Abstract
Multiple sclerosis (MS) is an autoimmune inflammatory disorder of the central nervous system (CNS) resulting in demyelination and axonal loss in the brain and spinal cord. The precise pathogenesis and etiology of this complex disease are still a mystery. Despite many studies that have been aimed to identify biomarkers, no protein marker has yet been approved for MS. There is urgently needed for biomarkers, which could clarify pathology, monitor disease progression, response to treatment, and prognosis in MS. Proteomics and metabolomics analysis are powerful tools to identify putative and novel candidate biomarkers. Different human compartments analysis using proteomics, metabolomics, and bioinformatics approaches has generated new information for further clarification of MS pathology, elucidating the mechanisms of the disease, finding new targets, and monitoring treatment response. Overall, omics approaches can develop different therapeutic and diagnostic aspects of complex disorders such as multiple sclerosis, from biomarker discovery to personalized medicine.
Keywords: Biomarker, proteomics, metabolomics, bioinformatics, multiple sclerosis, personalized medicine
Introduction
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system (CNS) that is considered the most common cause of non-traumatic neurological disability in young women.1 It is estimated that 2 million people between ages 17 and 65 will suffer from MS worldwide, which shows a remarkable increase in its prevalence over time.2
MS has 4 main pathological characteristics: First, the immunological changes in MS are considered by increasing proinflammatory cell infiltration, consisting of CD4+ T cells with the T helper 1 (Th1)/Th17 phenotypes, B cells, monocytes, macrophages, natural killer (NK) cells. There is also decreases in CD8+ T cells, CD4+ CD25+ forkhead box P3 (FoxP3+) Treg cells, and impaired Treg function3; second, the inflammatory process destroys myelin sheath or the oligodendrocyte cell body, which leads to demyelination; third, inflammation also causes axonal damage and loss; fourth, astrocytic response to inflammation-induced neural injury so-called “gliosis.”4
The first case report of MS back in 1421, but serious studies of this demyelinating disease began in the 19th century.5 Considering inflammation’s role in MS pathogenesis, scientists have used experimental autoimmune encephalomyelitis (EAE) animal models to reproduce many of the clinical and immunological aspects of MS.4 In the 1970s, scientists studying EAE in animals speculated that some myelin protein fragments could prevent the disease. Therefore, they synthesized a mix of protein fragments and used it for treating animals with EAE and humans with MS.6
Diagnosis of MS currently relies on the McDonald criteria, which combines clinical evidence supported by paraclinical investigations, including magnetic resonance imaging (MRI) to visualize typical lesions and identification of the oligoclonal band (s) of IgG in cerebrospinal fluid (CSF). The initial step in MS diagnosis is to find clinically isolated syndrome (CIS) or radiologically isolated syndrome (RIS). Consequently, the final diagnosis of MS depends on evidence of lesions dissemination in the CNS, as well as the exclusion of other potential neurological disorders.7
From 1996, the US National Multiple Sclerosis Society (NMSS) Advisory Committee on Clinical Trials in Multiple Sclerosis has classified MS according to its clinical course into 4 categories: Relapsing-Remitting (RR), Progressive-Relapsing (PR), Primary-Progressive (PP), and Secondary-Progressive (SP).8 These 4 categories handle the course that MS will follow during a person’s lifetime. Within each of these 4 types of MS, there are varying degrees of severity. About 80% to 85% of patients take the RR subtype; new symptoms can appear during relapses, and old ones recur or worsen.9 Relapses are followed by a full or partial recovery period, marked by a lack of disease progression. Most of these patients progress into an SP phase, while 10% to 15% of MS cases follow a PP course that patients accumulate a progressive disability from the onset.10
Clinicians mainly take subjective and objective approaches for diagnosing and staging MS. However, MRI, clinical manifestations, and other assessments cannot specifically and accurately define the activity, progression, responses to treatments, and MS recurrence. In order to gain better insight into MS behavior, several different approaches have been used to investigate the different biological processes and affected biomarkers during MS. Based on the National Institute of Health definition, biomarkers are described as “. . .characteristic that is objectively measured and evaluated as an indicator of a normal biological process, pathogenic process, or pharmacologic responses to a therapeutic intervention.”11
Conventionally, scientists use 2 techniques to identify appropriate biomarkers: hypothesis-based and discovery-based methods. The hypothesis-based approach focuses on understanding disease mechanisms. The discovery-based biomarker method attempts to detect a change in concentration of the specific molecules such as metabolites and proteins.12 The complicated mechanisms and etiopathology of MS limit identifying suitable biomarkers for diagnosis and prognosis prediction. However, by appearing new approaches including genomics, transcriptomics, epigenetics, proteomics, and metabolomics, which provide high throughput of information about the pathogenesis of diseases, scientists are approaching the achievement of the new and appropriate biomarker.13,14 Finding a new biomarker is a multi-step process that includes the primary discovery phase, preclinical validation, and clinical validation. In the initial step, large omics investigations are performed to mine promising candidate molecules. The possible biomarkers are then selected for preclinical and clinical evaluations.15 Omics investigations provide large quantitative datasets surrounding abundant molecules in each subtype or stage of MS, potentially correlates with the disease susceptibility, severity, and pathogenesis. Scientists can explore these datasets to identify novel and efficient biomarkers for MS.
Despite these advances, the diagnostic and prognostic biomarkers are challenged due to the complexity of MS.1 There is a major unmet need for specific and reliable biomarkers that help better understand molecular mechanisms and the etiopathogenesis of MS. These biomarkers could be used in all patient care stages to diagnose MS, predict treatment response outcomes, avoid unnecessary research, and guide further management.12 This manuscript will focus on proteomics and metabolomics’ potential role in MS, from biomarker discovery to personalized medicine.
Proteomics and Metabolomics Workflow
Over the past 2 decades, “omics” approaches have emerged as a promising tool for recognizing molecular pathways, just as distinguishing differentially expressed molecules, independent of several causative agents of disease. Therefore, proteomics may be utilized for biomarker discovery, potential drug targets, and novel adjusting mechanisms.16 Up to date, an impressive number of proteomic and metabolomic studies have been performed on laboratory model samples and patients with MS, even though all therapeutic methodologies are essentially founded on the inflammatory aspect of the disease.17 Moreover, there have been increasing efforts to characterize the connection between genome and phenotype in cells and organisms. It has been revealed that even a complete comprehension of the genome’s status and proteome in a living system does not reflect its phenotype. Subsequently, in addition to the proteome, metabolome study is essential; thus, metabolomics is presently used as an independent and widespread approach, as well as proteomic-supplementing research.18
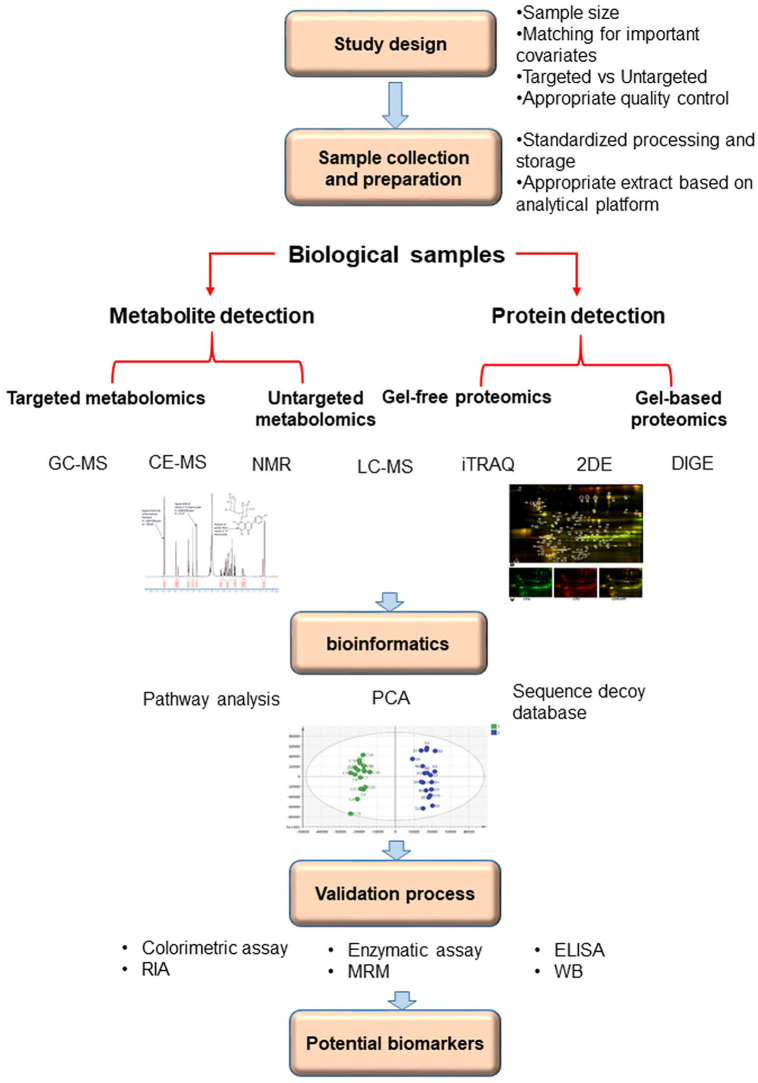
Identifying the most reliable biomarker arises from evaluating the “normal” state compared to the “diseased” state of the same subjects. The initial step in the workflow of most proteomics and metabolomics experiments is sample description and study design. An ideal sample for biomarker evaluation should possess important features, including non-invasive collection, acceptable sensitivity that allows early detection of disease with minor false-negative rate, high specificity that ensures the desired pathology, and rapid response to treatments. The selected sample type is also important, mainly depending on the available analysis methods and the disease in question.19 Appropriate study design necessitates planning a study question, hypothesis, appropriate selection of test and control groups, and conducting a study in an unbiased manner, resulting in proper conclusions. The results may support or refuse the proposed hypothesis. The second step is sample analysis that provides information on all molecular identities and quantities in the selected samples. Identification of proteins and metabolites requires analysis platforms including gas chromatography coupled to mass spectrometry (GC-MS), liquid chromatography coupled with single-stage mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR), isobaric tags for relative abundance and quantitation (iTRAQ), 2-dimensional gel electrophoresis (2DE), and difference gel electrophoresis (DIGE).20 As the third step, bioinformatics helps select protein or metabolite biomarker candidates by using complicated statistical methods at the discovery and validation phases.16 Utilizing standard workflow in omics studies design would improve the quality of results and find more appropriate biomarkers. The workflow of most proteomics and metabolomics experiments shown in Figure 1. It is important to realize that the discovery process might be different and can follow various routes that choice depends on the study question, as well as technologies available and expense. For instance, selecting an analysis platform or sample type depends on the main question and direction of the study.21,22
Figure 1.
