Abstract
To date, most antibodies recognizing the SARS-CoV-2 spike (S) protein target the receptor binding domain (RBD). Three recent studies (Cerutti et al., 2021; McCallum et al., 2021; and Suryadevara et al., 2021) report on highly protective antibodies that are specific to the N-terminal domain (NTD) and target a conserved supersite.
To date, most antibodies recognizing the SARS-CoV-2 spike (S) protein target the receptor binding domain (RBD). Three recent studies (Cerutti et al., 2021; McCallum et al., 2021; and Suryadevara et al., 2021) report on highly protective antibodies that are specific to the N-terminal domain (NTD) and target a conserved supersite.
Main text
The emergence and rapid spread of a new coronavirus, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has essentially paralyzed the world and resulted in millions of deaths since early 2020. Therefore, there is an urgent need to study the human immune response against SARS-CoV-2 to facilitate the development of vaccines and therapeutics. One area of therapeutic development is the identification of potent human monoclonal antibodies that can be used to either prevent or treat infection.
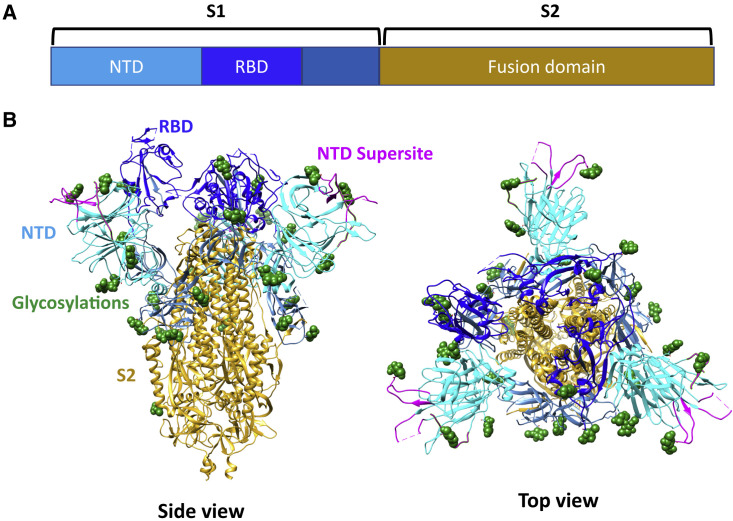
The major protein on the surface of SARS-CoV-2 virus particle is the spike (S) protein (Figure 1 ). The S protein exists as trimers and facilitates entry of virus into the host cell by binding to host cell receptors (angiotensin-converting enzyme 2 [ACE2]) and inducing fusion of the virus membrane to the host cell membrane. S protein contains two domains: S1 and S2. S1 is comprised of the N-terminal domain (NTD) and the receptor binding domain (RBD), whereas S2 contains the fusion domain. Analysis of the human monoclonal antibody repertoire from sera of recovered patients has shown that the majority of anti-S antibodies recognize the RBD and a smaller proportion of antibodies recognize the NTD (McCallum et al., 2021). While RBD antibodies are very well characterized and have been shown to be protective against disease in animal models (Baum et al., 2020), less is known about NTD-specific antibodies. Here, we summarize three different studies (Cerutti et al., 2021; McCallum et al., 2021; Suryadevara et al., 2021) reporting on NTD-specific antibodies. All showed that these NTD antibodies can be as potent as RBD antibodies, and Suryadevara et al. (2021), McCallum et al. (2021), and Cerutti et al. (2021) focused on characterizing two, six, and seven potent NTD antibodies, respectively.
Figure 1.
SARS-CoV2 spike (S) protein and the NTD supersite
(A) Schematic diagram showing the domains on the S protein.
(B) Side and top views of the S protein (PDB code: 7C2L). Domains/motifs/NTD-supersite/glycosylations are colored in the same color scheme as their respective font labels.
Due to its stabilizing effect on the NTD, a previous study on NTD antibody 4A8 complexed with S protein (Chi et al., 2020) determined the entire S protein structure, including five loops on NTD which were previously not visible in uncomplexed S protein structures. These loops are hereafter referred to as N1 (residues 14–26), N2 (residues 67–79), N3 (residues 141–156), N4 (residues 177–186), and N5 (residues 246–260). All potent antibodies identified by the three studies (Cerutti et al., 2021; McCallum et al., 2021; Suryadevara et al., 2021) and also antibodies 4A8 (Chi et al., 2020) and 4-8 (Liu et al., 2020) recognize an epitope comprising the N1, N3, and N5 loops, hence dubbed “the NTD supersite” (Figure 1).
The epitopes were identified by high resolution cryoelectron microscopy (cryo-EM) studies (Cerutti et al., 2021; McCallum et al., 2021) and also by superimposing low-resolution negatively stained EM maps (Suryadevara et al., 2021) onto a previously determined high-resolution cryo-EM structure of antibody 4-8 (Liu et al., 2020), which binds to N3 and N5, to show that they are similar. Additionally, Suryadevara et al. (2021) conducted alanine-scanning loss-of-binding experiments in a cell surface antigen display system, and both Suryadevara et al. (2021) and McCallum et al. (2021) selected escape mutants from potent antibodies. All results are consistent with the epitope identified by structural methods. Interestingly, McCallum et al. (2021) observed that some escape mutants contain mutations also present in SARS-CoV-2 clinical isolates; most of them in B.1.1.7, B.1.351, and P1 lineages, thus recapitulating naturally occurring variants. Both McCallum et al. (2021) and Cerutti et al. (2021) showed that the center of the NTD supersite is largely free of glycan (Figure 1B), while the boundaries contain glycans. Cerutti et al. (2021) also demonstrated that there are different ways Fabs can engage the same epitope. Analysis of the charges of the epitope and Fab paratope showed that the NTD supersite is strongly positively charged, while the paratope is strongly negatively charged.
