Abstract
Background:
Since vaccination against coronavirus disease 2019 (COVID-19) became available, risks related to vaccinating patients with multiple sclerosis (MS) need to be carefully assessed.
Objective:
Characterize safety and occurrence of immediate relapses following COVID-19 vaccination in a large cohort of MS patients.
Methods:
We assessed the safety of BNT162b2 COVID-19 vaccination in adult MS patients.
Results:
Between 20 December 2020 and 25 January 2021, 555 MS patients received the first dose of BNT162b2 vaccine and 435 received the second dose. There were three cases of COVID-19 infection encountered after the first dose. Safety profile of COVID-19 vaccine was characterized by pain at the injection site, fatigue, and headache. No increased risk of relapse activity was noted over a median follow-up of 20 and 38 days after first and second vaccine doses, respectively. The rate of patients with acute relapse was 2.1% and 1.6% following the first and second doses, respectively, similar to the rate in non-vaccinating patients during the corresponding period. Mild increase in the rate of adverse events was noted in younger patients (18–55 years), among patients with lower disability (Expanded Disability Status Scale (EDSS) ⩽3.0), and in patients treated with immunomodulatory drugs.
Conclusion:
COVID-19 BNT162b2 vaccine proved safe for MS patients. No increased risk of relapse activity was noted.
Keywords: Multiple sclerosis, COVID-19, vaccination, adverse events, acute relapse, immune response
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), has caused a devastating disease with high rates of morbidity and mortality spreading all over the world.1
It is not known how immunization against SARS-CoV-2 affects patients with multiple sclerosis (MS) and whether the vaccination might induce immunological response that can activate the disease. The exact mechanisms through which autoimmune reactions can be triggered by vaccination are not understood, although they probably vary according to the type of vaccine and individual genetic susceptibility.2,3
Vaccines are considered safe for MS patients
Regarding the effects of vaccination on disease activity, MS was reported to worsen following various live attenuated or inactivated vaccines like yellow fever and H1N1 swine flu vaccine4,5 though a recent small study was reassuring.6 Recent literature reviews summarizing the role of vaccines regarding the risk of developing MS and MS relapse reported no change in the risk of developing MS after vaccination against hepatitis B virus, human papillomavirus, seasonal influenza, measles-mumps-rubella, variola, tetanus, Bacillus Calmette–Guérin (BCG), polio, typhoid fever, or diphtheria. No change in risk of MS relapse was found for influenza, while for many other vaccines, there is currently insufficient evidence to assess the risk of post-vaccine relapse.7,8
The lipid nanoparticle-formulated Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is a new type of vaccine, based on nucleoside-modified mRNA vector vaccine encoding the prefusion spike glycoprotein of SARS-CoV-2.9 Currently, there is no data related to efficacy and safety of the vaccine in patients with MS.
In this study, we evaluated the adverse event profile and immediate risk for acute MS relapses in MS patients who received COVID-19 vaccination. In this study, we aimed to shed light on whether the innovative mRNA COVID-19 vaccination in patients with MS is associated with increased rates of adverse events, determine factors related to the safety response, and evaluate whether vaccination increased immediate disease activity.