Workflow for biomarkers discovery process for multiple sclerosis through proteomics and metabolomics approaches.
Proteomics Approach in Multiple Sclerosis
In recent years, the widespread growth of mass spectrometry techniques toward greater accuracy, resolution, sensitivity, and high throughput methods have driven noteworthy advances in the proteomics field.23 These developments lead to significant advances in proteomics processes, including sample collection and preparation, strategies of fractionation, instrumentation, bioinformatics analysis, and validation studies.24 New insights into molecular mechanisms of the pathophysiology of MS might be achieved by a proteomic approach that could be integrated and translated into medical neurology. These methods have advantages over conventional genomic/transcriptomic approaches because they can detect post-translational modifications such as phosphorylation and glycosylation associated with the pathogenesis of MS.14
Several proteomic studies have been released in the MS disease field that mainly focus on individual proteins for their biomarkers’ potential role.25 Mass spectrometry of proteins necessitates an ionized form of soluble or solid proteins in the gas phase before injection and acceleration in an analytic electric or magnetic field. The 2 approaches for ionizing proteins are electrospray ionization (ESI) and matrix-assisted laser desorption/ionization (MALDI). ESI creates the ions from proteins in a soluble state that permits intact ionization of fragile molecules; however, MALDI produces ionized proteins in a solid form by pulses of laser light. ESI application results in higher multiply-charged ions than MALDI, allowing higher mass protein analysis and better fragmentation, whereas MALDI is more rapid.26 The 2 main mass spectrometry approaches to identify and characterize ionized proteins include bottom-up or shotgun, which analyzes peptides by enzymatic digestion, and top-down approaches, which describe intact proteins.27,28 Based on the prerequisites of each method, bottom-up and top-down approaches have different advantages and disadvantages. The bottom-up approach can analyze high-complex samples and provide more sensitive results. On the other hand, the top-down analysis provides high sequence coverage, valuable information about post-translational modifications, and improved quantification compared to the bottom-up method.29 Many studies used 2-dimensional polyacrylamide gel electrophoresis (2D-PAGE) as the study’s central methodology.30 This method has been the base of proteomics from the launch, but to compare large-scale proteomics, 2DE has gradually been switched by shotgun proteomics techniques.31 Such approaches tend to be more sensitive, reproducible, and automated. Depending on the available and necessary technologies, the discovery method may be somewhat different and may follow different directions. These studies have indicated an excessive variety of biomarker candidates, probably due to methodology variations and MS heterogeneity.32 Different proteomic approaches in EAE animal models and biological samples such as CNS tissues, CFS, and blood of MS patients have been used to identify biomarkers and causative agents in the disease’s pathophysiology.25,33 EAE is an acceptable means for studying brain inflammation, CNS tissue injury, and potential drug responses in MS. Considering several common immunological features between MS and EAE, utilizing the EAE animal model can help suggest novel biomarkers for MS diagnosis, staging, and evaluating drug response.4,33 Several proteomic studies for identifying the panel of biomarkers for MS are summarized in Table 1.
Table 1.
Biofluid/tissue proteomic studies in MS.
| Studies | Biofluid/tissue | Method | Main results | Ref. |
|---|---|---|---|---|
| Early MS markers | CSF | 2D-LC-MS/MS | CSF proteins in first-attack patients were differentially enriched for gray matter components | Schutzer et al34 |
| Diagnostic autoantibody peptide markers | CSF | Label-free LC-MS/MS | Five complementarity-determining regions specific to MS | Singh et al35 |
| MS clinical courses | CSF | MALDI-TOF | Secretogranin II, Protein 7B2 upregulated in RRMS | Liguori et al36 |
| Diagnosis, prognosis, and disease course | CSF | iTRAQ, SRM | Secretogranin-1 to be increased in early-MS patients compared to RRMS and neurological controls | Kroksveen et al86 |
| Diagnostic and prognostic biomarkers MS | CSF | TMT-LC-MS/MS, ELISA | CHI3L1, CHI3L2 | Hinsinger et al89 |
| Prognostic MS markers | CSF | Label-free LC-MS/MS | Increased abundance of CNS gray matter-related proteins | Singh et al37 |
| Predicting clinical relapse of MS | Serum | MALDI-TOF, immunoblotting, and real-time PCR | Autophagy-related gene16L2 | Yin et al38 |
| Biomarkers of disease progression | Serum | iTRAQ-MALDI-TOF/TOF | A panel of 11 proteins has been identified. These proteins related to inflammation, opsonization, and complement activation | Tremlett et al39 |
| Diagnostic and prognostic markers | Serum | 2D-MALDI TOF, Redox proteomics | DBP, Apo A4 levels raised with the disease progression | Wallin et al40 |
| Marker for characterization of a patient with MS | Plasma | Protein microarrays, immunofluorescence | ANO2 (Anoctamin 2) | Beyoglu et al41 |
| Protein biomarkers of brain atrophy in SPMS | Serum | SELDI-TOF, ELISA | Serum-free hemoglobin | Lewin et al42 |
| Biomarker for SPMS | Serum | 2D-LC-MS/MS, Western blot | Galectin-3 | Nishihara et al43 |
| Pregnancy-related MS markers | Urine | Label-free LC-MS/MS | Pregnancy-related peptides were significantly elevated in MS compared with controls. | Singh et al99 |
| Biomarkers distinguishing SPMS from RRMS | Urine | Immunoassays and ELISA | Galectin-9, monocyte chemoattractant protein-1 (MCP-1), transforming growth factor alpha (TGF-α), tumor necrosis factor alpha (TNF-α), soluble CD40L (sCD40L) and platelet-derived growth factor AA (PDGF-AA) | Herman et al44 |
| Neuroprotection | Brain | LC-MS/MS, neuronal cultures, IHC | Hemoglobin β subunit (Hbb) interacting proteins | Brown et al45 |
| Remyelination promoting proteins | Brain | SDS-PAGE nLC-MS/MS LCM, in vitro/in vivo research | EphrinB3 | Syed et al105 |
| Pathogenesis | Brain | SDS-PAGE nLC-MS/MS peptide microarrays, NGS | Mutated forms of proteolipid protein 1, mutant-specific immune response | Qendro et al46 |
| Pathogenesis and remyelination markers | Brain | H&E, MALDI-IMS, LC-MS/MS, IHC | Thymosin β-4 in remyelinated lesions | Maccarrone et al47 |
| Early MS markers | Salvia | HPLC-ESI-MS, top-down proteomics | The lower level of mono- and di-oxidized cystatin SN, mono- and di-oxidized cystatin S1, monooxidized cystatin SA and mono-phosphorylated statherin. And upper levels of antileukoproteinase, 2 proteoforms of Prolactin-Inducible Protein, P-C peptide (Fr. 1-14, Fr. 26-44, and Fr. 36-44), Statherin SV1, Cystatin SN (P→L), Cystatin A (T96→M) in MS patients | Manconi et al97 |
| Diagnosis of MS | Tear | Iso-electrophoresis (IEF), | oligoclonal bands of IgG | Hagan et al48 |
| Discovery of new biomarkers | Tear | MS/MS, western blot (WB) | α-1-antichymotrypsin | Salvisberg et al102 |
Plasma and CSF
Plasma and CSF contain valuable proteins that may vary in different conditions. These biological fluids share a wide spectrum of molecules and protein composition due to multiple connections. Changes in CSF can indicate neurological inflammation, metabolic damage, BBB dysfunction, or brain tissue damage that could help predict MS progression.49 Considering easy access to the plasma and CSF, clinicians could benefit from utilizing these body fluid types to evaluate the patient with MS before the onset of symptoms and the possibility of long-term follow-up during the MS disease course.
