Abstract
Humanized liver mouse models are crucial tools in liver research, specifically in the fields of liver cell biology, viral hepatitis and drug metabolism. The livers of these humanized mouse models are repopulated by 3-dimensional islands of fully functional primary human hepatocytes (PHH), which are notoriously difficult to maintain in vitro. As low efficiency and high cost hamper widespread use, optimization is of great importance. In the present study, we analyzed experimental factors associated with Hepatitis E virus (HEV) infection and PHH engraftment in 2 xenograft systems on a Nod-SCID-IL2Ry-/- background: the alb-urokinase plasminogen activator mouse model (uPA-NOG, n=399); and the alb-HSV thymidine kinase model (TK-NOG, n = 198). In a first analysis, HEV fecal shedding in liver humanized uPA-NOG and TK-NOG mice with comparable human albumin levels was found to be similar irrespective of the mouse genetic background. In a second analysis, sex, mouse age at transplantation and hepatocyte donor were the most determinant factors for xenograft success in both models. The sexual imbalance for xenograft success was related to higher baseline ALT levels and lower thresholds for ganciclovir induced liver morbidity and mortality in males. These data call for sexual standardization of human hepatocyte xenograft models, but also provide a platform for further studies on mechanisms behind sexual dimorphism in liver diseases.
Keywords: sexual dimorphism, primary human hepatocyte transplantation, human liver chimeric mouse models, liver disease, NOG mice, TK-NOG, uPA-NOG
Introduction
Liver humanized mouse models have been developed 2 decades ago after transplanted primary human hepatocytes (PHH) were found to repopulate a genetically diseased mouse liver on an immunodeficient background1,2. In these models the murine liver is reconstituted by 3-dimensional islands of fully functional and differentiated PHH. As PHH are notoriously difficult to maintain in vitro, liver humanized mouse models have contributed significantly to our understanding of diverse areas of liver infectious diseases, drug and lipid metabolism and liver cell biology (reviewed in3–5).
The prerequisites for liver humanization are an immunodeficient background to prevent the rejection of xenografts6; and severe liver damage to create a favorable environment for PHH engraftment, expansion and repopulation7. Three major xenograft models have been developed so far in which hepatocellular injury is caused by different mechanisms: (1) hepatocyte-specific over-expression of a protease under the albumin (alb-) promotor in urokinase-type plasminogen activator (uPA)-transgenic mice8,9; (2) the accumulation of a toxic metabolite by a genetic defect in the tyrosine catabolic pathway in Fumarylacetoacetate Hydrolase (FAH) deficient mice10; (3) the accumulation of a toxic ganciclovir metabolite in hepatocytes that express the Herpes Simplex Virus type 1 thymidine kinase (HSV-TK) under the alb-promotor11. Liver damage starts perinatally and continues throughout the lifespan in uPA-transgenic mice; but is controllable in the latter 2 models. The accumulation of the toxic tyrosine catabolite can be prevented by the administration of a drug that blocks this pathway in FAH-deficient mice. Liver damage in HSV-TK mice is absent unless ganciclovir is injected8–10,12. Initial humanized liver models were backcrossed on a severe combined immune deficient (SCID) mouse line that carries dysfunctional T and B lymphocytes, but required additional depletion of human NK cells, macrophages and complement for optimal PHH engraftment13,14. Since then, most liver humanized models are profound immune deficient lacking at least a functional γ-chain of the IL-2 receptor (IL-2Rγcnull) on a NOD/Shi-scid background or are additionally deficient in the recombination activating gene 1 or 2 (Rag2-/- vs Rag1-/-). Combined these genetic defects result in dysfunctional T, B and NK cells and leave the monocyte-macrophage system severely crippled15,16.
Despite optimization of the immunodeficiency, initial low efficacy, high cost and PHH repopulation variability has hampered widespread use of these interesting models3–5,17. Given their importance to the liver research community and the heterogeneity of model systems, standardization of humanized mice should nevertheless be a high priority18. We and others have examined strategies and experimental conditions that determine PHH engraftment success and led to standardized criteria for Hepatitis B and C virus infections in liver humanized mice1,16,17. Higher engraftment levels were obtained after transplanting plateable cryopreserved PHH and increasing the cell dose to more than 3 million PHH/mouse17,19,20. Other optimizations included the induction of additional liver damage by transferring an adenoviral vector that encodes for uPA in FAH-deficient mice10 and the inhibition of endogenous mouse hepatocyte proliferation with retrorsine20.
Most of these optimization studies examine experimental factors in only 1 xenograft system1,4,9,10,12–14,17,19. In the current paper we perform a head-to-head comparison of xenografts in uPA- and HSV-TK-transgenic mice on an identical NOD/Shi-scid/IL-2Rγcnull (NOG) background, to eliminate any difference in residual immune system activities. In a comprehensive overview of 597 PHH-transplanted uPA-NOG and TK-NOG mice, we systematically examine the relative contribution of different factors to xenograft success. We found that irrespective of the model used, Hepatitis E Virus (HEV) faecal shedding correlated with serum human albumin levels (hALB) levels and male sex was the most determinant factor for the latter. Besides mouse sex, the mouse age at transplantation and the type of PHH donor contributed significantly to PHH engraftment. Sexual imbalance for xenograft success was related to higher baseline ALT levels and lower thresholds for ganciclovir induced liver morbidity and mortality in males. These data call for sexual standardization of human hepatocyte xenograft models, but also provide a platform for further studies on mechanisms behind sexual dimorphism in liver diseases.
Materials and Methods
Animals
uPA-NOG and TK-NOG mice embryos were provided by dr. Suemizu, Central Institute for Experimental Animals, Kawasaki, Japan9,12 and mice were bred at the Central Animal Facility of the Erasmus Medical Center (DEC nr 141-12-11). For genotyping of TK-NOG, a copy number duplex qPCR was applied using custom designed primer-probe mix F: CGATTCGCCGCGTTTACG, R: CGCCGCCCTGCAGATA and probe: [6FAM]CCGCACCGTATTGGCAA[BHQ1] targeting the TK gene. TaqMan Genotyping master mix (Life Technologies), TaqMan uPA genotyping assay (Mm00422051_cn; Life technologies, Carlsbad, CA, USA) and Tert gene references mix (Life technologies, Carlsbad, CA, USA) were used according to the manufacturer’s protocol. Assays were performed on phenol-chloroform-isoamyl alcohol (Sigma-Aldrich, St. Louis, MO, USA)-extracted genomic mouse DNA from toe snip.
