Abstract
Introduction:
Ischemic stroke is one of the leading causes of morbidity and mortality worldwide. Neuroprotective strategies were reported to attenuate cognitive deficits after ischemic incidents. Here we studied the neuroprotective potential of chrysin in a rat model of cerebral Ischemia/Reperfusion (I/R) in the presence or absence of Estrogen Receptors (ERs).
Methods:
Adult male Wistar rats were pretreated with chrysin (CH) (CH; 30 mg/kg; gavage; for 21 consecutive days) alone or with selective ERs antagonists (ERα antagonist MPP; ERβ antagonist PHTPP; IP) or nonselective ERs antagonist (ICI182780; IP). Then, the bilateral common carotid arteries were occluded for 20 min, which was followed by 72 h reperfusion. Subsequently, cognitive performance was evaluated by Morris Water Maze (MWM) and shuttle box tasks, and afterward, their hippocampi were removed for ELISA assays and H&E staining. Oxidative indicators Malondialdehyde (MDA) and Glutathione Peroxidase (GPx), as well as inflammation mediators interleukin (IL)-1β and tumor necrosis factor-alpha (TNFα), were measured using commercial kits.
Results:
Results of the current study showed that the anti-oxidative and anti-inflammatory properties of CH are possible mechanisms that could improve cognitive deficits and prevent neuronal cell death following I/R (P<0.001). These effects were reversed by ICI182780 (P>0.05). Furthermore, when chrysin was co-treated with ERβ antagonist, PHTPP showed a weak neuroprotective effect in I/R rats. However, these parameters were not significantly different when chrysin was combined with ERα antagonist MPP.
Conclusion:
Our data confirm that chrysin could potentially serve as a neuroprotective agent against devastating effects of cerebral I/R injury, which may be mediated via its interaction with ERs, especially ERβ.
Keywords: Chrysin, Ischemia/reperfusion, Estrogen receptor antagonists, Oxidative stress, Inflammation, Rat
Highlights
Chrysin attenuates inflammation and oxidative stress after cerebral ischemia reperfusion.
Chrysin modulated cognition deficit following cerebral ischemia reperfusion.
The anti-inflammatory and antioxidant effects of chrysin might be mediated by interaction with Estrogen Receptors (ERs) preferably ERβ.
Plain Language Summary
Cerebral ischemia, also called stroke, is one of the leading causes of mortality worldwide. This cerebrovascular disease occurs at the brain secondary to a pathological disorder of blood vessels (usually of arterial origin) or blood supply. Chrysin (5,7-dihydroxyflavone) is an important member of the flavonoid family. Therefore, it was hypothesized that chrysin could be a phytoestrogen that can improve the cognitive deficit induced by I/R via interaction with ERs; thus in the present work, it was used after administration of general and selective estrogen receptors antagonists. The current findings indicate that chrysin could significantly improve memory impairment and neuronal cell injury induced by cerebral hypoperfusion and reperfusion in rats. The decreased oxidative damage, reduced inflammatory responses, and improved cell brain lesions may all contribute to the neuroprotective effect of chrysin. Moreover, according to these results, it is hypothesized that the beneficial effects of chrysin in I/R-induced injury may be mediated via its interaction with ERs, preferably ERβ.
1. Introduction
Cerebral ischemia/reperfusion (I/R) injury is one of the major causes of neurological deterioration, mortality, and disability worldwide (El Khashab, Abdelsalam, Elbrairy, & Attia, 2019; Khoshnam, Sarkaki, Rashno, & Farbood, 2018; Yao et al., 2014; Zhang Yan, Li, & Yang, 2017). This injury is distinguished by a transient diminution of localized blood flow to brain tissue due to either arterial occlusion or systemic hypoperfusion (Khoshnam, Winlow, Farzaneh, Farbood, & Moghaddam, 2017). Various biomechanisms participate in the pathology of cerebral ischemia/reperfusion injury, such as excitotoxicity, inflammation, oxidative stress, and apoptotic cell death ( El Khashab et al., 2019). Hippocampus, one of the most sensitive regions regard to ischemia, is the cortical structure that controls behaviors looking like cognition, locomotion, exploratory activity, and anxiety. Hippocampal neurons, specifically the CA1 neurons are exclusively vulnerable to ischemia and reperfusion ( Sun et al., 2018; Wu et al., 2018). In addition, it has been documented that oxidative stress and inflammatory damage following I/R have a potent relationship with hippocampal neurons’ lesion, which is a result of severe learning and memory impairments (Fernandes, Mori, Ekuni, Oliveira, & Milani, 2008; Ge et al., 2017; Qi et al., 2016; Tao et al., 2017). After brain ischemic injury, activated microglia, as the “first line of defense” of the Brain, produce excessive pro-inflammatory cytokines such as tumor necrosis factor TNF-α and interleukin IL-1β and other pro-inflammatory mediators that can induce secondary damage, which leads to worse outcomes ( Fang et al., 2016; Ma, Wang, Wang, & Yang, 2017; Zarruk, Greenhalgh, & David, 2018). Oxidative stress and Reactive Oxygen Species (ROS) play key roles in the pathogenesis of I/R injury because they are released during the reperfusion that follows cerebral ischemia. After ischemic attacks, increased lipid peroxidation and decreased enzymatic and or non-enzymatic antioxidants defense causes tissue injury in the brain ( Durak et al., 2016; Ma et al., 2017).
In ischemia, however, oxygen becomes depleted prior to glucose, as shown by the fact that interstitial oxygen pressure in the penumbra, the brain region partially irrigated by collateral vessels that surrounds the hardly injured core, decreases to 33% only 1 h after ischemia. This condition favors the glycolytic pathway as the means of anaerobic ATP production. The outcome is an accumulation of lactic acid resulting in acidosis, which promotes pro-oxidant and harmful changes in neurons such as release of oxidant iron from proteins, increased glutamate toxicity, and the inactivation of antioxidant defenses. Other contributors to ROS in ischemia are the enzyme xanthine oxidase and mitochondrial depolarization (Manzanero, Santro, & Arumugam, 2013). It has been demonstrated that phytoestrogens are plantderived chemicals with estrogen-like activities that may carry out beneficial roles in human estrogen deficiency. In particular, phytoestrogens are commonly consumed by humans as a healthy food supplement or a food additive. The most potent phytoestrogens are members of the flavonoid family (Lebesgue, Chevaleyre, Zukin, & Etgen, 2009). These compounds have some structural similarities with the natural estrogen 17-β-estradiol (E2) also other steroid hormones and steroid hormone antagonists (Santizo, Anderson, Ye, Koenig, & Pelligrino, 2000), and they can interact with ERs and induce a gene expression similar to that induced by estrogens, albeit at a lower affinity ( Zhu et al., 2007). The ERs can bind to various compounds with a degree of structural diversity (Jiang, Gong, Zhao, & Li, 2014). However, the potency of each substance may be due to its affinity for the ERs (Resende, de Oliveira, de Camargo, Vilegas, & Varanda, 2013).