Immune-related proteins exist in variable body parts and participate in innate and adaptive immune responses in different ways. The immune-related protein, including complement chemokines, and cytokines are the proposed candidates as biomarkers for MS. However, many of these immune-related protein participates in the many inflammatory processes. Therefore, it seems that these proteins could not be considered as specific biomarkers for MS.30 Bottom-up proteomics evaluation on CSF of the EAE model showed significant elevation of complements such as Lysozyme C1 and C3 proteins.50 These proteins enhance opsonization and phagocytosis or create a cellular transmembrane channel that results in invasive cell death.51 The higher concentration of CXCL10 and CXCL13 in the CSF of MS patients than the control group indicated the involvement of these chemokines in immunopathogenesis mechanisms in MS.52,53 Considering the myelin sheath of CNS (gray and white matter) as the primary target in MS, the release of proinflammatory cytokines such as interleukin 17 (IL-17), IL-4, IL-10, IL-12, IL-23, TNF-α, and activation of macrophages and microglia damage the myelin sheath of neurons through secretion of toxic substances such as reactive oxygen species (ROS), reactive nitrogen species (RNS), and glutamate.54-56 Prostaglandins (PGs) are mediators of acute and chronic inflammation via intensification of cytokine signalings.57 One study has discovered the increased presence of prostaglandin-H2 D employing 2-DE MS/MS in MS patients’ CSF.58 PGD2 prompted apoptosis of mouse oligodendrocyte precursor cells, which could meddle in the demyelination cycle that happens in multiple sclerosis.59
Heat shock proteins (HSPs) are a group of cell protector agents that appear when cells are exposed to environmental stress. These proteins also contribute to protein folding, cell cycle regulation, and intracellular signaling.60 A proteomics study on peripheral blood mononuclear cells (PBMCs) protein profile by employing the 2-DE method coupled to MALDI indicated that HSP β-1 differentially was expressed in correlation with MS patients’ MRI findings.61 Altered Alpha-crystallin B chain (CRYAB) expression, the small heat shock protein, has been implicated in many neurological diseases like MS.62 CRYAB expression in astrocytes and oligodendrocytes has been displayed to protect these cells from apoptosis via binding to cell death mediators and inhibiting caspase activity.63,64 Additionally, CRYAB prevents NF-κB and STAT3 signaling, resulting in reduced neuroinflammation.65
Neurodegeneration-related proteins play an important role in the pathogenesis of MS. Proteomics studies suggested variable neurodegeneration-related proteins, including neurofilament, proteins with enzymatic properties, and glial fibrillary acidic protein (GFAP) as biomarkers for MS. Studies attribute long-term clinical outcomes of MS to the level of neurofilament.66 Two important types of neurofilament participate in MS pathogenesis: neurofilament light (NFL) and neurofilament heavy (NFH).67 A few investigations likewise uphold a prognostic part for the light chain subunit of NFL in the beginning stages of MS.68 In a longitudinal report, CSF NF-L levels were prescient of conversion to MS in CIS patients.69 Another proteomics study revealed that NFL level is elevated in patients with MS compared to healthy controls.70
Matrix metalloproteinases (MMPs), a group of enzymes, participate in the degradation of extracellular matrix (ECM) during some physiological processes, including angiogenesis.71 However, some studies shed light on the immunopathologic role of MMPs in MS.72 Studies on the serum, CSF, and brain tissue of MS patients have revealed an increase in MMPs activity such as MMP-1, -2, -3, -7, -9, -12, and -14.73 Some information proposes that microglial-derived MMPs may mediate the turnover of the ECM of CNS under typical conditions in microglial nodules; however, in many neuroinflammatory conditions such as brain tumors, encephalitis, meningitis, cerebral ischemia, and MS, these enzymes are significantly upregulated.74 In MS disease, monocytes participate in the neuroinflammatory process through a mechanism that includes the high expression of various MMPs and diminished expression of tissue inhibitors of metalloproteinases (TIMPs) such as TIMP-1 and TIMP-2.75 Cumulative evidence has demonstrated that MMP-9 is a key matrix metalloproteinase in multiple sclerosis pathogenesis, enhancing blood-brain-barrier disruption, leukocyte migration, and myelin lysis.76 Various endeavors have been made to create MMPs inhibitors for the potential treatment of diseases in which MMPs assume a critical role. In one study, managing MS patients with natalizumab by inhibiting MMP-9 reduced the risk of progressive multifocal encephalopathy.77 Since increased MMP activity and reduced TIMP levels contribute to a loss of the BBB integrity and infiltration of inflammatory immune cells to the CNS; therefore, MMPs and their TIMPs play a key role in the immunopathogenesis of MS.72 In contrast, one proteomics study utilized a multiplex aptamer proteomics platform to detect 1129 proteins in the plasma of patients with MS. This method uses base pairs for binding target proteins or peptides with higher specificity and affinity than common proteomics methods. Proteomics evaluation of MS patients revealed that MMP3 is lower all MS subtypes than healthy controls.78,79 Thus, using MMPs as specific and sensitive biomarkers for MS requires further studies. Studies recommended that serum levels of GFAP related to MRI lesion count, clinical seriousness scores, and disease progression in MS might be considered an effectively quantifiable biomarker of CNS pathology associated with disease progression.80,81
Neurotransmitters, chemical messengers, participate in CNS function by transmitting a neuron signal across the target cell’s synapse. Impaired neurotransmitter function was observed in many neurological disorders. For instance, dysfunctional glutamatergic excitation and gamma-aminobutyric acid (GABA)ergic prevention were reported in patients with MS.82 Some proteomics studies revealed lower levels of GABA in RR patients compared to healthy control. These data suggested the implication of GABA in suppressing regulatory T cells and enhancement of Th17-type immunity. GABAergic dysfunction may be involved in MS’s pathogenesis, suggesting a strong association between regional GABA levels and cognitive impairment in patients with RR MS.83
Targeting specific signaling proteins is another approach for identifying new biomarkers. Interestingly, a comparison of protein profile between MS subtypes indicates activation of notch signaling pathway through NOTCH2, PSEN1, EP300, JAG2, and DTX1 proteins, in the PP and RR clinical subtypes.84,85 Extra detailed analysis of individual protein players of Notch, such as the NOTCH2, PIK, and JAG2 proteins, shows the higher expression of these proteins in PP patients rather than RR (2.2 fold, P < .05).85 An increase in levels of TUBA4A, NCL, ACTB, FYN, KRT18, CTNNB1 proteins was displayed in RR subtype patients, which are a part of the pathogenic E.coli infection pathway.85 These findings elucidate the critical molecular pathways associated with MS’s inflammatory and neurodegenerative mechanisms.85
Some candidate biomarkers such as chitinase-3-like protein 1 (CHI3L1), Sirtuin 1 (SIRT1), transthyretin (TTR), and brain-derived neurotrophic factor (BDNF) were identified from MS analysis of CSF samples of MS patients.86 Proteins CHI3L1 promise prognostic biomarkers in clinically isolated syndrome (CIS) patients to predict conversion to MS and development of neurological disability.68 The prognostic role of the astrocyte-derived CHI3L1, which was first proposed in a proteomic study directed in CIS patients grouped by limits of change to MS,87 was later approved in an enormous cohort study including CSF tests from 813 CIS patients.88 High CSF CHI3L1 levels brought about a risk factor for change to MS independent of solid indicators. More critically, CSF increased CHI3L1 concentrations were the main noteworthy independent risk factors related to the advancement of disability in multivariate Cox relapse models.88 In this regard, by utilizing a proteomic approach, CHI3L1 was identified as perhaps the best indicator of change to MS in CIS patients.89 Tegla et al90 suggested that SIRT1 might symbolize a biomarker of relapses and a potential new target for therapeutic intervention in MS. Notably, TTR differentially expressed in different stages of the disease and indicating a significant role as a biomarker of MS.91 Studies indicated TTR might undergo oxidative post-translational modifications (PTMs) that may considerably alter its folding and function.92-94 BDNF plays a neuroprotective role in neurodegeneration, and reduced levels have been reported in MS patients.95 Afterward, more proteomics investigations are required to suggest novel and reliable biomarkers from body fluids in MS patients.
Urine
Another body fluid that can be collected non-invasively is urine with an enriched metabolic profile. In one proteomics study, 1699 tryptic peptides related to 402 proteins were evaluated in urine from 31 MS and 8 healthy control throughout the third trimester of pregnancy and the first postpartum period by employing the LC−MS method. Assessment of pregnancy-associated proteins revealed alteration of 531 peptides level between the third trimester and the postpartum period. They also found that placenta-derived pregnancy-associated immunoregulatory proteins such as pregnancy specific beta-1-glycoprotein 1, 9, and 11 were 10-fold higher in the third trimester than the postpartum period compared to healthy controls. These proteins play a crucial role in innate immunoregulation by promoting anti-inflammatory cytokine secretion.
Additionally, evaluation of disease-associated proteins indicated that the expression of 12 proteins was different between 2 periods. Among these proteins, lysosomal-associated membrane protein 2 (LAMP 2) and trefoil factor 3, which play crucial roles in the innate immune system regulation and eukaryotic translation initiation factor 6, and hemoglobin subunit alpha, which have no important role in immunomodulation, were elevated. Plasma glutamate carboxypeptidase, Ig mu chain C region, and osteoclast-associated immune-like receptor were the other 3 proteins with a dramatically decrease ratio in MS. It seems that the increase of immunosuppressive proteins and decrease of proteins related to the immunopathology of MS improve disease relapse during pregnancy.96 However, more proteomics studies are required to shed light on urine-derived proteins’ role as biomarkers for the diagnosis and prognosis of MS disease.
Saliva
Saliva is an attractive biofluid that can be easily collected and stored without an invasive process. Recently, Manconi et al. using top-down proteomics to evaluate 119 salivary proteins from 49 MS patients and 54 healthy controls. The study showed significant differences of 23 proteins between salivary proteomic profiles in MS patients and healthy controls.97 Among these altered proteins, 8 proteins displayed lower levels in MS patients, including mono- and di-oxidized cystatin SN, mono- and di-oxidized cystatin S1, mono-oxidized cystatin SA, and mono-phosphorylated statherin. On the other hand, fifteen proteins showed increased level, including cystatin SN P11 → L variant, ntileukoproteinase, P-C peptide (Fr.1-14, Fr. 26-44, and Fr. 36-44), SV1 fragment of statherin, 2 proteoforms of Prolactin-Inducible Protein, cystatin SN Des1-4, and cystatin A T96 → M variant. These identified proteins in patients belonged to inflammation and immune response, a typical MS pathology.97 Further studies are needed to evaluate these identified proteins’ role in MS pathology and treatment response as a biomarker for MS.
