Abstract
Preexisting hypertension is a known risk factor for severe COVID-19. Abnormal activation of RAS upregulates angiotensin II (Ang-II) and contributes to severe manifestations of COVID-19. Although RAS inhibitors (RASi) are a mainstay of antihypertensive therapy, they have been associated (in some animal studies) with an increase in angiotensin converting enzyme 2 (ACE2) receptors that facilitate cellular entry of the SARS-CoV-2 virus. Nonetheless, current medical practice does not recommend curtailing RASi to protect hypertensive patients from COVID. On the contrary, there is clinical evidence to support a beneficial effect of RASi for hypertensive patients in the midst of a COVID-19 pandemic, although the precise mechanism for this is unclear. In this paper, we hypothesize that RASi reduces the severity of COVID-19 by promoting ACE2-AT1R complex formation at the cell surface, where AT1R mediates the major vasopressor effects of Ang-II. Furthermore, we propose that the interaction between ACE2 and AT1R impedes binding of SARS-CoV-2 to ACE2, thereby allowing ACE2 to convert Ang-II to the more beneficial Ang(1–7), that has vasodilator and anti-inflammatory activity. Evidence for ACE2-AT1R complex formation during reduced Ang-II comes from receptor colocalization studies in isolated HEK293 cells, but this has not been confirmed in cells having endogenous expression of ACE2 and AT1R. Since the SARS-CoV-2 virus attacks the kidney, as well as the heart and lung, our hypothesis for the effect of RASi on COVID-19 could be tested in vitro using human proximal tubule cells (HK-2), having ACE2 and AT1 receptors. Specifically, colocalization of fluorescent labelled: SARS-CoV-2 spike protein, ACE2, and AT1R in HK-2 cells can be used to clarify the mechanism of RASi action in renal and lung epithelia, which could lead to protocols for reducing the severity of COVID-19 in both hypertensive and normotensive patients.
Keywords: SARS-CoV-2, Hypertension, Angiotensin, Renin, RAS, ACE2, Kidney
Introduction
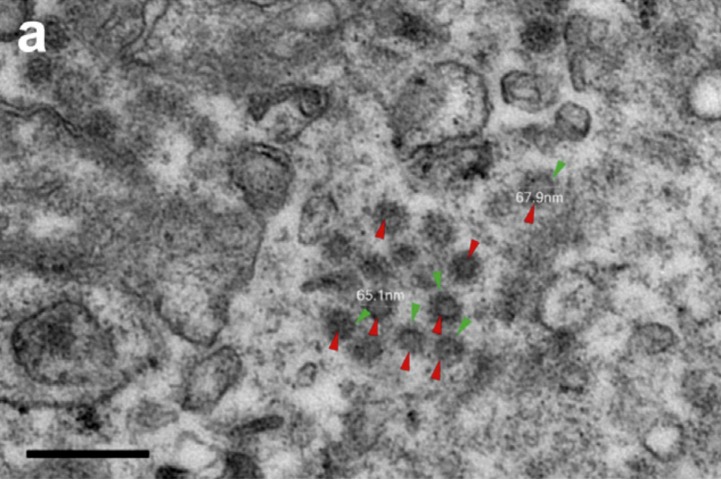
Recent evidence suggests that the SARS-CoV-2 virus targets the kidney, as well as the lung and heart. Postmortem studies of 26 Chinese COVID-19 patients have positively identified SARS-CoV-2 virus particles (Fig. 1 ) in proximal and distal tubules, as well as glomerular basement membrane [1]. Direct attack of SARS-CoV-2 on the kidney is enabled by an abundance of angiotensin-converting enzyme 2 (ACE2) receptors in human proximal tubular cells [2]. In the renin angiotensin system (RAS), angiotensin converting enzyme (ACE) catalyzes conversion of angiotensin I to the potent vasopressor, angiotensin II (Ang-II). Angiotensin-II can bind to either AT1 receptors (AT1R), resulting in vasoconstriction and aldosterone secretion; or to ACE2 receptors that catalyze conversion of Ang-II to Ang (1–7), resulting in vasodilator and anti-inflammatory activity. The anti-hypertensive action of RAS inhibitors (RASi) derives from their ability to decrease vasoconstriction and blood pressure by either reducing Ang-II levels (via ACEi) or reducing Ang-II binding to AT1R (via ARBs).
Fig. 1.