McCallum et al. (2021) conducted competitive bio-layer interferometry assays with 41 NTD antibodies and showed that apart from the supersite, there are five other sites on the NTD that can be bound by less potent antibodies. They also determined the cryo-EM structures of these antibodies in complex with S protein and found that their epitopes lie adjacent to the supersite.
All three studies then examined the variety of antibody genes encoding the NTD antibodies. Suryadevara et al. (2021) showed that the NTD supersite antibodies COV2-2489 and COV2-2676 are encoded by heavy-chain variable gene segments IGHV-4-39 and IGHV1-69, respectively. McCallum et al. (2021) showed that the 41 NTD antibodies use a large repertoire of variable (V) genes with an over-representation of IGHV 3-21 and IGK3-15 genes, which harbor few somatic hypermutations compared to the germline. Thus, they concluded that the antibody response to the SARS-CoV-2 NTD is largely polyclonal. Cerutti et al. (2021) showed the 17 NTD antibodies derived from only 9 variable heavy (VH) genes with antibodies originating from 5 genes (VH1-24, VH1-69, VH3-30, VH1-8, and VH4-39) observed in multiple donors. This suggests the convergent development of similar antibodies in different individuals. They also reverted the paratope-region somatic hypermutations of seven NTD antibodies and showed that the germline-reverted antibodies have reduced binding affinities and neutralization capabilities. This suggests that affinity maturation is essential for their potencies.
As SARS-CoV-2 variants are rapidly emerging, it is important to determine the ability of the NTD supersite antibodies to bind/neutralize these naturally occurring variants. Both Suryadevara et al. (2021) and McCallum et al. (2021) showed reduced binding/neutralizing capability of their NTD supersite antibodies to the B.1.1.7 (United Kingdom) or B.1B1.351 (South Africa) variants, indicating that these variants likely could partially or completely escape neutralization.
Suryadevara et al. (2021) and McCallum et al. (2021) determined the neutralization mechanisms of the NTD supersite antibodies. They showed that these antibodies (1) are unable to block S protein binding to its ACE2 receptor, (2) can inhibit a step post-virus attachment to cells, and (3) likely block fusion of virus to host cell membrane, and that (4) the preservation of the whole immunoglobulin structure is important for their neutralization properties.
Suryadevara et al. (2021) and McCallum et al. (2021) also tested the protective effect of NTD supersite antibodies in infected mice. Both groups showed that the NTD supersite antibodies have strong prophylactic properties. Suryadevara et al. (2021) showed that administration of antibodies prior to virus infection in mice (K18-hACE2-transgenic mouse model) prevented weight loss and reduced viremia, cytokine, and chemokine levels. McCallum et al. (2021) showed that low doses of anti-NTD antibodies provide strong prophylactic activity in an infected Syrian hamster model, but their results also indicate that, potentially, escape mutants can be generated. Suryadevara et al. (2021) also showed that these antibodies have good therapeutic efficacies in mice. Both groups showed, in mice and also cell culture, that antibody Fc-mediated effector functions play an important role in protection.
One of the ways to prevent generation of virus escape mutants from antibody therapy could be to administer a cocktail of NTD and RBD antibodies. Indeed, McCallum et al. (2021) showed that combinatory antibodies could prevent entry of SARS-CoV-2 S-MLV pseudotyped virus in Vero cells, and Suryadevara et al. (2021) showed that in mice, an antibody cocktail has good therapeutic efficacies.
The three papers therefore characterize a class of very important NTD supersite binding antibodies that are highly protective but are also probably the source of selection pressure leading to the generation of variants, supported by the fact that naturally circulated variants carry mutations within the NTD supersite. Treatment with a cocktail combining potent RBD and NTD antibodies may therefore prevent the emergence of escape mutants.
Acknowledgments
The work in this publication was supported by a National Research Foundation Investigatorship award (NRF-NRFI2016-01) and National Research Foundation Competitive Research Project grants (NRF2016NRF-CRP001-063 and NRF2017NRF-CRP001-027) awarded to S.-M.L., and the Duke-NUS Signature Research Programme funded by the Ministry of Health, Singapore.
References
- Baum A., Ajithdoss D., Copin R., Zhou A., Lanza K., Negron N., Ni M., Wei Y., Mohammadi K., Musser B., et al. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370:1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti G., Guo Y., Zhou T., Gorman J., Lee M., Rapp M., Reddem E.R., Yu J., Bahna F., Bimela J., et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe. 2021;29:819–833. doi: 10.1016/j.chom.2021.03.005. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi X., Yan R., Zhang J., Zhang G., Zhang Y., Hao M., Zhang Z., Fan P., Dong Y., Yang Y., et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science. 2020;369:650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- McCallum M., De Marco A., Lempp F., Tortorici M.A., Pinto D., Walls A.C., Beltramello M., Chen A., Liu Z., Zatta F., et al. N-terminal domain antigenic mapping reveals a site of vulnerability for SARS-CoV-2. Cell. 2021;184 doi: 10.1101/2021.01.14.426475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suryadevara N., Shrihari S., Gilchuk P., VanBlargan L.A., Binshtein E., Zost S.J., Nargi R.S., Sutton R.E., Winkler E.S., Chen E.C., et al. Neutralizing and protective human monoclonal antibodies recognizing the N-terminal domain of the SARS-CoV-2 spike protein. Cell. 2021;184 doi: 10.1101/2021.01.19.427324. [DOI] [PMC free article] [PubMed] [Google Scholar]