A numeral of studies have demonstrated that the steroid hormone E2 is neuroprotective in a variety of neurodegenerative disorders, such as stroke, Alzheimer’s disease, and Parkinson’s disease ( Zhang et al., 2009; Zhang et al., 2017). It is now well established that E2 exerts profound protective effects in animal models of focal and global ischemia ( Lebesgue et al., 2009; Santizo et al., 2000). E2 has also been implicated to act in the hippocampus and enhance cognitive function and synaptic plasticity ( Zhang et al., 2009). The findings confirm that the neuroprotection produced by chronic treatment with E2 is likely to involve activation of classical receptors ERα and ERβ ( Lebesgue et al., 2009). Although estrogen is well known to exert direct effects on the brain, the molecular mechanisms involved in the protective actions in the brain are not fully understood. Because of the side effects of estrogen pointed out in clinical evidence, the naturally occurring flavonoids are expected to be substitutes for this hormone. Unlike estrogen, flavonoids cannot induce cancer ( Zhu et al., 2007).
Flavonoids are a large group of natural compounds, which contain multiple potent biological effects, including anti-allergic, anti-thrombotic, anti-inflammatory, antioxidant, anti-viral, and anti-cancer activities. They are considered suitable substitutes for estrogen and have neuroprotective effects ( Durak et al., 2016; El Khashab et al., 2019; Jiang et al., 2014; Zhu et al., 2007). Besides, phytoestrogen properties of numerous flavonoids have been revealed as well (Zeng, Yan, Zhang, Zhang, & Liang 2013). Chrysin (5,7-dihydroxyflavone) is an important natural dietary phytochemical flavonoid that be extant in various foods and plants, such as passion flower, mushroom, chamomile, propolis, and honey (Mani & Natesan, 2018; Zeinali, Rezaee, & Hosseinzadeh 2017; Zeng et al., 2013). Several studies have reported that chrysin, with its anti-inflammatory and antioxidant properties, has a beneficial effect in improving the cognitive deficits induced by cerebral hypoperfusion and reperfusion in rats ( Durak et al., 2016; El Khashab et al., 2019; Sarkaki et al., 2019). Therefore, it was hypothesized that chrysin could be a phytoestrogen that can improve the cognitive deficit induced by I/R via interaction with ERs. Thus in the present work, it was used after administration of general and selective estrogen receptor antagonists. Then, passive avoidance memory, spatial memory, inflammation, and oxidative stress were assayed in this study.
2. Materials and Methods
2.1. Drugs and chemicals
Chrysin (purity >98% by HPLC analysis) was purchased from Sigma-Aldrich Co. (Sigma-Aldrich, St. Louis, MO, the USA). 17β-estradiol was purchased from Aburaihan Pharmaceutical Company (Iran). ERα antagonist 1,3bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy) phenol]-1N-pyrozoledihydrochloride (MPP), ERβ antagonist (4-[2-phenyo-5,7-bis (trifluoromethyl) pyrazole (1,5-a) pyrimidin-3-yl] phenol (PHTPP), and non-selective ER antagonist ICI182780 (ICI) were purchased from Tocris (Tocris Bioscience Co., Bristol, the United Kingdom). Assay kits for malondialdehyde (MDA) and glutathione peroxidase (GPx), as well as one ELISA kit for interleukin 1-beta (IL-1β), were obtained from ZellBio GmbH (ZellBio Co., Germany). Tumor necrosis factor-alpha (TNF-α) ELISA kit was purchased from Diaclone (Diaclone, France). All the other reagents were of analytical grade and purchased locally.
2.2. Animals and experimental design
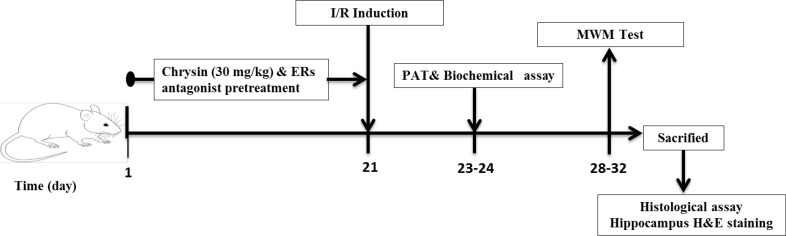
A total of 60 male Wistar rats (Six months old, weighing 250–300 g) were purchased from Ahvaz Jundishapur University of Medical Science (AJUMS) animal care and breeding center (Ahvaz, Iran). All animals were single-housed in a room with standard air-conditioning with constant humidity (50%±10%) and temperature (220C±20C) under a 12/12-h light/dark cycle. The animals were fed on standard commercial food pellets and allowed access to tap water ad libitum. All the experiments were done between 09:00 and 17:00. Animals handling and experimental protocols were performed according to the institutional guidelines for animal research and were approved by the Animal Experiments Ethics Committee of AJUMS (under ethical code IR.AJUMS. REC.1395.680). The animals were randomly divided into 7 groups (each contained 14 rats): 1) sham-operated group “Sham”, 2) ischemia/reperfusion group “I/R”, 3) chrysin (30 mg/kg, gavage) pretreated group plus I/R “CH30+ I/R”, 4) 17-βestradiol (100 µg/kg, SC); ( Cai et al., 2014) pretreated group plus I/R “E2+ I/R”; chrysin (30 mg/kg, gavage) and ERα antagonist (MPP; 150 µg/kg, IP; (Naderi, Khaksari, Abbasi, & Maghool 2015) pretreated group plus I/R “MPP”; 5) chrysin (30 mg/kg, gavage) and ERβ antagonist (PHTPP; 150 µg/kg, IP; ( Naderi et al., 2015) pretreated group plus I/R “PHTPP”, 6) chrysin (30 mg/kg, gavage) and non-selective ER antagonist (ICI182780, 150 µg/kg, IP; ( Naderi et al., 2015) pretreated group plus I/R “ICI”. “Sham” and “I/R” groups were pretreated with 5% DMSO as the vehicle. The dose of chrysin was selected from our previous study ( Sarkaki et al., 2019). The experimental design is illustrated in Figure 1.
Figure 1.
A schematic diagram of the study design and treatment schedule
2.3. Induction of cerebral ischemia/reperfusion model
The surgical procedure for I/R induction was adapted from previous studies ( Mori et al., 1998; Sarkaki et al., 2019). In brief, for the induction of ischemia, rats of all groups were anesthetized on the 21st day of the experiment by intraperitoneal injection of ketamine and xylazine (90, 10 mg/kg, IP, respectively). Then, the animal’s head was fixed, a neck ventral midline incision was made, and both Common Carotid Arteries (CCAs) were exposed and separated from the adjacent vagus nerves. The temporary occlusion of CCAs was performed using two microvascular clamps for 20 min. Afterward, the clamps were removed to allow reperfusion. Sham-operated surgery was conducted to show the effects of anesthesia and surgical manipulation on the results, and this procedure involved exposure of CCAs without any occlusion. During the surgical procedure, the heating pad’s temperature was maintained at 37˚C to 38˚C until the rat woke up.
2.4. Behavioral tests
2.4.1. Passive Avoidance Test (PAT)
We performed the Passive Avoidance Test (PAT) by the shuttle box to evaluate memory retention on the 23rd and 24th days after I/R induction. The passive avoidance procedure has been previously described ( Sarkaki et al., 2015; Sarkaki et al., 2019). Briefly, the test apparatus (27×14.5×14 cm, Borj Sanat Co, Tehran-Iran) consisted of two equal light and dark chambers that were separated by a guillotine doorway (8×8 cm) with a grid floor made of stainless steel rods (2 mm in diameter), while the floor of the dark chamber was connected to a shock generator. For habituation to the apparatus, on the first day of the experiment, each rat was located in the lit chamber while the guillotine door was opened and the animal was allowed to explore both chambers for 5 min. The acquisition phase was conducted 10 min after the habituation trial. In this phase, the latency of entrance to the dark chamber was recorded as Initial Latency (IL), which was considered from the time the door was opened, and when all 4 feet crossed the threshold, the guillotine door was closed, and a foot shock was applied to the rat (50 Hz, 1.3 mA, 3s) for 3 s. The retention trial was performed 24 hours after the acquisition phase, in which each rat was placed in the lit chamber again, and the guillotine door was raised 10 s later.