Ultrastructural features of kidneys from postmortems of patients with severe coronavirus disease 2019. Virus particles (red arrowheads) with distinctive spikes (green arrowheads) were present in the cytoplasm of the proximal tubular epithelium. EM preparation: osmium tetroxide post fixation and gradient dehydration, Epon-embedded, toluidine blue-stained “semi-thin” sections cut and stained with uranyl acetate and lead citrate. Viewed with a transmission electron microscope (HT-7800; Hitachi, Tokyo, Japan). Bar = 200 nm. From Ref [1].
Clinical dysfunction of the renin-angiotensin aldosterone axis (RAAS) together with elevated levels of angiotensin II (Ang-II) have been associated with severe COVID-19 in elderly hypertensive patients [3], [4], [5], [6]. Therefore, it stands to reason that reduction of Ang-II by ACE inhibitors (ACEi) or block of Ang-II binding to AT1R by angiotensin receptor blockers (ARB) could be beneficial to hypertensive COVID-19 patients. On the other hand, recent publications have questioned the therapeutic use of ARBs for treatment of cardiovascular, kidney and metabolic disorders arising from COVID-19 [7], [8]. Rodent studies, showing an increase in surface ACE2 receptors after ARBs, have raised the possibility that ARBs might enhance viral uptake; and consequently, RAS inhibitors should be withdrawn from COVID-19 patients [9], [10], [11], [12].
However, the majority of human studies and meta-analyses do not support withdrawal of RAS inhibitors (either ACE inhibitors or ARBs) from hypertensive patients [13], [14], [15], [16], [17], [18], [19], [20]. In fact, there is clinical evidence that RAS inhibitors might actually be beneficial to both hypertensive and normotensive COVID-19 patients [20]. One such study, having a small sample of 42 Chinese patients, found that ACEi or ARB administration produced a lower rate of severe disease, a decreased peak viral load, and a lower level of IL-6 in peripheral blood [21]. In large cohort studies, administration of an ACEi or ARB to COVID-19 patients with hypertension was associated with lower risk of all-cause mortality, compared to ACEi/ARB non-users [22], [23], [24]. Several reviews support these clinical observations and discuss possible ways that ARB's like losartan might alleviate the severity of COVID-19 [5], [19], [20], [25], [26]. Nonetheless, a coherent theory is still lacking for the cellular mechanism of RAS inhibitor activity on COVID-19. Our hypothesis (see below) addresses this deficiency by proposing that RASi’s mitigate both viral infection and disease severity by altering a cell surface interaction between ACE2 and the angiotensin receptor type one (AT1R). This model for the effect of RASi on COVID-19 is amenable to testing using an in vitro human kidney cell line.
Hypothesis
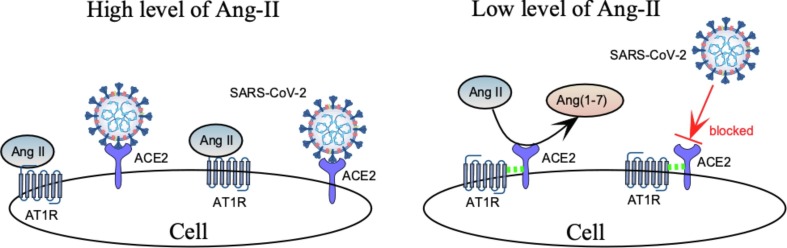
In light of the above clinical studies, we hypothesize that ACE inhibitors decrease SARS-CoV-2 access to its cellular ACE2 receptor according to the scheme of Fig. 2 . In this model, high levels of Ang-II (Left side of Fig. 2) favor binding of Ang-II to AT1R, thereby allowing the SARS-CoV-2 virus unfettered access to ACE2 on the cell surface. Application of RAS inhibitors (i.e. ACEi’s) would decrease Ang-II levels (Right side of Fig. 2). As a result, there would be less Ang-II bound to AT1R, greater likelihood of AT1R-ACE2 complex formation, less virus binding to ACE2, and more conversion of Ang-II to Ang (1–7), a beneficial vasodilator.
Fig. 2.
At high levels of Ang-II (left), increased binding of Ang-II to AT1R enhances availability of ACE2 to the invading virus. Conversely, low levels of Ang-II (right) frees AT1R to form complexes with ACE2 (dashed green lines) that increase conversion of Ang-II to Ang(1–7) and decrease interaction of the virus with ACE2.
