Abstract
Fyn kinase plays crucial roles in hematology and T cell signaling; however, there are currently limited tools to visualize the dynamic Fyn activity in live cells. Here we developed and characterized a highly sensitive Fyn biosensor based on fluorescence resonance energy transfer (FRET) to monitor Fyn kinase activity in live cells. Our results show that Fyn kinase activity can be induced in both mouse embryonic fibroblasts (MEFs) and T cells by ligand engagement. Two different motifs were further introduced to target the biosensor at the cellular membrane microdomains in MEFs, revealing that the Fyn-tagged biosensor had 70% greater response to growth factor stimulation than the Lyn-tagged version. This suggests that the plasma membrane microdomains can be categorized into different functional subdomains. Further experiments show that while the membrane accessibility is necessary for Fyn activation, the localization of Fyn outside of its microdomains causes its hyperactivity, indicating that membrane microdomains provide a suppressive microenvironment for Fyn regulation in MEFs. Interestingly, a relatively high Fyn activity can be observed at perinuclear regions, further supporting the notion that the membrane microenvironment has a significant impact on the local molecular functions. Our work hence highlights a novel Fyn FRET biosensor for live cell imaging and its application in revealing an intricate submembrane regulation of Fyn in live MEFs.
Keywords: Fyn kinase, membrane microdomain, fluorescence resonance energy transfer (FRET), protein palmitoylation, T cells
Graphical Abstract
Fyn is a nonreceptor tyrosine kinase in the Src family, which plays critical roles in a variety of pathophysiological processes including T cell regulation, neuronal learning and memory, and cell adhesion.1 The precise activation of Fyn together with Lck in space and time is essential for the tyrosine phosphorylation of the T cell receptor (TCR) complex and the propagation of signals to downstream molecules in T-cells.1c,d Dysregulation of the Fyn function can lead to autoimmune diseases, neurodegenerative diseases, and cancer.2 Like other Src family members, Fyn consists of a unique N-terminal SH4 domain, an SH3 domain, an SH2 domain, a proline-rich linker, an SH1 kinase domain, and an inhibitory C-tail containing a regulatory tyrosine site Y528.1d,3 The unique Fyn N-terminal sequence GCVQCKDK allows myristic (at the Gly site) and palmitic (at the two Cys sites) fatty acylations, which can target Fyn at the plasma membrane.4 Early work also showed that the activated Fyn is associated with endosomes as well as on plasma membranes in fibroblasts.4a Active Fyn can phosphorylate the transmembrane adapter molecule PAG (phosphoprotein associated with glycosphingolipid-enriched microdomains) and allows the recruitment of Csk (C-terminal Src kinase), which in-turn negatively regulates Src family kinases including Fyn itself.1d Although PAG has been shown to be membrane-bound,5 how Fyn activity is dynamically regulated at the submembrane compartments remains unclear.
The kinase activity of Fyn is mainly controlled by the intramolecular interaction between its SH2 domain and the phosphorylated cytoplasmic tail and a weak binding between the SH3 domain and the proline-rich linker.3d,6 Phosphorylation of the C-tail tyrosine site by Csk deactivates, while dephosphorylation by the phosphatase CD45 activates Fyn.3d,6,7 Alternatively, Fyn can also be activated by intermolecular interaction between its SH2 or SH3 domain with other tyrosine-phosphorylated sequences or proline-rich motifs, respectively.8 In CD4+ T cells, active Lck kinase can translocate to membrane lipid rafts and activate Fyn downstream of TCR/CD4 signaling.9 Despite the importance of membrane localization in the regulation of Fyn function, there is a lack of imaging tools that can directly visualize Fyn activities at the plasma membrane and its submembrane microdomains.
FRET-based technology has risen as a powerful approach to monitor dynamic molecular activities in live cells during the past decades.10 The genetically encoded FRET-based biosensors can be expressed either in the cytoplasm or targeted to the inside or outside of functional microdomains at the plasma membrane, allowing precise visualization of molecular activities in subcellular compartments.10b,11 Our group has optimized FRET donor and acceptor pair consisting of an enhanced cyan fluorescent protein (ECFP) and a variant of yellow fluorescence protein (YPet) for FRET biosensor engineering, which can form a weak dimer and subsequently enhance the FRET efficiency and sensitivity of the biosensors.10a Subsequently, this FRET pair has also been applied to engineer highly sensitive Rac1, Syk, and Zap70 FRET biosensors.10a,12
While the choice of the donor and acceptor fluorescent proteins contribute significantly to the sensitivity of the biosensor, the SH2 domain and substrate tyrosine peptide are a crucial determinant of the biosensor specificity and sensitivity. Therefore, utilizing the above design strategy and the ECFP/YPet FRET pair, we engineered new Fyn FRET biosensors and characterized the sensitivity and specificity of these designs in vitro and in MEFs to select the optimal biosensor. Fyn activity was also detected by the FRET response of the biosensor in T cells. By targeting the FRET-based biosensor to membrane microdomains in MEF cells, we observed a substantial population of active Fyn kinase around the perinuclear region. Further mutational experiments on the membrane-targeting sequence of Fyn kinase revealed that Fyn kinase activity is fine-tuned and precisely regulated by its submembrane localization. Therefore, our work provides a highly sensitive Fyn FRET biosensor for live-cell imaging although it also displays a certain reaction to Src and Yes kinase activities in vitro and further reveals a Fyn activity regulation mechanism by different membrane compartments in MEFs.
RESULTS
Biosensor Design and in Vitro Characterization.
Here we constructed a genetically encoded FRET biosensor to measure Fyn kinase activity, which has a design similar to the previously reported Src FRET biosensor.10a,b The biosensor consists of an ECFP at the N-terminus, followed by an SH2 domain from Src, a flexible linker with 15 amino acids, a Fyn substrate peptide, and a YPet at the C-terminus (Figure 1A). The Fyn substrate peptide (EKIEGTYGVV) was derived from p34cdc2, an established downstream effector molecule of Fyn kinase.13 As shown in Figure 1B, when the substrate is not phosphorylated, because of the spatial proximity between N- and C-termini of SH2 domain in the three-dimensional structure,14 the donor ECFP is positioned close to the acceptor YPet with a high FRET efficiency. Upon tyrosine phosphorylation by kinase, the phosphorylated substrate binds to the SH2 pocket located at the opposite site of its two termini, which leads to a separation of YPet from ECFP and a decrease of FRET efficiency. These phosphorylations and FRET changes are reversible upon the dephosphorylation of the substrate within the biosensor. As a result, the ECFP/FRET emission ratio of the biosensor can be applied to represent Fyn kinase activity in vitro and in live cells.
Figure 1.
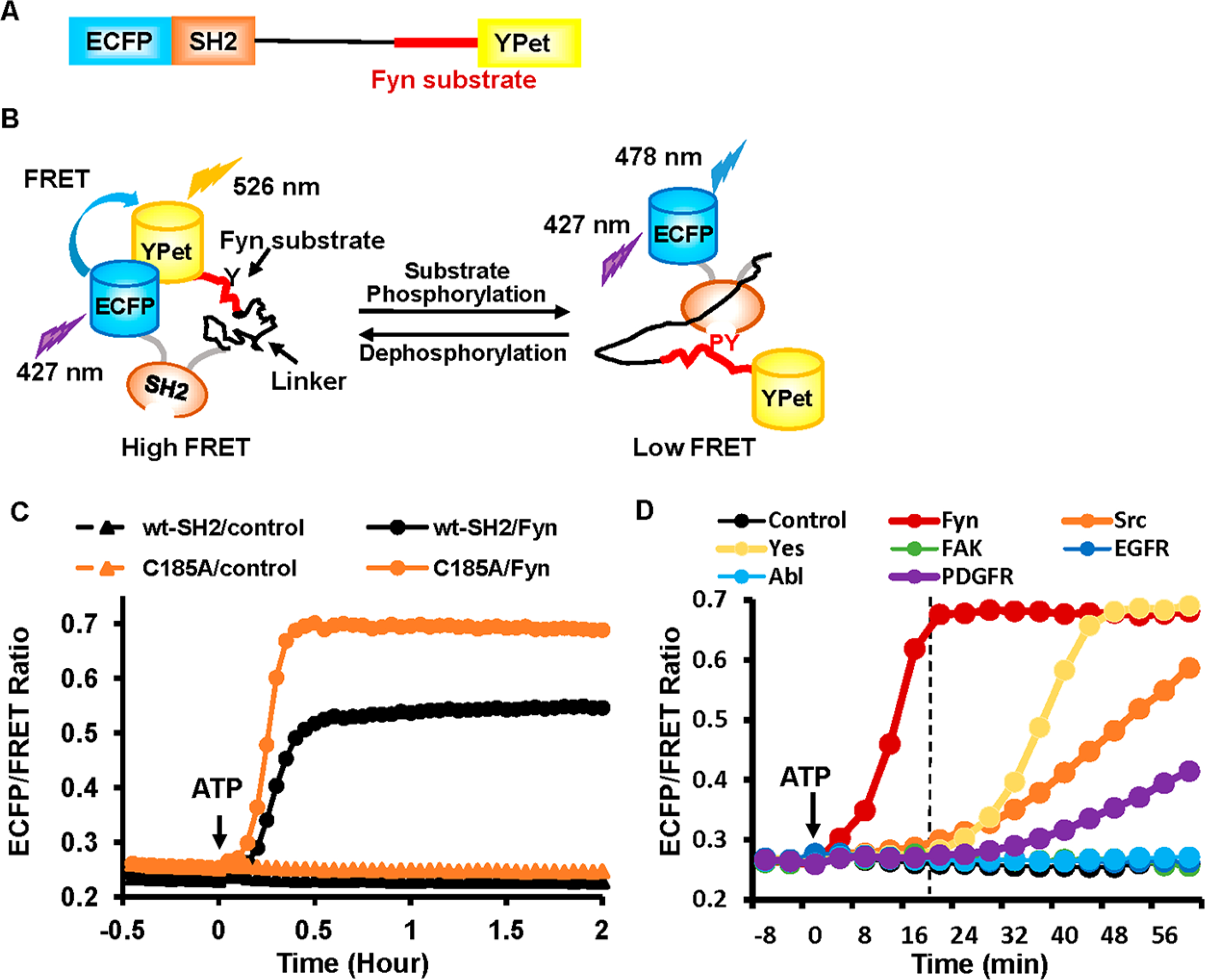
Design of Fyn biosensor and in vitro kinase assay. (A) The schematics of the biosensor. (B) The schematic drawing depicting the conformational change of the biosensor upon tyrosine phosphorylation at the substrate. (C) The in vitro activation of the biosensors with wild-type SH2 (wt-SH2) or SH2 with mutation (C185A) by adding active Fyn kinase and ATP at 0 min. The control groups were in the absence of active Fyn kinase. (D) Comparison of the in vitro activation of the C185A-SH2 biosensor by different active tyrosine kinases. These reaction assays were performed simultaneously on a fluorescence plate reader. The dashed line indicates the time point at ~20 min after adding ATP to initiate the enzymatic reactions.
