Abstract
Long non-coding RNAs (lncRNAs), a type of cellular RNA, play a critical regulatory role in several physiological developments and pathological processes, such as tumorigenesis and tumor progression. Obesity is a risk factor for a number of serious health conditions, including breast cancer (BC). However, the underlying mechanisms behind the association between obesity and increased BC incidence and mortality remain unclear. Several studies have reported changes in lncRNA expression due to obesity and BC, independently encouraging further investigation of the relationship between the two in connection with lncRNAs. The present study was designed to first screen for the expression of 29 selected lncRNAs that showed a link to cancer or obesity in the blood of a selected cohort of 6 obese and 6 non-obese patients with BC. The expression levels of significantly expressed lncRNAs, AP001429.1, PCAT6, P5549, P19461 and P3134, were further investigated in a larger cohort of 69 patients with BC (36 obese and 33 non-obese), using reverse transcription-quantitative polymerase chain reaction. Results showed not only that AP001429.1 remained significantly downregulated in the larger cohort (P=0.002), but also that it was associated with several clinicopathological characteristics, such as negative HER2 status, negative E-cadherin expression, negative vascular invasion, negative margin invasion and LCIS. These findings suggest that obesity may have a role in inhibiting AP001429.1 expression, which may serve as a novel potential biomarker and therapeutic target for BC.
Keywords: breast cancer, obesity, long non-coding RNA, AP001429.1, molecular biomarker, therapeutic target
Introduction
Breast cancer (BC) is the most common type of cancer, having the highest incidence and being the leading cause of death from cancer in women worldwide. Globally, in 2018, more than 2 million cases of BC were newly diagnosed in women, with >625,000 deaths due to this disease. It was also reported that, in 2018, BC accounted for 31.6% of all newly diagnosed cancer cases in women in Saudi Arabia (1). Obesity poses a serious growing public health problem worldwide (2). According to estimates by the World Health Organization (WHO), in 2016, there were ~2 billion overweight adults, of whom >650 million were considered obese (3). In Saudi Arabia, the prevalence of obesity is higher among women than men (4).
Obesity is one of the risk factors of cancer and may be involved with ~20% of several types of cancer, including colorectal, postmenopausal breast, endometrial, renal and prostate cancers (5). Obesity has been reported to be a risk factor in BC, especially in postmenopausal women, and may associate with an increased incidence, a poor prognosis and decreased survival rate (6–8). Focusing on the molecular connection between BC and obesity could provide an important tool for researchers to clarify the underlying mechanisms, which may help identify novel prognostic biomarkers and therapeutic targets for BC.
Long non-coding RNAs (lncRNAs) are a class of untranslated regulatory RNA consisting of >200 nucleotides, which are considered important cellular RNA types that play critical regulatory roles in numerous biological processes, including genomic imprinting, chromatin modeling and post-transcriptional regulation (9); they have also been associated with various human diseases, including a variety of cancer types, such as breast, gastric and colorectal cancers (10,11). Although numerous lncRNAs have differential expression levels that may act as oncogenes or tumor suppressors (12), their biological functions and molecular mechanisms remain largely unknown (13).
Obesity involves profound epigenetic changes and affects the expression level of obesity-associated lncRNAs, which may be involved in cancer initiation and/or progression and affect the outcome of cancer therapy (14). Moreover, the expression levels of several lncRNAs, such as lncRNA P5549 (P5549), lncRNA P19461 (P19461) and lncRNA P3134 (P3134), are differentially expressed in obesity (15). However, the contribution of these lncRNAs to obesity in relation to BC is still unclear. Although several mechanisms have been proposed (16), the molecular association between obesity and BC is still not well understood and remains under investigation (17). Moreover, the role of lncRNAs in obesity-related cancer also remains unclear (16). Therefore, the present study was designed to evaluate the expression level of 29 selected lncRNAs that have previously been linked to cancer or obesity (Table I)(15,16,18–52) in the whole blood of obese patients with BC compared with that in non-obese patients with BC, using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). Subsequently, the expression levels of significantly differentially expressed lncRNAs were assayed in a larger cohort and the associations with the baseline and clinicopathological characteristics of the patients were assessed.
Table I.
Selected lncRNAs associated with different cancer types, BC or obesity.
| lncRNA | Full name | Expression status | Biological functions | Associated diseases | (Refs.) |
|---|---|---|---|---|---|
| AC011891.5 | lncRNA AC011891.5 | Upregulated | Positively correlated with BMI | Obesity | (18) |
| ANRIL | lncRNA ANRIL | Upregulated | Homeostatic regulator | Several cancer types | (16) |
| B4GALT1-AS1 | lncRNA B4GALT1-AS1 | Upregulated | Promotes cancer cell stemness and migration | Colon cancer | (19) |
| BCAR4 | BC anti-estrogen resistance 4 | Upregulated | Induces cancer cell proliferation and migration | BC | (20) |
| Blnc1 | lncRNA Blnc1 | Upregulated | Controls adipocyte differentiation | Energy homeostasis | (21,22) |
| CCAT1 | Colon cancer-associated transcript 1 | Upregulated | Promotes cancer cell proliferation, migration and invasion | Cancer cell | (23–25) |
| CCAT2 | Colon cancer-associated transcript 2 | Upregulated | Promotes cancer cell proliferation, migration and invasion | Several carcinomas | (26) |
| H-19 | H19, imprinted maternally expressed transcript | Upregulated | Inhibits adipocyte differentiation and improves insulin sensitivity and mitochondrial biogenesis | Obesity and numerous cancer types, including BC | (16,27) |
| HOTAIR | HOX transcript antisense RNA | Upregulated | Abdominal preadipocyte differentiation | Several cancer types | (16) |
| LINC00968 | Long intergenic non-protein coding RNA 968 | Upregulated | Positively correlated with BMI | Obesity | (18) |
| LINCADL | lincRNA adipogenesis- and lipogenesis-associated | Upregulated | Regulates adipocyte differentiation and fatty acid synthesis | Obesity | (28) |
| MALAT-1 | Metastasis-associated lung adenocarcinoma transcript 1 | Upregulated | Promotes cancer cell proliferation, migration and invasion, and plays a role in tumorigenesis and/or metastasis | Various cancer types | (29–31) |
| NEAT1 | Nuclear-enriched abundant transcript 1 | Upregulated | Regulates adipogenic differentiation | Obesity | (32,33) |
| PANDAR-1 | Promoter of CDKN1A antisense DNA damage-activated RNA 1 | Upregulated | Induces cancer cell proliferation, invasion and activation of cell epithelial-mesenchymal transition pathway | Gastric cancer | (34–36) |
| PCAT6 | Prostate cancer-associated ncRNA transcript 6 | Upregulated | Promotes cancer cell growth | Numerous cancer types | (37–40) |
| RP11-20G13.3 | LincRNA RP11-20G13.3 | Upregulated | Attenuates adipogenesis of preadipocytes | Obesity | (18) |
| ZFAS1 | Zinc finger antisense 1 | Upregulated | Promotes cancer cell proliferation and metastasis | Various cancer types | (41,42) |
| AP001429.1 | LncRNA AP001429.1 | Upregulated | Negatively correlated with BMI | Obesity | (43) |
| GAS5 | Growth arrest-specific 5 | Downregulated | Inhibits cancer cell proliferation and promotes apoptosis | Obesity and numerous types of cancer | (44–47) |
| GYG2P1 | Glycogenin 2 pseudogene 1 | Downregulated | Negatively associated with BMI, fasting insulin and triglycerides, and may play a role in the pathogenetic mechanism | Obesity | (18) |
| MEG3 | Maternally expressed gene 3 | Downregulated | Inhibits adipogenesis | Obesity | (48) |
| OLMALINC | Oligodendrocyte maturation-associated lincRNA | Downregulated | Increases expression of lipid metabolism genes | Obesity | (18) |
| P19461 | lncRNA P19461 | Downregulated | Negatively correlated with BMI | Obesity | (15) |
| P21015 | lncRNA P21015 | Downregulated | Negatively correlated with BMI | Obesity | (15) |
| P5549 | lncRNA P5549 | Downregulated | Negatively correlated with BMI | Obesity | (15) |
| RP11-529H2.1 | lincRNA RP11-529H2.1 | Downregulated | Negatively correlated with BMI | Obesity | (18) |
| RP11-559N14.5 | lncRNA RP11-559N14.5 | Downregulated | Involve in the AMPK signaling pathway, adipocytokine signaling pathway and insulin resistance | Obesity | (18) |
| SAR1 | lncRNA steroid receptor RNA activator 1 | Downregulated | Regulates adipogenesis and insulin sensitivity | Obesity | (49) |
| UCA1 | Urothelial carcinoma-associated 1 | Downregulated | Promotes cancer cell migration and invasion | Multiple cancer types | (50–52) |
lncRNA, long non-coding RNAs; lincRNA, long intergenic ncRNA; BC, breast cancer; BMI, body mass index.