Tear
Tear analysis, a less invasive procedure, may help the lumbar puncture is not recommended or refused by the patient. Devos et al. showed that the sensitivity and specificity of oligoclonal IgG bands in tears from MS patients were similar to CSF, while is the collecting procedure is less invasive.98 The team indicated that tear fluid should be considered a valuable biological material for measuring MS biomarkers.98 Salvisberg et al. carried out 3 independent quantitative experiments between MS patients’ tears and healthy control.99 They found among 42 differential proteins, alpha-1 antichymotrypsin was the only protein to be dramatically increased in all experiments (P < .05). The authors concluded that tear proteomic modification could reflect biological abnormalities related to MS and other inflammatory conditions influencing the CNS.99 The elevated level of tear alpha-1 antichymotrypsin emerges as a promising MS biomarker, replacing traditional lumbar punctures.100 Later on, more clinical investigations are required to identify and verify novel and reliable biomarkers from CNS tissue and body fluids in MS patients.
Brain tissue
Biopsy and autopsy of the brain represent a critical avenue for MS research to explore demyelination, axonal degeneration, perivascular inflammation, and gliosis. A proteomics study utilized a 2-DE-based proteomics approach to evaluate the pattern of protein expression in the brain tissues of EAE models. Among approximately 1000 proteins analyzed, 70 proteins displayed significant alteration in the EAE model compared to controls. These proteins participate in the variable biological process, including ionic and neurotransmitter release, apoptosis, and signal transduction. Additionally, these proteins involve blood barriers and mitochondria formation and function.101 The presence of ephrinB3 and oligodendrocyte differentiation inhibitor in demyelinated white matter lesions was reported using LCM and proteomic analysis methods.102 Future studies require investigation of differentially expressed proteins’ role in the initiation and progression of MS and their probable function during the different stages of the disease.
Although several proteomics approaches were conducted to identify efficient biomarkers, they have not reached any reliable protein molecules as a biomarker. Nevertheless, these studies have suggested many potential protein candidates. Differentially expressed proteins can precisely guide studies to suggest more appropriate biomarkers that would be used in clinical practice. The entire therapeutic option for MS targets the disease’s inflammatory aspects; thus, understanding the pathobiology of MS and finding new targets in the neurodegenerative aspect will help scientists suggest new treatments.
Metabolomics Approach in Multiple Sclerosis
Metabolites are low molecular weight (<900 Da) products of enzyme-mediated biochemical reactions. The heterogeneous composition of biological metabolites and specific alterations in different pathological processes have brought them up as a biomarker. Metabolomics is the comprehensive investigation of metabolites within biochemical processes that can provide immediate clues of the physiology of the metabolic framework.103 There are 3 most commonly used analytical techniques in metabolomics: gas chromatography coupled to GC–MS, LC-MS, and NMR. GC-MS and LC-MS combine the features of gas-chromatography and liquid-chromatography with and mass spectrometry, respectively. NMR is an important tool in which nuclei of specific molecules produce an electromagnetic signal as an exclusive characteristic of the nucleus in a strong magnetic field. NMR has considerable advantages, such as quantitative and exceptionally reproducible, fast measurement, and minimal sample preparation. This tool gives insight into finding out the disease’s pathogenesis and mechanisms.104 In MS, identifying sensitive and specific metabolite markers would help diagnose and separate patients with different disease stages and use appropriate medications. Additionally, these markers will provide better insight into drug response and prognosis of MS. Studies used various body samples, such as CSF, plasma, urine, and brain tissue, to discover novel metabolite biomarkers. Several metabolomics studies for identifying the panel of biomarkers for MS are summarized in Table 2.
Table 2.
Biofluid/tissue metabolomics studies in MS.
| Metabolite | Biofluid/tissue | Method | Trend in MS | Disease’s stage | Ref |
|---|---|---|---|---|---|
| Citrate | CSF, serum, urine | NMR | Down | Relapse | Sinclair et al,120 Gebregiworgis et al119 and Reinke et al108 |
| 2-Hydroxyisovalerate | CSF | NMR | Down | Relapse | Reinke et al108 |
| 3-Hydroxybutyrate | CSF, Serum | NMR | Down | Relapse | Sinclair et al120 and Reinke et al108 |
| Phenylalanine | CSF | NMR | Down | Relapse | Zhao et al110 |
| Urine | NMR | Up | Relapse | Gebregiworgis et al119 | |
| Mannose | CSF | NMR | Down | Relapse | Del Boccio et al110 and Reinke et al108 |
| Oxaloacetate | CSF | NMR | Down | Relapse | Reinke et al108 |
| Choline | CSF | NMR | Up | Relapse | Skripuletz et al121 |
| Myo-inositol | CSF | NMR | Up | Relapse | Reinke et al108 |
| Threonate | CSF | NMR | Up | Relapse | Reinke et al108 |
| Lactate | CSF, serum, urine | NMR | Up | Relapse | Singh et al122 |
| CSF, urine | NMR | Down | Relapse | Kim et al108 | |
| Lysophosphatidylcholine | Serum | LC-MS and MS/MS | Down | Relapse | Stoessel et al123 |
| Arginine | CSF | LC-MS and GC-MS | Down | Onset | Noga et al112 |
| Alanine | CSF | LC-MS and GC-MS | Down | Onset | Noga et al112 |
| Branched-chain amino acid | CSF | MALDI-TOF-MS, LC-MS/MS | Up | Relapse | Singh et al122 |
| Glutamine | CSF | MALDI-TOF-MS, LC-MS/MS, GC-MS | Up | Relapse | Hassan-Smith et al117 |
| O-phosphoethanolamine | CSF | LC-MS and GC-MS | Up | Relapse | Noga et al112 |
| Putrescine | CSF | LC-MS and GC-MS | Up | Relapse | Noga et al112 |
| Fructose | CSF, serum, urine | NMR | Up | CIS | Hassan-Smith et al117 |
| Acetate | CSF, serum, urine | NMR | Down | CIS | Hassan-Smith et al117 |
| Serum | NMR | Up | CIS | Sinclair et al120 | |
| Creatinine | CSF, serum | NMR | Up | CIS | Hassan-Smith et al117 |
| Urine | NMR | Down | Relapse | Gebregiworgis et al119 | |
| β-Glucose | CSF, serum, urine | NMR | Up | CIS | Sinclair et al120 and Singh et al122 |
| Nitric oxide | CSF, urine | Clinical and MRI measurements | Up | Relapse | Del Boccio et al110 |
| Phosphatidylcholines | CSF | MALDI-TOF-MS, LC-MS/MS | Down | Relapse | Del Boccio et al113 |
| Aminoacids and acylcarnitines | Tear | LC-MS/MS | Down | Relapse | Cicalini et al124 |
CSF and plasma
CSF and plasma contain different metabolites that may provide better insight into the diagnosis and prognosis of MS. Reinke et al. reported 8 differentially metabolites between MS patients and healthy controls in CSF by NMR spectroscopy analysis.105 They showed a significant decrease in phenylalanine, citrate, 3-hydroxybutyrate, mannose, and 2-hydroxyisovalerate in MS patients.105,106 As it has been reported by Zhao et al.107 after methionine enkephalin treatment, an accumulation of the intracellular metabolites suggested their neuron protective effect. Kim et al.108 demonstrated a comparative metabolome analysis in CSF from MS and neuromyelitis optica spectrum disorder (NMOSD) patients. The authors reported higher amounts of 2-hydroxybutyrate, acetone, fumarate, and pyroglutamate and lower acetate and glucose in MS and NMOSD. They revealed a lower level of citrate, isoleucine, and valine in MS patients but a whole higher lactate level in NMOSD, specifically.109 The authors speculated these metabolite changes are related to altered fatty acid biosynthesis and energy metabolism in the brain and could help understand the biological background of MS.108 Significant upregulation of glutamine and increase of O-phosphoethanolamine, putrescine, and branched-chain amino acids in the case of MS was described by Noga et al.109 Actually, unlike the genome or proteome, the metabolome reacts to stimuli almost instantaneously, thereby making it possible to evaluate the disease response to environmental disturbances such as surgical resection or drug therapy on a nearly real-time basis.110 For example, lactate concentration may be considered a disease biomarker because it increased significantly in MS patients.111 As oxidative stress plays in MS disease, nitric oxide, and its metabolites, including nitrates and nitrites, also increased in CSF and urine during acute exacerbations and related to disability and inflammatory activity progression.112
Boccio et al. found in their study the altered serum phosphatidylcholine (PC) and LysoPC levels in MS subjects and a significant decrease of the total LysoPC/PC ratio the total LysoPC/PC ratio in MS compared to controls and other neurological diseases patients.113 Moreover, several studies indicated the upregulation of fructose, creatinine, glutamine, glucose, and acetate downregulation in patients with CIS.114,115 Lutz et al. assumed that the lactate level in CSF is correlated with inflammatory plaques number, while the β-hydroxyisobutyrate level is related to the presence of these plaques.115
Urine
Urine is an easy-collecting sample that may contain valuable metabolites related to MS diagnosis and prognosis. Urine metabolites in MS patients compared to healthy and NMOSD controls were evaluated by NMR.116 Findings illustrated different alterations in 27 metabolites associated with synthesis and degradation of amino acids, ketone bodies, tricarboxylic acid cycle, glycolysis, propionate, and pyruvate metabolism. Urine metabolites change in MS patients are related to known pathogenic processes relevant to the disease, comprising changes in mitochondrial activity, energy and fatty acid metabolism, and the gut microbiota.116
Brain tissue
Tisell et al. demonstrated elevated glutamine in MS patients. They speculated that elevated glutamine might be associated with the destruction of oligodendrocytes and a diffuse neurodegenerative MS process.117 Additionally, the authors reported an increased level of Myo-inositol and creatine in patients. Considering that these metabolites are glial cell-specific markers, the authors concluded that the increased concentrations result from increased glial cell density in MS patients.117 Wheeler et al.118 completely studied phosphatidylethanolamines of normal white and gray matter from MS and control brain tissues using electrospray ionization tandem MS (ESI-MS/MS). Results showed a higher phospholipid and lower sphingolipid content in these tissues’ lipid composition.118 However, the level of phosphatidylethanolamine in inactive MS appears to be higher than inactive tissues, suggesting that the metabolic may reflect different states in the same disease, a significant feature for a useful biomarker in prognosis.118
In metabolomics analysis, standardization of sample collection and processing methods are important; because they can cause changes in metabolite concentration that can disrupt biological differences. The metabolite’s extremely responsive nature can be exploited to monitor treatment effectiveness in response to drug or surgical interventions.110,119 Identifying specific biomarkers, markers of therapeutic efficacy, and other pharmacodynamic endpoints may improve therapeutic efficacies while avoiding toxicity and unnecessary side effects. Additionally, metabolomics analysis may suggest new pathways and therapeutic targets that can help pharmacologists and biotechnology experts design new agents for the targeted treatment of MS.