Recently our group discovered that introducing a single mutation (C185A) in the SH2 domain results in a significant increase of FRET sensitivity for a Src biosensor.15 Two versions of SH2 domain including wild-type (wt) and C185A mutant were hence tested in the Fyn biosensor. In vitro assays revealed that the biosensor with C185A-SH2 had a much larger and quicker FRET response (160% FRET ratio change at 21 min) to active Fyn kinase than that with wt-SH2 (108% FRET ratio change at 30 min) based on the FRET ratio of emission 478 nm/emission 528 nm (Figure 1C).
We further examined the specificity of the biosensors toward Fyn kinase activity by applying and comparing the effect of multiple tyrosine kinases with in vitro kinase assays. The biosensor with either wt- or C185A-SH2 showed a clear preference to active Fyn over other kinases tested, including the Src family kinases Src, Yes, and Abl (Figures 1D and S1B). Within ~20 min after adding ATP (when the two versions of biosensor display nearly the largest difference in FRET response to active Fyn), the wt-SH2 and C185A-SH2 biosensors displayed about 50% and 140% increases in the ECFP/FRET ratio from their basal levels toward active Fyn, respectively, but <8% increase toward all other kinases (Figures 1D and S1B). Indeed, as shown by the biosensor spectra in Figure S2, the biosensor with C185A-SH2 had significant changes of the FRET emission ratio (~60% decrease) within ~20 min when incubated with active Fyn but much less with active Yes or Src kinase. The active domains of PDGFR (platelet-derived growth factor receptor), EGFR (epidemic growth factor receptor), FAK (focal adhesion kinase), or Abl did not have a significant effect on the responses of both wt-SH2 and C185A-SH2 biosensors during this period (Figure 1D). As noted, it may be difficult though to draw firm conclusions about in situ specificity from in vitro assays, as the concentrations/activities of kinases may be different in different types of cells.
Since Fyn and Lck are both Src family kinases critical for TCR signaling during T cell activation,1c,d the Fyn biosensor was also specifically evaluated and compared in response to Fyn or Lck phosphorylation by the in vitro kinase assay. After ATP addition, Fyn caused a much quicker and larger response of the biosensor than Lck (Figure S3). This result demonstrates that the Fyn biosensor is highly sensitive with relative specificity toward Fyn kinase activity. The Fyn biosensor containing C185A-SH2 has better sensitivity than that containing wt-SH2, although both versions have a significant improvement in sensitivity compared to our previously developed Src FRET biosensors.10a,b Therefore, the C185A-SH2 version is chosen as the optimal Fyn biosensor and referred to as the Fyn biosensor in the remainder of the work.
Characterizing the Sensitivity, Activation Mechanism, and Specificity of the Fyn Biosensor in Mammalian Cells.
The C185A-SH2 Fyn biosensor was further examined in live mammalian cells.4a Since tyrosine kinase activations and the subsequent substrate phosphorylation are relatively fast reactions, typically reaching plateau in growth factors-stimulated cells within 10–15 min,16 we focus on this physiologically relevant period and monitor the biosensor within 30 min of activation in live cells. In fact, as shown in MEFs which express the PDGF receptor, Fyn kinase, and other Src family kinases, the FRET response of the Fyn biosensor mostly completed within 5 min upon kinase activation by PDGF stimulation (Figure 2A and Movie S1). These results suggest that the observed biosensor response in MEFs is mainly due to the specific activation of Fyn since other kinases have much slower kinetics in phosphorylating the biosensor in in vitro kinase assays (Figure 1D). The ECFP/FRET emission ratio, which represents Fyn kinase activity, increased from 0.23 to 0.5 and reached a stable plateau in about 5 min (Figure 2B). A mutant biosensor with a single tyrosine (Y) mutation to unphosphorylatable phenylalanine (F) in its peptide substrate had almost no change upon PDGF stimulation, in contrast to the ~130% ECFP/FRET ratio increase of the wild-type C185A-SH2 biosensor (Figures 2C, S4). These results suggest that the FRET response of the biosensor is dependent on the tyrosine phosphorylation of the Fyn substrate sequence as designed (Figure 1B). Furthermore, coexpression of Fyn kinase-dead mutant Fyn(K299M) also inhibited the FRET response of the wild-type Fyn biosensor in MEFs (Figures 2C, S4) but not that of the Src biosensor (Figure S5B). These results suggest that the FRET response of the Fyn biosensor is mainly attributed to the activity of Fyn kinase in MEFs.
Figure 2.
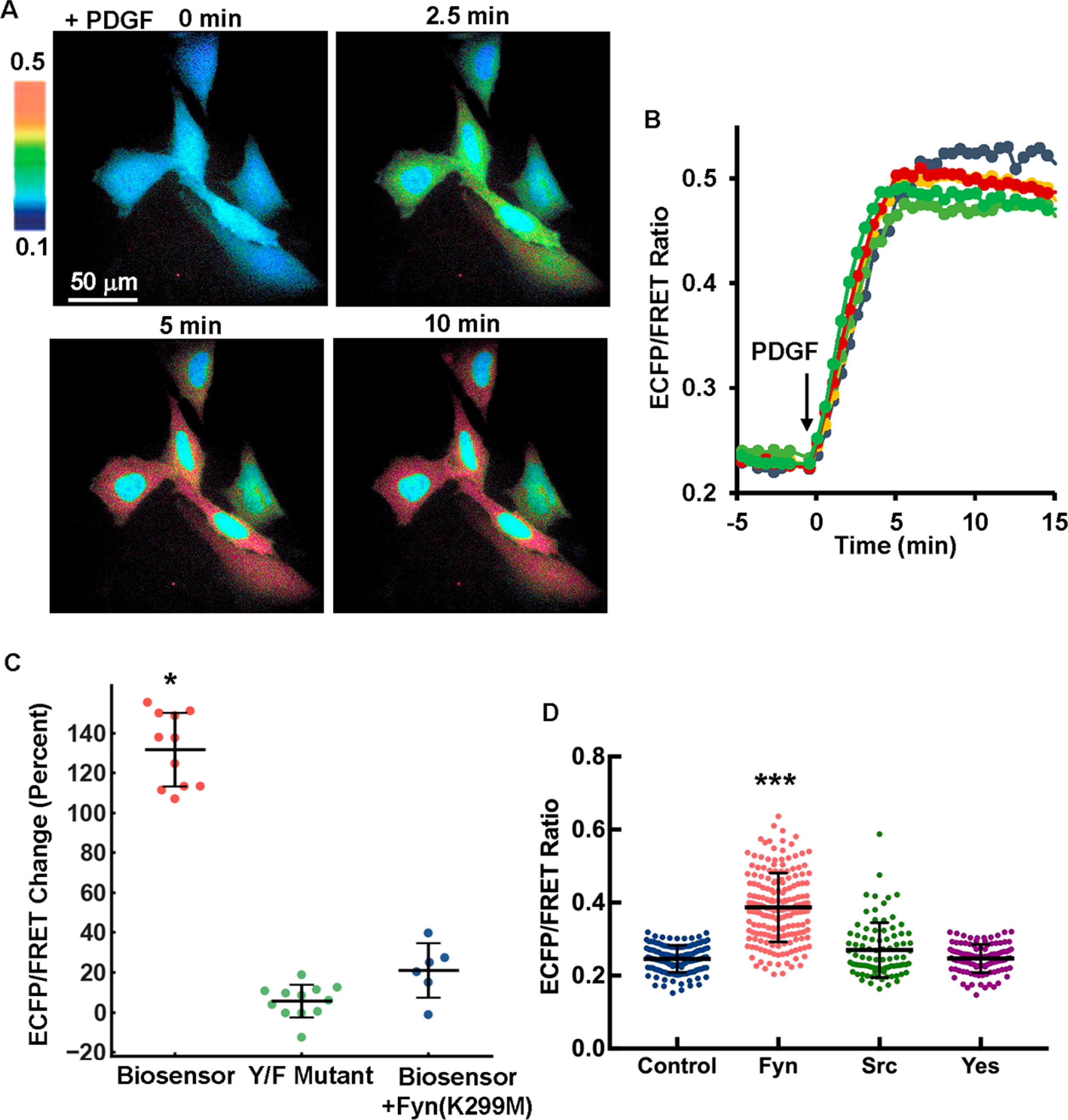
PDGF induced a fast and dramatic FRET response of the Fyn biosensor in MEF cells. (A) The ECFP/FRET ratio images of MEFs treated with PDGF. The color bar from blue to red represents the emission ratio (ECFP/FRET) of biosensors from low to high, respectively. (B) The time courses of the ECFP/FRET emission ratio were quantified for the cells shown in (A). (C) The scatter plots (showing all individual data points plus mean ± SD) show quantified FRET changes of the C185A-SH2 Fyn biosensor, its negative Y/F mutant, and with coexpression of dominant-negative Fyn(K299M) in response to 25 μg/mL PDGF in MEFs (n = 12, 12, 5 for each group, and p-value <10−8). (D) The biosensor ECFP/FRET emission ratio is compared among SYF(−/−) cells cotransfected with the control vector (scatter plots with mean ± SD, 0.246 ± 0.0037), Fyn (0.387 ± 0.094), Src kinase (0.270 ± 0.075), or Yes kinase (0.247 ± 0.039) at the same amount for each (1.0 μg of biosensor + 0.3 μg of kinase DNA per well in 24-well plate). The cells were seeded on 10 μg/mL fibronectin-coated glass-bottom dishes. *** indicates statistically significant difference from all other groups with p-value <1.0e-19, n = 133, 187, 87, 109 respectively.