Consequently, Step-Through Latency (STL) was recorded as a measure of retention performance. If the animal avoided entrance into the dark chamber within 300s, the retention test was terminated and a ceiling score of 300s was assigned No electric shock was delivered in the retention test. Short latency illustrated lower cognition.
2.4.2. Morris Water Maze (MWM) test
One week after I/R, hippocampus-dependent spatial learning and memory abilities of the rats were investigated by subjecting them to the Morris Water Maze (MWM) test. The apparatus consisted of a circular water tank (150 cm in diameter and 60 cm in height) divided into 4 equal quadrants and was filled with water (23°C ±1°C) to a depth of 40 cm. The tank was located in a dark room with dim light. Within the tank, a submerged platform (black, round, 10 cm diameter, 2 cm below surface) was hidden in the center of a designated target quadrant in the course of 4 training trials per day for 4 consecutive days, with 1-min inter-trial intervals. The rats were given a maximum of 60 s to find the hidden platform, and each trial started at one of the 4 starting locations in a different order each day. If a rat failed to reach the platform in the specified time, the animal was guided to the platform. Then, the rats were allowed to stay on the platform for 30 s. The time each rat spent on finding the platform was measured as escape latency. On the fifth day of the experiment, the hidden platform was removed from the tank, and the rats were subjected to a probe trial (60 s). Time (recorded in seconds) spent in the correct quadrant was recorded and used as a measurement of memory. The mean escape latency and the percentage of time spent in the correct quadrant were calculated and used for statistical analyses. The swimming speed was also recorded to assess whether the group differences in escape latency were due to their differences in swimming ability ( Farbood et al., 2019; Kiray, Bagriyanik, Pekcetin, Ergur, & Uysal, 2008; Sarkaki et al., 2019).
2.5. Biochemical assay
2.5.1. Hippocampus samples collection and homogenate
Seventy-two hours after I/R induction, 5 rats from each group were killed by cervical dislocation under deep anesthesia. Their brains were quickly removed, and hippocampi were immediately dissected on ice, rinsed with saline, and then stored in a freezer (−80°C) for ELISA analysis. The hippocampi were homogenized (100 mg tissue per 1.2 mL of cold PBS plus protease inhibitor cocktail) and centrifuged at 10000×g for 20 min at 4°C, and thereafter, the supernatant was collected and aliquoted in a tube for biochemical estimation. The protein concentration of the supernatant was determined using the Bio-Rad protein assay kit (based on the Bradford dye-binding method) ( He et al., 2012; Khoshnam et al., 2018).
2.5.2. Measurement of hippocampal oxidative stress biomarkers
The oxidant-antioxidant status of the rat brains that were subjected to cerebral I/R was assessed by determining the level of Malondialdehyde (MDA) as a biomarker of lipid peroxidation and Glutathione Peroxidase (GPx) activity as an antioxidant enzyme ( El Khashab et al., 2019; Rashno et al., 2019). GPx activity and MDA level in the hippocampus were analyzed by commercial ELISA kits, which were purchased from ZellBio GmbH (Germany), and the assay was performed according to the manufacturer’s guidelines.
2.5.3. Measurement of hippocampal inflammatory mediators
Values of pro-inflammatory cytokines, including TNF-α and IL-1β in the hippocampus, were assessed and quantified using ELISA kits according to the manufacturer’s instructions ( El Khashab et al., 2019). An ELISA kit for TNF-α was purchased from Diaclone SAS (France), and the kit for IL-1β was purchased from Zell-Bio GmbH (Germany).
2.6. Histological assay
At the end of the MWM test, 5 rats from each group were used for hematoxylin and eosin (H&E) staining. After being anesthetized, the rats were perfused intracardially with an isotonic sodium chloride solution, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer (pH = 7.4). The brain samples were immediately removed and fixed in 10% paraformaldehyde for 48 h and were then embedded in paraffin. Subsequently, the coronal sections were sliced (4 μm thick) and stained with H&E. Then, the intact neurons (normal cells) and dark neurons (dead cells) from the hippocampus CA1 region were observed under a light microscope (Olympus BX50 F3; Olympus Co., Tokyo, Japan) ( Wicha et al., 2017).
2.7. Statistical analyses
The study results are presented as Mean ±SEM. All data except MWM were analyzed by 1-way analysis of variance (ANOVA) followed by Tukey’s HSD for multiple comparisons. Acquisition data from the MWM tests were analyzed using the 2-way repeated measures ANOVA followed by Tukey’s Post Hoc test. P <0.05 were considered to be statistically significant. Statistical analyses were performed using GraphPad Prism 6.0 Software (GraphPad Software Inc. version 6, San Diego, USA).
3. Results
3.1. Inhibition of ERs reduced chrysin-mediated improvements in learning and memory deficits induced by I/R
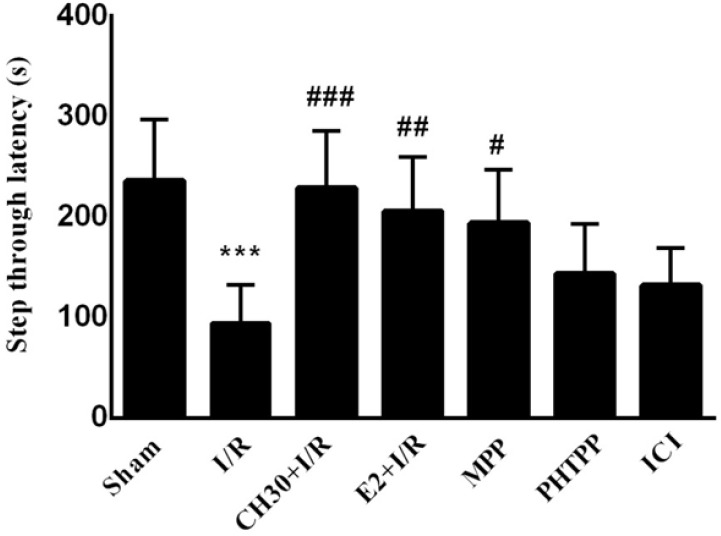
Passive Avoidance Tests (PAT) and Morris Water Maze (MWM) paradigms were performed to examine hippocampus-related learning and memory abilities. Whereas chrysin is known to improve cerebral ischemia-induced hippocampal injury and cognitive deficits ( Sarkaki et al., 2019), its impact on the estrogen receptors of hippocampal neurons is yet unclear. To address this issue in this work, the impact of co-treatment of chrysin and ERs antagonists (ERα antagonist MPP, ERβ antagonist PHTPP, and non-selective ER antagonist ICI82780) on the learning and memory of adult male rats subjected to sham operation or I/R was studied. The data obtained from the acquisition phase of PAT showed that IL was similar in all the experimental groups (data not shown). As shown in Figure 2, the STL time was decreased in the “I/R” group compared to the “Sham” group (F6, 42= 7.806; P<0.01) in the retention phase. Pretreatment of rats with CH in the “CH30+ I/R” group increased STL time in comparison with the “I/R” group (P<0.001), while co-treatment of chrysin and ERα antagonist MPP in the «MPP» group caused an increase in STL time compared to the «I/R» group (P<0.05). However, in co-treatment of chrysin and ERβ antagonist PHTPP, there was no significant difference in the STL time in the «PHTPP» group compared to the «I/R» group (P>0.05). Co-treatment of chrysin and nonselective ER antagonist ICI in the «ICI» group did not increase the STL time compared to the «I/R» group (P>0.05).
