Abstract
Background:
Differentiating multiple sclerosis (MS) from vascular risk factor (VRF)-small vessel disease (SVD) can be challenging.
Objective and Methods:
In order to determine whether or not pontine lesion location is a useful discriminator of MS and VRF-SVD, we classified pontine lesions on brain magnetic resonance imaging (MRI) as central or peripheral in 93 MS cases without VRF, 108 MS patients with VRF and 43 non-MS cases with VRF.
Results:
MS without VRF were more likely to have peripheral pons lesions (31.2%, 29/93) than non-MS with VRF (0%, 0/43) (Exp(B) = 29.8; 95% confidence interval (CI) = (1.98, 448.3); p = 0.014) but there were no significant differences regarding central pons lesions between MS without VRF (5.4%, 5/93) and non-MS with VRF patients (16.3%, 7/43) (Exp(B) = 0.89; 95% CI = (0.2, 3.94); p = 0.87). The presence of peripheral pons lesions discriminated between MS and VRF-SVD with 100% (95% CI = (91.8, 100)) specificity. The proportion of peripheral pons lesions in MS with VRF (30.5%, 33/108) was similar to that seen in MS without VRF (31.2%, 29/93, p = 0.99). Central lesions occurred in similar frequency in MS with VRF (8.3%, 9/108) and non-MS with VRF (16.3%, 7/43, p = 0.15).
Conclusion:
Peripheral pons lesion location is a good discriminator of MS from vascular lesions.
Keywords: Multiple sclerosis, cerebral small vessel disease, imaging, differential diagnosis
Introduction
Lesions located at the periphery of the pons have been described in multiple sclerosis (MS),1 and in theory, due to a rich vascularization, this area is less prone to vascular risk factor (VRF)-related small vessel disease (SVD). On the contrary, the central pons is supplied by perforating end-arterioles and is prone to ischaemic hypoxia and demyelination which underlies T2 magnetic resonance imaging (MRI) hyperintensities seen in this area in patients with VRF-SVD.2 However, these assumptions are based on limited observations and the role of these markers in discriminating MS from SVD-related lesions has not been tested so far. We aim to explore whether VRFs and MS associate with pontine lesions in different locations, defined as either central or peripheral, and to determine the specificity of pontine lesions in these locations for MS and VRF-SVD.
Materials and methods
Study design and cohorts
A multicentre, cross-sectional (2006–2017) comparative study assessed people with MS (2010 McDonald criteria3) without VRF (N = 93, mean age = 47.2 ± 8.2 years, 63.4% females), with VRF (N = 108, mean age = 48.5 ± 8.5 years, 62.0% females) and a non-MS group with VRF (N = 43, mean age = 58.5 ± 13.8 years, 61.7% females). The MS patients were seen in five MAGNIMS network centres (www.magnims.eu) (Amsterdam, Barcelona, Graz, London, Rome) and the non-MS cases derived from brain imaging studies on diabetic cohorts from two of these centres (Amsterdam and Barcelona). The VRF cases were asymptomatic because patients with transient ischemic attack (TIA) and stroke were excluded, and the MS cases were imaged outside of relapse and did not have new relevant symptoms at the time of MRI although the detailed chronic symptoms of each MS patient was not available.
Cases with known brain lesions unrelated to either MS or SVD were excluded. Anonymized clinical data were collected: sex, age and disease duration at the time of MRI scan, presence of the following VRFs (yes/no): (1) arterial hypertension (HT) (ever); (2) dyslipidaemia (ever); (3) diabetes mellitus (DM); and (4) self-reported smoking status – yes, if patients smoked more than 10 cigarettes a day for at least 6 months. Cases were considered to be in the VRF group when one or more of the above VRFs were present.
