Abstract
Algal toxicity studies are required by regulatory agencies for a variety of purposes including classification and labeling and environmental risk assessment of chemicals. Algae are also frequently the most sensitive taxonomic group tested. Acute to chronic ratios (ACRs) have been challenging to derive for algal species because of the complexities of the underlying experimental data including: a lack of universally agreed upon algal inhibition endpoints; evolution of experimental designs over time and by different standardization authorities; and differing statistical approaches (e.g., regression versus hypothesis-based effect concentrations). Experimental data for developing globally accepted algal ACRs have been limited because of data availability, and in most regulatory frameworks an ACR of 10 is used regardless of species, chemical type or mode of action. Acute and chronic toxicity (inhibition) data on 17 algal species and 442 chemicals were compiled from the EnviroTox database (https://envirotoxdatabase.org/) and a proprietary database of algal toxicity records. Information was probed for growth rate, yield, and final cell density endpoints focusing primarily on studies of 72 and 96 h duration. Comparisons of acute and chronic data based on either single (e.g., growth rate) and multiple (e.g., growth rate, final cell density) endpoints were used to assess acute and chronic relationships. Linear regressions of various model permutations were used to compute ACRs for multiple combinations of taxa, chemicals, and endpoints, and showed that ACRs for algae were consistently around 4 (ranging from 2.43 to 5.62). An ACR of 4 for algal toxicity is proposed as an alternative to a default value of 10, and recommendations for consideration and additional research and development are provided.
Introduction:
Algal toxicity studies are required by regulatory agencies for a variety of purposes including hazard determination, classification and labeling, and prioritization for environmental risk assessment of chemicals. Algae are often the most sensitive taxonomic group (approximately forty percent of the time) and often drive assessments (Hutchinson, Barrett et al. 2003, Jeram, Sintes et al. 2005, Rawlings et al. 2019). Application of acute to chronic ratios (ACR) are a common approach to estimate chronic toxicity values using generally more available acute toxicity data (Raimondo et al. 2007). Understanding ACRs can be informative for developing risk assessments but should be viewed cautiously as ACR magnitude can be a function of chemical mode of action, chemical class or taxon assessed (Raimondo et al. 2007). In the context of developing protective screening values, ecoTTCs (ecological Threshold of Toxicological Concern) (Belanger et al. 2015, Connors et al. 2019), ACRs can be useful to extrapolate potential chronic effects from acute data which dominate the ecotoxicological literature and regulatory databases. In most regulatory frameworks, an ACR of 10 is commonly used for algae, invertebrates and fish regardless of species, chemical type or mode of action (ECHA 2014, Nabholz and Zeeman 1993). However, ACRs for algae can be lower than for fish and invertebrates, thus algae chronic endpoint extrapolation may be more conservative than necessary (Mayo-Bean et al. 2012).
In contrast to algae, acute and chronic toxicity values for both invertebrates and fish are generated in separate tests measuring acute lethality and sublethal effects. Test designs, replication and statistics are distinctly different when comparing acute and chronic tests. However, a very different situation exists for algae, where acute and chronic toxicity (growth inhibition) are based on the same measurements of cell density and are interpreted in chemicals management as either acute or chronic based on a statistical protocol (Figure 1). Algal growth inhibition tests are mostly focused on fast growing green algal species in the family Oocystaceae such as Scenedesmus, Desmodesmus, and the Raphidocelis (formerly Pseudokirchneriella or Selenastrum). In algal tests, smaller effect concentration (e.g., EC10, EC20) values and no observed effect concentrations (NOEC) are interpreted as chronic responses whereas median effect concentrations (EC50) are generally interpreted as acute responses even though all are associated with population growth within the same short-term test (although spanning numerous generations). Algal toxicologists recognize the conundrum that occurs when using a microbial species which proceeds through several generations during a 72-hr (or longer) assay as having an acute exposure interpretation. As a matter of practice, however, regulatory frameworks use the EC50 as representative of acute (sometimes referred to as short term) response and the EC10/NOEC as representative of chronic (sometimes referred to as long term) in determining safe concentrations (PNEC, Predicted No Effect Concentration) of a chemical for ecosystems (Nabholz et al. 1993).
Figure 1.
Flow-chart for determining if an algal toxicity endpoint is defined as acute or chronic.
It is unlikely that the same default ACR for invertebrates and fish is directly applicable to algal species because of differences in sensitivity and measures of effect. To date experimental data for algal toxicity has been less available than for invertebrates and fish (Connors et al. 2019) even though algae can exhibit greater sensitivity for many chemicals (Rawlings et al. 2019). Development of algal ACRs has been limited due to both data availability and the complexity of the standard approach to determining effect levels for algal acute and chronic inhibition (Figure 1). However, some evidence suggests that ACRs for algae appear to vary between 3 to 5 depending on the toxicological endpoint and statistical metric being applied (Ahlers et al. 2006; Raimondo et al. 2007; Mayo-Bean et al 2012). In this work, we utilized the newly developed EnviroTox database (www.envirotoxdatabase.org) and a proprietary algal database to transparently compile acute and chronic toxicity data on 18 algae species (Table 1) and 442 chemicals to compute ACRs for algal growth inhibition. These ACRs can be further utilized in the development of ecoTTCs and Chemical Toxicity Distributions (Belanger et al. 2015 Connors et al. 2019, Kienzler et al. 2017, 2019).
