Abstract
Recent studies suggest that the accumulation of atypical, 1-deoxysphingolipids that lack the C1 hydroxyl group may be associated with diabetic neuropathy (DN). We hypothesized that specific plasma 1-deoxysphingolipids associate with DN severity, and that alterations in plasma serine and alanine associate with 1-deoxysphingolipid elevation in patients with type 2 diabetes (T2D). We examined individual 1-deoxysphingolipid species using LC/MS/MS in plasma samples from 75 individuals including lean controls (LC, n = 19), those with obesity (n = 19), obesity with T2D without DN (ob/T2D, n = 18), and obesity with T2D with DN (Ob/T2D/DN, n = 19). We observed a step wise increase in 1-deoxydihydroceramides across these four groups (spearman correlation coefficient r = 0.41, p = 0.0002). Mean total concentrations of 1-deoxydihydroceramides, and most individual 1-deoxydihydroceramide species, were higher in ob/T2D/DN versus LC group (8.939 vs. 5.195 pmol/100 μL for total 1-deoxydihydroceramides p = 0.005). No significant differences in 1-deoxydihydroceramides were observed between the ob/T2D and ob/T2D/DN groups. L-alanine was higher and L-serine lower in ob/T2D/DN versus LC groups (326.2 vs. 248.0 μM, p = 0.0086 and 70.2 vs. 89.8 μM, p = 0.0110), consistent with a potential contribution of these changes to the observed 1-deoxysphingolipids profiles. 1-deoxydihydroceramides correlated inversely with leg intraepidermal nerve fiber density (CC −0.40, p = 0.003). These findings indicate that 1-deoxydihydroceramides may be important biomarkers and/or mediators of DN.
Keywords: Sphingolipids, Neuropathy, Obesity, Type 2 diabetes, Mass spectrometry, Metabolomics
1. Introduction
There are currently no disease modifying treatments available for DN and even stringent glycemic control often fails to prevent the progression of neuropathy in individuals with type 2 diabetes.1 Recent studies have suggested that 1-deoxysphingolipids may have a pathogenic relationship with diabetes and DN and could therefore serve as a novel therapeutic target. Increased plasma 1-deoxysphingolipids have been observed in individuals with metabolic syndrome, diabetes (with more pronounced elevations in type 2 diabetes than type 1 diabetes), and DN.2–7 Moreover, 1-deoxysphingolipids were disproportionately elevated in individuals with type 1 diabetes and DN versus those without DN among a subset of study participants from The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC; n = 80).8
The hypothesis that 1-deoxysphingolipids may contribute to the development of diabetic neuropathy (DN) has emerged from studying Hereditary Sensory Autonomic Neuropathy Type 1 (HSAN1). HSAN1 is a rare, inherited peripheral neuropathy that shares several clinical features with DN, including disproportionate injury to sensory axons, neuropathic pain, and a propensity to limb ulceration.9 HSAN1 results from missense mutations in serine palmitoyltransferase (SPT), which catalyzes the first and rate-limiting step in sphingolipid formation.10 Dominant mutations to SPTLC1 or SPTLC2 shift the enzyme’s substrate preference from serine to alanine and glycine, thereby generating 1-deoxysphingolipids. 1-deoxysphingolipids lack the hydroxyl head group of typical sphingolipids and, therefore, cannot be metabolized to more complex sphingolipids or degraded by canonical cellular pathways.10 The cytotoxicity of 1-deoxysphingolipids is established, particularly to neurons and cells of neuronal origin, including β-cells and retinal cells.10–14
The reason that 1-deoxysphingolipids are elevated in diabetes, or the specific 1-deoxysphingolipid molecules that correlate with DN severity are unknown. Prior studies have shown that plasma L-serine (referred to as serine from here on) is reduced in individuals with type 2 diabetes2,15–19 and that serine deficiency, regardless of the cause, can result in 1-deoxysphingolipid formation (Fig. 1A).11,17,20 It has, therefore, been postulated that serine deficiency contributes to 1-deoxysphingolipid accumulation in diabetes. Importantly, serine supplementation suppressed 1-deoxysphingolipid formation and improved neuropathic symptoms in a preclinical type I diabetes rat model,21 and in both preclinical and clinical HSAN1 studies,17,22 suggesting that the sphingolipid pathway can be targeted to prevent nerve degeneration.
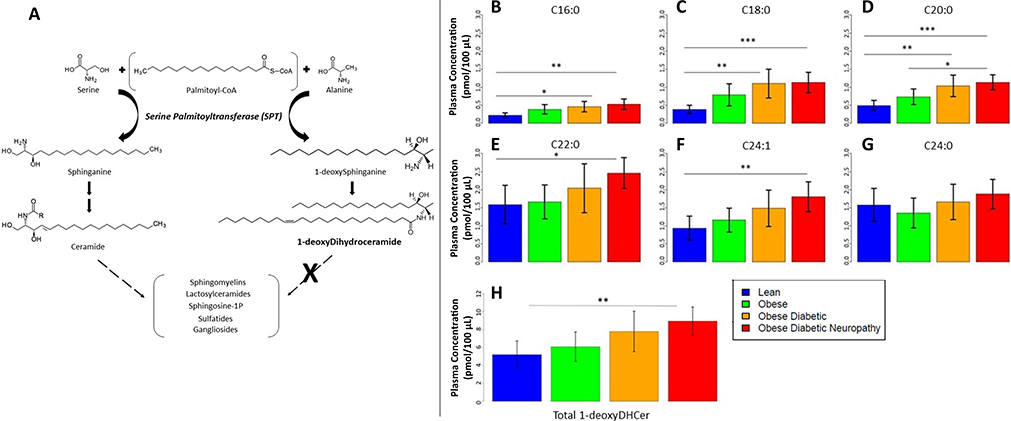
Fig. 1.
Schematic of key steps in the sphingolipid pathway & plasma 1-deoxydihydroceramide concentrations (A) Incorporating alanine instead of serine in the first step of de novo sphingolipid synthesis by SPT generates atypical 1-deoxysphingolipids that cannot be metabolized to downstream endogenous sphingolipids. (B–H) Quantification of plasma concentrations of individual 1-deoxydihydroceramide species in in lean (n = 19, blue), obese (n = 19, green), ob/T2D (n = 18, orange), and ob/T2D/DN (n = 19, red) participants: (B) C16:0 1-deoxydihydroceramide, (C) C18:0 1-deoxydihydroceramide, (D) C20:0 1-deoxydihydroceramide, (E) C22:0 1-deoxydihydroceramide, (F) C24:l 1-deoxydihydroceramide, and (G) C24:0 1-deoxydihydroceramide. Panel (H) shows total 1-deoxydihydroceramide concentrations for the four groups. Values are expressed as mean ± standard deviation (SD). *p < 0.05, **p < 0.01, ***p < 0.001. Spearman correlation coefficient (rs) for 1-deoxydihydroceramides across the four groups (lean, obese, ob/T2D, ob/T2D/DN) was 0.41, p = 0.0002.
