aged 18–25 years (61days), males (46 days) and Blacks (46 days). Individuals living in the moderate socioeconomically disadvantaged neighborhoods spent the least time living with VL > 1500 copies/ml (mean 31 days). We did not find a difference in median time living with VL > 1500 copies/ml between rural and urban residents (46 days). Further examination showed statistically significant differences in median days by transmission risk, and proportion of time spent in-care. Individuals reporting an HIV transmission risk group of IDU spent the longest time living with VL > 1500 copies/ml, means 76 days. Median number of VL tests was 7 during the study period. Heterosexuals (8 tests) and MSM (7 tests) recorded the largest number of VL tests (Table 2).
Table II:
Characteristics and Profile of Time Spent Living with Increased Transmission Risk (Detectable Viral Load) by Newly Diagnosed Adult PLWHs in South Carolina
| Characteristic | Detectable (n=1,028) | |||
|---|---|---|---|---|
| Median days | p-Value | Median # viral load tests | p-Value | |
| Age Group | <0.0001 | 0.3342 | ||
| 18–25 years | 61 | 6 | ||
| 26–50 years | 46 | 7 | ||
| >50 years | 31 | 7 | ||
| Sex | 0.6905 | 0.1826 | ||
| Male | 46 | 7 | ||
| Female | 45 | 7 | ||
| Race | 0.0022 | 0.2565 | ||
| Black | 46 | 7 | ||
| Non-Black | 31 | 7 | ||
| State Neighborhood ADI Rank | 0.0723 | 0.1498 | ||
| Least Disadvantaged Neighborhood | 46 | 7 | ||
| Moderate Disadvantaged Neighborhood | 31 | 6 | ||
| Most Disadvantaged Neighborhood | 46 | 7 | ||
| Residence | 0.1335 | 0.3516 | ||
| Rural | 46 | 7 | ||
| Urban | 46 | 7 | ||
| Transmission Risk Group | 0.0268 | 0.0435 | ||
| Non-identified risk (NIR) | 31 | 6 | ||
| Heterosexual | 46 | 8 | ||
| Men who have sex with men (MSM) | 42 | 7 | ||
| Injection drug user (IDU) | 76 | 6 | ||
| Proportion of Time In-Care | <0.0001 | <0.0001 | ||
| 0–25% | 31 | 2 | ||
| 25–75% | 213 | 7 | ||
| 100% | 45 | 7 | ||
| Initial CD4+ Count | 0.2771 | <0.0001 | ||
| ≤ 200 cells/mm3 | 31 | 8 | ||
| 200–500 cells/mm3 | 46 | 6 | ||
| > 500 cells/mm3 | 46 | 7 | ||
| Timely Linkage to care | 0.0955 | 0.1960 | ||
| No | 60 | 6 | ||
| Yes | 46 | 7 | ||
PLWH: People Living with HIV
Predictors of time spent living with viral load above 1500 copies/ml
Multivariable analyses showed age, race, transmission risks and proportion of time spent in care as significant predictors of time spent living with VL > 1500 copies/ml. Table 3 shows unadjusted and adjusted coefficients for the proportion of time spent living with detectable VL. Compared to individuals older than 50 years, younger individuals, 18–24 years [β=3.86; P<0.05] and 25–50 years [β=2.23; P =0.01] spent significantly higher proportion of time living with detectable VL. Blacks, compared to non-blacks, [β=1.72; P = 0.01] also spent higher proportions of time living with detectable VL. Among transmission risk groups, IDU [β=5.89; P <0.05]) spent significantly higher proportions of time living with detectable VL. The proportion of time in care was the strongest predictor for time spent living with VL > 1500 copies/ml. Compared to those with longer time in care, PLWH who were in care for 25–75% of time spent the longest time living with VL >1500 copies/ml [β=10.77; P <0.05]. We did not find statistically significant differences in time living with VL >1500 copies/ml by ADI. Table 3 shows the unadjusted and adjusted predictors of time spent living with VL >1500 copies/ml. We conducted a sensitivity analysis by state rank in deciles and found little change in predictors of time spent living with VL >1500 copies/ml.