This study could lead to a better understanding of the expression status of circulating lncRNAs and provide new insights into the lncRNAs involved in the interaction between obesity and BC, which could serve as a potential biomarker in BC prognosis.
Materials and methods
Study subjects
The study included 69 BC female patients who attended between October 2016 and September 2017 the Unit of Mammography, Department of Radiography at King Abdulaziz University Hospital (KAUH; Jeddah, Saudi Arabia), where they were diagnosed with BC. No patient had yet undergone any treatment and patients with recurrent BC were also excluded. Depending on the body mass index (BMI) differentiation (53), the BC patients were categorized as non-obese, which included lean and overweight (BMI <30 kg/m2; n=33), and obese (BMI ≥30 kg/m2; n=36). All patients provided written informed consent. The KAUH Unit of Biomedical Ethics Research Committee approved the study (approval number, HA-02-J-008). The patient information and sociodemographic characteristics were obtained through a standard questionnaire by interview. A standard well-established method was used to collect anthropometric data following WHO recommendations (53). The clinicopathological characteristics and clinical interpretation were provided by the consultants, radiologist and pathologist, as described previously (54,55).
Blood sample collection and storage
According to the manufacturer's instruction, whole blood samples were collected in PAXgene™ blood RNA tubes (Qiagen, Inc.), and then stored at −80°C until being used for RNA extraction.
RNA extraction
Total RNA was isolated from the whole blood of 69 patients with BC using the PAXgene blood RNA kit (Qiagen, Inc.). The quantity and quality of the extracted RNA were verified by DeNovix DS-11 Spectrophotometer (DeNovix, Inc.). The RNA samples were also separated in 1.2% agarose gel electrophoresis to check the quality. The RNA samples were aliquoted and stored at −80°C until being used for complementary DNA (cDNA) synthesis.
Complementary DNA (cDNA) synthesis
Total RNA (400 ng) from each BC sample was reverse transcribed to generate cDNA using a QuantiTect Reverse Transcription (RT) kit (Qiagen, Inc.) following the manufacturer's protocols. The cDNA was stored at −20°C until required.
Quantitative polymerase chain reaction (qPCR)
The gene expression levels of 29 lncRNAs, selected according to a suggested role in cancer or obesity as reported by the literature, including by our previous study (43) (Table I), were evaluated in the blood of obese and non-obese patients with BC by qPCR. Each experiment was run in duplicate in 96-well plates using a Bio-Rad IQ SYBR Green mix and the CFX Connect™ Real-Time PCR Detection system (both Bio-Rad Laboratories, Inc.), according to the manufacturers' protocols and guidelines. The qPCR reactions were carried out as follows: Initial cycle for 30 sec at 95°C; followed by 40 cycles of 15 sec at 98°C, and 30 sec at 60°C. The amplification product was checked at the end of each cycle, and the purity of amplification products was checked by the analysis of melting curves. The lncRNA expression levels were normalized using the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control for relative expression quantification. The primer pairs of target lncRNAs and reference genes were designed over two different exons using the Primer3 web tool and assessed using the In-Silico PCR tool for human genome assembly GRCh38 (hg38), provided by the University of California, Santa Cruz Genome Browser (http://genome.ucsc.edu/index.html). The sequences of the primers are presented in Table II. The relative expression quantification was calculated using the relative expression software tool (REST 2009) version 2.0.13 (56) and the comparative Cq method (2−ΔΔCq) (57).
Table II.
PCR primer sequences for target lncRNAs and reference genes.
| Gene symbol | Gene name | Gene type | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|---|---|
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Reference | TCACCAGGGCTGCTTTTAAC | GATGATCTTGAGGCTGTTGTCA |
| AP001429.1 | lncRNA AP001429.1 | Non-coding | AATATGACTGGGCCCTGCAA | CCGTTGGCCATTTCGTGATT |
| P5549 | lncRNA P5549 | Non-coding | CTTTTCCGGCTGAGGTGTTC | TGAACCAGCCATCTCTCACA |
| P21015 | lncRNA P21015 | Non-coding | ACCCCAGAAGTGACAAGAGG | AGATAAACCGCTGCCTTGTG |
| P19461 | lncRNA P19461 | Non-coding | CAGCCTCCTCCTGTGATGTA | CGTTCTTCTTGTTTGGACCCA |
| Blnc1 | lncRNA Blnc1 | Non-coding | CCTTCTCCAACCATCTGCCT | CTCTTCCCTCTGCCTCTGAC |
| SRA1 | lncRNA steroid receptor RNA activator 1 | Non-coding | GGAGGATGTGCTGAGACCTT | CAACTTTCCTCCAGCCCACT |
| B4GALT1-AS1 | lncRNA B4GALT1-AS1 | Non-coding | CTAGCCCACCGTCTGTTTTGGCAG | GGAAACTAGCCAACCT |
| LINCADL | lincRNA adipogenesis-and lipogenesis-associated | Non-coding | ATATGACCCAAGACCAGGCC | TCACAGCGAATCACTCCCTT |
| ANRIL | lncRNA ANRIL | Non-coding | ACGAAGCTCTACACACTTGAAG | GGATCACAGACCATACTTGCAC |
| RP11-20G13.3 | lincRNA RP11-20G13.3 | Non-coding | TCTGGAAGGAGTGTCGGTCT | CGTGTTCACAGATTGGGAGA |
| LINC00968 | long intergenic non-protein coding RNA 968 | Non-coding | ACCATCCCATTGAGAACCAA | CGAAAGGCTGGAAGTGTCAT |
| AC011891.5 | lncRNA AC011891.5 | Non-coding | CGAAAGGCTGGAAGTGTCAT | TGACCCAATTCTGACATTTGC |
| GYG2P1 | Glycogenin 2 pseudogene 1 | Non-coding | TCAGCCTCCCAAGTAGCTGT | CAGCCTGTGTCTCCTCAGTG |
| RP11-529H2.1 | lincRNA RP11-529H2.1 | Non-coding | AGGAGAATGGTGAAGGCAGA | TGCCGAAGCAGTTTAATCCT |
| OLMALINC | Oligodendrocyte maturation-associated lincRNA | Non-coding | AGACCCAGGACAGGAGGACT | ATTGGCAAGATGTTCCTTGG |
| MALAT1 | Metastasis-associated lung adenocarcinoma transcript 1 | Non-coding | GCAGGGAGAATTGCGTCATT | TTCTTCGCCTTCCCGTACTT |
| PCAT6 | Prostate cancer-associated ncRNA transcript 6 | Non-coding | CTCCATCCTCATTCGGTCCA | GAAGGGTGGTGGTAGAAGCA |
| UCA1 | Urothelial carcinoma-associated 1 | Non-coding | TTTGCCAGCCTCAGCTTAAT | TTGTCCCCATTTTCCATCAT |
| MEG3 | Maternally expressed 3 | Non-coding | TCACCTGCTAGCAAACTGGA | CATGCTCATTCCAGAAGCCC |
| CCAT2 | Colon cancer-associated transcript 2 | Non-coding | ATGAAGGCGTCGTCCAAATG | TCAGGCAATTGGTCAGAGGT |
| BCAR4 | BC anti-estrogen resistance 4 | Non-coding | CGATGCTTGTCTTGCTCTGA | CCGCTTTTTCGTATCACTCC |
| CCAT1 | Colon cancer-associated transcript 1 | Non-coding | TTGCTCACCTTACTGCCTGA | CCTGCAACTAGACACTCCCA |
| PANDAR | Promoter of CDKN1A antisense DNA damage-activated RNA 1 | Non-coding | TTGTAGCTCCTCCCATGTCG | AGGAACAGGCAATGGGATCA |
| HOTAIR | HOX transcript antisense RNA | Non-coding | GAGTTCCACAGACCAACACC | AATCCGTTCCATTCCACTGC |
| NEAT1 | Nuclear-enriched abundant transcript 1 | Non-coding | CCAGTGTGAGTCCTAGCATTGC | CCTGGAAACAGAACATTGGAGAAC |
| GAS5 | Growth arrest-specific 5 | Non-coding | CCCAAGGAAGGATGAGAATAGC | CTGTCTAATGCCTGTGTGCC |
| H19 | H19 imprinted maternally expressed transcript | Non-coding | ATCCGGACACAAAACCCTCT | AGAGCCGATTCCTGAGTCAG |
| ZFAS1 | ZNFX1 antisense RNA1 | Non-coding | AAGCCACGTGCAGACATCTAC | CTACTTCCAACACCCGCATTCA |
| P3134 | lncRNA P3134 | Non-coding | GTGGTGAGATCTCGGGGAAA | GTGCCAGAATTTCCTCACCC |
lncRNA, long non-coding RNAs; lincRNA, long intergenic ncRNA.