Figure 2.
Effects of chrysin on passive avoidance memory following I/R in combination with estrogen receptor antagonist, step-through latency in different experimental groups during the passive avoidance memory test
Data are presented as Mean±SEM. (n = 7).
I/R: Ischemia/Reperfusion; CH: Chrysin (30 mg/kg); E2, 17-βestradiol; MPP: ERα antagonist; PHTPP, ERβ antagonist; ICI, non-selective estrogen receptors antagonist.
***P< 0.001; vs. sham group; #P<0.05; ##P<0.001; ###P< 0.001; vs. I/R group.
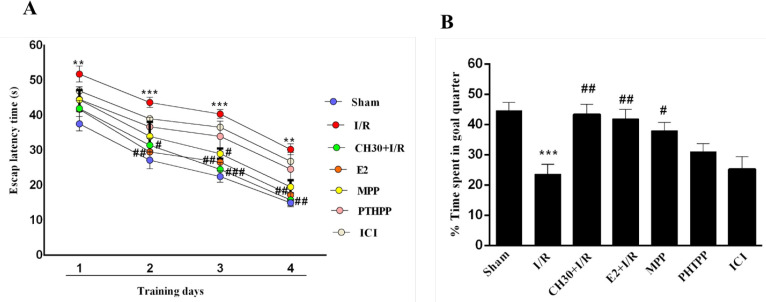
Furthermore, to evaluate the changes in spatial learning and memory, the MWM paradigm was applied. All rats were exposed to 4 days of training. As shown in Figure 3A, the latency time to find the hidden platform during the four-day training trials in MWM task was diminished in all the experimental groups, suggesting that all rats learned to find the hidden platform. Two-way repeated measures ANOVA analysis of escape latency to find the hidden platform showed significant difference in pretreatment (F6, 36 =8.559; P<0.001) and day (F3, 18 =149.4; P<0.001). However, there were no significant effects of day × pretreatment interaction (F18, 108 =0.267; P > 0.05) in all training trial days. The “Sham” group showed a rapid reduction in escape latency during the 4-day training sessions. In contrast, the rats subjected to I/R showed prolonged latency to find the platform (day 1, P<0.01; day 2, P<0.001; day 3, P< 0.001; day 4, P<0.01; Figure 3A). Chrysin pretreatment remarkably shortened the escape latency in the “CH30+ I/R” group compared to the “I/R” group from day 2 (P<0.05, P<0.001, P<0.01; Figure 3A), indicating that pretreatment with CH significantly improved spatial learning impairments in the I/R rats. However, co-treatment of chrysin and ERα antagonist MPP in the «MPP» group shortened the escape latency compared to the «I/R» group (P<0.05) on day 3.
Figure 3.
Effects of chrysin on spatial memory following I/R in combination with estrogen receptor antagonist.
Data are presented as Mean±SEM; (n = 7). A. Escape latency of each group to find the hidden platform during the four consecutive days of the acquisition trial; B. Percentage of time spent in the target quadrant during the probe trials.
I/R: Ischemia/reperfusion; CH, chrysin (30 mg/kg); E2, 17-βestradiol; MPP, ERα antagonist; PHTPP, ERβ antagonist; ICI: non-selective estrogen receptors antagonist.
***P<0.001; vs. sham group; #P<0.05; ## P<0.001; ### P<0.001, vs. I/R group.
Moreover, co-treatment of chrysin and ERβ antagonist PHTPP in the «PHTPP» group shortened the escape latency compared to the «I/R» group, but it was not significant (P>0.05). However, co-treatment of chrysin with nonselective ER antagonist ICI in the «ICI» group could not improve spatial learning impairments in the I/R rats (P>0.05). Probe tests were conducted without the hidden platform, which evaluated the memory of the trained rats, twenty-four hours after the last training test. In the probe test, a significant effect was observed on the time spent in the target quadrant (F6, 42= 7.422; P <0.001). As demonstrated in Figure 3B, compared with the
“Sham” group, the rats in the “I/R” group spent less time in the target quadrant (P<0.001). Pretreatment with CH in the “CH30+I/R” group caused the rats to spend more time in the target quadrant compared to the rats in the “I/R” group (P<0.01). Furthermore, pretreatment with CH in the “CH30+I/R” group alone and then with ERα antagonist MPP in the «MPP» group led to more spent time in the target quadrant in comparison with the «I/R» group (P<0.05). Besides, co-treatment of chrysin and ERβ antagonist in the «PHTPP» group increased the time spent in the target quadrant compared to the «I/R» group, but it was not significant (P>0.05). However, co-treatment of chrysin with nonselective ER antagonist ICI in the «ICI» group could not increase the time spent in the target quadrant compared to the «I/R» group (P>0.05).
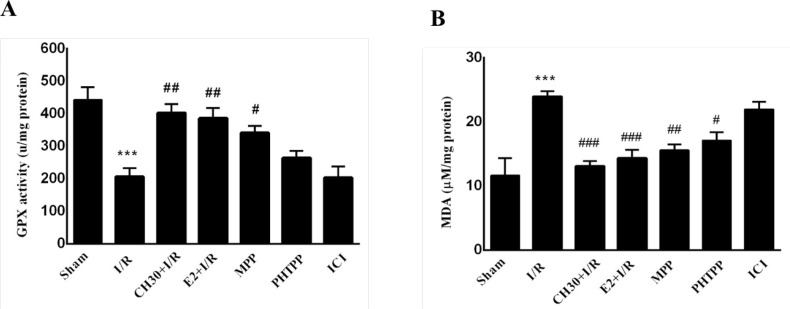
3.2. Antioxidant actions of chrysin were changed by inhibition of ERs
As shown in Figure 4A, hippocampal GPX activity significantly decreased in the “I/R” group (F6, 28= 10.84; P<0.001) compared to the “Sham” group, while pretreatment of rats with CH significantly increased the level of GPX activity (P<0.01) in the “CH30+I/R” group compared to the “I/R” group. Furthermore, when CH was pretreated with ER antagonist MPP, this caused an increase in basal GPX activity level in the “MPP” group compared to the “I/R” group (P<0.05); but when CH was pretreated with ERβ antagonist PHTPP, an increase was observed in basal GPX activity level in the “PHTPP” group compared to the “I/R” group. Nonetheless, the difference was not significant (P>0.05). Moreover, cotreatment of chrysin with nonselective ER antagonist ICI in the “ICI” group did not increase GPX activity level compared to the “I/R” group (P>0.05). On the other hand, based on the results shown in Figure 4B, there was a significant increase in the hippocampal MDA level of the “I/R” group in comparison with the “Sham” group (F6, 28= 10.24; P< 0.001). Three weeks of pretreatment with CH significantly decreased the hippocampal MDA level in the “CH30+I/R” group compared to the “I/R” group (P<0.001). Co-treatment of CH with ERβ antagonist MPP or ERβ antagonist
Figure 4.
Effects of chrysin on oxidative stress biomarkers following I/R in combination with estrogen receptor antagonist.
Data are presented as Mean±SEM, (n = 5). A. Hippocampal GPx contents in different experimental groups; B. Hippocampal MDA contents in different experimental groups.