Personalized Medicine in Multiple Sclerosis
The terms ‘personalized medicine’ and ‘precision medicine’ may often be used mutually in several fields, including MS. It is pivotal to distinguish between these 2 terms concerning MS care. Personalized medicine is defined as a strategy to diagnose, treat, and follow up disease regarding an individual’s characteristics, environment, disease features, and biomolecular traits. Personalized medicine researches aim to ensure that patients get the right amounts of the right medicine at the right time. On the other hand, precision medicine contains the same principles, but genomics and pharmacogenomics are the crucial players.125-127
Utilizing personalized medicine in MS requires 3 crucial components; (1) Assessment of prognosis soon after diagnosis, (2) Considering an early therapeutic plan based on risk-benefit and patient predilections, (3) An early assessment of response to therapy and taking alternative therapies in the case of failure.127 As described before, MS is a highly heterogeneous disease with different phenotypes evident in various disease stages and without a certain cure.128 In order to ensure appropriate treatment for patients with MS, considering the numerous clinical and immunopathological subtypes of the disease, profiling and the individual patient’s characterization are critical.125,128 Despite extensive researches on MS, there is no effective treatment for this disease. For RR subtype patients, several disease-modifying drugs (DMDs), classified into first- and second-line therapies, are accessible with different efficacy and safety outlines.129 The standard injectable first-line therapies with a good safety profile are interferon beta-1a (IFN-β 1a), IFN-β 1b, and glatiramer acetate.129,130 Also, oral first-line therapies such as teriflunomide and dimethyl fumarate with diverse activity mechanisms, similar efficacy, and various security profiles are used. When first-line therapies fail, second-line therapies such as fingolimod, natalizumab, mitoxantrone, and alemtuzumab are recommended.131 The treatment strategy for MS mainly targets early inflammatory processes that are more effective than other treatments; however, anti-inflammatory agents have greater side effects than first-line DMDs.132 Conventional clinical and radiological predictors poorly predict the progression of neurological and neuropsychological disability in MS due to the lack of vigorous biomarkers and detailed understanding of MS pathogenesis.133 Therefore, the achievement of more exact evaluations of valuable predictors in MS is needed. With more accurate and earlier diagnoses, MS patients will be treated sooner, along with increased opportunities for early preventive interventions.134,135 In this context, personalized medicine is recently considered beyond the precision medicine concept in the therapeutic approach in MS.1,125,135 Biomarkers are important for understanding MS patient disease profile, prognosis, diagnosis, and disease course prediction. They are also critical in identifying the benefits and side effects of new therapies on patients.
Future Directions and Conclusion
The results demonstrate the potential of integrated “omics” approaches to characterize the phenotype that places discoveries into a biological context. The proteomics and metabolomics approach may allow personalizing treatment for MS patients. The complexity of the disease itself and its effect on the nervous system and immune system makes it difficult to identify its underlying pathogenic mechanisms. Therefore, it seems that MS does not have a single biomarker that might distinguish and fully reflect the pathology of this disease. In this context, proteomics and metabolomics analysis of CNS tissue and body fluids may be great tools to distinguish biomarkers and therapeutic candidates.
The vital step in biomarker identification is reducing the number of supposed biomarkers’ possibilities from a large omics dataset by utilizing appropriate methods such as traditional statistics and artificial intelligence.136,137 Traditional statistics’ main concept is that significant expression changes in some molecules bring them up as disease- or treatment-related biomarker candidates. Additionally, significant alteration of some molecules can predict the prognosis and stage of the disease. This approach identifies molecules from omics datasets that have not been formerly related to disease’s different aspects.138 Machine learning is a subfield of artificial intelligence that includes various algorithms and techniques to create an accurate model that mimics a procedure and predicts new samples’ outputs. Creating the model will be done by combining computing and machine learning algorithms without any explicit function and based on learning from the relation between features of recorded samples as inputs and labels as outputs of the procedure.137 A variety of machine learning methods have been utilized to analyze transcriptomics and metabolomics datasets to classify unknown samples and identify disease-associated molecules.139,140 However, developing efficient new approaches for integrating datasets of different omics investigations will help identify more appropriate biomarkers for MS. For example, the development of novel gene sequencing technologies at the beginning of the 2000s results in bulk RNA-sequencing (RNA-seq) or DNA-based microarrays to evaluate cellular pathways and molecular alteration at the transcript level. The development of next-generation sequencing (NGS) improved transcriptomic approaches by discovering new RNA variants and splice sites or evaluating epigenetic factors, including DNA methylation patterns and DNA-protein interactions.141 Despite the development of many “omics” technologies, there is a lack of integrating and linking studies to see the full picture of cellular pathways in the future. This association would improve our insight into human pathologies that result in a revolution in preclinical and clinical studies of the disease diagnosis, prognosis, drug response, and new drug development.142
In conclusion, we would like to highlight that future biomarkers’ discovery and execution will face numerous challenges. Consequently, serious cooperative attempts among scientists, clinicians, and industry are needed. This collaboration will move us one step forward from hope to the ultimate goal of personalized medicine in MS. We hope that standardizing critical elements of proteomics studies, as well as metabolomics signature with different omics such as genomics, microbiome, and transcriptomic information, provide insights into the pathogenesis of MS shortly.
Acknowledgments
This study is related to project NO 1398/4014 From the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We appreciate the “Student Research Committee” and “Research & Technology Chancellor” in Shahid Beheshti University of Medical Sciences for their financial support of this study. We also thank Dr. Ali Askary for his valuable contribution to language editing.
Footnotes
Authors’ Contributions: AJ conceived the idea, wrote and revised the article. AB wrote and revised the article. MRT designed and supervised the project. All authors read and approved the manuscript.