Human Hepatocyte Transplantation
Homozygous uPA+/+ or TK+ mice were anesthetized and transplanted with 0.4 to 2×106 viable commercially available cryopreserved PHH (Lonza, Lot:9F3003, Basel, Switzerland, and BD Gentest, Lot:342 and Lot:345, Corning, Corning, NY, USA) via the intrasplenic injection route9,12,17,21. Based on our and others’ previous experience plateable cryopreserved hepatocytes from neonatal and pediatric donors were selected for liver humanization studies17,22,23. Cell viability was assessed immediately after thawing using trypan blue and a cell viability of ≥ 50% was set as threshold for further transplantation. TK+ mice were challenged with an intraperitoneal ganciclovir (GCV) (stock solution is 750 µg/mL) injection at day 7 and 5 before transplantation12. At indicated time points mouse blood samples (50 μL) were obtained via a tail vein bleed.
Except for the GCV preconditioning in TK-NOG mice, all housing, handling and experimental procedures were identical between TK-NOG and uPA-NOG mice. In addition, both models were mostly transplanted in parallel, but inherent variation in offspring numbers with the required uPA+/+ and TK+ zygosity led to some differences in the age at transplatation and number of recipients per PHH donor. Hepatocyte engraftment was determined by measuring hALB in mouse serum with a sandwich ELISA method as previously described (Bethyl laboratories, Montgomery, TX, USA). Human serum and non-transplanted mouse serum samples were used as positive and negative controls, respectively. Human reference serum (RS10-110, Bethyl laboratories, Montgomery, TX, USA) dilutions were used to create a standard curve for quantification12,21.
HEV Infection
Successfully engrafted mice were intravenously inoculated with at least 106 geq or IU HEV gt3c isolated from a chronically infected patient or the prototype HEV gt3a Kernow strain (a kind gift from Dr Zongdi Feng, Nationswide Children Hospital, Columbus, Ohio, USA). After inoculation mice were housed individually. HEV RNA levels in weekly collected mouse fecal samples and a liver sample, collected at euthanasia, were determined using an ISO15189:2012-validated, internally controlled qRT-PCR, as described previously21,24. Detection limit of the assay is 148 IU/mL and results were normalized using tissue weight and dilution factor.
High Fat Diet
Successfully engrafted mice were housed in individually ventilated cages, with a maximum of 4 mice per cage. Mice were fed al libitum with rodent chowcontaining 0.3% cholesterol (Altromin Spezialfutter GmbH & Co. KG, Lage, Germany) up to 10 weeks16.
Histology, Immunohistochemistry, and Oil-Red-O (ORO) Staining
Mouse livers were fixed in 4% formaldehyde (Merck Millipore, MA, USA). Standard hematoxylin and eosin (H&E) staining was performed using 4-5 µm cuts from paraffin embedded blocks. An Oil-Red-O staining method was performed to identify steatosis on 5 µm cuts from N2 snap frozen humanized liver fragments25. Human hepatocytes were stained using goat anti-human albumin cross-adsorbed antibody (Bethyl Laboratories Inc., TX, USA) and slides were visualized using substrate 3,3′-diaminobenzidine (DAB; Dako, Copenhagen, Denmark) detection.
RNA Isolation, cDNA Synthesis and qPCR Analysis
RNA isolation was performed using whole liver tissues stored in RNAlater RNA stabilization reagent (Qiagen, Hilden, Germany) with Qiazol (Qiagen, Hilden, Germany). Complementary DNA (cDNA) was generated by using PrimeScriptTM reverse transcriptase master mix (Takara Bio Inc., Kusatsu, Japan) according to manufacturer’s protocol. For the mRNA expression analysis following TaqMan probes were used: F: CGATTCGCCGCGTTTACG, R: CGCCGCCCTGCAGATA and probe: [6FAM]CCGCACCGTATTGGCAA[BHQ1] targeting the HSVTK mRNA and Mm00422051_cn TaqMan probe (Life technologies, Carlsbad, CA, USA) for plau mRNA. Tert gene mRNA level was used to calculate the relative expression.
ALT Measurement
Enzymatic quantification of mouse serum ALT levels was performed at the Erasmus University Medical Center Clinical Diagnostic Laboratory (TrialLab, ISO15189:2012 - M098).
Statistical Analysis
Survival analysis, correlation analysis and t-test were performed using GraphPad Prism version 5.00 for Windows, (GraphPad Software, San Diego, CA, USA) and P < 0.1 was accepted as statistically significant. *P < 0.1, **P < 0.05, ***P < 0.001.
Multivariate regression and correlation analysis were performed using Statistical Package for the Social Sciences (SPSS) for Windows, version 25.0 (SPSS Inc., Chicago, IL, USA). Multivariate regression analysis (list wise exclusion of independent variables, with both an enter and backward approach) was applied to study the effect of PHH engraftment on fecal HEV shedding upon HEV inoculation, using fecal HEV titers as dependent variable and mouse serum hALB level (µg/mL), the mouse genetic background and HEV strain as independent variables. Thereafter, linear regression was performed with the identified significant independent variable from the multivariate analysis.
To examine the relative contribution of different experimental parameters on PHH engraftment in a second multivariate regression analysis, post-transplantation (postTx) week 8 mouse serum hALB level (µg/mL) was set as dependent variable and the following parameters as independent variables: hepatocyte donor, transplanted PHH cell count, sex of the acceptor mouse, the mouse genetic background and offspring generation. As we performed transplantation with three different hepatocyte donors, we first performed a multivariate analysis without including hepatocyte donor as variable. Second, dummy data transformation was applied for each of the hepatocyte donors, using the Donor345 as our baseline, as this hepatocyte donor was applied most extensively in both xenograft models (Fig. 2B). Forward analysis was applied to see the effect of each significant parameter (SPSS, IBM SPSS Inc., Chicago, IL, USA).
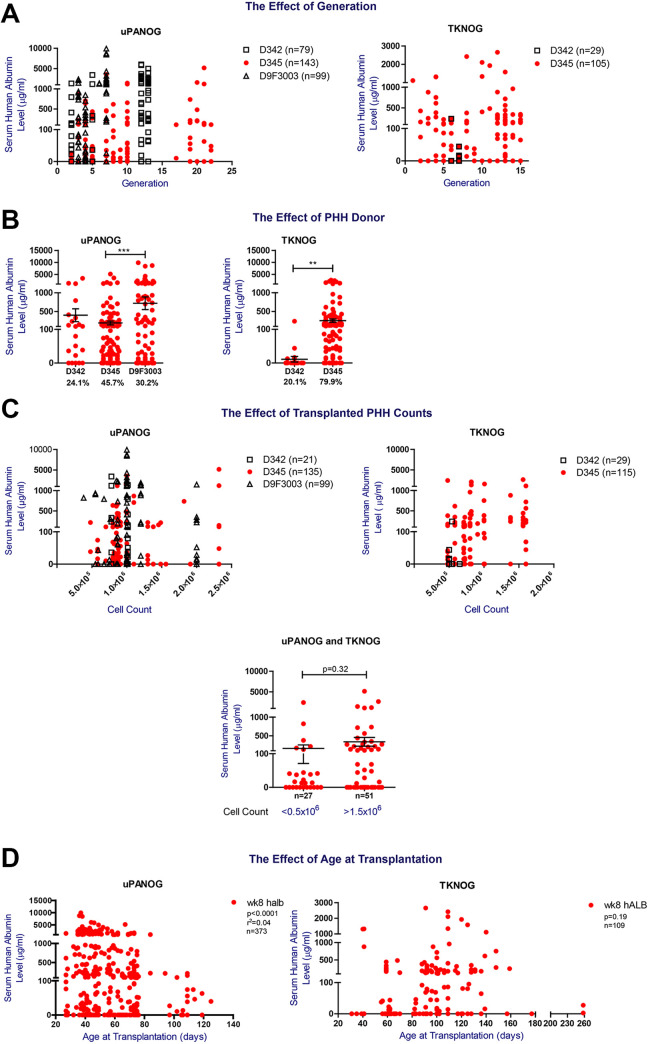
Figure 2.