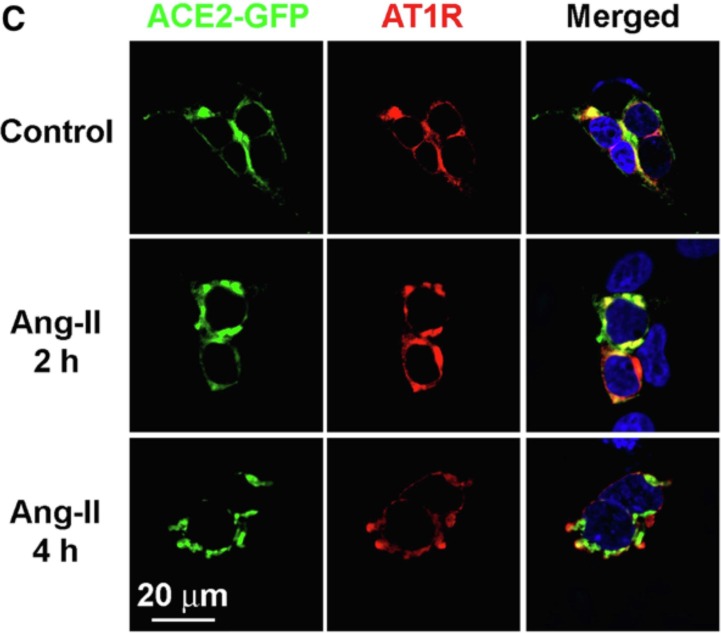
The basic elements of this hypothesis rely on the demonstration of AT1R-ACE2 complexes at the cell membrane and their modulation by the renin-angiotensin system. Evidence supporting an effect of Ang-II on putative AT1R-ACE2 surface complexes comes from confocal experiments (Fig. 3) in which sustained exposure to Ang-II decreased colocalization of AT1R and ACE2 in the HEK293T cell line [27]. In these experiments the initial association of AT1R and ACE2 (yellow, Fig. 3) was followed by decreased colocalization after a 4 h exposure to Ang-II, suggesting that Ang-II decreases AT1R-ACE2 association. However, interpretation of Fig. 3 requires the caveat that receptor colocalization does not necessarily prove a physical interaction between the two receptors.
Fig. 3.
Colocalization of ACE2 and AT1R in HEK293T cells in control conditions (top), or after 2 hrs (middle) and 4 hrs (bottom) treatment with Ang-II (100 nmol/L). HEK293T cells were serum-starved for 24 hrs and treated with Ang-II (100 nmol/L) for the indicated time periods [27]. In the merged panel, yellow indicates colocalization of ACE2 and AT1R. There is much less colocalization after 4 h of Ang-II treatment. Figure is from Ref [27].
Even though the data of Fig. 3 suggest an Ang-II effect on AT1R-ACE2 association, possible degradation of AT1R at 4 h complicates interpretation of the data [27]. No explanation was provided for the decrease in labeled AT1R at 4 h (3rd row of Fig. 3), although this did not appear to result from chronic angiotensin exposure [28]. Moreover, co-immunoprecipitation experiments performed as part of the same study [27] indicated that Ang-II reduced the interaction between AT1R and ACE2.
Nonetheless, we suggest repeating both the confocal and co-immunoprecipitation Ang-II experiments in the HK-2 human kidney cell line rather than HEK293T (Fig. 3 ), since HEK cells require co-transfection of ACE2 and AT1 receptors which introduces an additional level of uncertainty when extrapolating to real patients [27]. HK-2 cells display a phenotype consistent with human proximal tubules [29] and possess native ACE2 and AT1 surface receptors, although the apical vs. basolateral distribution of these receptors is unknown [30].
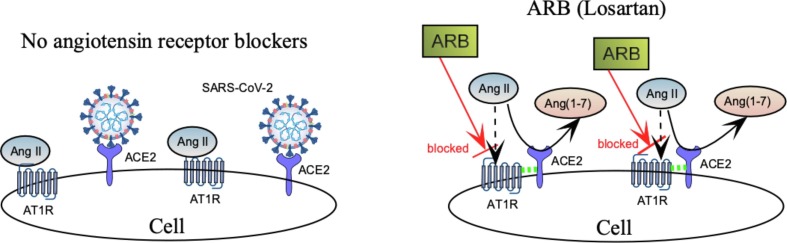
The second part of our hypothesis addresses the possible role of ARBs on AT1R-ACE2 complex formation (Fig. 4). We hypothesize that, by blocking Ang-II binding to AT1R, angiotensin receptor blockers (ARBs) facilitate AT1R-ACE2 complex formation at the cell surface, allowing ACE2 to convert Ang-II to Ang(1–7), rather than bind SARS-CoV-2 (right side of Fig. 4).
