Abstract
Objective:
To describe the mortality difference between acute ischemic stroke (AIS) and non-AIS groups within COVID-19 patients.
Materials & methods:
We included observational studies through September 2020 that categorized COVID-19 patients into two groups (with and without AIS).
Results:
Eight studies with a total sample size of 19,399 COVID-19 patients were included. The pooled risk difference showed that patients with COVID-19 who developed AIS had significantly higher mortality than those without AIS by a risk difference of 24% (95% CI: 0.10–0.39; p = 0.001). In two studies, the COVID-19+AIS group had significantly higher lymphocytes, procalcitonin and creatinine levels.
Conclusion:
Developing AIS significantly adds to the mortality of COVID-19. Timely interventions to manage those patients are strongly recommended.
Keywords: : biomarkers, coronavirus, COVID-19, ischemic stroke, mortality rate, stroke
Lay abstract
We systematically searched for COVID-19 studies that categorized patients into two groups: with and without acute ischemic stroke (AIS). Of 5100 unique records, eight studies with a total of 19,399 COVID-19 patients were included. The overall mortality rate of COVID-19 patients who developed AIS was 29.6% compared with 2.6% in those without AIS. We therefore conclude that development of AIS increases the mortality rate of COVID-19, and recommend timely intervention for such patients.
The emergence of SARS-CoV-2 in China at the end of 2019 and its subsequent global spread caused unprecedented health and economic challenges. It is characterized by its rapid contagion, reaching more than 134 million confirmed cases, including more than 2.91 million deaths globally by 10 April 2021 [1]. The clinical presentation of COVID-19 varies, ranging from asymptomatic infection to severe complications, including acute respiratory distress syndrome, neurological and cardiovascular complications and even death [2–4].
COVID-19 is also associated with inflammatory coagulopathy that causes disseminated vascular obstructions, including but not limited to acute ischemic stroke (AIS) [5–7]. Several hypotheses have been proposed to explain the increased risk of AIS, such as alteration in the stability of atherosclerotic plaques [8], tissue hypoxia caused by inflammation [9], and the more recent concept of cerebral vasculitis [10]. Antiphospholipid antibodies have also been positive in severely infected patients with AIS, regardless of their genetic predisposition [5,6].
Although healthcare resources including personnel, hospital and intensive care unit beds and physicians should be directed toward caring for COVID-19 patients, this should not compromise the care of patients presenting with AIS [11]. A German nationwide cohort study using all hospitalized patients' administrative database showed that the absolute number of stroke admissions declined during the pandemic. However, patients presenting with AIS continued to receive tissue plasminogen activator and mechanical thrombectomy according to standards [12].
Patients with AIS are usually at higher risk of mortality and morbidity than healthy controls [13–16]. However, the mortality rates of COVID-19 patients who develop AIS compared with those without AIS remain unanswered. This systematic review and meta-analysis aimed to compare the mortality rates of COVID-19 patients who developed AIS with those who did not.
Materials & methods
The study was conducted following the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist for systematic reviews [17]. The review underwent critical appraisal following the A MeaSurement Tool to Assess systematic Reviews 2 (AMSTAR 2) checklist [18].
Search Strategy
We systematically searched PubMed, Scopus and Web of Science from database inception to August 2020. The search strategy was: (“brain” OR “cerebral” OR “cerebrovascular” OR “CNS” OR “large vessel” OR “central nervous system”) AND (“infarction” OR “occlusion” OR “thrombosis” OR “thrombotic” OR “cerebrovascular accident” OR “stroke”) AND (“coronavirus” OR “COVID” OR “SARS-COV 2” OR “severe respiratory distress syndrome”). A manual search was performed to minimize results bias by searching for relevant missed publications in included articles' references. The reference lists of the studies included in our study were searched to trace potential further studies. The search was updated in September 2020 to include articles published after the first search.
Inclusion & exclusion criteria
Studies satisfying the following criteria were included:
Study design: studies that were described as observational studies (cohort or case–control studies);
Population: studies on COVID-19 patients categorized into two groups, with and without AIS, with confirmed temporality between COVID-19 and stroke;
Outcome: studies reporting the mortality rate and demographic, clinical, and laboratory findings; and
Language: only studies written in the English language were included.
We excluded in vitro or animal studies; reviews, thesis, books, conference papers or articles without available full texts; duplicated, overlapping, unreliably extracted or incomplete data; and preprints that were not peer-reviewed.
Screening & study selection process
Two independent reviewers screened all records for eligibility. Eligibility screening was performed in two steps: in the first step, titles and abstracts were screened, and in the second step, full-text articles of the selected abstracts were retrieved and assessed for eligibility. Disagreements were resolved by discussion with a third reviewer.
Data extraction
We extracted data on patients' demographic characteristics, past medical history, clinical presentation, laboratory values, treatments and clinical outcomes. Two independent reviewers extracted data to a uniform Microsoft Excel sheet. A third independent reviewer performed a further check on the retrieved data to confirm data accuracy. All disagreements were resolved through discussion.
Assessing the risk of bias in the included studies
We used the Newcastle Ottawa Scale (NOS) to assess the risk of bias in the included observational studies. The NOS is a star-based score in which studies are judged on three domains: the selection of the study groups, the comparability of the groups and the ascertainment of either the exposure or outcome of interest for case–control or cohort studies, respectively.
Data analysis
The differences between COVID-19 with AIS and COVID-19 without AIS groups were pooled as risk difference (RD) with the corresponding 95% CIs in the random effects meta-analysis model. For continuous outcomes, the standardized mean difference (SMD) between the study groups was pooled with the corresponding 95% CI in the random effect meta-analysis model. All analysis was done by the RevMan software for Windows (Review Manager Version 5.3. Copenhagen: The Nordic Cochrane Centre, 2014). A p-value <0.05 was considered for statistical significance.
Heterogeneity
Heterogeneity was assessed by visual inspection of the forest plots to check the pooled estimates' overlapping 95% CIs. Heterogeneity was tested by the chi-square test and quantified by the I2 test. The outcomes were considered heterogeneous when the p-value was <0.1 and I2 >50%.