The effect of generation, PHH donor, transplanted PHH counts and age at transplantation on PHH xenografting success in uPA-NOG and TK-NOG models. (A) The effect of generation on PHH engraftment. Left Panel: week 8 postTx serum hALB levels (µg/mL) of uPA-NOG mice transplanted with 342, D345 and D9F3003. (Right panel) TK-NOG mice transplanted with D342 and D345. (B) The effect of PHH donor on transplantation success. Week 8 postTx serum hALB levels (µg/mL) of uPA-NOG mice (n = 328, left) and TK-NOG mice (n = 144, right) transplanted with PHH donors 342, 345, and 9F3003. (C) The effect of the number of transplanted PHH cells on serum hAlb levels. Week 8 postTx serum hALB levels (µg/mL) of uPA-NOG (n = 263, left) and TK-NOG (n = 170, right) mice transplanted with PHH donors 342, 345, and 9F3003. Cell counts are also split into two categories irrespective of the recipient background and donors: <0.5 × 106/mouse and >1.5 × 106/mouse. Groups are compared using a t-test. (D) The correlation between week 8 serum hALB level (µg/mL) and age at transplantation for 373 uPA-NOG (left) and 109 TK-NOG (right) mice. GraphPad Prism was used for statistical analysis and *P < 0.1, **P < 0.05, ***P < 0.001.
Results
UPA-NOG and TK-NOG Mice with Comparable Serum Human Albumin Levels Show Similar HEV Fecal Shedding, Irrespective of the Mouse Genetic Background.
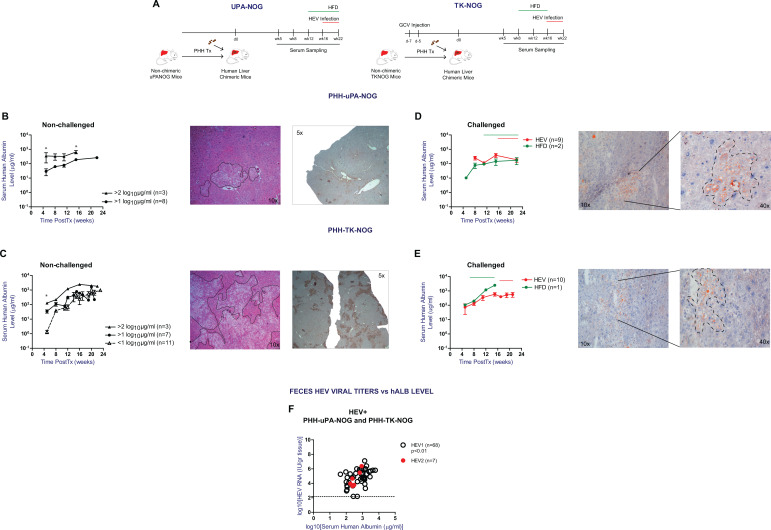
In this large scale comparison of 2 PHH xenograft models, we initially wanted to determine the impact of the model system on HEV infection and fecal shedding, similar to our previous optimization studies for HBV and HCV infections in humanized uPA-SCID mice17. For this purpose, we first compared serum hALB levels, as established marker for PHH engraftment, at different time points after PHH transplantation between both model systems10,17,19. Experimental design and summary of the analyzed samples and PHH recipients is illustrated in Fig. 1A and Supplemental Table S1.
To examine the differences in serum hALB kinetics, PHH transplanted animals were divided into three categories according to serum hALB levels at week 5 postTx: <1 log10 µg/mL, >1 log10 µg/mL, and >2 log10 µg/mL. Serum hALB levels were monitored in unchallenged animals up to 24 weeks postTx. As shown in Fig. 1B, increases in serum hALB levels of PHH transplanted uPA-NOG mice are small over the course of 16 weeks. Compared to animals with early hALB levels >1 log10 µg/mL, those with >2 log10 µg/mL show less pronounced increases in serum hALB levels (mean 9.8-fold vs 2.8-fold, respectively), but at week 16 postTx the latter (initial levels >2 log10 µg/mL) still remain significantly higher than the former (initial levels >1 log10 µg/mL) (P-value < 0.05). PHH transplanted TK-NOG mice show a different serum hALB kinetics pattern (Fig. 1C); overall week 5 post Tx serum hALB levels were lower compared to uPA-NOG animals. However, serum hALB levels in animals with lowest week 5 post Tx levels (<1 log10 µg/mL hALB) progressively increased and reached levels that were not significantly different at week 20–24 post Tx from animals that showed highest week 5 post Tx serum hALB levels (>2 log10 µg/mL hALB). Serum hALB increments of up to 3 log10 µg/mL were observed in these animals, which is significantly higher than the increments observed for uPA-NOG (mean ± SEM hALB change is 175.1 ± 66.35 µg/mL and 708.6 ± 148.6; for uPA-NOG and TK-NOG resp; P < 0.05). These serum hALB increments paralleled true PHH repopulation as shown by representative H&E and hALB stains of liver fragments (Fig. 1B, C). Combined these data indicate that week 5 post Tx serum hALB levels in uPA-NOG mice is higher, but serum hALB increase until week 20–24 post Tx is significantly lower compared to TK-NOG mice.
We next compared serum hALB kinetics under external stress factors, such as a HEV infection or high fat diet (HFD) induced steatosis. Under these conditions, both TK-NOG and uPA-NOG models were shown to keep their characteristic serum hALB kinetics (Fig. 1D, E): Average serum hALB increments upon HEV infections were +114.8 and +161.7 µg/mL in uPA-NOG and TK-NOG respectively. Similarly, a HFD resulted in comparable serum hALB changes of +154.4 and +2272.3 µg/mL (10 weeks of HFD) in uPA-NOG and TK-NOG, respectively, despite histologically confirmed steatosis in the xenograft (Fig. 1D, E).