In this study, we tested the hypotheses that specific 1-deoxysphingolipids are elevated in people with obesity and type 2 diabetes, with further elevations in those with neuropathy. We also hypothesized that 1-deoxysphingolipids associate with DN severity, and, that alterations in serine and alanine concentrations predict 1-deoxysphingolipid elevation in people with obesity and type 2 diabetes. To examine this question, we employed state of the art lipidomic and amino acid analysis on plasma from individuals who were previously formally assessed for neuropathy. We examined four groups including 1) lean controls, 2) individuals with obesity without diabetes or DN, 3) individuals with obesity and type 2 diabetes without DN, and 4) individuals with obesity, type 2 diabetes and clinically defined DN. Specifically, we evaluated 1-deoxysphingolipid and amino acid concentrations across these four groups, compared concentrations between those with obesity, type 2 diabetes and DN to those without DN, and looked at correlations between concentrations and neuropathy severity.
2. Material and methods
2.1. Subjects and study overview
Our study involved a secondary analysis of plasma from an established cohort from the University of Michigan’s bariatric surgery clinic.23 The study was approved by the University of Michigan Institutional Review Board and all participants provided written informed consent. De-identified plasma samples were collected between November 2010 and December 2014. All samples were collected prior to participants having bariatric surgery. Inclusion criteria were age 18 years or older, and a body mass index (BMI) ≥ 35 kg/m2. Exclusion criteria were BMI >70 (bariatric surgery clinic criterion), anticoagulant therapy, current tobacco, marijuana, or nicotine use, active cancer within the last year, suicide attempt in the last year or multiple suicide attempts, reliance on a wheelchair or scooter, high dose steroids, cardiac stent within the last year, history of open Nissen surgery or esophagectomy, and cirrhosis of the liver. Plasma samples from lean controls recruited from a research website (no metabolic syndrome components as defined by the NCEP/ATPIII criteria including no medication for blood pressure, cholesterol, diabetes, or triglycerides) were also collected at the University of Michigan. The presence of neuropathy was determined by the Toronto consensus definition of probable polyneuropathy, which requires two or more of the following: neuropathy symptoms, abnormal sensory examination, and abnormal reflexes as determined by one of four neuromuscular specialists.24 Diabetes was defined according to the Expert Committee on the diagnosis and classification of diabetes mellitus.25
2.2. Clinical and neuropathy assessments
Blood pressure, height, weight, BMI, and lipid panels were available for all participants. HbA1c was available for all participants other than the lean controls. All participants without a pre-existing history of diabetes had glucose tolerance testing. Validated neuropathy measures included, (i) the Michigan Neuropathy Screening Instrument Questionnaire (MNSI-Q), a 15 point “yes or no” questionnaire, with higher scores indicating increased DN severity26; (ii) the Michigan Neuropathy Screening Instrument Examination Score (MNSI-E), an 8 point score based on a brief clinical examination; (iii) routine sensory nerve conduction studies of the sural nerve; and (iv) intraepidermal nerve fiber density (IENFD) on skin biopsy at the distal leg and thigh, analyzed by bright field immunohistochemistry.27
2.3. Lipidomics
Sphingolipids were analyzed by the lipidomics core of the Nutrition Obesity Research Center at the University of Colorado. Sphingolipids were extracted from plasma (100 μL) and typical sphingolipids were detected and quantitated by liquid chromatography tandem mass spectrometry (LC-MS/MS), as previously described.28 A cocktail of internal standards for each sphingolipid subclass was added to the samples to evaluate extraction recovery and enable quantitation. Comprehensive 1-deoxysphingolipid analysis was conducted as previously described7,29 with some modifications, as stated below. C12-deoxyceramide, C12-deoxyDHceramide, C12-deoxyMeceramide, and C12-deoxyMeDHceramide were added as internal standards (1 pmol each). LC was performed on a Kinetex C18, 50 × 3 mm, 2.6 μm column (Phenomenex) at 300 μL/min with a gradient from 50% solvent B (isopropanol/acetonitrile/5 mM ammonium formate in formic acid, v/ v/v, 32.5/66.5/1) to 98% in 1.5 min, held at 98% for 12.5 min, and reequilibrated at 50% for 3 min (solvent A was methanol/water/5 mM ammonium formate in formic acid, v/v/v, 58/41/1). MS/MS was performed on a Sciex QTRAP 5500 Triple Quad mass spectrometer using positive ionization in the MRM mode. Standard curves were built using C16-deoxyDHceramide reference standards to quantitate C16-, C18-, and C20-deoxyDHceramides, and C24:1-deoxyDHceramide reference standards to quantitate C22-, C24:1-, and C24-deoxyDHceramides. Prior studies evaluating 1-deoxysphingolipids in human plasma have varied in their methodology, with some studies measuring 1-deoxysphingolipid concentrations after total hydrolysis and reporting elevations in deoxysphinganine and deoxymethylsphinganine (the sum of free 1-deoxysphinganine or 1-deoxysphingosine and 1-deoxysphinganine or 1-deoxysphingosine derived from hydrolysis of 1-deoxydihydroceramide species). Analysis of non-hydrolyzed plasma samples was performed in this study in order to distinguish between specific 1-deoxysphingolipid molecules.