Results
Literature search results
Our literature search yielded 5100 unique records. Following the title and abstract screening, 31 studies remained eligible for full-text screening. Finally, eight cohort studies (n = 19,399 patients) were eligible for inclusion in this meta-analysis [19–26]. The flow diagram of the study selection process is shown in Figure 1.
Figure 1. . PRISMA flow diagram of the study selection process.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Baseline characteristics of included studies
We included eight studies with a total population of 19,399 COVID-19 patients, including 288 with AIS versus 19,111 without AIS. Three studies were conducted in China [20–22], one in Spain [19], one in Italy [23], two in the USA [24,25] and one was multinational [26]. The summary of the included studies and their population characteristics are shown in Table 1.
Table 1. . Summary of the included studies and their population characteristics.
| Study | Type of study | Country | Patients (n) | Age (years), mean (± SD) | Gender (males) | Medical history | Severe COVID-19 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AIS | Non-AIS | AIS | Non-AIS | AIS | Non-AIS | AIS | Non-AIS | ||||
| Annie et al. (2020) | Retrospective observational study | USA | AIS = 64 non-AIS = 9294 total = 9358 |
39.3 (± 9.0) | 36.7 (8.5) | 25 | 3680 | Smoking (22), HTN (39), DM (21), obesity (30) | Smoking (548), HTN (1087), DM (604), obesity (1617) | NA | NA |
| Fan et al. (2020) | Retrospective observational study | China | AIS = 6, non-AIS = 80 total = 86 |
68.2 (± 2.1) | 66.5 ± 11.5 | 5 | 49 | Smoking (1), HTN (3), DM (2) | Smoking (11), HTN (41), DM (17) | NA | NA |
| Hernández-Fernández et al. (2020) | Retrospective observational study | Spain | AIS = 17, non-AIS = 1667 total = 1684 |
68.2 (± 13.0) | NA | 13 | NA | Smoking (2), HTN (10), DM (6), dyslipidemia (7) | NA | NA | NA |
| Zhang et al. (2020) | Single-center case series | China | AIS = 39, non-AIS = 612 total = 651 |
Median (IQR) 70 (67–84) | Median (IQR) 55 (37–67) | 33 | 259 | HTN (43), DM (19) | HTN (183), DM (93) | 6 | 167 |
| Li et al. (2020) | Retrospective observational study | China | AIS = 10, non-AIS = 209 total = 219 |
75.7 (± 10.8) | 52.1 (15.3) | 5 | 83 | Smoking (3), HTN (9), DM (6), heart disease (3), malignancy (1) | HTN (46), DM (25), heart disease (14), malignancy (13) | 9 | 83 |
| Lodigiani et al. (2020) | Retrospective observational study | Italy | AIS = 9, non-AIS = 353 yotal = 362 |
NA | NA | NA | NA | NA | NA | 3 | 58 |
| Bach et al. (2020) | Retrospective observational study | USA | AIS = 20, non-AIS = 663 total = 683 |
63 (± 10.7) | 13 | 384 | Smoking (5), HTN (18), DM (13), dyslipidemia (8), obesity (17) | NA | NA | NA | |
| Shahjouei et al. (2020) | Retrospective observational study | Multiple countries | AIS = 123, non-AIS = 6233 total = 6356 |
68.6 (13.9) | 58 (14) | 67 | 3688 | Smoking (15), HTN (61), DM (32) | Smoking (385), HTN (1912), DM (1312) | NA | NA |
AIS: Acute ischemic stroke; DM: Diabetes milletus; HTN: Hypertension; IQR: Interquartile range; NA: Not available.
Quality of the included studies
As assessed by the NOS, the included studies' overall quality was acceptable (Table 2). Regarding selection bias, all studies had good quality in the exposed cohort's representativeness, selection of the nonexposed cohort and ascertainment of exposure. Four studies did not clarify whether the outcome was presented initially or not [22,24–26]. As for comparability, all studies presented comparable cohorts except Annie et al. [24]. All studies showed good quality in outcome assessment, sufficiently long follow-up and the cohort's adequacy.
Table 2. . Newcastle Ottawa Scale quality assessment of the included studies.
| Study | Selection | Comparability |
Outcomes | |||||
|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the nonexposed cohort | Ascertainment of exposure | The outcome was not present at the start of the study | Comparable cohort | Assessment of outcome | Follow-up long enough | Adequacy of follow-up of the cohort | |
| Hernández-Fernández et al.(2020) | * | * | * | * | * | * | * | * |
| Li et al. (2020) | * | * | * | * | * | * | * | * |
| Lodigiani et al. (2020) | * | * | * | * | * | * | * | * |
| Zhang et al. (2020) | * | * | * | * | * | * | * | * |
| Annie et al. (2020) | * | * | * | – | – | * | * | * |
| Fan et al. (2020) | * | * | * | – | * | * | * | * |
| Shahjouei et al. (2020) | * | * | * | – | * | * | * | * |
| Bach et al. (2020) | * | * | * | – | * | * | * | * |
Mortality rates
Six studies reported the mortality rate in both COVID-19 with AIS and COVID-19 without AIS patients [20–25]. Mortality rates tended to be higher in the COVID-19 with AIS group than the COVID-19 without AIS group; however, the pooled effect estimate was not statistically significant (RD: 0.08; 95% CI: -0.14–0.29; p = 0.48; Figure 2A). Pooled studies were not homogenous (p < 0.001, I2 = 85%). After doing sensitivity analysis by excluding small studies (those reporting data from fewer than 10 patients with COVID-19 with AIS), heterogeneity was resolved, and the overall risk difference showed higher mortality in the COVID-19 with AIS group compared with the COVID-19 without AIS group (RD: 0.24; 95% CI: 0.10–0.39; p = 0.001; Figure 2B). The COVID-19 with AIS group's overall mortality rate was 29.6% compared with 2.6% in the COVID-19 patients without AIS.
Figure 2. . The forest plot of the risk difference in mortality and the corresponding 95% CIs (right graph area suggests higher mortality in the COVID-19 with AIS group).
