Abstract
Acute pulmonary embolism (APE) is one of the prominent causes of death in patients with cardiovascular disease. Currently, reliable biomarkers to predict the prognosis of patients with APE are limited. The present study aimed to investigate the association of blood urea nitrogen to serum albumin (B/A) ratio and intensive care unit (ICU) mortality in critically ill patients with APE. A retrospective cohort study was performed using data extracted from a freely accessible critical care database (MIMIC-III). Adult (≥18 years) patients of first ICU admission with a primary diagnosis of APE in the database were enrolled in the study. The primary endpoint was the ICU mortality rate while the 28-day mortality after ICU admission was the secondary endpoint. The data of survivors and non-survivors were compared. A total of 1048 patients with APE were enrolled in this study, of which 131 patients died in ICU and 169 patients died within 28 days after ICU admission. The B/A ratio in the non-survivors group was significantly higher compared to the survivors group (P < 0.001). The multivariate analysis revealed that the B/A ratio was an independent predictor of ICU mortality (odds ratio [OR] 1.10, 95% CI 1.07-1.14, P < 0.001) and all-cause mortality within 28 days after ICU admission (hazard ratio [HR] 1.07, 95% CI 1.05-1.09, P < 0.001) in APE patients. The B/A ratio showed a greater area under the curve (AUC) of ICU mortality prediction (0.80; P < 0.001) than simplified acute physiology score II (SAPSII) (0.79), systemic inflammatory response syndrome score (SIRS) (0.62), acute physiology score III (APSIII) (0.76) and sequential organ failure assessment (SOFA) score (0.71). The B/A ratio could be a simple and useful prognostic tool to predict mortality in critically ill patients with APE.
Keywords: acute pulmonary embolism, critical care, hospital mortality, blood urea nitrogen to serum albumin ratio
Introduction
Acute pulmonary embolism (APE) is one of the common acute cardiovascular diseases with a 30-day mortality rate as high as 30%.1,2 Patients with APE have various clinical presentation and prognosis. The prognosis of APE primarily depends on the stability of hemodynamics. Patients with low blood pressure and right ventricular dysfunction could have a high risk of in-hospital mortality.3,4 However, because of the complexity of the disease, predicting the clinical outcome of APE is a major challenge. Serum cardiac troponin and brain natriuretic peptide have been used to evaluate the prognosis of patients.5,6 However, a favorable prognostic biomarker has not been established for patients with APE.
Hypoalbuminemia has been shown in the literature to be an independent risk factor for mortality and prognosis. An acute decline in albumin levels after infection predicts a poor prognosis.7 Blood urea nitrogen (BUN) is an important parameter reflecting the relationship between kidney condition, protein metabolism level and nutritional status of patients. Studies have shown that urea nitrogen levels are closely related to mortality.8 Recent studies reported that blood urea nitrogen to serum albumin (B/A) ratio was a useful prognostic biomarker of mortality for community-acquired pneumonia and aspiration pneumonia.9,10 However, there are a handful of data on the relationship between the B/A ratio and mortality in patients with APE. In this study, we aimed to determine whether the B/A ratio is a valuable prognostic biomarker for intensive care unit (ICU) patients with APE.
Materials and Methods
Study Design and Data Source
This retrospective study reviewed data from the Multiparameter Intelligent Monitoring in Intensive Care Database III (MIMIC-III) version 1.4, a large and free critical database for researchers globally.11 The database comprised of over 40000 clinical data of patients who stayed in the ICU of Beth Israel Deaconess Medical Center from 2001 to 2012. Access to the database is only approved following a successful training on the protection of human subjects. The database was created with the approval of the Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA) institutional review boards. The data for this study were extracted by the author (Xu B.), who completed the online training course of the National Institutes of Health and was approved to obtain the original data (certification number: 35936744). The data were extracted using PostgreSQL tools (V.9.6.18).
