Abstract
Introduction
Programmed cell death 1 ligand 1 (PD-L1) can be upregulated in cancer cells via interferon gamma (IFNγ) in the tumor microenvironment. IFNγ/PD-L1 signaling is associated with the response to immune checkpoint blockade in melanoma patients. Our previous investigation indicated that the microsatellite instability-high (MSI-H) cell line might exhibit selective hyperresponsiveness to IFNγ treatment, which contributes to increased PD-L1 expression and may be a mechanism of response to anti-PD-1 therapy in colorectal cancer.
Methods
The present study evaluated the expression of PD-L1 in a set of MSI and microsatellite stability (MSS) cell lines with IFNγ treatment. The differential signaling molecules associated with signal transducer and activator of transcription (STAT) contributing to hyperresponsiveness to IFNγ exposure were also investigated. Furthermore, we established a coculture assay containing CT26 cells with higher expression of PD-L1 and peripheral blood mononuclear cells (PBMCs) in vitro. Changes in cancer cell viability as well as apoptosis status in response to anti-PD-1 therapy were demonstrated. We further observed changes in the percentage of CD4+ and CD8+ lymphocytes after PD-1 immunotherapy in the coculture assay. Finally, the average extent of inflammation and adaptive immunity factors in the assay was also investigated.
Results
This in vitro study revealed that the MSI cell line might exhibit hyperresponsiveness to IFNγ exposure, and IFNγ induced upregulation of PD-L1 mainly through increased STAT1 and decreased STAT3 signaling. IFNγ/PD-L1 signaling participated in the response to anti-PD-1 therapy mainly through the CTL profile.
Discussion
Our findings reinforce previous knowledge of the fact that the response to immune checkpoint blockade occurs mainly in patients with a preexisting intratumoral IFNγ/PD-L1 signal, thus suggesting potential therapeutic strategies to enhance responsiveness to PD-1 blockade immunotherapy in most patients with colorectal cancer.
Keywords: IFNγ, PD-L1, CTL, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer in the world.1 With the aging of the population and changes in lifestyle, the incidence of colorectal cancer in China has gradually increased.2 Tumor surgery and chemotherapy are the main treatments for patients with colorectal cancer.3 However, innumerable side effects induced by chemotherapy-based treatment cannot be ignored. Currently, growing numbers of targeted drugs are also available to treat metastatic/progressed colorectal cancer,4 including epidermal growth factor receptor (EGFR)-targeted therapy, vascular endothelial growth factor receptor (VEGFR)-targeted therapy and immune checkpoint (IC) inhibitor immunotherapy. These monoclonal antibodies targeting IC have drawn much attention and displayed promising therapeutic effects in the field of solid tumor therapy.5–7
Programmed cell death 1 ligand 1 (PD-L1) is a critical IC molecule. After binding to its receptor, programmed death (PD-1), PD-L1 delivers a suppressive signal to T cells and an anti-apoptotic signal to tumor cells, leading to T cell dysfunction and tumor survival. Blockade of PD-1/PD-L1 results in the preferential activation of T cells with specificity for cancer and restoration of antitumor activity.8 PD-1 inhibitors have become the second-line or third-line treatment recommended by the National Comprehensive Cancer Network guidelines for patients with advanced colorectal cancer who display mismatch repair deficiency (dMMR)/microsatellite instability-high (MSI-H). However, only 5% to 15% of colorectal cancers display the MSI-H genotype,9 which leaves the majority of patients with CRC of the microsatellite stability (MSS) genotype, without immunotherapy options.10,11 If the mechanism for the heterogeneity of the responses to anti-PD-1 therapy is elucidated, it could guide clinicians to identify other potentially targetable factors and determine therapeutic strategies to enhance responsiveness to PD-1 blockade immunotherapy in most CRC patients.
PD-L1 may be induced by oncogenic signals or can be upregulated via interferon gamma (IFNγ). In the tumor microenvironment, tumor-infiltrated lymphocytes produce IFNγ and drive PD-L1 expression in cancer cells; however, the extent of PD-L1 induction is not uniform.12–14 IFNγ/PD-L1 signaling is an important marker for predicting the response to immune checkpoint blockade in NSCLC and melanoma patients.15 Defects in the IFNγ signaling pathway are one of the main mechanisms of resistance to anti-PD-1 immunotherapy.16,17 It is currently accepted that differential expression of the PD-L1 protein exists between MSI-H and MSS genotypes for CRC patients.18–20 There seems to be some evidence to indicate that PD-L1 expression shows more signals of antitumor activity during PD-1 blockade therapy.21,22 Our previous investigation23 indicated that an MSI cell line might exhibit selective hyperresponsiveness to IFNγ treatment, which contributes to increased PD-L1 expression and may be a mechanism of the response to anti-PD-1 therapy in colorectal cancer. Furthermore, the differential molecular mechanisms associated with the signal transducer and activator of transcription (STAT) signaling pathway contributing to the high PD-L1 expression induced by IFNγ exposure were not investigated in the MSI cell line, and the profiles of adaptive immunity factors for PD-L1 overexpression treated with PD-1 blockade, in general, remain to be determined.
Accordingly, the present study investigated partial differential signaling molecules associated with STATs contributing to hyperresponsiveness to IFNγ exposure in an MSI cell line. The profiles of adaptive immunity factors for PD-L1 overexpression treated with PD-1 blockade in a coculture assay in vitro were also observed. Our results provide novel insights into the mechanism by which anti-PD-1 therapy focuses on IFNγ/PD-L1 signaling, thus suggesting potential therapeutic strategies to enhance responsiveness to PD-1 blockade immunotherapy in most patients with CRC.