The biosensor was further introduced into SYF(−/−) cells in which Src, Yes, and Fyn kinases are deficient, and the biosensor activation was measured in these cells with Fyn, Src, or Yes kinase reconstituted during integrin-mediated adhesion. SYF(−/−) cells cotransfected with the biosensor and kinase plasmids were seeded on fibronectin(Fn)-coated glass-bottom dishes overnight before imaging, hence the kinases can be sufficiently activated by Fn-dependent integrin signals. Indeed, the expression of Fyn kinase in SYF(−/−) cells led to a 57% increase of the biosensor ECFP/FRET ratio from the cells without Fyn, which is significantly higher than that resulting from Src or Yes expression (Figure 2D). The large cell–cell heterogeneity of the FRET level in Figure 2D could be partly due to the cotransfection efficiency in addition to the intrinsic noise at single cell levels. Specifically, exogenous kinase DNA was introduced into the cells together with the Fyn biosensor by cotransfection, and individual cells might uptake varying levels of DNA plasmids, which together with different translational and transcriptional activities in individual cells may lead to the difference in kinase expressions and hence biosensor measurements. As control, the expression of Src kinase in SYF(−/−) cells led to a ~70% increase of the ECFP/FRET ratio of a Src biosensor (Figure S5A).10a These results demonstrate that the Fyn biosensor has a relatively specific preference toward Fyn kinase over Src or Yes in mammalian cells, which is consistent with the results from the in vitro assay (Figure 1D).
Detection of Fyn Activation in Jurkat T Cells.
During T cell activation, Fyn can be activated in the TCR signaling pathways, which is crucial in further activating downstream target molecules such as ERK, PI3K, and PLC.1d We hence examined our biosensor in monitoring Fyn activation in Jurkat T cell lines. CD3 (one type of subunits of the TCR complex) antibody binding to cell surface can cause clustering of TCR molecules and activate TCR signaling.17 Indeed, when Jurkat T cells expressing the Fyn biosensor adhered onto the CD3 antibody-coated glass surface, an obvious FRET response was observed (Figure 3A,B). In contrast, no FRET change was detected in cells expressing the Fyn(Y/F) mutant biosensor, indicating the FRET response of our biosensor can reflect the kinase-mediated phosphorylation (largely from Fyn as characterized above) in the biosensor substrate sequence (Figure 3A,B).
Figure 3.
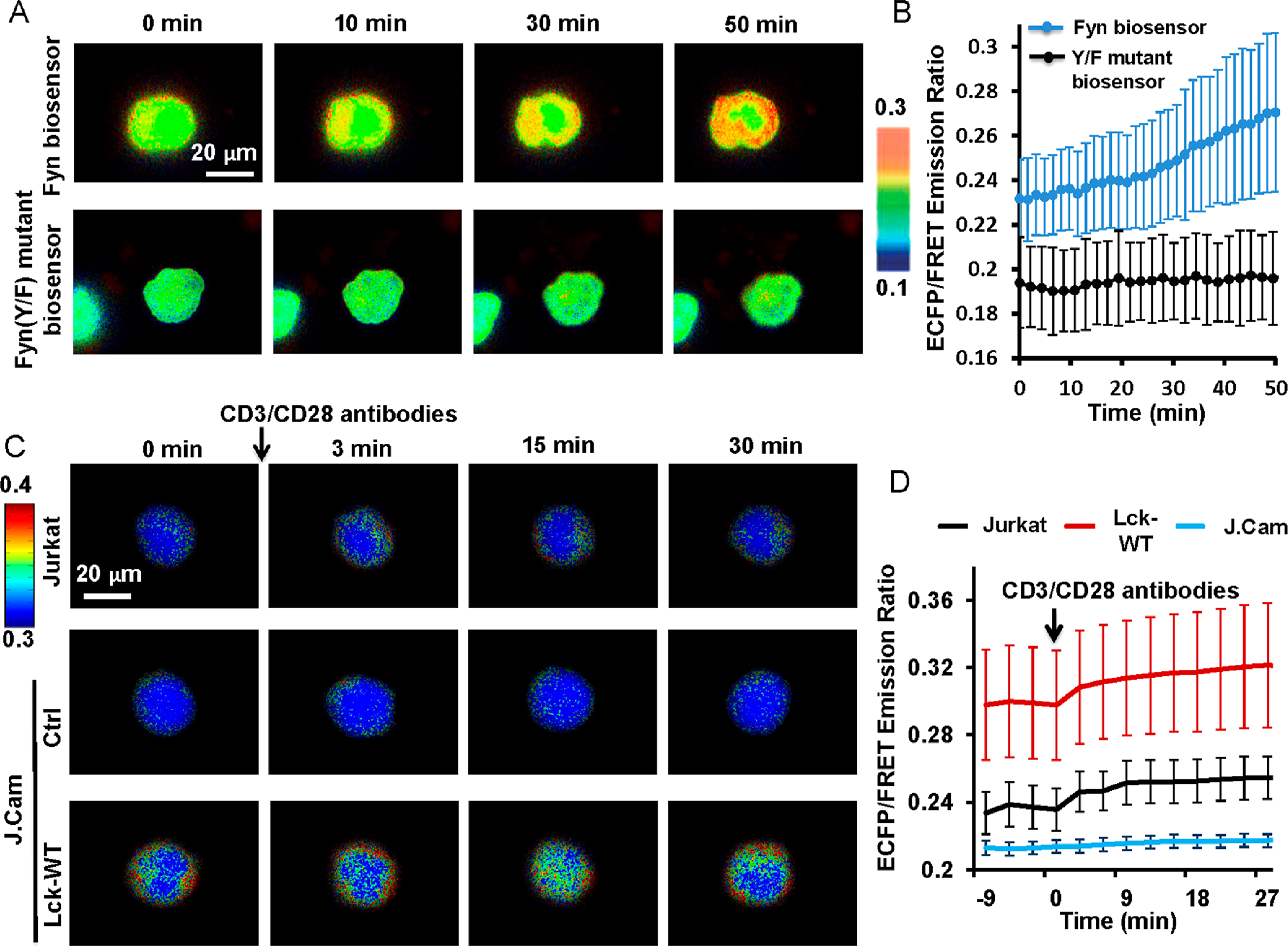
Fyn activity in Jurkat cells. The Fyn biosensor construct was introduced into cells by electroporation. (A) The FRET response of the wild-type biosensor (top panel) or the mutant Fyn(Y/F) biosensor (bottom panel) in Jurkat cells when they were adhered on CD3 antibody-coated (10 μg/mL) glass-bottom dishes. Imaging started within about 10 min after cells were settled onto the coated surface. (B) The quantified FRET changes (mean ± SD, n = 16, 18) from the groups of cells in (A). (C) The FRET response of the Fyn biosensor in Jurkat or JCam cells treated with the preclustered CD3/CD28 antibody complexes. Cells were transfected with the biosensor alone or together with the Lck construct (Lck-WT) by electroporation. (D) The quantified FRET changes (mean ± SD, n = 26, 22, 21) from the groups of cells in (C).
We further examined whether our biosensor can monitor the Fyn activation mediated by Lck in T cells, a crucial signaling pathway as previously reported.9a The wild-type Lck (Lck-WT) was introduced together with the Fyn biosensor into Lck-deficient J.Cam1.6 (J.Cam) cells, a derivative from the E6–1 clone of the Jurkat T cell line (ATCC).18 The adherent cells were then stimulated with the preclustered CD3/CD28 antibody complexes to activate TCR signals.19 J.Cam cells reconstituted with Lck had a higher Fyn activity both before and after the antibody stimulation, whereas the J.Cam control cells had low Fyn activity throughout the experiment (Figure 3C,D). These results indicate a positive impact of Lck on Fyn activation in T cells, consistent with the previous report for Fyn regulation by Lck.9a Therefore, our Fyn biosensor can be used to monitor the dynamic Fyn activities in live T cells and verify the underlying regulatory signaling pathway.
Detection of Subcellular Distribution of Fyn Kinase Activity.