Study Population
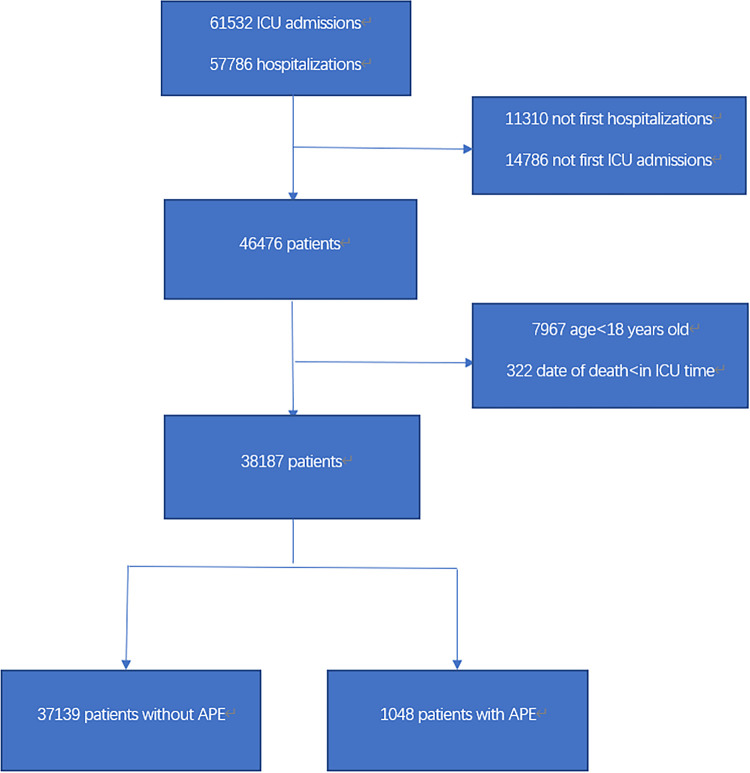
This study enrolled adult (≥18 years) patients of the first ICU admission and with a primary diagnosis of APE in the database. Patients were excluded if the date of death was less than the date of ICU hospitalization to avoid potential typos in the original data. The selection procedure of study participants is summary is in Figure 1.
Figure 1.
Flow chart of the study participants.
Data Extraction
tructured query language and codes to extract the data from the MIMIC Code Repository were used to extract data from the database. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (41511, 41512, 41519) were used to identify APE. The data were extracted as age, gender, heart rate, respiratory rate, systolic blood pressure (SBP), pulse oxygen saturation (SpO2), comorbidities, hospital length of stay (LOS), ICU length of stay (LOS), ICU mortality, 28-day mortality after ICU admission, vasopressin use, statin use, thrombolytic therapy. Data were also extracted on disease severity as assessed by simplified acute physiology score (SAPSII),12 systemic inflammatory response syndrome score (SIRS),13 acute physiology score (APSIII)14 and sequential organ failure assessment (SOFA) score.15 Moreover, laboratory parameters were extracted as serum levels of the white blood cell (WBC), hemoglobin, platelet, glucose, creatinine, albumin, blood urea nitrogen (BUN), K+, Na+ and Ca2+. The B/A ratio (mg/g) was calculated as blood urea nitrogen (mg/dL) divide serum albumin (g/dL).
Definitions and Outcomes
The ICU mortality rate, defined as death during ICU hospitalization, was used as the primary endpoint. The secondary endpoint was 28-day mortality after ICU admission. For patients with more than one ICU admission, only the first ICU stay was considered. The use of the vasopressor during ICU hospitalization for any reason was defined as vasopressor use.
Statistical Analysis
The mean with standard deviation (SD) or median with interquartile ranges was used to present continuous variables. The Student’s t-test and Wilcoxon rank-sum test were applied accordingly. Categorical variables were presented as percentages and the X2 test was used for comparison. A stepwise multiple logistic regression analysis was used to assess the independent factors associated with ICU mortality while a stepwise multivariate Cox regression analysis was used to assess the independent factors associated with 28-day mortality after ICU admission. Analysis of a receiver-operating characteristic (ROC) curve was performed to identify the best cutoff value of the B/A ratio and determine the ability of the B/A ratio to discriminate between survivors and non-survivors. A comparison between survival distributions was achieved using the Kaplan-Meier survival and Log-rank test. All statistical analyses were performed using Stata V.16.0. All tests were performed at a significance level of 5% and were double-sided. P-values of less than 0.05 were considered statistically significant.