Materials and Methods
Cell Line Culture and IFNγ Treatment in vitro
Human colorectal cancer cell lines of the MSI genotype (HCT116, LOVO, HCT15) and MSS genotype (SW480, SW620, SW1116) were obtained from the Institute of Zoology, Chinese Academy of Sciences (Kunming, China), and mouse colorectal cancer CT26 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The PD-L1-overexpressing CT26 cell line was generated as previously described.23 In brief, the cDNA sequence of the mouse PD-L1 was generated by PCR using 5ʹ-CCGGAATTCGCCACCATGAGGATATTTGCTGGCATTATATTC-3ʹ as the forward primer and 5ʹ-CCGCTCGAGTTACGTCTCCTCGAATTGTGTATCA-3ʹ as the reverse primer. Lentiviruses were produced by transient transfection of 293T cells using PGMLV-GTP-PD-L1 constructed plasmid. After transfection, virus samples were harvested and concentrated. Transduction of CT26 cells was performed by 6 h of exposure to dilutions of the viral supernatant in the presence of polybrene (5 µg/mL). The abovementioned cell lines were plated in 6-well plates at a density of 2×105 cells/well and cultured in the presence of IFNγ (100 IU/mL, PeproTech, USA).
Isolation and Activation of Peripheral Blood Mononuclear Cells
PBMCs were isolated out of blood from healthy donors, which was obtained from the blood transfusion center in Kunming, Yunnan. A total of 10 mL of EDTA-anticoagulant peripheral blood was collected. The source of peripheral blood was mouse. Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Hypaque (Sigma, St. Louis, MO, USA) density gradient centrifugation from anticoagulant peripheral blood samples. PBMCs were activated by the addition of 1% PHA (Sigma, St. Louis, MO, USA) and IL-2 (2000 IU/mL, PeproTech, USA) to cultures.
Cancer Cells and PBMC Coculture Experiments
IFNγ pretreated cancer cells were seeded in a 96-well plate (5×104 per well) in complete RPMI and cocultured with activated PBMCs (1×105 per well). The PD-1 antibody was used at a final concentration of 10 μg/mL to block the PD-1/PD-L1 interaction.
Cell Proliferation Viability Assay
Cell proliferation viability was measured using Cell Counting Kit-8 (CCK-8; Beyotime, China) according to the manufacturer’s instructions. Cell viability (%)=OD450 (experiment - No-treatment control)/OD450 (Conditional control - No-treatment control) ×100%.
RT-qPCR
Total cell RNA was extracted using TRIzol reagent (Thermo, USA) and then reverse transcribed with the Revert First Strand cDNA Synthesis Kit (Thermo, USA) according to the manufacturer’s instructions. Reverse-transcription quantitative polymerase chain reaction (RT-qPCR) was performed using ABI-7500 (Applied Biosystems, USA). The primer sequences are described as previously.23
Western Blot
The cell lysates (80 μg) were harvested, separated and then transferred onto PVDF membranes as previously described.23 The membranes were blocked with 5% bovine serum albumin for 1 h at room temperature before the addition of 5 mL of primary antibody. The antibodies used in this study included anti-PD-L1 (1:500; Proteintech) and anti-β-actin (1:2000; Abmart). The membrane was then washed with PBS and incubated with rabbit anti-HRP-conjugated secondary antibody (1:2000; Abmart)) for 1 h at room temperature. The bands were visualized using a chemiluminescence reagent (Millipore, USA).
Flow Cytometry
The following antibodies were used for FACS analysis: CD274-PE, CD4-FITC, and CD8-FITC (eBioscience). The carboxyfluorescein succinimidyl ester (CFSE) for the proliferation assays was purchased from eBioscience. Cell samples were harvested, washed and stained with 5 μL of antibody for 30 min to avoid light exposure. Cells were washed, resuspended in 500 μL of PBS and transferred to FACS tubes for analysis. Cells were analyzed using a Canto II (BD Biosciences) FACS machine.
TUNEL Assay
TUNEL staining was performed using an in situ cell death detection kit (Roche), and nuclei were stained with DAPI for 10 min. The number of TUNEL-positive cells (red color) and total number of cells (blue color) were captured with a fluorescence microscope (Olympus). The rate of cell apoptosis was measured by the number of red cells divided by the number of blue cells.
Statistical Analysis
All statistical analyses were performed using SPSS software version 21.0. Values are given as the mean ± SDs, and the differences were assessed with 2-sided tests. Statistical significance was set at p values less than 0.05.
Results
IFNγ Induced a Higher Intensity of PD-L1 Expression in MSI Cell Lines
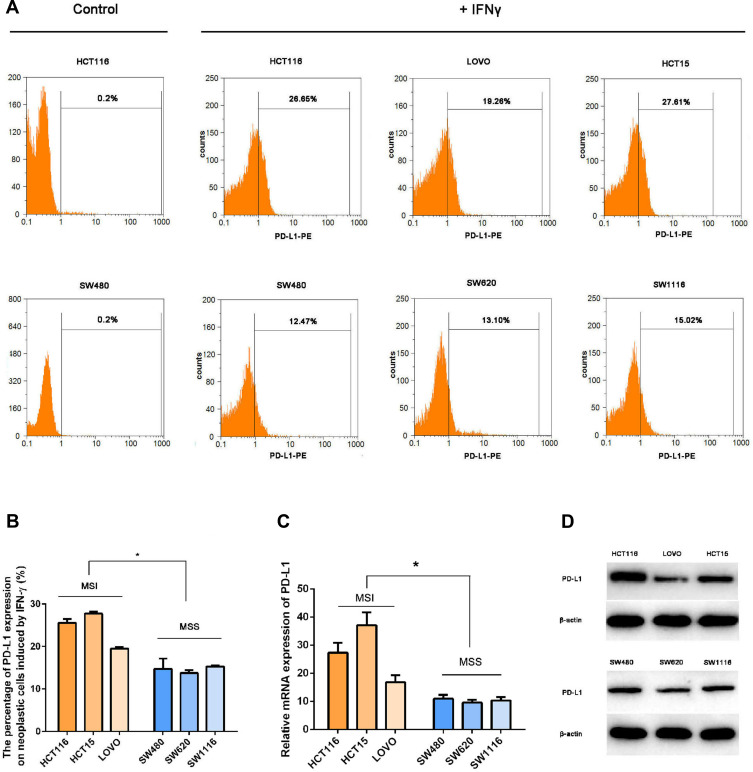
To investigate whether IFNγ responsiveness is related to MSI status, we evaluated the expression of PD-L1 in different cell lines induced by IFNγ via RT-qPCR, Western blot and FCM. The basic level of PD-L1 oncogenic expression in CRC cell lines was extremely low. Treatment with the same concentrations of IFNγ in MSI (HCT116, LOVO, HCT15) cell lines displayed a higher intensity of PD-L1 expression compared with MSS (SW480, SW620, SW1116) status (Figure 1).