We previously found that HEV RNA titers in liver humanized uPA-NOG correlated with the degree of liver chimerism21. In addition, we and others identified mouse feces as a straight-forward non-invasive compartment to monitor HEV infections in vivo26–28. To determine the HEV infection efficiency in humanized uPA-NOG and TK-NOG mice, we next examined the association between serum hALB levels and HEV fecal shedding in both xenograft models. As shown in Fig. 1F, week 4 fecal HEV titers positively correlated with serum hALB levels (P < 0.01; Supplemental Table S2A). In a multivariate regression analysis, neither the model background nor the infecting HEV strain were independent predictors for the observed fecal HEV RNA loads (Supplemental Table S2B). Therefore, uPA-NOG and TK-NOG models behave similar in HEV infection studies, once a minimal level of serum human albumin is reached. Only 2 animals out of 50 were found to have HEV fecal shedding with serum hALB levels < 2 log10 µg/mL, which therefore qualifies as an optimal engraftment criterion for HEV infection studies.
Multivariate Analysis Identifies PHH Donor Type and Male Sex to Be the Most Important Parameters for PHH Engraftment
Next, to examine the relative contribution of donor and host characteristics to serum human albumin levels, as established marker of PHH engraftment, we performed a comprehensive analysis of 399 uPA-NOG and 198 TK-NOG PHH transplanted mice. Offspring were backcrossed onto the previous generation or crossed within the same generation to maintain genomic stability29,30. High human serum albumin levels were observed up to generation 22 and 15 in uPA- and TK-NOG respectively, indicating no loss of phenotype with further inbreeding (Fig. 2A; P = 0.36).
Figure 1.
Serum hALB kinetics after PHH transplantation and the correlation with HEV RNA feces shedding. (A) Experimental design and summary of the analyzed samples. (B) Serum hALb levels of non-challenged uPA-NOG mice starting with >1 log10 µg/mL (n = 8) and >2 log10 µg/mL (n = 3) at week 5 postTx resp. Liver H&E and hALB staining of a representative uPA-NOG mouse which had 216 µg/mL and 251 µg/mL serum hALB at week 8 and 18 postTx resp. (C) Serum hALb levels of non-challenged TK-NOG mice starting with <1 log10 µg/mL (n = 11), >1 log10 µg/mL (n = 7) and >2 log10 µg/mL (n = 3) at week 5 postTx resp. Liver H&E and hALB staining of a representative TK-NOG mouse which had >1 log10 µg/mL and 2836.5 µg/mL hALB by week 8 and 16 postTx resp. (D) hALB levels of uPA-NOG mice following HEV infection (n = 9) and high fat diet (HFD) (0.3% cholesterol, n = 2). Liver ORO staining following 10 weeks of HFD. (E) Serum hALB level change of TK-NOG mice following HEV infection (n = 10) and HFD (0.3% cholesterol, n = 1). Liver ORO staining following 10 weeks of HFD. Data shown in B, C, D, and E were obtained from mice transplanted with PHH D345. (F) Correlation analysis between week 4 feces HEV RNA titers and serum human albumin levels of xenografted mice infected with a clinical HEV gt3c strain (HEV1, n = 68) and the prototype HEV gt3a Kernow strain (HEV2, n = 7). Mice transplanted with D342, D345, or D3F3003. Statistical analysis was performed using GraphPad Prism and *P < 0.1.
Previously, the contribution of donor type to engraftment success has been reported by us and others17,20. Details of the PHH donors are provided in Table 1. Analysis of week 8 postTx serum hALB levels of uPA-NOG and TK-NOG mice transplanted with three different PHH donors (Fig. 2B, Table 2), demonstrated significant differences between the donors but also a large variation within different acceptor mice, suggesting that factors other than donor type add to the serum human albumin levels and engraftment success in these mice. As a high number of transplanted cells was identified to contribute to humanization ratios19,31, we next analyzed the effect of the transplanted PHH counts for each PHH donor. Except for higher serum human albumin levels in uPA-NOG animals receiving > 106 donor345 cells, this effect was not observed in TK-NOG mice; nor with donor9F3003 (Supplemental Fig. S1A). Therefore, we regard 0.5–1 × 106 PHH/mouse to be the most optimal transplanted cell dose (Fig. 2C). We additionally examined the mouse age at PHH transplantation. As shown in Fig. 2D, we identified the optimal age at transplantation to be <80 days for uPA-NOG (P < 0.0001). For TK-NOG mice age was not significantly associated with serum human albumin levels (P = 0.19). Overall, in this univariate analysis PHH donor, transplanted cell number and recipient age at transplantation are associated with serum human albumin levels, as marker for PHH engraftment success.
Table 1.
Demographic Data of Cryopreserved Primary Human Hepatocyte (PHH) Donors.
| Lot number | Sex | Age | Race |
|---|---|---|---|
| 342 | Female | 2 years | Caucasian |
| 345 | Female | 7 months | Caucasian |
| 9F3003 | Male | 2 years | Caucasian |
Table 2.
The Distribution of Male and Female Sex in Recipient uPANOG and TKNOG Mice Transplanted with PHH D342, D342, or D9F3003.
| Genetic Background of Mice | Total | ||||||
|---|---|---|---|---|---|---|---|
| uPANOG | TKNOG | ||||||
| PHH Donor | D342 | D345 | D9F3003 | D342 | D345 | ||
| Sex | Female | 4 | 24 | 36 | 9 | 21 | 94 |
| Male | 75 | 126 | 63 | 20 | 94 | 378 | |
| Total | 328 | 144 | 472 | ||||
To further quantify the relative contributions of donor and host characteristics, we performed a multivariate regression analysis (Supplemental Table S3A, S3B). First, we performed a multivariate analysis to study the contributions of recipient sex, age at transplantation, transplanted PHH count, generation and genetic background to predict week 8 postTx serum hALb levels (µg/mL). Interestingly, and confirming our previous data, neither the background of the mouse strain, nor the mouse generation determined serum hALB levels. Recipient sex was found to be the first most significant independent variable (adjusted R 2 0.020, P = 0.003) and age at transplantation the second (adjusted R 2 addition 0.016, P = 0.011) (Supplemental Table S3A, 3B). To be able to include the PHH donor as a variable, we performed dummy data transformation. Donor345 was set as baseline for comparison and we re-performed the multivariate regression analysis (Supplemental Table S3C). Again recipient sex was one of the most significant variables (P < 0.0001), with female recipient sex resulting in an average 442.1 fewer units serum hAlb (expressed in μg/mL) compared to male mice. Donor9F3003 was shown to increase the serum hALB level on average with 683.9 units μg/mL relative to PHH donor345 (P < 0.0001). Other significant independent variables were age at transplantation (P < 0.05, –4.5 units serum hAlb per day). The transplanted PHH count was found to significantly contribute to engraftment, but its effect size was low, suggesting that 1 unit μg/mL serum hAlb increase would require 9.6 × 105 more initially transplanted PHH (P < 0.05). Our data show that male recipient sex and a good PHH donor (in our case PHH Donor9F3003) can result in serum hALB levels that are up to 1000 µg/mL higher.
In summary, both PHH donor type and recipient sex are the most important factors contributing to serum human albumin levels, as marker for PHH engraftment in uPANOG and TKNOG mice.