Visual scoring
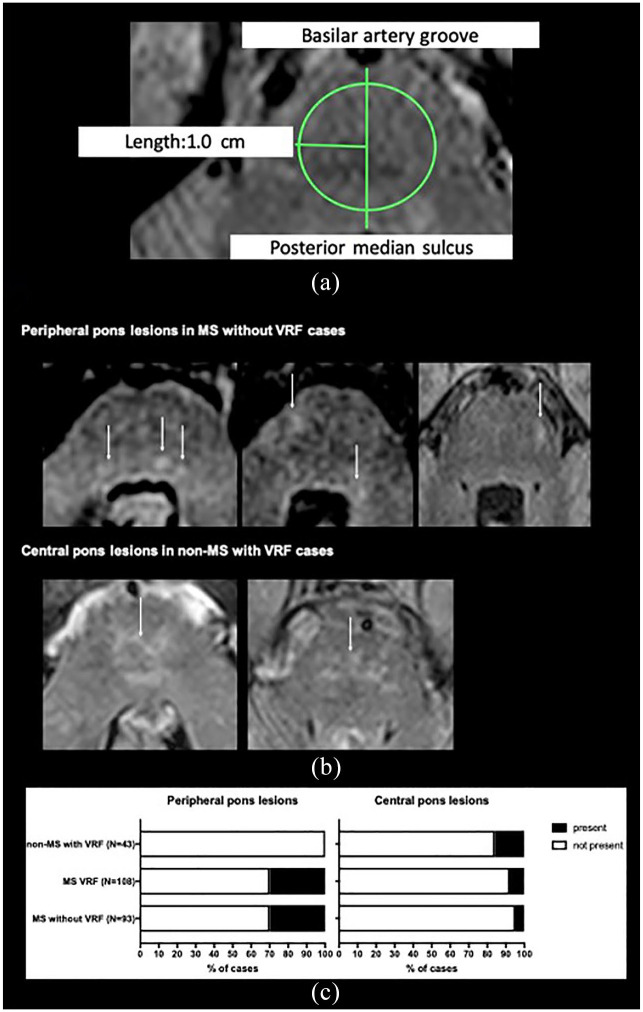
Investigators (R.G., M.J., G.R.d.P.) were blinded to clinical information, performed visual assessment of the brain MRIs (3T) using the following sequences: 2D/3D T2-weighted fluid-attenuated inversion recovery and or 2D T2-weighted fast spin echo (Supplemental Table 1). The total number of infratentorial lesions – defined as any lesion in the brainstem or cerebellum – was counted. When present, pontine lesions were classified as central if they were present with a 1.0 cm radius of the pons midpoint or peripheral if beyond that circle, as depicted in Figure 1(a).
Figure 1.
(a) Pons lesions were classified as central if they were present with a 1.0 cm radius of the midpoint (midpoint of a line from the basilar groove to the posterior median sulcus, as seen on axial sections) of the pons, or peripheral if beyond that circle. If crossing the circle line, lesions would be considered central if more than ¾ of the lesion is inside the circle.a,b (b) Examples of the patterns of pons lesion location in MS without VRF and non-MS with VRF cases are shown, white arrows pointing to peripheral and central pons lesions, respectively. (c) Proportion of peripheral and central pons lesions in MS only, MS with VRF and VRF only groups.
aConsidering that pontine anterior–posterior length did not differ between 10 MS only and 10 VRF only cases, the authors used an absolute cut-off instead of a discriminatory cut-off. A discriminatory threshold, independent of pontine size, may be preferable in cohorts with significant pontine size variability.
bRaters blinded to clinical information, were given a mixture of MS with and without VRF and non-MS with VRF, and were instructed to ignore the supratentorial areas.
All MRI and clinical data sets had initially been collected, anonymized and stored at the contributing sites in accordance with the local research ethics regulations.