Methods
Establishing communication during the COVID-19 pandemic
During the early phase of COVID-19 pandemic, we established an educational program aimed to provide updated medical information related to COVID-19 infection, safety concerns, and treatment decisions for MS patients treated and followed in our MS Center. The program was initiated in mid-March 2020 when the COVID-19 pandemic spread in Israel, and many patients searched guidance and medical advice. During the following months, we set up acommunication network with patients via face-to-face meetings, Zoom meetings, teleconferences, webinars, and explanatory lectures via the Internet. Using this network, we initiated in early December 2020 a campaign to the MS community stressing the importance of vaccination. The campaign was a part of the initiative of the health authorities in Israel that promoted COVID-19 vaccine suggesting to the population that vaccination is the order of the day and the only solution to overcome the devastating SARS-CoV-2 infection. In accordance, we established the following recommendations to MS-treating neurologists regarding COVID-19 vaccination for MS patients: (1) promote vaccination for all untreated MS patients; (2) promote vaccination for MS patients treated with beta-interferons, natalizumab, or glatiramer acetate; (3) perform pre-vaccination lymphocyte count for patients treated with Fingolimod, Dimethyl fumarate, Teriflunomide, Alemtuzumab, Ocrelizumab, and Cladribine (for future evaluation of vaccination success and proper immune response under low (<1000) lymphocyte count), and regardless of the lymphocyte count, patients were recommended to be vaccinated without stopping their immunomodulatory treatment; (4) vaccinate patients recently treated with B-cell depletion therapies like Alemtuzumab, Ocrelizumab, and Cladribine, at least 3 months after the last treatment; and (5) postpone vaccination for patients who have recently received high-dose steroids due to acute relapse until 1 month after the steroid treatment.
COVID-19 vaccination
Patients received two intramuscular injections, 21 days apart, delivered in the deltoid muscle. Each injection contained 30 μg of BNT162b2 (0.3 mL volume per dose). Patients with previous COVID-19 disease were not vaccinated.
Study design
This study is an observational study.
Data collection
Our planned target was to vaccinate 500 MS patients (~12% of the whole MS Center population), to achieve a large enough cohort to study the adverse events profile and occurrence of immediate relapses following COVID-19 vaccination. Patients were approached randomly after prioritization for age and appropriate time interval related to B-cell depletion therapies. In accordance, 750 MS patients were contacted by phone, emails, WhatsApp, or face-to-face encounters, and 574 agreed and received the first vaccine dose, yielding a compliance rate of 76.5%. Demographic, clinical, and medications data were obtained from electronic health records using the Sheba MS Computerized Database Registry. The digital database was updated to include COVID-19-related metrics consisting of the date of administration of first and second vaccine doses, symptoms following each vaccine dose according to a pre-specified list comprising the occurrence of any of the following: (1) no vaccine-associated symptoms; (2) pain at the injection site; (3) fever; (4) muscle and/or joint pain; (5) flu-like symptoms; (6) fatigue and/or general weakness; (7) headache; (8) dizziness; (9) gastrointestinal symptoms, for example, nausea, vomiting, or diarrhea; (10) face tingling; (11) facial weakness; (12) post-vaccination COVID-19 infection; (13) acute MS relapse; and (14) worsening of MS symptomatology. As the dates for the first and second vaccine doses were pre-scheduled, data were collected within 7–21 days after each vaccine dose by the treating neurologists during face-to-face encounters, contact using emails, phone calls, or WhatsApp application. Specifically, use of WhatsApp was a continuation of the interactive networking between physicians and patients developed during the COVID-19 pandemic. Patients were systematically queried about the occurrence of any adverse events, the severity of symptoms, and the duration they persisted. In the event of a possible relapse or sustained neurological worsening, patients underwent neurological examination to verify or exclude the event. Vaccination dates were pre-planned with prioritization of older patients and patients scheduled to receive B-cell depletion therapies. Data cut-off date for vaccinations was set to 3 February 2021, and cut-off date for safety evaluations and relapse assessment was set to 10 February 2021.
Ethics
The study was approved by the Sheba Medical Center Institutional Review Board. Each patient record was coded anonymously to ensure confidentiality during statistical analyses and the need for informed consent was waived.
Statistical analysis
Categorical variables were described as frequency and percentage, and continuous variables were reported by their median and interquartile range (IQR). Descriptive data analyses were performed using Python software (version 3.0). For the evaluation of relapse rate following the vaccine, we used historical controls and a group of MS patients who were not vaccinated within the comparable time periods.
Results
Patient population
COVID-19 vaccination was administered to 574 MS patients followed in our MS Center between 21 December 2020 and 3 February 2021. Nineteen patients (3.4%) with missing data were excluded from the analysis. A total of 555 patients, 65.6% female, 74.6% treated with various immunomodulatory drugs (IMDs), received the first BNT162b2 vaccine injection, and of them, 435 patients, 65.3% female, 74.9% treated, received the second vaccination by the defined cut-off date. Demographic and clinical data of vaccinated MS patients are presented in Table 1.