Table 1.
Species represented in evaluating acute:chronic ratios (ACR) for algal inhibition to chemical exposure.
| Division | Ecology | Species |
|---|---|---|
| Chlorophyta | Freshwater | Raphidocelis subcapitata |
| Freshwater | Chlorella vulgaris viridis | |
| Chlorella kessleri | ||
| Chlorella pyrenoidosa | ||
| Chlorella saccharophila | ||
| Chlorella sp. | ||
| Chlorella vulgaris | ||
| Freshwater | Desmodesmus subspicatus | |
| Chlamydomonas reinhardtii | ||
| Freshwater | Microcystis aeruginosa | |
| Microcystis anacystis | ||
| Microcystis flos-aquae | ||
| Marine | Dunaliella bioculata | |
| Dunaliella tertiolecta | ||
| Bacillariophyta | Marine | Phaeodactylum tricornutum |
| Marine | Skeletonema costatum | |
| Freshwater | Nitzschia closterium | |
| Cyanobacteria | Freshwater | Anabaena inaequalis |
Materials and Methods
Understanding algal inhibition endpoints
Algal inhibition tests yield both acute and chronic effect concentrations from the same short 72 to 96 - hour assay. Several generations of algae can be assessed despite the brief test duration (OECD 2013). Algal inhibition is generally expressed as Y-hr ECx. Where Y is the duration of exposure, EC the effective inhibition concentration and X the percent reduction relative to the control condition. All measures of inhibition are correlated in some way to living biomass. Depending on the test design, the effect may be on inhibition of growth rate or intrinsic rate of increase (ECr), cell yield (final cell density minus inoculation density) (ECx) or terminal cell density at test end (EC) (Fig. 1, 2). An older interpretation of yield was biomass area under the curve, indicated as “b” (OECD 2006); however, international discussions concluded “y” was scientifically appropriate to replaced biomass area under the curve or “b” (OECD 2013). Large values of x, such as 50%, indicate acute inhibition and small levels of x, such as 10% or a NOEC determination indicate chronic inhibition. Because yield has only recently been endorsed as the better interpretation of total biomass (versus b) the number of citations of y in both literature and regulatory databases is limited and b is somewhat more prevalent. The full expression of growth rate inhibition, as an example, would be 72-hr ErC50. This is the concentration of chemical that reduces the growth rate of an algal population by 50% in 72 hours. The preferred experimental duration and endpoint depends on the regulatory organization, with USEPA guidelines recommending 96 hr terminal cell density (96 hr ECx) and OECD guidelines recommending 72 hr ECr results. However, if the requisite raw data is available the relevant endpoint can be calculated to meet the different regulatory preferences.
Figure 2.
Representation of typical inhibition of algal growth curves along with interpretation of inhibition metrics regarding growth rate (r) and biomass area under the curve (b) and final cell density.
An algal effect concentration should not be cited without specification of endpoint such as EC50, ErC50, EyC50 or EbC50. Growth rate is a logarithmic term, and small changes in growth rate may lead to great changes in biomass. EbC and ErC values are therefore not numerically comparable. These have different meanings, different regulatory connotations and acceptabilities and quantitatively impact chemical assessments. In 2002, OECD officially endorsed growth rate as the preferred endpoint over biomass. Growth rate tends to be the least sensitive as an inhibition endpoint by 3-5 times. The biomass parameter (b) generally provides a lower effect (more conservative) level compared with the growth rate for systematic and mathematical reasons (Eberius et al. 2002). However, the parameter growth rate is scientifically more appropriate and robust against deviations in test conditions because it allows for clearer interpretation and comparison among studies (Bergtold, 2010).
Constructing algal inhibition acute and chronic database
Derivation of ACRs for algae as an alternative to a default value of 10 has been limited because of inconsistently reported endpoints in the literature and in available databases. Recently the EnviroTox database (https://envirotoxdatabase.org/) was developed to provide access to a robust and curated acute and chronic toxicity data traceable to the original source (Connors et al. 2019). The EnviroTox database was designed to include acute and chronic endpoints for algae, invertebrates and fish that have regulatory relevance (i.e., the endpoints are identified as useful for typical chemical hazard assessment). The publicly available database was used in the current study in deriving algal ACRs by querying algal toxicity entries with test durations (72 and 96 h) considered usable as acute and chronic toxicity records. For the purpose of this study, algal toxicity entries in EnviroTox that were between 72 and 96 h in duration were considered usable as acute and chronic toxicity records. Bounded effects values (> or < values), LOECs or MATCs were excluded in the derivation of ACRs. Algal effect concentrations recorded as EC50 were identified as acute, and EC10 values and NOECs were identified as chronic. For ACR determination, EC10 and NOEC values were not used interchangeably. But for the purpose of defending the equivalence of the EC10 and NOEC we pulled data for chemicals with citations that derived both an EC10 and NOEC value. In some citations, there were multiple experiments for the same species, chemical, duration and statistical endpoint and those values were averaged. A linear regression was developed to establish the equivalence of EC10 and NOEC. The ratio of the EC10/NOEC is generally around 1 thus denominator doesn’t matter being EC10 or NOEC.