2.4. Amino acid determination
Plasma serine and alanine concentrations were measured by the Metabolomics Core Facility at the University of Colorado. Plasma samples (20 μL) were thawed on ice and extracted with 480 μL of ice-cold lysis/extraction buffer (methanol/acetonitrile/water, v/v/v, 5/3/2) containing 1 μM of stable isotope labeled amino acid mix (13C-, 15N-labeled; Cambridge Isotope Laboratories, cat no MSK-A2–1.2). Samples were extracted and analyzed on a Vanquish ultrahigh performance LC (UHPLC) coupled online to a Q Exactive mass spectrometer (Thermo Scientific) with data analyzed by the software Maven, exactly as previously described.30
2.5. Statistical methods
Means for 1-deoxysphingolipids, typical sphingolipids, amino acids, and neuropathy measures were compared by ANOVA type models among the groups: lean, obese, obese with T2D (ob/T2D), and obese with T2D and DN (ob/T2D/DN). Different residual variances were allowed for different groups. Overall F tests and Tukey-Kramer adjustments of the pair-wise comparisons were performed. 1-deoxyshigolipids were also analyzed with ANCOVA type models, with the differences between groups adjusted for ordinary sphingolipids. Correlations between the different variables were investigated. Spearman correlations were used for correlating the ordinal groups with other variables. The threshold for statistical significance was alpha = 0.05. The mediation of the relationship between amino acids and neuropathy by 1-deoxyshigolipids was studied by comparing unadjusted correlations between amino acids and neuropathy measures with partial correlations adjusted for 1-deoxysphingolipids. For the obese group, without diabetes or neuropathy, leg NFD was classified as either normal or abnormal, according to the standards for each age group and sex, and 1-deoxyshingolipids were compared between the normal and abnormal groups with a t-test.
2.6. Data and resource availability
Data not provided in the article because of space limitations as well as the statistical analysis will be shared at the request of any qualified investigator for purposes of replicating procedures and results.
3. Results
We analyzed plasma from 75 subjects including those who were obese (n = 19), ob/T2D (n = 18), and ob/T2D/DN (n = 19), and from lean controls (n = 19) (Table 1). Subjects had a mean age of 50.5 years (SD = 8.1) and were 63.2% (n = 48) female and 36.8% (n = 28) male. Subjects in the four groups were similar in sex and age, and those with diabetes with and without DN were similar in BMI (p value = 0.800) and %HbA1c (p value = 0.643). Individuals in the obese, ob/T2D, and ob/T2D/DN groups had reduced IENFD in both the leg and thigh as compared to lean controls (Table 1), suggesting that participants who did not yet meet clinical criteria for DN already demonstrated evidence of subclinical neuropathy.
Table 1.
Clinical characteristics and neuropathy outcome measures (mean ± SD)
| Lean (n = 19) | Ob (n = 19) | Ob/T2D (n = 18) | Ob/T2D/DN (n = 19) | |
|---|---|---|---|---|
| Sex (%M/F) | 37/63 | 37/63 | 39/61 | 37/63 |
| Age (years) | 48.4 ± 10.0 | 51.6 ± 7.3 | 48.2 ± 6.7 | 53.7 ± 7.3 |
| Weight (kg) | 68.4 ± 11.8 | 134.8 ± 24.7*** | 131.8 ± 24.9*** | 132.6 ±29.6*** |
| BMI (kg/m2) | 23.5 ± 1.5 | 48.1 ± 8.9*** | 45.3 ± 6.0*** | 46.1 ± 8.3*** |
| %HbA1C | N/A | 5.7 ± 0.31 | 7.18 ± 1.2††† | 7.4 ± 0.85††† |
| Total cholesterol (mg/dL) | 184.4 ±46.7 | 168.7 ± 38.0 | 155.0 ±44.7 | 135.8 ± 34.5**,† |
| HDL (mg/dL) | 66.5 ± 20.3 | 45.3 ± 8.7** | 40.3 ± 11.6*** | 38.8 ± 10.9*** |
| LDL (mg/dL) | 108.2 ± 25.3 | 99.6 ±31.4 | 101.8 ± 64.3 | 67.5 ± 31.7***,† |
| TAG (mg/dL) | 80.0 ± 19.7 | 123.4 ±61.6* | 163.4 ± 127.4 | 185.8 ± 158.3* |
| Diabetes medication use (yes/no) | 0/19 | 0/19 | 15/3 | 18/1 |
| Statin use (yes/no) | 0/19 | 4/15 | 11/7 | 15/4 |
| MNSI-Q | 0.37 ± 0.76 | 2.5 ± 2.4** | 3.2 ± 2.5** | 6.9 ± 2.6***,†††,‡‡‡ |
| MNSI-E | 0.34 ± 0.88 | 1.0 ± 1.28 | 0.97 ± 1.29 | 2.79 ± 2.12***,†,‡ |
| Sural amplitude (pV) | 17.3 ±6.6 | 10.3 ± 5.3** | 9.4 ± 4.9** | 5.4 ± 5.0***,† |
| Leg IENFD (fibers/mm) | 11.4 ±5.0 | 6.1 ± 3.5** | 5.6 ± 6.0* | 3.0 ± 3.5*** |
| Thigh IENFD (fibers/mm) | 25.82 ± 8.21 | 12.44 ± 8.07*** | 16.28 ± 8.08** | 11.68 ± 7.27*** |
Values are expressed as mean ± standard deviation (SD).
statistically significant compared to lean (Tukey-Kramer adjusted p value: *p < 0.05, **p < 0.01, ***p < 0.001).
statistically significant compared to obese (Tukey-Kramer adjusted p value: †p < 0.05, ††p < 0.01 †††p < 0.001).
statistically significant compared to ob/T2D (Tukey-Kramer adjusted p value: ‡p < 0.05, ‡‡p < 0.01, ‡‡‡p < 0.001).
statistically significant compared to ob/T2D/DN (Tukey-Kramer adjusted p value: §p < 0.05, §§p < 0.01, §§§ p < 0.001).
3.1. 1-deoxysphingolipid profiles differ in individuals with obesity, T2D, and DN
We evaluated plasma samples from all subjects using LC-MS/MS for 1-deoxysphingolipid species (deoxysphinganine, deoxysphingosine, 1-deoxyceramides, 1-deoxydihydroceramides, 1-deoxymethylceramides, and 1-deoxymethyldihydroceramides), but only detected 1-deoxydihydroceramides. An increase in 1-deoxydihydroceramides was observed across the four ordinal groups (lean, obese, ob/T2D, ob/T2D/DN), with a Spearman correlation coefficient (CC) of rs = 0.41 and p = 0.0002 for total 1-deoxydihydroceramides (Fig. 1B–H, Supplemental Table 1). Adjusting for pair-wise comparisons, mean total 1-deoxydihydroceramide concentrations, as well as most individual 1-deoxydihydroceramide species, were higher in ob/T2D/DN versus lean control group (8.939 vs. 5.195 pmol/100 μL for total 1-deoxydihydroceramides, p = 0.005) (Fig. 1B–H). No significant differences in 1-deoxydihydroceramides were observed between the ob/T2D and ob/T2D/DN groups (p = 0.8127 for total 1-deoxydihydroceramides). 1-deoxydihydroceramides positively correlated with triglyceride levels (CC of 0.42, p = 0.0001, for total 1-deoxydihydroceramides), data not shown.