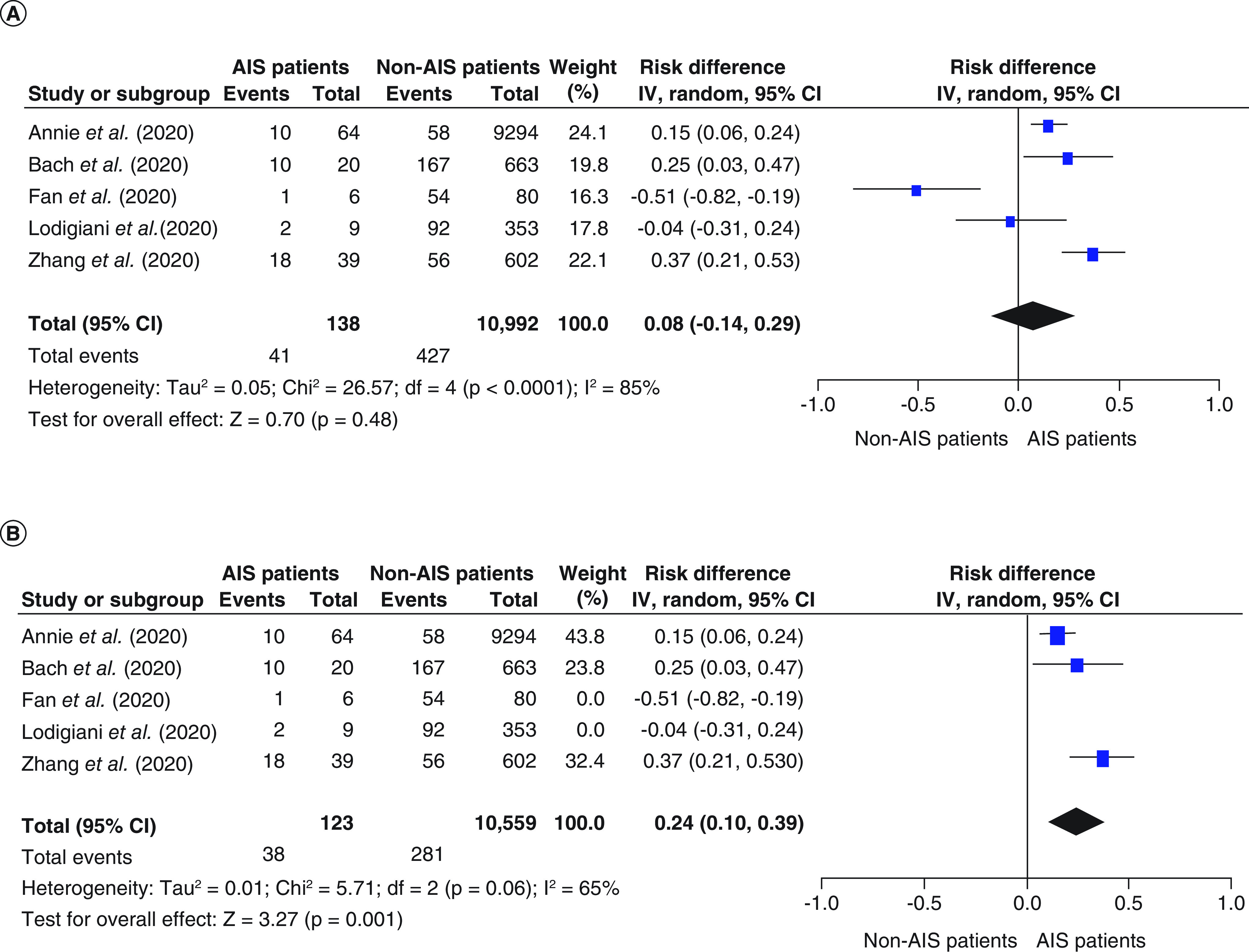
AIS: Acute ischemic stroke; df: Degrees of freedom.
Laboratory findings
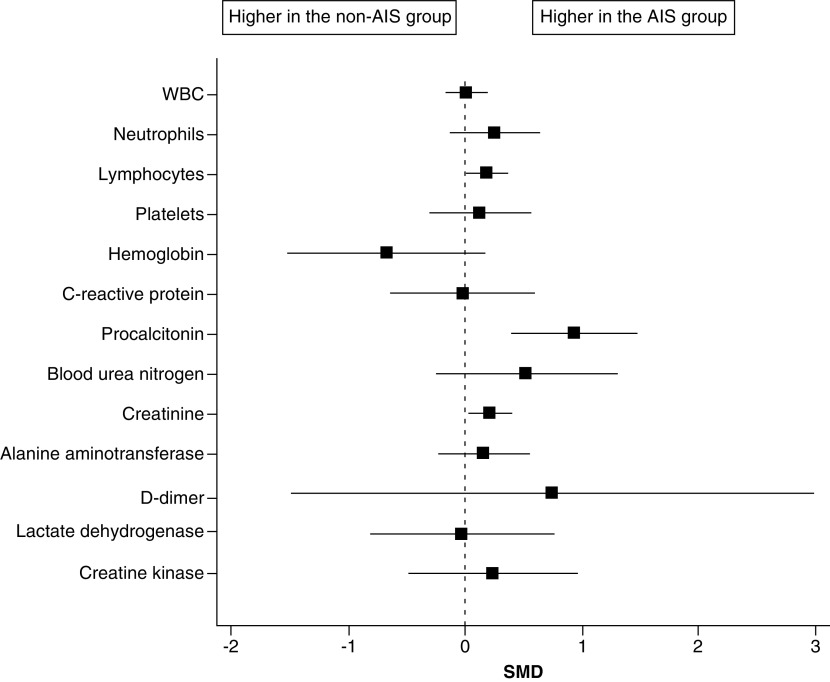
Two studies compared biomarker levels between COVID-19 with AIS (n = 129) and COVID-19 without AIS patients (n = 6,280) [22,26]. When data of the laboratory findings were pooled in the meta-analysis model, the overall SMD between the two groups did not favor either group in terms of the white blood cells, hemoglobin, platelets, C-reactive protein, blood urea nitrogen, alanine aminotransferase, d-dimer, lactate dehydrogenase or creatine kinase. Patients in the COVID-19 with AIS group had significantly higher lymphocyte count (SMD: 0.19; 95% CI: 0.02–0.36, p = 0.03), procalcitonin level (SMD: 0.94; 95% CI: 0.41–1.47; p < 0.001), and creatinine level (SMD: 0.21; 95% CI: 0.04–0.39; p = 0.02) compared with those in the COVID-19 without AIS group (Figure 3 & Table 3).