Statistical analysis
Agreement was measured as previously reported.4 The number of infratentorial lesions and the proportion of cases with peripheral and central pontine lesions were compared between MS without VRF, MS with VRF, and non-MS with VRF. Variables are presented as median and interquartile range (IQR). Chi-square or Fisher’s exact test and nonparametric tests were applied as appropriate. Considering potential differences between MAGNIMS sites, a ‘site’ factorial variable was built. Comparisons between MS without VRF and non-MS with VRF regarding the presence of central and peripheral pons lesions, adjusting for age and site, were made using a generalized linear model with methods for bias reduction and maximum penalized likelihood; results are presented as odds ratios (exponential coefficients EXP(B)) and 95% confidence intervals (CI). A p value < 0.05 was considered statistically significant. The specificity (number of true negative cases/number of false positive cases + true negative cases) of peripheral pontine lesions for MS and of central pontine lesions for VRF-SVD was assessed by comparing MS without VRF and non-MS with VRF cases. Specificity and 95% CI were calculated using MEDCALC statistical software. All other analyses were performed with SPSS version 25, R software version 3.6.2, and GraphPad Prism version 7.
Results
The VRF profile of the non-MS cases was as follows: 100% with DM, 41.9% ever-smokers, 23.3% with HT, 25.6% with dyslipidaemia; 37.2% with more than one VRF; and in the MS with VRF: 78.7% ever-smokers, 40.7% with HT, 23.1% with dyslipidaemia, and 9.2% with DM; 25.9% with more than one VRF. The non-MS with VRF group was older (N = 43, mean age = 58.5 ± 13.8 years) than the MS without VRF group (N = 93, mean age = 47.2 ± 8.2 years) (p < 0.001), but age did not differ between MS without and MS with VRF (N = 108, mean age = 48.5 ± 8.5 years) (p = 0.51).
Moderate agreement (0.5) was found for the total exact number of infratentorial lesions and substantial for pontine lesion location (0.61). Infratentorial lesions were more commonly seen in people with MS without VRF (52.7%, 49/93, per patient: median number = 1, IQR = 2) and with MS with VRF (50.9%, 55/108, per patient: median number = 1, IQR = 2) compared to non-MS with VRF cases (20.9%, 9/43, per patient: median number = 0, IQR = 0), p = 0.001.
Peripheral pontine lesions (Figure 1(b)) were observed in 31.2% (29/93) MS without VRF patients and in none of non-MS with VRF cases (p < 0.0001), thus showing 100% specificity for MS (95% CI = (91.78, 100)) (Figure 1(c)).
Central pontine lesions (Figure 1(b)) were seen in 16.3% (7/43) of non-MS with VRF cases and only in 5.4% (5/93) of MS without VRF cases (p = 0.02) (Figure 1(c)), showing a 94.6% (95% CI = (87.9, 98.2)) specificity for VRF-SVD. The proportion of central pontine lesions was not different in MS with VRF cases (8.3%, 9/108) compared with MS without VRF (5.4%, 5/93), (p = 0.57).
VRFs in MS did not impact the presence of peripheral pontine lesions which were seen in 30.5% (33/108) of MS with VRF and in 31.2% (29/93) of MS without VRF (p = 0.99). The proportion of central pontine lesions in MS with VRF (8.3% (9/108)) did not significantly differ from non-MS with VRF (16.3%, 7/43), p = 0.15 (Figure 1(c)).
In a model including age and site as covariates, MS without VRF were more likely to have peripheral pons lesions than non-MS with VRF (Exp(B) = 29.8; 95% CI = (1.98, 448.3); p = 0.014). Using the same model, there were no significant differences regarding central pons lesions between MS without VRF and VRF patients (Exp(B) = 0.89; 95% CI = (0.2, 3.94); p = 0.87).
Discussion
Differentiating MS lesions from SVD lesions on MRI may be challenging, especially in cases with atypical clinical presentations5 and where both MS and VRFs are present.