It has been reported that Fyn kinase can localize to membrane microdomains via its N-terminal palmitoylation and myristoylation.4a,b,9b To position the Fyn biosensor in a close proximity of Fyn kinase at intracellular plasma membrane or the outer side of cellular organelle membranes in MEFs, a peptide “MGCIKSKRKDNLNDDE” derived from the N-terminus of Lyn kinase (Lyn tag) was added to the N-terminus of the Fyn biosensor, named as the Lyn-Fyn biosensor4a,b,9b (Figure 4A). Upon PDGF stimulation, the Lyn-Fyn biosensor in MEF cells showed an immediate increase in the ECFP/FRET ratio (Figure 4B and Movie S2) but at a lower level (~60% increase) (Figure 5) than that reported by the cytosolic biosensor (Figure 2A).
Figure 4.
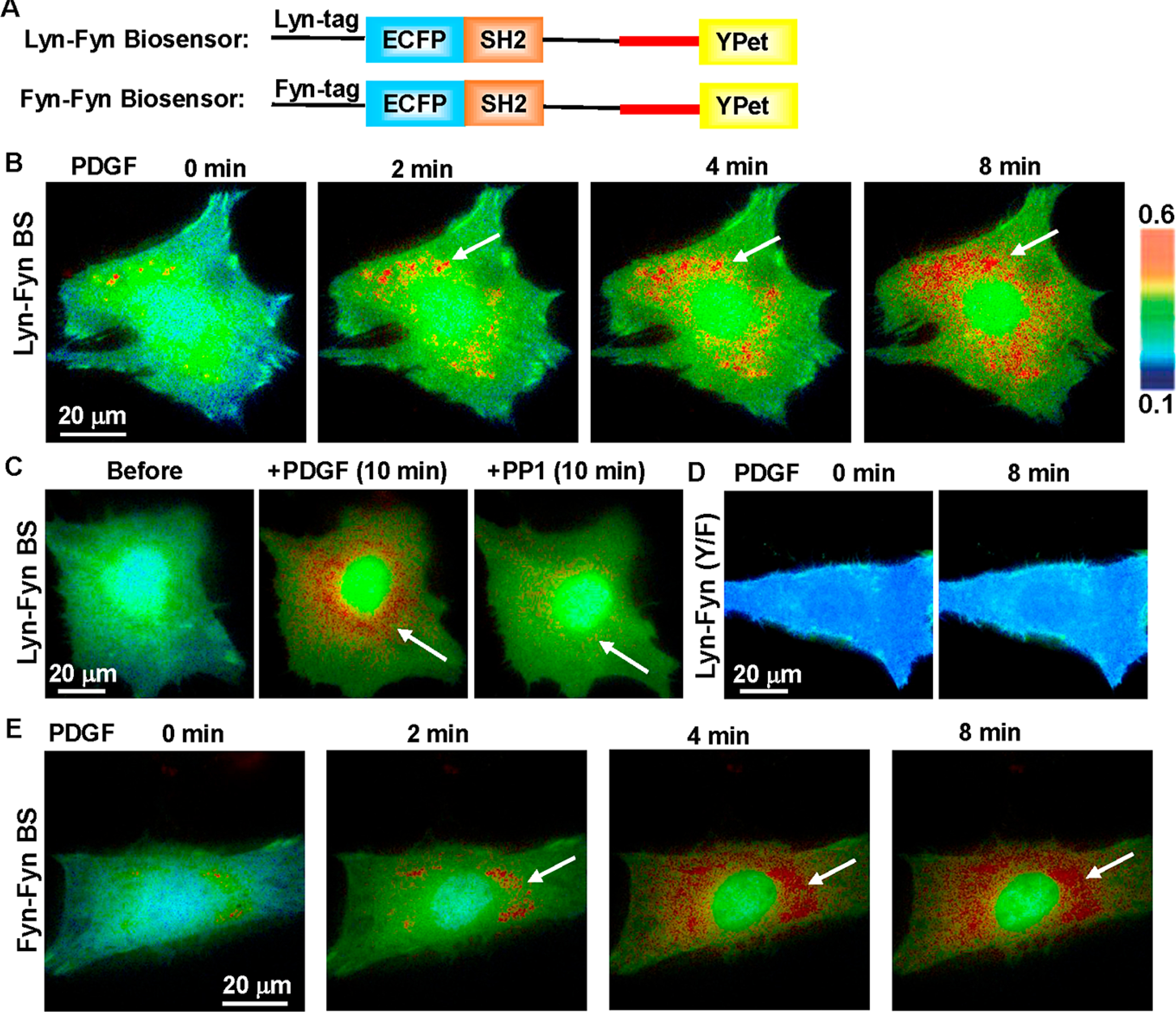
Detection of Fyn activity around the perinuclear area by the membrane-targeted biosensor. (A) The schematics of Lyn- and Fyn N-terminal peptide-tagged (Fyn-Fyn) biosensors. (B) The ratiometric (ECFP/FRET) images of the Lyn-Fyn biosensor in a representative MEF before and after PDGF stimulation. The arrows point to the perinuclear regions of high FRET activity. (C) The high FRET of the Lyn-Fyn biosensor around the perinuclear area was inhibited by Src family inhibitor PP1 (10 μM). (D) The Y/F mutant of the Lyn-Fyn biosensor displays low and relatively uniform FRET distribution in MEF, distinct from the wild-type biosensor. (E) The Fyn-tagged Fyn biosensor displays a similar FRET distribution pattern as the Lyn-Fyn biosensor, with high FRET activity at perinuclear regions (arrows) in MEFs.
Figure 5.
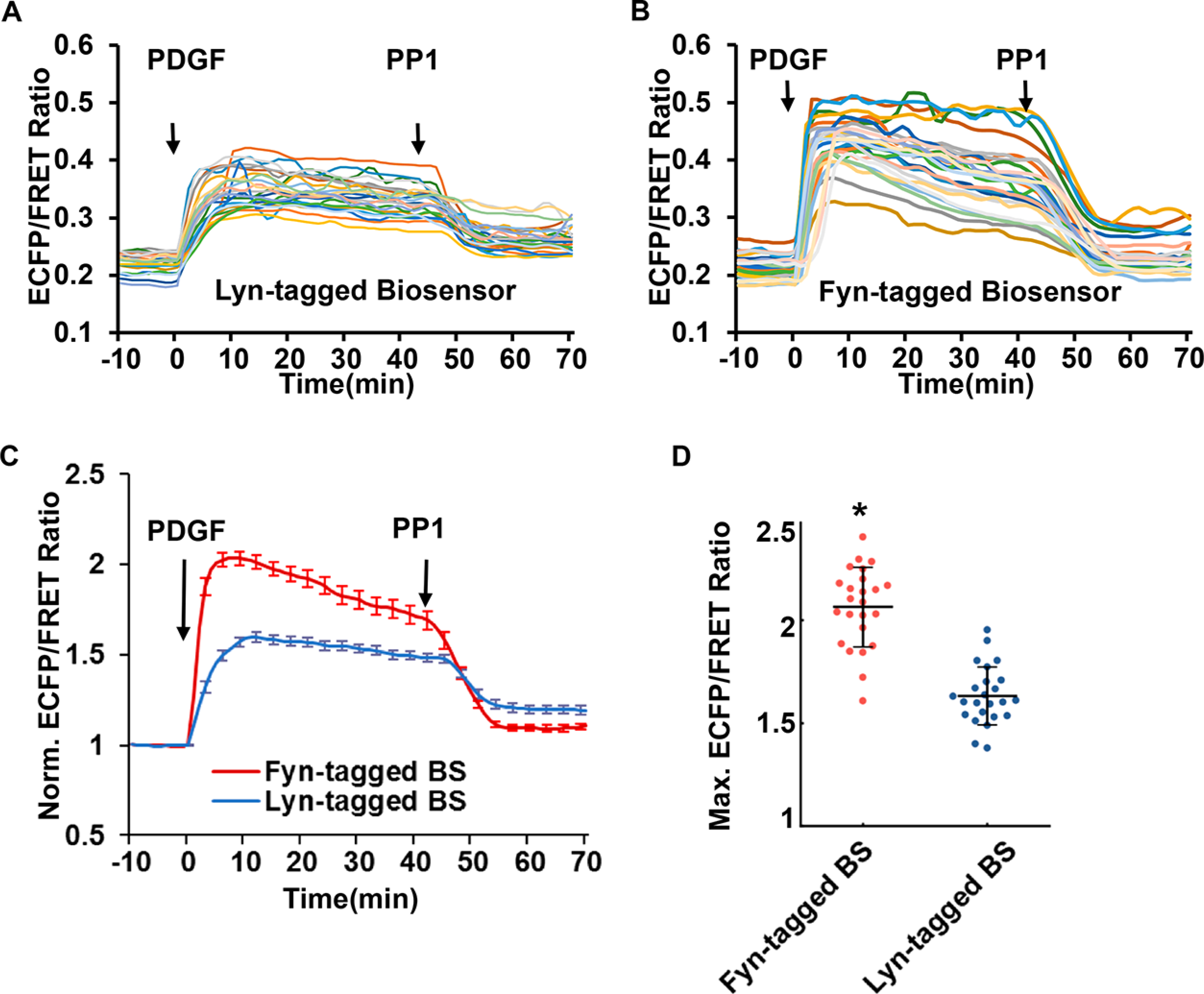
Detection of distinct Fyn activity by Lyn-tagged or Fyn-tagged biosensor. The time course curves show the PDGF-induced FRET response of (A) the Lyn-tagged biosensor or (B) the Fyn-tagged biosensor from a group of MEF cells, followed by the addition of Src family inhibitor PP1 (10 μM). Each colored line was quantified from one single cell. (C) The normalized time courses of the ECFP/FRET ratio from the group of cells in (A) and (B). Error bars: SEM. (D) Compare the normalized maximal ECFP/FRET ratio reported by the Fyn-tagged (scatter plots with mean ± SD: 2.06 ± 0.19) and Lyn-tagged (1.63 ± 0.14) biosensors after PDGF stimulation (* p < 0.001 by Student’s t test, n = 24).