Figure 3. . The forest plot of the SMD and the corresponding 95% CI for the laboratory findings (SMD >0 suggests higher values in the COVID-19 with A IS group).
AIS: Acute ischemic stroke; SMD: Standardized mean difference.
Table 3. . The effect size and statistical significance of the laboratory findings.
| Effect size | CI | p-value | |
|---|---|---|---|
| White blood cells | 0.01 | (-0.16–0.18) | 0.91 |
| Neutrophils | 0.25 | (-0.12–0.63) | 0.19 |
| Lymphocytes | 0.19 | (0.02–0.36) | 0.03 |
| Platelets | 0.13 | (-0.3–0.56) | 0.54 |
| Hemoglobin | -0.67 | (-1.5–0.16) | 0.11 |
| C-reactive protein | -0.02 | (-0.62– 0.58) | 0.94 |
| Procalcitonin | 0.94 | (0.41–1.47) | <0.001 |
| Blood urea nitrogen | 0.52 | (-0.25–1.29) | 0.18 |
| Creatinine | 0.21 | (0.04–0.39) | 0.02 |
| Alanine aminotransferase | 0.16 | (-0.22–0.55) | 0.40 |
| D-dimer | 0.75 | (-1.48–2.97) | 0.51 |
| Lactate dehydrogenase | -0.03 | (-0.81–0.76) | 0.95 |
| Creatine kinase | 0.24 | (-0.47–0.95) | 0.51 |
Discussion
Overall, 19,399 COVID-19 patients were included in the qualitative and quantitative evidence synthesis. This study shows that COVID-19 patients who developed AIS were at a 24% higher risk of mortality than non-AIS COVID-19 patients. In addition, specific laboratory values as lymphocyte count, procalcitonin level and creatinine level were higher in the COVID-19 with AIS group. There was no significant difference in the remaining laboratory values, which could be explained by the limited number of available studies.
The high mortality rate in COVID-19 with AIS group in our meta-analysis (29.6%) is concordant with recent studies on COVID-19 [27–29]. A recent systematic review showed that the proportion of patients with COVID-19 who developed stroke was 1.8% (95% CI: 0.9–3.7%), and the in-hospital mortality rate was 34.4% (95% CI: 27.2–42.4%) [27]. However, this study is different from our meta-analysis in that it included patients with all types of stroke and not only ischemic stroke. It also did not directly compare the mortality of COVID-19 patients with stroke versus those without stroke [27]. In a recent systematic review by Tan et al. investigating the clinical features, neuroimaging findings and outcomes of AIS in COVID-19 patients, the pooled incidence of AIS in COVID-19 patients from observational studies was 1.2%, with a high mortality rate of 38% [28]. This study did not include a direct comparison between COVID-19 with AIS against those without AIS. Dmytriw et al. investigated the effects of race differences among COVID-19 with AIS patients and found that the mortality rate was significantly higher in African–Americans than other races (55.6% vs 28.6%; p = 0.05) [29]. Another study by Yaghi et al. found that 0.9% of all COVID-19 hospitalized patients experienced an ischemic stroke, and these patients had a higher inpatient mortality rate than contemporary and historical ischemic stroke controls before the COVID-19 pandemic (63.6% vs 9.3%; p < 0.001) [30]. Even patients with a history of stroke before their COVID-19 infection had higher mortality, as evidenced by a recent study in Wuhan, China, on 1875 patients, which found that patients with a history of stroke had worse outcomes compared with those without a history of stroke, with lower rates of discharge and higher mortality risks (24.0 vs 43.3%, 14.0 vs 8.3%, respectively) [31].
Compared with the previously reported mortality rates of ischemic stroke patients in the general population, COVID-19 patients with AIS in our meta-analysis had a higher mortality rate (29.6%) [15,32,33]. Saposnik et al. reported the mortality rates for 12,262 AIS patients ranged from 12.2 to 12.6% at 30 days and 22.5 to 22.9% at 1 year [15]. A previous systematic review and meta-analysis showed that from 2000 to 2008, early (21 days to 1 month) case fatality of ischemic stroke ranged from 13 to 23% in high-income countries and from 13 to 19% in low to middle-income countries [32]. Ganesh et al. conducted a retrospective study on 319,972 stroke/TIA hospitalized patients and found that the crude 30-day mortality rate decreased from 15.8% in 2003/2004 to 12.7% in 2012/2013 in provinces with stroke systems while remained 14.5% in those without such systems [33]. By 8 April 2020, the total number of confirmed COVID-19 cases globally was 133.9 million, with 2.9 million deaths (mortality rate of 2.2%) [34]. A systematic review and meta-analysis of published evidence on COVID-19 until July 2020 showed that the COVID-19 infection fatality rate across populations was 0.68% (0.53–0.82%) [35]. Thus, this meta-analysis shows that the mortality rate of COVID-19 patients with AIS is greater than the mortality rate of either COVID-19 patients in general or patients with acute ischemic stroke alone, suggesting a compounded mortality effect of stroke and COVID-19 when they co-occur.