Results
Study Population and Baseline Characteristics
A total of 1048 APE patients met the inclusion criteria. Of these, 131 (12.5%) were non-survivors and 917 (87.5%) were survivors during ICU hospitalization (Table 1). The number of women (57.3% vs 44.7%, P = 0.007) and the elderly (70.5 ± 14.5 vs 61.1 ± 17.2, P < 0.001) was significantly higher in the non-survivors group compared with the survivors group. Compared with the survivors group, the non-survivors group had significantly higher respiratory rate (21.3 [18.1-24.7] vs 19.7 [17.3-22.3], P < 0 .001), lower SBP (110.1 [101.2-122.3] vs 118.4 [108.3-129.4], P < 0.001) and lower SpO2 (96.7 [94.6-98.3] vs 97.0 [95.7-98.5], P = 0.048). Moreover the non-survivors group had significantly higher SAPSII (4736-60 vs 30,22-40 P < 0.001), SIRS (33–4 vs 3,2–4 P < 0.001), SOFA (53–8 vs 3,1–5 P = 0.006) and APSIII (5440-75 vs 36,28-48 P < 0.001) than the survivors group. Similarly, when compared to the survivors group, the non-survivors group had significantly higher creatinine (1.2 [0.8-2.0] vs 0.8 [0.7 -1.1], P < 0.001), blood urea nitrogen (BUN) (30.8 [19.0-46.5] vs 16.1 [11.8-22.8], P < 0.001), blood glucose (139.4 [119.5-165.0] vs 119.2 [107.0-136.5], P<0.001), WBC (13.9 [10.6-18.2] vs 9.7 [7.7-12.3], P<0.001), K+ (4.1 [3.9-4.5] vs 4.0 [3.9-4.2], P = 0.002) and B/A ratio (11.7 [7.4-18.2] vs 5.5 [4.0-8.2], P<0.001), and lower albumin (26.5 [23.0-28.5] vs 28.5 [27.8-29.9], P < 0.001), platelet (203.8 [122.2-292.1] vs 256.8 [198-336.1], P < 0.001) and hemoglobin (10.1 [9.2-11.0] vs 10.4 [9.5-11.7], P = 0.004). In addition, compared with survivors, the number of non-survivors associated with malignancy (47.3% vs 28.2%, P < 0.001), renal failure (13.7% vs 7.7%, P = 0.021), vasopressin use (41.2% vs 23.0%, P < 0.001) and albumin use (16.0% vs 8.7%, P = 0.008) was higher.
Table 1.
Comparison of Baseline Characteristics Between the Survivors Group and Non-Survivors Group.
| Variable | Survivors (n = 917) | Non-survivors (n = 131) | P value |
|---|---|---|---|
| Age (years) | 61.1 ± 17.2 | 70.5 ± 14.5 | <0.001 |
| Female (n (%)) | 410 (44.7%) | 75 (57.3%) | 0.007 |
| Clinical parameters | |||
| Heart rate (beats/min) | 91.0 (80.1-101.9) | 92.4 (78.0-106.5) | 0.444 |
| SBP (mmHg) | 118.4(108.3-129.4) | 110.1 (101.2-122.3) | <0.001 |
| Respiratory Rate (beats/min) | 19.7 (17.3-22.3) | 21.3 (18.1-24.7) | <0.001 |
| SpO2 (%) | 97.0 (95.7-98.5) | 96.7 (94.6-98.3) | 0.048 |
| Comorbidities | |||
| Coronary (n (%)) | 116 (12.6%) | 15 (11.5%) | 0.698 |
| COPD | 22 (2.4%) | 3 (2.3%) | 0.939 |
| Diabetes (n (%)) | 182 (19.8%) | 25 (19.1%) | 0.837 |
| Hypertension (n (%)) | 387 (42.2%) | 54 (41.2%) | 0.831 |
| Malignancy (n (%)) | 259 (28.2%) | 62 (47.3%) | <0.001 |
| Congestive heart failure (n (%)) | 130 (14.2%) | 25 (19.1%) | 0.139 |
| Renal failure (n (%)) | 71 (7.7%) | 18 (13.7%) | 0.021 |
| Liver disease (n (%)) | 44 (4.8%) | 5 (3.8%) | 0.619 |