Statistical analysis
GraphPad Prism version 8.0.1 (GraphPad Software) was used to evaluate the statistical analyses of the obtained data using an unpaired, two-tailed t-test to determine the significant differences in the gene expression between groups. Moreover, χ2 and Kruskal-Wallis tests (one-way ANOVA) with a two-tailed P-value were used to test the distribution of categorical baseline and clinicopathological characteristics between obese and non-obese patients with BC. P≤0.05 was used to indicate a statistically significant difference. Bonferroni's correction was applied and the corrected P-value of ≤0.05 used for multiple comparisons of AP001429.1 expression level and patient baseline and clinicopathological characterizations. The data are presented as the mean ± standard error of the mean (SEM). Receiver operating characteristic (ROC) curves were generated to evaluate the sensitivity and specificity of AP001429.1 as a potential biomarker, using its gene expression values (2−ΔCq) of obese and non-obese patients with BC in the easyROC web-tool (ver.1.3.1; http://www.biosoft.hacettepe.edu.tr/easyROC/).
Results
General and clinicopathological characterization of the studied patients
The study cohort consisted of 69 newly diagnosed female patients with BC. The mean age ± SEM of the patients at the time of diagnosis was 52.3±1.51 (age range, 29–80 years). Over half (50.7%) were <50 years old, of which 29.0% were between 41 and 50 years old. The mean BMI ± SEM of the patients was 30.0±0.67 kg/m2; 52.2% of the patients were obese at the time of diagnosis and 47.8% were not obese, with a mean BMI ± SEM of 33.9±0.74 and 25.8±0.51 kg/m2, respectively (Table III).
Table III.
Baseline characteristics of studied patients with BC.
| Parameters | Total | Non-obese BC | Obese BC |
|---|---|---|---|
| Number of patients, n (%) | 69 (100.0) | 33 (47.8) | 36 (52.2) |
| Age, yearsa | 52.3±1.51 | 46.5±1.55 | 57.5±2.20 |
| BMI, kg/m2a | 30.0±0.67 | 25.8±0.51 | 33.9±0.74 |
| Waist circumference, cma | 90.2±2.84 | 87.1±4.56 | 93.1±3.48 |
| Hip circumference, cma | 104.5±2.94 | 101.8±4.51 | 106.9±3.84 |
| W/H ratioa | 0.87±0.01 | 0.85±0.02 | 0.88±0.01 |
| Age of first menstruation, yearsa | 13.36±0.16 | 13.22±0.23 | 13.49±0.23 |
| Age since menopause, yearsa | 50.30±0.89 | 48.37±1.20 | 51.43±1.20 |
| Age of first pregnancy, yearsa | 22.37±0.54 | 22.10±0.79 | 22.62±0.76 |
Data presented as mean ± SEM. BC, breast cancer; BMI, body mass index; W/H, waist/hip ratio.
Overall, 84.1% of the patients were married with three children or less, 46.4% had experienced a miscarriage and 81.1% were breastfeeding mothers. The mean age of first pregnancy was 22.7±0.65 years. A total of 40 patients had reached menopause at the time of diagnosis, with a mean age ± SEM 49.9±0.99 years, while the first appearance of menstruation for most patients was at a mean age ± SEM of 13.41±0.19 years, with only 5.8% experiencing first menstruation when <12 years of age. Most patients did not have any family history of BC or other cancer types, nor polycystic fibrosis or diabetes mellitus. In total, 92.8% of the patients were non-smokers, of which 33.3% performed physical activity. Moreover, most of the patients (75.4%) did not have diet rich in fat, and a few of the patients (18.8%) took omega-3 supplements (Table IV).
Table IV.
Distribution of general information characteristics of the studied patients with BC.
| Categories | Total, n (%) | Non-obese BC, n (%) | Obese BC, n (%) | P-value |
|---|---|---|---|---|
| Patients | 69 (100) | 33 (47.8) | 36 (52.2) | |
| Age of patients, years | 0.004 | |||
| ≤40 | 15 (21.7) | 10 (66.7) | 5 (33.3) | |
| 41-60 | 38 (55.1) | 21 (55.3) | 17 (44.7) | |
| >60 | 16 (23.2) | 2 (12.5) | 14 (87.5) | |
| Marital status | 0.56 | |||
| Single | 7 (10.1) | 2 (28.6) | 5 (71.4) | |
| Married | 58 (84.1) | 29 (50.0) | 29 (50.0) | |
| Divorced | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| Education level | 0.45 | |||
| Illiterate | 19 (27.5) | 7 (36.8) | 12 (63.2) | |
| School | 25 (36.2) | 12 (48.0) | 13 (52.0) | |
| First and higher degree | 25 (36.2) | 14 (56.0) | 11 (44.0) | |
| Nationality | 0.57 | |||
| Saudi | 38 (55.1) | 17 (44.7) | 21 (55.3) | |
| Non-Saudi | 31 (44.9) | 16 (51.6) | 15 (48.4) | |
| Age of first menstruation, years | 0.53 | |||
| <12 | 4 (5.8) | 3 (75.0) | 1 (25.0) | |
| 12-15 | 61 (88.4) | 28 (45.9) | 33 (54.1) | |
| >15 | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| Menopausal status | 0.003 | |||
| Postmenopausal | 40 (58.0) | 13 (32.5) | 27 (67.5) | |
| Premenopausal | 29 (42.0) | 20 (69.0) | 9 (31.0) | |
| Age of menopause, years | 0.44 | |||
| <48 | 3 (7.5) | 0 (0.0) | 3 (100.0) | |
| 48-55 | 32 (80.0) | 11 (34.4) | 21 (65.6) | |
| >55 | 5 (12.5) | 2 (40.0) | 3 (60.0) | |
| Hormone replacement therapy | 0.17 | |||
| Yes | 2 (2.9) | 0 (0.0) | 2 (100.0) | |
| No | 67 (97.1) | 33 (49.3) | 34 (50.7) | |
| Number of children | 0.14 | |||
| None | 8 (11.6) | 2 (25.0) | 6 (75.0) | |
| ≤3 | 31 (44.9) | 19 (61.3) | 12 (38.7) | |
| 4-6 | 18 (26.1) | 6 (33.3) | 12 (66.7) | |
| >6 | 12 (17.4) | 6 (50.0) | 6 (50.0) | |
| Number of miscarriages | 0.48 | |||
| None | 27 (39.1) | 14 (51.9) | 13 (48.1) | |
| 1 or 2 | 24 (34.8) | 13 (54.2) | 11 (45.8) | |
| ≥3 | 8 (11.6) | 2 (25.0) | 6 (75.0) | |
| No answer | 10 (14.5) | 4 (40.0) | 6 (60.0) | |
| Age of pregnancy, years | 0.79 | |||
| ≤20 | 22 (36.1) | 12 (54.5) | 10 (45.5) | |
| 21-30 | 34 (55.7) | 16 (47.1) | 18 (52.9) | |
| >30 | 5 (8.2) | 3 (60.0) | 2 (40.0) | |
| Breast feeding | 0.45 | |||
| Never | 13 (18.8) | 5 (38.5) | 8 (61.5) | |
| Yes | 56 (81.2) | 28 (50.0) | 28 (50.0) | |
| Family history of BC | 0.89 | |||
| Yes | 13 (18.8) | 6 (46.2) | 7 (53.8) | |
| No | 56 (81.2) | 27 (48.2) | 29 (51.8) | |
| Family history of other cancer | 0.89 | |||
| Yes | 13 (18.8) | 6 (46.2) | 7 (53.8) | |
| No | 56 (81.2) | 27 (48.2) | 29 (51.8) | |
| Polycystic fibrosis status | 0.35 | |||
| Yes | 9 (13.0) | 3 (33.3) | 6 (66.7) | |
| No | 60 (87.0) | 30 (50.0) | 30 (50.0) | |
| Diabetes mellitus status | 0.92 | |||
| Yes | 15 (21.7) | 7 (46.7) | 8 (53.3) | |
| No | 54 (78.3) | 26 (48.1) | 28 (51.9) | |
| Physical activities performance | 0.31 | |||
| Yes | 23 (33.3) | 13 (56.5) | 10 (43.5) | |
| No | 46 (66.7) | 20 (43.5) | 26 (56.5) | |
| Smoking status | 0.2 | |||
| Yes | 5 (7.2) | 1 (20.0) | 4 (80.0) | |
| No | 64 (92.8) | 32 (50.0) | 32 (50.0) | |
| Omega-3 supplements | 0.17 | |||
| Yes | 13 (18.8) | 4 (30.8) | 9 (69.2) | |
| No | 56 (81.2) | 29 (51.8) | 27 (48.2) | |
| Diet rich in fat | 0.23 | |||
| Yes | 17 (24.6) | 6 (35.3) | 11 (64.7) | |
| No | 52 (75.4) | 27 (51.9) | 25 (48.1) |
BC, breast cancer.