Figure 1.
IFNγ induced a higher intensity of PD-L1 expression in MSI cell lines. To investigate whether IFNγ responsiveness is related to MSI status, we evaluated the expression of PD-L1 in different cell lines induced by IFNγ via RT-qPCR, Western blot and FCM. (A and B) PD-L1 expression was measured by flow cytometry in MSI (the upper line: HCT116, LOVO and HCT15) and MSS (the lower line: SW480, SW620 and SW1116) cell lines 12 h after treatment with 100 IU/mL IFNγ. DMSO was used as a negative control (*P < 0.05). (C) PD-L1 mRNA relative expression induced by IFNγ (100 IU/mL IFNγ for 12 h) was measured by qPCR (*P < 0.05). (D) Expression levels of the PD-L1 protein stimulated with 100 IU/mL IFNγ for 12 h.
Upregulation of PD-L1 by IFNγ in CRC Cell Lines Was Mainly Associated with STAT1 Activation, and STAT3 Signaling Molecules Might Contribute to Hyporesponsiveness to IFNγ in MSS Cell Lines in vitro
PD-L1 expression levels may be associated with intrinsic oncogenic changes and exogenous cytokine induction. We observed that the basic level of PD-L1 oncogenic expression was extremely low, and IFNγ treatment induced high level of PD-L1 expression on neoplastic cells. Therefore, the expression induced by exogenous cytokines including IFNγ, was the main source of expression of PD-L1 on CRC cells. It is well known that PD-L1 expression stimulated by IFNγ was mainly controlled by the JAK-STAT, MAPK or PI3K-AKT pathways. Therefore, we used the ERK1/2 inhibitor PD98059 (APExBIO, 50 μmol/L), the AKT inhibitor wortmannin (APExBIO, 10 μmol/L), or the STAT1 inhibitor fludara (APExBIO, 50 μmol/L) to explore the signaling mechanism of PD-L1 expression induced by IFNγ (100 IU/mL) in HCT15 and SW620 cells. We evaluated the expression of PD-L1 in human colorectal cancer cells pretreated with the ERK1/2 inhibitor PD98059 (APExBIO, 50 μmol/L), the AKT inhibitor wortmannin (APExBIO, 10 μmol/L), or the STAT1 inhibitor fludara (APExBIO, 50 μmol/L) by FCM. As shown in Figure 2 in detail, PD-L1 expression was significantly downregulated in cell lines with differential MSI status when pretreated with fludara. With IFNγ treatment, both MSI and MSS cell lines displayed significant phosphorylation of STAT1. Twelve hours later, the expression of STAT1 in both types of CRC cell lines significantly increased (Figure 2D). In contrast, there was no significant alteration in STAT3 expression after treatment with IFNγ in HCT116 and HCT15 cell lines that displayed the MSI genotype. Interestingly, only MSS cell lines (SW480 and SW620) displayed obviously enhanced expression of STAT3 signaling molecules after IFNγ exposure, which showed significantly increased phosphorylation and protein levels, respectively.
Figure 2.
Effects of IFNγ and kinase inhibitors on PD-L1 expression in the MSI and MSS cell lines. (A–C) PD-L1 expression was measured by flow cytometry in HCT15 (the upper line) and SW620 (the lower line) cell lines 12 h after treatment with 100 IU/mL IFNγ, with 1 μmol/L wortmannin (AKT inhibitor), 50 μmol/L PD98059 (ERK1/2 inhibitor), or 50 μmol/L fludara (STAT1 inhibitor). DMSO was used as a vehicle and negative control (*P < 0.05 vs cells treated with IFNγ and DMSO). (D) Expression levels of STAT1, p-STAT1, STAT3 and p-STAT3 in MSI cell lines (HCT116 and HCT15) as well as MSS cell lines (SW480 and SW620) with 100 IU/mL IFNγ for 15 min and 12 h, respectively.
