Abstract
Atrial fibrillation (AF) is the most common type of arrhythmia. Warfarin reduces the incidence and mortality of strokes in patients with AF. Edoxaban reduces the bleeding risk in patients with AF. This study evaluates the efficacy and safety of edoxaban versus warfarin in preventing clinical events in patients with AF through a meta-analysis of randomized controlled trials (RCTs). RCTs were retrieved from medical literature databases. Risk ratios (RRs) and 95% confidence intervals (CIs) were calculated to compare the primary and safety endpoints. In total, five articles (10 trial comparisons) containing 24,836 patients were retrieved. Of these patients, 16,268 (65.5%) received edoxaban and 8,568 (34.5%) received warfarin. Compared with warfarin, edoxaban significantly reduced the incidence of cardiovascular death (CVD), major bleeding, and non-major bleeding (RR: 0.86, 95% CI: 0.80–0.93, I2: 0.0%; RR: 0.65, 95% CI: 0.59–0.71, I2: 75.6%; and RR: 0.80, 95% CI: 0.77–0.84, I2: 79.3%, respectively). Edoxaban did not increase the incidence of stroke, systemic embolic events, myocardial infarction, and adverse events compared with warfarin (RR: 1.00, 95% CI: 0.90–1.11, I2: 42.8%; RR: 1.00, 95% CI: 0.67–1.49, I2: 0.0%; RR: 1.08, 95% CI: 0.93–1.27, I2: 0.0%; RR: 1.00, 95% CI: 0.91–1.10, I2: 46.4%, respectively). This meta-analysis indicated that compared with warfarin, edoxaban can significantly reduce the incidence of CVD and major and non-major bleeding. The anticoagulant effect and safety of edoxaban may be better than those of warfarin.
Keywords: edoxaban, warfarin, atrial fibrillation, meta-analysis
Introduction
Atrial fibrillation (AF) is the most common arrhythmia observed in outpatient clinics; with the increase in the aging population, the occurrence of AF is also increasing (1). Some studies have found an association between AF and embolism, stroke, myocardial infarction (MI), and cardiovascular death (CVD) (2–4). The most serious complication of AF is embolism, where the dislodged thrombus can be carried to different parts of the body via the blood circulation, leading to the occurrence of various systemic complications such as stroke, pulmonary embolism, and even death (5). AF increases the incidence of stroke by 4–5 times in all age groups, with a significant increasing trend with age (6). Presently, the relationship between AF and MI is unclear (7). However, in outpatient clinics, AF complicated with MI is common, and acute MI commonly leads to cardiogenic shock (8). Thus, AF is an independent risk factor for mortality in patients with coronary heart disease. Embolism is the main cause of death and disability in patients with AF (9); therefore, standardized anticoagulation therapy is an important aspect in comprehensively treating patients with AF.
Warfarin, (9) a widely used coumarin anticoagulant, inhibits the synthesis of coagulation factors II, VII, IX, and X in the liver and is a vitamin K antagonist, thus inhibiting the formation of thrombi in vivo (10). When administering warfarin, measuring the international standardized ratio (INR) regularly is necessary, aiming for 2–3 times the control value (11). This ensures a better antithrombotic effect and a lower risk of bleeding. Studies have shown that warfarin can significantly reduce the incidence and mortality of stroke in patients with AF, but individual differences may lead to different effective doses of warfarin (12, 13). Frequent measurement of INR levels is necessary to adjust to the appropriate warfarin dosage (14).
Edoxaban, the free base of DU-176b, is a highly specific direct inhibitor of coagulation factor Xa (15). In the coagulation process, activated coagulation factor Xa activates prothrombin to thrombin, and thrombin cleaves fibrin monomer to fibrin to form a thrombus (16). As a new oral anticoagulant (NOAC), edoxaban inhibits thrombus formation by selective and reversible direct inhibition of factor Xa (17). Studies have shown that edoxaban significantly reduces the risk of bleeding in patients with AF (18). However, its role in reducing the incidence of CVD, MI, systemic embolism events (SEE), and stroke in patients with AF is controversial.
Thus, we performed this meta-analysis to compare the effects and safety of edoxaban with those of warfarin in preventing clinical events in patients with AF to provide evidence for clinical use.
Methods
Search strategy
Two researchers searched for published articles comparing the efficacy and safety of edoxaban with those of warfarin in preventing clinical events in patients with AF following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The RCTs were systematically searched in databases such as PubMed, Embase, Cochrane Library, Web of Science, and Google Scholar, with no restrictions on language or publication date (from inception to April 11, 2019). The following keywords and Medical Subject Heading terms were used: “atrial fibrillation”; “atrial fibrillations”; “fibrillation, atrial”; “fibrillations, atrial”; “auricular fibrillation”; “auricular fibrillations”; “fibrillation, auricular”; “fibrillations, auricular”; “persistent atrial fibrillation”; “atrial fibrillation, persistent”; “atrial fibrillations, persistent”; “fibrillation, persistent atrial”; “fibrillations, persistent atrial”; “persistent atrial fibrillations”; “familial atrial fibrillation”; “atrial fibrillation, familial”; “atrial fibrillations, familial”; “familial atrial fibrillations”; “fibrillation, familial atrial”; “fibrillations, familial atrial”; “paroxysmal atrial fibrillation”; “atrial fibrillation, paroxysmal”; “atrial fibrillations, paroxysmal”; “fibrillation, paroxysmal atrial”; “fibrillations, paroxysmal atrial”; and “paroxysmal atrial fibrillations” in combination with atrial fibrillation. “Apo-Warfarin”; “Aldocumar”; “Warfarin”; “Warfant”; “4-Hydroxy-3-(3-oxo-1-phenylbutyl)-2H-1-benzopyran-2-one”; “Gen-Warfarin”; “Marevan”; “Coumadin”; “warfarin potassium”; “potassium, warfarin”; “warfarin sodium”; “sodium, warfarin”; “Coumadine”; and “Tedicumar” were used in combination with warfarin. “edoxaban,” “DU-176b,” “edoxaban tosylate,” and “DU-176” were used in combination with edoxaban. Additional relevant studies were retrieved from reviews, meta-analyses, and other literature. Two authors screened and double-reviewed the retrieved studies. If disputes were encountered, they were resolved by consulting a third author.