The cytosolic biosensor can diffuse in 3D throughout the whole cell body, while the Lyn-Fyn biosensor is constrained to the microdomains along the 2D cellular membrane.20 This difference in local topology where the biosensors are localized may contribute to the response dynamic range. Interestingly, a group of more activated Lyn-Fyn biosensors (higher ECFP/FRET ratio) was observed to localize around perinuclear regions in MEFs (Figure 4B and Movie S2). Adding Src family inhibitor PP1 to these cells immediately caused the decrease of the ECFP/FRET ratio and eliminated the spatial pattern of the high biosensor signals concentrated at perinuclear regions (Figures 4C and 5A). Consistently, this perinuclear high FRET ratio was not observed in cells with the Y/F mutant biosensor (Figure 4D). These results suggest that the perinuclear pool of biosensors with the higher ECFP/FRET ratio is due to the specific tyrosine phosphorylation but not from nonspecific biosensor aggregation or misfolding at perinuclear organelles.
In order to assess the impact of the Lyn tag itself, we further fused the N-terminal sequence from Fyn to the biosensor to target the biosensor more precisely at the location of Fyn kinase. The Fyn-tagged biosensor showed a prominent subcellular pattern of high ECFP/FRET ratios at perinuclear regions, similar to that of the Lyn-tagged version (Figure 4E and Movie S3). This result suggests that the perinuclear high FRET activity observed is unlikely due to targeting tags but a possible Fyn activation occurring at the membrane of perinuclear organelles. Our results hence demonstrate that, although we cannot completely exclude the contributions of other localized kinases, the membrane-targeted Fyn biosensor can highlight the perinuclear-localized activation pattern of Fyn kinase at perinuclear regions upon PDGF stimulation, which is not possible with the cytosolic biosensor. Although the membrane-targeted biosensor did not detect obvious Fyn activation on the cell boundaries which was documented by antiphospho-Y416 Src antibody,4a we reasoned that the biosensor may be diffusible and not have the resolution needed for the detection of Fyn activation occurring on the local plasma membranes of fibroblasts.
Characterization of Fyn Activity at the Membrane Microdomains.
The quantified ECFP/FRET ratio time courses showed that the Fyn-tagged biosensor had a significantly stronger response (106% increase) to PDGF stimulation than the Lyn-tagged biosensor (63% increase, Figure 5C). The length of the Lyn tag is not a significant factor affecting the FRET response in comparison, as the biosensors with 16aa- or 21aa-Lyn tag showed FRET changes at a similar scale (~50–60%) in response to PDGF stimulation (Figures 5B and S6). Although both Lyn and Fyn tags can target the biosensor at the intracellular membrane, one possible reason for the difference of the FRET response is the closer localization of the Fyn-tagged biosensor to Fyn kinase. The Lyn tag has one Cys (C3) and the Fyn tag has two (C3 and C6) at their N-termini. Palmitoylation at two Cys residues in the Fyn tag could cause its different spatial partition in the membrane microdomains from the Lyn tag. As such, the Fyn-tagged biosensor may locate closer to Fyn kinase with higher stability anchoring at the membrane than the Lyn-Fyn biosensor.
In Fyn kinase, the N-terminal starting sequence MGCVQCKDK is important for its membrane localization through fatty acylation. Myristoylation occurs at the first glycine (G2) and palmitoylation at the third and sixth cysteines (C3 and C6), which are required for membrane localization of Fyn and its interaction with the TCR complex in T-cells.21 While myristoylation can help the target proteins to interact with intracellular membrane, palmitoylation facilitates the stable anchoring of the protein.21b To examine the roles of specific residues within the Fyn-tag in regulating the membrane localization of Fyn kinase, the palmitoylation sites, C3 and C6, were mutated to serine,4a and the Fyn kinase activity was monitored by the cytosolic Fyn biosensor (Figure 6). Surprisingly, the mutant Fyn C3S/C6S (Fyn(GSS)) displays much more elevated activity than wild-type Fyn both before and after PDGF stimulation in MEFs (Figure 6). In this case, the overall Fyn kinase activities at both basal level and that with PDGF stimulation measured with the cytosolic-Fyn-BS ratio value were detected to be higher for the mutant Fyn(GSS) compared to the Fyn (WT) kinase. This result suggests that Fyn may become more active outside of its microdomains. When the myristoylation site (G2) was further mutated to serine, this triple mutant Fyn kinase (G2S/C3S/C6S) (Fyn(SSS)) lost plasma membrane anchoring and became less active (Figure 6). The activities of Fyn mutants were further confirmed in SYF(−/−) cells coexpressing the cytosolic biosensor: Fyn(GSS) can activate the biosensor much more than the wild-type Fyn, while Fyn(SSS) is relatively inactive (Figures 7A and 7B). Indeed, the Lyn-tagged biosensor did not detect the hyperactivity of Fyn(GSS) in either MEF or SYF(−/−) cells (Figure S7), supporting a spatial separation between Fyn(GSS) localization and the Lyn-tagged membrane microdomains (Figure 7C). Collectively, our results suggest that the membrane accessibility is critical for Fyn activation; however, Fyn localization in the specific membrane microdomains may provide a suppressive regulation of Fyn activity.
Figure 6.
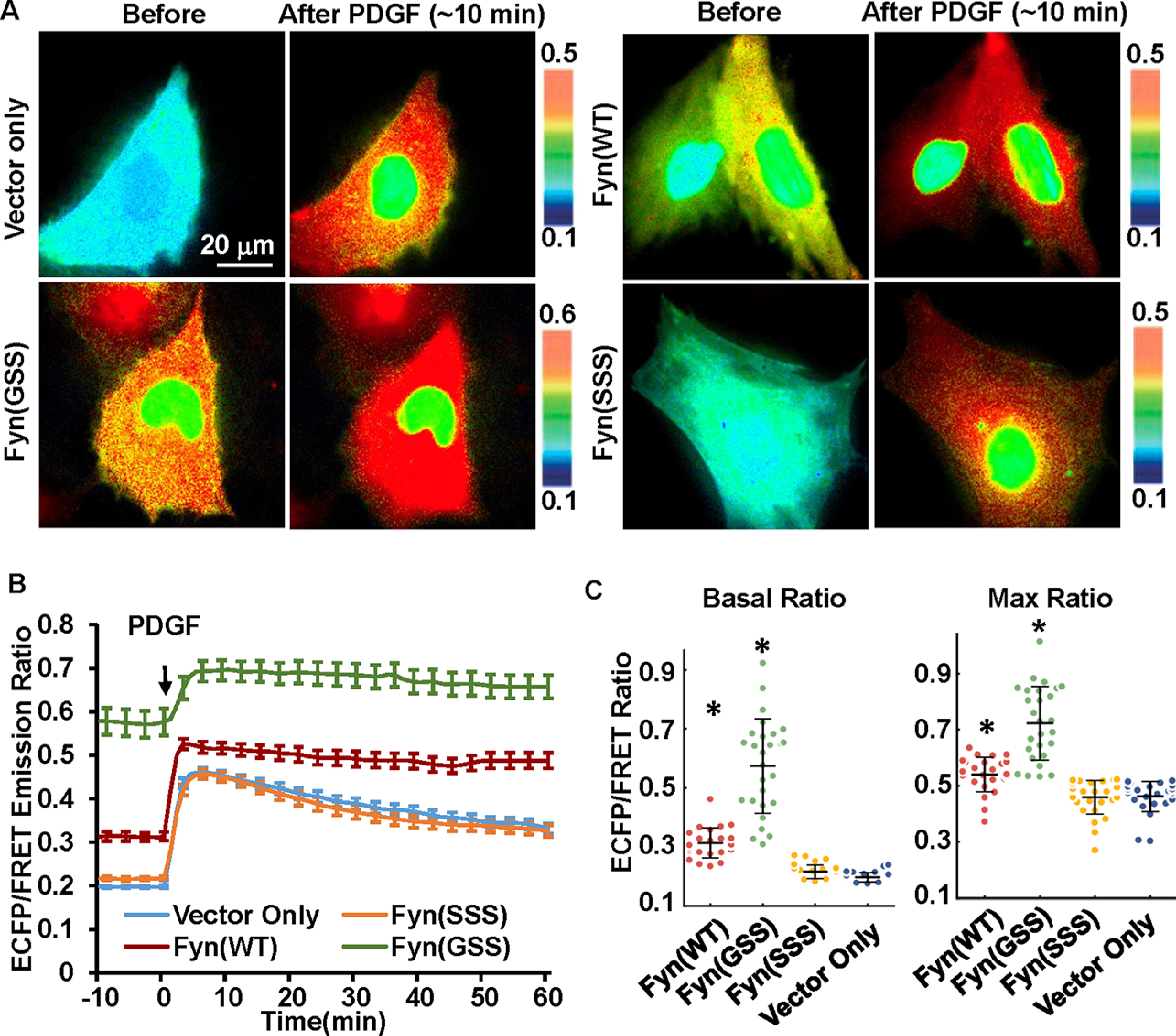
The effect of Fyn N-terminal fatty acylation on Fyn activation in MEF cells. Change of palmitoylation or myristoylation modification at the Fyn N-terminal was introduced by mutation of wild-type Fyn(GCC) to Fyn(GSS) or Fyn(SSS). (A) Representative ECFP/FRET ratio images of PDGF-stimulated MEF cells cotransfected with the cytosolic Fyn biosensor (1.0 μg of DNA on a 24-well plate) with vector only or the indicated Fyn mutants (0.3 μg of DNA each). (B) The average time courses of the ECFP/FRET ratio of the cytosolic Fyn biosensor in cells coexpressing the Fyn mutants or control vector in (A) when they are treated with PDGF (mean ± SEM). (C) The scatter plots with mean ± SD compare the basal level and maximal ECFP/FRET ratio in cells with time courses shown in (B). (* indicates a statistically significant difference when compared with the control vector group by Student’s t test, p < 1.0e-4, n = 25, 27, 29, 27). In cells cotransfected with Fyn(WT), Fyn(GSS), Fyn(SSS), or the control vector, the ECFP/FRET ratio values (mean ± SD) were, respectively, 0.31 ± 0.05, 0.57 ± 0.16, 0.22 ± 0.023, or 0.20 ± 0.016 at the basal level and 0.54 ± 0.06, 0.72 ± 0.13, 0.46 ± 0.06, or 0.46 ± 0.05 at peak after PDGF stimulation.