Declaration of Conflicting Interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amirhesam Babajani  https://orcid.org/0000-0002-8853-4343
https://orcid.org/0000-0002-8853-4343
References
- 1. Ziemssen T, Kern R, Thomas K. Multiple sclerosis: clinical profiling and data collection as prerequisite for personalized medicine approach. BMC Neurol. 2016;16(1):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hauser SL. Multiple lessons for multiple sclerosis. Mass Med Soc. 2008;359:1838-1841. [DOI] [PubMed] [Google Scholar]
- 3. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545-558. [DOI] [PubMed] [Google Scholar]
- 4. Constantinescu CS, Farooqi N, O’Brien K, Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol. 2011;164(4):1079-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Filippo M, Portaccio E, Mancini A, Calabresi P. Multiple sclerosis and cognition: synaptic failure and network dysfunction. Nat Rev Neurosci. 2018;19(10):599-609. [DOI] [PubMed] [Google Scholar]
- 6. Harris C. Nursing practice in Multiple Sclerosis: A Core Curriculum. Demos Medical Publishing; 2003. [Google Scholar]
- 7. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50(1):121-127. [DOI] [PubMed] [Google Scholar]
- 8. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis. The 2013 revisions. Neurology. 2014;83(3):278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502-1517. [DOI] [PubMed] [Google Scholar]
- 10. Rosenling T, Attali A, Luider TM, Bischoff R. The experimental autoimmune encephalomyelitis model for proteomic biomarker studies: from rat to human. Clin Chim Acta. 2011;412(11-12):812-822. [DOI] [PubMed] [Google Scholar]
- 11. Group BDW, Atkinson AJ, Jr, Colburn WA, et al. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Therap. 2001;69(3):89-95. [DOI] [PubMed] [Google Scholar]
- 12. McDermott JE, Wang J, Mitchell H, et al. Challenges in biomarker discovery: combining expert insights with statistical analysis of complex omics data. Exp Opin Med Diagn. 2013;7(1):37-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet. 2013;45(11):1353-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Singh V, Tripathi A, Dutta RJP. Proteomic approaches to decipher mechanisms underlying pathogenesis in multiple sclerosis patients. Proteomics. 2019;16:1800335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goossens N, Nakagawa S, Sun X, Hoshida Y. Cancer biomarker discovery and validation. Transl Cancer Res. 2015;4(3):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Amiri-Dashatan N, Koushki M, Abbaszadeh HA, Rostami-Nejad M, Rezaei-Tavirani M. Proteomics applications in health: biomarker and drug discovery and food industry. Iran J Pharm Res. 2018;17(4):1523-1536. [PMC free article] [PubMed] [Google Scholar]
- 17. Farias AS, Santos LM. How can proteomics elucidate the complexity of multiple sclerosis? Proteomics Clin Appl. 2015;9(9-10):844-847. [DOI] [PubMed] [Google Scholar]
- 18. Goldsmith P, Fenton H, Morris-Stiff G, Ahmad N, Fisher J, Prasad KR. Metabonomics: a useful tool for the future surgeon. J Surg Res. 2010;160(1):122-132. [DOI] [PubMed] [Google Scholar]
- 19. Sas KM, Karnovsky A, Michailidis G, Pennathur S. Metabolomics and diabetes: analytical and computational approaches. Diabetes. 2015;64(3):718-732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orton DJ, Doucette AA. Proteomic workflows for biomarker identification using mass spectrometry—technical and statistical considerations during initial discovery. Proteomes. 2013;1(2):109-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mischak H, Kolch W, Aivaliotis M, et al. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin Appl. 2010;4(4):464-478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Füzéry AK, Levin J, Chan MM, Chan DW. Translation of proteomic biomarkers into FDA approved cancer diagnostics: issues and challenges. Clin Proteomics. 2013;10(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ghaste M, Mistrik R, Shulaev V. Applications of Fourier transform ion cyclotron resonance (FT-ICR) and orbitrap based high resolution mass spectrometry in metabolomics and lipidomics. Int J Mol Sci. 2016;17(6):816-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lapek JD, Jr, Greninger P, Morris R, et al. Detection of dysregulated protein-association networks by high-throughput proteomics predicts cancer vulnerabilities. Nat Biotechnol. 2017;35(10):983-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ziemssen T, Akgün K, Brück W. Molecular biomarkers in multiple sclerosis. J Neuroinflammation. 2019;16(1):272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chait BT. Mass spectrometry in the postgenomic era. Annu Rev Biochem. 2011;80:239-246. [DOI] [PubMed] [Google Scholar]
- 27. Aslam B, Basit M, Nisar MA, Khurshid M, Rasool MH. Proteomics: technologies and their applications. J Chromatogr Sci. 2017;55(2):182-196. [DOI] [PubMed] [Google Scholar]
- 28. Catherman AD, Skinner OS, Kelleher NL. Top down proteomics: facts and perspectives. Biochem Biophys Res Commun. 2014;445(4):683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yates JR, Ruse CI, Nakorchevsky A. Proteomics by mass spectrometry: approaches, advances, and applications. Annu Rev Biomed Eng. 2009;11:49-79. [DOI] [PubMed] [Google Scholar]
- 30. Farias AS, Pradella F, Schmitt A, Santos LM, Martins-de-Souza D. Ten years of proteomics in multiple sclerosis. Proteomics. 2014;14(4-5):467-480. [DOI] [PubMed] [Google Scholar]
- 31. Zhang Y, Fonslow BR, Shan B, Baek M-C, Yates JR, III. Protein analysis by shotgun/bottom-up proteomics. Chem Rev. 2013;113(4):2343-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lourenço AS, Baldeiras I, Grãos M, Duarte CB. Proteomics-based technologies in the discovery of biomarkers for multiple sclerosis in the cerebrospinal fluid. Curr Mol Med. 2011;11(4):326-349. [DOI] [PubMed] [Google Scholar]
- 33. Elkabes S, Li H. Proteomic strategies in multiple sclerosis and its animal models. Proteomics Clin Appl. 2007;1(11):1393-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stoop MP, Runia TF, Stingl C, van der Vuurst de Vries RM, Luider TM, Hintzen RQ. Decreased neuro-axonal proteins in CSF at first attack of suspected multiple sclerosis. Proteomics Clin Appl. 2017;11(11-12):1700005. [DOI] [PubMed] [Google Scholar]
- 35. Rosenling T, Stoop MP, Attali A, et al. Profiling and identification of cerebrospinal fluid proteins in a rat EAE model of multiple sclerosis. J Proteome Res. 2012;11(4):2048-2060. [DOI] [PubMed] [Google Scholar]
- 36. Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement system part I – molecular mechanisms of activation and regulation. Front Immunol. 2015;6:262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Iwanowski P, Losy J, Kramer L, Wójcicka M, Kaufman E. CXCL10 and CXCL13 chemokines in patients with relapsing remitting and primary progressive multiple sclerosis. J Neurol Sci. 2017;380:22-26. [DOI] [PubMed] [Google Scholar]
- 38. Chang K-H, Tseng M-Y, Ro L-S, et al. Analyses of haptoglobin level in the cerebrospinal fluid and serum of patients with neuromyelitis optica and multiple sclerosis. Clin Chim Acta. 2013;417:26-30. [DOI] [PubMed] [Google Scholar]
- 39. Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61(1):71-90. [DOI] [PubMed] [Google Scholar]
- 40. Barcelos IPD, Troxell RM, Graves JS. Mitochondrial dysfunction and multiple sclerosis. Biology. 2019;8(2):37-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jafari A, Rezaei-Tavirani M, Salimi M, Tavakkol R, Jafari Z. Oncological emergencies from pathophysiology and diagnosis to treatment: a narrative review. Soc Work Public Health. 2020;35(8):689-709. [DOI] [PubMed] [Google Scholar]
- 42. Aoki T, Narumiya S. Prostaglandins and chronic inflammation. Trends Pharmacol Sci. 2012;33(6):304-311. [DOI] [PubMed] [Google Scholar]
- 43. Dumont D, Noben JP, Raus J, Stinissen P, Robben J. Proteomic analysis of cerebrospinal fluid from multiple sclerosis patients. Proteomics. 2004;4(7):2117-2124. [DOI] [PubMed] [Google Scholar]
- 44. Lima IVDA, Bastos LFS, Limborço-Filho M, Fiebich BL, de Oliveira ACP. Role of prostaglandins in neuroinflammatory and neurodegenerative diseases. Mediators Inflamm. 2012;2012:946813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Babajani A, Soltani P, Jamshidi E, Farjoo MH, Niknejad H. Recent advances on drug-loaded mesenchymal stem cells with anti-neoplastic agents for targeted treatment of cancer. Front Bioeng Biotechnol. 2020;8:748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. De Masi R, Vergara D, Pasca S, et al. PBMCs protein expression profile in relapsing IFN-treated multiple sclerosis: a pilot study on relation to clinical findings and brain atrophy. J Neuroimmunol. 2009;210(1-2):80-86. [DOI] [PubMed] [Google Scholar]
- 47. van Noort JM, Bsibsi M, Gerritsen WH, et al. αB-crystallin is a target for adaptive immune responses and a trigger of innate responses in preactive multiple sclerosis lesions. J Neuropathol Exp Neurol. 2010;69(7):694-703. [DOI] [PubMed] [Google Scholar]
- 48. Shin J-H, Kim S-W, Lim C-M, Jeong J-Y, Piao C-S, Lee J-K. αB-crystallin suppresses oxidative stress-induced astrocyte apoptosis by inhibiting caspase-3 activation. Neurosci Res. 2009;64(4):355-361. [DOI] [PubMed] [Google Scholar]
- 49. Li R, Reiser G. Phosphorylation of Ser45 and Ser59 of αB-crystallin and p38/extracellular regulated kinase activity determine αB-crystallin-mediated protection of rat brain astrocytes from C2-ceramide-and staurosporine-induced cell death. J Neurochem. 2011;118(3):354-364. [DOI] [PubMed] [Google Scholar]