I/R: Ischemia-reperfusion; CH: Chrysin (30 mg/kg); E2, 17-β estradiol; MPP: ERα antagonist; PHTPP: ERβ antagonist; ICI: Nonselective estrogen receptors antagonist.
***P<0.001; vs. sham group; #P<0.05; ## P<0.001; ### P<0.001; vs. I/R group.
PHTPP decreased hippocampal MDA levels in the “MPP” and “PHTPP” groups compared to the “I/R” group (P<0.01; P<0.05, respectively). However, the decrease in the MDA level resulting from co-treatment of chrysin with nonselective ER antagonist in the ICI group was not significantly different from the I/R group (P>0.05).
3.3. Inhibition of ERs changed chrysin-mediated decreases in the level of inflammatory mediators
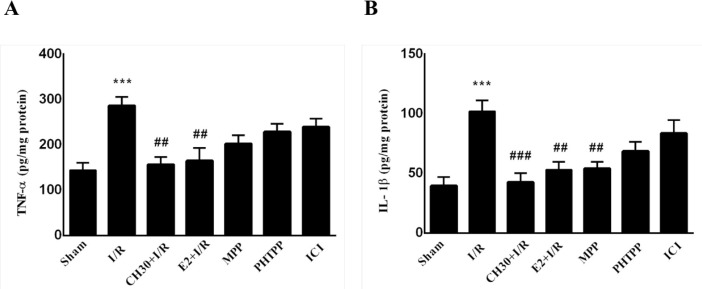
As demonstrated in Figure 5A, the performed analyses revealed a significant increase of TNF-α in the “I/R” group compared to the “Sham” group (F6, 28= 6.766; P<0.001). As demonstrated in our previous study, oral administration of CH (30 mg/kg) for 21 consecutive days before I/R in the “CH30+I/R” group significantly decreased hippocampal TNF-α content compared to the “I/R” group (P<0.01). However, when CH was pretreated with ER antagonist MPP or ERβ antagonist PHTPP, it caused a decrease in basal hippocampal TNF-α content in both “MPP” and “PHTPP” groups compared to the “I/R” group, but no significant difference was observed (P>0.05). Co-treatment of chrysin with nonselective ER antagonist ICI in the “ICI” group did not decrease hippocampal TNF-α content compared to the “I/R” group (P>0.05).
Figure 5.
Effects of chrysin on inflammation mediators following I/R in combination with estrogen receptor antagonist.
Data are presented as Mean±SEM, (n = 5); A. Hippocampal TNFα contents in different experimental groups; B. Hippocampal IL-1β contents in different experimental groups.
I/R: Ischemia/reperfusion; CH, Chrysin (30 mg/kg); E2, 17-β estradiol; MPP: ERα antagonist; PHTPP: ERβ antagonist; ICI: Nonselective estrogen receptors antagonist.
***P<0.001; vs. sham group; #P<0.05; ##P<0.001; ###P<0.001; vs. I/R group.
The current study’s analyses revealed a significant increase of IL-1β in the “I/R” group compared to the “Sham” group (F6,28=7.554; P<0.001; Figure 5B), while in the “CH30+I/R” group, IL-1β content decreased significantly compared to the “I/R” group (P<0.001). Besides, when CH was pretreated with ERα antagonist sidered to be indicative of normal neurons. In the “I/R” group, the neurons demonstrated obscure cell layers with a disordered arrangement. Moreover, in some neurons, the cytoplasm was darkly stained, and the cell bodies appeared smaller (Figure 6B). In the “CH30 +I/R” group, the alteration of the neuronal morphology and disordered neuronal arrangements were ameliorated in comparison with the “I/R” group (Figure 6C). Based on the results shown in (Figure 6H), there was a significant decline in the number of intact neurons in the hippocampal CA1 region in the “I/R” group in contrast to the “Sham” group MPP, it caused a decrease in basal hippocampal IL-1β (F6,28 = 13.85; P<0.001). In the “CH30+ I/R” group, CH content in the «MPP» group compared to the «I/R» group (P<0.01), and when CH was pretreated with ERβ antagonist PHTPP, it caused a decrease in basal hippocampal IL-1β content in the «PHTPP» group compared to the «I/R» group, albeit there was no significant difference (P>0.05). Also, co-treatment of chrysin with nonselective ER antagonist ICI in the «ICI» group could not decrease hippocampal IL-1β content compared to the «I/R» group (P>0.05).
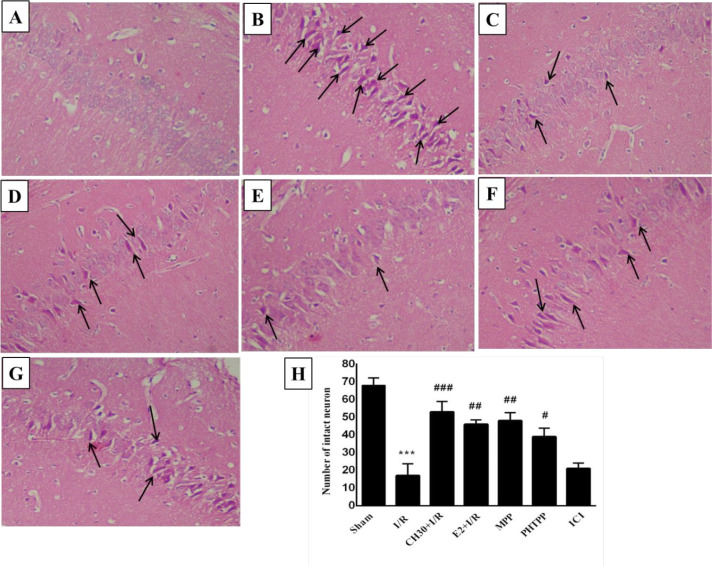
Figure 6.
Effects of chrysin on histological changes in the hippocampal CA1 regions (H & E staining) following I/R combination with estrogen receptors antagonist.
Data are presented as Mean±SEM, n = 5. A-G: Representative photomicrographs of H&E-stained coronal rat brain sections following I/R in different experimental groups; A. Sham group; B. I/R group; C. CH30+I/R group; D. E2 group; E. MPP group;
F. PHTPP group; G. ICI group; H. Quantitative data of the number of intact neurons in different experimental groups.
I/R: Ischemia/reperfusion; CH: Chrysin (30 mg/kg); E2, 17-β estradiol; MPP: ERα antagonist; PHTPP: ERβ antagonist; ICI: Non-selective estrogen receptors antagonist.
***P<0.001; vs. sham group; #P<0.05; ## P<0.001; ### P<0.001; vs. I/R group.
3.4. Inhibition of ERs changed the effects of chrysin on neuronal morphology and the number of neuron in the hippocampal CA1 region of I/R rats
In this study, H&E staining showed that hippocampal CA1 neurons in the “Sham” group were normal and arranged in an orderly and well-structured manner, and the neuronal cytoplasm was lightly stained (Figure 6A). Therefore, the neurons in the “Sham” group were conprotected the hippocampal CA1 neurons when compared to the “I/R” group (P<0.001). Furthermore, when CH was pretreated with ERβ antagonist MPP or ERβ antagonist PHTPP, it could protect the hippocampal CA1 neurons in “MPP” and “PHTPP” groups compared to the “I/R” group (P<0.001; P>0.05; respectively). However, co-treatment of chrysin with nonselective ER antagonist ICI in the “ICI” group could not protect the hippocampal CA1 neurons in comparison with the “I/R” group (P>0.05).