| Obesity (n (%)) | 71(7.7%) | 6 (4.6%) | 0.194 |
| Weight loss (n (%)) | 49 (5.3%) | 6 (4.6%) | 0.714 |
| Depression (n (%)) | 83 (9.1%) | 10 (7.6%) | 0.594 |
| Scoring systems | |||
| SAPSII | 30 (22-40) | 47 (36-60) | <0.001 |
| SIRS | 3 (2-4) | 3 (3-4) | <0.001 |
| SOFA | 3 (1-5) | 5 (3-8) | 0.006 |
| APSIII | 36 (28-48) | 54 (40-75) | <0.001 |
| Laboratory parameters | |||
| Albumin, g/L | 28.5 (27.8-29.9) | 26.5 (23.0-28.5) | <0.001 |
| Creatinine, mg/dL | 0.8 (0.7 -1.1) | 1.2 (0.8-2.0) | <0.001 |
| BUN, mg/dL | 16.1 (11.8-22.8) | 30.8 (19.0-46.5) | <0.001 |
| Glucose, mg/dL | 119.2 (107.0-136.5) | 139.4 (119.5-165.0) | <0.001 |
| WBC, K/uL | 9.7 (7.7-12.3) | 13.9 (10.6-18.2) | <0.001 |
| Platelet, K/uL | 256.8 (198-336.1) | 203.8 (122.2-292.1) | <0.001 |
| Hemoglobin, g/dL | 10.4 (9.5-11.7) | 10.1 (9.2-11.0) | 0.004 |
| Na+, mEq/L | 138.8 (136.8-140.5) | 139 (136.5-141.8) | 0.294 |
| K+, mEq/L | 4.0 (3.9-4.2) | 4.1 (3.9-4.5) | 0.002 |
| Ca2+mean, mEq/L | 1.12 (1.11 -1.13) | 1.12 (1.10 -1.15) | 0.740 |
| Albumin use (n (%)) | 80 (8.7%) | 21 (16.0%) | 0.008 |
| Statin use (n (%)) | 413 (45.0%) | 49 (37.4%) | 0.100 |
| Vasopressor use (n (%)) | 211 (23.0%) | 54 (41.2%) | <0.001 |
| Thrombolytic therapy (n (%)) | 52 (5.7%) | 13 (9.9%) | 0.059 |
| B/A ratio (mg/g) | 5.5 (4.0-8.2) | 11.7 (7.4-18.2) | <0.001 |
Abbreviations: SBP, systolic blood pressure; SpO2, pulse oxygen saturation; COPD, chronic obstructive pulmonary disease; SAPSII, simplified acute physiology score II; SIRS, systemic inflammatory response syndrome score; SOFA, sequential organ failure assessment; APSIII, acute physiology score III; BUN, blood urea nitrogen; WBC, white blood cell; B/A ratio, blood urea nitrogen to serum albumin ratio.
Factors Associated With Mortality
The stepwise multiple logistic regression analysis showed that gender, SpO2, malignancy, blood glucose, platelet, SAPSII and B/A ratio were independent factors associated with ICU mortality (Table 2). The stepwise multivariate Cox regression analysis revealed that Spo2, malignancy, renal failure, WBC, blood glucose, SAPSII, SOFA, B/A ratio, vasopressor use, hospital LOS and ICU LOS were independent factors associated with all-cause mortality within 28 days after ICU (Table 3).
Table 2.
Multiple Logistic Regression Analyses of Factors Affecting ICU Mortality With APE.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Female | 1.61 | 1.03-2.52 | 0.038 |
| Spo2 (%) | 0.91 | 0.85-0.98 | 0.008 |
| Malignancy | 2.24 | 1.44-3.50 | <0.001 |
| Glucose, mg/dL | 1.01 | 1.00-1.02 | 0.002 |
| Platelet, K/uL | 0.997 | 0.996-0.999 | 0.009 |
| SAPSII | 1.06 | 1.04-1.07 | <0.001 |
| B/A Ratio | 1.10 | 1.07-1.14 | <0.001 |
Abbreviations: SpO2, pulse oxygen saturation; SAPSII, simplified acute physiology score II; B/A ratio, blood urea nitrogen to serum albumin ratio; OR, odds ratio; CI, confidence interval.
Table 3.