Table 1.
Clinical and demographic variables of patients with multiple sclerosis who received COVID-19 vaccination.
| First COVID-19 vaccination dose | Second COVID-19 vaccination dose | |
|---|---|---|
| Study population | 555 | 435* |
| Duration of follow-up at data cut-off date, days | ||
| Median | 38 | 20 |
| 25–75 IQR | 33–43 | 15–22 |
| Gender, n (%) | ||
| Female | 364 (65.6) | 284 (65.3) |
| Male | 191 (34.4) | 151 (34.7) |
| Age group, n (%) | ||
| 18–55 years | 370 (66.7) | 274 (63) |
| >55 years | 185 (33.3) | 161 (37) |
| Disease duration, years | ||
| Median | 15.2 | 16.0 |
| 25–75 IQR | 8.2–22.5 | 8.4–23.4 |
| Disability by EDSS, n (%) | ||
| ⩽3.0 | 294 (53) | 224 (51.5) |
| 3.5–5.5 | 118 (21.3) | 93 (21.4) |
| ⩾6.0 | 143 (25.8) | 118 (27.1) |
| Disease type, n (%) | ||
| RIS | 4 (0.7) | 3 (0.6) |
| CIS | 24 (4.3) | 15 (3.4) |
| RRMS | 388 (69.9) | 306 (70.3) |
| SPMS | 91 (16.4) | 71 (16.3) |
| PPMS | 48 (8.6) | 40 (9.2) |
| IMD treatment, n (%) | ||
| Untreated | 141 (25.4) | 109 (25.1) |
| Beta-interferons (1a and 1b) | 59 (10.6) | 50 (11.5) |
| Glatiramer acetate | 16 (2.9) | 14 (3.2) |
| Teriflunomide | 44 (7.9) | 33 (7.6) |
| Dimethyl fumarate | 65 (11.7) | 46 (10.6) |
| Natalizumab | 52 (9.3) | 43 (9.9) |
| Fingolimod | 35 (6.3) | 27 (6.2) |
| Ocrelizumab | 62 (11.1) | 49 (11.3) |
| Alemtuzumab | 25 (4.5) | 18 (4.1) |
| Cladribine | 32 (5.8) | 28 (6.4) |
| Rituximab | 7 (1.2) | 4 (0.9) |
| IVIg | 17 (3.0) | 14 (3.2) |
EDSS: Expanded Disability Status Scale; IQR: interquartile range; IMD: immunomodulatory drug; IVIg: intravenous immunoglobulin immunoglobulin; RIS: Radiologically Isolated Syndrome; CIS: Clinically Isolated Syndrome; RRMS: Relapsing Remmiting Multiple Sclerosis; SPMS: Secondary Progressive Multiple Sclerosis; PPMS: Primary Progressive Multiple Sclerosis.
At the date of data cut-off, only 435 received the second vaccine dose; 109 patients did not complete the 21 days of inter-dose interval; 3 patients were infected by SARS-CoV-2 after the first vaccine dose; and 8 patients experienced an acute MS relapse and their second vaccine dose was postponed.