Fig. 4.
ARBs enhance AT1-ACE2 complex formation, increase conversion of Ang-II to Ang(1–7), and decrease availability of ACE2 receptors to invading virus. Dashed green lines indicate a putative linkage between AT1R and ACE2.
Evidence in support of this model comes from supplemental experiments reported in Ref [27] which demonstrated that the ARB, losartan, stabilized ACE2-AT1R complexes at the cell surface and prevented Ang-II induced endocytosis of ACE2. According to the model of Fig. 4 , ACE2-AT1R stabilization should make ACE2 less available for virus binding and more available to catalyze conversion of Ang-II to the vasodilator Ang(1–7), thereby providing a therapeutic benefit to hypertensive COVID-19 patients.
In vitro testing of the hypothesis
Our hypothesis for the beneficial effect of RAS inhibitors on COVID-19 could be tested in vitro using a stable, immortalized HK-2 cell line, that displays a phenotype characteristic of renal epithelial proximal tubules [29], and has both ACE2 and AT1 receptors [30]. A set of experimental protocols for hypothesis testing are outlined below.
Effect of Ang-II on spike binding
The effect of Ang-II on SARS-CoV-2 spike binding to ACE2 (Fig. 2) can be assessed by quantifying the colocalization of (mCherry red) fluorescent S1 spike protein with ACE2 (labeled with rabbit polyclonal anti-ACE2 primary antibody, and goat anti-rabbit IgG secondary antibody Alexa Fluor® 488 green). Specificity controls would test for the Alexa Fluor® 488 green signal in the absence of primary antibody. Use of the SARS-CoV-2 spike S1 subunit (which cannot enter the cell without its S2 component) avoids the complication of SARS-CoV-2 spike binding to ACE2, entering the cell, and being degraded. Measurements of S1-ACE2 colocalization as a function of Ang-II would have to be normalized to total ACE2 surface immunoreactivity since elevated Ang-II would probably reduce the total number of ACE2 receptors at the surface [31]. The model of Fig. 2 predicts that high Ang-II levels would increase ACE2 occupancy by S1-spike protein, as more ACE2 receptors become available to the virus. Conversely, a decrease in Ang-II level would be expected to decrease S1 spike colocalization with ACE2.
Effect of Ang-II on AT1R-ACE2 complex formation
The model of Fig. 2 predicts that elevated Ang-II would enhance binding of Ang-II to AT1R, while at the same time decreasing AT1R-ACE2 linkage and rendering ACE2 more receptive to incoming virus (Left side of Fig. 2). Conversely, the model predicts that reducing Ang-II would increase AT1R-ACE2 linkage, and make ACE2 less likely to bind incoming virus (Right side of Fig. 2). Association between AT1R and ACE2 could be determined by immunofluorescent colocalization, similar to what is shown in Fig. 3, but with HK-2 human proximal tubule cells that express endogenous ACE2 and AT1 receptors, rather than HEK 293T cells that require co-transfection of both ACE2 and AT1. Spatial correlation of these receptors could be quantified using primary antibodies to AT1R and ACE2, followed by secondary fluorescent antibodies conjugated to 2 different Alexa Fluors®. Controls could test for cross-reactivity between AT1R and ACE2 primary antibodies by checking for secondary antibody fluorescence in the absence of either primary antibody.
If elevated Ang-II destabilizes AT1R-ACE2 complexes at the HK-2 cell surface, we would expect very little fluorescent overlap between these two receptors at high Ang-II (Left side of Fig. 2). On the other hand, we predict that low Ang-II would enhance colocalization of AT1R and ACE2 and decrease SARS-CoV-2 spike binding to its ACE2 receptor (Right side, Fig. 2). Again, normalization of the AT1R-ACE2 colocalization data would be required to compensate for Ang-II induced changes in ACE2 surface density. One caveat in all experiments of this type is that colocalization does not unequivocally prove functional coupling between receptors. Parallel experiments looking at the effect of Ang-II on coimmunoprecipitation of AT1R and ACE2 would help to confirm a physical linkage between AT1R and ACE2.