Inclusion and exclusion criteria
The inclusion criteria were as follows: RCTs that involved edoxaban and warfarin; studies that allocated patients into two groups (edoxaban and warfarin groups); and all patients who had been diagnosed with AF according to the international diagnostic guidelines. Exclusion criteria were as follows: retrospective trials, animal experiments, non-RCTs, reviews, series and case reports, studies with erroneous or incomplete data, studies with results that were not focused on patients with AF, studies with patients with low coagulation function, and studies with patients allergic to edoxaban or warfarin.
Endpoints
The primary endpoints for this study were CVD, stroke, SEE, and MI. The safety endpoints included major bleeding, non-major bleeding (life-threatening bleeding, clinically relevant non-major bleeding, minor bleeding, any overt bleeding, etc.), and other adverse events (AEs).
Data extraction
Two authors independently reviewed the contents of the retrieved studies. The primary and safety endpoints were extracted by the authors and verified by a third author. The data extracted included the following primary information: the first author’s name, year of publication, test type/region, sample size, sex ratio, average age, intervention, CHADS-2 score [congestive heart failure, hypertension, age >75 years, diabetes (all 1 point each); previous stroke (2 points)], type of AF, follow-up time, and endpoints measured in each study. If the contents of the studies needed clarification, the first author of the study was contacted. Disagreements were resolved through consensus or by consulting a third author.
Risk-of-bias assessments
The methodological quality of the included studies was estimated independently by two authors based on the Cochrane Risk-of-Bias criteria. Each quality item was graded as low risk, high risk, or no clear risk. The seven items used to assess bias in each trial included randomization sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.
Statistical analysis
Stata (version 12.0; Stata Corp, College Station, Texas) was used to analyze and pool the individual research results. The pooled results were presented as risk ratios (RR) and 95% confidence intervals (CI) with two-sided p values. P values of <0.05 were used to denote statistical significance. Heterogeneity was evaluated using the I2 test. Heterogeneity was considered small when I2 <50% and substantial when I2 >50%. A funnel plot was generated to examine publication bias and to explore the sources of heterogeneity, if more than 10 studies were included to assess this endpoint. Subgroup analysis was performed according to the dosage of edoxaban (30 mg, 60 mg, and others according to the administration dose of edoxaban per day).
Results
Studies retrieved and characteristics
In total, 18,434 relevant studies were enrolled according to the PRISMA guidelines. The titles and abstracts of the studies were screened to exclude irrelevant studies. Furthermore, we eliminated the unsuitable studies by reading the full text of the articles. Finally, five studies (19–23) (10 trial comparisons) were included according to the inclusion and exclusion criteria with 24,836 patients (Fig. 1). Moreover, 16,268 patients (65.5%) were randomized into the edoxaban group and 8,568 (34.5%) to the warfarin group. All studies included in this meta-analysis were RCTs. The basic characteristics of the individuals from the trials are described in Table 1.
Figure 1.

Flow diagram of the study selection process.
Table 1.
Characteristics of studies included in the meta-analysis
| Author | Year | Test type/ region | Sample size | Women, No. (%) | Average age (years) | Intervention | CHADS2 score (mean) | Type of AF | Follow-up | Endpoints | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||||||
| Edoxaban | Warfarin | Edoxaban | Warfarin | Edoxaban | Warfarin | Edoxaban | Warfarin | Edoxaban | Warfarin | ||||||
| Chung et al.a (19) | 2010 | Multicentre | 79 | 75 | 28 (35.4) | 28 (37.3) | 64.9 | 64.5 | 30 mg qd | Adjusted based on INR values | 2.0 | 1.8 | Non- alvular AF | 3 months | Major bleeding, Non- major bleeding, AEs |
| Chung et al.b (19) | 2010 | Multicentre | 80 | 75 | 25 (31.2) | 28 (37.3) | 65.9 | 64.5 | 60 mg qd | Adjusted based on INR values | 1.9 | 1.8 | Non- valvular AF | 3 months | Major bleeding, Non- major bleeding, AEs |
| Weitz et al.a (20) | 2010 | Multicentre | 235 | 250 | 95 (40.4) | 99 (39.6) | 65.2±8.3 | 66.0±8.5 | 30 mg qd | Adjusted based on INR values | N/A | N/A | Non- valvular AF | 12 weeks | CVD, Stroke, MI, SEE, Major bleeding, Non- major bleeding, AEs |
| Weitz et al.b (20) | 2010 | Multicentre | 244 | 250 | 94 (38.5) | 99 (39.6) | 64.8±8.8 | 66.0±8.5 | 30 mg bid | Adjusted based on INR values | N/A | N/A | Non- valvular AF | 12 weeks | CVD, Stroke, MI, SEE, Major bleeding, Non- major bleeding, AEs |
| Weitz et al.c (20) | 2010 | Multicentre | 234 | 250 | 79 (33.7) | 99 (39.6) | 64.9±8.8 | 66.0±8.5 | 60 mg qd | Adjusted based on INR values | N/A | N/A | Non- valvular AF | 12 weeks | CVD, Stroke, MI, SEE, Major bleeding, Non-major bleeding, AEs |
| Yamashita et al.a (21) | 2011 | Japan | 130 | 125 | 21 (16.0) | 22 (17.0) | 69.4 | 68.8 | 30 mg qd | Adjusted based on INR values | 1.9 | 2.2 | Non-valvular AF | 12 weeks | Major bleeding, Non-major bleeding, AEs |
| Yamashita et al.b (21) | 2011 | Japan | 134 | 125 | 25 (18.6) | 22 (17.0) | 69.5 | 68.8 | 45 mg qd | Adjusted based on INR values | 2.1 | 2.2 | Non-valvular AF | 12 weeks | Major bleeding, Non-major bleeding, AEs |
| Giugliano et al.a (22) | 2013 | Multicentre | 7034 | 7036 | 2730 (38.8) | 2641 (37.5) | 72 | 72 | 30 mg qd | Adjusted based on INR values | 2.8±1.0 | 2.8±1.0 | N/A | 2.8 years | CVD, Stroke, MI, SEE, Major bleeding, Non- major bleeding |
| Giugliano et al.b (22) | 2013 | Multicentre | 7035 | 7036 | 2669 (37.9) | 2641 (37.5) | 72 | 72 | 60 mg qd | Adjusted based on INR values | 2.8±1.0 | 2.8±1.0 | N/A | 2.8 years | CVD, Stroke, MI, SEE, Major bleeding, Non- major bleeding |
| Goette et al. (23) | 2016 | Multicentre | 1067 | 1082 | 374 (34.0) | 382 (35.0) | 64.3 | 64.2 | 60 mg qd | Adjusted based on INR values | 2.6 | 2.6 | Non-valvular AF | 58 days | CVD, Stroke, MI, SEE, Major bleeding, Non-major bleeding, AEs |
represent different doses of Edoxaban compared with a same dose of Warfarin in a study.