Regarding the clinicopathological features (Table V), the majority of the patients (76.8%) had invasive ductal carcinoma, 7.2% had invasive lobular carcinoma and 10.1% were diagnosed with an invasive mixture of ductal and lobular carcinoma. Approximately 56.5% of the patients had grade II tumors, 53.6% had tumor size <2 cm, and 43.5% had negative lymph node involvement. Based on the hormone receptor phenotypes, 71.0% of the patients had a luminal BC subtype (ER+/PR+/HER2−): 69.6% ER+, 56.5% PR+ and 59.4% HER2−. By contrast, HER2+ was only found in 34.8% of the patients. Therefore, the ER+/PR+/HER2− phenotype was the most abundant in the patient cohort.
Table V.
Distribution of clinicopathological features of the studied patients with BC.
| Categories | Total, n (%) | Non-obese BC, n (%) | Obese BC, n (%) | P-value |
|---|---|---|---|---|
| Patients | 69 (100) | 33 (47.8) | 36 (52.2) | |
| Hormone receptor phenotype | 0.28 | |||
| Luminal | 49 (71.0) | 25 (51.0) | 24 (49.0) | |
| HER2-enriched | 10 (14.5) | 5 (50.0) | 5 (50.0) | |
| Triple negative/basal like | 6 (8.7) | 1 (16.7) | 5 (83.3) | |
| Unknown | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| ER status | 0.53 | |||
| ER− | 17 (24.6) | 7 (41.2) | 10 (58.8) | |
| ER+ | 48 (69.6) | 24 (50.0) | 24 (50.0) | |
| Unknown | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| PR status | 0.08 | |||
| PR− | 26 (37.7) | 9 (34.6) | 17 (65.4) | |
| PR+ | 39 (56.5) | 22 (56.4) | 17 (43.6) | |
| Unknown | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| HER2 status | 0.19 | |||
| HER2− | 41 (59.4) | 17 (41.5) | 24 (58.5) | |
| HER2+ | 24 (34.8) | 14 (58.3) | 10 (41.7) | |
| Unknown | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| Lymph node involvement | 0.67 | |||
| Negative | 30 (43.5) | 12 (40.0) | 18 (60.0) | |
| Positive | 15 (21.7) | 7 (46.7) | 8 (53.3) | |
| Unknown | 24 (34.8) | 14 (58.3) | 10 (41.7) | |
| Size of tumor, cm | 0.69 | |||
| <2 | 37 (53.6) | 17 (45.9) | 20 (54.1) | |
| 2-5 | 22 (31.9) | 9 (40.9) | 13 (59.1) | |
| >5 | 3 (4.3) | 2 (66.7) | 1 (33.3) | |
| Unknown | 7 (10.1) | 5 (71.4) | 2 (28.6) | |
| Tumor grade | 0.37 | |||
| I | 8 (11.6) | 4 (50.0) | 4 (50.0) | |
| II | 39 (56.5) | 16 (41.0) | 23 (59.0) | |
| III | 18 (26.1) | 11 (61.1) | 7 (38.9) | |
| Unknown | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| Histotype | 0.32 | |||
| DCIS | 53 (76.8) | 23 (43.4) | 30 (56.6) | |
| LCIS | 5 (7.2) | 3 (60.0) | 2 (40.0) | |
| Mixture of ductal and lobular | 7 (10.1) | 5 (71.4) | 2 (28.6) | |
| Unknown | 4 (5.8) | 2 (50.0) | 2 (50.0) | |
| Vascular invasion | 0.28 | |||
| Negative | 42 (60.9) | 19 (45.2) | 23 (54.8) | |
| Positive | 11 (15.9) | 3 (27.3) | 8 (72.7) | |
| Unknown | 16 (23.2) | 11 (68.8) | 5 (31.3) | |
| Margin | 0.40 | |||
| Negative | 41 (59.4) | 17 (41.5) | 24 (58.5) | |
| Positive | 1 (3.6) | 0 (0.0) | 1 (100.0) | |
| Unknown | 27 (39.1) | 16 (59.3) | 11 (40.7) |
BC, breast cancer; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; LCIS, lobular carcinoma in situ; PR, progesterone receptor.
The non-obese and obese BC groups were significantly different in terms of age and menopausal status (P=0.003); however, the results did not show any significant differences with regard to other general and clinicopathological characteristics (Tables IV and V).
Screening of lncRNAs in a selected cohort of obese versus non-obese BC patients
The gene expression levels of the 29 selected lncRNAs were initially measured in a selected cohort of BC patients, based on the greatest BMI differentiation: 6 obese patients with the highest BMI and 6 non-obese BC patients with the lowest BMI were selected from the overall BC patient cohort. The amplification products for lncRNAs and GAPDH were specific and pure in all samples as assessed by melting curve analysis and across the threshold within 30 cycles.
The expression level of lncRNAs in a selected cohort of obese compared with non-obese patients with BC is shown in Fig. 1. Among all selected lncRNAs, P5549, P19461, PCAT6, AP001429.1 and P3134 were significantly differentially expressed. The expression levels of circulating PCAT6, P19461 and P3134 were significantly upregulated [fold-change (FC), 2.526 and P≤0.02; FC, 1.361 and P≤0.008; and FC, 1.5 and P=0.05, respectively], whereas P5549 and AP001429.1 showed a significant decrease in expression within the same group of obese BC patients (FC, 0.56 and P=0.05; and FC, 0.6 and P=0.02, respectively). The rest of the studied lncRNAs did not show any significant differences in expression between the groups (Fig. 1).
Figure 1.
Relative expression fold of long non-coding RNAs in a selected cohort of obese compared with non-obese patients with BC. Gene expression was detected by reverse transcription-quantitative PCR and normalized according to GAPDH expression. Error bars represent SEM. *P≤0.05 and **P<0.01. BC, breast cancer.
Evaluation of lncRNA expression in a larger cohort of obese and non-obese BC patients
The gene expression levels of the significantly differentially expressed identified lncRNAs, (P5549, P19461, P3134, PCAT6 and AP001429.1) were evaluated in a larger cohort consisting of the study population of 36 obese and 33 non-obese BC patients, as shown in Fig. 2. Among these evaluated lncRNAs, AP001429.1 was significantly downregulated in obese compared with non-obese patients with BC (FC, 0.5; P=0.002). By contrast, P5549 (FC, 1.0; P=0.97), P19461 (FC, 1.1; P=0.56), P3134 (FC, 1.2; P=0.12) and PCAT6 (FC, 1.0; P=0.94) were not found to exhibit any significant differences in expression within the larger group of patients (Fig. 2).
Figure 2.
Long non-coding RNA relative expression fold in a larger cohort of obese compared with non-obese patients with BC. Gene expression was detected by reverse transcription-quantitative PCR and normalized according to GAPDH expression. Error bars represent SEM. **P<0.01. BC, breast cancer.
To evaluate AP001429.1 as a potential biomarker, a ROC curve was generated using the gene expression values of AP001429.1 in obese and non-obese BC patients. In the ROC curve analysis (Fig. 3 and Table SI), the area under the ROC curve was 0.684 (nearly 0.7), indicating that AP001429.1 expression enabled weak but significant differentiation of patients with BC based on obesity status (P=0.004) (58). Therefore, AP001429.1 may act as a potential biomarker in obese patients with BC.
Figure 3.

Receiver operating characteristic curve for the gene expression of AP001429.1, a suggested potential biomarker in obese patients with breast cancer.
