Abstract
Background
As a result of the COVID-19 pandemic first wave, reductions in ST-elevation myocardial infarction (STEMI) invasive care, ranging from 23% to 76%, have been reported from various countries. Whether this change had any impact on coronary angiography (CA) volume or on mechanical support device use for STEMI and post-STEMI mechanical complications in Canada is unknown.
Methods
We administered a Canada-wide survey to all cardiac catheterization laboratory directors, seeking the volume of CA use for STEMI performed during the period from March 1 2020 to May 31, 2020 (pandemic period), and during 2 control periods (March 1, 2019 to May 31, 2019 and March 1, 2018 to May 31, 2018). The number of left ventricular support devices used, as well as the number of ventricular septal defects and papillary muscle rupture cases diagnosed, was also recorded. We also assessed whether the number of COVID-19 cases recorded in each province was associated with STEMI-related CA volume.
Results
A total of 41 of 42 Canadian catheterization laboratories (98%) provided data. There was a modest but statistically significant 16% reduction (incidence rate ratio [IRR] 0.84; 95% confidence interval 0.80-0.87) in CA for STEMI during the first wave of the pandemic, compared to control periods. IRR was not associated with provincial COVID-19 caseload. We observed a 26% reduction (IRR 0.74; 95% confidence interval 0.61-0.89) in the use of intra-aortic balloon pump use for STEMI. Use of an Impella pump and mechanical complications from STEMI were exceedingly rare.
Conclusions
We observed a modest 16% decrease in use of CA for STEMI during the pandemic first wave in Canada, lower than the level reported in other countries. Provincial COVID-19 caseload did not influence this reduction.
Résumé
Introduction
Après la première vague de la pandémie de COVID-19, de nombreux pays ont déclaré une réduction de 23 % à 76 % des soins invasifs de l'infarctus du myocarde avec élévation du segment ST (STEMI). On ignore si ce changement a entraîné des répercussions sur le volume d'angiographies coronariennes (AC) ou sur l'utilisation des dispositifs d'assistance mécanique lors de STEMI et des complications mécaniques post-STEMI au Canada.
Méthodes
Nous avons réalisé un sondage pancanadien auprès de tous les directeurs de laboratoire de cathétérisme cardiaque pour obtenir le volume d'utilisation des AC lors des STEMI réalisées durant la période du 1er mars 2020 au 31 mai 2020 (période de pandémie) et durant 2 périodes témoins (1er mars 2019 au 31 mai 2019 et 1er mars 2018 au 31 mai 2018). Le nombre de dispositifs d'assistance ventriculaire gauche utilisés et le nombre de cas de communications interventriculaires et de ruptures du muscle papillaire diagnostiqués ont également été enregistrés. Nous avons aussi évalué si le nombre de cas de COVID-19 enregistrés dans chaque province était associé au volume d'AC liées aux STEMI.
Résultats
Au total, 41 des 42 laboratoires canadiens de cathétérisme (98 %) ont fourni des données. Lors de la comparaison de la première vague de la pandémie aux périodes témoins, nous avons noté une réduction modeste, mais significative, sur le plan statistique de 16 % (ratio du taux d'incidence [RTI] 0,84; intervalle de confiance à 95 % 0,80-0,87) des AC lors de STEMI. Le RTI n’était pas associé au nombre provincial de cas de COVID-19. Nous avons observé une réduction de 26 % (RTI 0,74; intervalle de confiance à 95 % 0,61-0,89) de l'utilisation de pompes à ballonnet intra-aortique lors de STEMI. L'utilisation d'une pompe Impella et les complications mécaniques après les STEMI étaient extrêmement rares.
Conclusions
Nous avons observé une diminution modeste de 16 % de l'utilisation des AC lors de STEMI durant la première vague de la pandémie au Canada, soit une diminution plus faible que ce que les autres pays ont signalé. Le nombre provincial de cas de COVID-19 n'a pas influencé cette réduction.