Multivariate Cox regression Analyses of Factors Affecting All-Cause Mortality Within 28 Days After ICU With APE.
| Variable | 28-day mortality, N = 169 (16.1%) | HR | 95% CI | P |
|---|---|---|---|---|
| Spo2 (%) | 96.7 (94.8-98.3) | 0.91 | 0.87-0.94 | <0.001 |
| Malignancy (n (%)) | 93 (55.0%) | 2.53 | 1.84-3.47 | <0.001 |
| Renal failure (n (%)) | 21 (12.4%) | 0.60 | 0.37-0.97 | 0.038 |
| WBC | 13.3 (10.4-17.6) | 1.01 | 1.00-1.01 | 0.008 |
| Glucose, mg/dL | 113.8 (117.0-153.5) | 1.01 | 1.004-1.008 | <0.001 |
| Vasopressor Use (n (%)) | 63 (37.3%) | 1.54 | 1.06-2.24 | 0.024 |
| SAPSII | 46 (37-59) | 1.07 | 1.06-1.09 | <0.001 |
| SOFA | 5 (3-7) | 0.93 | 0.88-1.00 | 0.043 |
| Hospital LOS (days) | 7.8 (3.9-12.4) | 0.89 | 0.86-0.92 | <0.001 |
| ICU LOS (days) | 3.1 (1.6-6.8) | 1.05 | 1.01-1.10 | 0.016 |
| B/A Ratio (mg/g) | 10.3 (7.0-15.9) | 1.07 | 1.05-1.09 | <0.001 |
Abbreviations: SpO2, pulse oxygen saturation; WBC, white blood cell; SAPSII, simplified acute physiology score II; SOFA, sequential organ failure assessment; LOS, length of stay; ICU, intensive care unit; B/A Ratio, blood urea nitrogen to serum albumin ratio; HR, hazard ratio; CI, confidence interval.
To determine the ability of predictors, we used the ROC curve graphics. The AUC value for the B/A ratio was 0.80. The cutoff point was 7.797, with the highest predictive performance for both specificity and sensitivity (Figure 2). Survival rates at high B/A levels were significantly worse than at low B/A levels (P < 0.001) (Figure 3).
Figure 2.
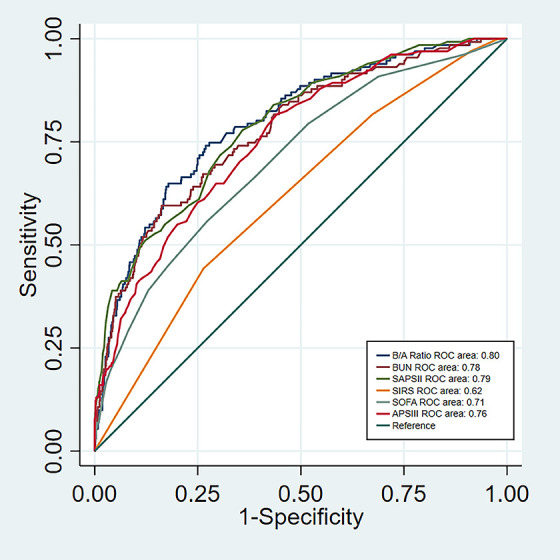
Receiver-operating characteristic curve of the B/A ratio, BUN, SAPSII, SIRS, SOFA and APSIII to predict ICU mortality of APE. Abbreviations: B/A ratio, blood urea nitrogen to serum albumin ratio; BUN, blood urea nitrogen; SAPSII, simplified acute physiology score II; SIRS, systemic inflammatory response syndrome score; SOFA, sequential organ failure assessment; APSIII, acute physiology score III; ICU, intensive care unit; APE, acute pulmonary embolism.
Figure 3.