PD-L1 Expression Influenced Neoplastic Cells in Response to PD-1 Blockade
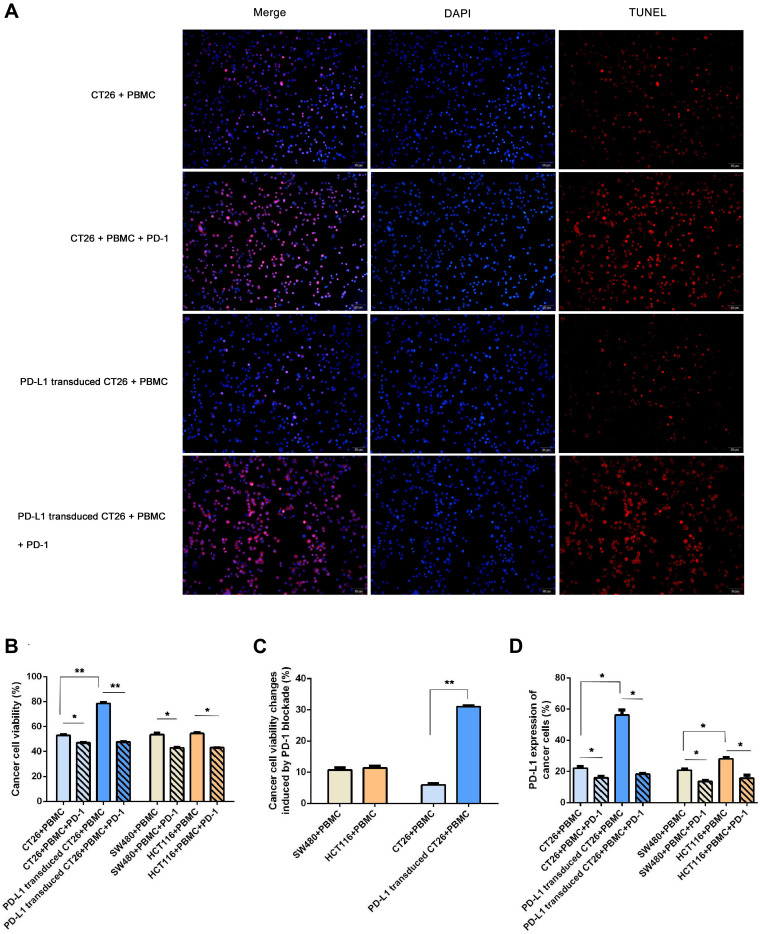
To investigate whether PD-L1 expression influences the response of PD-1 blockade immunotherapy in vitro, we performed two different profiles with higher PD-L1 expression in a coculture assay: PD-L1 transduced CT26 cell challenge (a higher extent of PD-L1 overexpression) and HCT116 enrolled (a lower degree of PD-L1 overexpression). As shown in Figure 3, higher PD-L1 expression in the PD-L1-transduced CT26 coculture system exhibited obviously enhanced neoplastic cell viability as well as decreased cell apoptosis. No significant difference was observed between the HCT116 and SW480 groups. Furthermore, immunotherapy with PD-1 blockade significantly inhibited cancer cell viability in line with enhanced cell apoptotic rate, especially in the coculture group enrolled with PD-L1 transduced CT26 cells (Figure 3C). All neoplastic cells in different coculture systems treated with PD-1 blockade displayed similar cell viability and apoptotic rates.
Figure 3.
PD-L1 expression influenced neoplastic cells in response to PD-1 blockade. To investigate whether PD-L1 expression influences the response of PD-1 blockade immunotherapy in vitro, we performed two different profiles with higher PD-L1 expression in a coculture assay: PD-L1 transduced CT26 cell challenge (a higher extent of PD-L1 expression) and HCT116 enrolled (a lower degree of PD-L1 expression). (A) Cell apoptosis shown by TUNEL staining (×40, scale bar is 20 μm) in the four coculture assays. Shown by fluorescence microscopy, red indicates apoptotic cells stained by TUNEL, and blue indicates nuclei stained by DAPI. The number of TUNEL-positive cells (red color) and total number of cells (blue color) were captured with a fluorescence microscope. The rate of cell apoptosis was measured by the number of red cells divided by the number of blue cells. The apoptotic rate was (26.00±3.61)% in group 1 (CT26+PBMC), (12.33±3.21)% in group 3 (PD-L1 transduced CT26+PBMC), (90.33±2.52)% in group 2 (CT26+PBMC+PD-1), and (92.00±2.65)% in group 4 (PD-L1 transduced CT26+PBMC+PD-1). The apoptosis rate of group 3 was significantly decreased compared with that of group 1 (p=0.001). PD-1 blockade significantly induced an increased apoptosis rate in CT26-challenged (p=0.003), or PD-L1-transduced CT26 cell-exposed (p=0.000). No significant difference was observed among the two PD-1 blockade groups. (B and C) Effect of PD-1 blockade on viability of CT26, PD-L1 transduced CT26, SW480 and HCT116 cells. Cells were treated with IFNγ plus PD-1 blockade for 12 hours. Cell proliferation viability was measured using Cell Counting Kit-8 assay. Data represented as mean ± SE of three independent experiments made in three replicates (*P < 0.05, **P < 0.001). (D) Effect of PD-1 blockade on PD-L1 expression of CT26, PD-L1 transduced CT26, SW480 and HCT116 cells. Cells were treated with IFNγ plus PD-1 blockade for 12 hours. PD-L1 expression of cancer cells was measured using flow cytometry (*P < 0.05, **P < 0.001).