Figure 7.
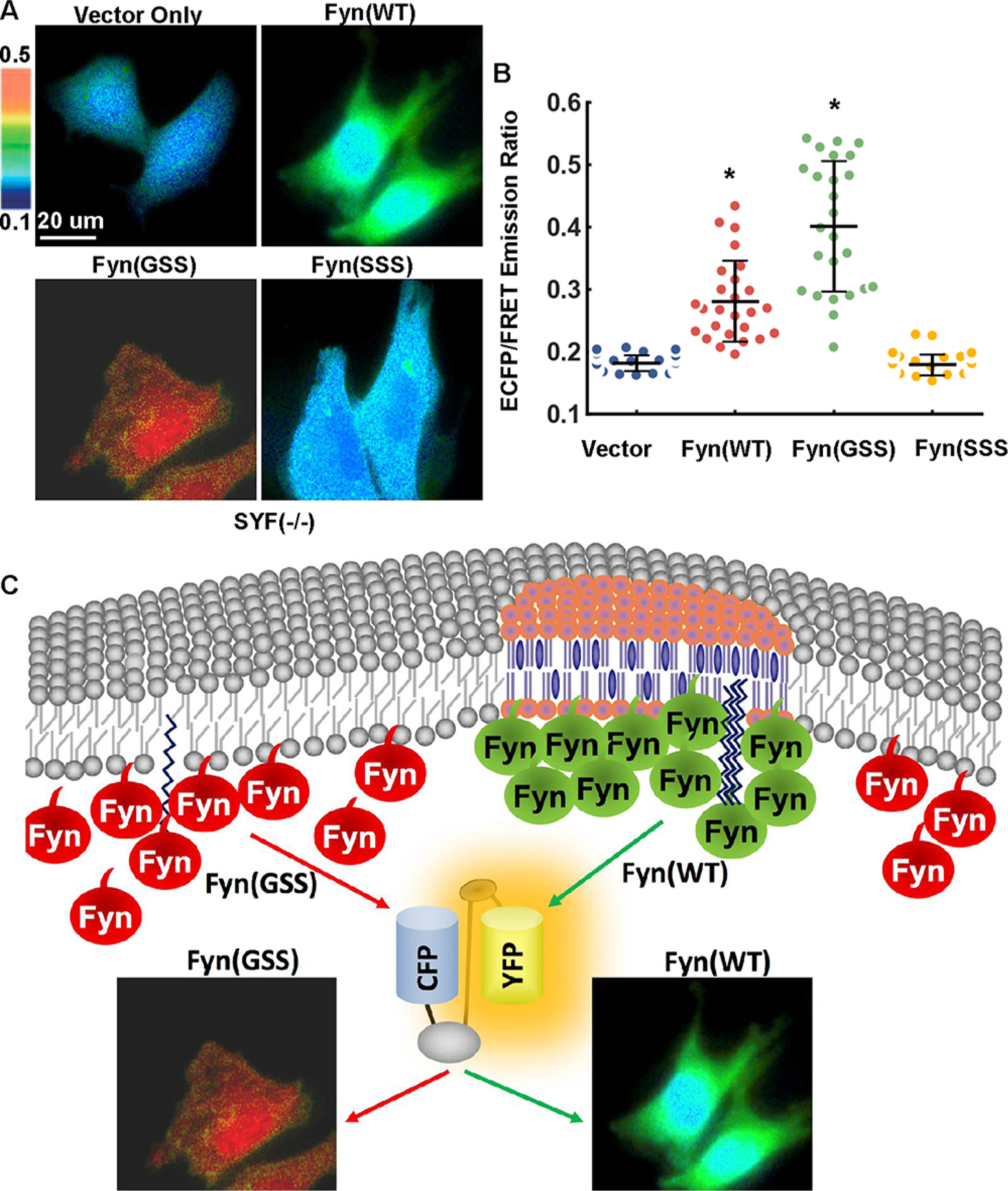
The activities of Fyn and its mutants in SYF(−/−) cells and an illustration of Fyn kinase activity at different membrane microdomains. Cells cotransfected with the cytosolic biosensor (1.0 μg of DNA on a 24-well plate) and vector only, Fyn(WT), Fyn(GSS), or Fyn(SSS) construct (0.3 μg of DNA each) were seeded on fibronectin-coated glass-bottom dishes overnight, followed by FRET measurement. (A) The representative ECFP/FRET ratio images and (B) the quantified cellular ECFP/FRET ratio (scatter plots with mean ± SD) are 0.17 ± 0.013 (vector), 0.281 ± 0.065 (Fyn WT), 0.399 ± 0.10 (Fyn GSS), or 0.179 ± 0.016 (Fyn SSS) in cells with the indicated Fyn variant. * indicates a statistically significant difference from all other groups by t test (n = 31, 26, 25, 33, p-value <10−4). (C) Fyn with N-terminal palmitoylation is located at submembrane microdomains and maintains relatively low activity, possibly due to phosphorylation of PAG/CBP (Csk-binding protein) followed by recruitment of Fyn negative regulator Csk. N-terminal nonpalmitoylated Fyn located outside these microdomains has loose association with plasma membrane and shows constitutively high activity. The Fyn FRET biosensor displays a normal or highly active status of Fyn respectively in the cotransfected SYF(−/−) cells.
DISCUSSION
The Fyn tyrosine kinase plays important physiological roles in the immune and neuronal systems. Our work developed and characterized the first Fyn FRET biosensor for monitoring the Fyn kinase activity in live-cells with high sensitivity. Utilizing the SH2 domain with tuned binding affinity to tyrosine-phosphorylated substrate peptide, an optimized biosensor containing the C185A-SH2 and a tyrosine peptide from Fyn substrate p34CDC2 is engineered, which shows a 140% increase of the ECFP/FRET ratio toward Fyn kinase activation (Figure 1D). The C185A-SH2 domain has also been applied to improve the sensitivity of a Src biosensor,15 highlighting the broad applicability of the C185A-SH2 domain to serve as a binding partner for tyrosine phosphorylated peptide motifs. Through the comparison among different tyrosine kinases, the biosensor displays a clear preference for Fyn activity over other kinases. This result is also consistent with a previous report that this specific substrate peptide employed in our biosensor displayed a higher sensitivity to Fyn than to Src or Lck.13b
Fyn is localized at the cell membrane via its SH4 domain with fatty acylation. Here, we positioned the Fyn biosensor close to Fyn kinase at cell membranes through a Lyn or Fyn tag, to monitor Fyn activity at different membrane microdomains. Surprisingly, a subpopulation of Fyn with high kinase activity is observed around the perinuclear area by the membrane-targeted biosensor. This result highlights the spatial resolution achievable by the genetically targeted and subcellularly anchored Fyn FRET biosensors, which should be difficult for cytosolic biosensors diffusible in the cell body with the reported signals averaged across space. The population of highly active Fyn is possibly localized on the surface of the perinuclear membrane structures such as ER compartments related to endosomes.4a
The existence of microdomains in the cell membrane has been well reported, although direct visualization of the microdomains in live cells has been difficult due to their nanoscale size and dynamic organization.22 Fyn kinase is known to localize at these membrane microdomains through N-terminal modification with palmitoylation and myristoylation.4b,21b The Fyn-tagged biosensor has more FRET response than that of the Lyn-tagged one in response to PDGF stimulation (Figure 5). This is surprising, considering that the two versions of biosensor are identical except for the small tags at the N-terminus. The double palmitoylation sites on the Fyn tag may bring the biosensor close to interact with Fyn kinase more stably than the single site of palmitoylation on the Lyn tag. Their sensitivity difference may also imply that signal molecules could be spatially separated in membrane microdomains through their fatty acid modifications. This observation is consistent with the study that the trafficking pathways of Fyn and Lyn kinases to the plasma membrane differ due to their specific N-terminal palmitoylation states.23 It will be interesting to further understand how these microdomains are sorted into different kinds of compartments based on their specific functional molecules. Biosensors with specific targeting tags hence may help to characterize the subtle difference among different membrane microdomains and to study the underlying mechanism of local molecular regulations.