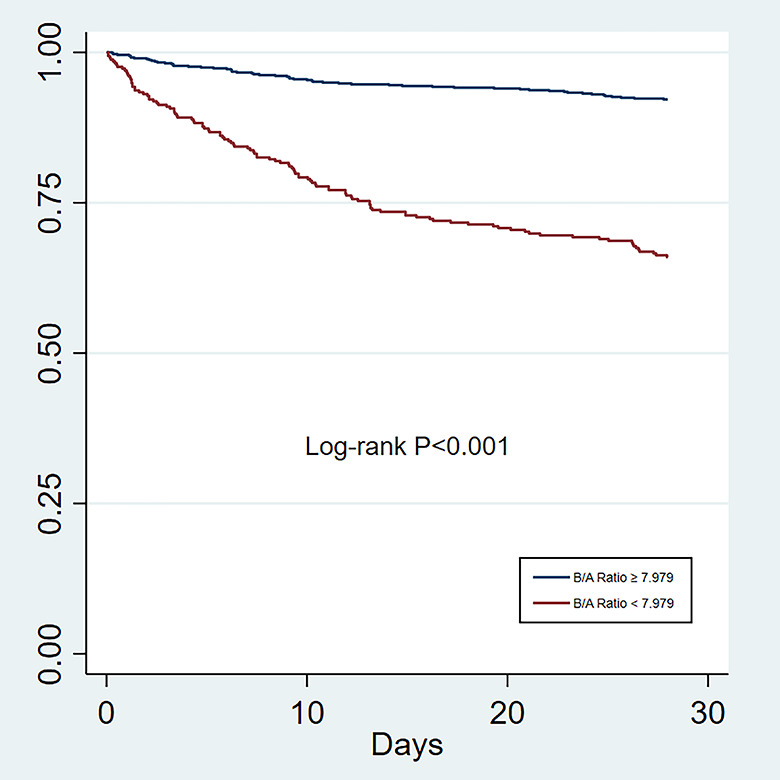
Kaplan-Meier curves of the B/A ratio for predicting 28-day mortality with APE. A high B/A ratio was significantly associated with higher mortality than a low B/A ratio (P < 0.001). Abbreviation: B/A ratio: blood urea nitrogen to serum albumin ratio.
Discussion
The B/A ratio was reported to be positively associated with 28-day mortality in patients with aspiration pneumonia.10 In another study that enrolled 1158 hospital-acquired pneumonia patients, the B/A ratio was reported as a risk factor for patients’ mortality.16 In our study, the B/A ratio was significantly increased in the non-survivors group with APE. The AUC for the B/A ratio was 0.80. In multivariate analysis, the B/A ratio was an independent predictor of ICU mortality in APE patients (odds ratio (OR) 1.10, 95% CI 1.07-1.14, P < 0.001) and an independent predictor of all-cause mortality within 28 days after ICU admission (hazard ratio (HR) 1.07, 95% CI 1.05-1.09, P < 0.001).
Serum albumin plays a vital role in maintaining physiological homeostasis, including maintenance of normal colloid osmotic pressure, improvement of arterial hyporeactivity, reduction of ischemia-reperfusion injury and contribution to anti-inflammatory action.17,18 Serum albumin can interact with nitric oxide (NO) to some extent and generate S-nitrosoproteins, which then promote vasomotor activity and inhibit platelet aggregation.19 Hoskin et al reported that hypoalbuminemia is an independent predictor of mortality following PE.20
Blood urea increases due to enhanced proximal tube reabsorption and enhanced water and sodium transport.21 A previous study documented the correlation between APE and the right ventricular (RV) dysfunction.22 BUN can reflect a state of hypoperfusion, which could be a good indicator of the RV systolic dysfunction because of renal hypoperfusion. Another study involving 252 patients showed that BUN could detect high-risk patients with APE.23 In our study, the non-survivors group had a significantly higher BUN (P < 0.001) and lower serum albumin (P < 0.001) than the survivors group during ICU hospitalization, which was consistent with previous findings. The non-survivors group was associated with renal failure, which could cause low serum albumin and high BUN. However, we suggest that the B/A ratio could be a favorable prognostic factor of mortality in APE patients than BUN alone.
Gender and age significantly differed between the non-survivors and survivors groups in ICU hospitalization in our study as reported by Keller et al.24 However, the reason for the disparity remains unclear. Nevertheless, older patients are prone to a series of basic diseases. Our results revealed that patients with malignancy had higher mortality. Alotaibi et al found that patients with cancer have a higher risk of short-term mortality,25 which was similar to our findings.
Rozjabek et al reported that vital signs, including respiratory rate, heart rate, systolic blood pressure and oxygen saturation, had high sensitivity and specificity to predict PE patients’ risk of 30-day all-cause mortality,26 which was consistent with our findings.