CVD - cardiovascular death, SEE - systemic embolism events, MI - myocardial infarction, AEs - adverse events
Literature quality evaluation
The Cochrane Risk-of-Bias criteria were used to evaluate the quality of the retrieved studies, which were assessed by two authors. All five studies (19–23) described random sequence generation and allocation concealment, blinding of participants and personnel, and blinding of outcome assessment. None described other biases. The literature quality score is shown in Table 2.
Table 2.
Assessment of methodological quality of included study
| Study | Random allocation | Hidden distribution | Blind method | Incomplete outcome data | Selective reporting of results | Other bias | Quality grade |
|---|---|---|---|---|---|---|---|
| Chung et al. (19) | Randomized | No clear | Double-blind | Low | Low | Low | B |
| Weitz et al. (20) | Randomized | No clear | Double-blind | Low | Low | Low | A |
| Yamashita et al. (21) | Randomized | No clear | Double-blind | Low | Low | Low | B |
| Giugliano et al. (22) | Randomized | No clear | Double-blind | Low | Low | Low | A |
| Goette et al. (23) | Randomized | No clear | Blinded-endpoint evaluation | Low | Low | Low | A |
Primary endpoints
The incidence of CVD
Three studies (19, 20, 22) (six trial comparisons) reported CVD. In total, 1,064 of 15,849 patients in the edoxaban group and 618 of 8,368 patients in the warfarin group developed CVD. The results showed that edoxaban could significantly reduce the incidence of CVD compared with warfarin (6.7% vs. 7.4%) (RR=0.86, 95% CI=0.80–0.93, I2=0.0%) (Fig. 2a). The fixed effects model was applied. The heterogeneity was low. Subgroup analysis was performed according to the dosage of edoxaban and showed that edoxaban significantly reduced the incidence of CVD at 30 and 60 mg dosages compared with warfarin (RR=0.86, 95% CI=0.77–0.97 and RR=0.86, 95% CI=0.77–0.96, respectively) (Fig. 2b). The cumulative meta-analysis result is shown in Figure 2c.
Figure 2. a–c.
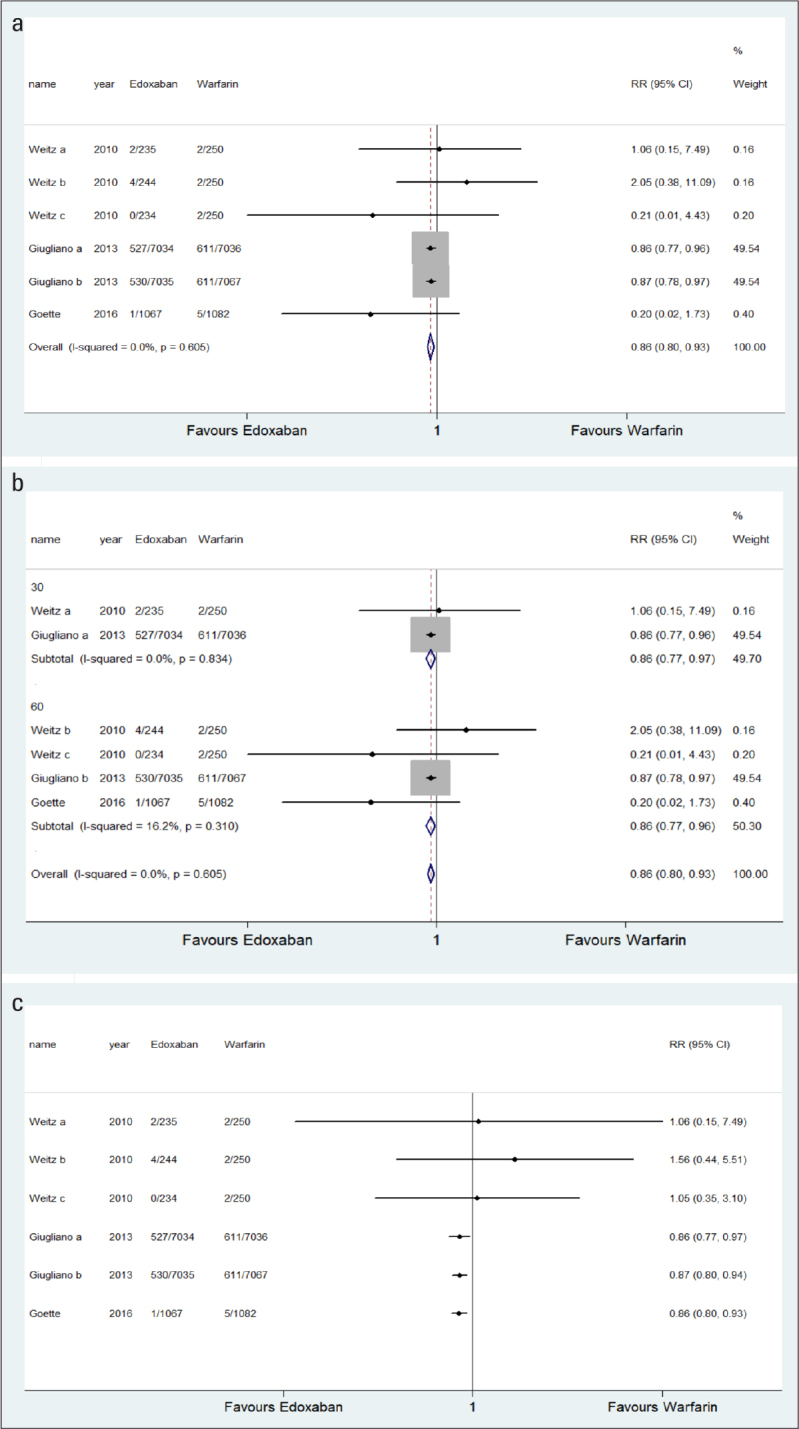
a. Comparison of CVD between the edoxaban group and the warfarin group. b. Comparison of CVD between the edoxaban group and the warfarin group (subgroup analysis according to edoxaban dosage). c. Comparison of CVD between the edoxaban group and the warfarin group (cumulative meta-analysis).