Table III.
Generalized Linear Model for percentage person- time spent living with increased transmission risk among Adult PLWHs in South Carolina (2014–2017)
| Characteristics | Full Model (N=2,076) | |||||
|---|---|---|---|---|---|---|
| Unadjusted Coeff. | Std. Error | p-Value | Adjusted Coeff. | Std. Error | p-Value | |
| Intercept | - | - | - | 4.39 | 1.43 | 0.0021 |
| Age Group | ||||||
| 18–25 years | 4.70 | 1.08 | <0.0001 | 4.42 | 1.09 | <0.0001 |
| 26–50 years | 2.76 | 1.00 | 0.0058 | 2.81 | 0.95 | 0.0031 |
| >50 years | Ref. | Ref. | ||||
| Sex | ||||||
| Male | Ref. | Ref. | ||||
| Female | −0.06 | 0.86 | 0.9411 | 0.07 | 1.04 | 0.9468 |
| Race | ||||||
| Black | Ref. | Ref. | ||||
| Non-Black | 2.71 | 0.76 | 0.0004 | 1.58 | 0.73 | 0.0308 |
| State Neighborhood ADI Rank | ||||||
| Least Disadvantaged Neighborhood | Ref. | Ref. | ||||
| Moderate Disadvantaged Neighborhood | 0.06 | 0.89 | 0.9445 | −0.49 | 0.83 | 0.5566 |
| Most Disadvantaged Neighborhood | 1.08 | 0.89 | 0.2233 | 0.46 | 0.87 | 0.5998 |
| Residence | ||||||
| Urban | Ref. | Ref. | ||||
| Rural | 1.05 | 0.82 | 0.1969 | 0.53 | 0.81 | 0.5119 |
| Transmission Risk Group | ||||||
| Non-identified risk (NIR) | Ref. | Ref. | ||||
| Heterosexual | −1.35 | 1.17 | 0.2501 | −1.60 | 1.13 | 0.1564 |
| Men who have sex with men (MSM) | −1.13 | 0.83 | 0.1723 | −1.79 | 0.93 | 0.0542 |
| Injection drug user (IDU) | 5.55 | 2.13 | 0.0091 | 5.69 | 2.01 | 0.0046 |
| Proportion of Time In-Care | Ref. | Ref. | ||||
| 0–25% | 15.93 | 1.10 | <0.0001 | 15.58 | 1.10 | <0.0001 |
| 25–75% | 11.25 | 1.10 | <0.0001 | 10.72 | 1.10 | <0.0001 |
| 100% | Ref. | Ref. | ||||
| Initial CD4+ Count | ||||||
| ≤ 200 cells/mm3 | Ref. | Ref. | ||||
| 200–500 cells/mm3 | 1.10 | 0.83 | 0.1873 | 0.04 | 0.80 | 0.9630 |
| > 500 cells/mm3 | 0.97 | 0.94 | 0.3053 | 0.14 | 0.90 | 0.8745 |
| Timely Linkage to care | ||||||
| No | Ref. | Ref. | ||||
| Yes | −0.94 | 0.81 | 0.2508 | −0.40 | 0.76 | 0.6010 |
PLWH: People Living with HIV
Discussion
Detectable HIV viral load is an important indicator for future high-risk transmissions. This analysis of 2,076 newly diagnosed, non-pregnant PLWH in SC who are linked to HIV medical care found that a considerable number of PLWH remain at risk of transmitting HIV infection based on the percentage of time spent with viral load above 1500 copies/ml. Our study’s finding of mean percentage time spent living with VL above 1500 (18%) is similar to findings previously reported by Mendoza et al. (14.4%) (9), Lesko et al. (12.6% - 27.2%) (8) and Crepaz et al. (19%) (3), but slightly lower compared to findings of Marks et al. (26.3%) (6). Possible explanations for the reduced time spent living with VL >1500 copies/ml in the current study are improved antiretroviral therapies and updated treatment guidelines emphasizing treatment for all (9). However, previous studies also show that PLWH who are in care but not virally suppressed pose an increased risk for transmission (33–34). We did not find an association with neighborhood ADI rank and proportion of time spent with detectable VL. A prior study showed that neighborhoods are important social determinants of health but did not account for the socioeconomic environment (15). South Carolina’s adult non-pregnant PLWHs living in the most socioeconomically disadvantaged areas and least disadvantaged neighborhoods both had the most median days living with detectable VL.