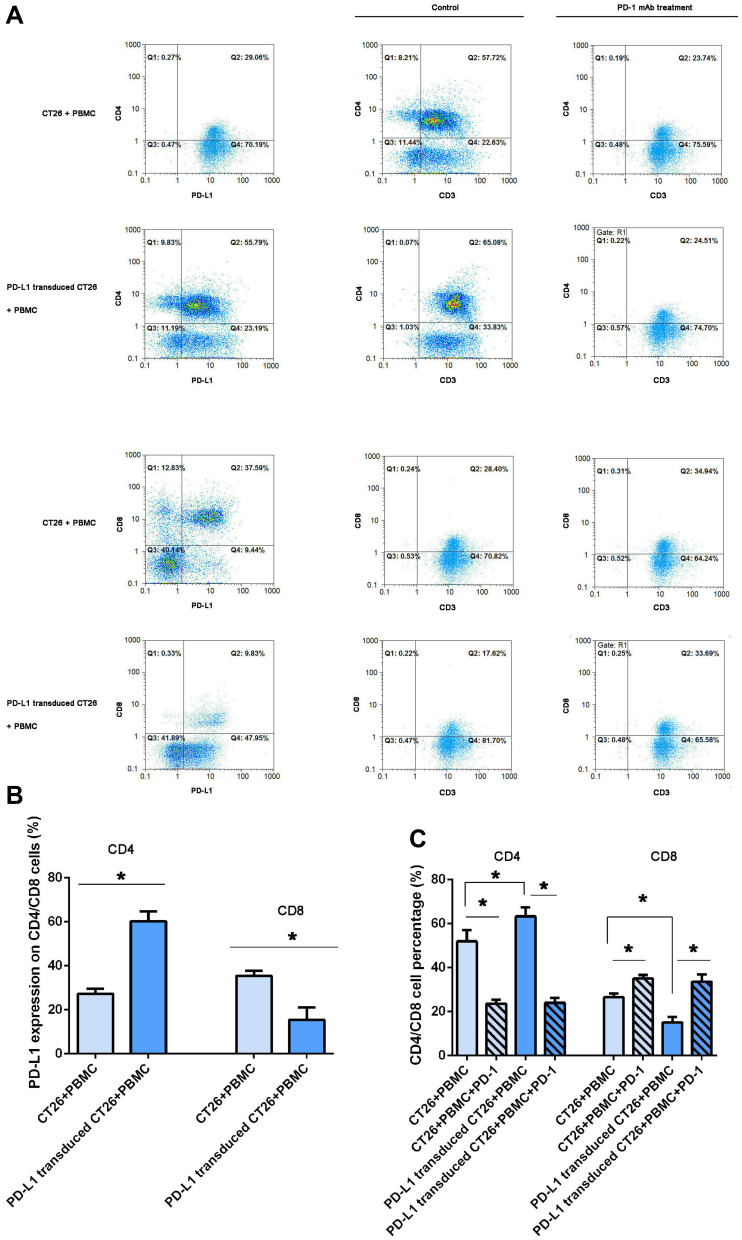
PD-L1 Overexpression on CT26 Cells Influenced PD-L1 Expression on CD4+ and CD8+ Lymphocytes
As shown in Figure 4A and B, we observed that PD-L1 protein was also extensively expressed on CD4+ and CD8+ lymphocytes in the coculture assay. To investigate whether the higher PD-L1 expression on CT26 cells influenced PD-L1 expression on cocultured mononuclear cells, we further performed fluorescence double staining for PD-L1 and CD4/CD8 by FCM analysis. The results demonstrated that PD-L1 expression on CD8+ lymphocytes was clearly decreased when challenged with PD-L1 overexpression. However, an inverse increase in PD-L1 expression on CD4+ lymphocytes was observed in the same coculture system.
Figure 4.
PD-L1 overexpression on CT26 cells influenced PD-L1 expression on CD4+/CD8+ lymphocytes and PD-1 blockade induced changes in CD4+/CD8+ cell counts. Analysis of PD-L1 expression on lymphocytes in a coculture assay and CD4+/CD8+ T cells by flow cytometry. (A) After 12 h following activation in the presence of 100 IU/mL IFNγ in the two coculture assays, the mononuclear cells were collected and PD-L1 expression was assessed by flow cytometry (the left column). CD4 and CD8 expression was, respectively, detected in the two coculture assays treated with IFNγ plus PD-1 blockade for 12 hours (the right column). One representative sample of three is shown. (B) The frequency of CD4+PD-L1+ and CD8+PD-L1+ T cells, and the frequency of CD4+CD3+ and CD8+CD3+ T cells (C) in different coculture assays was statistical analyzed (*P < 0.05).
PD-1 Blockade Induced Changes in CD4+ or CD8+ Cell Percentage
To observe changes in CD4+ or CD8+ cell percentage, we performed FCM analysis for CD4 or CD8 expression in the two coculture system, as shown in Figure 4A and C. PD-L1 overexpression on CT26 cells significantly decreased the percentage of CD8+ lymphocytes in line with increased CD4+ cells in the coculture assay. As expected, the percentage of CD8+ lymphocytes clearly increased in response to PD-1 blockade. However, the expression of CD4+ cells was interestingly decreased after anti-PD-1 immunotherapy (Figure 4). After PD-1 blockade, PD-L1 overexpression on CT26 cells did not influence the percentage of CD4+ or CD8+ cells in those coculture systems.
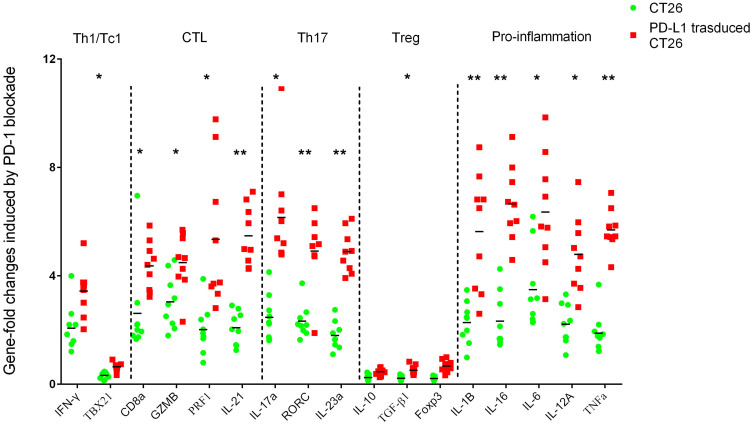
PD-L1 Overexpression Influenced Inflammation and Adaptive Immunity in Response to Anti-PD-1 Immunotherapy
To observe the effect of PD-L1 overexpression on the average extent of adaptive immunity in response to PD-1 blockade, we performed RT-qPCR for selected genes encoding signature T cell cytokines as well as core transcription factors for the major T helper subsets CD4, Th1/Tc1 (TBX21 and IFNγ), CTL (CD8A, GZMB, PRF1, IL21), Th17 (IL-17A, RORC, IL23A) and Treg (IL10, TGF-β1). The sets of genes associated with proinflammatory factors (IL1B, IL16, IL6, IL12A, TNF-α) were also analyzed (Figure 5). In response to immunotherapy with PD-1 mAb, genes encoding CD8, Th17 and pro-inflammatory factors were prominently enhanced, especially in cells harvested from the PD-L1 overexpression assay. However, associated genes of CD4, Th1/Tc1 and Treg were mostly decreased when challenged with anti-PD-1 immunotherapy.