Regarding laboratory findings, the difference between COVID-19 with AIS and COVID-19 without AIS was only significant for lymphocyte count, procalcitonin and creatinine, which were significantly higher in AIS patients. Creatinine level is one of the most critical indicators that correlates with ischemic and hemorrhagic strokes [36]. Snarska et al. have investigated the impact of kidney functions on in-hospital patients with ischemic and hemorrhagic strokes. They have found that in both, creatinine was a predictor for stroke outcome [36]. Our analysis of the available literature has shown that two studies [22,26] have assessed creatinine levels for COVID-19 stroke patients. The meta-analysis pooled results show that creatinine levels among COVID-19 with AIS patients were higher than the COVID-19 without AIS group. This finding suggests that patients with COVID-19 and high creatinine levels should be closely monitored for the development of any focal neurologic deficit that might indicate the occurrence of AIS for timely intervention. Current literature shows that severe COVID-19 is associated with lymphopenia [37,38]. The two studies included in the meta-analysis that compared lymphocyte count between COVID-19 patients with AIS against those without AIS showed low or low normal lymphocyte count in both groups, but absolute lymphocyte count was higher in COVID-19 with AIS group [22,26]. Elevated procalcitonin has been reported in patients with severe COVID-19, stroke with malignant cerebral edema, and stroke-associated pneumonia [39–41]. Scattered reports have documented an increase in procalcitonin in COVID-19 patients with AIS, with which findings of our study corroborate [42–45]. Other biomarkers, such as elevated D-dimer and fibrinogen, as well as detection of antiphospholipid antibodies, were reported to be significantly associated with AIS developing in COVID-19 patients [46].
Several studies indicated an association between COVID-19 and increased arterial and venous thromboses rates [47–63]. Reported rates of arterial thrombosis range from 2.8 to 3.8% [47]. Multiple mechanisms could explain the increased risk of ischemic strokes in COVID-19 patients. These include depletion and viral uptake of angiotensin-converting enzyme 2 (ACE2) receptors [48], hypercoagulability (Figure 4) [49–51], high systemic inflammatory response or ‘cytokine storm’ [52], neutrophil extracellular traps (NETs) [53,54], complement system activation [55], increased expression of tissue factor on endothelial cells and infiltrating macrophages and neutrophils [56], hypoxia [57], elevated antiphospholipid antibodies [58], elevated vWF activity, tissue factor, and factor VIII levels [59,60], vascular endothelial injury [61], hyperviscosity [62] and cardiac injury resulting in cerebral embolism [63]. Figure 5 summarizes the proposed mechanisms of AIS in COVID-19 patients.
Figure 4. . Mechanisms of COVID-19-associated hypercoagulability.
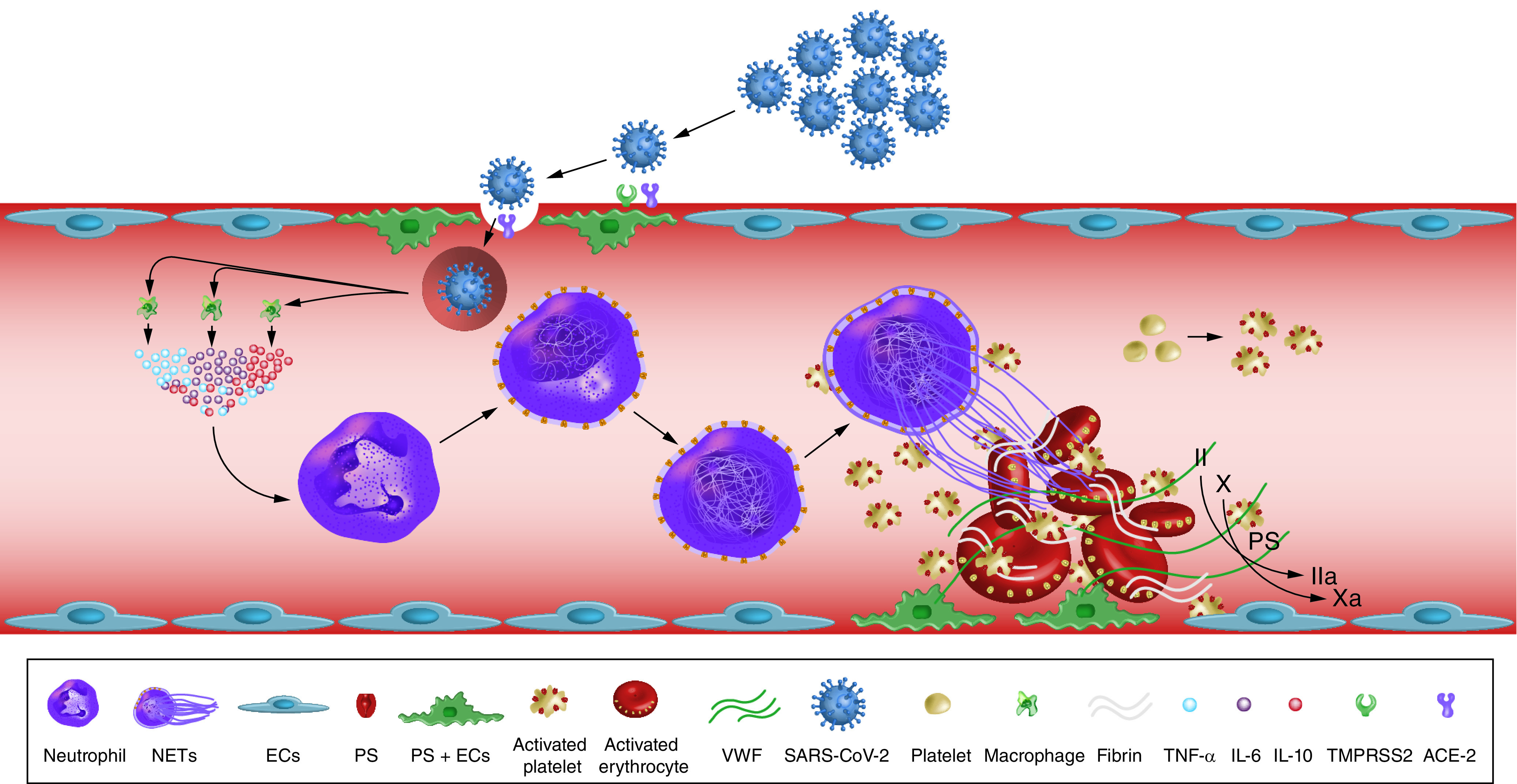
SARS-CoV-2 enters blood vessels through the ACE2 receptors on endothelial cells. Viral infection activates monocytes and macrophages to release a proinflammatory cytokine storm. This results in neutrophil activation and recruitment and tissue factor activation. Persistent neutrophil recruitment promotes a NET. NET and SARS-CoV-2 infection induce an endothelial cell injury, releasing vWF and activate platelets. The NET formation, TF activation and platelet aggregation induce coagulopathy and inhibit fibrinolysis. These processes represent the most recent data describing COVID-19-associated hypercoagulability.