Future studies are needed to identify the true effects and impact neighborhood socioeconomic disadvantages have even after newly diagnosed PLWH are linked to and engaged in HIV care. The finding of no significant difference in time spent with undetectable between the most disadvantaged and least disadvantaged neighborhoods is supported by recent literature (35). The lack of variation may be because PLWH in most disadvantaged neighborhoods are more likely to receive Ryan White services because of focused targeting of HIV care and case management services (36). Conversely, PLWH in most disadvantaged neighborhoods may be more likely to never have linked to care. While those that did link to care have a “resilience” that is not captured through a neighborhood measure like the ADI but predicts better HIV outcomes. It could also be that the neighborhood ADI may not be a good predictor of HIV outcomes. For the least disadvantaged neighborhoods, often there is a mixed population, some with more, others with less resources, thus differences are not clearly seen. The impact of neighborhood is further compounded with the presence of traditional social determinants for living with increased transmission risk (detectable VL) such as younger age, length of time in HIV care, and IDU.
As the field continues towards ending the epidemic or launching a new “Undetectable=Untransmittable [U=U]” campaign, further exploration is needed to account for non-traditional predictors such as neighborhood disadvantage. In addition, focus is needed on elucidating possible reasons for why 49.5% of a population in HIV medical care still live about 18% of their time in care with detectable VL. Our analysis did not consider disengaging patients (e.g. those with two or three VL results who dropped out of HIV medical care). These individuals probably have a VL greater than 1500 copies/ml and may be actively transmitting the virus in their neighborhoods. Therefore, our findings probably underestimate the percent of person-time in which newly HIV diagnosed non-pregnant adult SC PLWH live with detectable viral load above 1500 copies/ml.
Limitations
This study has a few limitations. The ADI used for this study does not account for PLWHs migrating in and out of neighborhoods. Such migration patterns could lead to an overestimation of population-level increased transmission risk. Although residential mobility could have affected the data, we believe that it is highly unlikely that significant proportions of newly diagnosed non-pregnant adult PLWH changed residence often between 2014 −2018. Second, we could not account for those who were never engaged in HIV medical care. The factors affecting the levels of increased transmission risk for that population may be different from our findings. Third, we could not ascertain viral load for those who dropped out of care after linkage to care beyond the available test results. Finally, since we were limited to using only HIV surveillance data, we could not include specific ART drug regimen/polypharmacy as independent variables during our analysis. Therefore, we cannot assess the extent to which the proportion of time spent living with detectable VL was a consequence of ART regimen or inappropriate HIV medical care. However, while we could not access individual health utilization data, the main goal for HIV care is viral suppression, which is continuously reported to the SC eHARS and accessible for this study.
Conclusions
Our analyses have several important implications for surveillance, clinical practices, prevention and care efforts. First, the evidence shows that the proportion of time spent in care is important for living with detectable VL. Second, residents living in moderately disadvantaged areas may do better based on median days spent living with detectable VL in South Carolina, it may be useful to examine the factors and resources that reduces the time spent living with detectable VL for this group. Third, IDU who are a significant transmission risk group spend significant amount of time living with detectable VL. Clinicians and case management workers serving patients across all neighborhoods need to focus treatment, prevention efforts and outreach efforts to reach these groups who are most at risk for living with detectable VL.