Association between AP001429.1 expression level and patient baseline characteristics
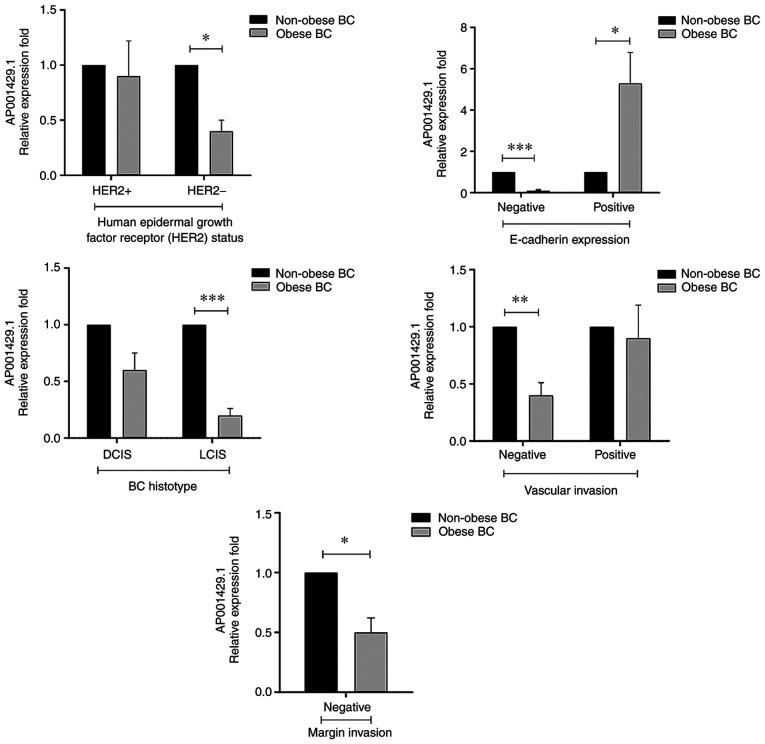
Differential expression patterns in AP001429.1 were observed when assessing the association with patient baseline features (Table SII). Significant differences in AP001429.1 expression with regard to patient baseline characteristics were assessed by Bonferroni's correction (P≤0.05) and are presented in Fig. 4. Significant decreases in AP001429.1 expression were detected in obese patients with BC who were at middle-aged (FC, 0.4; P=0.03), married (FC, 0.4; P=0.006), Saudi national (FC, 0.5; P=0.02) and patients who had low education level (FC, 0.2; P<0.0003). AP001429.1 also showed significant downregulation in relation to premenopausal obese BC patients (FC, 0.3; P=0.002), in those who were breastfeeding their children (FC, 0.4; P<0.001) and in those who experienced their first menstruation event between 12 and 15 years old (FC, 0.5; P=0.01) or had their first pregnancy aged between 21 and 30 (FC, 0.4; P=0.03). Moreover, the non-smoking obese BC patients, those who did not take omega-3 supplements and those who performed physical activity also showed a significantly decreased expression level of AP001429.1 (FC, 0.6 and P=0.01; FC, 0.5 and P=0.02; and FC, 0.2 and P<0.001, respectively). Furthermore, AP001429.1 showed significant downregulation in relation to diabetic obese patients with BC, as well as those who did not have hormone replacement therapy, those who did not have any family history of BC, other cancer types or polycystic fibrosis (FC, 0.2 and P<0.001; FC, 0.5 and P=0.01; FC, 0.4 and P=0.004; and FC, 0.5 and P=0.01, respectively). Moreover, the significantly decreased expression of AP001429.1 was also detected in obese patients with BC who had 4 to 6 children (FC, 0.2; P=0.03) and those who had miscarriages once or twice (FC, 0.4; P=0.03) (Fig. 4).
Figure 4.
Relative expression fold of AP001429.1 and its association with patient baseline characteristics within obese patients with BC compared with the same categories in non-obese patients with BC. Gene expression was detected by reverse transcription-quantitative-PCR and normalized according to GAPDH expression. Error bars represent SEM. The significance level is presented as assessed by Bonferroni's correction. *P≤0.05, **P<0.01 and ***P<0.001. BC, breast cancer.
Association between AP001429.1 expression level and patient clinicopathological characteristics
Associations in the expression levels of AP001429.1 in obese patients with BC compared with that in non-obese patients with BC were assessed with regard to patient clinicopathological characteristics (Table SIII). Significant differences in AP001429.1 expression with regard to patient clinicopathological characteristics were assessed by Bonferroni's correction (P≤0.05) and are presented in Fig. 5. AP001429.1 exhibited a significantly lower expression level in obese patients compared with that in non-obese patients with BC; however, the significantly decreased expression was detected with regard to negative HER2 status (FC, 0.4; P=0.02), negative E-cadherin expression (FC, 0.1; P<0.001), negative vascular invasion (FC, 0.4; P=0.004), negative margin invasion (FC, 0.5; P=0.02) and LCIS (FC, 0.2; P<0.001) BC patients. By contrast, a high expression level of AP001429.1 was only detected in relation to positive E-cadherin expression (FC, 5.3; P=0.04) within the obese patients with BC (Fig. 5).
Figure 5.
Relative expression fold of AP001429.1 and its association with patient clinicopathological parameters in obese patients with BC compared with the same categories in non-obese patients with BC. Gene expression was detected by reverse transcription-quantitative PCR and normalized according to GAPDH expression. Error bars represent SEM. The significance level is presented as assessed by Bonferroni's correction. *P≤0.05, **P<0.01 and ***P<0.001. BC, breast cancer; DCIS, ductal carcinoma in situ; LCIS, lobular carcinoma in situ.
Discussion
lncRNA, as a class of untranslated regulatory RNA, is considered an important type of cellular RNA that plays a critical regulatory role in a number of biological processes in normal development, as well as in tumorigenesis and tumor progression processes (59). lncRNA is regarded as a key regulator of diseases with tissue specificity (60). lncRNA controls the flux of genetic information modulating various cellular processes, such as modulation of chromosome structure, transcription, splicing, mRNA stability and availability, post-translational modifications (61) and epigenetic mechanisms (62). Obesity involves profound epigenetic changes and affects the expression of obesity-associated lncRNAs that may be involved in cancer initiation and/or progression and affect cancer therapy. To the best of our knowledge, the approach of the present study comparing differences between obese and non-obese patients with BC has so far not been applied. Previous studies investigated healthy non-obese versus obese patients (15,16,44,63,64) as well as healthy control cases versus patients with BC (65–68). Therefore, in the present study, lncRNA expression levels were evaluated in whole blood taken from BC patients by liquid biopsy, with obese patients being compared with non-obese patients, aiming to determine the expression status of lncRNAs in obese patients with BC and their associations with the general and clinicopathological attributes of the patients.
AP001429.1 is also known as novel transcript sense intronic lncRNA to tetratricopeptide repeat domain 3; it is located on the long arm of chromosome 21 (21q22.13) and is 530 nucleotides in length (69). Very limited information is available on the expression and biological functions of AP001429.1; however, its mRNA expression has been detected in a number of normal human tissues and cells, including whole blood, brain, cerebellum, endometrium, heart, ovary and testis (69). Furthermore, according to the RNAcentral resource (70) and the LncBase database (71), AP001429.1 is targeted by several miRNAs; notably, a number of AP001429.1-targeted miRNAs are downregulated and reported to have roles as tumor suppressors in BC, such as miR-124-3p (72), miR-196b-5p (73), the miR-34-5p family (74,75), miR-449b-5p (76), miR-940 (77) and miR-99a-3p (78,79). In addition, miR-196a-5p and miR-449a were upregulated and reported to be involved in oncogenesis in BC (80,81), suggesting that AP001429.1 may function as a potential tumor suppressor in BC by targeting those miRNAs. The present study showed that AP001429.1 was significantly downregulated in obese patients with BC compared with non-obese patients with BC. A significant decrease in AP001429.1 expression was detected in obese patients with BC who were middle-aged, premenopausal, married, had 4 to 6 children and who breastfed their newborn. Moreover, in the BC patient cohort, non-smoking status, performance of a physical activity, diabetes, the absence of hormone replacement therapy and the absence of a family history of cancer or polycystic fibrosis, was also associated with a significant decrease in the expression level of AP001429.1 (Fig. 3 and Table SII). Moreover, a significant association was also detected with regard to certain molecular and histological characteristic, including negative HER2 status, negative E-cadherin expression, negative vascular and margin invasion, and LCIS. Obese patients with BC also exhibited downregulation of AP001429.1 compared with non-obese patients with BC (Fig. 4 and Table SIII). The exact reasoning behind the significant associations with regard to these parameters is not clear.