RR - risk ratio
Stroke
Three studies (20, 22, 23) (six trial comparisons) reported strokes. In total, 647 of 15,849 patients in the edoxaban group and 324 of 8,368 patients in the warfarin group had stroke. The results showed that no significant differences were observed between the edoxaban and warfarin groups (4.1% vs. 3.9%, respectively) (RR=1.00, 95% CI=0.90–1.11, I2=42.8%) (Fig. 3a). The fixed effects model was applied. The heterogeneity was low. Subgroup analysis was performed according to the dosage of edoxaban, which showed that no significant differences at 30 and 60 mg dosages were observed between the edoxaban and warfarin groups (RR=1.13, 95% CI=0.97–1.30 and RR=0.87, 95% CI=0.75–1.02, respectively) (Fig. 3b). The cumulative meta-analysis result is shown in Figure 3c.
Figure 3. a–c.

a. Comparison of stroke between the edoxaban group and the warfarin group. b. Comparison of stroke between the edoxaban group and the warfarin group. (subgroup analysis according to edoxaban dosage). c. Comparison of stroke between the edoxaban group and the warfarin group. (cumulative meta-analysis).
RR - risk ratio
SEEs
Three studies (20, 22, 23) (six trial comparisons) reported SEE. In total, 47 of 15,849 patients in the edoxaban group and 24 of 8,368 patients in the warfarin group had an SEE. The results showed no significant differences between the edoxaban and warfarin groups (0.3% vs. 0.3%, respectively) (RR=1.00, 95% CI=0.67–1.49, I2=0.00%) (Fig. 4a). The fixed effects model was applied. The heterogeneity was low. Subgroup analysis was performed according to the dosage of edoxaban, which showed that no significant differences at 30 and 60 mg dosages were observed between the edoxaban and warfarin groups (RR=1.30, 95% CI=0.76–2.23 and RR=0.72, 95% CI=0.39–1.32, respectively) (Fig. 4b). The cumulative meta-analysis result is shown in Figure 4c.
Figure 4. a–c.

a. Comparison of SEE between the edoxaban group and the warfarin group. b. Comparison of SEE between the edoxaban group and the warfarin group. (subgroup analysis according to edoxaban dosage). c. Comparison of SEE between the edoxaban group and the warfarin group. (cumulative meta-analysis).
RR - risk ratio
Myocardial infarction
Three studies (20, 22, 23) (six trial comparisons) reported MI. In total, 309 of 15,849 patients in the edoxaban group and 144 of 8,368 patients in the warfarin group had MI. No significant differences were observed between the edoxaban and warfarin groups (1.9% vs. 1.7%, respectively) (RR=1.08, 95% CI=0.93–1.27, I2=0.0%) (Fig. 5a). The fixed effects model was applied. The heterogeneity was low. Subgroup analysis was performed according to the dosage of edoxaban and showed that no significant differences at 30 and 60 mg dosages were observed between the edoxaban and warfarin groups (RR=1.21, 95% CI=0.97–1.51 and RR=0.96, 95% CI=0.76–1.21, respectively) (Fig. 5b). The cumulative meta-analysis result is shown in Figure 5c.
Figure 5. a–c.

Comparison of MI between the edoxaban group and the warfarin group. b. Comparison of MI between the edoxaban group and the warfarin group (subgroup analysis according to edoxaban dosage). c. Comparison of MI between the edoxaban group and the warfarin group (cumulative meta-analysis).
RR - risk ratio
Safety endpoints
Major bleeding
Five studies (19–23) (10 trial comparisons) reported major bleeding. In total, 683 of 16,268 patients in the edoxaban group and 532 of 8,568 patients in the warfarin group experienced major bleeding. The result showed that edoxaban could significantly reduce the incidence of major bleeding compared with warfarin (4.2% vs. 6.2%, respectively) (RR=0.65, 95% CI=0.59–0.71, I2=75.6%) (Fig. 6a). The fixed effects model was applied. Subgroup analysis was performed according to the dosage of edoxaban and revealed that edoxaban significantly reduced the incidence of major bleeding at 30 and 60 mg dosages compared with warfarin (RR=0.48, 95% CI=0.42–0.56 and RR=0.81, 95% CI=0.71–0.91, respectively) (Fig. 6b). The cumulative meta-analysis result is shown in Figure 6c.