Figure 5.
Immune-related gene expression profiles were assessed using RT-qPCR for selected genes. Sets of genes were defined by functional relevance: CD4, Th1/Tc1, CTL, Th17, Treg and pro-inflammation related gene. To analyze the different responses to PD-1 blockade in a coculture assay with CT26 enrolled (the legend is “CT26”, blue) or PD-L1 overexpressing CT26 challenged (the legend is “PD-L1 transduced CT26”, red), we calculated the fold change in gene expression using the 2−ΔΔCt method. *Indicates statistically significant differences, p<0.05; **indicates p<0.001.
Discussion
In this study, we found that cancer cell lines with the MSI genotype displayed higher PD-L1 expression induced by IFNγ in vitro. Here, IFNγ responsiveness is related to MSI status, and this result is also consistent with most clinical investigations that show that MSI patients have a significantly higher rate of positivity for PD-L1 expression.22,24 Recent investigation25 have suggested that IFNγ treatment converts immunologically quiet tumors into “hot” tumors and works in concert with anti-PD1 therapy to provide patient benefit. The mechanism may be partly associated with the increased expression of PD-L1 on tumor cells.
PD-L1 expression on tumor cells may be generally induced by oncogenic signals26,27 or can be upregulated via IFNγ.28–30 Considering the similar low PD-L1 expression on CRC cells without IFNγ exposure in vitro, we then explored whether IFNγ/PD-L1 signaling molecules may have different effects on the response to IFNγ treatment in MSI cell lines. In our experiment, IFNγ exposure in vitro induced increased PD-L1 mRNA expression in line with the enhanced protein expression, which demonstrated that the upregulated PD-L1 molecules on CRC cell lines may be the result of IFNγ/PD-L1 signaling activation and new mRNA transcription, rather than translocation of preexisting intracellular PD-L1 protein stores to the cell surface. We also showed that IFNγ induces the expression of PD-L1 by increasing STAT1 signaling in all CRC cell lines. This raises the possibility that drugs targeting STAT1 might improve IFNγ-induced PD-L1 expression and enhance the efficiency of anti-PD-1 immunotherapy.
As shown in the current report, only MSS cell lines displayed obviously enhanced expression of STAT3 signaling molecules after IFNγ exposure. IFNγ decreased cytokine-induced activation of STAT3 while increasing STAT1 signaling.14,31–33 STAT1 may compete for STAT binding sites on the receptor, thereby interfering with the activation of STAT3. This demonstration of the involvement of distinct signaling pathways in driving PD-L1 expression in MSS genotype cells suggests new strategies for targeting STAT3 to improve the responsiveness of anti-PD-1 therapy in colorectal cancer. The broad spectrum of biological roles for STAT1/3 suggests that it could be difficult to target this factor specifically or selectively in MSS tumor cells, but these theoretical mechanisms should be kept in mind in future studies.
A literature review34 highlighted that blockade of PD-1/PD-L1 ligation results in the preferential activation of T-cells with specificity for cancer, restoring antitumor T cell activity. It is currently accepted that PD-1 blockade immunotherapy might play a remarkable role in tumor rejection through activated CD8+ cytotoxic T lymphocytes (CTLs). In our experiment, anti-PD-1 immunotherapy in the PD-L1-overexpressing CT26 cell coculture system in vitro displayed the highest extent of cancer cell viability rejection, in line with the increased apoptosis of CT26 cells, enhanced expression of CTL genes, Th17 genes and proinflammatory genes, and higher levels of CD8+ lymphocytes, compared with the CT26 cell coculture models treated with the same blockade. Consistent with our formal studies23 in vivo, the experiment suggested that experimental PD-L1 overexpression on CT26 cells might play a remarkable role in tumor cell viability rejection in response to PD-1 immunotherapy, possibly through infiltrating cytotoxic T lymphocytes as well as proinflammatory factors, which promote cell apoptosis in the colorectal cancer microenvironment. Extremely elevated enhancement of CTL genes was not observed in the PD-L1-overexpressing CT26 cell coculture system after PD-1 blockade. Furthermore, it was a bit depressed that neither terminal neoplastic cell viability nor apoptotic rate in coculture assays could be influenced by experimental PD-L1 overexpression on CT26 cells in the course of PD-1 blockade, which partly associated with the limited infiltrating cytotoxic T lymphocytes in the coculture assays in vitro. However, to the best of our knowledge, our present study is the first report observed that PD-1 blockade immunotherapy in PD-L1 higher-expression neoplastic cell interestingly displayed the higher extent of cancer cell viability rejection compared with the CT26 cell coculture models treated with the same blockade. Furthermore, the expression of Th17-associated genes also displayed an enhanced profile after PD-1 immunotherapy, especially in the PD-L1-overexpressing CT26 cell coculture assay. Published data35 have shown that the IL-17A level is increased in the tumor tissues of CRC patients. Interestingly, PD-1 blockade increased the expression of Th17-associated genes. The paradoxical tendency induced by anti-PD-1 therapy might be partly associated with the decreased expression of Treg-associated genes. Lessening Treg immunity is favorable for eliminating neoplastic cells that have undergone PD-1 immunotherapy. In response to PD-1 blockade, experimental PD-L1 overexpression on CT26 cells induced prominently enhanced expression of genes encoding Th17, in line with the significantly decreased level of Treg-associated genes. It is probably concluded that PD-L1 overexpression on CT26 cells has little effect on maintaining the balance of Th17/Treg cells in the coculture assay, which responds to PD-1 blockade.