- 50. Kuipers HF, Yoon J, Van Horssen J, et al. Phosphorylation of αB-crystallin supports reactive astrogliosis in demyelination. Proc Natl Acad Sci. 2017;114(9):E1745-E1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Thebault S, Abdoli M, Fereshtehnejad S-M, Tessier D, Tabard-Cossa V, Freedman MS. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10(1):10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Williams T, Zetterberg H, Chataway J. Neurofilaments in progressive multiple sclerosis: a systematic review. J Neurol. Published online May 23, 2020. doi: 10.1007/s00415-020-09917-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gil-Perotin S, Castillo-Villalba J, Cubas-Nuñez L, et al. Combined cerebrospinal fluid neurofilament light chain protein and chitinase-3 like-1 levels in defining disease course and prognosis in multiple sclerosis. Front Neurol. 2019;10:1008-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Varhaug KN, Torkildsen Ø, Myhr K-M, Vedeler CA. Neurofilament light chain as a biomarker in multiple sclerosis. Front Neurol. 2019;10:338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huang J, Khademi M, Fugger L, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci. 2020;117(23):12952-12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jafari A, Babajani A, Abdollahpour-Alitappeh M, Ahmadi N, Rezaei-Tavirani M. Exosomes and cancer: from molecular mechanisms to clinical applications. Med Oncol. 2021;38(4):45. [DOI] [PubMed] [Google Scholar]
- 57. Mirshafiey A, Asghari B, Ghalamfarsa G, Jadidi-Niaragh F, Azizi G. The significance of matrix metalloproteinases in the immunopathogenesis and treatment of multiple sclerosis. Sultan Qaboos Univ Med J. 2014;14(1):e13-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kurzepa J, Bartosik-Psujek H, Suchozebrska-Jesionek D, Rejdak K, Stryjecka-Zimmer M, Stelmasiak Z. Role of matrix metalloproteinases in the pathogenesis of multiple sclerosis. Neurol Neurochir Polska. 2005;39(1):63-67. [PubMed] [Google Scholar]
- 59. Rosenberg GA. Matrix metalloproteinases and neuroinflammation in multiple sclerosis. The Neuroscientist. 2002;8(6):586-595. [DOI] [PubMed] [Google Scholar]
- 60. Bar-Or A, Nuttall RK, Duddy M, et al. Analyses of all matrix metalloproteinase members in leukocytes emphasize monocytes as major inflammatory mediators in multiple sclerosis. Brain. 2003;126(12):2738-2749. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Y, Dong H, Seeburg DP, et al. Multimodal molecular imaging demonstrates myeloperoxidase regulation of matrix metalloproteinase activity in neuroinflammation. Mol Neurobiol. 2019;56(2):954-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fissolo N, Pignolet B, Matute-Blanch C, et al. Matrix metalloproteinase 9 is decreased in natalizumab-treated multiple sclerosis patients at risk for progressive multifocal leukoencephalopathy. Ann Neurol. 2017;82(2):186-195. [DOI] [PubMed] [Google Scholar]
- 63. Malekzadeh A, Leurs C, van Wieringen W, et al. Plasma proteome in multiple sclerosis disease progression. Ann Clin Transl Neurol. 2019;6(9):1582-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ngo D, Sinha S, Shen D, et al. Aptamer-based proteomic profiling reveals novel candidate biomarkers and pathways in cardiovascular disease. Circulation. 2016;134(4):270-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8(1):1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Högel H, Rissanen E, Barro C, et al. Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler J. 2018;26(2):210-219. [DOI] [PubMed] [Google Scholar]
- 67. Gao F, Yin X, Edden RAE, et al. Altered hippocampal GABA and glutamate levels and uncoupling from functional connectivity in multiple sclerosis. Hippocampus. 2018;28(11):813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cao G, Edden RA, Gao F, et al. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur Radiol. 2018;28(3):1140-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ascherio A, Munger KL, White R, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014;71(3):306-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Avsar T, Durası İM, Uygunoğlu U, et al. CSF proteomics identifies specific and shared pathways for multiple sclerosis clinical subtypes. PLoS One. 2015;10(5):e0122045-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kroksveen AC, Jaffe JD, Aasebø E, et al. Quantitative proteomics suggests decrease in the secretogranin-1 cerebrospinal fluid levels during the disease course of multiple sclerosis. Proteomics. 2015;15(19):3361-3369. [DOI] [PubMed] [Google Scholar]
- 72. Comabella M, Fernández M, Martin R, et al. Cerebrospinal fluid chitinase 3-like 1 levels are associated with conversion to multiple sclerosis. Brain. 2010;133(4):1082-1093. [DOI] [PubMed] [Google Scholar]
- 73. Cantó E, Tintoré M, Villar LM, et al. Chitinase 3-like 1: prognostic biomarker in clinically isolated syndromes. Brain. 2015;138(4):918-931. [DOI] [PubMed] [Google Scholar]
- 74. Hinsinger G, Galeotti N, Nabholz N, et al. Chitinase 3-like proteins as diagnostic and prognostic biomarkers of multiple sclerosis. Mult Scler J. 2015;21(10):1251-1261. [DOI] [PubMed] [Google Scholar]
- 75. Tegla CA, Azimzadeh P, Andrian-Albescu M, et al. SIRT1 is decreased during relapses in patients with multiple sclerosis. Exp Mol Pathol. 2014;96(2):139-148. [DOI] [PubMed] [Google Scholar]
- 76. Amin B, Maurer A, Voelter W, Melms A, Kalbacher H. New poteintial serum biomarkers in multiple sclerosis identified by proteomic strategies. Curr Med Chem. 2014;21(13):1544-1556. [DOI] [PubMed] [Google Scholar]
- 77. Pieragostino D, Del Boccio P, Di Ioia M, et al. Oxidative modifications of cerebral transthyretin are associated with multiple sclerosis. Proteomics. 2013;13(6):1002-1009. [DOI] [PubMed] [Google Scholar]
- 78. Hybeľová M, Svatoňová J, Sobek O, Adam P, Doležil D, Adam D. Cerebrospinal fluid and serum prealbumin (transthyretin) in patients with multiple sclerosis (MS): comparison of particular subgroups of MS patients. Folia Microbiol. 2009;54(2):173-176. [DOI] [PubMed] [Google Scholar]
- 79. Jiang S, Lu Q, Hu S, et al. Proteomics comparison of the sera from multiple sclerosis patients and neuromyelitis optica patients. Genet Mol Res. 2014;13(4):9292-9299. [DOI] [PubMed] [Google Scholar]
- 80. Semkina A, Alifirova V, Titova M, Maltseva A, Abadzhyan M. Brain-derived neurotrophic factor in multiple sclerosis. Zh Nevrol Psikhiatr Im S S Korsakova. 2019;119(2):28-35. [DOI] [PubMed] [Google Scholar]
- 81. Singh V, Stingl C, Stoop MP, et al. Proteomics urine analysis of pregnant women suffering from multiple sclerosis. J Proteome Res. 2015;14(5):2065-2073. [DOI] [PubMed] [Google Scholar]
- 82. Manconi B, Liori B, Cabras T, et al. Top-down proteomic profiling of human saliva in multiple sclerosis patients. J Proteomics. 2018;187:212-222. [DOI] [PubMed] [Google Scholar]
- 83. Lebrun C, Forzy G, Collongues N, Cohen M, De Seze J, Hautecoeur P. Tear analysis as a tool to detect oligoclonal bands in radiologically isolated syndrome. Rev Neurol. 2015;171(4):390-393. [DOI] [PubMed] [Google Scholar]
- 84. Salvisberg C, Tajouri N, Hainard A, Burkhard PR, Lalive PH, Turck NJPCA. Exploring the human tear fluid: discovery of new biomarkers in multiple sclerosis. Proteomics Clin Appl. 2014;8(3-4):185-194. [DOI] [PubMed] [Google Scholar]
- 85. Riemens A, Stoyanova E, Rothova A, Kuiper J. Cytokines in tear fluid of patients with ocular graft-versus-host disease after allogeneic stem cell transplantation. Mol Vis. 2012;18:797-802. [PMC free article] [PubMed] [Google Scholar]
- 86. Fazeli AS, Nasrabadi D, Sanati MH, et al. Proteome analysis of brain in murine experimental autoimmune encephalomyelitis. Proteomics. 2010;10(15):2822-2832. [DOI] [PubMed] [Google Scholar]
- 87. Syed YA, Zhao C, Mahad D, et al. Antibody-mediated neutralization of myelin-associated EphrinB3 accelerates CNS remyelination. Acta Neuropathol. 2016;131(2):281-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schutzer SE, Angel TE, Liu T, et al. Gray matter is targeted in first-attack multiple sclerosis. PLoS One. 2013;8(9):e66117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Singh V, Stoop MP, Stingl C, et al. Cerebrospinal-fluid-derived immunoglobulin G of different multiple sclerosis patients shares mutated sequences in complementarity determining regions. Mol Cell Proteomics. 2013;12(12):3924-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Liguori M, Qualtieri A, Tortorella C, et al. Proteomic profiling in multiple sclerosis clinical courses reveals potential biomarkers of neurodegeneration. PLoS One. 2014;9(8):e103984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh V, van Pelt ED, Stoop MP, et al. Gray matter–related proteins are associated with childhood-onset multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2015;2(5):e155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Yin L, Liu J, Dong H, et al. Autophagy-related gene16L2, a potential serum biomarker of multiple sclerosis evaluated by bead-based proteomic technology. Neurosci Lett. 2014;562:34-38. [DOI] [PubMed] [Google Scholar]
- 93. Tremlett H, Dai DL, Hollander Z, et al. Serum proteomics in multiple sclerosis disease progression. J Proteomics. 2015;118:2-11. [DOI] [PubMed] [Google Scholar]
- 94. Wallin M, Oh U, Nyalwidhe J, et al. Serum proteomic analysis of a pre-symptomatic multiple sclerosis cohort. Eur J Neurol. 2015;22(3):591-599. [DOI] [PubMed] [Google Scholar]
- 95. Ayoglu B, Mitsios N, Kockum I, et al. Anoctamin 2 identified as an autoimmune target in multiple sclerosis. Proc Natl Acad Sci. 2016;113(8):2188-2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lewin A, Hamilton S, Witkover A, et al. Free serum haemoglobin is associated with brain atrophy in secondary progressive multiple sclerosis. Wellcome Open Res. 2016;1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nishihara H, Shimizu F, Kitagawa T, et al. Identification of galectin-3 as a possible antibody target for secondary progressive multiple sclerosis. Mult Scler J. 2017;23(3):382-394. [DOI] [PubMed] [Google Scholar]