Figure 6. a–e.
a. Comparison of major bleeding between the edoxaban group and the warfarin group. b. Comparison of major bleeding between the edoxaban group and the warfarin group (subgroup analysis according to edoxaban dosage). c. Comparison of major bleeding between the edoxaban group and the warfarin group (cumulative meta-analysis). d. Comparison of major bleeding between the edoxaban group and the warfarin group (funnel plot) e. Comparison of major bleeding between the edoxaban group and the warfarin group (sensitivity analysis)
RR - risk ratio
Non-major bleeding
Five studies (19–23) (10 trial comparisons) reported non-major bleeding. In total, 3,552 of 16,268 patients in the edoxaban group and 2,216 of 8,568 patients in the warfarin group experienced non-major bleeding. The results showed that edoxaban could significantly reduce the incidence of non-major bleeding compared with warfarin (21.8% vs. 25.9%, respectively) (RR=0.80, 95% CI=0.77–0.84, I2=79.3%) (Fig. 7a). The fixed effects model was applied. Subgroup analysis was performed according to the dosage of edoxaban, which showed that edoxaban significantly reduced the incidence of non-major bleeding at 30 and 60 mg dosages compared with warfarin (RR=0.71, 95% CI=0.67–0.75 and RR=0.89, 95% CI=0.85–0.94, respectively) (Fig. 7b). The cumulative meta-analysis result was shown in Figure 7c.
Figure 7. a–e.
a. Comparison of non-major bleeding between the edoxaban group and the warfarin group. b. Comparison of non-major bleeding between the edoxaban group and the warfarin group. (subgroup analysis according to edoxaban dosage). c. Comparison of non-major bleeding between the edoxaban group and the warfarin group. (cumulative meta-analysis). d. Comparison of non-major bleeding between the edoxaban group and the warfarin group. (funnel plot) e. Comparison of non-major bleeding between the edoxaban group and the warfarin group. (sensitivity analysis)
RR - risk ratio
Adverse events
Four studies (19–21, 23) (eight trial comparisons) reported AEs. In total, 557 of 2,199 patients in the edoxaban group and 451 of 1,532 patients in the warfarin group had AEs. No significant differences were observed between the edoxaban and warfarin groups (25.3% vs. 29.4%, respectively) (RR=1.00, 95% CI=0.91–1.10, I2=46.4%) (Fig. 8a). The fixed effects model was applied. Subgroup analysis was performed according to the dosage of edoxaban, which showed that no significant differences at 30 and 60 mg dosages were observed between the edoxaban and warfarin groups (RR=1.03, 95% CI=0.85–1.25 and RR=1.00, 95% CI=0.90–1.11, respectively) (Fig. 8b). The cumulative meta-analysis result is shown in Figure 8c.
Figure 8. a–c.

a. Comparison of AEs between the edoxaban group and the warfarin group. b. Comparison of AEs between the edoxaban group and the warfarin group. (subgroup analysis according to edoxaban dosage). c. Comparison of AEs between the edoxaban group and the warfarin group. (cumulative meta-analysis).
RR - risk ratio
Publication bias and sensitivity analysis
The funnel plot showed that there was bias among retrieved articles (Figs. 6d and 7d). The results of the sensitivity analysis are shown in Figures 6e and 7e.
Discussion
Warfarin is the most commonly used traditional anticoagulant in patients with AF (24). However, due to its narrow therapeutic window and interactions with several drugs and foods, warfarin is more likely to fail to meet the appropriate INR ratio than to cause bleeding events. Time in therapeutic range (TTR) of INR, which was used to evaluate the effectiveness and safety of warfarin anticoagulation, is approximately half were suboptimal (25, 26). Edoxaban is a NOAC recently approved by the US Food and Drug Administration following dabigatran, rivaroxaban, and apixaban (27). In many clinical studies, edoxaban is superior to warfarin in preventing SEEs and reducing the risk of bleeding (28). However, there are still inconsistent conclusions with respect to prevention of strokes and SEEs (29) owing to incomplete research, the small sample size of studies, and limitations in the clinical reference value.
This is the first meta-analysis to compare the efficacy and safety of edoxaban with those of warfarin in preventing clinical events in patients with AF. The results of this meta-analysis show that the incidences of CVD, major bleeding, and non-major bleeding in the edoxaban group was significantly lower than that in the warfarin group. Edoxaban did not increase the incidence of stroke, SEE, MI, and AEs compared with warfarin.
Subgroup analyses were performed according to the dosage of edoxaban and showed that edoxaban did not increase the incidence of stroke, SEE, MI, or AEs at 30 mg, 60 mg, 120 mg, or other dosages compared with warfarin; edoxaban significantly reduced the incidence of CVD, major bleeding, and non-major bleeding at 30 and 60 mg dosages compared with warfarin. However, in other dosage groups (45 and 120 mg), the result was the opposite. No significant differences at a 120-mg dosage were observed between the edoxaban and warfarin groups when evaluating AEs; edoxaban can significantly decrease the incidence of major and non-major bleeding at other dosage levels compared with warfarin. This reversal of edoxaban’s effect may be due to its current suggested dosage (30 or 60 mg). The commonly used dosage of edoxaban is 60 mg a day, and a dosage of 30 mg a day might be only prescribed if the patient has complications such as kidney disease, a low body weight, or taking some specific drugs, such as ciclosporin, dronedarone, erythromycin, and ketoconazole (30). However, in one trial by Weitz et al. (20), patients were administered 60-mg edoxaban twice a day—much higher than the usual clinical dosage—which was proven to increase the risk of bleeding and might be a source of heterogeneity to our results.
When evaluating the safety endpoints, we found that the results were highly heterogeneous; therefore, we performed sensitivity analyses to deconstruct the results. The results showed that after excluding the study by Giugliano et al. (22), the overall effect on major and non-major bleeding was greatly affected, and after excluding the study by Goette et al. (23), the overall effect on AEs was greatly affected. The same results were obtained from the cumulative meta-analysis based on the sample size from small to large. This may be because the sample size in different studies was extremely unbalanced. The weight of the sample size in the studies by Giugliano et al. (22) and Goette et al. (23) was too large, which directly resulted in the overall effect changing with the results of the two studies.