For the management of ST-elevation myocardial infarction (STEMI), reperfusion therapy with primary percutaneous coronary intervention has become standard therapy when it is accessible and can be provided in a timely fashion.1 As a result of the first COVID-19 pandemic wave, several publications that gathered single-or multi-centre experience from diverse regions have reported significant reductions in STEMI invasive care, measured as catheterization laboratory activations, acute coronary angiography (CA), or hospitalization for STEMIs, ranging from 23% to 76%2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14 compared to control periods, with the sole exception of New Zealand,15 where no change was observed. Proposed explanatory hypotheses include over-observance of lockdown policies (although such policies were never meant to discourage patients from seeking medical attention in case of emergency), reduced or delayed emergency visits due to patient fears of contracting COVID-19 in the medical system, increased use of fibrinolytic therapy in a period with limited human and physical resources, and increased out-of-hospital mortality. Anecdotal evidence supports increased delayed or late presentations, leading to increased mechanical complications.16, 17, 18, 19, 20
The first wave of the COVID-19 pandemic has not occurred homogeneously throughout Canada. Although the province of British Columbia was affected first, Quebec and Ontario experienced the greatest infection burden during the first wave of the pandemic. Nevertheless, a countrywide lockdown was imposed by all provinces from mid-March to May, with progressive unlocking throughout May and June from region to region. Whether the regional caseload had any impact on patient or healthcare provider behaviour is not known.
Based on shared subjective impression among the interventional cardiology community, we hypothesized that invasive management for STEMI may have decreased during the worst phase of the pandemic (March 1 to May 31, 2020) compared with the same months in 2018 and 2019. We also hypothesized that use of mechanical support devices for STEMI would be greater, as a consequence of presentation delays or worse clinical features, and we assumed an increase in mechanical complications during the pandemic period compared with the previous months. Finally, we hypothesized that the regional intensity of the pandemic, as reflected by the COVID-19 caseload, may be associated with CA for STEMI volumes.
Methods
We performed an observational health-services research study utilizing a survey sent through the Canadian Association of Interventional Cardiology/Association Canadienne de Cardiologie d'Intervention office to all cardiac catheterization laboratory directors, requesting that they provide the volume of CA used for STEMI between March 1, 2020 and May 31, 2020 (pandemic period), and from March 1, 2019 to May 31, 2019, and March 1, 2018 to May 31, 2018 (control periods). Activation of the laboratory for other urgent indications, including unstable angina or non-STEMI issues, was not part of our research question. Data extraction from catherization laboratory databases, STEMI activation logs, administrative hospital databases, or detailed angiographic and/or chart reviews provided the source documentation. The questionnaire was constructed within an Excel spreadsheet, as the case report form could be downloaded and printed for ease of completion. Data was extracted by administrative assistants, research coordinators, or physicians, depending on location. However, each author verified and confirmed the accuracy of their local data, to the best of their knowledge. For the same periods, the number of left ventricular or circulatory support devices, such as an intra-aortic balloon pump (IABP), an Impella axial pump (any type) (Abiomed, Danvers, MA), or veno-arterial extra corporal membrane oxygenation (VA-ECMO), used for STEMI was also provided. For the pandemic period, laboratories provided the number of cases for which a ventricular septal defect or a papillary muscle rupture was diagnosed, as compared with a full year prior (as these events are rare). Due to the nature of the data collected (anonymized administrative data) and shared by centres, this study received a waiver for formal research ethics board review at McGill University Health Centre, with which one centre requested a data-sharing agreement. Data were collected through the Canadian Association of Interventional Cardiology/Association Canadienne de Cardiologie d'Intervention to populate the final dataset. Once all data were collected, aggregate data were transferred to the primary investigator for statistical analysis.
Data analysis
In each Canadian province, the number of STEMI cases during the first wave of the pandemic (2020) was compared to the average number of STEMI cases during the control period (ie, the same months during 2018-2019). For left ventricular or circulatory support, we compared the number of times an IABP, an Impella pump, or VA-ECMO was used for STEMI during the first wave of the pandemic vs during control periods. Canada-wide comparisons were also calculated for both STEMI and left ventricular/circulatory support. All comparisons were based on incidence rate ratios (IRRs), defined as the ratio of the number of cases in 2020 vs 2018-2019, per province, and then for the whole country, assuming the size of the underlying population remained the same in the 2 periods being compared. For each IRR, 95% confidence intervals (CIs) were calculated under the assumption that the number of cases follows a Poisson distribution in each time period. Because of low counts, we did not perform statistical comparison for mechanical complications.