3.2. Amino acid levels differ in individuals with obesity, T2D, and DN
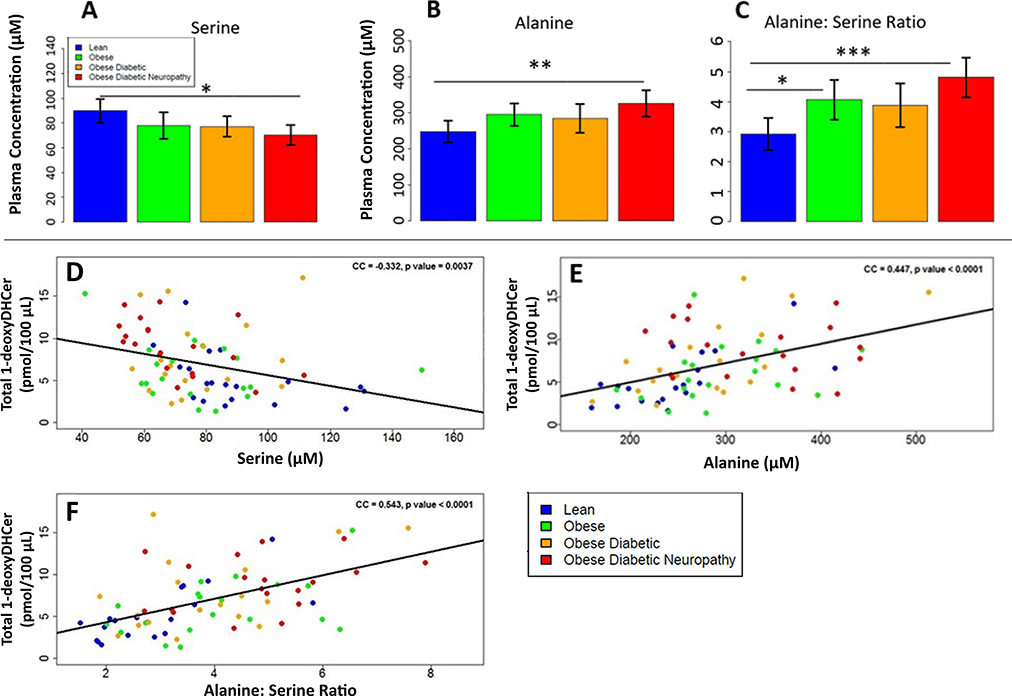
Plasma serine, alanine, and glycine concentrations were determined (Fig. 2A–C). Participants in the ob/T2D/DN group had lower serine levels than lean controls (70.3 vs. 89.9 μM, p = 0.0110). Plasma alanine and alanine:serine ratios were higher in ob/T2D/DN versus lean controls (p = 0.0086 for alanine and p = 0.0003 for alanine:serine ratio). No significant differences in serine, alanine or alanine:serine ratios were observed between the ob/T2D and ob/T2D/DN groups (3.88 vs. 4.81, p = 0.052 for alanine:serine ratio). Glycine, the other amino acid that SPT can metabolize to 1-deoxysphingolipids, was not detected, so, we could not evaluate differences between the groups.
Fig. 2.
Plasma amino acid concentrations and correlations of amino acid and 1-deoxydihydroceramide concentrations (A–C) Quantification of plasma concentrations of amino acids: (A) serine, (B) alanine, and (C) alanine:serine in lean (n = 19, blue), obese (n = 19, green), ob/T2D (n = 18, orange), and ob/T2D/DN (n = 19, red). Values are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001. (D–F) Correlation plots of amino acid with total plasma 1-deoxydihydroceramide concentrations: (D) serine, (E) alanine, and (F) alanine:serine within lean (n = 19, blue), obese (n = 19, green), ob/T2D (n = 18, orange), and ob/T2D/DN (n = 19, red). 1-deoxyDHcer = 1-deoxydihydroceramides.
To examine the relationship between amino acid profiles and 1-deoxysphingolipids across the spectrum of disease we examined the entire cohort. We observed a negative correlation between serine and all the 1-deoxydihydroceramide species (CC −0.332, p = 0.0037, for total 1-deoxydihydroceramides) (Fig. 2D, Supplemental Table 2). In contrast, alanine concentration and alanine:serine ratios correlated positively with total 1-deoxydihydroceramides (CC 0.447, p < 0.001, for alanine; CC 0.543, p < 0.001, for alanine:serine ratio) (Fig. 2E–F, Supplemental Table 2).