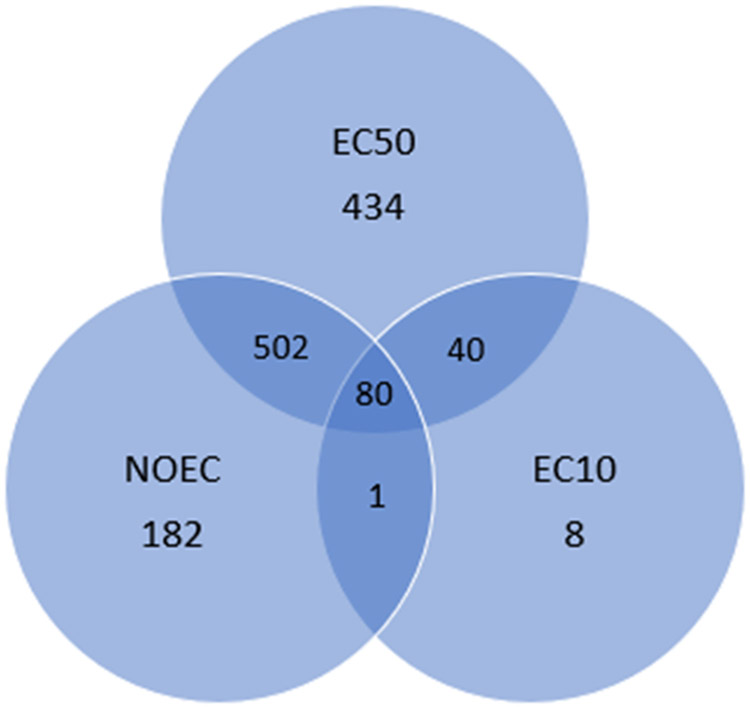
The final algal database used for this research included values from EnviroTox (66% of records), a proprietary algal database (30% of records), and a targeted search of the REACH portal (https://echa.europa.eu/web/guest/information-on-chemicals/registered-substances; (4% of records) to increase the diversity of chemicals evaluated. Studies were curated or quality controlled for adequacy as given in Connors et al. (2019) and Brill et al. (2016). A Venn diagram identifies the overlaps in statistical endpoints found within the dataset used here to investigate algal ACR relationships (Figure 3). Note here that as this is largely a literature compilation, not every statistical endpoint is always presented per study (this is also true for industry reports). Individual studies may not have the same experimental design (differences in replication, numbers of exposures, etc.) which can inhibit assessment for some endpoints, particularly chronic expressions of growth inhibition. Valid studies from literature may also only have interest in one aspect and not fully disclose all possible ecotoxicity metrics (this is very common). Envirotox is a robust, highly curated database containing high-quality aquatic toxicity studies that are traceable to the original information source. The 91,217 aquatic toxicity records represent 1563 species and 4016 unique chemicals (Connors et al. 2019). For example, when only acute or chronic data could be obtained for a specific chemical through EnviroTox, data available on the REACH registered substances portal was searched to identify the potential missing algal data for that same chemical. The following data criteria were used in developing an algal ACR: (1) the corresponding acute and chronic toxicity values were available for the same chemical and the same species, and (2) the metric of growth rate (r) and biomass (b) were defined within the record. The database was then probed for effect concentration statistics expressed as ErCx, EbCx, NOECr and NOECb focusing on 72- and 96-hour duration studies among common test species (Table 1, 2). In practical terms the databases are often confounded by lack of explicit algal endpoints (clear definition of ErC, EyC, EbC, and EC, for example). If the endpoint could not be explicitly identified the record was not included. Extracted data (chemical identity, species tested, duration, effect, percent inhibition) was curated (duplicate records removed, confirmed species and chemical identity), and comparable data were paired to allow for comparisons of acute and chronic information. Geometric means for acute and chronic inhibition of individual test results were calculated if multiple tests were available on the same taxon and same endpoint.
Figure 3.
Venn diagram indicating overlaps per statistical endpoint for studies included in the acute-chronic algal inhibition assessment.
Table 2.
Acute-chronic relationships for algal inhibition and sample sizes associated with each.