4. Discussion
Cerebral ischemia reperfusion is a multifactor injury, in which inflammation, oxidative stress, apoptosis, and excitotoxicity play major roles ( El Khashab et al., 2019). In our previous study, it was demonstrated that chrysin significantly improved the cognitive deficits induced by hypoperfusion and reperfusion in rats, and this is most likely related, at least in part, to its anti-inflammatory and antioxidant properties ( He et al., 2012; Sarkaki et al., 2019). Several studies in recent years have suggested that chrysin can protect neurons from oxidative insults, inflammatory mediators, and apoptosis in the I/R-induced cerebral ischemia in vivo and in vitro ( Durak et al., 2016; Li et al., 2019; Yao et al., 2014). The current study presents a step forward in understanding how chrysin, as a phytoestrogen, can protect the brain against an ischemic injury in the presence or absence of ERs using selective and nonselective ERs antagonist. The data showed that pretreatment of rats with CH (30 mg/kg) alone could ameliorate passive avoidance and spatial memory, evaluated by the shuttle box and MWM tasks.
However, as shown in Figure 1 and Figure 2, when chrysin was co-treated with ERβ antagonist PHTPP, it had weak neuroprotective effects in I/R rats; thus, it was hypothesized that chrysin might bind preferentially to ERβ. Numerous studies suggest estrogen receptors could be involved in estrogenor flavonoid-mediated neuroprotection ( Zhu et al., 2007). The ER binds to the numeral of compounds that exhibit remarkably diverse structural features. In fact, the estrogen receptor is probably unique among the steroid receptors in its ability to interact with a vast variety of compounds ( Kuiper et al., 1998; Paterni, Granchi, Katzenellenbogen, & Minutolo 2014). It is widely known that the estrogenic potency of phytoestrogens is significant, especially for ERβ. They may trigger many of the biological responses that are evoked by the physiological estrogens. A study showed that chrysin could induce the osteogenic differentiation of MC3T3-E1 cells mainly by activating ERK1/2 (a group of three Mitogen-Activated Protein (MAP) kinases) and ER. Chrysin treatment promoted the expression of transcription factors (Runx2 and Osx) and bone formation marker genes (Col1A1, OCN, and OPN) and also enhanced the formation of mineralized nodules. Furthermore, general ER antagonist ICI182780 efficiently reduced the chrysin-induced ERK1/2 activation. Therefore, the effect of chrysin on osteogenesis is ERK1/2-dependent and involves ER, and it demonstrated an estrogen-like effect on osteogenesis ( Yao et al., 2014). The efforts made within the last decade with regard to birds, rodents, monkeys, and humans have revealed that forebrain structures, in particular the hippocampus CA1-CA3 regions, can produce significant levels of E2. This finding suggests that brain-derived E2 may mediate beneficial neuroprotective and anti-inflammatory actions in the hippocampal CA1 region following global cerebral ischemia ( Zhang et al., 2014). The activation of MAP kinases, mediated by E2, is another possible explanation for this neuroprotective effect. E2 is known to activate different MAP kinases. In line with this hypothesis, studies have shown that the application of flavonoids in cultured neurons could activate the phosphorylation of MAP kinases. Whether this activation could lead to up-regulation of antiapoptotic genes has not yet been demonstrated ( Zhu et al., 2007). According to the results of this study and other studies, chrysin may activate the MAP kinase pathway by binding to the ERs (preferably ERβ) and exerts its neuroprotective effects in I/R rats. Although it has been stated that the neuroprotective effect of flavonoids could not be fully explained through their estrogen-like effect, which is not the only factor involved in neuroprotection. It is well known that oxidative injury probably an important part of events involved in the pathogenesis of numerous neurodegenerative diseases including Alzheimer’s disease and vascular dementia. In the I/R rats, the level of MDA in the hippocampus significantly increased; however, the GPx activity decreased. The scavenging of ROS formation is another possible mechanism to explicate the neuroprotective effect of flavonoids. The current work’s data showed that chrysin alone or when combined with ERα MPP antagonists diminished the MDA production and enhanced GPx activity in I/R rats. These results suggest that chrysin may ameliorate the abnormality of the free radical system and compensate the antioxidant ability secondary to hypoperfusion and reperfusion insult in vivo and in the presence of ERβ. These findings may suggest that the antioxidant actions of chrysin involve the classical activation of genomic responses by estrogen receptors. The results of another study showed that genistein (an isoflavone of similar structure to 17β-estradiol) decreased basal peroxide levels in MCF-7 cells via estrogen receptors. They stated that the antioxidant actions of genistein are mediated by estrogen receptors (Borrás et al., 2006). Following ischemia, inflammatory mechanisms increase brain damage and a poor outcome in stroke patients ( Khoshnam et al., 2018). In the nervous system, cytokines are found at low concentrations, but they quickly increase in pathological conditions such as ischemic stroke. An increase in producing of pro-inflammatory cytokines is correlated with a larger infarct size in animal models and a worse clinical outcome ( Khoshnam et al., 2017). Previous studies have shown that IL-1β and TNF-α levels increased within hours after transient global ischemia ( Jiang et al., 2014). The current study data showed that cerebral I/R could trigger an inflammatory response in rats, manifested by increased inflammatory cytokines, including IL-1β and TNF-α. These observations are in line with other prior reports. CH indicated protective effects due to its antioxidant and anti-inflammatory effects. Interestingly, it was found in this work that enhancements of hippocampal IL-1β and TNF-α contents in the I/R rats were significantly attenuated when chrysin was used alone or co-treated in ERα antagonist MPP and ERβ antagonist PHTPP pretreated animals; but when chrysin was co-treated with nonselective ER antagonist ICI, it efficiently reduced the anti-inflammatory activity of CH in I/R rats. Furthermore, it was found that neurons in the hippocampal CA1 area were degenerated after cerebral I/R ( Xuan et al., 2012).
These data showed that chrysin alone or with ERα MPP antagonists ameliorates the cerebral hypoperfusion and reperfusion-induced neuron damage, thereby indicating that ER is necessary for chrysin function, which may indicate the involvement of ER in chrysin-induced neuroprotection. Cerebral ischemia, even for a short period, results in selective neurodegeneration in vulnerable brain regions such as the CA1 region of the hippocampus. Particularly, pyramidal neurons of the CA1 field are among the most vulnerable cells to I/R injury. Severe loss of CA1 hippocampal neurons have been shown to happen after transient global cerebral ischemia as a consequence of immediate, maturational, and delayed neuronal death ( Kirino, 2000; Kirino, Tamura, & Sano, 1984). Chrysin protected against ischemia-induced CA1 neuronal death, indicating that this compound probably operate against the cascade of pathological events that lead to neuronal death. This finding agrees with the only available data about the protective effect against estradiol given chronically for 2 weeks before neuronal death induced by 30-min carotid occlusion in rats. Accordingly, chronic or acute 17β-estradiol treatment reduced some injury paradigms in rats and gerbils of both sexes (Petrone, Simpkins, & Barr, 2014; Wise & Dubal, 2000). Therefore, our data indicates the protective effect of Chrysin, when given before 20-min carotid occlusion in Rats, against neuronal death. This effect appears to be mediated through the activation of ERβ even if various other mechanisms cannot be excluded. These findings provide further grounds for the growing interest concerning the true potential of phytoestrogens as compounds to beneficially affect brain injury without having the disadvantage of estrogens (Vina, Sastre, Pallardo, Gambini, & Borras, 2006). Further studies are needed to verify if the beneficial effects can be seen after prolonged administration intervals.