3.3. Relationship between amino acids, 1-deoxydihydroceramides, and DN severity
3.3.1. 1-deoxydihydroceramides and DN severity
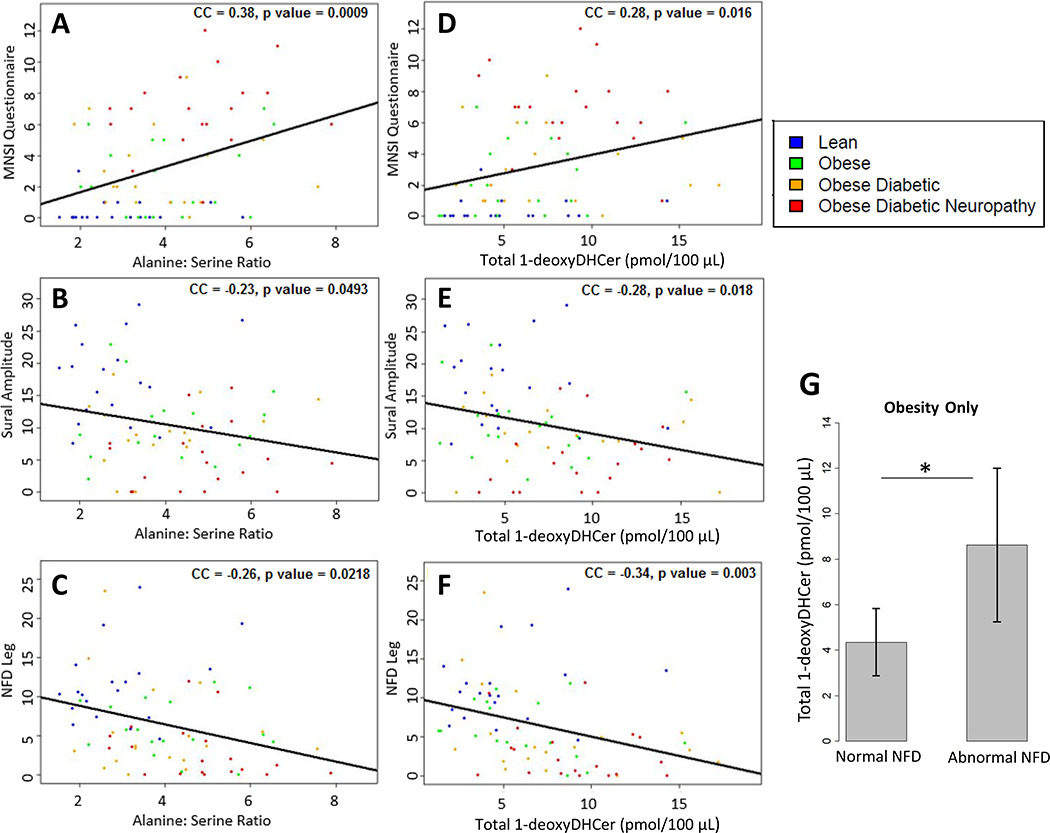
To test the relationship between amino acid profiles, 1-deoxysphingolipids, and DN severity, we evaluated a series of well-established DN outcomes, clinical and objective (Fig. 3, Supplemental Table 3). One participant did not have data for IENFD leg and two participants did not have sural amplitudes. Given that individuals in the obese and ob/T2D groups had evidence of subclinical neuropathy as assessed by IENFD, we first evaluated molecular-clinical correlations across all groups. All 1-deoxydihydroceramide species except for C24 1-deoxydihydroceramide correlated with DN severity as defined by MNSI-Q, sural amplitude, and leg IENFD [for total 1-deoxydihydroceramides: CC 0.28, p = 0.016 for MNSI-Q; CC −0.28, p = 0.02 for sural amplitude; CC −0.34, p < 0.003 for leg IENFD (Fig. 3D–F, Supplemental Table 3)]. Several 1-deoxydihydroceramide species also negatively correlated with the IENFD in the thigh, though the correlation of total 1-deoxydihydroceramides and IENFD in the thigh did not reach statistical significance (CC −0.21, P = 0.070). No correlation was appreciated between 1-deoxydihydroceramides and the MNSI-E (Supplemental Table 3).
Fig. 3.
Plasma amino acid and 1-deoxydihydroceramide concentrations and DN severity (A–C) Correlation plots of alanine:serine with: (A) MNSI-Q (higher score denotes more severe DN), (B) sural nerve amplitude, and (C) leg NFD for lean (n = 19, blue), obese (n = 19, green), ob/T2D (n = 18, orange), and ob/T2D/DN (n = 19, red) subjects. (D–F) Correlation plots of total plasma 1-deoxydihydroceramide concentrations with: (D) MNSI-Q (E) sural nerve amplitude, and (F) leg NFD. (G) Comparison of 1-deoxydihydroceramide concentrations in participants with obesity only and normal versus abnormal NFD. *p < 0.05. 1-deoxyDHCer = 1-deoxydihydroceramides. NFD = nerve fiber density.
3.4. Amino acids and DN severity
We observed a positive correlation between alanine and alanine:serine ratios with DN severity as measured by the MNSI-Q (CC 0.32, p = 0.006; CC 0.38, p = 0.0009; respectively), MNSI-E (CC 0.23, p = 0.04; CC 0.27, p = 0.0173; respectively), and sural sensory amplitudes (CC −0.25, p = 0.033; CC −0.23, p = 0.0493; respectively) (Fig. 3A–C). Alanine correlated negatively with IENFD leg (CC −0.26, p = 0.026), but did not show a significant correlation with IENFD thigh (−0.22, p = 0.057). Alanine:serine ratios also correlated negatively with both leg and thigh IENFDs (CC −0.32, p = 0.0056 and CC −0.26, p = 0.0218 respectively). Serine concentrations did not show a significant correlation with DN severity. A mediation analysis revealed that controlling for 1-deoxydihydroceramides attenuated the correlation of alanine:serine ratios and DN severity (CC −0.32, p = 0.006 versus CC −0.17, p = 0.157 for IENFD leg), supporting their role in the causal pathway.
3.5. Molecular-clinical correlations in individuals with obesity, T2D and DN
In order to minimize skewing of the data resulting from the inclusion of lean controls, we next examined only those individuals at risk for DN (obese, ob/T2D, and ob/T2D/DN). We observed a negative correlation between total 1-deoxydihydroceramide concentrations and alanine:serine ratios and distal leg IENFD only (CC −0.40, p = 0.003; CC −0.27, p = 0.04, respectively). No correlation was evident between either 1-deoxydihydroceramides or alanine: serine ratios and IENFD in the thigh, MNSI-Q, MNSI-E or sural amplitudes (NS P-values). It is worth noting that although prior studies and our findings demonstrate a positive correlation between 1-deoxydihydroceramide and triglycerides (CC 0.42, p-0.0001), the relationship between 1-deoxydihydroceramides and distal leg IENFD persisted after adjusting for triglycerides in this cohort (CC −0.35, p = 0.010).
Given that obesity alone is an increasingly recognized driver of neuropathy,31–33 we also compared 1-deoxydihydroceramides in participants with obesity only and normal versus abnormal IENFD leg, as previously defined.34 We found that total 1-deoxydihydroceramies were increased in those with reduced IENFD (n = 7) as opposed to those with normal values (n = 11), (8.626 versus 4.353 pmol/100 μL, p = 0.021) (Fig. 3G).