Presently, there are many meta-analyses and RCTs on NOACs in patients with AF. However, whether NOACs can reduce the incidence of CVD, strokes, SEE, MI, major bleeding, non-major bleeding, and AEs in patients with AF is still unclear. These studies mention edoxaban as a NOAC; however, no meta-analysis had compared edoxaban with warfarin. A report by Almutairi et al. (31) has shown that edoxaban can significantly reduce the incidence of CVD and MI, and edoxaban and warfarin had no difference in effect on the incidence of SEE, major bleeding, and stroke events. Another study (32) has shown that edoxaban can significantly reduce the incidence of CVD and major bleeding; however, no difference in the incidence of SEE and stroke events was observed between edoxaban and warfarin (3). However, only one RCT (33) was included in these two meta-analyses. The study by Bruins Slot et al. (34) has reported that factor Xa inhibitors can significantly reduce the incidence of CVD, stroke, SEE, major bleeding, non-major bleeding, and AEs (except for MI) compared with warfarin. The registration studies, ENGAGE AF-TIMI 48 trial and Hokusai-VTE study, all have confirmed that the efficacy and safety of edoxaban are not lower than those of warfarin, with a superior trend (35). Similarly, a network meta-analysis (36) has reported on the efficacy of five anticoagulants on preventing clinical events in patients with AF and showed that no difference in reducing the incidence of CVD, stroke, SEE, MI, major bleeding, non-major bleeding, and AEs was observed between edoxaban and warfarin. In addition, the dosage of edoxaban is important; however, meta-analyses did not analyze the dose. The ENGAGE AF-TIMI 48 trial (22) has suggested that the clinical benefits of edoxaban at 30 mg and 60 mg doses were consistent. However, according to clinical guidelines, different doses have different effects on patients. Therefore, a systematic review of the clinical use of edoxaban is urgently needed.
NOACs included dabigatran, rivaroxaban, apixaban, and edoxaban, which were all factor Xa inhibitors except for the first one. The study by Lee et al. (37) has demonstrated that the efficacy and safety of edoxaban and rivaroxaban were similar. In addition, the research by Sherrill et al. (38) has supported that a high dose of edoxaban has a similar effect with other NOACs and has a significant advantage in reducing the incidence of hemorrhage as well. The 2016 American College of Cardiology Annual Scientific Sessions (ACC 16) has put forward that compared with other NOACs, edoxaban is an agent with larger renal elimination and should be avoided in patients with a creatinine clearance (CrCl) of more than 95 ml/min. Compared with rivaroxaban, edoxaban seems to be more cost effective according to Miller et al. (39).
The strengths of this meta-analysis are as follows: (1) This is the first study focusing on patients with AF in a meta-analysis of the efficacy and safety of edoxaban versus warfarin. Articles have compared several NOACs to warfarin in preventing clinical events; however, no specific meta-analysis comparing edoxaban and warfarin exists. Our article fills this gap. (2) Five RCTs were retrieved with a sample size of 24,836 patients, which is much larger than previous meta-analysis. (3) Subgroup analyses were conducted according to the dosage of edoxaban to explain the heterogeneity among the included studies and increase the reliability of these results. (4) Sensitivity analyses and cumulative meta-analyses were conducted to deconstruct heterogeneity and explore the influence of sample size on the overall effect. (5) Several different bleeding definitions, other than major bleeding, were observed among the retrieved studies (life-threatening bleeding, clinically relevant non-major bleeding, minor bleeding, any overt bleeding, etc.). Such inconsistent definitions may affect the interpretation of the results. This study used major bleeding and non-major bleeding as the safety endpoints to represent the aforementioned definitions.
Study limitations
The limitations of this study are as follows: (1) We retrieved only five articles, and the sample sizes were unbalanced. The study by Giugliano et al. (22) accounted for more than 80% of the total sample size. Through sensitivity analysis and cumulative meta-analysis, we are aware that this had a great impact on several endpoints. The RCTs we included lack TTR analyses of warfarin, which might render the result about the effectiveness of the warfarin inaccurate. (2) Several baseline characteristics (i.e., diabetes, hypertension, older age, and other drug use) were not considered, which may have led to a mixed bias. (3) We used the outcome events reported in the retrieved studies to integrate the results of this meta-analysis. Therefore, assessing the effect of these baseline characteristics on the results was difficult. (4) This study could not explore the interactions among the subgroup analysis because of the limitations inherent in the included studies. (5) Although the definition of non-major bleeding as an endpoint was used to summarize all types of bleeding (except for major bleeding) observed among the retrieved studies, the differences may still have impacted the results of our meta-analysis. (6) Large differences in the follow-up duration used were observed among the studies. Therefore, the results on the long-term effect and safety of edoxaban were not comprehensive. (7) Although the assessment of safety endpoints was significantly heterogeneous, due to the shortage of retrieved articles, we still chose a fixed effects model.
Conclusion
This meta-analysis revealed that compared with warfarin, edoxaban can significantly reduce the incidence of CVD, major bleeding, and non-major bleeding in patients with AF. The anticoagulant effect and safety of edoxaban may be better than those of warfarin.
HIGHLIGHTS.
This is the first study focusing on patients with AF in a meta-analysis of the efficacy and safety of edoxaban versus warfarin. No specific meta-analysis comparing edoxaban and warfarin exists. Our article fills this gap. Compared with warfarin, edoxaban can significantly reduce the incidence of CVD and major and non-major bleeding. The anticoagulant effect and safety of edoxaban may be better than those of warfarin.
Acknowledgments
This study was supported by The First People’s Hospital of Yulin and the Sixth Affiliated Hospitals of Guangxi Medical University.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Author contributions: Concept – X.L., W.X., M.L.; Design – W.X., M.L.; Supervision – W.X., M.L.; Fundings – W.X., Z.L.; Materials – W.X., Z.L.; Data collection &/or processing – X.L., Z.L.; Analysis &/or interpretation – X.L., Z.L.; Literature search – X.L., Z.L.; Writing – X.L., W.X., Z.L., Critical review – X.L., M.L.