Supplementary Material
Acknowledgements
The authors thank South Carolina Department of Health and Environmental Control (DHEC) for providing the data on People Living with HIV in South Carolina. This study is supported by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Number 1R01AI127203-01A1. Drs. Li and Olatosi are the PI for the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: All authors declare that they have no conflict of interest.
Ethical Approval: Ethical approval for this study has been obtained from the University of South Carolina Institutional Review Board.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Centers for Disease Control and Prevention (CDC). Vital signs: HIV prevention through care and treatment--United States. MMWR Morbidity and mortality weekly report. 2011;60(47):1618. [PubMed] [Google Scholar]
- 2.DHHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [Google Scholar]
- 3.Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012–2013. Clinical Infectious Diseases. 2016;63(7):976–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall HI, Holtgrave DR, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. Aids. 2012;26(7):893–6. [DOI] [PubMed] [Google Scholar]
- 5.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. Aids. 2006;20(10):1447–50. [DOI] [PubMed] [Google Scholar]
- 6.Marks G, Gardner LI, Rose CE, Zinski A, Moore RD, Holman S, et al. Time above 1500 copies: a viral load measure for assessing transmission risk of HIV-positive patients in care. AIDS (London, England). 2015;29(8):947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. Aids. 2009;23(11):1397–404. [DOI] [PubMed] [Google Scholar]
- 8.Lesko CR, Lau B, Chander G, Moore RD. Time Spent with HIV Viral Load> 1500 Copies/mL Among Persons Engaged in Continuity HIV Care in an Urban Clinic in the United States, 2010–2015. AIDS and Behavior. 2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendoza MC, Gardner L, Armon C, Rose CE, Palella FJ Jr, Novak RM, et al. Time spent with HIV viral load above 1500 copies/ml among patients in HIV care, 2000–2014. AIDS. 2018;32(14):2033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. New England journal of medicine. 2000;342(13):921–9. [DOI] [PubMed] [Google Scholar]
- 11.Ragni MV, Faruki H, Kingsley LA. Heterosexual HIV-1 transmission and viral load in hemophilic patients. Journal of acquired immune deficiency syndromes and human retrovirology: official publication of the International Retrovirology Association. 1998;17(1):42–5. [DOI] [PubMed] [Google Scholar]
- 12.Tovanabutra S, Robison V, Wongtrakul J, Sennum S, Suriyanon V, Kingkeow D, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. Journal of acquired immune deficiency syndromes (1999). 2002;29(3):275–83. [DOI] [PubMed] [Google Scholar]
- 13.Burke-Miller JK, Weber K, Cohn SE, Hershow RC, Sha BE, French AL, et al. Neighborhood community characteristics associated with HIV disease outcomes in a cohort of urban women living with HIV. AIDS care. 2016;28(10):1274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castel AD, Befus M, Willis S, Griffin A, West T, Hader S, et al. Use of the community viral load as a population-based biomarker of HIV burden. Aids. 2012;26(3):345–53. [DOI] [PubMed] [Google Scholar]
- 15.Shacham E, Lian M, Önen N, Donovan M, Overton E. Are neighborhood conditions associated with HIV management? HIV medicine. 2013;14(10):624–32. [DOI] [PubMed] [Google Scholar]
- 16.Latkin CA, Curry AD, Hua W, Davey MA. Direct and indirect associations of neighborhood disorder with drug use and high-risk sexual partners. American journal of preventive medicine. 2007;32(6):S234–S41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maas B, Fairbairn N, Kerr T, Li K, Montaner JS, Wood E. Neighborhood and HIV infection among IDU: place of residence independently predicts HIV infection among a cohort of injection drug users. Health & Place. 2007;13(2):432–9. [DOI] [PubMed] [Google Scholar]
- 18.Acevedo-Garcia D, Lochner KA, Osypuk TL, Subramanian SV. Future directions in residential segregation and health research: a multilevel approach. American journal of public health. 2003;93(2):215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Biello KB, Niccolai L, Kershaw TS, Lin H, Ickovics J. Residential racial segregation and racial differences in sexual behaviours: an 11-year longitudinal study of sexual risk of adolescents transitioning to adulthood. J Epidemiol Community Health. 2013;67(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boutrous ML, Ta AL, Tian Y, Freeman CA, Smeds MR. Area Deprivation Index Score Is Associated With Lower Rates of Long-Term Follow-up After Upper Extremity Vascular Injuries. Journal of Vascular Surgery. 2018;68(3):e63–e4. [DOI] [PubMed] [Google Scholar]
- 21.WWAMI RUCA Map Classifications Available at: https://depts.washington.edu/uwruca/. Accessed October 17, 2019.