5. Conclusion
The current findings indicate that chrysin could significantly improve memory impairment and neuronal cell injury induced by cerebral hypoperfusion and reperfusion in rats. The decreased oxidative damage, reduced inflammatory responses, and improved cell brain lesions may all contribute to the neuroprotective effect of chrysin. Moreover, according to these results, it is hypothesized that the beneficial effects of chrysin in I/R-induced injury may be mediated via its interaction with ERs, preferably ERβ. Therefore, it seems that different molecular mechanisms are involved, and further studies are required to fully address this issue in the future.
Acknowledgments
We gratefully thank Ahvaz Jundishapur University of Medical Sciences for supporting this study.
Footnotes
Conflict of interest
The authors declared no conflict of interest.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences (Code: IR.AJUMS.REC.1395.680).
Funding
This study was financially supported by Research burough of Ahvaz Jundishapur University of Medical Sciences (Grant No.: APRC-94-04 in Ahvaz Physiology Research Center).
Authors’ contributions
Conceptualization, supervision, and writing – review & editing: Alireza Sarkaki; Methodology, Investigation and preparing materials: Alireza Sarkaki, Yghoub Farbood, Seyed Mohammad Taghi Mansouri, Mohammad Badavi, Layasadat Khorsandi; Investigation, Data collection, Data analysis, and writing – original draft: Maryam Khombi Shooshtari; Participation in experimental procedures: All authors.
References
- Borrás C., Gambini J., Gómez-Cabrera M. C., Sastre J., Pallardó F. V., Mann G. E., et al. (2006). Genistein, a soy isoflavone, upregulates expression of antioxidant genes: involvement of estrogen receptors, ERK1/2, and NFκB. The FASEB Journal, 20(12), 2136–8. [DOI: 10.1096/fj.05-5522fje] [PMID ] [DOI] [PubMed] [Google Scholar]
- Cai M., Ma Y. L., Qin P., Li Y., Zhang L. X., Nie H., et al. (2014). The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neuroscience letters, 558, 115–9. [DOI: 10.1016/j.neulet.2013.11.007] [PMID ] [DOI] [PubMed] [Google Scholar]
- Durak M. A., Öztanir M. N., Türkmen N. B., Ciftci O., Taşlidere A., Tecellioğlu M., et al. (2016). Chrysin prevents brain damage caused by global cerebral ischemia/reperfusion in a C57BL/J6 mouse model. Turkish Journal of Medical Sciences, 46(6), 1926–33. [DOI: 10.3906/sag-1508-119] [PMID ] [DOI] [PubMed] [Google Scholar]
- El Khashab I. H., Abdelsalam R. M., Elbrairy A. I., Attia A. S. (2019). Chrysin attenuates global cerebral ischemic reperfusion injury via suppression of oxidative stress, inflammation and apoptosis. Biomedicine & Pharmacotherapy, 112, 108619. [DOI: 10.1016/j.biopha.2019.108619] [PMID ] [DOI] [PubMed] [Google Scholar]
- Fang T., Zhou D., Lu L., Tong X., Wu J., Yi L. (2016). LXW7 ameliorates focal cerebral ischemia injury and attenuates inflammatory responses in activated microglia in rats. Brazilian Journal of Medical and Biological Research, 49(9), 1–9. [DOI: 10.1590/1414-431x20165287] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbood Y., Rashno M., Ghaderi S., Khoshnam S. E., Sarkaki A., Rashidi K., et al. (2019). Ellagic acid protects against diabetes-associated behavioral deficits in rats: Possible involved mechanisms. Life sciences, 225, 8–19. [DOI: 10.1016/j.lfs.2019.03.078] [PMID ] [DOI] [PubMed] [Google Scholar]
- Fernandes J. S., Mori M. A., Ekuni R., Oliveira R. M. W., Milani H. (2008). Long-term treatment with fish oil prevents memory impairments but not hippocampal damage in rats subjected to transient, global cerebral ischemia. Nutrition research, 28(11), 798–808. [DOI: 10.1016/j.nutres.2008.09.004] [PMID ] [DOI] [PubMed] [Google Scholar]
- Ge X. H., Zhu G. J., Geng D. Q., Zhang H. Z., He J.M., Guo A.Z., et al. (2017). Metformin protects the brain against ischemia/reperfusion injury through PI3K/Akt1/JNK3 signaling pathways in rats. Physiology & Behavior, 170, 115–23. [DOI: 10.1016/j.physbeh.2016.12.021] [PMID ] [DOI] [PubMed] [Google Scholar]
- He X. L., Wang Y. H., Bi M.G., Du G. H. (2012). Chrysin improves cognitive deficits and brain damage induced by chronic cerebral hypoperfusion in rats. European Journal of Pharmacology, 680(1–3), 41–8. [DOI: 10.1016/j.ejphar.2012.01.025] [PMID ] [DOI] [PubMed] [Google Scholar]
- Jiang Y., Gong F. L., Zhao G. B., Li J. (2014). Chrysin suppressed inflammatory responses and the inducible nitric oxide synthase pathway after spinal cord injury in rats. International Journal of Molecular Sciences, 15(7), 12270–9. [DOI: 10.3390/ijms150712270] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnam S. E., Sarkaki A., Rashno M., Farbood Y. (2018). Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sciences, 211, 126–32. [DOI: 10.1016/j.lfs.2018.08.065] [PMID ] [DOI] [PubMed] [Google Scholar]
- Khoshnam S. E., Winlow W., Farzaneh M., Farbood Y., Moghaddam H. F. (2017). Pathogenic mechanisms following ischemic stroke. Neurological Sciences, 38(7), 11671186. [DOI: 10.1007/s10072-017-2938-1] [PMID ] [DOI] [PubMed] [Google Scholar]
- Kiray M., Bagriyanik H. A., Pekcetin C., Ergur B. U., Uysal N. (2008). Protective effects of deprenyl in transient cerebral ischemia in rats. The Journal of Physiology, 51(5), 275–81. https://cps.org.tw/issues/file/30b806d3954a385eac78786e01255da9.pdf [PubMed] [Google Scholar]
- Kirino T. (2000). Delayed neuronal death. Neuropathology, 20, 95–7. [DOI: 10.1046/j.1440-1789.2000.00306.x] [PMID ] [DOI] [PubMed] [Google Scholar]
- Kirino T., Tamura A., Sano K. (1984). Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathologica, 64(2), 139–47. [DOI: 10.1007/BF00695577] [PMID ] [DOI] [PubMed] [Google Scholar]
- Kuiper G. G., Lemmen J. G., Carlsson B., Corton J. C., Safe S. H., Van Der Saag P. T. (1998). Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology, 139(10), 4252–63. [DOI: 10.1210/endo.139.10.6216] [PMID ] [DOI] [PubMed] [Google Scholar]
- Lebesgue D., Chevaleyre V., Zukin R. S., Etgen A. M. (2009). Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids, 74(7), 555–61. [DOI: 10.1016/j.steroids.2009.01.003] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. F., Ma J., Han X. W., Jia Y. X., Yuan H. F., Shui S. F., et al. (2019). Chrysin ameliorates cerebral ischemia/reperfusion (I/R) injury in rats by regulating the PI3K/Akt/mTOR pathway. Neurochemistry International, 129(October 2019), 104496. [DOI: 10.1016/j.neuint.2019.104496] [PMID ] [DOI] [PubMed] [Google Scholar]
- Ma Y., Wang J., Wang Y., Yang G.-Y. (2017). The biphasic function of microglia in ischemic stroke. Progress in Neurobiology, 157, 247–72. [DOI: 10.1016/j.pneurobio.2016.01.005] [PMID ] [DOI] [PubMed] [Google Scholar]