We found that the non-survivors group had significantly higher WBC (P < 0.001) compared with the survivors group during ICU hospitalization. The right-heart dysfunction associated with PE could lead to an elevated WBC count, which is a known factor for poor prognosis in patients with PE.27
SAPSII, SIRS, APSIII and SOFA have been widely used to assess disease severity and predict in-hospital mortality in ICU. However, these scoring systems were more complex and less readily available than the B/A ratios. In our study, the B/A ratio had a higher AUC value (0.80) for predicting ICU mortality, which was superior to SAPSII, SIRS, APSIII and SOFA, although SAPSII, SIRS, APSIII and SOFA were significant factors of ICU mortality. A previous meta-analysis reported that high mortality was associated with vasopressin use.28 Vasopressin use was also found to be a risk factor of ICU mortality in APE patients in our study.
Limitations
This study, however, had some limitations. First, a measurement error caused by identifying APE or other comorbidities using ICD-9-CM codes might have occurred. Second, we conducted this study retrospectively; this might include selection bias and the issue of missing data. Third, the internal validation approach of the prediction model could not include all the risk variables associated with APE, including hypovolemia, echocardiography, the severity of illness scores and drugs. Nevertheless, we still believe that this method can be used in clinical studies and achieve the purpose.
Conclusion
In conclusion, we found that the B/A ratio is an independent prognostic factor of ICU mortality and 28-day mortality after ICU admission in critically ill APE patients. The B/A ratio is a simple but potentially valuable prognostic biomarker in patients with APE.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval to report this case series was obtained from Laboratory for Computational Physiology at the Massachusetts Institute of Technology.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed Consent: Informed consent for patient information to be published in this article was not obtained because the data of this article was based on the Multiparameter Intelligent Monitoring in Intensive Care Database III (MIMIC-III) version 1.4, a large and free critical database for researchers globally.
ORCID iD: Bin Xu  https://orcid.org/0000-0001-8258-4414
https://orcid.org/0000-0001-8258-4414
References
- 1. Konstantinides SV, Torbicki A, Agnelli G, et al. ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033–3080. doi:10.1093/eurheartj/ehu283 [DOI] [PubMed] [Google Scholar]
- 2. Bach AG, Taute BM, Baasai N, et al. 30-day mortality in acute pulmonary embolism: prognostic value of clinical scores and anamnestic features. PLoS One. 2016;11(2):e0148728. doi:10.1371/journal.pone.0148728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kucher N, Rossi E, De Rosa M, et al. Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher. Arch Intern Med. 2005;165(15):1777–1781. doi:10.1001/archinte.165.15.1777 [DOI] [PubMed] [Google Scholar]
- 4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronicthromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788–1830. doi:10.1161/CIR.0b013e318214914 f. [DOI] [PubMed] [Google Scholar]
- 5. El-Menyar A, Sathian B, Al-Thani H. Elevated serum cardiac troponin and mortality in acute pulmonary embolism: Systematic review and meta-analysis. Respir Med. 2019;157: 26–35. doi:10.1016/j.rmed.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 6. Coutance G, Le Page O, Lo T, Hamon M. Prognostic value of brain natriuretic peptide in acute pulmonary embolism. Crit Care. 2008;12(4):R109. doi:10.1186/cc6996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magnussen B, Oren Gradel K, Gorm Jensen T, et al. Association between hypo-albuminaemia and mortality in patients with community-acquired bacteraemia is primarily related to acute disorders. PLOS ONE. 2016;11(9):e0160466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Sun LL, Wang J, Ji G. Blood urea nitrogen in the prediction of in-hospital mortality of patients with acute aortic dissection. Cardiol J. 2018;25(3):371–376. [DOI] [PubMed] [Google Scholar]