- 22.Health UoWSoMaP. Area Deprivation Index 2018. Available at: https://www.neighborhoodatlas.medicine.wisc.edu/. Accessed July 12, 2018
- 23.Hu J, Kind AJ, Nerenz D. Area Deprivation Index Predicts Readmission Risk at an Urban Teaching Hospital. American Journal of Medical Quality. 2018:1062860617753063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tuliani TA, Shenoy M, Parikh M, Jutzy K, Hilliard A. Impact of Area Deprivation Index on Coronary Stent Utilization in a Medicare Nationwide Cohort. Population health management. 2017;20(4):329–34. [DOI] [PubMed] [Google Scholar]
- 25.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, et al. Neighborhood socioeconomic disadvantage and 30 day rehospitalizations: an analysis of Medicare data. Annals of internal medicine. 2014;161(11):765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh GK. Area deprivation and widening inequalities in US mortality, 1969–1998. American journal of public health. 2003;93(7):1137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh GK, Siahpush M. Increasing inequalities in all-cause and cardiovascular mortality among US adults aged 25–64 years by area socioeconomic status, 1969–1998. International Journal of Epidemiology. 2002;31(3):600–13. [DOI] [PubMed] [Google Scholar]
- 28.Sundquist K, Malmström M, Johansson S, Sundquist J. Care Need Index, a useful tool for the distribution of primary health care resources. Journal of Epidemiology & Community Health. 2003;57(5):347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Insititute. SAS 9.4 for Windows. SAS Institue Inc, Cary, NC, USA. 2012. [Google Scholar]
- 30.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. The Lancet. 2009;373(9657):48–57. [DOI] [PubMed] [Google Scholar]
- 31.Walensky RP, David Paltiel A, Losina E, Morris BL, Scott CA, Rhode ER, et al. Test and treat DC: forecasting the impact of a comprehensive HIV strategy in Washington DC. Clinical Infectious Diseases. 2010;51(4):392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryan White Care RYW eligibility 2018. Available at: https://hab.hrsa.gov/sites/default/files/hab/Global/pcn1303eligibilityconsiderations.pdf Accessed July 12, 2018
- 33.Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable viral suppression and transmission risk potential among persons with diagnosed HIV infection: United States, 2012– 2013. Clinical Infectious Diseases. 2016; 63:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crepaz N, Tang T, Marks G, Hall HI. Viral suppression patterns among persons in the United States with diagnosed HIV infection in 2014. Annals of Internal Medicine 2017; 167:446–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rebeiro PF, Howe CJ, Rogers WB, Bebawy SS, Turner M, Kheshti A, … & Sterling TR (2018). The relationship between adverse neighborhood socioeconomic context and HIV continuum of care outcomes in a diverse HIV clinic cohort in the Southern United States. AIDS care, 30(11), 1426–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crockett KB, Edmonds A, Johnson MO, Neilands TB, Kempf MC, Konkle-Parker D, … & Logie CH (2019). Neighborhood Racial Diversity, Socioeconomic Status, and Perceptions of HIV-Related Discrimination and Internalized HIV Stigma Among Women Living with HIV in the United States. AIDS patient care and STDs, 33(6), 270–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