Sexual Imbalance in PHH Xenografting is Related to Higher Baseline ALT Levels and Sensitivity for Ganciclovir in Males
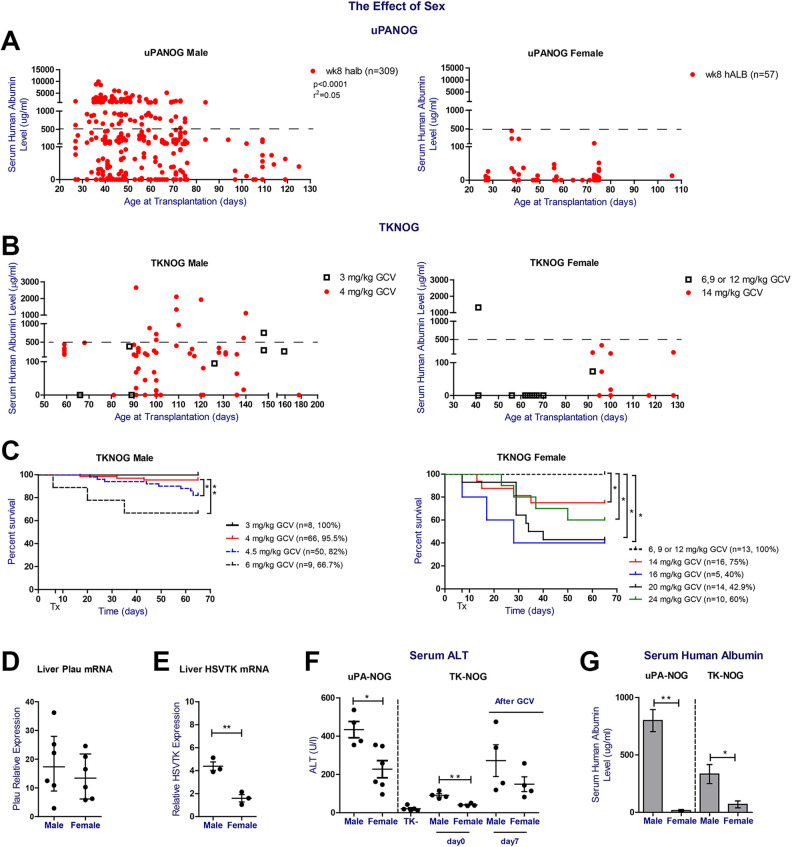
To further examine the effect of recipient sex in xenografting success, we listed serum hALB levels per recipient sex and xenograft model (Fig. 3A, B). Except for 1 young TKNOG female transplanted at 40 days of age (Fig. 3B), maximum serum human albumin levels remained well below 500 μg/mL, irrespective of the applied model (Supplemental Fig. S1B). We recorded no differences in serum human albumin levels in male UPANOG recipients transplanted with either male or female PHH donors, indicating that the observed difference stems from a host gender imbalance (Supplemental Fig. S1C). We also analyzed GCV induced morbidity (Supplemental Fig. S2) and mortality for both male and female TK-NOG mice separately. Again, recipient sex was the major determinant of survival (Fig. 3C). Mortality after GCV reached more than 30% at doses of 6 mg/kg in males, while TK-NOG females tolerated doses up to 12 mg/kg. Increasing the GCV posology in female TK-NOG mice to 14 mg/kg resulted in significantly lower survival rates of 75% and serum hAlb levels <500 μg/mL that remained well below those of males challenged with 4 mg/kg GCV (Fig. 3B, C). Substantial increases in female mortality prohibited the use of even higher GCV doses (Fig. 3C). On the other hand, excellent survival rates of > 95.5% and serum hAlb upto 2650 μg/mL levels were seen in male TK-NOG animals after applying GCV at 3 or 4 mg/kg. (Fig. 3B, C).
Figure 3.
Sexual dimorphism in serum hAlb levels after PHH transplantation, GCV tolerance and liver damage parameters in uPA-NOG and TK-NOG models. (A) The correlation between week 8 serum hALb levels (µg/mL) and age at transplantation (days) for male (left, n = 309) and female (right, n = 59, respectively) uPA-NOG mice. (B) Age (days) and week 8 postTx serum hALB level (µg/mL) correlation of male TK-NOG mice which received 3mg/kg (n = 8) and 4mg/kg GCV (n = 64) 7 and 5 days prior to transplantation (left). Age (days) and week 8 post Tx serum hALB level (µg/mL) correlation of TK-NOG female mice which received 6, 9 or 12mg/kg (n = 13) and 14mg/kg (n = 12) GCV at 7 and 5 days prior to transplantation (right). (C) Survival plots of male (left.) and female (right) TK-NOG mice. Both sexes received different GCV doses i.p. 7 and 5 days prior to transplantation, as indicated in the graph legend. Survival plots show the response of animals until week 8 postTx. Time point of first GCV dose is set at 0. (D) Liver plau relative mRNA expression 40–60 days old non-transplanted uPA-NOG male (n = 6) and female mice (n = 6). (E) Liver HSVTK relative mRNA expression of 130–160 days old male (n = 3) and female (n = 3) non-transplanted TK-NOG mice. Tert gene mRNA level was used to calculate the relative expression and relative expression level was calculated using 2-deltaCt transformation. (F) Serum ALT analysis of 40-60 days old non-transplanted uPA-NOG male (n = 6) and female mice (n = 6) (left) and 130–160 days old male and female non-transplanted TK+ and TK- mice (right). Baseline serum samples of TK-NOG mice were obtained before treatment and then TK+ mice were challenged with GCV at day 0 and 2 and post-challenge serum samples were obtained at day 7 (n = 4 mice/group). (G) The comparison of week 8 postTx serum hALB levels (µg/mL) of uPA-NOG male mice (n = 150) and female mice (n = 25), at age 40–60 days at transplantation (left) and 90–130 days old female (n = 12) and male (n = 48) TK-NOG mice (right). Female TK-NOG mice received 14 mg/GCV and TK-NOG male received 4 mg/kg GCV prior to Tx. Correlation analysis and t test were performed using GraphPad Prism. *P < 0.1, **P < 0.05 and ***P < 0.001.
To understand the sexual imbalance in both models, we examined the relative transgene expression and baseline serum ALT levels of non-transplanted uPA-NOG and TK-NOG mice. While liver Plasminogen activator (Plau) mRNA levels were not significantly different between both uPA-NOG sexes, HSVTK mRNA levels were higher in male compared to female TK-NOG mice (Fig. 3D, E). Nevertheless, serum ALT levels at baseline were significantly higher in males than their female counterparts, irrespective of the examined model. Furthermore, the optimal GCV dose in TKNOG mice (cfr supra) led to higher ALT peaks in males compared to female mice (Fig. 3F). Parallel to higher ALT levels, uPA-NOG and TK-NOG males had significantly higher levels of serum hALB at week 8 postTx (Fig. 3G). In conclusion, despite titrating GCV to highest tolerated doses in females, TK-NOG males remain better PHH acceptors. Therefore, irrespective of the applied xenograft model, recipient males present higher ALT levels, which parallel serum hAlb levels after PHH transplantation.