Presence of infratentorial (brainstem and cerebellum) lesions is one of the criteria required to demonstrate dissemination in space according to the McDonald diagnostic criteria for MS,6 but few studies have described the characteristics of brainstem lesions. Half of our MS cohort showed one or more lesions in this location. The frequency of brainstem lesions has been reported to range from 6% to 82% in MS case series with specific clinical symptoms and a tendency for lesions to occur closer to the ventricular surface or the periphery of the pons especially where cranial nerves emerge.1,7–10 We have directly compared pontine lesions locations in MS and SVD-VRF cases and have shown that the presence of peripheral pons lesions (seen in about one-third of people with MS) is a useful discriminator for MS from VRF-associated lesions. Furthermore, the presence of concomitant VRF in MS does not appear to associate with increased peripheral pons lesions, further suggesting the specificity of this finding. Central pontine lesions can be seen both in MS and with VRF-related SVD. In our study, there was a poor age and VRF matching between MS (mainly smokers) and non-MS groups (all diabetic), and the frequency of central pontine lesions in age-matched healthy controls and age- and VRF-matched asymptomatic and symptomatic patients with VRF needs to be further explored. Nevertheless, our findings seem to be consistent despite the heterogeneity of MRI protocols across centres, which were not systematically different between MS and VRF groups (see Supplementary Table 1), suggesting that they may be applicable in the clinical setting. The fact that extra-pontine areas were not masked while scoring pons lesions is a limitation although the scorers were aware that mixed lesion groups were included making it impossible for them to be sure whether patients had VRF or not. Despite the limitations, our data suggest that peripheral pontine lesions may have a different pathogenesis from central lesions, some of which may be related to vascular disease. In clinical practice, new lesions in MS are assumed to be related to disease activity, and differentiating new vascular from inflammatory lesions would be useful in decisions around escalating disease-modifying treatments. Prospective studies are warranted to confirm pontine lesion location as a discriminator of MS from vascular to determine the impact of specific VRF on lesion accumulation in MS.
Supplemental Material
Supplemental material, MSJ943777_supplemental_material for The role of pontine lesion location in differentiating multiple sclerosis from vascular risk factor-related small vessel disease by Ruth Geraldes, Maciej Juryńczyk, Giordani Rodrigues dos Passos, Alexander Pichler, Karen Chung, Marloes Hagens, Serena Ruggieri, Cristina Auger, Jaume Sastre-Garriga, Christian Enzinger, Declan Chard, Frederik Barkhof, Claudio Gasperini, Alex Rovira, Gabriele DeLuca and Jacqueline Palace in Multiple Sclerosis Journal
Acknowledgments
The authors are grateful to Adriana Roca-Fernández for her support with the statistical analysis. Statistical analysis was conducted by Dr R.G., MD, Nuffield Department of Clinical Neurosciences, John Radcliffe Hospital, Oxford, UK.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: R.G. has received travel expenses from Bayer, Biogen, Merck, Teva, Novartis and Wolfson College; has also received honoraria for advisory work and as an invited speaker for Biogen, Novartis and the EAN. M.J. reports no disclosures. G.R.d.P. has received scholarships from the ECTRIMS, the World Federation of Neurology and Novartis; funding for research from Biogen, Novartis and Roche; travel grants from Roche, Sanofi-Genzyme and Teva; and fees for editorial content from Bayer, Merck Serono and Roche. A.P. reports no disclosures. K.C. has received honoraria for speaking at meetings, and advisory work or support to attend meetings from Teva, Biogen Idec and Roche. M.H. reports no disclosures. S.R. reports no disclosures. C.A. has received honoraria for speaking at meeting from Novartis. J.S.