3.6. Alterations in typical ceramides in individuals with obesity, T2D, and DN
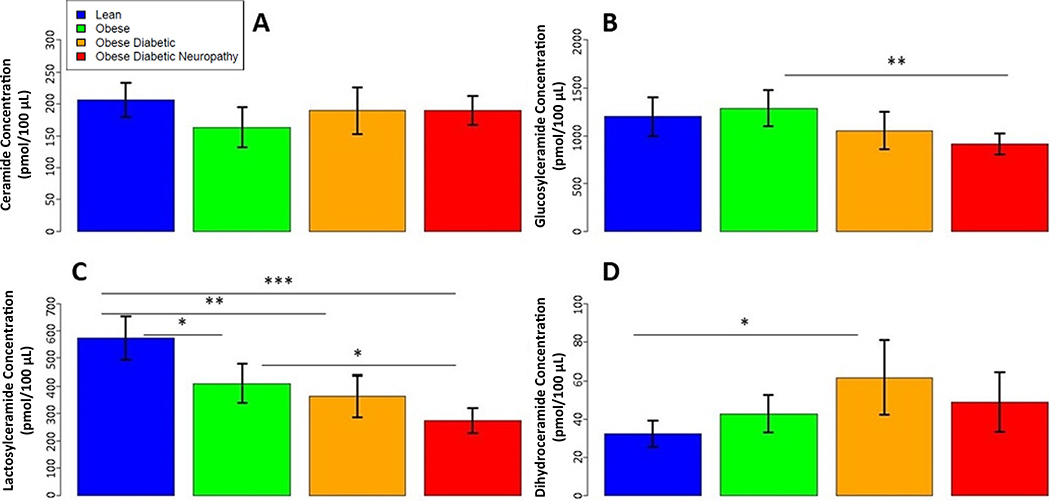
To garner a better sense of the overall plasma sphingolipid distribution, we evaluated typical ceramide, glucosylceramide, lactosylceramide, and dihydroceramide species, (Table 1, Fig. 4). No differences in ceramide concentrations were observed between the four groups in our cohort. Estimate mean lactosylceramides decreased with each ordinal group, from lean, to obese, to ob/T2D, to ob/T2D/DN, with significant reductions observed in the latter three groups as compared to lean controls (ob/T2D/DN vs. lean: 274.04 vs. 573.15 pmol/100 μL, p < 0.0001). (FIG. 4). Adjusting for lactosylceramides, in ANCOVA type models, also increased the observed differences in mean total 1-deoxydihydroceramides between the groups (Supplemental Table 4).
Fig. 4.
Plasma concentrations of typical ceramide species Quantification of plasma concentrations of: (A) total ceramides, (B) glucosylceramides, (C) lactosylceramides, and (D) dihydroceramides, in lean (n = 19, blue), obese (n = 19, green), ob/T2D (n = 18, orange), and ob/T2D/DN (n = 19, red). Values are expressed as mean ± SD. *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
Our study revealed an increase in specific 1-deoxydihydroceramides across the four groups, from lean controls, to obesity only, to obesity and type 2 diabetes without DN, to obesity, type 2 diabetes and clinically defined DN. It also uncovered a previously unknown relationship between increased alanine:serine ratios with 1-deoxydihydroceramide elevation in T2D. This study adds to our current knowledge by examining the relationship of the 1-deoxysphingolipid pathway and objective DN biomarkers, including intraepidermal nerve fiber density (IENFD). We identified a correlation between 1-deoxydihydroceramide concentrations and multiple markers of DN severity, consistent with the potential pathogenicity of these molecules in DN. Plasma 1-deoxydihydroceramide concentrations did not differ between individuals with diabetes with and without clinical DN in this cohort.
Taken together, our results provide evidence supporting the importance of 1-deoxydihydroceramides in DN and longitudinal studies are needed to verify these results. Given that similar alterations in the sphingolipid pathway have been observed in other neuropathic conditions, and that 1-deoxysphingolipids have been effectively targeted to improve neuropathy outcomes, our findings may have implications for the development of future DN treatments.
Emerging evidence reveals that 1-deoxysphingolipids are elevated in a number of neuropathic conditions. In addition to HSAN1, 1-deoxysphingolipids are associated with paclitaxel- and mitochondrial disease-induced neuropathy.20,35 Additionally, Ganter et al. recently demonstrated elevated 1-deoxysphingolipids in individuals with macular telangiectasia type 2, with and without peripheral neuropathy.11 1-deoxysphingolipids are not universally elevated in neuropathies, but rather appear to be disease-specific.4 The observation that 1-deoxysphingolipids correlate with objectively measured DN in this study is consistent with a potential pathogenic role as reported in other neuropathies.
Prior reports examining 1-deoxysphingolipids have employed varied technical methods. Herein, we have employed targeted sphingolipid analysis of non-hydrolyzed plasma in order to enable the identification of specific 1-deoxydihydroceramide species in individuals with obesity, type 2 diabetes, and DN. This is a useful strategy as specific species may be responsible for pathological events, e.g., the 1-deoxydihydroceramides identified in this study are known to disrupt the cytoskeletal stability of cultured sensory neurons13 and are the predominant species responsible for neurotoxic effects in macular telangiectasia type 2.11 Due to the use of different lipidomics methods, we cannot directly compare our study results to previously reported levels of 1-deoxysphingolipids in diabetes or other neuropathies. However, the sum of all 1-deoxydihydroceramides herein are comparable to deoxysphinganine concentrations in HSAN1 and MacTel type 211,17 and exceed the levels required for neurotoxic effects in vitro.35,36 The 1-deoxydihydroceramide species we detected in our cohort are distinct from the C24:0 and C24:1 deoxyceramides observed in individuals with self-reported symptoms of DN in the DCCT/EDIC type 1 diabetes study.8 These unique lipidomic profiles may reflect the varied ways in which DN was assessed in the two studies, and/or the distinct pathophysiology of DN in type 1 and type 2 diabetes.
The use of validated clinical DN outcome assessments in this study is unique and demonstrates a negative relationship between 1-deoxydihydroceramides and IENFD in the distal leg. Dhorn et al. previously demonstrated elevations in 1-deoxysphinganine and 1-deoxysphingosine in individuals with type 2 diabetes and DN compared to age-matched healthy controls, but did not observe a correlation between these molecules and the clinical stage of DN as defined by the Dyck classification.4 The discrepancy in these findings may be attributable to the use of non-hydrolyzed plasma samples and skin biopsy IENFD in our study. IENFD is increasingly used as a DN outcome measure because many people with early DN experience isolated small fiber neuropathy, which is not detected by nerve conduction studies.37 The molecular-clinical correlations in our study are modest and will need to be validated in larger studies of similarly phenotyped individuals with DN.
DN in this report was defined by clinical examination according to the Toronto consensus definition of probable neuropathy. Interestingly, we found that 7 of the 18 subjects with obesity and no diabetes that did not meet our neuropathy definition had an abnormal IENFD leg, and that these subjects had higher levels of 1-deoxydihydroceramides that those with normal IENFD (p = 0.02). This finding is consistent with prior observations that 1-deoxysphingolipids are elevated in metabolic syndrome,7 and supports the possibility that 1-deoxydihydroceramides are associated with the development of neuropathy related to obesity, independently of glycemic status. Given the mounting evidence that obesity is an independent risk factor for neuropathy,31–33 and the pathological evidence for neuropathy in our obese only cohort, we cannot say with certainty that the neuropathy examined in our study is purely DN, rather than an obesity related or “metabolic” neuropathy.