Effect of ARBs
As indicated in Fig. 4, our hypothesis also predicts that angiotensin receptor blockers (ARBs) like losartan, which block binding of Ang-II to AT1R, would strengthen the interaction between AT1R and ACE2 receptors at the cell surface (Right side, Fig. 4). Colocalization of ACE2 and AT1 receptors could be quantified as a function of losartan in renal HK-2 immortalized cells, using primary and secondary antibodies to ACE2 and AT1R. Normalization to the total number of surface ACE2 receptors would also be required since these could be altered by losartan.
In addition, our hypothesis predicts that losartan block of Ang-II binding to AT1R should also decrease spike binding to ACE2 since more ACE2 would now be linked to AT1R, making ACE2 less available to the virus (Right side, Fig. 4). This aspect of our hypothesis could be tested by comparing S1 spike-ACE2 colocalization as a function of losartan, using (mCherry red) fluorescent SARS-CoV-2 S1 spike protein, and immunofluorescent ACE2 (labeled with rabbit polyclonal anti-ACE2 primary antibody).
Conclusions
Dysfunction of the renin-angiotensin-aldosterone system aggravates the severity of COVID-19, and renin-angiotensin system inhibitors (RASi) have been suggested as a possible treatment for hypertensive COVID-19 patients. A number of large-scale cohort studies have confirmed the benefit of RASi treatments in hypertensive COVID patients, although the reason for this is still unclear [22], [23], [24]. Our hypothesis proposes a mechanistic cellular basis for the benefits of RASi treatment of COVID-19 patients. Specifically, we believe that RASi facilitates ACE2-AT1R complex formation at the epithelial cell surface, making ACE2 less available to bind SARS-Cov-2, and making it more available for catalyzing conversion of Ang-II to Ang(1–7), which lowers blood pressure (vasodilation), enhances excretion of sodium, and reduces inflammation. Our hypothesis for a RASi effect on ACE2 and AT1 receptors is consistent with other models that have been proposed [5], [19], [26], and suggests that readily available anti-hypertensive medications (ACEi’s and ARBs) could be repurposed as a medical treatment for COVID-19. As such, RASi would not only be useful for hypertensive COVID patients but possibly for normotensives as well, until the successful distribution of SARS-CoV-2 vaccines obviates the need for medical therapies. Since ACE2 and AT1R are present in lung epithelia, our hypothesis would also be relevant for understanding the respiratory benefits of RASi therapy. Finally, our theory for RASi modulation of ACE2-AT1R linkage and its effect on SARS-Cov-2 binding is amenable to in vitro testing with immunocytochemistry, using fluorescent labeled: spike protein, ACE2, and AT1R in the human kidney (HK-2) cell line.
Funding
This hypothesis was based on work supported by National Institutes of Health grant DK046950 to Henry Sackin.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Sparks MA, Hiremath S et al. “The Coronavirus Conundrum: ACE2 and Hypertension Edition” NephJC. http://www.nephjc.com/news/covidace2.
Glossary
- ACE1
Angiotensin-converting enzyme: converts angiotensin 1 (Ang-I) to angiotensin 2 (Ang-II).
- Ang-II
Angiotensin 2: increases blood pressure (BP) by vasoconstriction, stimulates adrenals to secrete aldosterone.
- ACEi
ACE1 inhibitor: binds and inhibits ACE1, not ACE2.
- ACE2
Angiotensin-converting enzyme 2: converts Ang-II to Ang(1–7). ACE2 is also the cellular entry receptor for SARS-CoV-2.
- AT1R
Angiotensin receptor type 1: cell receptor mediating the major effects of Ang-II.
- Ang(1–7)
Angiotensin-(1–7): activates MAS receptor, lowering BP, effects opposite to AT1R.
- ARB
Angiotensin receptor blocker: prevents Ang-II from binding to AT1R and producing its effect.
- RAS
Renin-angiotensin system, or RAAS (renin-angiotensin-aldosterone system): a hormone system that regulates BP via vascular resistance, as well as fluid & electrolyte balance.