Safety
No events of anaphylaxis or life-threatening responses occurred immediately after either the first or second doses of vaccination. There was a report of death in a 52-year-old male patient with a long-standing MS and severe disability (Expanded Disability Status Scale (EDSS) = 9.0), indwelling catheter and percutaneous endoscopic gastrostomy for the last 4 years. He lived in a caring facility and was found dead in his bed after a night sleep, 21 days after the second vaccination. The patient had no comorbidities, but was hospitalized several times during the preceding year due to urinary sepsis and pressure sores in his buttocks and right leg. We do not think the death of this severely ill patient was related to the vaccination, and it is important to note that based on data from the UK Office for National Statistics, for every 100,000 doses given to people aged 80 years or over, around 200 people die of natural causes within a week.10 The safety profile of COVID-19 vaccine in MS patients reported within a median of 38 days after the first BNT162b2 vaccination dose and 20 days after the second dose was characterized by short-term, mild-to-moderate adverse events, none necessitating hospitalization. Overall, these adverse events were transient and resolved within a few days after onset. The frequency of patients with any adverse event was 29.7% and 40.2% after the first and second doses, respectively. None of the patients declined the second vaccine because of ill effects after the first dose. The most common reported adverse events were local pain at the injection site, fatigue, headaches, muscle or joint pain, and flu-like symptoms manifested as either fever, chills or a combination of both. After the second dose, the frequency of fatigue, headaches, muscle or joint pain, and flu-like symptoms increased, while local pain at the injection site slightly decreased. The list of common adverse events reported by MS patients following each dose of COVID-19 vaccine is presented in Table 2.
Table 2.
Adverse events reported in MS patients following COVID-19 vaccination.
| First COVID-19 vaccination dose | Second COVID-19 vaccination dose | |
|---|---|---|
| Study population | 555 | 435 |
| Any adverse events, n (%) | 165 (29.7) | 175 (40.2) |
| Pain at the injection site | 89 (16) | 62 (14.2) |
| Fever/chills, flu-like symptoms | 11 (2) | 52 (11.9) |
| Fatigue | 51 (9.2) | 69 (15.9) |
| Headache | 25 (4.5) | 32 (7.3) |
| Muscle or joint pain | 13 (2.2) | 40 (9.2) |
| Infection with SARS-CoV-2 after vaccination, n (%) | 3 (0.5) | 0 |
| New or worsening neurological symptomatology, n (%) | 11 (2) | 21 (4.8) |
| Face tingling | 3 (0.5) | 5 (1.1) |
| Acute MS relapses, n (%)* | 8 (2.1) | 5 (1.6) |
| Time to relapse, days | ||
| Median | 16 | 15 |
| Range | 10–19 | 14–21 |
MS: multiple sclerosis.
Calculated as the number of patients who presented with an acute relapse out of the vaccinated group of RRMS patients.
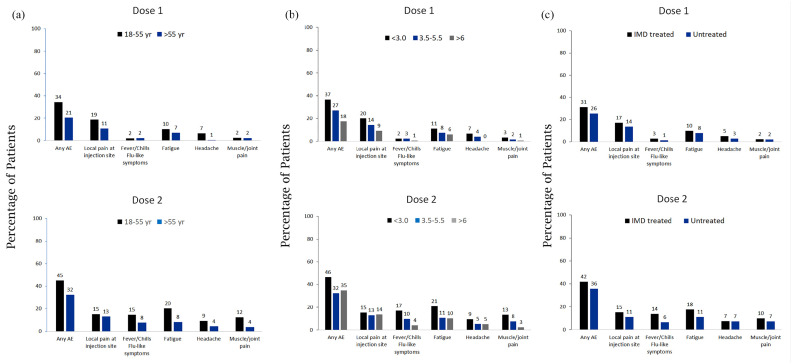
Both local and systemic events were reported more often by younger patients (18–55 years of age) than by older patients (more than 55 years of age), by patients with lower disability (EDSS ⩽3.0) than by patients with moderate (EDSS = 3.5–5.5) or severe (EDSS ⩾6.0) disability, and by IMD treated MS patients than by untreated patients, and more often after dose 2 than dose 1 (Figure 1(a)–(c)).
Figure 1.
Adverse events reported by MS patients after BNT162b2 COVID-19 vaccination according to (a) age, (b) neurologic disability, and (c) IMD treatment.
Numbers above the bars are the percentage of participants who reported the specified adverse event.
SARS-CoV-2 infection
Between the first dose and the second vaccination dose, three patients (0.5%) were infected by SARS-CoV-2; all had no or mild symptomatology. These patients were tested for SARS-CoV-2 RNA by nasopharyngeal swab because they were in close contact with a subject that was identified as COVID-19 positive. According to the Health Care Policy in Israel, infected subjects need to report the people they were in contact with and the identified subjects are than tested. No SARS-CoV-2 infections were reported after the second vaccination dose.