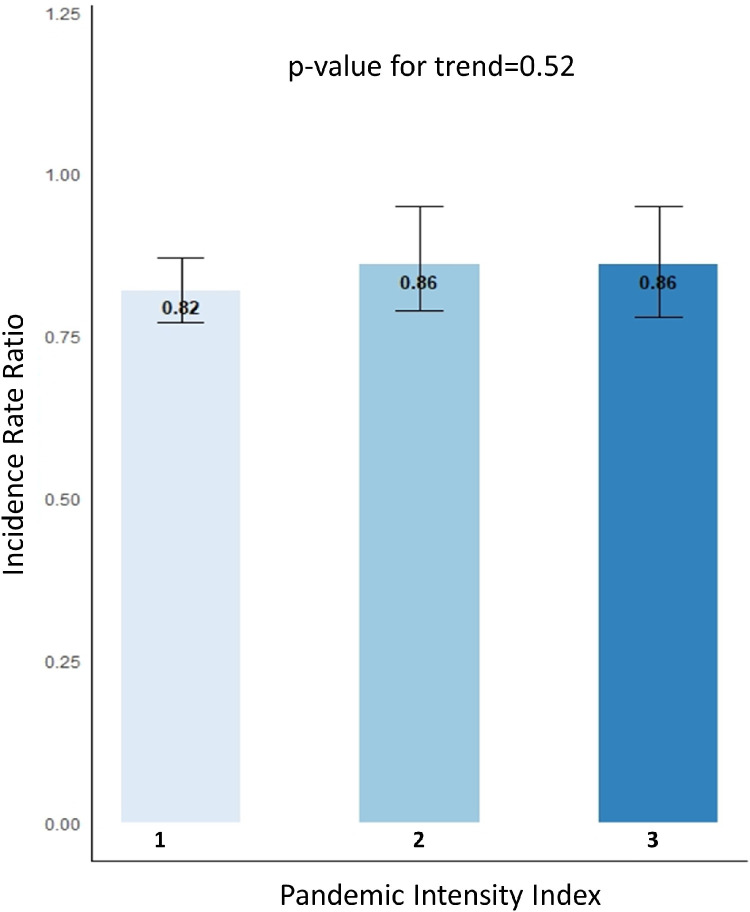
We finally assessed whether the intensity of the pandemic in each province was associated with the volume of STEMI cases. To answer this question, we divided provinces in 3 pandemic-intensity groups: provinces with the highest COVID-19 caseload (Quebec and Ontario) were assigned an intensity index of 3; provinces with a moderate caseload (Alberta and British Columbia) were assigned an index of 2; and lower-caseload provinces (all others) were assigned an index of 1. To test for a trend in lower IRR as a function of the pandemic-intensity index, a χ2 test of trend was performed.
Results showing 95% CIs excluding the null value or P values < 0.05 were considered statistically significant. Analyses were performed using the R Statistical Software environment (Vienna, Austria).
Results
Questionnaires were sent on June 1, 2020. By October 15, 2020, we had received data from 41 (98%) of the 42 Canadian hospitals equipped with a cardiac catheterization laboratory. Only one laboratory did not provide data within the allocated time frame.
Coronary angiography for STEMI
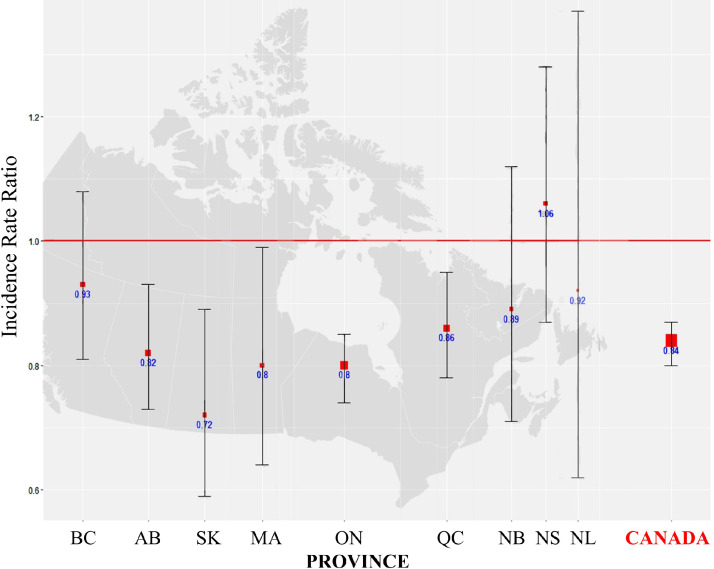
Table 1 and Figure 1 show the main study results. The size of the square in Figure 1 indicates the sample size. Overall, there was a 16% (IRR 0.84; 95% CI 0.80-0.87) reduction in CA performed for STEMI during the first 3 months of the pandemic, compared to control periods. From west to east, Alberta (IRR 0.82; 95% CI 0.73-0.93), Saskatchewan (IRR 0.72; 0.59-0.89), Manitoba (IRR 0.80; 95% CI 0.64, 0.99), Ontario (IRR 0.80; 95% CI 0.74-0.85), and Quebec (IRR 0.86; 95% CI 0.78-0.95) observed significant reductions. Variation in other provinces did not reach statistical significance. Figure 2 shows a comparison by provincial severity of the pandemic. As displayed, we did not observe any significant trend (P = 0.52) in decrease of the IRR by COVID-19 caseload.