On the other hand, we observed that experimental PD-L1 overexpression on CT26 cells in the coculture assay was accompanied by a decreased amount of CD8+ lymphocytes in line with enhanced CD4+ lymphocytes. Recently, investigations36 in gastric cancer patients demonstrated that positive PD-L1 status in tumor cells (tPD-L1) was associated with a higher density of CD8+ TILs. Nevertheless, tPD-L1 was associated with an increased number of CD4+ TILs only in the nonmetastasis group. The discrepancy in CD8+ cell changes might be partly attributed to the profile of higher PD-L1 expression on cancer cells. These data suggest that experimentally overexpressed PD-L1 in CT26 cells probably acted like a high-level molecular shield, resulting in anti-apoptosis of cells as well as decreased CD8+ lymphocytes. Although recent studies37 were compelling as to whether the higher density of PD-L1 expressed in tumor cells or in tumor-infiltrating myeloid cells in the stroma was featured more prominently in anti-PD-1 therapy in CRC, this study also extensively observed PD-L1 expression on CT26 neoplastic cells and CD4+/CD8+ lymphocytes in a coculture system in vitro. Specifically, PD-L1 expression on CD4+/CD8+ lymphocytes might be associated with changes in the number of lymphocytes.
In conclusion, this in vitro study revealed that the MSI cell line might exhibit hyperresponsiveness to IFNγ exposure, and IFNγ induced upregulation of PD-L1 mainly through increased STAT1 and decreased STAT3 signaling. Furthermore, IFNγ/PD-L1 signaling also participated in the response to anti-PD-1 therapy mainly through the CTL profile. Our findings reinforce previous knowledge of the fact that the response to immune checkpoint blockade occurs mainly in patients with a preexisting intratumoral IFNγ/PD-L1 signal. Understanding the complex mechanisms will open new opportunities for developing pharmaceutical targeted combination therapies to enhance the effects of anti-PD-1 immunotherapy.
Acknowledgments
We would like to thank Xiaojin Li (Central laboratory, The Affiliated Hospital of Yunnan University, The second hospital of Yunnan Province) for his technical assistance in flow cytometry.
Funding Statement
This study was partially supported by the National Natural Science Foundation of China (grant number: 82002259), by the Association Foundation Program of Yunnan Provincial Science and Technology Department and Kunming Medical University (grant number: 2019FE001 (−256)) and by the Foundation of Yunnan Health training Project of High level talents (grant number: D-2017053 and H-2019046).
Cell Line Authentication
Human CRC cell lines were obtained from the Institute of Zoology, Chinese Academy of Sciences (Kunming, China), and rat CRC CT26 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). Cell Bank, Type Culture Collection, Chinese Academy of Sciences (CBTCCCAS) can provide the Certificate of STR Analysis.
Ethical Approval and Ethical Standards
This article does not contain any studies with human participants performed by any of the authors.
Disclosure
The authors declare no conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Zhang J, Chen G, Li Z, et al. Colonoscopic screening is associated with reduced Colorectal Cancer incidence and mortality: a systematic review and meta-analysis. J Cancer. 2020;11(20):5953–5970. doi: 10.7150/jca.46661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349 [DOI] [PubMed] [Google Scholar]
- 4.Hull R, Francies FZ, Oyomno M,et al. Incidence and risk factors: in search for targeted therapies. Cancer Manag Res. 2020;12(12):9869–9882. doi: 10.2147/CMAR.S251223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nasser NJ, Gorenberg M, Agbarya A. First line immunotherapy for non-small cell lung cancer. Pharmaceuticals (Basel). 2020;13(11):E373. doi: 10.3390/ph13110373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuryk L, Bertinato L, Staniszewska M, et al. From conventional therapies to immunotherapy: melanoma treatment in Review. Cancers (Basel). 2020;12(10):3057. doi: 10.3390/cancers12103057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aly R, Aujla AS, Gupta S, et al. Evolving paradigms in the management and outcomes of sarcomatoid renal cell carcinoma in the era of immune checkpoint inhibitors. World J Oncol. 2020;11(5):183–187. doi: 10.14740/wjon1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balar AV, Weber JS. PD-1 and PD-L1 antibodies in cancer: current status and future directions. Cancer Immunol Immunother. 2017;66(5):551–564. doi: 10.1007/s00262-017-1954-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers. 2004;20(4–5):199–206. doi: 10.1155/2004/368680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marmorino F, Boccaccino A, Germani MM, et al. Immune checkpoint inhibitors in pMMR metastatic colorectal cancer: a tough challenge. Cancers (Basel). 2020;12(8):2317. doi: 10.3390/cancers12082317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jácome AA, Eng C. Role of immune checkpoint inhibitors in the treatment of colorectal cancer: focus on nivolumab. Expert Opin Biol Ther. 2019;19(12):1247–1263. doi: 10.1080/14712598.2019.1680636 [DOI] [PubMed] [Google Scholar]
- 12.Ritprajak P, Azuma M. Intrinsic and extrinsic control of expression of the immunoregulatory molecule PD-L1 in epithelial cells and squamous cell carcinoma. Oral Oncol. 2015;51(3):221–228. doi: 10.1016/j.oraloncology.2014.11.014 [DOI] [PubMed] [Google Scholar]