-G. reports, in the last 36 months, grants and personal fees from Genzyme; personal fees from Biogen, Bayer, Merck, Almirall, Bial, Novartis, Roche, TEVA and Celgene; he is director of Revista de Neurologia, for which he does not receive any compensation; and serves on the editorial board of Multiple Sclerosis Journal, for which he receives a compensation. C.E. has received funding for travel and speaker honoraria from Biogen, Bayer, Genzyme, Merck, Novartis, Shire and Teva; research support from Biogen, Merck and Teva; and has served on scientific advisory boards for Bayer, Biogen, Merck, Novartis, Roche and Teva. D.C. has, in the last 3 years, received honoraria from EXCEMED for faculty-led education work. He is a consultant for Biogen and Hoffmann-La Roche. He has received research funding from the International Progressive MS Alliance, the MS Society and the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre. F.B. serves as a consultant for Biogen, Bayer, Genzyme, Jansen Research, Merck, Novartis, Roche, Synthon BV and Teva. C.G. has received compensation for consulting from Bayer and Biogen, and speaker’s fees for lectures from Biogen, Bayer, Genzyme, Merck, Novartis and Teva. A.R. serves on scientific advisory boards for Novartis, Sanofi-Genzyme, SyntheticMR, Bayer, Roche, Biogen, Neurodiem and OLEA Medical; and has received speaker honoraria from Bayer, Sanofi-Genzyme, Bracco, Merck-Serono, Teva Pharmaceutical Industries Ltd, Novartis, Roche and Biogen. G.D. was supported by the NIHR Biomedical Research Centre (BRC), Oxford; and has research funding from the Oxford BRC, MRC (UK) and Merck-Serono. He has received travel expenses from Bayer Schering, Biogen Idec, Genzyme, Merck Serono and Novartis; and honoraria as an invited speaker for Bayer Schering and Novartis. J.P. is partly funded by highly specialized services to run a national congenital myasthenia service and a neuromyelitis service. She has received support for scientific meetings and honorariums for advisory work from Merck Serono, Biogen Idec, Novartis, Teva, Chugai Pharma and Bayer Schering, Alexion, Roche, Genzyme, MedImmune, EUROIMMUN, MedDay, Abide and argenx; and grants from Merck Serono, Novartis, Biogen Idec, Teva, Abide and Bayer Schering. She has received grants from the MS Society, Guthy-Jackson Charitable Foundation, NIHR, Oxford Health Services Research Committee, EDEN, MRC, GMSI and John Fell for research studies.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
ORCID iDs: Ruth Geraldes  https://orcid.org/0000-0001-5829-808X
https://orcid.org/0000-0001-5829-808X
Serena Ruggieri  https://orcid.org/0000-0001-5287-3290
https://orcid.org/0000-0001-5287-3290
Jaume Sastre-Garriga  https://orcid.org/0000-0002-1589-2254
https://orcid.org/0000-0002-1589-2254
Declan Chard  https://orcid.org/0000-0003-3076-2682
https://orcid.org/0000-0003-3076-2682
Frederik Barkhof  https://orcid.org/0000-0003-3543-3706
https://orcid.org/0000-0003-3543-3706
Claudio Gasperini  https://orcid.org/0000-0002-3959-4067
https://orcid.org/0000-0002-3959-4067
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Ruth Geraldes, Nuffield Department of Clinical Neurosciences, Oxford, UK.
Maciej Juryńczyk, Nuffield Department of Clinical Neurosciences, Oxford, UK.
Giordani Rodrigues dos Passos, Nuffield Department of Clinical Neurosciences, Oxford, UK.
Alexander Pichler, Department of Neurology, Medical University of Graz, Graz, Austria/Division of Neuroradiology, Vascular & Interventional Radiology, Medical University of Graz, Graz, Austria.
Karen Chung, NMR Research Unit, Queen Square Multiple Sclerosis Centre, University College London Institute of Neurology, London, UK.
Marloes Hagens, MS Center Amsterdam, Department of Neurology, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Serena Ruggieri, Multiple Sclerosis Center, Department of Neurosciences, San Camillo-Forlanini Hospital, Rome, Italy.
Cristina Auger, Section of Neuroradiology, Department of Radiology, Hospital Universitari Vall d’Hebron, Universitat Autonoma de Barcelona, Barcelona, Spain.