It is surprising that nonmicrodomain-targeted Fyn has higher kinase activity than wild-type Fyn (Figures 5B and 6B). Although previously antibody staining (a less quantitative method) showed palmitoylation-deficient Fyn can still be moderately activated in cells,4a its significantly enhanced activity measured by our FRET biosensor provides new insights on the regulation of cellular Fyn activity. Our observation suggests that when located outside of these microdomains, Fyn can be highly active, probably due to segregation from deactivator such as Csk via biophysical properties or lipids ionic interactions.1d,24 This result again highlights the critical biological function of the membrane microdomains which not only provide docking sites for Fyn kinase but also the spatial platforms for appropriate regulation of functional Fyn activity. Palmitoylation-deficiency may cause malfunction of Fyn in cells by location of Fyn outside of the microdomains, with the elevated Fyn activity potentially leading to diseases, including immunological disorders and cancers.3a,25 For instances, overexpression of Fyn is detected in multiple cancers3a and in T cells with a lymphoproliferative disorder;24 hyperactivity of Fyn is also connected with brain migration disorder and T cell activation deficiency.25a
Therefore, we hypothesize the following model based on the current data (Figure 7C): Fyn with N-terminal palmitoylation is located at specific membrane microdomains and maintains relatively low activity, possibly due to the local phosphorylation of PAG/CBP (Csk-binding protein) followed by recruitment of Csk, a negative regulator of Fyn kinase, whereas N-terminal nonpalmitoylated Fyn located outside these microdomains has loose association with plasma membrane and shows constitutively high activity.5,26 The highly active signals of the Fyn biosensor at the perinuclear regions also suggest that membrane compositions at these perinuclear organelles can be significantly different from those of the plasma membrane. In fact, it was reported that there is a lack of rafts in mitochondria.27 Therefore, a negative control mechanism of Fyn kinase may be lacking in perinuclear membranes. In considering that the contribution of other localized kinases to the biosensor activation cannot be completely excluded in this study, alternative models could be possible.
MATERIALS AND METHODS
DNA Constructs.
The Fyn biosensor was first constructed into the vector pRSETb for bacterial expression. The DNA fragment including SH2 domain, a 15 amino acids linker and the substrate peptide EKIEGTYGVV, was amplified by PCR based on the template of a Src biosensor.10a The PCR fragment with SH2 domain containing a single-site mutation (C185A) was amplified similarly from two newly improved Src biosensors as the template.15 The individual PCR fragment was ligated between ECFP and YPet fluorescent proteins from the Src biosensor by SphI/SacI restriction sites. The biosensors with the two versions of SH2 are the same except for the mutation in SH2 domain. The biosensor in pRSETb was further amplified by PCR and ligated into the mammalian expression vector pCAGGS28 by using EcoRI/SalI sites. The negative Y/F mutation in the substrate of the biosensor was introduced by site-directed mutagenesis (following the protocol from Agilent Technologies) in the pRSETb version, and the mutated biosensor was further subcloned into the pCAGGS vector. The membrane-targeted biosensors were constructed by PCR amplification of the biosensor while adding the Lyn tag (MGCIKSKRKDNLNDDE), the longer Lyn tag with 21aa (MGCIKSKRKDNLNDDGVDMKT) or the Fyn tag (MGCVQCKDKEATKLTEERDGSLNQ) at the N-terminus of the biosensor and then by ligation into the pCAGGS vector with EcoRI/SalI sites. The DNA constructs containing wild-type Fyn, Fyn(K299M) mutant, and c-Src were described previously.10b,29
Fyn N-terminal mutation from G2C3C6 (GCC) to G2S3S6 (GSS) or S2S3S6 (SSS) was generated by site-directed mutagenesis (following the protocol from Agilent Technologies).
The other experimental procedures are available in the Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Jia Guo and Zhili Qian (Changzhou University) for technical help. This work is supported by grants from NIH HL121365, GM125379, CA204704, and CA209629 (Y.W. and S.Lu), the Natural Science Foundation of Jiangsu Province (Grant Nos. BK20181464) (M.O.), and NSFC11532003 (L.D.), National Science Foundation (NSF) CBET1360341 (Y.W.), NSF under NSF/NIH Math/Bio Initiative DMS1361421 (S.Lu), and UC FISP undergraduate research fellowship (S.Laub). This research was also supported by the China Scholarship Council (R.W.).
Footnotes
The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare the following competing financial interest(s): Y.W. and S.Lu are scientific co-founders of and have an equity interest in Cell E&G Inc. However, these financial interests do not affect the design, conduct, or reporting of this research.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssensors.8b00896.
REFERENCES
- (1).(a) Semba K; Nishizawa M; Miyajima N; Yoshida MC; Sukegawa J; Yamanashi Y; Sasaki M; Yamamoto T; Toyoshima K yes-related protooncogene, syn, belongs to the protein-tyrosine kinase family. Proc. Natl. Acad. Sci. U. S. A. 1986, 83 (15), 5459–63. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Resh MD Fyn, a Src family tyrosine kinase. Int. J. Biochem. Cell Biol. 1998, 30 (11), 1159–62. [DOI] [PubMed] [Google Scholar]; (c) Palacios EH; Weiss A Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene 2004, 23 (48), 7990–8000. [DOI] [PubMed] [Google Scholar]; (d) Salmond RJ; Filby A; Qureshi I; Caserta S; Zamoyska R T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol. Rev. 2009, 228 (1), 9–22. [DOI] [PubMed] [Google Scholar]; (e) Lee G; Thangavel R; Sharma VM; Litersky JM; Bhaskar K; Fang SM; Do LH; Andreadis A; Van Hoesen G; Ksiezak-Reding H Phosphorylation of tau by fyn: implications for Alzheimer’s disease. J. Neurosci. 2004, 24 (9), 2304–12. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ittner LM; Ke YD; Delerue F; Bi M; Gladbach A; van Eersel J; Wolfing H; Chieng BC; Christie MJ; Napier IA; Eckert A; Staufenbiel M; Hardeman E; Gotz J Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer’s disease mouse models. Cell 2010, 142 (3), 387–97. [DOI] [PubMed] [Google Scholar]; (g) James JR; Vale RD Biophysical mechanism of T-cell receptor triggering in a reconstituted system. Nature 2012, 487 (7405), 64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Yu CC; Yen TS; Lowell CA; DeFranco AL Lupus-like kidney disease in mice deficient in the Src family tyrosine kinases Lyn and Fyn. Curr. Biol. 2001, 11 (1), 34–8. [DOI] [PubMed] [Google Scholar]; (b) Kim JE; Roh E; Lee MH; Yu DH; Kim DJ; Lim TG; Jung SK; Peng C; Cho YY; Dickinson S; Alberts D; Bowden GT; Einspahr J; Stratton SP; Curiel-Lewandrowski C; Bode AM; Lee KW; Dong Z Fyn is a redox sensor involved in solar ultraviolet light-induced signal transduction in skin carcinogenesis. Oncogene 2016, 35 (31), 4091–101. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Yang K; Belrose J; Trepanier CH; Lei G; Jackson MF; MacDonald JF Fyn, a potential target for Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 27 (2), 243–52. [DOI] [PubMed] [Google Scholar]
- (3).(a) Saito YD; Jensen AR; Salgia R; Posadas EM Fyn: a novel molecular target in cancer. Cancer 2010, 116 (7), 1629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sicheri F; Moarefi I; Kuriyan J Crystal structure of the Src family tyrosine kinase Hck. Nature 1997, 385 (6617), 602–9. [DOI] [PubMed] [Google Scholar]; (c) Williams JC; Weijland A; Gonfloni S; Thompson A; Courtneidge SA; Superti-Furga G; Wierenga RK The 2.35 A crystal structure of the inactivated form of chicken Src: a dynamic molecule with multiple regulatory interactions. J. Mol. Biol. 1997, 274 (5), 757–75. [DOI] [PubMed] [Google Scholar]; (d) Xu W; Harrison SC; Eck MJ Three-dimensional structure of the tyrosine kinase c-Src. Nature 1997, 385 (6617), 595–602. [DOI] [PubMed] [Google Scholar]
- (4).(a) Sandilands E; Brunton VG; Frame MC The membrane targeting and spatial activation of Src, Yes and Fyn is influenced by palmitoylation and distinct RhoB/RhoD endosome requirements. J. Cell Sci. 2007, 120 (15), 2555–2564. [DOI] [PubMed] [Google Scholar]; (b) Liang X; Lu Y; Wilkes M; Neubert TA; Resh MD The N-terminal SH4 region of the Src family kinase Fyn is modified by methylation and heterogeneous fatty acylation: role in membrane targeting, cell adhesion, and spreading. J. Biol. Chem. 2004, 279 (9), 8133–9. [DOI] [PubMed] [Google Scholar]; (c) Alland L; Peseckis SM; Atherton RE; Berthiaume L; Resh MD Dual myristylation and palmitylation of Src family member p59fyn affects subcellular localization. J. Biol. Chem. 1994, 269 (24), 16701–5. [PubMed] [Google Scholar]
- (5).Brdicka T; Pavlistova D; Leo A; Bruyns E; Korinek V; Angelisova P; Scherer J; Shevchenko A; Hilgert I; Cerny J; Drbal K; Kuramitsu Y; Kornacker B; Horejsi V; Schraven B Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J. Exp. Med. 2000, 191 (9), 1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Xu W; Doshi A; Lei M; Eck MJ; Harrison SC Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol. Cell 1999, 3 (5), 629–38. [DOI] [PubMed] [Google Scholar]