As outlined in Fig. 1A, 1-deoxyshingolipids are known to increase under conditions of L-serine depletion, and lower serine and higher alanine concentrations have been reported in diabetic and normoglycemic obese individuals.2,16 Similarly, we found that serine was lowest and alanine highest in obese, type 2 diabetes and DN subjects. Consistent with prior findings in macular telangiectasia type 2,11 we also found that 1-deoxydihydroceramides negatively correlated with serine and positively correlated with alanine in this type 2 diabetes cohort. Our findings also demonstrate a novel relationship between alanine:serine ratios and DN severity, as defined by IENFD, which may have clinical use as a biomarker. Furthermore, mediation analysis showed an attenuated correlation between alanine:serine ratios and IENFD when controlling for 1-deoxydihydroceramines, supporting their role in the causal pathway.
The observation that alanine:serine ratios may be contributing to elevated 1-deoxysphingolipids in DN is supported in interventional studies. Preclinical studies in HSAN1, macular telangiectasia type 2, and animal models of insulin resistant and insulin deficient diabetes with DN have demonstrated that the 1-deoxysphingolipid pathway can be manipulated to affect neuropathy and glycemic outcomes.11,17,21,38 In a clinical trial of HSAN1, we demonstrated that serine supplementation reduced 1-deoxysphingolipid levels and improved neuropathy severity without significant adverse effects.22 These findings suggest that the sphingolipid pathway can be targeted, and that serine supplementation could potentially be used to reduce 1-deoxysphingolipids and ameliorate clinical symptoms in DN.
In addition to evaluating atypical 1-deoxysphingolipds, we also analyzed typical plasma sphingolipids. We examined a series of glycosphingolipids, which are composed of a ceramide and sugar moiety. Plasma lactosylceramides were significantly lower in obese, ob/T2D, and ob/T2D/DN participants compared to lean controls. To our knowledge, this is the first time that reduced plasma lactosylceramides have been observed in T2D and DN; however, lowered plasma lactosylceramides have been shown to predict macroalbuminuria in diabetic individuals, a separate but frequent microvascular complication.39 Lactosylceramides are upstream of ganglioside formation, which is required to support axon-myelin interactions. It is therefore conceivable that reductions in lactosylceramides prevent the formation of select gangliosides, resulting in nerve injury or, alternatively, that an increase in ganglioside formation in individuals with obesity and type 2 diabetes depletes plasma lactosylceramides.
Our study is limited by small sample size and, perhaps owing to that, we did not detect significant differences in 1-deoxysphingolipids between type 2 diabetes participants with and without DN. However, the objective increase observed across the groups and the correlations with DN severity align with a potential pathogenic contribution of 1-deoxysphingolipids to DN. Furthermore, DN is known to occur over a continuum and 1-deoxysphingolipid accumulation could precede the onset of clinical DN. Importantly, our study also only examined 1-deoxydihydroceramide concentrations in plasma, which may not reflect that found in neurons.11,17 Our analysis of amino acids was limited by our inability to detect plasma glycine, or to account for dietary amino acid intake. Another consideration is that the use of a morbidly obese population (BMI > 40) in this study may limit generalizability to individuals with diabetes with overweight or stage 1 obesity, as well as to lean diabetics. Our study included individuals who had undergone extensive clinical outcome assessments for neuropathy and sets the stage for larger studies evaluating the deoxysphingolipid pathway in DN.
5. Conclusion
In summary, our findings indicate that 1-deoxydihydroceramides may be important biomarkers and/or mediators of DN. The correlations between plasma 1-deoxysphingolipids, circulating serine and alanine levels, and DN severity are also intriguing in the context of other “serineopathies”. Furthermore, the selective vulnerability of peripheral nerves in numerous nerve disorders and retinal cells in Macular Telangiectasia Type 2 to low serine concentrations and elevations in 1-deoxydihydroceramides makes a compelling case for their potential role in these microvascular complications of type 2 diabetes. Future mechanistic studies will be needed to determine whether these atypical molecules are truly pathogenic in DN, and to further evaluate their potential utility as biomarkers and therapeutic targets.
Supplementary Material
Acknowledgements
The authors would like to thank all of the patients who participated in this study. The authors also thank Jacob Bockhorst, BA for his assistance with manuscript preparation.
Funding
The study described was supported by Grant Number P30DK020572 (MDRC) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Dr. Callaghan is currently funded by a NIH NIDDK R-01 award (DK115687). The study was also supported by the Colorado Clinical and Translational Science Institute Co-Pilot Award (CO-M-19–34 CCTSI to V·F) and the Colorado Nutrition Obesity Research Center grant P30DK048520.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: V·F, Z.S, S·S, H·K, B·C·B, E.L.F, and R.J.E.B have no conflicts of interest.
C·B Dr. Callaghan consults for a PCORI grant, DynaMed, and performs medical legal consultations including consultations for the Vaccine Injury Compensation Program.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jdiacomp.2021.107852.