MS worsening
Worsening of MS symptomatology defined as transient increase in MS symptoms following vaccination (pseudo-relapse) was reported in 2% of patients after the first vaccination dose and in 4.8% of patients after the second vaccination dose, was short-term, lasting 1–2 days, and mainly associated with the flu-like symptomatology. We noted in few patients the occurrence of face tingling that quickly resolved. No symptomatology of facial palsy was recorded.
Acute relapses
Within 10–19 days following the first vaccination dose, eight patients presented with an acute relapse (2.1% of the 388 vaccinated RRMS patients), and within 14–21 days following the second vaccine dose, five patients (1.6% of the 306 vaccinated RRMS patients) presented with an acute relapse. It is of note that 36 patients (6.5%) who received the first vaccine dose, and 57 patients (13.1%) who received the second vaccine dose were followed less than 14 days, and therefore, the rate of acute relapse following the vaccine could be higher than recorded. Analysis of the rate of relapsing-remitting MS patients who presented with an acute relapse during the corresponding periods in 2017, 2018, 2019, and 2020, disclosed that the rate of acute relapses in non-vaccinated MS patients, calculated as the number of patients who presented with an acute relapse divided by the number of relapsing-remitting patients followed in the corresponding time period, was 2.7%, 2.9%, 2.6%, and 2.3%, respectively.
Discussion
In this study, we evaluated whether COVID-19 vaccination is associated with increased rates of adverse events and whether vaccination increased immediate acute relapse disease activity. Our findings demonstrated that MS patients had similar rates of adverse reactions to what has been reported in the general population following BNT162b2 vaccination against SARS-CoV-2 infection.11
The reported adverse events were more common after the second dose than after the first dose, however to a much lesser extent in the MS population, suggesting that MS patients do not show higher risk for vaccine-induced adverse events. We also studied whether age, disability, or IMD treatment were contributing factors that determine the safety response following vaccination.
The most commonly reported systemic events in COVID-19 vaccinated non-MS population were fatigue and headache (59% and 52%, respectively);11 in our MS population, fatigue was reported only in 20%, and headache in 9%, both after the second dose, among younger vaccine recipients. Although fatigue is a common complaint in many MS patients, rate of fatigue associated with COVID-19 vaccination was lower in more disabled patients.
Fever was reported after the second dose by 15% of younger MS patients and by 8% of older patients, while in the non-MS subjects, the rates were 16% and 11%, respectively. Similar to younger MS patients, adverse events were more commonly reported by less disabled patients and by IMD treated patients. This distribution suggests that younger MS patients tend to be less disabled and more treated and therefore the worse adverse event profile in mainly attributed to age.
After the first vaccine dose, three patients had evidence of SARS-CoV-2 infection, and none were reported after the second dose suggesting a high vaccine efficacy, although longer follow-up is recommended to better evaluate the longevity of the early observed protection by the vaccine. This is especially of importance for patients treated with B-cell depletion therapies or Fingolimod that are known to develop weaker immune response to non-live vaccines like tetanus toxoid, pneumovax, and influenza.12–14
Although this study included a large cohort of MS patients, the limitation of our findings relates to the relatively short follow-up period. This could result in lower rate of adverse events and immediate relapses, as 6.5% and 13.1% of patients were follows less than 14 days after the first and second vaccine doses, respectively.
BNT162b2 COVID-19 vaccine, in contrary to the above-mentioned vaccines, is RNA-based vaccine containing mRNA that encodes the SARS-CoV-2 full-length spike, modified by two proline mutations to lock it in the prefusion conformation. Therefore, the risk of integration with the host cell genome is avoided, and as the viral mRNA is transiently expressed, the vaccine is expected to produce high immunogenicity associated with favorable safety profile. Indeed, vaccinated MS patients did not show increased disease activity and the rate of relapsing patients was similar to that of non-vaccinated patients during the corresponding periods in the last 4 years. Taken together, our findings provide safety information related to COVID-19 vaccination in patients with MS and support to recommendation to promote and not to delay vaccination during the still expanding SARS-CoV-2 pandemic.