- (7).(a) Hurley TR; Hyman R; Sefton BM Differential effects of expression of the CD45 tyrosine protein phosphatase on the tyrosine phosphorylation of the lck, fyn, and c-src tyrosine protein kinases. Mol. Cell. Biol. 1993, 13 (3), 1651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Thomas ML; Brown EJ Positive and negative regulation of Src-family membrane kinases by CD45. Immunol Today 1999, 20 (9), 406–11. [DOI] [PubMed] [Google Scholar]
- (8).(a) Alvarado JJ; Betts L; Moroco JA; Smithgall TE; Yeh JI Crystal structure of the Src family kinase Hck SH3-SH2 linker regulatory region supports an SH3-dominant activation mechanism. J. Biol. Chem. 2010, 285 (46), 35455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cowan-Jacob SW; Fendrich G; Manley PW; Jahnke W; Fabbro D; Liebetanz J; Meyer T The crystal structure of a c-Src complex in an active conformation suggests possible steps in c-Src activation. Structure 2005, 13 (6), 861–71. [DOI] [PubMed] [Google Scholar]; (c) Trible RP; Emert-Sedlak L; Smithgall TE HIV-1 Nef selectively activates Src family kinases Hck, Lyn, and c-Src through direct SH3 domain interaction. J. Biol. Chem. 2006, 281 (37), 27029–38. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kypta RM; Goldberg Y; Ulug ET; Courtneidge SA Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell 1990, 62 (3), 481–92. [DOI] [PubMed] [Google Scholar]; (e) Fry MJ; Panayotou G; Booker GW; Waterfield MD New insights into protein-tyrosine kinase receptor signaling complexes. Protein Sci. 1993, 2 (11), 1785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Boggon TJ; Eck MJ Structure and regulation of Src family kinases. Oncogene 2004, 23 (48), 7918–27. [DOI] [PubMed] [Google Scholar]
- (9).(a) Filipp D; Zhang J; Leung BL; Shaw A; Levin SD; Veillette A; Julius M Regulation of Fyn through translocation of activated Lck into lipid rafts. J. Exp. Med. 2003, 197 (9), 1221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Filipp D; Moemeni B; Ferzoco A; Kathirkamathamby K; Zhang J; Ballek O; Davidson D; Veillette A; Julius M Lck-dependent Fyn activation requires C terminus-dependent targeting of kinase-active Lck to lipid rafts. J. Biol. Chem. 2008, 283 (39), 26409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Ouyang M; Sun J; Chien S; Wang Y Determination of hierarchical relationship of Src and Rac at subcellular locations with FRET biosensors. Proc. Natl. Acad. Sci. U. S. A. 2008, 105 (38), 14353–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang Y; Botvinick EL; Zhao Y; Berns MW; Usami S; Tsien RY; Chien S Visualizing the mechanical activation of Src. Nature 2005, 434 (7036), 1040–5. [DOI] [PubMed] [Google Scholar]; (c) Seong J; Ouyang M; Kim T; Sun J; Wen PC; Lu S; Zhuo Y; Llewellyn NM; Schlaepfer DD; Guan JL; Chien S; Wang Y Detection of focal adhesion kinase activation at membrane microdomains by fluorescence resonance energy transfer. Nat. Commun. 2011, 2, 406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Seong J; Lu S; Ouyang M; Huang H; Zhang J; Frame MC; Wang Y Visualization of Src activity at different compartments of the plasma membrane by FRET imaging. Chem. Biol. 2009, 16 (1), 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Xiang X; Sun J; Wu J; He HT; Wang Y; Zhu C A FRET-Based Biosensor for Imaging SYK Activities in Living Cells. Cell. Mol. Bioeng. 2011, 4 (4), 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Li K; Xiang X; Sun J; He HT; Wu J; Wang Y; Zhu C Imaging Spatiotemporal Activities of ZAP-70 in Live T Cells Using a FRET-Based Biosensor. Ann. Biomed. Eng. 2016, 44, 3510–3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Cheng HC; Litwin CM; Hwang DM; Wang JH Structural basis of specific and efficient phosphorylation of peptides derived from p34cdc2 by a pp60src-related protein tyrosine kinase. J. Biol. Chem. 1991, 266 (27), 17919–25. [PubMed] [Google Scholar]; (b) Cheng HC; Nishio H; Hatase O; Ralph S; Wang JH A synthetic peptide derived from p34cdc2 is a specific and efficient substrate of src-family tyrosine kinases. J. Biol. Chem. 1992, 267 (13), 9248–56. [PubMed] [Google Scholar]
- (14).Waksman G; Kominos D; Robertson SC; Pant N; Baltimore D; Birge RB; Cowburn D; Hanafusa H; Mayer BJ; Overduin M; Resh MD; Rios CB; Silverman L; Kuriyan J Crystal structure of the phosphotyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature 1992, 358 (6388), 646–53. [DOI] [PubMed] [Google Scholar]
- (15).Wang PZ; Liang J; Shi LZ; Wang Y; Zhang P; Ouyang MX; Preece D; Peng Q; Shao LA; Fan J; Sun J; Li SS; Berns MW; Zhao HM; Wang YX Visualizing Spatiotemporal Dynamics of Intercellular Mechanotransmission upon Wounding. ACS Photonics 2018, 5 (9), 3565–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Hamamura K; Tsuji M; Hotta H; Ohkawa Y; Takahashi M; Shibuya H; Nakashima H; Yamauchi Y; Hashimoto N; Hattori H; Ueda M; Furukawa K; Furukawa K Functional activation of Src family kinase yes protein is essential for the enhanced malignant properties of human melanoma cells expressing ganglioside GD3. J. Biol. Chem. 2011, 286 (21), 18526–37. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yoder SM; Dineen SL; Wang Z; Thurmond DC YES, a Src family kinase, is a proximal glucose-specific activator of cell division cycle control protein 42 (Cdc42) in pancreatic islet beta cells. J. Biol. Chem. 2014, 289 (16), 11476–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Arndt B; Poltorak M; Kowtharapu BS; Reichardt P; Philipsen L; Lindquist JA; Schraven B; Simeoni L Analysis of TCR activation kinetics in primary human T cells upon focal or soluble stimulation. J. Immunol. Methods 2013, 387 (1–2), 276–83. [DOI] [PubMed] [Google Scholar]
- (18).Straus DB; Weiss A Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell 1992, 70 (4), 585–93. [DOI] [PubMed] [Google Scholar]
- (19).Trickett A; Kwan YL T cell stimulation and expansion using anti-CD3/CD28 beads. J. Immunol. Methods 2003, 275 (1–2), 251–5. [DOI] [PubMed] [Google Scholar]
- (20).Lu S; Ouyang M; Seong J; Zhang J; Chien S; Wang Y The spatiotemporal pattern of Src activation at lipid rafts revealed by diffusion-corrected FRET imaging. PLoS Comput. Biol. 2008, 4 (7), No. e1000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).(a) van’t Hof W; Resh MD Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J. Cell Biol. 1999, 145 (2), 377–389. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Resh MD Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta, Mol. Cell Res. 1999, 1451 (1), 1–16. [DOI] [PubMed] [Google Scholar]; (c) Timson Gauen LK; Linder ME; Shaw AS Multiple features of the p59fyn src homology 4 domain define a motif for immune-receptor tyrosine-based activation motif (ITAM) binding and for plasma membrane localization. J. Cell Biol. 1996, 133 (5), 1007–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Allen JA; Halverson-Tamboli RA; Rasenick MM Lipid raft microdomains and neurotransmitter signalling. Nat. Rev. Neurosci. 2007, 8 (2), 128–40. [DOI] [PubMed] [Google Scholar]
- (23).Sato I; Obata Y; Kasahara K; Nakayama Y; Fukumoto Y; Yamasaki T; Yokoyama KK; Saito T; Yamaguchi N Differential trafficking of Src, Lyn, Yes and Fyn is specified by the state of palmitoylation in the SH4 domain. J. Cell Sci. 2009, 122 (7), 965–975. [DOI] [PubMed] [Google Scholar]
- (24).Yang W; Pan W; Chen S; Trendel N; Jiang S; Xiao F; Xue M; Wu W; Peng Z; Li X; Ji H; Liu X; Jiang H; Wang H; Shen H; Dushek O; Li H; Xu C Dynamic regulation of CD28 conformation and signaling by charged lipids and ions. Nat. Struct. Mol. Biol. 2017, 24, 1081–1092. [DOI] [PubMed] [Google Scholar]
- (25).(a) Katagiri T; Urakawa K; Yamanashi Y; Semba K; Takahashi T; Toyoshima K; Yamamoto T; Kano K Overexpression of src family gene for tyrosine-kinase p59fyn in CD4-CD8-T cells of mice with a lymphoproliferative disorder. Proc. Natl. Acad. Sci. U. S. A. 1989, 86 (24), 10064–8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Del Giudice E; Gaetaniello L; Matrecano E; Cosentini E; Ursini MV; Racioppi L; Arrigo G; Pignata C Brain migration disorder and T-cell activation deficiency associated with abnormal signaling through TCR/CD3 complex and hyperactivity of Fyn tyrosine kinase. Neuropediatrics 2000, 31 (5), 265–8. [DOI] [PubMed] [Google Scholar]
- (26).(a) Yasuda K; Nagafuku M; Shima T; Okada M; Yagi T; Yamada T; Minaki Y; Kato A; Tani-Ichi S; Hamaoka T; Kosugi A Cutting edge: Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J. Immunol. 2002, 169 (6), 2813–7. [DOI] [PubMed] [Google Scholar]; (b) Webb Y; Hermida-Matsumoto L; Resh MD Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 2000, 275 (1), 261–70. [DOI] [PubMed] [Google Scholar]
- (27).(a) Zheng YZ; Berg KB; Foster LJ Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J. Lipid Res. 2009, 50 (5), 988–98. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Matsushima S; Kuroda J; Zhai P; Liu T; Ikeda S; Nagarajan N; Oka S; Yokota T; Kinugawa S; Hsu CP; Li H; Tsutsui H; Sadoshima J Tyrosine kinase FYN negatively regulates NOX4 in cardiac remodeling. J. Clin. Invest. 2016, 126 (9), 3403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Komatsu N; Aoki K; Yamada M; Yukinaga H; Fujita Y; Kamioka Y; Matsuda M Development of an optimized backbone of FRET biosensors for kinases and GTPases. Mol. Biol. Cell 2011, 22 (23), 4647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Mariotti A; Kedeshian PA; Dans M; Curatola AM; Gagnoux-Palacios L; Giancotti FG EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J. Cell Biol. 2001, 155 (3), 447–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