References
- 1.Callaghan BC, Little AA, Feldman EL, Hughes RA. Enhanced glucose control for preventing and treating diabetic neuropathy. Cochrane Database Syst Rev 2012;6 (6) 10.1002/14651858.CD007543.pub2.CD007543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertea M, Rutti MF, Othman A, et al. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis 2010;9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Othman A, Saely CH, Muendlein A, et al. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res Care 2015;3, e000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dohrn MF, Othman A, Hirshman SK, et al. Elevation of plasma 1-deoxy-sphingolipids in type 2 diabetes mellitus: a susceptibility to neuropathy? Eur J Neurol 2015;22: 806–14. [e855]. [DOI] [PubMed] [Google Scholar]
- 5.Wei N, Pan J, Pop-Busui R, et al. Altered sphingoid base profiles in type 1 compared to type 2 diabetes. Lipids Health Dis 2014;13:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mwinyi J, Bostrom A, Fehrer I, et al. Correction: plasma 1-deoxysphingolipids are early predictors of incident type 2 diabetes mellitus. PLoS One 2017;12, e0179313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Othman A, Rutti MF, Ernst D, et al. Plasma deoxysphingolipids: a novel class of biomarkers for the metabolic syndrome? Diabetologia 2012;55:421–31. [DOI] [PubMed] [Google Scholar]
- 8.Hammad SM, Baker NL, El Abiad JM, et al. Increased plasma levels of select deoxyceramide and ceramide species are associated with increased odds of diabetic neuropathy in type 1 diabetes: a pilot study. Neuromolecular Med 2016;19:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houlden H, King R, Blake J, et al. Clinical, pathological and genetic characterization of hereditary sensory and autonomic neuropathy type 1 (HSAN I). Brain 2006;129: 411–25. [DOI] [PubMed] [Google Scholar]
- 10.Penno A, Reilly MM, Houlden H, et al. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem 2010;285: 11178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gantner ML, Eade K, Wallace M, et al. Serine and lipid metabolism in macular disease and peripheral neuropathy. N Engl J Med 2019;381:1422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuellig RA, Hornemann T, Othman A, et al. Deoxysphingolipids, novel biomarkers for type 2 diabetes, are cytotoxic for insulin-producing cells. Diabetes 2014;63:1326–39. [DOI] [PubMed] [Google Scholar]
- 13.Guntert T, Hanggi P, Othman A, et al. 1-Deoxysphingolipid-induced neurotoxicity involves N-methyl-d-aspartate receptor signaling. Neuropharmacology 2016;110: 211–22. [DOI] [PubMed] [Google Scholar]
- 14.Wilson ER, Kugathasan U, Abramov AY, et al. Hereditary sensory neuropathy type 1-associated deoxysphingolipids cause neurotoxicity, acute calcium handling abnormalities and mitochondrial dysfunction in vitro. Neurobiol Dis 2018;117:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bervoets L, Massa G, Guedens W, Louis E, Noben JP, Adriaensens P. Metabolic profiling of type 1 diabetes mellitus in children and adolescents: a case-control study. Diabetol Metab Syndr 2017;9:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drabkova P, Sanderova J, Kovarik J, kandar R. An assay of selected serum amino acids in patients with type 2 diabetes mellitus. Adv Clin Exp Med 2015;24:447–451. [DOI] [PubMed] [Google Scholar]
- 17.Garofalo K, Penno A, Schmidt BP, et al. Oral L-serine supplementation reduces production of neurotoxic deoxysphingolipids in mice and humans with hereditary sensory autonomic neuropathy type 1. J Clin Invest 2011;121:4735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamakado M, Nagao K, Imaizumi A, et al. Plasma free amino acid profiles predict four-year risk of developing diabetes, metabolic syndrome, dyslipidemia, and hypertension in Japanese population. Sci Rep 2015;5:11918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihalik SJ, Michaliszyn SF, de las Heras J, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care 2012;35:605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer R, Bielawski J, Kistner-Griffin E, et al. Neurotoxic 1-deoxysphingolipids and paclitaxel-induced peripheral neuropathy. FASEB J 2015;29:4461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Othman A, Bianchi R, Alecu I, et al. Lowering plasma 1-deoxysphingolipids improves neuropathy in diabetic rats. Diabetes 2015;64:1035–45. [DOI] [PubMed] [Google Scholar]
- 22.Fridman V, Suriyanarayanan S, Novak P, et al. Randomized trial of l-serine in patients with hereditary sensory and autonomic neuropathy type 1. Neurology 2019;92: e359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callaghan BC, Reynolds EL, Banerjee M, et al. The prevalence and determinants of cognitive deficits and traditional diabetic complications in the severely obese. Diabetes Care 2020;43:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tesfaye S, Boulton AJ, Dyck PJ, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010;33: 2285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26:3160–7. [DOI] [PubMed] [Google Scholar]
- 26.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994;17:1281–9. [DOI] [PubMed] [Google Scholar]
- 27.Lauria G, Hsieh ST, Johansson O, et al. European Federation of Neurological Societies/peripheral nerve society guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol 2010;17:903–12. [e944–909]. [DOI] [PubMed] [Google Scholar]
- 28.Harrison KA, Bergman BC. HPLC-MS/MS methods for diacylglycerol and sphingolipid molecular species in skeletal muscle. Methods Mol Biol 1978;2019:137–52. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz NU, Mileva I, Gurevich M, Snider J, Hannun YA, Obeid LM. Quantifying 1-deoxydihydroceramides and 1-deoxyceramides in mouse nervous system tissue. Prostaglandins Other Lipid Mediat 2019;141:40–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nemkov T, D’Alessandro A, Hansen KC. Three-minute method for amino acid analysis by UHPLC and high-resolution quadrupole orbitrap mass spectrometry. Amino Acids 2015;47:2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Callaghan BC, Reynolds E, Banerjee M, Chant E, Villegas-Umana E, Feldman EL. Central obesity is associated with neuropathy in the severely obese. Mayo Clin Proc 2020;95:1342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callaghan BC, Xia R, Reynolds E, et al. Association between metabolic syndrome components and polyneuropathy in an obese population. JAMA Neurol 2016;73:1468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callaghan BC, Xia R, Banerjee M, et al. Metabolic syndrome components are associated with symptomatic polyneuropathy independent of glycemic status. Diabetes Care 2016;39:801–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauria G, Bakkers M, Schmitz C, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. J Peripher Nerv Syst 2010;15:202–7. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira CR, Goorden SMI, Soldatos A, et al. Deoxysphingolipid precursors indicate abnormal sphingolipid metabolism in individuals with primary and secondary disturbances of serine availability. Mol Genet Metab 2018;124:204–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zitomer NC, Mitchell T, Voss KA, et al. Ceramide synthase inhibition by fumonisin B1 causes accumulation of 1-deoxysphinganine: a novel category of bioactive 1-deoxysphingoid bases and 1-deoxydihydroceramides biosynthesized by mammalian cell lines and animals. J Biol Chem 2009;284:4786–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pittenger GL, Ray M, Burcus NI, McNulty P, Basta B, Vinik AI. Intraepidermal nerve fibers are indicators of small-fiber neuropathy in both diabetic and nondiabetic patients. Diabetes Care 2004;27:1974–9. [DOI] [PubMed] [Google Scholar]
- 38.Holm LJ, Haupt-Jorgensen M, Larsen J, Giacobini JD, Bilgin M, Buschard K. L-serine supplementation lowers diabetes incidence and improves blood glucose homeostasis in NOD mice. PLoS One 2018;13, e0194414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopes-Virella MF, Baker NL, Hunt KJ, et al. Glycosylated sphingolipids and progression to kidney dysfunction in type 1 diabetes. J Clin Lipidol 2019;13:481–91. [e481]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.