Table 1.
Coronary angiography for ST-elevation myocardial infarction
| Province | Pandemic volume | Pre-pandemic volume | IRR | 95% CI |
|---|---|---|---|---|
| British Columbia (BC) | 348 | 373.0 | 0.93 | 0.81–1.08 |
| Alberta (AB) | 497 | 604.5 | 0.82 | 0.73–0.93 |
| Saskatchewan (SK) | 154 | 214.0 | 0.72 | 0.59–0.89 |
| Manitoba (MB) | 148 | 185.0 | 0.80 | 0.64–0.99 |
| Ontario (ON) | 1346 | 1692.5 | 0.80 | 0.74–0.85 |
| Quebec (QC) | 723 | 841.5 | 0.86 | 0.78–0.95 |
| New Brunswick (NB) | 136 | 152.5 | 0.89 | 0.71–1.12 |
| Nova Scotia (NS) | 208 | 197.0 | 1.06 | 0.87–1.28 |
| Newfoundland (NL) | 46 | 50.0 | 0.92 | 0.62–1.37 |
| Canada | 3606 | 4310.0 | 0.84 | 0.80–0.87 |
CI, confidence interval; IRR, incidence rate ratio.
Figure 1.
Variation in coronary angiography in March, April, and May of 2020 compared to the same months in 2018 and 2019, by province providing the service. Box and “whiskers” plot: box size reflects sample size, and whiskers indicate the 95% confidence interval around the incidence rate ratio estimates. AB, Alberta; BC, British Columbia; MA, Manitoba; NB, New Brunswick; NL, Newfoundland-Labrador; NS, Nova Scotia; ON, Ontario; QC, Quebec; SK, Saskatchewan.
Figure 2.
Incidence rate ratios as a function of the pandemic intensity index.
Mechanical left ventricular or circulatory support
Table 2 shows IRRs for the whole country, as counts were small for each centre. We did not observe any significant variation in the use of Impella pumps or VA-ECMO support during the pandemic, compared to control periods, although counts were very low and clinically meaningful increases or decreases cannot be excluded. However, there was a significant 26% reduction (IRR 0.74; 95% CI 0.61-0.89) in the use of IABPs in STEMI during the pandemic, compared to the same months in 2018-2019.
Table 2.
Mechanical support for ST-elevation myocardial infarction
| Type of support | Number of units used during pandemic | Number of units used in 3-month control period | IRR for Canada | 95% CI |
|---|---|---|---|---|
| IABP | 194 | 263.0 | 0.74 | 0.61–0.89 |
| Impella | 14 | 7.5 | 1.87 | 0.77–4.53 |
| VA-ECMO | 17 | 14.4 | 1.17 | 0.58–2.36 |
CI, confidence interval; IABP, intraaortic balloon pump; IRR, incidence rate ratio; VA-ECMO, veno-arterial extra corporeal membrane oxygenation.
Mechanical complications post-STEMI
In the whole country, 7 cases of ventricular septal defect and 9 cases of papillary muscle ruptures were identified during the 3 first months of the pandemic, compared to 30 and 13 cases, respectively, in the year prior to the pandemic, which amount to 7.5 cases of ventricular septal defect and 3.25 cases of papillary muscle rupture for a similar 3-month control period. Because of the statistical instability associated with these low counts, we did not perform any statistical comparisons.