- 13.Kursunel MA, Esendagli G. The untold story of IFN-γ in cancer biology. Cytokine Growth Factor Rev. 2016;31:73–81. doi: 10.1016/j.cytogfr.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 14.Mimura K, Teh JL, Okayama H, et al. PD-L1 expression is mainly regulated by interferon gamma associated with JAK-STAT pathway in gastric cancer. Cancer Sci. 2018;109(1):43–53. doi: 10.1111/cas.13424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karachaliou N, Gonzalez-Cao M, Crespo G, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol. 2018;10:1758834017749748. doi: 10.1177/1758834017749748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7(2):188–201. doi: 10.1158/2159-8290.CD-16-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eriksen AC, Sørensen FB, Lindebjerg J, et al. Programmed death ligand-1 expression in stage II colon cancer - experiences from a nationwide population based cohort. BMC Cancer. 2019;19(1):142. doi: 10.1186/s12885-019-5345-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salem ME, Puccini A, Grothey A, et al. Landscape of tumor mutation load, mismatch repair deficiency, and PD-L1 expression in a large patient cohort of gastrointestinal cancers. Mol Cancer Res. 2018;16(5):805–812. doi: 10.1158/1541-7786.MCR-17-0735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts J, Salaria SN, Cates J, et al. PD-L1 expression patterns in microsatellite instability-high intestinal adenocarcinoma subtypes. Am J Clin Pathol. 2019;152(3):384–391. doi: 10.1093/ajcp/aqz052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emambux S, Tachon G, Junca A, et al. Results and challenges of immune checkpoint inhibitors in colorectal cancer. Expert Opin Biol Ther. 2018;18(5):561–573. doi: 10.1080/14712598.2018.1445222 [DOI] [PubMed] [Google Scholar]
- 22.Yaghoubi N, Soltani A, Ghazvini K, et al. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019;110:312–318. doi: 10.1016/j.biopha.2018.11.105 [DOI] [PubMed] [Google Scholar]
- 23.Yuan W, Deng D, Jiang H, et al. Hyperresponsiveness to interferon gamma exposure as a response mechanism to anti-PD-1 therapy in microsatellite instability colorectal cancer. Cancer Immunol Immunother. 2019;68(2):257–268. doi: 10.1007/s00262-018-2270-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei XL, Wu QN, Chen DL, et al. The clinical and biomarker association of programmed death ligand 1 and its spatial heterogeneous expression in colorectal cancer. J Cancer. 2018;9(23):4325–4333. doi: 10.7150/jca.27735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang S, Kohli K, Black RG, et al. Systemic interferon-γ increases MHC class I expression and T-cell infiltration in cold tumors: results of a Phase 0 Clinical Trial. Cancer Immunol Res. 2019;7(8):1237–1243. doi: 10.1158/2326-6066.CIR-18-0940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lastwika KJ, Wilson W, Li QK, et al. Control of PD-L1 expression by oncogenic activation of the AKT-mTOR pathway in non-small cell lung cancer. Cancer Res. 2016;76(2):227–238. doi: 10.1158/0008-5472.CAN-14-3362 [DOI] [PubMed] [Google Scholar]
- 27.Saigi M, Alburquerque-Bejar JJ, Mc Leer-Florin A, et al. MET-oncogenic and JAK2-inactivating alterations are independent factors that affect Regulation of PD-L1 expression in lung cancer. Clin Cancer Res. 2018;24(18):4579–4587. doi: 10.1158/1078-0432.CCR-18-0267 [DOI] [PubMed] [Google Scholar]
- 28.Imai Y, Chiba T, Kondo T, et al. Interferon-gamma induced PD-L1 expression and soluble PD-L1 production in gastric cancer. Oncol Lett. 2020;20(3):2161–2168. doi: 10.3892/ol.2020.11757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao T, Li Y, Zhang J, et al. PD-L1 expression increased by IFN-gamma via JAK2-STAT1 signaling and predicts a poor survival in colorectal cancer. Oncol Lett. 2020;20(2):1127–1134. doi: 10.3892/ol.2020.11647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hänze J, Wegner M, Noessner E, et al. Co-regulation of immune checkpoint PD-L1 with interferon-gamma signaling is associated with a survival benefit in renal cell cancer. Target Oncol. 2020;15(3):377–390. doi: 10.1007/s11523-020-00728-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwasa M, Harada T, Oda A, et al. PD-L1 upregulation in myeloma cells by panobinostat in combination with interferon-gamma. Oncotarget. 2019;10(20):1903–1917. doi: 10.18632/oncotarget.26726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tong L, Li J, Li Q, et al. ACT001 reduces the expression of PD-L1 by inhibiting the phosphorylation of STAT3 in glioblastoma. Theranostics. 2020;10(13):5943–5956. doi: 10.7150/thno.41498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathi SC, Peters HL, Taguchi A, et al. Immunoproteasome deficiency is a feature of non-small cell lung cancer with a mesenchymal phenotype and is associated with a poor outcome. Proc Natl Acad Sci U S A. 2016;113(11):E1555–1564. doi: 10.1073/pnas.1521812113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamanishi J, Mandai M, Matsumura N, et al. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21(3):462–473. doi: 10.1007/s10147-016-0959-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razi S, Baradaran Noveiry B, Keshavarz-Fathi M, et al. IL-17 and colorectal cancer: from carcinogenesis to treatment. Cytokine. 2019;116:7–12. doi: 10.1016/j.cyto.2018.12.021 [DOI] [PubMed] [Google Scholar]
- 36.Yan R, Yang X, Wang X, et al. Association between intra-tumoral immune response and programmed death ligand 1 (PD-L1) in Gastric Cancer. Med Sci Monit. 2019;25:6916–6921. doi: 10.12659/MSM.916432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]