ACE2: Angiotensin-converting enzyme 2; EC: Endothelial cell; NET: Neutrophil extracellular trap; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; TF: Tissue factor; vWF: von Willebrand factor.
Redrawn from [49].
Figure 5. . Proposed mechanisms thought to result in acute ischemic stroke in COVID-19 patients.
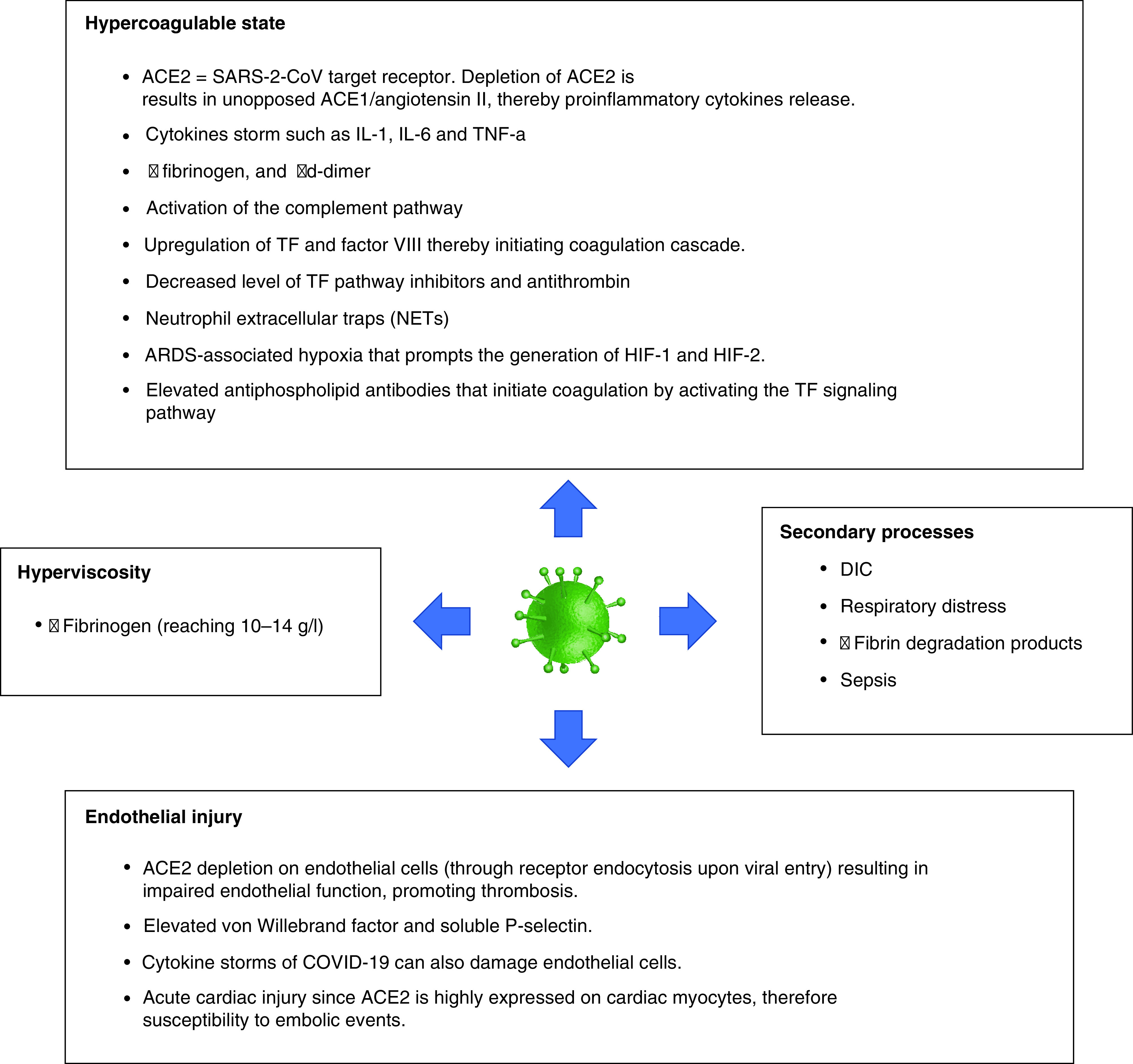
ACE: Angiotensin-converting enzyme; DIC: Disseminated intravascular coagulation; HIF: Hypoxia-inducible factor; TF: Tissue factor.
Our meta-analysis is limited by the relatively small number of available studies, the small sample size in some reports and a limited number of available parameters compared between groups. This is because studies did not consistently report AIS outcomes in the literature, and only a few studies provided head-to-head comparisons between COVID-19 with AIS and COVID-19 without AIS populations. Pooled studies were not homogenous, which was resolved by doing sensitivity analysis, excluding small studies. Although there might be a suggestion that mortality in COVID-19 with AIS was not necessarily higher due to AIS per se but rather the severity or co-occurring complications of COVID-19 such as renal failure and pneumonia, the included studies' data did not consistently provide such manifestations, complications or severity of COVID-19 for their patients, so including such variables in the meta-analysis was not possible. However, because this study compares groups of patients (COVID-19 with AIS vs those without AIS), the aim was not to establish direct causation between AIS and mortality. Another limitation is that only two studies investigated laboratory findings associated with AIS in COVID-19 patients [22,26]. Although Fan et al.'s study had a substantially small sample size of six AIS patients compared to 80 COVID-19 patients without AIS [22], this was compensated by Shahjouei et al. large sample size with a relatively higher number of COVID-19 with AIS patients (n = 123) [26].
Nonetheless, this meta-analysis expands the literature by providing evidence on the increased mortality in COVID-19 patients with AIS, which requires medical attention and prompt management to reduce the mortality.