| Metric | Based on | Comparison | # of chemicals | ACR geomean: |
|---|---|---|---|---|
| Biomass | All Data | EbC50 vs EbC10 | 87 | 4.85 |
| Biomass | All Data | EbC50 vs NOECb | 94 | 4.29 |
| Biomass | Over-all | Biomass data | - | 4.56 |
| Growth Rate | All Data | ErC50 vs ErC10 | 61 | 3.22 |
| Growth Rate | All Data | ErC50 vs NOECr | 433 | 2.43 |
| Growth Rate | Over-all | Growth rate data | - | 3.93 |
| Growth Rate | Endpoint Over-all | All data combined | - | 3.57 |
| Growth Rate | Raphidocelis subcapitata | ErC50 vs NOECr | 349 | 4.64 |
| Growth Rate | Desmodesmus subspicatus | ErC50 vs NOECr | 16 | 4.21 |
| Growth Rate | Chlorella pyrenoidosa | ErC50 vs NOECr | 7 | 3.06 |
| Growth Rate | Skeletonema costatum | ErC50 vs NOECr | 9 | 3.12 |
| Growth Rate | Chlamydomonas reinhardtii | ErC50 vs NOECr | 7 | 3.06 |
| Growth Rate | Single Species Over-all | ErC50 vs NOECr | - | 3.56 |
| Growth Rate | Chemical Class: Neutral Organics | ErC50 vs NOECr | 56 | 4.32 |
| Growth Rate | Chemical Class: Phenols | ErC50 vs NOECr | 42 | 5.35 |
| Growth Rate | Chemical Class: Aliphatic Amines | ErC50 vs NOECr | 33 | 4.71 |
| Growth Rate | Chemical Class: Anilines | ErC50 vs NOECr | 29 | 4.85 |
| Growth Rate | Chemical Class: Esters | ErC50 vs NOECr | 26 | 4.26 |
| Growth Rate | Chemical Class Over-all | ErC50 vs NOECr | - | 4.68 |
| Growth Rate | MOA: Narcotics | ErC50 vs NOECr | 218 | 4.82 |
| Growth Rate | MOA: Unspecified | ErC50 vs NOECr | 110 | 3.99 |
| Growth Rate | MOA: Specifics | ErC50 vs NOECr | 65 | 5.62 |
| Growth Rate | MOA: Herbicides | ErC50 vs NOECr | 26 | 4.18 |
| Growth Rate | MOA Over-all | ErC50 vs NOECr | - | 4.61 |
| Overall ACR | - | - | - | 4.07 |
Understanding possible variation in relationships based on chemical class and mode of action was considered important to investigate. Chemical categories as identified in ECOSAR (Mayo-Bean et al. 2011) were used based on chemical structure, CAS number and SMILES notation. In addition, consensus mode of action (MOA) assignments from EnviroTox were associated to each compound (Kienzler et al. 2017, 2019). Both ECOSAR and MOA categories were used to subset algal inhibition data to investigate influence on acute-chronic ratios. Consensus MOA assignments were devised by Kienzler et al. (2019) to address the diversity of MOA classification systems with varying degrees of specificity and include narcotic, specific-acting, and non-classifiable MOAs; the latter category created for those compounds that classify differently across several mode of action schemes.
In terms of chronic inhibition of algal population growth, NOEC and EC10 statistical outcomes are viewed as interchangeable (OECD 2011). To provide additional support for this interpretation, a subset of data where NOEC and EC10 were available were directly compared via least squares regression. The data were further supplemented by 33 additional internal studies from Procter & Gamble where NOECr and ErC10 were readily available to augment 55 studies from the compiled algal toxicity information could be verified that the same test was the source for both NOEC and EC10 findings.
Statistical Analyses
Least squares linear regressions were calculated using various model permutations to establish ACRs for various combinations of species, endpoints and chemical groupings and NOEC-EC10 relationships using JMP Pro v 15.0.0 (SAS Institute 2019). Only significant regressions are reported (slope significant at α = 0.05). Acute:chronic comparisons were made in the context of endpoint only, based on chemical class and mode of toxic action (MOA) when possible. Analyses of variance (ANOVA) was used to identify if ACR relationships were taxon-specific for a subset of data where sufficient studies per taxon were available. Significance was inferred at α=0.05.
Results
The relationship between acute (EC50) and chronic (NOEC, EC10) algal inhibition were explored for each data type (r, b, y) (Figure 4). Endpoints that contained sufficient data to make acute to chronic comparisons include: ErC50 and ErC10, EbC50 and EbC10, ErC50 and NOECr, and EbC50 and NOECb. A minimum sample size criterion of seven along with statistical evidence for a significant relationship for any given paired comparison was previously shown to provide acceptable levels of interpretation for algal toxicity in Brill et al. (2016). Overall, growth rate data were the most abundant and encompassed the greatest diversity of chemicals while comparisons based on biomass endpoints were fewer in number (Table 2). The relationships between ErC50-ErC10, EbC50-EbC10, ErC50-NOECr, and EbC50-NOECb were all highly significant (p<0.001, R2 = 0.698-0.901 (Figure 4)). All comparisons indicate a strong correlation and consistent relationship between acute and chronic algal inhibition regardless of metric (r, b, y) and statistics (NOEC versus EC10). Due to lack of corresponding data, comparisons were not able to be made to distinguish NOEC and EC10 as comparable. This is due to the fact that studies tend to report a NOEC or an EC10, but rarely both.
Figure 4.

Regressions relating acute algal inhibition (EC50) and chronic effects (NOEC, EC10) for growth rate (r) and biomass area under the curve (b). (A) ErC10 vs ErC50 (n=61). (B) EbC10 vs EbC50 (n=87). (C) NOECr vs ErC50 (n=433). (D) NOECb vs EbC50 (n=94).