Numerous lncRNAs have been detected as differentially expressed in different cells and tissues associated with cancer and/or obesity (82). Moreover, the differential expression of lncRNAs may contribute to the initiation, development, invasion and metastasis of various types of cancer, including BC, as well as obesity development, brown adipocyte differentiation and the function of adipose tissue (83), through both activation and inhibition of the expression of other genes (84) that could affect various cancer-related physiological processes (85). Therefore, lncRNAs may serve as BC prognostic and diagnostic biomarkers as well as being useful as therapeutic targets for BC treatments. Despite the existence of studies considering lncRNAs in BC, there is still an urgent need for more studies focusing on the role of lncRNAs in BC with obesity in order to provide a better understanding of their involvement and offer new insights into the role of lncRNAs in obesity-related BC.
In conclusion, the present results demonstrated the downregulation of AP001429.1 in obese patients with BC, suggesting that obesity may have a role in inhibiting the expression of AP001429.1, which could be considered as a potential tumor suppressor of BC. This information may help improve our understanding and provide an important research tool with regard to the molecular associations between obesity and BC. Therefore, the expression of AP001429.1 could serve as a potential biomarker for BC prognosis and a target for therapy. Further study is needed to confirm these findings and elucidate the underlying mechanism for the effects of AP001429.1 with regard to connections between obesity and BC.
The current study has certain limitations, including the small sample size, which needs to be increased to confirm and validate the findings. Further control cross-sectional studies using healthy obese and non-obese patients with an increase in sample size will be conducted in the near future. Finally, further investigation is required to elucidate the expression profile and functional role of AP001429.1 in BC tissue.
Supplementary Material
Acknowledgements
Not applicable.
Funding Statement
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU) Jeddah, Kingdom of Saudi Arabia (grant no. G: 638/130/1438).
Funding
This project was funded by the Deanship of Scientific Research (DSR) at King Abdulaziz University (KAU) Jeddah, Kingdom of Saudi Arabia (grant no. G: 638/130/1438).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
MAH, HC, KAAS and ALAM designed and coordinated the experiments. KAAS obtained the ethical approval, patients' consent and blood samples. MAH performed the experiments and analyzed the data. HC contributed to laboratory facilitates and project funding. MAH wrote the original manuscript draft. KAAS and HC edited the manuscript. HC, MAH and KAAS confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Unit of Biomedical Ethics Research Committee, KAUH (approval no. HA-02-J-008). All patients signed a consent form to engage in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Smith KB, Smith MS. Obesity statistics. Prim Care. 2016;43:121–135. doi: 10.1016/j.pop.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 3.WHO, corp-author. Obesity and overweight. https://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight. [Dec 15;2020 ]; [Google Scholar]
- 4.Memish ZA, El Bcheraoui C, Tuffaha M, Robinson M, Daoud F, Jaber S, Mikhitarian S, Al Saeedi M, AlMazroa MA, Mokdad AH, Al Rabeeah AA. Obesity and associated factors-Kingdom of Saudi Arabia, 2013. Prev Chronic Dis. 2014;11:E174. doi: 10.5888/pcd11.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argolo DF, Hudis CA, Iyengar NM. The impact of obesity on breast cancer. Curr Oncol Rep. 2018;20:47. doi: 10.1007/s11912-018-0688-8. [DOI] [PubMed] [Google Scholar]
- 7.Iyengar NM, Hudis CA, Dannenberg AJ. Obesity and cancer: Local and systemic mechanisms. Annu Rev Med. 2015;66:297–309. doi: 10.1146/annurev-med-050913-022228. [DOI] [PubMed] [Google Scholar]
- 8.Chan DS, Norat T. Obesity and breast cancer: Not only a risk factor of the disease. Curr Treat Options Oncol. 2015;16:22. doi: 10.1007/s11864-015-0341-9. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Zhang X, Chen W, Hu X, Li J, Liu C. Regulatory roles of long noncoding RNAs implicated in cancer hallmarks. Int J Cancer. 2020;146:906–916. doi: 10.1002/ijc.32277. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Shi J, Zhang Y, Xie A, Yu L, Zhang C, Lei J, Xu H, Leng Z, Li T, et al. LncTarD: A manually-curated database of experimentally-supported functional lncRNA-target regulations in human diseases. Nucleic Acids Res. 2019;48:D118–D126. doi: 10.1093/nar/gkz985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Zhao W, Wang M, Zhou X. Gene Expression Profiling in Cancer IntechOpen. London, UK: 2019. The Role of Long Noncoding RNAs in Gene Expression Regulation. [DOI] [Google Scholar]
- 12.Zhuang C, Ma Q, Zhuang C, Ye J, Zhang F, Gui Y. LncRNA GClnc1 promotes proliferation and invasion of bladder cancer through activation of MYC. FASEB J. 2019;33:11045–11059. doi: 10.1096/fj.201900078RR. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Yang X, Kang X, Liu S. The regulatory roles of long noncoding RNAs in the biological behavior of pancreatic cancer. Saudi J Gastroenterol. 2019;25:145–151. doi: 10.4103/sjg.SJG_465_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohde K, Keller M, la Cour Poulsen L, Blüher M, Kovacs P, Böttcher Y. Genetics and epigenetics in obesity. Metabolism. 2019;92:37–50. doi: 10.1016/j.metabol.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Ruan Y, Wang M, Chen R, Yu N, Sun L, Liu T, Chen H. Differentially expressed circulating LncRNAs and mRNA identified by microarray analysis in obese patients. Sci Rep. 2016;6:35421. doi: 10.1038/srep35421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yau MY, Xu L, Huang CL, Wong CM. Long non-coding RNAs in obesity-induced cancer. Noncoding RNA. 2018;4:19. doi: 10.3390/ncrna4030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng J, Sauter ER, Li B. FABP4: A new player in obesity-associated breast cancer. Trends Mol Med. 2020;26:437–440. doi: 10.1016/j.molmed.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Ji Y, Li M, Wang M, Yi X, Yin C, Wang S, Zhang M, Zhao Z, Xiao Y. Integrated analysis of long noncoding RNA and mRNA expression profile in children with obesity by microarray analysis. Sci Rep. 2018;8:8750. doi: 10.1038/s41598-018-27113-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Fang ZX, Guo X, Dong H, Zhou K, Huang Z, Xiao Z. lncRNA B4GALT1-AS1 promotes colon cancer cell stemness and migration by recruiting YAP to the nucleus and enhancing YAP transcriptional activity. J Cell Physiol. 2019;234:18524–18534. doi: 10.1002/jcp.28489. [DOI] [PubMed] [Google Scholar]
- 20.Ouyang S, Zhou X, Chen Z, Wang M, Zheng X, Xie M. LncRNA BCAR4, targeting to miR-665/STAT3 signaling, maintains cancer stem cells stemness and promotes tumorigenicity in colorectal cancer. Cancer Cell Int. 2019;19:72. doi: 10.1186/s12935-019-0784-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mi L, Zhao XY, Li S, Yang G, Lin JD. Conserved function of the long noncoding RNA Blnc1 in brown adipocyte differentiation. Mol Metab. 2016;6:101–110. doi: 10.1016/j.molmet.2016.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun L, Lin JD. Function and mechanism of long noncoding RNAs in adipocyte biology. Diabetes. 2019;68:887–896. doi: 10.2337/dbi18-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li GH, Ma ZH, Wang X. Long non-coding RNA CCAT1 is a prognostic biomarker for the progression of oral squamous cell carcinoma via miR-181a-mediated Wnt/β-catenin signaling pathway. Cell Cycle. 2019;18:2902–2913. doi: 10.1080/15384101.2019.1662257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu M, Zhang Q, Tian XH, Wang JL, Niu YX, Li G. lncRNA CCAT1 is a biomarker for the proliferation and drug resistance of esophageal cancer via the miR-143/PLK1/BUBR1 axis. Mol Carcinog. 2019;58:2207–2217. doi: 10.1002/mc.23109. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zhu G, Ma Y, Qu H. lncRNA CCAT1 contributes to the growth and invasion of gastric cancer via targeting miR-219-1. J Cell Biochem. 2019;120:19457–19468. doi: 10.1002/jcb.29239. [DOI] [PubMed] [Google Scholar]