- RASi
Renin-angiotensin system inhibitors: (ACEi and ARBs)
References
- 1.Su H., Yang M., Wan C., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun M., Yang J., Sun Y., Su G. Inhibitors of RAS Might Be a Good Choice for the Therapy of COVID-19 Pneumonia. Chin J Tuberc Respir Dis. 2020;43 doi: 10.3760/cma.j.issn.1001-0939.2020.0014. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Sriram K., Insel P.A. A hypothesis for pathobiology and treatment of COVID-19: The centrality of ACE1/ACE2 imbalance. Br J Pharmacol. 2020;177(21):4825–4844. doi: 10.1111/bph.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;1–4 doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo J., Huang Z., Lin L., Lv J. Coronavirus Disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J Am Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.120.016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz JH. Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19 J Travel Med. 2020;27(3): Mar 18, ahead of print. [DOI] [PMC free article] [PubMed]
- 8.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30116-8. 11 ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cüre E., Cumhur C.M. Comment on ‘Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic?’. J Hypertens. 2020;38(6):1189–1198. doi: 10.1097/HJH.0000000000002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esler M., Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens. 2020;38(5):781–782. doi: 10.1097/HJH.0000000000002450. [DOI] [PubMed] [Google Scholar]
- 11.Ferrario C.M., Jessup J., Chappell M.C., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111(20):2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 12.Soler M.J., Ye M., Wysocki J., William J., Lloveras J., Batlle D. Localization of ACE2 in the renal vasculature: amplification by angiotensin II type 1 receptor blockade using telmisartan. Am J Physiol Renal Physiol. 2009;296(2):F398–405. doi: 10.1152/ajprenal.90488.2008. [DOI] [PubMed] [Google Scholar]
- 13.Vaduganathan M., Vardeny O., Michel T., McMurray J.J.V., Pfeffer M.A., Solomon S.D. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med. 2020;382(17):1653–1659. doi: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morales D.R., Conover M.M., You S.C., et al. Renin-angiotensin system blockers and susceptibility to COVID-19: an international, open science, cohort analysis. Lancet Digit Health. 2021;3(2):e98–e114. doi: 10.1016/S2589-7500(20)30289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta N., Kalra A., Nowacki A.S., et al. Association of use of angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers with testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(9):1020–1026. doi: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Wang X., Chen J., Zhang H., Deng A. Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825–830. doi: 10.1001/jamacardio.2020.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes R.D., Macedo A.V.S., de Barros E.S.P.G.M., et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325(3):254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grover A., Oberoi M. A systematic review and meta-analysis to evaluate the clinical outcomes in COVID-19 patients on angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. Eur Heart J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreutz R., Algharably E.A.E., Azizi M., et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020 doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sparks M.A., South A., Welling P., et al. Sound science before quick judgement regarding RAS blockade in COVID-19. Clin J Am Soc Nephrol. 2020;15(5):714–716. doi: 10.2215/CJN.03530320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng J., Xiao G., Zhang J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang P., Zhu L., Cai J., et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126(12):1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salah H.M., Calcaterra G., Mehta J.L. Renin-angiotensin system blockade and mortality in patients with hypertension and COVID-19 infection. J Cardiovasc Pharmacol Ther. 2020;25(6):503–507. doi: 10.1177/1074248420947628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xue Y, Sun S, Cai J, et al. Effects of ACEI and ARB on COVID-19 patients: A meta-analysis. J Renin-Angiotensin-Aldosterone System. 2020;21(4):1470320320981321. [DOI] [PMC free article] [PubMed]
- 25.South A.M., Tomlinson L., Edmonston D., Hiremath S., Sparks M.A. Controversies of renin–angiotensin system inhibition during the COVID-19 pandemic. Nat RevNephrol. 2020 doi: 10.1038/s41581-020-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saavedra J. Angiotensin receptor blockers and COVID-19. Pharmacol Res. 2020;156 doi: 10.1016/j.phrs.2020.104832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deshotels M.R., Xia H., Sriramula S., Lazartigues E., Filipeanu C.M. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64(6):1368–1375. doi: 10.1161/HYPERTENSIONAHA.114.03743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison-Bernard L.M., Zhuo J., Kobori H., Ohishi M., Navar L.G. Intrarenal AT(1) receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol. 2002;282(1):F19–25. doi: 10.1152/ajprenal.00335.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan M.J., Johnson G., Kirk J., Fuerstenberg S.M., Zager R.A., Torok-Storb B. HK-2: an immortalized proximal tubule epithelial cell line from normal adult human kidney. Kidney Int. 1994;45(1):48–57. doi: 10.1038/ki.1994.6. [DOI] [PubMed] [Google Scholar]
- 30.Zhu Y., Cui H., Lv J., et al. AT1 and AT2 receptors modulate renal tubular cell necroptosis in angiotensin II-infused renal injury mice. Sci Rep. 2019;9(1):19450. doi: 10.1038/s41598-019-55550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koka V., Huang X., Arthur C.K., et al. Angiotensin II up-regulates angiotensin I converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172(5):1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]