Discussion
In this national survey, which gathered data from 98% of Canadian cardiac catheterization laboratories, we observed a modest but statistically significant 16% decrease in use of CA for STEMI during the first wave of the pandemic, compared to the same months in the 2 previous years, a decrease lower than that reported in other countries. We could not demonstrate any association between higher CA use reductions and higher COVID-19 caseloads. Also, we did not observe any increase in the use of mechanical support for STEMI, but rather a decrease in the use of IABPs, following the reduction in CA use for STEMI. This does not support our original hypothesis that more higher-risk STEMIs from delayed presentation would require more invasive mechanical support. Because of low counts, we could not draw any conclusion about the variation in Impella pump or VA-ECMO use, or in mechanical complications.
The 16% reduction in Canada is among the lowest reductions in CA use reported in the world. Geographically closer to Canada, a more important reduction in the number of activations for STEMI (29%), CA (34%), and primary percutaneous coronary intervention (20%) were observed in 18 high-volume US centres.21 Due to these concerns, a reappraisal of STEMI care in the context of COVID-19 has been suggested.22,23 Although it would be tempting to conclude that universal medical access mitigated risk-averse behaviour from patients in Canada, data from the large British Cardiovascular Intervention Society registry in the UK, also in a public healthcare system, reported a larger 43% decline.24 A lower absolute number of COVID-19 cases in Canada may have resulted in fewer Canadian patients being discouraged from seeking medical attention compared to UK or US patients, where the level of COVID-19 mortality was higher. Our data show the modest reduction to be similar across provinces, despite the different COVID-19 caseload. Thus, the intensity of the pandemic did not seem to influence the observed reduction in CA use.
Beyond reduction in incidence of CA for STEMI, delayed presentations have been more common during the first wave,25,26 with increases in overall symptom-to-hospital delays,24,27 along with increased out-of-hospital cardiac arrest rates,11 and anecdotal evidence of increased delayed presentations and mechanical complications.16, 17, 18, 19, 20 However, the incidence of cardiogenic shock has not increased in a large Danish registry.28 In one Canadian study, the incidence of STEMI admission was not reduced during the pandemic period in Montreal, but unstable STEMI presentations and worse in-hospital course were more frequent,29 data we could not replicate with a much larger and broader sample size. Our study is the first to gather usage data for left ventricular or circulatory support devices. We did not observe any significant increase in the use of left ventricular or circulatory support, but rather a consistent decrease in the use of IABPs across all provinces, larger than the decrease in use of CA, which is surprising, to some extent, given the increase in shock patients during the pandemic. Such reduction cannot be explained by preferential usage of the Impella pump, which remained low across the country. Although our data suggest a true reduction in cardiogenic shock patients reaching the catheterization laboratory, the phenomenon is unlikely to be explained by a true reduction in the incidence of severe STEMI. A more plausible explanation is an increased mortality rate among the sickest patients who did not present at the catheterization laboratory during the pandemic. Although an increase in mechanical complication may have occurred, especially for papillary muscle rupture, the very low number of events precludes any strong conclusion.
Our study has other limitations to acknowledge. First, it is a survey on resource use, without patient-level data. Patient-related outcomes and patient perspectives were not captured by the design of this study. Although such a design alleviated the need for research contracts and approval nationwide, it limited the scope of possible analyses. Second, data source verification was not performed, and we could rely on only best estimates from the various laboratories. Despite this issue, systematic bias is unlikely. Third, the COVID-19 pandemic is now affecting all provinces more uniformly, except for the so-called Maritime bubble (NB, NS, and NL), where caseload remains low. A survey performed at this time of the year could have yielded different results. Fourth, we relied on catheterization laboratory data. As mentioned earlier, the adverse effect of the pandemic may have increased mortality from STEMI prior to patients presenting in the hospital. Sixth, mechanical complications and the need of an Impella pump and VA-ECMO were too infrequent to use these as a surrogate for medical assistance delay. Moreover, the unequal access to Impella pumps or VA-ECMO throughout the country, which are available in less than 20% of laboratories, further reduced the power to detect any real change in use during the pandemic. Finally, our study design could not assess whether the pandemic altered the type of patient presenting for STEMI care. As mentioned earlier, although no increase in IABP use was observed, we cannot exclude the possibility of an increase in late presenters and higher-risk STEMIs leading to a higher level of out-of-hospital death. Also, the volume of CA might not accurately reflect patient behaviour in seeking medical attention, as some patients might have presented to centres without primary percutaneous coronary intervention capacity and been managed with thrombolytics rather than transferred for primary percutaneous coronary intervention. Only a detailed per province evaluation of hospitalization for STEMI, including lethal cases, could answer that question.