References
- 1.Juhász V, Hornyik T, Benák A, Nagy N, Husti Z, Pap R, et al. Comparison of the effects of IK,ACh, IKr, and INa block in conscious dogs with atrial fibrillation and on action potentials in remodeled atrial trabeculae. Can J Physiol Pharmacol. 2018;96:18–25. doi: 10.1139/cjpp-2017-0342. [DOI] [PubMed] [Google Scholar]
- 2.Larsson SC, Åkesson A, Wolk A. Egg consumption and risk of heart failure, myocardial infarction, and stroke: results from 2 prospective cohorts. Am J Clin Nutr. 2015;102:1007–13. doi: 10.3945/ajcn.115.119263. [DOI] [PubMed] [Google Scholar]
- 3.Hachet O, Guenancia C, Stamboul K, Daubail B, Richard C, Béjot Y, et al. Frequency and predictors of stroke after acute myocardial infarction: specific aspects of in-hospital and postdischarge events. Stroke. 2014;45:3514–20. doi: 10.1161/STROKEAHA.114.006707. [DOI] [PubMed] [Google Scholar]
- 4.He W, Chu Y. Atrial fibrillation as a prognostic indicator of myocardial infarction and cardiovascular death: a systematic review and meta-analysis. Sci Rep. 2017;7:3360. doi: 10.1038/s41598-017-03653-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer DE, Hughes RA, Gress DR, Sheehan MA, Oertel LB, Maraventano SW, et al. The effect of aspirin on the risk of stroke in patients with nonrheumatic atrial fibrillation: The BAATAF Study. Am Heart J. 1992;124:1567–73. doi: 10.1016/0002-8703(92)90074-6. [DOI] [PubMed] [Google Scholar]
- 6.Yazdan-Ashoori P, Baranchuk A. Obstructive sleep apnea may increase the risk of stroke in AF patients: refining the CHADS2 score. Int J Cardiol. 2011;146:131–3. doi: 10.1016/j.ijcard.2010.10.104. [DOI] [PubMed] [Google Scholar]
- 7.Rodseth R, Devereaux PJ ACP Journal Club. Review: Perioperative statins reduce perioperative MI and AF in statin-naïve patients. Ann Intern Med. 2012;156:JC6–2. doi: 10.7326/0003-4819-156-12-201206190-02002. [DOI] [PubMed] [Google Scholar]
- 8.Ng R, Yeghiazarians Y. Post myocardial infarction cardiogenic shock: a review of current therapies. J Intensive Care Med. 2013;28:151–65. doi: 10.1177/0885066611411407. [DOI] [PubMed] [Google Scholar]
- 9.Zhu J, Zhang X, Shi G, Yi K, Tan X. Atrial Fibrillation Is an Independent Risk Factor for Hospital-Acquired Pneumonia. PLoS One. 2015;10:e0131782. doi: 10.1371/journal.pone.0131782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miletich JP, Broze GJ, Jr, Majerus PW. The synthesis of sulfated dextran beads for isolation of human plasma coagulation factors II, IX, and X. Anal Biochem. 1980;105:304–10. doi: 10.1016/0003-2697(80)90462-5. [DOI] [PubMed] [Google Scholar]
- 11.Adcock DM, Johnston M. Evaluation of frozen plasma calibrants for enhanced standardization of the international normalized ratio (INR): a multi-center study. Thromb Haemost. 2002;87:74–9. doi: 10.1055/s-0037-1612946. [DOI] [PubMed] [Google Scholar]
- 12.Beinema M, Brouwers JR, Schalekamp T, Wilffert B. Pharmacogenetic differences between warfarin, acenocoumarol and phenprocoumon. Thromb Haemost. 2008;100:1052–7. doi: 10.1160/TH08-04-0116. [DOI] [PubMed] [Google Scholar]
- 13.Li Y, Zhu J, Ding J. VKORC1 -1639G/A and 1173 C/T Genetic Polymorphisms Influence Individual Differences in Warfarin Maintenance Dose. Genet Test Mol Biomarkers. 2015;19:488–93. doi: 10.1089/gtmb.2015.0097. [DOI] [PubMed] [Google Scholar]
- 14.Kumar M, Upreti RK. In vitro effect of azadirachtin on aerobic bacteria of rat intestine. Bull Environ Contam Toxicol. 2003;70:1205–12. doi: 10.1007/s00128-003-0110-5. [DOI] [PubMed] [Google Scholar]
- 15.Samama MM, Kunitada S, Oursin A, Depasse F, Heptinstall S. Comparison of a direct Factor Xa inhibitor, edoxaban, with dalteparin and ximelagatran: a randomised controlled trial in healthy elderly adults. Thromb Res. 2010;126:e286–93. doi: 10.1016/j.thromres.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 16.Horan JT, Francis CW. Fibrin degradation products, fibrin monomer and soluble fibrin in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27:657–66. doi: 10.1055/s-2001-18870. [DOI] [PubMed] [Google Scholar]
- 17.Morishima Y, Kamisato C, Honda Y. Treatment of venous thrombosis with an oral direct factor Xa inhibitor edoxaban by single and multiple administrations in rats. Eur J Pharmacol. 2014;742:15–21. doi: 10.1016/j.ejphar.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 18.O’Donoghue ML, Ruff CT, Giugliano RP, Murphy SA, Grip LT, Mercuri MF, et al. Edoxaban vs. warfarin in vitamin K antagonist experienced and naive patients with atrial fibrillation†. Eur Heart J. 2015;36:1470–7. doi: 10.1093/eurheartj/ehv014. [DOI] [PubMed] [Google Scholar]
- 19.Chung N, Jeon HK, Lien LM, Lai WT, Tse HF, Chung WS, et al. Safety of edoxaban, an oral factor Xa inhibitor, in Asian patients with non-valvular atrial fibrillation. Thromb Haemost. 2011;105:535–44. doi: 10.1160/TH10-07-0451. [DOI] [PubMed] [Google Scholar]
- 20.Weitz JI, Connolly SJ, Patel I, Salazar D, Rohatagi S, Mendell J, et al. Randomised, parallel-group, multicentre, multinational phase 2 study comparing edoxaban, an oral factor Xa inhibitor, with warfarin for stroke prevention in patients with atrial fibrillation. Thromb Haemost. 2010;104:633–41. doi: 10.1160/TH10-01-0066. [DOI] [PubMed] [Google Scholar]