Taxa diversity in the models was somewhat limited because of trends in toxicity testing towards a few standard test species (Table 2). Collected data used for this study covers 17 algal species and 534 unique chemicals. The five most commonly tested species were the green algae Raphidocelis (formerly Pseudokirchneriella or Selenastrum) subcapitata, Desmodesmus (formerly Scenedesmus) subspicatus, Skeletonema costatum, Chlorella pyrenoidosa, and Chlamydomonas reinhardtii accounting for 68.9%, 7.0%, 4.4%, 7.5% and 3.1% of the studies, respectively. Chemical coverage was diverse and spanned a wide range of mode of actions (i.e., polar and non-polar narcosis, chemical-specific mechanisms including herbicidal, and unspecified reactivity) and chemical class (for representative examples see Fig. 5, 6).
Figure 5.
Regressions of Acute (ErC50) versus chronic (NOECr) inhibition for the most common ECOSAR chemical classes in the database.
Figure 6.
Regressions for broad mode of action classifications (based on Kienzler et al. 2017)
ACRs based on the geometric means for each effect endpoint (r, b) were 4.85, 4.29, 3.22 and 2.43 for EbC50-EbC10, EbC50-NOECb, ErC50-ErC10 and ErC50-NOECr, respectively (Table 2). The coefficient of determination (r2) ranged from 0.698 to 0.901 showing high correlations between acute and chronic endpoints derived from the same underlying data. However, the ACR correlations are not expected to be identical across r and b because they are based on different aspects of the underlying exposure-response curves. Data availability (number of chemicals) did not have a substantial influence on the regression. For example, slopes and intercepts were similar for regressions with 61 chemicals comparing ErC50 versus ErC10 and 433 chemicals comparing ErC50 versus NOECr. The similarity of acute-chronic slopes for EC10 and NOEC endpoints is further supported by a very strong relationship between NOEC and EC10 (Figure 7). The NOEC-EC10 regression has a slope near 1 and an r2 of 0.968 spanning nearly 8 orders of magnitude of potency.
Figure 7.

Relationship between NOEC and EC10 statistical endpoints indicating these can be considered equally useful in developing Acute-chronic ratios when only one of these may be available.
It is difficult to calculate robust single species and class specific acute:chronic ratios due to the small number of comparable tests available. For these comparisons, ACRs were generated using ErC50 and NOECr data. ACRs based on single species yielded ACRs of 4.64, 4.21, 3.06, 3.12 and 3.06 for Raphidocelis subcapitata, Desmodesmus subspicatus, Chlorella pyrenoidosa, Skeletonema costatum and Chlamydomonas reinhardtii, respectively (Supplementary data). Raphidocelis subcapitata was the species with the greatest information available, however, taxa with somewhat fewer data showed similar ACR comparisons. One-way analysis of variance (ANOVA) was not significant indicating single species ACRs were not taxon-specific (F-value = 0.3675; p = 0.8318).
Figure 5 presents the acute-chronic relationships for several chemical classes identified using ECOSAR for all studies in this research. Chemical class assignments identified five major chemical classes for which algal inhibition data were available including: neutral organics (n=56), phenols (n=42), aliphatic amines (n=33), anilines (n=29) and esters (n=26). The resulting group-specific ACRs for neutral organics, phenols, aliphatic amines, analines, and esters were 4.32, 5.35, 4.71, 4.85 and 4.26, respectively. The data suggest that ACRs generally covered a narrow range from slightly greater than 4 to slightly less than 5 regardless of chemical class. An ANOVA test indicated there was no significant differences in median ACRs among chemical class data sets (F = 0.2951 and p = 0.8809), and these relationships appear to be independent of substance type. Worth noting is that data on metal toxicity has not been presented in this paper. It is well established that hardness, alkalinity and pH modify metal toxicity extensively (e.g., Fawza et al. 2018). Algal media, while it is often defined at the level of nutrient application, is very often not assayed with standard methods for hardness and alkalinity. The net result was that it is especially difficult to reconcile different studies with each other.
MOA-based ACRs were derived using the same ErC50 and NOECr data subset. MOAs were assigned according to the consensus MOA framework outlined in Kienzler et al (2019). Narcotic, specific-acting, and non-classifiable MOAs contained 218, 65, and 110 chemicals, with ACRs of 4.82, 5.62, 3.99, respectively. When herbicidal chemicals were assessed separately, the ACR was 4.18 for ErC50-NOECr comparisons. The ACR was slightly lower compared to narcotics and specific-acting chemicals, which is consistent with the higher potency of herbicides for plant species. No significant differences were found among the median ACRs for the MOA category. Despite the numerical difference between narcotic ACRs (4.82) versus ACRs for specifically-acting compounds (5.62), one-way ANOVA indicated these were not significantly different (F-value = 0.2229 ; p = 0.8805). Figure 6 presents the regressions for each group (narcotic, specific-acting, non-classifiable and herbicide based MOA) and Table 2 summarizes geometric mean ACRs associated for each comparison. The median ACR of 3.57 for endpoint, 3.56 for single species, 4.68 for chemical class and 4.61 for MOA were established. The ACR for all tests, chemicals and modes of action was 4.07 (Table 2). ACRs were approximately 4 regardless of dataset used or combination of endpoints.