- 26.Cai Y, He J, Zhang D. Long noncoding RNA CCAT2 promotes breast tumor growth by regulating the Wnt signaling pathway. Onco Targets Ther. 2015;8:2657–2664. doi: 10.2147/OTT.S90485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidt E, Dhaouadi I, Gaziano I, Oliverio M, Klemm P, Awazawa M, Mitterer G, Fernandez-Rebollo E, Pradas-Juni M, Wagner W, et al. LincRNA H19 protects from dietary obesity by constraining expression of monoallelic genes in brown fat. Nat Commun. 2018;9:3622. doi: 10.1038/s41467-018-05933-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Xue CY, Line J, Ferguson JF, Weiner A, Liu W, Han Y, Hinkle C, Li W, Jiang H, et al. Interrogation of nonconserved human adipose lincRNAs identifies a regulatory role of linc-ADAL in adipocyte metabolism. Sci Transl Med. 2018;10:eaar5987. doi: 10.1126/scitranslmed.aar5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kong X, Wang J, Cao Y, Zhang H, Lu X, Wang Y, Bo C, Wang T, Li S, Tian K, et al. The long noncoding RNA MALAT-1 functions as a competing endogenous RNA to regulate MSL2 expression by sponging miR-338-3p in myasthenia gravis. J Cell Biochem. 2019;120:5542–5550. doi: 10.1002/jcb.27838. [DOI] [PubMed] [Google Scholar]
- 30.Han X, Xu Z, Tian G, Tang Z, Gao J, Wei Y, Xu X. Suppression of the long non-coding RNA MALAT-1 impairs the growth and migration of human tongue squamous cell carcinoma SCC4 cells. Arch Med Sci. 2019;15:992–1000. doi: 10.5114/aoms.2018.73343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tripathi V, Ellis JD, Shen Z, Song DY, Pan Q, Watt AT, Freier SM, Bennett CF, Sharma A, Bubulya PA, et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gernapudi R, Wolfson B, Zhang Y, Yao Y, Yang P, Asahara H, Zhou Q. MicroRNA 140 promotes expression of long noncoding RNA NEAT1 in adipogenesis. Mol Cell Biol. 2015;36:30–38. doi: 10.1128/MCB.00702-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper DR, Carter G, Li P, Patel R, Watson JE, Patel NA. Long non-coding RNA NEAT1 associates with SRp40 to temporally regulate PPARγ2 splicing during adipogenesis in 3T3-L1 cells. Genes (Basel) 2014;5:1050–1063. doi: 10.3390/genes5041050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Y, Su X, Pan H. Inhibition of lncRNA PANDAR reduces cell proliferation, cell invasion and suppresses EMT pathway in breast cancer. Cancer Biomark. 2019;25:185–192. doi: 10.3233/CBM-182251. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Ben Q, Lu E, He X, Yang X, Ma J, Zhang W, Wang Z, Liu T, Zhang J, Wang H. Long noncoding RNA PANDAR blocks CDKN1A gene transcription by competitive interaction with p53 protein in gastric cancer. Cell Death Dis. 2018;9:168. doi: 10.1038/s41419-017-0246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Fang L, Jiang J, Kuang Y, Wang B, Shang X, Han P, Li Y, Liu M, Zhang Z, Li P. The cisplatin-induced lncRNA PANDAR dictates the chemoresistance of ovarian cancer via regulating SFRS2-mediated p53 phosphorylation. Cell Death Dis. 2018;9:1103. doi: 10.1038/s41419-018-1148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xin Y, He X, Zhao W, Zhan M, Li Y, Xiao J, He K, Lu L. LncRNA PCAT6 increased cholangiocarcinoma cell proliferation and invasion via modulating miR-330-5p. Am J Transl Res. 2019;11:6185–6195. [PMC free article] [PubMed] [Google Scholar]
- 38.Wu H, Zou Q, He H, Liang Y, Lei M, Zhou Q, Fan D, Shen L. Long non-coding RNA PCAT6 targets miR-204 to modulate the chemoresistance of colorectal cancer cells to 5-fluorouracil-based treatment through HMGA2 signaling. Cancer Med. 2019;8:2484–2495. doi: 10.1002/cam4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang WM, Su G, Huang XX, Zou A, Wu J, Yang Y, Zhu Y, Liang S, Li D, Ma F, Guo L. Long noncoding RNA PCAT6 inhibits colon cancer cell apoptosis by regulating anti-apoptotic protein ARC expression via EZH2. Cell Cycle. 2019;18:69–83. doi: 10.1080/15384101.2018.1558872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong F, Ruan S, Wang J, Xia Y, Le K, Xiao X, Hu T, Wang Q. M2 macrophage-induced lncRNA PCAT6 facilitates tumorigenesis and angiogenesis of triple-negative breast cancer through modulation of VEGFR2. Cell Death Dis. 2020;11:728. doi: 10.1038/s41419-020-02926-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong H, Zhuang X, Cai J, Ding Y, Si Y, Zhang H, Shen M. Long noncoding RNA ZFAS1 promotes progression of papillary thyroid carcinoma by sponging miR-590-3p and upregulating HMGA2 expression. Onco Targets Ther. 2019;12:7501–7512. doi: 10.2147/OTT.S209138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong D, Mu Z, Wei N, Sun M, Wang W, Xin N, Shao Y, Zhao C. Long non-coding RNA ZFAS1 promotes proliferation and metastasis of clear cell renal cell carcinoma via targeting miR-10a/SKA1 pathway. Biomed Pharmacother. 2019;111:917–925. doi: 10.1016/j.biopha.2018.12.143. [DOI] [PubMed] [Google Scholar]
- 43.Hassan MA, Al-Sakkaf K, Shait Mohammed MR, Dallol A, Al-Maghrabi J, Aldahlawi A, Ashoor S, Maamra M, Ragoussis J, Wu W, et al. Integration of transcriptome and metabolome provides unique insights to pathways associated with obese breast cancer patients. Front Oncol. 2020;10:804. doi: 10.3389/fonc.2020.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mansoori Y, Tabei MB, Askari A, Izadi P, Daraei A, Bastami M, Naghizadeh MM, Nariman-Saleh-Fam Z, Mansoori B, Tavakkoly-Bazzaz J. Expression levels of breast cancer-related GAS5 and LSINCT5 lncRNAs in cancer-free breast tissue: Molecular associations with age at menarche and obesity. Breast J. 2018;24:876–882. doi: 10.1111/tbj.13067. [DOI] [PubMed] [Google Scholar]
- 45.Ni W, Yao S, Zhou Y, Liu Y, Huang P, Zhou A, Liu J, Che L, Li J. Long noncoding RNA GAS5 inhibits progression of colorectal cancer by interacting with and triggering YAP phosphorylation and degradation and is negatively regulated by the m6A reader YTHDF3. Mol Cancer. 2019;18:143. doi: 10.1186/s12943-019-1079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji J, Dai X, Yeung SJ, He X. The role of long non-coding RNA GAS5 in cancers. Cancer Manag Res. 2019;11:2729–2737. doi: 10.2147/CMAR.S189052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun M, Jin FY, Xia R, Kong R, Li JH, Xu TP, Liu YW, Zhang EB, Liu XH, De W. Decreased expression of long noncoding RNA GAS5 indicates a poor prognosis and promotes cell proliferation in gastric cancer. BMC Cancer. 2014;14:319. doi: 10.1186/1471-2407-14-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li Z, Jin C, Chen S, Zheng Y, Huang Y, Jia L, Ge W, Zhou Y. Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Mol Cell Biochem. 2017;433:51–60. doi: 10.1007/s11010-017-3015-z. [DOI] [PubMed] [Google Scholar]
- 49.Xu B, Gerin I, Miao H, Vu-Phan D, Johnson CN, Xu R, Chen XW, Cawthorn WP, MacDougald OA, Koenig RJ. Multiple roles for the non-coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PLoS One. 2010;5:e14199. doi: 10.1371/journal.pone.0014199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong P, Qiao F, Wu H, Cui H, Li Y, Zheng Y, Zhou M, Fan H. LncRNA UCA1 promotes tumor metastasis by inducing miR-203/ZEB2 axis in gastric cancer. Cell Death Dis. 2018;9:1158. doi: 10.1038/s41419-018-1170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bian Z, Jin L, Zhang J, Yin Y, Quan C, Hu Y, Feng Y, Liu H, Fei B, Mao Y, et al. LncRNA-UCA1 enhances cell proliferation and 5-fluorouracil resistance in colorectal cancer by inhibiting miR-204-5p. Sci Rep. 2016;6:23892. doi: 10.1038/srep23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao F, Wang Q, Wu Q. The prognostic value and mechanisms of lncRNA UCA1 in human cancer. Cancer Manag Res. 2019;11:7685–7696. doi: 10.2147/CMAR.S200436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Onis M, Habicht JP. Anthropometric reference data for international use: Recommendations from a world health organization expert committee. Am J Clin Nutr. 1996;64:650–658. doi: 10.1093/ajcn/64.4.650. [DOI] [PubMed] [Google Scholar]