Acknowledgments
The authors thank Ms Shany Tomer, Mrs Yamit Titngi, Mrs Ortal Fyorentino, Mrs Nurit Amsalem, Mrs Hagit Zabari, Ms Neta Sella, Ms Hen Eisikovitz, Ms Maria Didkin, and Mrs Sue Mayust for their outstanding technical help in collecting the data.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Anat Achiron  https://orcid.org/0000-0002-2020-3126
https://orcid.org/0000-0002-2020-3126
Contributor Information
Anat Achiron, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Mark Dolev, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Shay Menascu, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Daniela-Noa Zohar, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Sapir Dreyer-Alster, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Shmuel Miron, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Emanuel Shirbint, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
David Magalashvili, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Shlomo Flechter, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Uri Givon, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Diana Guber, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Yael Stern, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Michael Polliack, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Rina Falb, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
Michael Gurevich, Multiple Sclerosis Center, Sheba Medical Center, Ramat-Gann, Israel/Laura Schwarz-Kipp Chair of Research of Autoimmune Diseases, Sackler School of Medicine, Tel-Aviv University, Tel-Aviv, Israel.
References
- 1. Shih HI, Wu CJ, Tu YF, et al. Fighting COVID-19: A quick review of diagnoses, therapies, and vaccines. Biomed J 2020; 43(4): 341–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen RT, Pless R, Destefano F. Epidemiology of autoimmune reactions induced by vaccination. J Autoimmun 2001; 16(3): 309–318. [DOI] [PubMed] [Google Scholar]
- 3. Kivity S, Agmon-Levin N, Blank M, et al. Infections and autoimmunity: Friends or foes? Trends Immunol 2009; 30: 409–414. [DOI] [PubMed] [Google Scholar]
- 4. Farez MF, Correale J. Yellow fever vaccination and increased relapse rate in travelers with multiple sclerosis. Arch Neurol 2011; 68(10): 1267–1271. [DOI] [PubMed] [Google Scholar]
- 5. Huttner A, Eperon G, Lascano AM, et al. Risk of MS relapse after yellow fever vaccination: A self-controlled case series. Neurol Neuroimmunol Neuroinflamm 2020; 7(4): e726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oikonen M, Laaksonen M, Aalto V, et al. Temporal relationship between environmental influenza A and Epstein-Barr viral infections and high multiple sclerosis relapse occurrence. Mult Scler 2011; 17(6): 672–680. [DOI] [PubMed] [Google Scholar]
- 7. Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: A systematic review. J Neurol 2017; 264(6): 1035–1050. [DOI] [PubMed] [Google Scholar]
- 8. Farez MF, Correale J. Immunizations and risk of multiple sclerosis: Systematic review and meta-analysis. J Neurol 2011; 258(7): 1197–1206. [DOI] [PubMed] [Google Scholar]
- 9. World Health Organization. Background document on mRNA vaccine BNT162b2 (Pfizer-BioNTech) against COVID-19, 2021. (WHO/2019-nCoV/vaccines/SAGE_recommendation/BNT162b2/background/2021.1), https://apps.who.int/iris/handle/10665/338671
- 10. Torjesen I. Covid-19: First UK vaccine safety data are “reassuring,” says regulator. BMJ 2021; 372: n363. [DOI] [PubMed] [Google Scholar]
- 11. Polack FP, Thomas SJ, Kitchin N, et al. C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology 2020; 95: e1999–e2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ciotti JR, Valtcheva MV, Cross AH. Effects of MS disease-modifying therapies on responses to vaccinations: A review. Mult Scler Relat Disord 2020; 45: 102439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kappos L, Mehling M, Arroyo R, et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology 2015; 84: 872–879. [DOI] [PubMed] [Google Scholar]