Discussion
Acute-chronic ratios (ACRs) are fundamental to chemical hazard assessment when chronic data are lacking. Extrapolation factors are used at each step of the risk assessment process to generate safe concentrations for ecosystems (Predicted No Effect Concentrations, PNECs). In the case of data sets lacking chronic toxicity information, an assessment or extrapolation factor of 10 is used to predict chronic toxicity (Nabholz et al. 1993; ECHA 2014). In Europe, the AF to derive PNECs based on acute or chronic toxicity are 1000 and 10, respectively, when fish, Daphnia, and algal inhibition data are available (ECHA 2014; see details in Table R.10.4 of ECHA 2014 for a complete overview). In the US, the AF are 100 and 10 when all taxa are available. The more conservative nature of the European assessment includes additional factors impacting uncertainty beyond acute-chronic extrapolation including policy objectives, the need for additional data, suitability of conclusions as to the order of taxonomic sensitivity, and perceived importance of sensitivity information for invertebrates and fish versus microbes. The US assessment process places more emphasis on the extrapolation of acute to chronic toxicity with a nominal ACR of 10 which is generally assumed to be useful in chemical toxicity extrapolations. Raimondo et al. (2007) assessed variabilities in ACR as a function of invertebrate and fish species tested, chemical class and mode of action. ACRs were found to be quite variable with larger ACRs associated with specifically acting toxicants. Interestingly, the central tendency (all chemicals considered simultaneously) indicated ACRs of 7.5 and 9.3 for invertebrates and fish, respectively. Algae, however, were not considered. Because certain modes of action are often associated with a large ACR and because MOA is a key determinant of chemical toxicity, it is relevant to note that MOA classifications are most often based on fish and invertebrate toxicity, and less frequently on algae. Different MOA frameworks have different degrees of coverage within a dataset and there is currently no standard for MOA assignment (Kienzler et al. 2017). Several systems are available varying in specificity, inclusivity and accessibility. Kienzler et al. (2017) described various MOA classifications assignment approaches (ECOSAR, MOATox, ASTER and Verhaar for example), and devised a consensus classification system. However, the relevance for algae is somewhat problematic (specific neurotoxicity, for example) when established on the basis of fish and invertebrates. Chemicals identified as herbicides in this research were assessed separately producing an overall ACR of 4.18.
The central-tendency ACRs of Ahlers et al. (2006) match well with those of Raimondo et al. (2007) and also found considerable variability depending on the chemical assessed. Ahlers also determined an algal median ACR of 5.4. The lowest to highest ACRs in the Ahlers et al. (2006) work were for algae, Daphnia magna, and fish at 5.4, 7.0, and 10.5. Importantly “algae” and “fish” are mixtures of numerous taxa, so the determination of ACRs at the same level of taxonomic resolution of Daphnia magna should be inspected. In our research presented in this paper, algal ACRs ranged from slightly above 3 to slightly greater than 5, depending on the toxicity metric and chemical class.
The use of NOECs versus EC10 values to interpret chronic ecotoxicity has been a subject of significant discussion at the international level (OECD 1998) and in the scientific literature (Beasley et al. 2015). Taxa, other than algae, have also shown strong relationship between EC10 and NOEC values including Daphnia (Beasley et al. 2015), fish (Oris et al. 2012), and even for specific chemical categories regardless of taxon (Belanger and Dorn 2004). This, along with observations of the greater scientific community, has resulted in the clear recommendation that EC10s are a valid and often preferred statistical endpoint in OECD test guidelines (OECD 2011, 2012, 2013). Of course, EC10 and NOEC determinations are also dependent on meeting statistical assumptions and validity criteria.
Many reasons contribute to a lower ACR for algae versus other taxonomic groups. Both the acute and chronic effects are characterized by population growth and do not reflect individual responses of survival (as occurs in fish and daphnid assays). A higher ACR in algae tests then directly reflects that the exposure-response profile for the compound is rather shallow versus small ACRs being rather steep. Thus, the ACR is somewhat pre-determined in a mathematical sense, much more so than for invertebrate and fish assessments. It is worth noting here that the use of the terms “acute” and “chronic” for algal responses to chemical contaminants is something of a misnomer. It is well understood that even at the EC50 level, numerous generations of algae have been chronically exposed. For convenience, regulatory assessment schemes have made the distinction between acute and chronic based on the statistical, not the biological, interpretation. In the early days of algal toxicity testing, this distinction was appreciated and one of the testing paradigms was designed to identify concentrations that were algicidal (i.e., where cell death occurred widely enough in the population such that negative growth occurred), algistatic (cells go into stasis, not increasing in abundance further, but also not dead), and true growth (the typical interpretation) (Payne and Hall 1979; ASTM 2017). The design is more intricate and takes longer than the OECD 201 or USEPA 8500.4500 test guidelines owing to the utilization of a post-exposure recovery phase to discern the algicidal-algistatic boundary concentration.
Noise in the ACR assessment for algae can be attributed to several factors beyond simple biological variation. Algal inhibition responses have been quantified using several metrics including intrinsic rate of increase, yield, biomass area under the curve, and final cell density. Each metric has played a role at various times throughout the history of algal toxicity test method development. Different regulatory authorities place different emphases on each metric (Europe prioritizing growth rate, the US prioritizing final cell density). In addition, assays have been performed for various durations. The earliest algal tests were 10 days in duration, 5 days of exposure and 5 days of subculturing to assay for exposure at which toxicants were algistatic or algicidal effects (Payne and Hall 1979). By 1984, when OECD adopted the 72-h exposure duration as the standard for algae, the US had already codified the 96-h approach (OECD 1984; US EPA 1974, 1982). This leads to enormous difficulty in comparing algal responses to chemicals used on the global market where the principle of testing only once is conceptually feasible (OECD Mutual Acceptance of Data; https://www.oecd.org/env/ehs/mutualacceptanceofdatamad.htm). The analysis presented here supports the contention that acute-chronic relationships are still relatively immune to which metric is chosen (i.e., analysis being conducted on r, b, y, or final cell density for both acute and chronic responses without mixing unlike metrics in the analysis). ACRs for algae are similar regardless.