- 54.Al-Maghrabi J, Al-Sakkaf K, Qureshi IA, Butt NS, Damnhory L, Elshal M, Al-Maghrabi B, Aldahlawi A, Ashoor S, Brown B, et al. AMPK expression patterns are significantly associated with poor prognosis in breast cancer patients. Ann Diagn Pathol. 2017;29:62–67. doi: 10.1016/j.anndiagpath.2017.05.012. [DOI] [PubMed] [Google Scholar]
- 55.Khabaz MN, Al-Sakkaf K, Qureshi IA, Butt NS, Damnhory L, Elshal M, Al-Maghrabi B, Aldahlawi A, Ashoor S, Brown B, et al. Expression of p-AMPK is associated with hormone receptor phenotypes and lymph node metastasis in breast cancer. Int J Clin Exp Patho. 2017;10:7044–7051. [Google Scholar]
- 56.Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 58.Mandrekar JN. Receiver operating characteristic curve in diagnostic test assessment. J Thorac Oncol. 2010;5:1315–1316. doi: 10.1097/JTO.0b013e3181ec173d. [DOI] [PubMed] [Google Scholar]
- 59.Soudyab M, Iranpour M, Ghafouri-Fard S. The role of long non-coding RNAs in breast cancer. Arch Iran Med. 2016;19:508–517. [PubMed] [Google Scholar]
- 60.Zhu Y, Mao D, Gao W, Han G, Hu H. Analysis of lncRNA expression in patients with eosinophilic and neutrophilic asthma focusing on LNC_000127. Front Genet. 2019;10:141. doi: 10.3389/fgene.2019.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernandes JCR, Acuña SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long non-coding RNAs in the regulation of gene expression: Physiology and disease. Noncoding RNA. 2019;5:17. doi: 10.3390/ncrna5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wei JW, Huang K, Yang C, Kang CS. Non-coding RNAs as regulators in epigenetics (Review) Oncol Rep. 2017;37:3–9. doi: 10.3892/or.2016.5236. [DOI] [PubMed] [Google Scholar]
- 63.Ghafouri-Fard S, Taheri M. The expression profile and role of non-coding RNAs in obesity. Eur J Pharmacol. 2021;892:173809. doi: 10.1016/j.ejphar.2020.173809. [DOI] [PubMed] [Google Scholar]
- 64.Butler AE, Hayat S, Dargham SR, Malek JA, Abdullah SA, Mahmoud YA, Sathyapalan T, Atkin SL. Long non-coding RNA expression in non-obese women with polycystic ovary syndrome and weight-matched controls. Reprod Biomed Online. 2020;41:579–583. doi: 10.1016/j.rbmo.2020.06.018. [DOI] [PubMed] [Google Scholar]
- 65.Liang Y, Song X, Li Y, Chen B, Zhao W, Wang L, Zhang H, Liu Y, Han D, Zhang N, et al. LncRNA BCRT1 promotes breast cancer progression by targeting miR-1303/PTBP3 axis. Mol Cancer. 2020;19:85. doi: 10.1186/s12943-020-01206-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Sun Z, Liu J, Liu J. The expression of lncRNA-MALAT1 in breast cancer patients and its influences on prognosis. Cell Mol Biol (Noisy-le-grand) 2020;66:72–78. doi: 10.14715/cmb/2020.66.3.11. [DOI] [PubMed] [Google Scholar]
- 67.Lv D, Xu K, Jin X, Li J, Shi Y, Zhang M, Jin X, Li Y, Xu J, Li X. LncSpA: LncRNA spatial atlas of expression across normal and cancer tissues. Cancer Res. 2020;80:2067–2071. doi: 10.1158/0008-5472.CAN-19-2687. [DOI] [PubMed] [Google Scholar]
- 68.Mohebi M, Ghafouri-Fard S, Modarressi MH, Dashti S, Zekri A, Kholghi-Oskooei V, Taheri M. Expression analysis of vimentin and the related lncRNA network in breast cancer. Exp Mol Pathol. 2020;115:104439. doi: 10.1016/j.yexmp.2020.104439. [DOI] [PubMed] [Google Scholar]
- 69.Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et al. The genecards suite: From gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1–1.30.33. doi: 10.1002/cpbi.5. [DOI] [PubMed] [Google Scholar]
- 70.The Rnacentral Consortium, Petrov AI, Kay SJE, Kalvari I, Howe KL, Gray KA, Bruford EA, Kersey PJ, Cochrane G, Finn RD, et al. RNAcentral: A comprehensive database of non-coding RNA sequences. Nucleic Acids Res. 2017;45:D128–D134. doi: 10.1093/nar/gkw1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paraskevopoulou MD, Vlachos IS, Karagkouni D, Georgakilas G, Kanellos I, Vergoulis T, Zagganas K, Tsanakas P, Floros E, Dalamagas T, Hatzigeorgiou AG. DIANA-LncBase v2: Indexing microRNA targets on non-coding transcripts. Nucleic Acids Res. 2016;44:D231–D238. doi: 10.1093/nar/gkv1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Chen L, Wu Z, Wang M, Jin F, Wang N, Hu X, Liu Z, Zhang CY, Zen K, et al. miR-124-3p functions as a tumor suppressor in breast cancer by targeting CBL. BMC Cancer. 2016;16:826. doi: 10.1186/s12885-016-2862-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu X, Rao X, Yao W, Zou X. Downregulation of MiR-196b-5p impedes cell proliferation and metastasis in breast cancer through regulating COL1A1. Am J Transl Res. 2018;10:3122–3132. [PMC free article] [PubMed] [Google Scholar]
- 74.Wang B, Li D, Kovalchuk I, Apel IJ, Chinnaiyan AM, Wóycicki RK, Cantor CR, Kovalchuk O. miR-34a directly targets tRNAiMet precursors and affects cellular proliferation, cell cycle, and apoptosis. Proc Natl Acad Sci USA. 2018;115:7392–7397. doi: 10.1073/pnas.1703029115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang L, Wang L, Dong D, Wang Z, Ji W, Yu M, Zhang F, Niu R, Zhou Y. MiR-34b/c-5p and the neurokinin-1 receptor regulate breast cancer cell proliferation and apoptosis. Cell Prolif. 2019;52:e12527. doi: 10.1111/cpr.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang J, Yang X, He X, Ma W, Wang J, Zhou Q, Li M, Yu S. MicroRNA-449b-5p suppresses the growth and invasion of breast cancer cells via inhibiting CREPT-mediated Wnt/β-catenin signaling. Chem Biol Interact. 2019;302:74–82. doi: 10.1016/j.cbi.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Hou L, Chen M, Yang H, Xing T, Li J, Li G, Zhang L, Deng S, Hu J, Zhao X, Jiang J. MiR-940 inhibited cell growth and migration in triple-negative breast cancer. Med Sci Monit. 2016;22:3666–3672. doi: 10.12659/MSM.897731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hu Y, Zhu Q, Tang L. MiR-99a antitumor activity in human breast cancer cells through targeting of mTOR expression. PLoS One. 2014;9:e92099. doi: 10.1371/journal.pone.0092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang X, Li Y, Qi W, Zhang N, Sun M, Huo Q, Cai C, Lv S, Yang Q. MicroRNA-99a inhibits tumor aggressive phenotypes through regulating HOXA1 in breast cancer cells. Oncotarget. 2015;6:32737–32747. doi: 10.18632/oncotarget.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang YW, Zhang W, Ma R. Bioinformatic identification of chemoresistance-associated microRNAs in breast cancer based on microarray data. Oncol Rep. 2018;39:1003–1010. doi: 10.3892/or.2018.6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi W, Bruce J, Lee M, Yue S, Rowe M, Pintilie M, Kogo R, Bissey PA, Fyles A, Yip KW, Liu FF. MiR-449a promotes breast cancer progression by targeting CRIP2. Oncotarget. 2016;7:18906–18918. doi: 10.18632/oncotarget.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dahariya S, Paddibhatla I, Kumar S, Raghuwanshi S, Pallepati A, Gutti RK. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol Immunol. 2019;112:82–92. doi: 10.1016/j.molimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 83.Xu S, Chen P, Sun L. Regulatory networks of non-coding RNAs in brown/beige adipogenesis. Biosci Rep. 2015;35:e00262. doi: 10.1042/BSR20150155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9:1354–1366. [PMC free article] [PubMed] [Google Scholar]
- 85.Lo PK, Wolfson B, Zhou X, Duru N, Gernapudi R, Zhou Q. Noncoding RNAs in breast cancer. Brief Funct Genomics. 2016;15:200–221. doi: 10.1093/bfgp/elv055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.