Conclusion
We observed a modest but significant 16% decrease in use of CA for STEMI during the first wave of the pandemic, lower than that reported in other countries. Such a decrease may be attributable to patient behaviour, reduction in volume of transfer for CA, or increased mortality prior to CA. Although instances of mechanical complications were higher, we could not draw any conclusion regarding the effect of the pandemic on mechanical complications from STEMI or use of Impella pumps, given low counts. The reduction in use of CA for STEMI was accompanied by a decrease in the use of IABPs, which most likely suggests an increased mortality level among the sickest patients—that is, that they did not ever reach the catheterization laboratory. Finally, the intensity of the pandemic in each province, which was much less lethal than in other parts of the world at that time, was not associated with the reduction in CA use observed. A Canadian study using pre-hospital and hospital clinical or medico-administrative patient-level data, gathering all treatments, including fibrinolytics and primary angioplasty, with related outcomes would be required to further illuminate our findings.
Acknowledgments
Funding Sources
Funding for the work was provided by the McGill Interdisciplinary Initiative in Infection and Immunity (MI4) and by the Emergency COVID-19 Research Funding (ECRF) program, grant number ECRF-R2-62.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Due to the nature of the data collected (anonymized administrative data, and shared by centres), this study received a waiver for formal research ethics board review at McGill University Health Centre.
See page 1130 for disclosure information.
References
- 1.Gershlick AH, Banning AP, Myat A, Verheugt FW, Gersh BJ. Reperfusion therapy for STEMI: Is there still a role for thrombolysis in the era of primary percutaneous coronary intervention? Lancet. 2013;382:624–632. doi: 10.1016/S0140-6736(13)61454-3. [DOI] [PubMed] [Google Scholar]
- 2.Fileti L, Vecchio S, Moretti C. Impact of the COVID-19 pandemic on coronary invasive procedures at two Italian high-volume referral centers. J Cardiovasc Med (Hagerstown) 2020;21:869–873. doi: 10.2459/JCM.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Leor O, Cid-Álvarez B, Pérez de Prado A. Impact of COVID-19 on ST-segment elevation myocardial infarction care. The Spanish experience. Rev Esp Cardiol (Engl Ed) 2020;73:994–1002. doi: 10.1016/j.rec.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papafaklis MI, Katsouras CS, Tsigkas G. "Missing" acute coronary syndrome hospitalizations during the COVID-19 era in Greece: medical care avoidance combined with a true reduction in incidence? Clin Cardiol. 2020;43:1142–1149. doi: 10.1002/clc.23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daoulah A, Hersi AS, Al-Faifi SM. STEMI and COVID-19 pandemic in Saudi Arabia. Curr Probl Cardiol. 2020;46 doi: 10.1016/j.cpcardiol.2020.100656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mesnier J, Cottin Y, Coste P. Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health. 2020;5:e536–e542. doi: 10.1016/S2468-2667(20)30188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piuhola J, Kerkelä R, Laine M. Lower ST-elevation myocardial infarction incidence during COVID-19 epidemic in Northern Europe. Scand Cardiovasc J. 2020;54:358–360. doi: 10.1080/14017431.2020.1820563. [DOI] [PubMed] [Google Scholar]
- 8.Claeys MJ, Argacha JF, Collart P. Impact of COVID-19-related public containment measures on the ST elevation myocardial infarction epidemic in Belgium: a nationwide, serial, cross-sectional study [epub ahead of print] Acta Cardiol. 2020 doi: 10.1080/00015385.2020.1796035. doi: 10.1080/00015385.2020.1796035, accessed April 12, 2021. [DOI] [PubMed] [Google Scholar]
- 9.Erol MK, Kayıkçıoğlu M, Kılıçkap M. Treatment delays and in-hospital outcomes in acute myocardial infarction during the COVID-19 pandemic: a nationwide study. Anatol J Cardiol. 2020;24:334–342. doi: 10.14744/AnatolJCardiol.2020.98607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Little CD, Kotecha T, Candilio L. COVID-19 pandemic and STEMI: pathway activation and outcomes from the pan-London heart attack group. Open Heart. 2020;7 doi: 10.1136/openhrt-2020-001432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rashid M, Gale CP, Curzen N. Impact of COVID19 pandemic on the incidence and management of out of hospital cardiac arrest in patients presenting with acute myocardial infarction in England. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mayol J, Artucio C, Batista I. An international survey in Latin America on the practice of interventional cardiology during the COVID-19 pandemic, with a particular focus on myocardial infarction. Neth Heart J. 2020;28:424–430. doi: 10.1007/s12471-020-01440-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oikonomou E, Aznaouridis K, Barbetseas J. Hospital attendance and admission trends for cardiac diseases during the COVID-19 outbreak and lockdown in Greece. Public Health. 2020;187:115–119. doi: 10.1016/j.puhe.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mafham MM, Spata E, Goldacre R. COVID-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396:381–389. doi: 10.1016/S0140-6736(20)31356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott JM, Crozier IG. Decreases in cardiac catheter laboratory workload during the COVID-19 level 4 lockdown in New Zealand. Intern Med J. 2020;50:1000–1003. doi: 10.1111/imj.14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parikh M, Busman M, Dickinson M, Wohns D, Madder RD. Ventricular septal rupture in 2 patients presenting late after myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2:2013–2015. doi: 10.1016/j.jaccas.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atreya AR, Kawamoto K, Yelavarthy P. Acute Myocardial infarction and papillary muscle rupture in the COVID-19 era. JACC Case Rep. 2020;2:1637–1641. doi: 10.1016/j.jaccas.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed T, Nautiyal A, Kapadia S, Nissen SE. Delayed presentation of STEMI complicated by ventricular septal rupture in the era of COVID-19 pandemic. JACC Case Rep. 2020;2:1599–1602. doi: 10.1016/j.jaccas.2020.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otero D, Singam NSV, Barry N. Complication of late presenting STEMI due to avoidance of medical care during the COVID-19 pandemic. JACC Case Rep. 2020;2:1610–1613. doi: 10.1016/j.jaccas.2020.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albiero R, Seresini G. Subacute left ventricular free wall rupture after delayed STEMI presentation during the COVID-19 pandemic. JACC Case Rep. 2020;2:1603–1609. doi: 10.1016/j.jaccas.2020.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia S, Stanberry L, Schmidt C. Impact of COVID-19 pandemic on STEMI care: an expanded analysis from the United States. Catheter Cardiovasc Interv. 2021;98:217–222. doi: 10.1002/ccd.29154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bainey KR, Bates ER, Armstrong PW. ST-segment-elevation myocardial infarction care and COVID-19: the value proposition of fibrinolytic therapy and the pharmacoinvasive strategy. Circ Cardiovasc Qual Outcomes. 2020;13 doi: 10.1161/CIRCOUTCOMES.120.006834. [DOI] [PubMed] [Google Scholar]
- 23.Boukhris M, Hillani A, Moroni F. Cardiovascular implications of the COVID-19 pandemic: a global perspective. Can J Cardiol. 2020;36:1068–1080. doi: 10.1016/j.cjca.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwok CS, Gale CP, Kinnaird T. Impact of COVID-19 on percutaneous coronary intervention for ST-elevation myocardial infarction. Heart. 2020;106:1805–1811. doi: 10.1136/heartjnl-2020-317650. [DOI] [PubMed] [Google Scholar]
- 25.Fu XY, Shen XF, Cheng YR. Effect of COVID-19 outbreak on the treatment time of patients with acute ST-segment elevation myocardial infarction. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.09.038. S0735-6757(20)30833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moroni F, Gramegna M, Ajello S. Collateral damage: medical care avoidance behavior among patients with myocardial infarction during the COVID-19 pandemic. JACC Case Rep. 2020;2:1620–1624. doi: 10.1016/j.jaccas.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reinstadler SJ, Reindl M, Lechner I. Effect of the COVID-19 pandemic on treatment delays in patients with ST-segment elevation myocardial infarction. J Clin Med. 2020;9:2183. doi: 10.3390/jcm9072183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lauridsen MD, Butt JH, Østergaard L. Incidence of acute myocardial infarction-related cardiogenic shock during corona virus disease 19 (COVID-19) pandemic. Int J Cardiol Heart Vasc. 2020;31 doi: 10.1016/j.ijcha.2020.100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haddad K, Potter BJ, Matteau A, Gobeil F, Mansour S. Implications of COVID-19 on time-sensitive STEMI care: a report from a North American epicenter. Cardiovasc Revasc Med. 2021;30:33–37. doi: 10.1016/j.carrev.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]