In addition to biological variability and test method considerations, algae inhibition results are also subjected to various statistical summarization techniques. OECD (2006) Test Guideline 201 was the first aquatic test guideline to be revised where the EC10 was directly suggested as the preferred statistical endpoint over the No-observed-effect-concentration (NOEC) in accordance with other scientific recommendations (Bruce and Versteeg 1992; OECD 1998). The ecotoxicological debate continues with respect to the preference of point estimation versus regression estimation of effects (Tanaka et al. 2018; Warne and van Dam 2008). Depending on the quality of the study, choice of test concentrations, and effect level chosen, the NOEC and EC10 will deliver different quantitative results thereby affecting the apparent ACR. Algae appear to be somewhat less impervious to differences between NOEC and EC10 determinations relative to Daphnia (Beasley et al. 2015) and fish (Oris et al. 2012) based on results within this research and our laboratory’s experience. Nevertheless, this does further contribute to noise in the overall ACR variability.
Algae are frequently the most sensitive taxon in chemical hazard assessment, thus the application factor applied to algal data becomes very important. In cases where algae are most sensitive (about 40% of the time; Rawlings et al. 2018; Hutchinson et al. 2003; Jeram et al. 2005), the use of an application factor of 10 clearly is a conservative approach as the ACR is significantly less than 10, closer to 4 on average. Mayo-Bean et al. (2012) identified the algal ACR of 4 was applicable for acute-chronic predictions in USEPA’s ECOSAR program. We suggest that an ACR of 4 is supportable by the analysis presented here and can be useful when only acute data are available or to estimate acute responses when only chronic data is provided.
Acknowledgements
This work was supported by the HESI Animal Alternatives in Environmental Risk Assessment Technical Committee during the development of the EnviroTox database (www.EnviroToxdatabase.org) and accompanying PNEC derivation and ecoTTC analytical tools. This manuscript has been subjected to review by U.S. EPA’s National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Ahlers J, Riedhammer C, Vogliano M, Ebert R, Kühne R, Schüürmann G. 2006. "Acute to chronic ratios in aquatic toxicity - variation across trophic levels and relationship with chemical structure." Environmental Toxicology and Chemistry 25(11): 2937–2945. [DOI] [PubMed] [Google Scholar]
- Beasley A, E Belanger S and Otter RR. 2015. Stepwise Information-Filtering Tool (SIFT): A method for using risk assessment metadata in a nontraditional way. Ecotoxicology and Environmental Safety 34(6):1436–1422. [DOI] [PubMed] [Google Scholar]
- Belanger S, Sanderson H, Embry M, Coady K, DeZwart D, Farr B, Gutsell S, Halder M, Sternberg R, Wilson P. 2015. "It is time to develop ecological thresholds of toxicological concern to assist environmental hazard assessment." Environmental Toxicology and Chemistry 34(12): 2864–2869. [DOI] [PubMed] [Google Scholar]
- Belanger SE And Dorn PB. 2004. Chronic aquatic toxicity of alcohol ethoxylate surfactants under Canadian exposure conditions. Canadian Technical Report of Fisheries and Aquatic Sciences. pp. 95–104. In, Burridge LE, Haya K, and Niimi AJ (eds.). Proceedings of the 31st Annual Aquatic Toxicity Workshop, October 24-27, 2004, Charlottetown, Prince Edward Island, Candian Technical Report of Fisheries and Aquatic Sciences No. 2562. [Google Scholar]
- Bergtold M, Dohmen G. 2010. "Biomass or Growth Rate Endpoint for Algae and Aquatic Plants: Relevance for the Aquatic Risk Assessment of Herbicides." Integrated Environmental Assessment and Management 7 (2): 237–247 [DOI] [PubMed] [Google Scholar]
- Brill J, Belanger S, Chaney J, Dyer D, Raimondo S, Barron M, Pittinger C. 2016. "Development of algal interspecies correlation estimation models for chemical hazard assesssment". Environmental Toxicology and Chemistry 35 (9): 2368–2378. [DOI] [PubMed] [Google Scholar]
- Connors K, Beasley A, Barron M, Belanger S, Bonnell M, Brill J, DeZwart D, Kienzler A, Krailler J, Otter R, Philips J, Embry M. 2019. "Creation of a Curated Aquatic Toxicology Database: EnviroTox". Environmental Toxicology and Chemistry DOI: 10.1002/etc.4382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberius M, Mennicken G, Reuter I, Vandenhirtz J. 2002. Sensitivity of different growth inhibition tests – Just a matter of mathematical calculation? Theory and practice for algae and duckweed. Ecotoxciology 11: 293–297. [DOI] [PubMed] [Google Scholar]