Discussion
Over the last years, liver humanized mice have been crucial tools in diverse areas of liver disease and liver biology research, including viral hepatitis, drug and lipid metabolism16,17,19,26–28,31–33. Because of initial engraftment variability and high cost, further refinement and standardization of these xenograft models is a highly endorsed research priority5,17,18,33. After a comprehensive head-to-head analysis of almost 600 PHH xenografted mice, we here show that the optimal engraftment requirement for robust fecal HEV shedding to be serum hALB levels > 2 log µg/mL. Once this humanization level is reached, viral shedding is independent of the type of xenograft model. In addition, we demonstrate that not the model background, but the recipient sex and hepatocyte donor are the most important parameters for serum hAlb as marker for PHH engraftment success.
The identification of optimal engraftment criteria for HEV studies is important for further viral infectivity and antiviral efficacy studies, as has been shown for both HBV and HCV infections in liver humanized mice1,2,10,17,19,20,34. Previously, PHH engraftment criteria for HBV and HCV infection studies have been found to differ, with higher humanization ratios and serum hALB levels of 1 mg/mL required for HCV infections, while serum hALB levels of 0.5 mg/mL suffice for HBV propagation in these humanized mice. This might relate to intrinsic viral infectivity differences, but also to the respective preferential viral secretory pathways. Indeed both HBV and HCV are secreted basolaterally, while canalicular secretion is thought to be more important for HAV and HEV. The fact that we study HEV fecal shedding as an easy sampling compartment and not serum viremia, poses additional quality criteria to the PHH graft: not only a minimal humanization ratio, but also optimal communication of human inter-hepatocytic biliary canaliculi with the mouse biliary tractus is required.
Previous studies have examined experimental factors for xenograft success mostly in one particular mouse model. In addition, the relative contribution of sex to PHH engraftment has not been convincingly shown. Indeed, previous studies showed that FAH-deficient females seem more robust than males20, while for HSV-TK mice males seem to be better PHH acceptors12. The sexual imbalance for uPA-transgenic mice is not systematically reported20. Contributing to this lack of knowledge is that initial uPA-transgenic mouse strains have an early postnatal liver dysfunction and therefore short transplantation window before mice reach 3 weeks of age, related to a high integrated copy number of upto 10 uPA-transgenes in tandem array1,2,13. At this early age before weaning, ascertainment of sex is very difficult. The more recent uPA-transgenic strain that we apply, harbor a transgene array of only 3 uPA-copies in their genome, resulting in a lower degree of liver damage and allowing a transplantation after weaning, when sex can be more easily determined9. As we compared two different xenograft models with similar immunodeficient backgrounds we could additionally eliminate variation in residual mouse immune system activities. We found by multivariate regression analysis that not the model system, but male recipient sex and an optimal PHH donor are the most important determinants for serum hAlb levels, as marker for engraftment success. Applying these conditions might lead to a serum hALB level increment of 1000 µg/mL (or 10% more humanization ratio), which would suffice for all types of viral hepatitis experimental studies. Sex dimorphism in disease models is increasingly being recognized and recently included in the National Institutes of Health (NIH) recommendations for experimental study designs35. Our data further supports that this is also essential in liver xenografting studies.
Sexual imbalance in liver diseases are found for drug pharmacokinetics, liver inflammation and oncogenesis36–40 and mostly regulated by growth hormone (GH)-related signaling cascades41,42. The transcription factor STAT5b is one of the key players of GH-related sex biased liver phenotypes43. Other sex related modifications include epigenetic alterations such as Ezh1 and Ezh2 and histone H3K27-trimethylation modification which are involved in liver regeneration mechanisms44–46. In uPA-transgenic mice, endoplasmic reticulum (ER) stress from accumulating plasminogen leads to an unfolded protein response, which results in chronic liver damage9,13,47. A similar mechanism of α-1-Antitrypsin (α-1-AT) accumulation in the ER has been described for α-1-AT deficiency48. Chronic liver disease in this model is almost exclusively seen in male α-1-AT deficient mice, which have a higher hepatocellular proliferation and risk for hepatocellular carcinoma compared to female α-1-AT deficient mice48. Interestingly, this phenotype is reflected in the clinical disease presentation as homozygous ZZ α-1-AT deficient boys require more often a liver transplantation than homozygous ZZ α-1-AT deficient girls. The identified sexual imbalance in xenografted uPA-NOG and TK-NOG mice seems therefore clinically relevant and both models would provide a platform for further studies on mechanisms behind sexual dimorphism in liver diseases.
In conclusion, after conducting this large head-to-head comparison of PHH xenografting in two different models, we here identify the minimal serum hALB requirement for HEV infection studies. Moreover, we demonstrate that both male sex and the PHH donor are the dominant experimental factors related to serum hAlb levels as marker for xenograft success in the systems that we tested. Our data call for sexual standardization in human hepatocyte xenograft models.
Supplemental Material
Supplemental Material, sj-docx-1-cll-10.1177_09636897211006132 for Sexual Dimorphism in Hepatocyte Xenograft Models by Gulce Sari, Gertine W. van Oord, Martijn D.B. van de Garde, Jolanda J.C. Voermans, Andre Boonstra and Thomas Vanwolleghem in Cell Transplantation
Supplemental Material, sj-jpg-1-cll-10.1177_09636897211006132 for Sexual Dimorphism in Hepatocyte Xenograft Models by Gulce Sari, Gertine W. van Oord, Martijn D.B. van de Garde, Jolanda J.C. Voermans, Andre Boonstra and Thomas Vanwolleghem in Cell Transplantation
Supplemental Material, sj-jpg-2-cll-10.1177_09636897211006132 for Sexual Dimorphism in Hepatocyte Xenograft Models by Gulce Sari, Gertine W. van Oord, Martijn D.B. van de Garde, Jolanda J.C. Voermans, Andre Boonstra and Thomas Vanwolleghem in Cell Transplantation
Acknowledgments
The authors thank Dr Zongdi Feng (Nationswide Children Hospital, Columbus, Ohio) for providing the HEV Kernow strain; Claudia E. Mulders (Erasmus University Medical Center, Department of Viroscience, Rotterdam, Netherlands) and Anthonie Z.M.A. Groothuismink (Erasmus University Medical Center, Department of Gastroenterology and Hepatology, Rotterdam, Netherlands) for excellent technical assistance in HEV studies and microscopy imaging. Authors also thank Juan Rodríguez-Coira Villanueva who was involved in the initial parts of the study. TV is a Senior Clinical Investigator of the Research Foundation—Flanders (Belgium) (FWO).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: This study was approved by Central Animal Facility of the Erasmus Medical Center, Rotterdam, Netherlands (DEC nr 141-12-11).