- 98. Herman S, Khoonsari PE, Tolf A, et al. Integration of magnetic resonance imaging and protein and metabolite CSF measurements to enable early diagnosis of secondary progressive multiple sclerosis. Theranostics. 2018;8(16):4477-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Brown N, Alkhayer K, Clements R, et al. Neuronal hemoglobin expression and its relevance to multiple sclerosis neuropathology. J Mol Neurosci. 2016;59(1):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Qendro V, Bugos GA, Lundgren DH, Glynn J, Han MH, Han DK. Integrative proteomics, genomics, and translational immunology approaches reveal mutated forms of Proteolipid Protein 1 (PLP1) and mutant-specific immune response in multiple sclerosis. Proteomics. 2017;17(6):1600322. [DOI] [PubMed] [Google Scholar]
- 101. Maccarrone G, Nischwitz S, Deininger S-O, et al. MALDI imaging mass spectrometry analysis—a new approach for protein mapping in multiple sclerosis brain lesions. J Chromatogr B. 2017;1047:131-140. [DOI] [PubMed] [Google Scholar]
- 102. Hagan S, Martin E, Enríquez-de-Salamanca A. Tear fluid biomarkers in ocular and systemic disease: potential use for predictive, preventive and personalised medicine. EPMA J. 2016;7(1):15-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Dunn WB, Broadhurst DI, Atherton HJ, Goodacre R, Griffin JL. Systems level studies of mammalian metabolomes: the roles of mass spectrometry and nuclear magnetic resonance spectroscopy. Chem Soc Rev. 2011;40(1):387-426. [DOI] [PubMed] [Google Scholar]
- 104. Emwas AH, Roy R, McKay RT, et al. NMR spectroscopy for metabolomics research. Metabolites. 2019;9(7):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Reinke S, Broadhurst D, Sykes B, et al. Metabolomic profiling in multiple sclerosis: insights into biomarkers and pathogenesis. Mult Scler. 2014;20(10):1396-1400. [DOI] [PubMed] [Google Scholar]
- 106. Mathur D, López-Rodas G, Casanova B, Marti MB. Perturbed glucose metabolism: insights into multiple sclerosis pathogenesis. Front Neurol. 2014;5:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Zhao C, Du H, Xu L, et al. Metabolomic analysis revealed glycylglycine accumulation in astrocytes after methionine enkephalin administration exhibiting neuron protective effects. J Pharm Biomed Anal. 2015;115:48-54. [DOI] [PubMed] [Google Scholar]
- 108. Kim H-H, Jeong IH, Hyun J-S, Kong BS, Kim HJ, Park SJ. Metabolomic profiling of CSF in multiple sclerosis and neuromyelitis optica spectrum disorder by nuclear magnetic resonance. PLoS One. 2017;12(7):e0181758-e0181778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Noga MJ, Dane A, Shi S, et al. Metabolomics of cerebrospinal fluid reveals changes in the central nervous system metabolism in a rat model of multiple sclerosis. Metabolomics. 2012;8(2):253-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Del Boccio P, Rossi C, di Ioia M, Cicalini I, Sacchetta P, Pieragostino D. Integration of metabolomics and proteomics in multiple sclerosis: From biomarkers discovery to personalized medicine. Proteomics Clin Appl. 2016;10(4):470-484. [DOI] [PubMed] [Google Scholar]
- 111. Regenold WT, Phatak P, Makley MJ, Stone RD, Kling MA. Cerebrospinal fluid evidence of increased extra-mitochondrial glucose metabolism implicates mitochondrial dysfunction in multiple sclerosis disease progression. J Neurol Sci. 2008;275(1-2):106-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Rejdak K, Eikelenboom M, Petzold A, et al. CSF nitric oxide metabolites are associated with activity and progression of multiple sclerosis. Neurology. 2004;63(8):1439-1445. [DOI] [PubMed] [Google Scholar]
- 113. Del Boccio P, Pieragostino D, Di Ioia M, et al. Lipidomic investigations for the characterization of circulating serum lipids in multiple sclerosis. J Proteomics. 2011;74(12):2826-2836. [DOI] [PubMed] [Google Scholar]
- 114. Hassan-Smith G, Wallace GR, Douglas MR, Sinclair AJ. The role of metabolomics in neurological disease. J Neuroimmunol. 2012;248(1-2):48-52. [DOI] [PubMed] [Google Scholar]
- 115. Lutz NW, Viola A, Malikova I, et al. Inflammatory multiple-sclerosis plaques generate characteristic metabolic profiles in cerebrospinal fluid. PLoS One. 2007;2(7):e595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Gebregiworgis T, Nielsen HH, Massilamany C, et al. A urinary metabolic signature for multiple sclerosis and neuromyelitis optica. J Proteome Res. 2016;15(2):659-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Tisell A, Leinhard OD, Warntjes JBM, et al. Increased concentrations of glutamate and glutamine in normal-appearing white matter of patients with multiple sclerosis and normal MR imaging brain scans. PLoS One. 2013;8(4):e61817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wheeler D, Bandaru VVR, Calabresi PA, Nath A, Haughey NJ. A defect of sphingolipid metabolism modifies the properties of normal appearing white matter in multiple sclerosis. Brain. 2008;131(11):3092-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bhargava P, Calabresi PA. Metabolomics in multiple sclerosis. Mult Scler J. 2016;22(4):451-460. [DOI] [PubMed] [Google Scholar]
- 120. Sinclair AJ, Viant MR, Ball AK, et al. NMR-based metabolomic analysis of cerebrospinal fluid and serum in neurological diseases–a diagnostic tool? NMR Biomed. 2010;23(2):123-132. [DOI] [PubMed] [Google Scholar]
- 121. Skripuletz T, Manzel A, Gropengießer K, et al. Pivotal role of choline metabolites in remyelination. Brain. 2015;138(2):398-413. [DOI] [PubMed] [Google Scholar]
- 122. Singh V, Tripathi A, Dutta R. Proteomic approaches to decipher mechanisms underlying pathogenesis in multiple sclerosis patients. Proteomics. 2019;19(16):1800335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stoessel D, Stellmann J-P, Willing A, et al. Metabolomic profiles for primary progressive multiple sclerosis stratification and disease course monitoring. Front Hum Neurosci. 2018;12:226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Cicalini I, Rossi C, Pieragostino D, et al. Integrated lipidomics and metabolomics analysis of tears in multiple sclerosis: an insight into diagnostic potential of lacrimal fluid. Int J Mol Sci. 2019;20(6):1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hansen MR, Okuda DT. Precision medicine for multiple sclerosis promotes preventative medicine. Ann N Y Acad Sci. 2018;1420(1):62-71. [DOI] [PubMed] [Google Scholar]
- 126. Willard HF, Ginsburg GS. Genomic and Personalized Medicine. Elsevier; 2009. [DOI] [PubMed] [Google Scholar]
- 127. Rotstein D, Montalban X. Reaching an evidence-based prognosis for personalized treatment of multiple sclerosis. Nat Rev Neurol. 2019;15(5):287-300. [DOI] [PubMed] [Google Scholar]
- 128. Disanto G, Berlanga AJ, Handel AE, et al. Heterogeneity in multiple sclerosis: scratching the surface of a complex disease. Autoimmune Dis. 2011;2011:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Liblau R. Glatiramer acetate for the treatment of multiple sclerosis: evidence for a dual anti-inflammatory and neuroprotective role. J Neurol Sci. 2009;287:S17-S23 [DOI] [PubMed] [Google Scholar]
- 130. Murray T. Diagnosis and treatment of multiple sclerosis. BMJ. 2006;332(7540):525-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Mellergård J, Edström M, Vrethem M, Ernerudh J, Dahle C. Natalizumab treatment in multiple sclerosis: marked decline of chemokines and cytokines in cerebrospinal fluid. Mult Scler J. 2010;16(2):208-217. [DOI] [PubMed] [Google Scholar]
- 132. Stankiewicz JM, Kolb H, Karni A, Weiner HL. Role of immunosuppressive therapy for the treatment of multiple sclerosis. Neurotherapeutics. 2013;10(1):77-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. D’Amico E, Patti F, Zanghì A, Zappia M. A personalized approach in progressive multiple sclerosis: the current status of disease modifying therapies (DMTs) and future perspectives. Int J Mol Sci. 2016;17(10):1725-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Butterworth SE, Ingram G, Robertson NP. Advances in biomarker research in multiple sclerosis. J Neurol. 2016;263(3):621-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Harris VK, Tuddenham JF, Sadiq SA. Biomarkers of multiple sclerosis: current findings. Degener Neurol Neuromuscul Dis. 2017;7:19-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Ray S, Reddy PJ, Jain R, Gollapalli K, Moiyadi A, Srivastava S. Proteomic technologies for the identification of disease biomarkers in serum: advances and challenges ahead. Proteomics. 2011;11(11):2139-2161. [DOI] [PubMed] [Google Scholar]
- 137. Swan AL, Mobasheri A, Allaway D, Liddell S, Bacardit J. Application of machine learning to proteomics data: classification and biomarker identification in postgenomics biology. Omics. 2013;17(12):595-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wilm M. Quantitative proteomics in biological research. Proteomics. 2009;9(20):4590-4605. [DOI] [PubMed] [Google Scholar]
- 139. Huizar CC, Raphael I, Forsthuber TG. Genomic, proteomic, and systems biology approaches in biomarker discovery for multiple sclerosis. Cell Immunol. 2020;358:104219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sun CS, Markey MK. Recent advances in computational analysis of mass spectrometry for proteomic profiling. J Mass Spectrom. 2011;46(5):443-456. [DOI] [PubMed] [Google Scholar]
- 141. Jäkel S, Williams A. What have advances in transcriptomic technologies taught us about human white matter pathologies? Front Cell Neurosci. 2020;14:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Misra BB, Langefeld CD, Olivier M, Cox LA. Integrated omics: tools, advances, and future approaches. J Mol Endocrinol. Published online July13, 2018. doi: 10.1530/jme-18-0055. [DOI] [PubMed] [Google Scholar]