Conclusion
COVID-19 patients with AIS have higher rates of mortality compared with COVID-19 patients without AIS. There is a suggestion that COVID-19 with AIS patients have higher levels of lymphocytes, procalcitonin and creatinine than those without AIS. Future prospective, well-designed studies should provide more data on the predictors of mortality among COVID with AIS patients. Also, future clinical trials are needed to evaluate interventions to reduce mortality in this population.
Future perspective
On the basis of this study's findings, the management of COVID-19 patients should follow a risk stratification model. The healthcare for COVID-19 patients who developed AIS or at risk of developing AIS should be maximized to lower their mortality. Specific biomarkers such as lymphocyte count and levels of procalcitonin and creatinine should be monitored closely as they can predict the occurrence of AIS in COVID-19 patients. Future prospective, well-designed studies should provide more data on the predictors of mortality among this population. Future clinical trials are needed to evaluate interventions to reduce mortality in COVID-19 with AIS patients.
Summary points.
COVID-19 is an evolving disease with varying clinical courses, ranging from asymptomatic infection to severe complications, including death.
Previous reports indicated an association between COVID-19 and inflammatory coagulopathy that causes disseminated vascular obstructions, including but not limited to acute ischemic stroke (AIS).
This systematic review and meta-analysis aimed to compare the mortality rates of COVID-19 patients who developed AIS versus those who did not and determine the significant demographic, clinical and laboratory characteristics of COVID-19 patients who developed AIS.
A total of eight studies with 19,399 COVID-19 patients, including 288 with AIS versus 19,111 without AIS, were included in this study.
Six studies reported the mortality rates in both COVID-19 with AIS and COVID-19 without AIS patients. Two studies compared biomarkers' levels between COVID-19 with AIS (n = 129) and COVID-19 without AIS patients (n = 6,280).
The meta-analysis showed higher mortality in the COVID-19 with AIS group compared with the COVID-19 without AIS group (RD: 0.24; 95% CI: 0.10–0.39; p = 0.001).
Higher lymphocytes, procalcitonin and creatinine levels were seen in COVID-19 patients with AIS compared with those without AIS.
The overall standardized mean difference between the two groups did not favor either of the two groups in terms of the white blood cells, hemoglobin, platelets, C-reactive protein, blood urea nitrogen, alanine aminotransferase, D-dimer, lactate dehydrogenase or creatine kinase.
COVID-19 patients with AIS in our meta-analysis had a higher mortality rate than previously reported mortality rates of AIS patients in the general population.
In conclusion, COVID-19 patients who develop AIS have higher mortality than COVID-19 patients without AIS; thus, they need timely interventions to lower their mortality. The biomarkers mentioned herein should be monitored closely in COVID-19 patients as they are associated with AIS development.
Acknowledgments
The authors wish to acknowledge the COVID-19 MENA Response Research Team.
Footnotes
Author contributions
All authors have seen and approved the content, fulfilled the authorship criteria and contributed significantly to work. All authors presented substantial contributions to the article and participated in the version's final approval to be submitted.
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that this article is a systematic review and meta-analysis that does not require informed consent.
Availability of data & materials
The datasets generated and analyzed during the current study are available on request from the corresponding authors.
Open access
This work is licensed under the Creative Commons Attribution 4.0 License. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time [published correction appears in Lancet Infect Dis. 2020 20(9), e215]. Lancet Infect. Dis. 20(5), 533–534 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 382(8), 727–733 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samrah SM, Al-Mistarehi AW, Ibnian AM et al. COVID-19 outbreak in Jordan: epidemiological features, clinical characteristics, and laboratory findings. Ann. Med. Surg. (Lond). 57, 103–108 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yassin A, Nawaiseh M, Shaban A et al. Neurological manifestations and complications of coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. BMC Neurol. 21(1), 138 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyrouti R, Adams ME, Benjamin L et al. Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 91(8), 889–891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martirosyan A, Aminov R, Manukyan G. Environmental triggers of autoreactive responses: induction of antiphospholipid antibody formation. Front. Immunol. 10, 1609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz JM, Libman RB, Wang JJ et al. Cerebrovascular complications of COVID-19. Stroke 51(9), e227–e231 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartmann P, Schober A, Weber C. Chemokines and microRNAs in atherosclerosis. Cell Mol. Life Sci. 72(17), 3253–3266 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li S, Hafeez A, Noorulla F et al. Preconditioning in neuroprotection: from hypoxia to ischemia. Prog. Neurobiol. 157, 79–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Becker RC. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis 50(3), 499–511 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dafer RM, Osteraas ND, Biller J. Acute stroke care in the coronavirus disease 2019 pandemic. J. Stroke Cerebrovasc. Dis. 29(7), 104881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richter D, Eyding J, Weber R et al. Analysis of nationwide stroke patient care in times of COVID-19 pandemic in Germany. Stroke 52(2), 716–721 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigin VL, Forouzanfar MH, Krishnamurthi R et al. Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2014 Jan 18;383(9913):218]. Lancet 383(9913), 245–254 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 8, 355–369 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Saposnik G, Kapral MK, Liu Y et al. IScore: a risk score to predict death early after hospitalization for an acute ischemic stroke. Circulation 123(7), 739–749 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Ganesh A, Lindsay P, Fang J et al. Integrated systems of stroke care and reduction in 30-day mortality: A retrospective analysis. Neurology 86(10), 898–904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J. Clin. Epidemiol. 62(10), 1006–1012 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Shea BJ, Reeves BC, Wells G et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358, j4008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA et al. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain 143(10), 3089–3103 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Li M, Wang M et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc. Neurol. 5(3), 279–284 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Sun W, Wang Y et al. Clinical course and mortality of stroke patients with coronavirus disease 2019 in Wuhan, China. Stroke 51(9), 2674–2682 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan S, Xiao M, Han F et al. Neurological manifestations in critically ill patients with COVID-19: a retrospective study. Front. Neurol. 