Jaume Sastre-Garriga, Servei de Neurologia/Neuroimmunologia, Multiple Sclerosis Centre of Catalonia (Cemcat), Hospital Universitari Vall d’Hebron, Universitat Autònoma de Barcelona, Barcelona, Spain.
Christian Enzinger, Department of Neurology, Medical University of Graz, Graz, Austria/Division of Neuroradiology, Vascular & Interventional Radiology, Medical University of Graz, Graz, Austria.
Declan Chard, NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK/National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre, London, UK.
Frederik Barkhof, MS Center Amsterdam, Department of Radiology and Nuclear Medicine, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands/NMR Research Unit, Queen Square MS Centre, Department of Neuroinflammation, UCL Institute of Neurology, Faculty of Brain Sciences, University College London, London, UK/National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre, London, UK.
Claudio Gasperini, Multiple Sclerosis Center, Department of Neurosciences, San Camillo-Forlanini Hospital, Rome, Italy.
Alex Rovira, Section of Neuroradiology, Department of Radiology, Hospital Universitari Vall d’Hebron, Universitat Autonoma de Barcelona, Barcelona, Spain.
Gabriele DeLuca, Nuffield Department of Clinical Neurosciences, Oxford, UK.
Jacqueline Palace, Nuffield Department of Clinical Neurosciences, Oxford, UK.
References
- 1. Nakashima I, Fujihara K, Kimpara T, et al. Linear pontine trigeminal root lesions in multiple sclerosis. Arch Neurol 2001; 58: 101–104. [DOI] [PubMed] [Google Scholar]
- 2. Pullicino P, Ostrow P, Miller L, et al. Pontine ischemic rarefaction. Ann Neurol 1995; 37(4): 460–466. [DOI] [PubMed] [Google Scholar]
- 3. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Geraldes R, Juryńczyk M, Dos Passos G, et al. Distinct influence of different vascular risk factors on white matter brain lesions in multiple sclerosis. J Neurol Neurosurg Psychiatry 2020; 91: 388–391. [DOI] [PubMed] [Google Scholar]
- 5. Geraldes R, Ciccarelli O, Barkhof F, et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nat Rev Neurol 2018; 14(4): 199–213. [DOI] [PubMed] [Google Scholar]
- 6. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17(2): 162–173. [DOI] [PubMed] [Google Scholar]
- 7. Comi G, Filippi M, Martinelli V, et al. Brain stem magnetic resonance imaging and evoked potential studies of symptomatic multiple sclerosis patients. Eur Neurol 1993; 33(3): 232–237. [DOI] [PubMed] [Google Scholar]
- 8. Renard D, Castelnovo G, Bousquet PJ, et al. Brain MRI findings in long-standing and disabling multiple sclerosis in 84 patients. Clin Neurol Neurosurg 2010; 112(4): 286–290. [DOI] [PubMed] [Google Scholar]
- 9. Di Stadio A, Dipietro L, Ralli M, et al. Clinical and radiological findings of facial paralysis in multiple sclerosis. Mult Scler Relat Disord 2020; 37: 101456. [DOI] [PubMed] [Google Scholar]
- 10. Frohman EM, Zhang H, Kramer PD, et al. MRI characteristics of the MLF in MS patients with chronic internuclear ophthalmoparesis. Neurology 2001; 57(5): 762–768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ943777_supplemental_material for The role of pontine lesion location in differentiating multiple sclerosis from vascular risk factor-related small vessel disease by Ruth Geraldes, Maciej Juryńczyk, Giordani Rodrigues dos Passos, Alexander Pichler, Karen Chung, Marloes Hagens, Serena Ruggieri, Cristina Auger, Jaume Sastre-Garriga, Christian Enzinger, Declan Chard, Frederik Barkhof, Claudio Gasperini, Alex Rovira, Gabriele DeLuca and Jacqueline Palace in Multiple Sclerosis Journal