- ECHA 2014. Guidance on information requirements and chemical safety assessment Chapter R.10: Characterisation of dose [concentration]-response for environment. Helsinki, Finland. 65 p. [Google Scholar]
- Fawza EG, Salam DA, Kamareddine. 2018. Evaluation of copper toxicity using site specific algae and water chemistry: Field validation of laboratory bioassays. Ecotoxicology and Environmental Safety 155:59–65. [DOI] [PubMed] [Google Scholar]
- Hutchinson T, Barrett S, Buzby M, Constable D, Hartmann A, Hayes E, Huggett D, Laenge R, Lillicrap A, Straub J, Thompson R. 2003. "A strategy to reduce the numbers of fish used in acute ecotoxicity testing of pharmaceuticals." Environmental Toxicology and Chemistry 22(12): 3031–3036. [DOI] [PubMed] [Google Scholar]
- Jeram S, Riego Sintes J, Halder M, Fentanes B, Soküll-Kluttgen B, Hutchinson T 2005. "A strategy to reduce the use of fish in acute ecotoxicity testing of new chemical substances notified in the European Union,." Regulatory Toxicology and Pharmacology 42(2): 218–224. [DOI] [PubMed] [Google Scholar]
- Kienzler A, Barron MG, Belanger SE, Beasley A, Embry M. 2017. Mode of action (MOA) assignment classifications for ecotoxicology: An evaluation of approaches. Environmental Science and Technology 51: 10202–10211. [DOI] [PubMed] [Google Scholar]
- Kienzler A, Connors KA, Bonnell M, Barron MG, Beasley A, Inglis CG, Norberg-King TJ, Martin T, Sanderson H, Vallotton N, Wilson P, Embry MR. 2019. Mode of action (MOA) classifications in the EnviroTox database: Development and implementation of a consensus MOA classification. Environmental Toxicology and Chemistry. doi: 10.1002/etc4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo-Bean K, Nabholz JV, Clements R, Zeeman M, Henry T, Rodier D, Moran K, Meylan B, Ranslow P. 2011. EcoSAR class program for estimating toxicity of industrial chemicals to aquatic organisms using the EcoSAR (Ecological Structure Activity Relationship) class program. MSWindows Ver 1.1. Office of Pollution Prevention and Toxics, US Environmental Protection Agency, Washington, DC. [Google Scholar]
- OECD 1984. OECD Guideline for Testing of Chemicals 201, Alga, Growth Inhibition Test. 7 June 1984, Paris France. 14p. [Google Scholar]
- OECD (Organization for Economic Cooperation and Development). 1998. Report of the OECD Workshop on Statistical Analysis of Aquatic Toxicity Data. OECD Series on Testing and Assessment No. 10. Paris, France, 26 p. [Google Scholar]
- OECD Guidelines for the Testing of Chemicals. Guideline 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. 2006. [Google Scholar]
- OECD (Organization for Economic Cooperation and Development). 2011. OECD Guidelines for the Testing of Chemicals - Freshwater Alga and Cyanobacteria, Growth Inhibition Test, Test Guideline 201. Paris, France, 26 p. [Google Scholar]
- OECD (Organization for Economic Cooperation and Development). 2012. OECD Guidelines for the Testing of Chemicals – Daphnia magna Reproduction Test, Test Guideline 211. Paris, France. 25p. [Google Scholar]
- OECD (Organization for Economic Cooperation and Development). 2013a. OECD Guidelines for the Testing of Chemicals – Fish Early Life Stage Toxicity Test, Test Guideline 210. Paris, France. 24p. [Google Scholar]
- Payne AG and Hall RH 1979. A Method for Measuring Algal Toxicity and Its Application to the Safety Assessment of New Chemicals, Aquatic Toxicology, ASTM STP 667, Marking LL and KimerJe RA, Eds., American Society for Testing and Materials, 1979, pp. 171–180. [Google Scholar]
- [USEPA] U.S. Environmental Protection Agency. 1996. Ecological Effects Test Guidelines: OPPTS 850.5400 Algal Toxicity, Tiers I and II. EPA 712-C-96-164, April 1996. Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- Raimondo S, Montague B, Barron M. 2007. Determinants of variability in acute to chronic toxicity ratios for aquatic invertebrates and fish. Environmental Toxicology and Chemistry 26:2019–2023. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakamura K, Yokomizo H. 1996. Relative robustness of NOEC and ECx against large uncertainties in data. PLoS ONE 13 (11): e0206901. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA Ecological Effects Test Guidelines. OPPTS 850.5400: Algal Toxicity, Tiers I and II. [Google Scholar]
- Warne M, van Dam R. 2008. NOEC and LOEC Data Should No Longer be Generated or Used. Australasian Journal of Ecotoxicology Vol. 14, No. 1, January 2008: pp 1–5. ISSN: 1323:3475 [Google Scholar]
- Zeeman M 1995. Ecotoxicity testing and estimation methods developed under Section 5 of the Toxic Substances Control Act (TSCA). In: Rand GM (Ed.), Fundamentals of Aquatic Toxicology. Taylor & Francis, Bristol, PA, pp. 703–716. [Google Scholar]