- 9. Ugajin M, Yamaki K, Iwamura N, Yagi T, Asano T. Blood urea nitrogen to serum albumin ratio independently predicts mortality and severity of community-acquired pneumonia. Int J Gen Med. 2012;5:583–589. doi:10.2147/IJGM.S33628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ryu S, Oh SK, Cho SU, et al. Utility of the blood urea nitrogen to serum albumin ratio as a prognostic factor of mortality in aspiration pneumonia patients. Am J Emerg Med. 2020;S0735-6757(20)30118-2. doi:10.1016/j.ajem.2020.02.045 [DOI] [PubMed] [Google Scholar]
- 11. Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Sci Data. 2016;3:160035. doi:10.1038/sdata.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. Apache ii: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi:10.1097/00003246-198510000-00009 [PubMed] [Google Scholar]
- 13. Rangel-Frausto MS, Pittet D, Costigan M, Wenzel RP, Hwang T, Davis CS. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995;273(2):117–123. doi:10.1001/jama.1995.03520260039030. [PubMed] [Google Scholar]
- 14. Pollack MM, Patel KM, Ruttimann UE. The pediatric risk of mortality iii-acute physiology score (prism iii-aps): a method of assessing physiologic instability for pediatric intensive care unit patients. J Pediatr. 1997;131(4):575–581. doi:10.1016/S0022-3476(97)70065-9 [DOI] [PubMed] [Google Scholar]
- 15. Vincent JL, de Mendonça A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi:10.1097/00003246-199811000-00016 [DOI] [PubMed] [Google Scholar]
- 16. Feng DY, Zhou YQ, Zou XL, et al. Elevated blood urea nitrogen-to-serum albumin ratio as a factor that negatively affects the mortality of patients with hospital-acquired pneumonia. Can J Infect. Dis. Med. Microbiol. 2019;2019:1547405. doi:10.1155/2019/1547405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Meziani F, Kremer H, Tesse A, et al. Human serum albumin improves arterial dysfunction during early resuscitation in mouse endotoxic model via reduced oxidative and nitrosative stresses. Am J Pathol. 2007;171(6):1753–1761. doi:10.2353/ajpath.2007.070316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85(4):599–610. doi:10.1093/bja/85.4.599 [DOI] [PubMed] [Google Scholar]
- 19. Caraceni P, Domenicali M, Tovoli A, et al. Clinical indications for the albumin use: still a controversial issue. Eur J Intern Med. 2013;24(8):721–728. doi:10.1016/j.ejim.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 20. Hoskin S, Chow V, Kritharides L, Ng ACC. Incidence and impact of hypoalbuminaemia on outcomes following acute pulmonary embolism. Heart Lung Circ. 2020;29(2):280–287. doi:10.1016/j.hlc.2019.01.007 [DOI] [PubMed] [Google Scholar]
- 21. Lin J, Denker BM. Azotemia and urinary abnormalities. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson SL, Loscalzo J, eds. 18th Harrison’s principles of internal medicine. McGraw-Hill Education; 2012:334–341. [Google Scholar]
- 22. Lankeit M, Jiménez D, Kostrubiec M, et al. Validation of N-terminal pro-brain natriuretic peptide cut-off values for risk stratification of pulmonary embolism. Eur Respir J. 2014;43(6):1669–1677. doi:10.1183/09031936.00211613 [DOI] [PubMed] [Google Scholar]
- 23. Tatlisu MA, Kaya A, Keskin M, et al. The association of blood urea nitrogen levels with mortality in acute pulmonary embolism. J Crit Care. 2017;39:248–253. doi:10.1016/j.jcrc.2016.12.019 [DOI] [PubMed] [Google Scholar]
- 24. Keller K, Hobohm L, Munzel T, Konstantinides SV, Lankeit M. Sex-specific and age-related seasonal variations regarding incidence and in-hospital mortality of pulmonary embolism in Germany. ERJ Open Res. 2020;6(2):181–2012. doi:10.1183/23120541.00181-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alotaibi G, Wu C, Senthilselvan A, McMurtry MS. Short- and long-term mortality after pulmonary embolism in patients with and without cancer. Vasc Med. 2018;23(3):261–266. doi:10.1177/1358863X18754692 [DOI] [PubMed] [Google Scholar]
- 26. Rozjabek HM, Coleman CI, Weeda ER, et al. Effect of vital sign measurement timing on Pulmonary Embolism Severity Index (PESI) and simplified PESI 30-day mortality risk determination. Thromb Res. 2016;141:8–10. doi:10.1016/j.thromres.2016.02.017 [DOI] [PubMed] [Google Scholar]
- 27. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569–1577. doi:10.1093/eurheartj/ehn208 [DOI] [PubMed] [Google Scholar]
- 28. Dettmer MR, Damuth E, Zarbiv S, Bartock JL, Trzeciak S. Prognostic factors for long-term mortality in critically ill patients treated with prolonged mechanical ventilation: a systematic review. Crit Care Med. 2017;45(1):69–74. doi:10.1097/CCM.0000000000002022 [DOI] [PubMed] [Google Scholar]