- 21.Yamashita T, Koretsune Y, Yasaka M, Inoue H, Kawai Y, Yamaguchi T, et al. Randomized, multicenter, warfarin-controlled phase II study of edoxaban in Japanese patients with non-valvular atrial fibrillation. Circ J. 2012;76:1840–7. doi: 10.1253/circj.CJ-11-1140. [DOI] [PubMed] [Google Scholar]
- 22.Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 23.Goette A, Merino JL, Ezekowitz MD, Zamoryakhin D, Melino M, Jin J, et al. ENSURE-AF investigators. Edoxaban versus enoxaparin-warfarin in patients undergoing cardioversion of atrial fibrillation (ENSURE-AF): a randomised, open-label, phase 3b trial. Lancet. 2016;388:1995–2003. doi: 10.1016/S0140-6736(16)31474-X. [DOI] [PubMed] [Google Scholar]
- 24.Jaakkola S, Nuotio I, Kiviniemi TO, Virtanen R, Issakoff M, Airaksinen KEJ. Incidence and predictors of excessive warfarin anticoagulation in patients with atrial fibrillation-The EWA study. PLoS One. 2017;12:e0175975. doi: 10.1371/journal.pone.0175975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dlott JS, George RA, Huang X, Odeh M, Kaufman HW, Ansell J, et al. National assessment of warfarin anticoagulation therapy for stroke prevention in atrial fibrillation. Circulation. 2014;129:1407–14. doi: 10.1161/CIRCULATIONAHA.113.002601. [DOI] [PubMed] [Google Scholar]
- 26.Pokorney SD, Simon DN, Thomas L, Fonarow GC, Kowey PR, Chang P, et al. Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) Investigators. Patients’ time in therapeutic range on warfarin among US patients with atrial fibrillation: Results from ORBIT-AF registry. Am Heart J. 2015;170:141–8. doi: 10.1016/j.ahj.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Scheen AJ, Lancellotti P. Edoxaban (LIXIANA®) : new oral anticoagulant forthetreatment and secondary prevention of thromboembolic disease. Rev Med Liege. 2016;71:517–24. [PubMed] [Google Scholar]
- 28.Vaughan Sarrazin MS, Rose A. Safety of new oral anticoagulants. BMJ. 2015;350:h1679. doi: 10.1136/bmj.h1679. [DOI] [PubMed] [Google Scholar]
- 29.Szucs TD, Bramkamp M. Pharmacoeconomics of anticoagulation therapy for stroke prevention in atrial fibrillation: a review. J Thromb Haemost. 2006;4:1180–5. doi: 10.1111/j.1538-7836.2006.01890.x. [DOI] [PubMed] [Google Scholar]
- 30.Roberts A. Anticoagulation therapy: Edoxaban noninferior to warfarin in patients with AF. Nat Rev Cardiol. 2014;11:66. doi: 10.1038/nrcardio.2013.206. [DOI] [PubMed] [Google Scholar]
- 31.Almutairi AR, Zhou L, Gellad WF, Lee JK, Slack MK, Martin JR, et al. Effectiveness and Safety of Non-vitamin K Antagonist Oral Anticoagulants for Atrial Fibrillation and Venous Thromboembolism: A Systematic Review and Meta-analyses. Clin Ther. 2017;39:1456–78. doi: 10.1016/j.clinthera.2017.05.358. [DOI] [PubMed] [Google Scholar]
- 32.Yoshioka H, Sato H, Hatakeyama H, Hisaka A. Model-based meta-analysis to evaluate optimal doses of direct oral factor Xa inhibitors in atrial fibrillation patients. Blood Adv. 2018;2:1066–75. doi: 10.1182/bloodadvances.2017013805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Savarese G, Giugliano RP, Rosano GM, McMurray J, Magnani G, Filippatos G, et al. Efficacy and Safety of Novel Oral Anticoagulants in Patients With Atrial Fibrillation and Heart Failure: A Meta-Analysis. JACC Heart Fail. 2016;4:870–80. doi: 10.1016/j.jchf.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 34.Bruins Slot KM, Berge E. Factor Xa inhibitors versus vitamin K antagonists for preventing cerebral or systemic embolism in patients with atrial fibrillation. Cochrane Database Syst Rev. 2018;3:CD008980. doi: 10.1002/14651858.CD008980.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hokusai-VTE Investigators. Büller HR, Décousus H, Grosso MA, Mercuri M, Middeldorp S, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 36.Guo L, Li S, Wang P, Zhong X, Hong Y. Comparative Efficacy of Clinical Events Prevention of Five Anticoagulants in Patients With Atrial Fibrillation (A Network Meta-Analysis) Am J Cardiol. 2017;119:585–93. doi: 10.1016/j.amjcard.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Lee SR, Choi EK, Han KD, Jung JH, Oh S, Lip GYH. Comparison of Once-Daily Administration of Edoxaban and Rivaroxaban in Asian Patients with Atrial Fibrillation. Sci Rep. 2019;9:6690. doi: 10.1038/s41598-019-43224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sherrill B, Fernandez M, Wang J, Ye X, Kwong W, Sherif B, et al. Network meta-analysis of relative efficacy and safety of edoxaban versus other novel oral anticoagulants (NOACS) among atrial fibrillation patients with CHADS2 score ≥ 2. J Am Coll Cardiol. 2015;65(10_Supplement):A346. doi: 10.1016/S0735-1097(15)60346-1. [DOI] [Google Scholar]
- 39.Miller JD, Ye X, Lenhart GM, Farr AM, Tran OV, Kwong WJ, et al. Cost-effectiveness of edoxaban versus rivaroxaban for stroke prevention in patients with nonvalvular atrial fibrillation (NVAF) in the US. Clinicoecon Outcomes Res. 2016;8:215–26. doi: 10.2147/CEOR.S98888. [DOI] [PMC free article] [PubMed] [Google Scholar]