11, 806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lodigiani C, Iapichino G, Carenzo L et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 191, 9–14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Annie F, Bates MC, Nanjundappa A, Bhatt DL, Alkhouli M. Prevalence and outcomes of acute ischemic stroke among patients ≤50 years of age with laboratory confirmed COVID-19 infection. Am. J. Cardiol. 130, 169–170 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bach I, Surathi P, Montealegre N et al. Stroke in COVID-19: a single-centre initial experience in a hotspot of the pandemic. Stroke Vasc. Neurol. 5(4), 331–336 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shahjouei S, Naderi S, Li J et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine 59, 102939 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fridman S, Bres Bullrich M, Jimenez-Ruiz A et al. Stroke risk, phenotypes, and death in COVID-19: systematic review and newly reported cases. Neurology 95(24), e3373–e3385 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Tan YK, Goh C, Leow AST et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J. Thromb. Thrombolysis 50(3), 587–595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dmytriw AA, Phan K, Schirmer C et al. Ischaemic stroke associated with COVID-19 and racial outcome disparity in North America. J. Neurol. Neurosurg. Psychiatry 91(12), 1362–1364 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Yaghi S, Ishida K, Torres J et al. SARS-CoV-2 and stroke in a New York healthcare system [published correction appears in Stroke. 2020 Aug;51(8):e179]. Stroke 51(7), 2002–2011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qin C, Zhou L, Hu Z et al. Clinical characteristics and outcomes of COVID-19 patients with a history of stroke in Wuhan, China. Stroke 51(7), 2219–2223 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feigin VL, Lawes CM, Bennett DA et al. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 8, 355–369 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Ganesh A, Lindsay P, Fang J et al. Integrated systems of stroke care and reduction in 30-day mortality: a retrospective analysis. Neurology 86(10), 898–904 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Our World in Data. Mortality risk of COVID-19. https://ourworldindata.org/mortality-risk-covid
- 35.Meyerowitz-Katz G, Merone L. A systematic review and meta-analysis of published research data on COVID-19 infection fatality rates. Int. J. Infect. Dis. 101, 138–148 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snarska K, Kapica-Topczewska K, Bachórzewska-Gajewska H, Małyszko J. Renal function predicts outcomes in patients with ischaemic stroke and haemorrhagic stroke. Kidney Blood Press Res. 41(4), 424–433 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Henry BM, de Oliveira MHS, Benoit S et al. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 58(7), 1021–1028 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Wagner J, DuPont A, Larson S, Cash B, Farooq A. Absolute lymphocyte count is a prognostic marker in Covid-19: a retrospective cohort review. Int. J. Lab. Hematol. 42(6), 761–765 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu R, Han C, Pei S et al. Procalcitonin levels in COVID-19 patients. Int. J. Antimicrob. Agents 56(2), 106051 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Liu G, Wang Y et al. Procalcitonin as a biomarker for malignant cerebral edema in massive cerebral infarction. Sci. Rep. 8(1), 993 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi G, Li M, Zhou R et al. Procalcitonin related to stroke-associated pneumonia and clinical outcomes of acute ischemic stroke after IV rt-PA treatment Cell. Mol. Neurobiol. (2021) (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Avula A, Nalleballe K, Narula N et al. COVID-19 presenting as stroke. Brain Behav. Immun. 87, 115–119 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, Xiao M, Zhang S et al. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 382(17), e38 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barrios-López JM, Rego-García I, Muñoz Martínez C et al. Ischaemic stroke and SARS-CoV-2 infection: acausal or incidental association? Neurología (English Edition) 35(5), 295–302 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christian Oliver C, Yu JRT, Laxamana LC, David-Ona DIA. Intravenous thrombolysis for stroke in a COVID-19 positive Filipino patient. A case report. J. Clin. Neurosci. 77, 234–236 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan YK, Goh C, Leow AST et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J. Thromb. Thrombolysis 50(3), 587–595 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackman N, Antoniak S, Wolberg AS et al. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler. Thromb. Vasc. Biol. 40, ATVBAHA120314514 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl. Stroke Res. 11, 322–325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang S, Zhang J, Wang C et al. COVID-19 and ischemic stroke: mechanisms of hypercoagulability [review]. Int. J. Mol. Med. 47(3), 1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mullaguri N, Hepburn M, Gebel JM Jr. et al. COVID-19 Disease and Hypercoagulability Leading to Acute Ischemic Stroke. Neurohospitalist 11(2), 131–136 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zakeri A, Jadhav AP, Sullenger BA, Nimjee SM. Ischemic stroke in COVID-19-positive patients: an overview of SARS-CoV-2 and thrombotic mechanisms for the neurointerventionalist. J. Neurointerv. Surg. 13(3), 202–206 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Mehta P, McAuley DF, Brown M et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395(10229), 1033–1034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou P, Li T, Jin J et al. Interactions between neutrophil extracellular traps and activated platelets enhance procoagulant activity in acute stroke patients with ICA occlusion. EBioMedicine 53, 102671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zuo Y, Yalavarthi S, Shi H et al. Neutrophil extracellular traps in COVID-19. JCI Insight 5(11), e138999 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Magro C, Mulvey JJ, Berlin D et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 220, 1–13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McGonagle D, O'Donnell JS, Sharif K et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2, e437–e445 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cameron SJ, Mix DS, Ture SK et al. Hypoxia and ischemia promote a maladaptive platelet phenotype. Arterioscler. Thromb. Vasc. Biol. 38(7), 1594–1606 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Xiao M, Zhang S et al. Coagulopathy and antiphospholipid antibodies in patients with covid-19. N. Engl. J. Med. 382(17), e38 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anigada M, Bottino N, Tagliabue P et al. Hypercoagulability of COVID-19 patients in intensive care unit: a report of thromboelastography findings and other parameters of hemostasis. J. Thromb. Haemost. 18, 1738–1742 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Becker RC. COVID-19 update: covid-19-associated coagulopathy. J. Thromb. Thrombolysis 50, 54–67 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gavriilaki E, Anyfanti P, Gavriilaki M et al. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr. Hypertens. Rep. 22(9), 63 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maier CL, Truong AD, Auld SC et al. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia?. Lancet 395(10239), 1758–1759 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Akhmerov A, Marbán E. COVID-19 and the Heart. Circ. Res. 126(10), 1443–1455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]