Statement of Animal and Human Rights: This article does not contain any studies with human subjects.
Statement of Informed Consent: In the current study, informed consent is not applicable.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Gulce Sari  https://orcid.org/0000-0002-8585-5889
https://orcid.org/0000-0002-8585-5889
Thomas Vanwolleghem  https://orcid.org/0000-0002-0572-8741
https://orcid.org/0000-0002-0572-8741
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, et al. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7(8):927–933. [DOI] [PubMed] [Google Scholar]
- 2. Dandri M, Burda MR, Torok E, Pollok JM, Iwanska A, Sommer G, Rogiers X, Rogler CE, Gupta S, Will H, Greten H, et al. Repopulation of mouse liver with human hepatocytes and in vivo infection with hepatitis b virus. Hepatology. 2001;33(4):981–988. [DOI] [PubMed] [Google Scholar]
- 3. Bissig KD, Han W, Barzi M, Kovalchuk N, Ding L, Fan X, Pankowicz FP, Zhang QY, Ding X. P450-Humanized and human liver chimeric mouse models for studying xenobiotic metabolism and toxicity. Drug Metab Dispos. 2018;46(11):1734–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foquet L, Wilson EM, Verhoye L, Grompe M, Leroux-Roels G, Bial J, Meuleman P. Successful engraftment of human hepatocytes in uPA-SCID and FRG<ovid: sup> R</ovid: sup> KO Mice. Methods Mol Biol. 2017;1506:117–130. [DOI] [PubMed] [Google Scholar]
- 5. Grompe M, Strom S. Mice with human livers. Gastroenterology. 2013;145(6):1209–1214. [DOI] [PubMed] [Google Scholar]
- 6. Fox IJ, Roy-Chowdhury J. Hepatocyte transplantation. J Hepatol. 2004;40(6):878–886. [DOI] [PubMed] [Google Scholar]
- 7. Shafritz DA, Oertel M. Model systems and experimental conditions that lead to effective repopulation of the liver by transplanted cells. Int J Biochem Cell Biol. 2011;43(2):198–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heckel JL, Sandgren EP, Degen JL, Palmiter RD, Brinster RL. Neonatal bleeding in transgenic mice expressing urokinase-type plasminogen activator. Cell. 1990;62(3):447–456. [DOI] [PubMed] [Google Scholar]
- 9. Suemizu H, Hasegawa M, Kawai K, Taniguchi K, Monnai M, Wakui M, Suematsu M, Ito M, Peltz G, Nakamura M. Establishment of a humanized model of liver using NOD/Shi-scid IL2Rgnull mice. Biochem Biophys Res Commun. 2008;377(1):248–252. [DOI] [PubMed] [Google Scholar]
- 10. Azuma H, Paulk N, Ranade A, Dorrell C, Al-Dhalimy M, Ellis E, Strom S, Kay MA, Finegold M, Grompe M. Robust expansion of human hepatocytes in Fah-/-/Rag2-/-/Il2rg-/- mice. Nat Biotechnol. 2007;25(8):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Camus MC, Chapman MJ, Forgez P, Laplaud PM. Distribution and characterization of the serum lipoproteins and apoproteins in the mouse, Mus musculus. J Lipid Res. 1983;24(9):1210–1228. [PubMed] [Google Scholar]
- 12. Hasegawa M, Kawai K, Mitsui T, Taniguchi K, Monnai M, Wakui M, Ito M, Suematsu M, Peltz G, Nakamura M, Suemizu H. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem Biophys Res Commun 2011;405(3):405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meuleman P, Libbrecht L, De Vos R, de Hemptinne B, Gevaert K, Vandekerckhove J, Roskams T, Leroux-Roels G. Morphological and biochemical characterization of a human liver in a uPA-SCID mouse chimera. Hepatology. 2005;41(4):847–856. [DOI] [PubMed] [Google Scholar]
- 14. Tateno C, Yoshizane Y, Saito N, Kataoka M, Utoh R, Yamasaki C, Tachibana A, Soeno Y, Asahina K, Hino H, Asahara T, et al. Near completely humanized liver in mice shows human-type metabolic responses to drugs. Am J Pathol. 2004;165(3):901–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–191. [PubMed] [Google Scholar]
- 16. Sari G, Meester EJ, van der Zee LC, Wouters K, van Lennep JR, Peppelenbosch M, Boonstra A, Van der Heiden K, Mulder MMT, Vanwolleghem T. A mouse model of humanized liver shows a human-like lipid profile, but does not form atherosclerotic plaque after western type diet. Biochem Biophys Res Commun. 2020;524(2):510–515. [DOI] [PubMed] [Google Scholar]
- 17. Vanwolleghem T, Libbrecht L, Hansen BE, Desombere I, Roskams T, Meuleman P, Leroux-Roels G. Factors determining successful engraftment of hepatocytes and susceptibility to hepatitis B and C virus infection in uPA-SCID mice. J Hepatol. 2010;53(3):468–476. [DOI] [PubMed] [Google Scholar]
- 18. Stripecke R, Munz C, Schuringa JJ, Bissig KD, Soper B, Meeham T, Yao LC, Di Santo JP, Brehm M, Rodriguez E, Wege AK, et al. Innovations, challenges, and minimal information for standardization of humanized mice. EMBO Mol Med. 2020;12(7):e8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bissig KD, Wieland SF, Tran P, Isogawa M, Le TT, Chisari FV, Verma IM. Human liver chimeric mice provide a model for hepatitis B and C virus infection and treatment. J Clin Invest. 2010;120(3):924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Michailidis E, Vercauteren K, Mancio-Silva L, Andrus L, Jahan C, Ricardo-Lax I, Zou C, Kabbani M, Park P, Quirk C, Pyrgaki C, et al. Expansion, in vivo-ex vivo cycling, and genetic manipulation of primary human hepatocytes. Proc Natl Acad Sci U S A. 2020;117(3):1678–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. van de Garde MD, Pas SD, van der Net G, de Man RA, Osterhaus AD, Haagmans BL, Boonstra A, Vanwolleghem T. Hepatitis e virus (HEV) genotype 3 infection of human liver chimeric mice as a model for chronic HEV infection. J Virol. 2016;90(9):4394–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kawahara T, Toso C, Douglas DN, Nourbakhsh M, Lewis JT, Tyrrell DL, Lund GA, Churchill TA, Kneteman NM. Factors affecting hepatocyte isolation, engraftment, and replication in an in vivo model. Liver Transplantation. 2010;16(8):974–982. [DOI] [PubMed] [Google Scholar]
- 23. Tolosa L, Pareja-Ibars E, Donato MT, Cortés M, López S, Jiménez N, Mir J, Castell JV, Gómez-Lechón MJ. Neonatal livers: a source for the isolation of good-performing hepatocytes for cell transplantation. Cell Transplantation. 2014;23(10):1229–1242. [DOI] [PubMed] [Google Scholar]
- 24. Pas SD, de Man RA, Mulders C, Balk AH, van Hal PT, Weimar W, Koopmans MP, Osterhaus AD, van der Eijk AA. Hepatitis e virus infection among solid organ transplant recipients, the Netherlands. Emerg Infect Dis. 2012;18(5):869–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc. 2013;8(6):1149–1154. [DOI] [PubMed] [Google Scholar]
- 26. Allweiss L, Gass S, Giersch K, Groth A, Kah J, Volz T, Rapp G, Schobel A, Lohse AW, Polywka S, Pischke S, et al. Human liver chimeric mice as a new model of chronic hepatitis e virus infection and preclinical drug evaluation. J Hepatol. 2016;64(5):1033–1040. [DOI] [PubMed] [Google Scholar]
- 27. Sayed IM, Verhoye L, Cocquerel L, Abravanel F, Foquet L, Montpellier C, Debing Y, Farhoudi A, Wychowski C, Dubuisson J, Leroux-Roels G, et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut. 2017;66(5):920–929. [DOI] [PubMed] [Google Scholar]
- 28. van de Garde MDB, Pas SD, van Oord GW, Gama L, Choi Y, de Man RA, Boonstra A, Vanwolleghem T. Interferon-alpha treatment rapidly clears Hepatitis E virus infection in humanized mice. Sci Rep. 2017;7(1):8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. van Zutphen LF, van der Valk JB. Developments on the implementation of the Three Rs in research and education. Toxicol In Vitro. 2001;15(4-5):591–595. [DOI] [PubMed] [Google Scholar]
- 30. van Zutphen LF. Focus on animal welfare. Comp Med. 2001;51(2):110–111. [PubMed] [Google Scholar]
- 31. Burm R, Collignon L, Mesalam AA, Meuleman P. Animal Models to Study Hepatitis C Virus Infection. Front Immunol. 2018;9:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sari G, van de Garde MDB, van Schoonhoven A, Voermans JJC, van der Eijk AA, de Man RA, Boonstra A, Vanwolleghem T, Pas SD. hepatitis e virus shows more genomic alterations in cell culture than in vivo. Pathogens. 2019;8(4):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Katoh M, Tateno C, Yoshizato K, Yokoi T. Chimeric mice with humanized liver. Toxicology. 2008;246(1):9–17. [DOI] [PubMed] [Google Scholar]
- 34. Lindenbach BD, Meuleman P, Ploss A, Vanwolleghem T, Syder AJ, McKeating JA, Lanford RE, Feinstone SM, Major ME, Leroux-Roels G, Rice CM. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc Natl Acad Sci U S A. 2006;103(10):3805–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature. 2014;509(7500):282–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Buzzetti E, Parikh PM, Gerussi A, Tsochatzis E. Gender differences in liver disease and the drug-dose gender gap. Pharmacol Res. 2017;120:97–108. [DOI] [PubMed] [Google Scholar]
- 37. Marcos R, Correia-Gomes C, Miranda H, Carneiro F. Liver gender dimorphism--insights from quantitative morphology. Histol Histopathol. 2015;30(12):1431–1437. [DOI] [PubMed] [Google Scholar]
- 38. Hanna D, Riedmaier AE, Sugamori KS, Grant DM. Influence of sex and developmental stage on acute hepatotoxic and inflammatory responses to liver procarcinogens in the mouse. Toxicology. 2016;373:30–40. [DOI] [PubMed] [Google Scholar]
- 39. Clocchiatti A, Cora E, Zhang Y, Dotto GP. Sexual dimorphism in cancer. Nat Rev Cancer. 2016;16(5):330–339. [DOI] [PubMed] [Google Scholar]
- 40. Lonardo A, Nascimbeni F, Ballestri S, Fairweather D, Win S, Than TA, Abdelmalek MF, Suzuki A. Sex differences in nonalcoholic fatty liver disease: state of the art and identification of research gaps. Hepatology. 2019;70(4):1457–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Waxman DJ, Holloway MG. Sex differences in the expression of hepatic drug metabolizing enzymes. Mol Pharmacol. 2009;76(2):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lichanska AM, Waters MJ. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet. 2008;24(1):41–47. [DOI] [PubMed] [Google Scholar]
- 43. Clodfelter KH, Holloway MG, Hodor P, Park SH, Ray WJ, Waxman DJ. Sex-dependent liver gene expression is extensive and largely dependent upon signal transducer and activator of transcription 5b (STAT5b): STAT5b-dependent activation of male genes and repression of female genes revealed by microarray analysis. Mole Endocrinol. 2006;20(6):1333–1351. [DOI] [PubMed] [Google Scholar]
- 44. Sugathan A, Waxman DJ. Genome-wide analysis of chromatin states reveals distinct mechanisms of sex-dependent gene regulation in male and female mouse liver. Mol Cell Biol. 2013;33(18):3594–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lau-Corona D, Bae WK, Hennighausen L, Waxman DJ. Sex-biased genetic programs in liver metabolism and liver fibrosis are controlled by EZH1 and EZH2. PLoS Genet 2020;16(5):e1008796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bae WK, Kang K, Yu JH, Yoo KH, Factor VM, Kaji K, Matter M, Thorgeirsson S, Hennighausen L. The methyltransferases enhancer of zeste homolog (EZH) 1 and EZH2 control hepatocyte homeostasis and regeneration. FASEB J. 2015;29(5):1653–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Fomin ME, Beyer AI, Publicover J, Lu K, Bakkour S, Simmons G, Muench MO. Higher serum alanine transaminase levels in male urokinase-type plasminogen activator-transgenic mice are associated with improved engraftment of hepatocytes but not liver sinusoidal endothelial cells. Cell Med. 2017;9(3):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rudnick DA, Liao Y, An JK, Muglia LJ, Perlmutter DH, Teckman JH. Analyses of hepatocellular proliferation in a mouse model of alpha-1-antitrypsin deficiency. Hepatology. 2004;39(4):1048–1055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-cll-10.1177_09636897211006132 for Sexual Dimorphism in Hepatocyte Xenograft Models by Gulce Sari, Gertine W. van Oord, Martijn D.B. van de Garde, Jolanda J.C. Voermans, Andre Boonstra and Thomas Vanwolleghem in Cell Transplantation
Supplemental Material, sj-jpg-1-cll-10.1177_09636897211006132 for Sexual Dimorphism in Hepatocyte Xenograft Models by Gulce Sari, Gertine W. van Oord, Martijn D.B. van de Garde, Jolanda J.C. Voermans, Andre Boonstra and Thomas Vanwolleghem in Cell Transplantation
Supplemental Material, sj-jpg-2-cll-10.1177_09636897211006132 for Sexual Dimorphism in Hepatocyte Xenograft Models by Gulce Sari, Gertine W. van Oord, Martijn D.B. van de Garde, Jolanda J.C. Voermans, Andre Boonstra and Thomas Vanwolleghem in Cell Transplantation