- Mani R., Natesan V. (2018). Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry, 145, 187–96. [DOI: 10.1016/j.phytochem.2017.09.016] [PMID ] [DOI] [PubMed] [Google Scholar]
- Manzanero S., Santro T., Arumugam T. V. (2013). Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochemistry International, 62(5), 712–8. [DOI: 10.1016/j.neuint.2012.11.009] [PMID ] [DOI] [PubMed] [Google Scholar]
- Mori K., Yoshioka M., Suda N., Togashi H., Matsumoto M., Ueno K. I., et al. (1998). An incomplete cerebral ischemia produced a delayed dysfunction in the rat hippocampal system. Brain Research, 795(1–2), 221–6. [DOI: 10.1016/S0006-8993(98)00295-9] [DOI] [PubMed] [Google Scholar]
- Naderi V., Khaksari M., Abbasi R., Maghool F. (2015). Estrogen provides neuroprotection against brain edema and blood brain barrier disruption through both estrogen receptors α and β following traumatic brain injury. Iranian Journal of Basic Medical Sciences, 18(2), 138–44. [PMCID ] [PMID ] [PMC free article] [PubMed] [Google Scholar]
- Paterni I., Granchi C., Katzenellenbogen J. A., Minutolo F. (2014). Estrogen Receptors Alpha (ERα) and Beta (ERβ): subtype-selective ligands and clinical potential. Steroids, 90, 13–29. https://www.sciencedirect.com/science/article/abs/pii/S0039128X14001512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrone A. B., Simpkins J. W., Barr T. L. (2014). 17β-estradiol and inflammation: Implications for ischemic stroke. Aging and Disease, 5(5), 340–5. [PMCID ] [PMID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi D. S., Tao J. H., Zhang L.Q., Li M., Wang M., Qu R., et al. (2016). Neuroprotection of Cilostazol against ischemia/reperfusion-induced cognitive deficits through inhibiting JNK3/caspase-3 by enhancing Akt1. Brain Research, 1653, 67–74. [DOI: 10.1016/j.brainres.2016.10.017] [PMID ] [DOI] [PubMed] [Google Scholar]
- Rashno M., Sarkaki A., Farbood Y., Rashno M., Khorsandi L., Naseri M. K. G., et al. (2019). Therapeutic effects of chrysin in a rat model of traumatic brain injury: A behavioral, biochemical, and histological study. Life Sciences, 228, 285–94. [DOI: 10.1016/j.lfs.2019.05.007] [PMID ] [DOI] [PubMed] [Google Scholar]
- Resende F. A., de Oliveira A. P. S., de Camargo M. S., Vilegas W., Varanda E. A. (2013). Evaluation of estrogenic potential of flavonoids using a recombinant yeast strain and MCF7/BUS cell proliferation assay. PloS One, 8(10), e74881. [DOI: 10.1371/journal.pone.0074881] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santizo R. A., Anderson S., Ye S., Koenig H. M., Pelligrino D. A. (2000). Effects of estrogen on leukocyte adhesion after transient forebrain ischemia. Stroke, 31(9), 22312234. [DOI: 10.1161/01.STR.31.9.2231] [PMID ] [DOI] [PubMed] [Google Scholar]
- Sarkaki A., Farbood Y., Gharib-Naseri M. K., Badavi M., Mansouri M. T., Haghparast A., et al. (2015). Gallic acid improved behavior, brain electrophysiology, and inflammation in a rat model of traumatic brain injury. Canadian Journal of Physiology and Pharmacology, 93(8), 687–94. [DOI: 10.1139/cjpp-2014-0546] [PMID ] [DOI] [PubMed] [Google Scholar]
- Sarkaki A., Farbood Y., Mansouri S. M. T., Badavi M., Khorsandi L., Dehcheshmeh M. G., et al. (2019). Chrysin prevents cognitive and hippocampal long-term potentiation deficits and inflammation in rat with cerebral hypoperfusion and reperfusion injury. Life Sciences, 226, 202–9. [DOI: 10.1016/j.lfs.2019.04.027] [PMID ] [DOI] [PubMed] [Google Scholar]
- Sun D., Wang W., Wang X., Wang Y., Xu X., Ping F., et al. (2018). bFGF plays a neuroprotective role by suppressing excessive autophagy and apoptosis after transient global cerebral ischemia in rats. Cell Death & Disease, 9(2), 172. https://www.nature.com/articles/s41419-017-0229-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J., Cui Y., Duan Y., Zhang N., Wang C., Zhang F. (2017). Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β signaling pathway in an in vivo model of cerebral ischemia. Oncotarget, 8(63), 106283. [DOI: 10.18632/on-cotarget.22290] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vina J., Sastre J., Pallardo F., Gambini J., Borras C. (2006). Role of mitochondrial oxidative stress to explain the different longevity between genders. Protective effect of estrogens. Free Radical Research, 40(12), 1359–65. [DOI: 10.1080/10715760600952851] [PMID ] [DOI] [PubMed] [Google Scholar]
- Wicha P., Tocharus J., Janyou A., Jittiwat J., Changtam C., Suksamrarn A., et al. (2017). Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PloS One, 12(12), e0189211. [DOI: 10.1371/journal.pone.0189211] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise P. M., Dubal D. B. (2000). Estradiol protects against ischemic brain injury in middleaged rats. Biology of Reproduction, 63(4), 982–5. [DOI: 10.1095/biolreprod63.4.982] [PMID ] [DOI] [PubMed] [Google Scholar]
- Wu H., Tang C., Tai L. W., Yao W., Guo P., Hong J., et al. (2018). Flurbiprofen axetil attenuates cerebral ischemia/reperfusion injury by reducing inflammation in a rat model of transient global cerebral ischemia/reperfusion. Bioscience Reports, 38(4), BSR20171562. [DOI: 10.1042/BSR20171562] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan A., Long D., Li J., Ji W., Hong L., Zhang M., et al. (2012). Neuroprotective effects of valproic acid following transient global ischemia in rats. Life Sciences, 90(1112), 463–8. [DOI: 10.1016/j.lfs.2012.01.001] [PMID ] [DOI] [PubMed] [Google Scholar]
- Yao Y., Chen L., Xiao J., Wang C., Jiang W., Zhang R., et al. (2014). Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. International Journal of Molecular Sciences, 15(11), 20913–26. [DOI: 10.3390/ijms151120913] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarruk J. G., Greenhalgh A. D., David S. (2018). Microglia and macrophages differ in their inflammatory profile after permanent brain ischemia. Experimental Neurology, 301, 120132. [DOI: 10.1016/j.expneurol.2017.08.011] [PMID ] [DOI] [PubMed] [Google Scholar]
- Zeinali M., Rezaee S. A., Hosseinzadeh H. (2017). An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomedicine & Pharmacotherapy, 92, 998–1009. [DOI: 10.1016/j.biopha.2017.06.003] [PMID ] [DOI] [PubMed] [Google Scholar]
- Zeng W., Yan Y., Zhang F., Zhang C., Liang W. (2013). Chrysin promotes osteogenic differentiation via ERK/MAPK activation. Protein & Cell, 4(7), 539–47. [DOI: 10.1007/s13238013-3003-3] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Yan H., Li S., Yang J. (2017). Rosmarinic acid protects rat hippocampal neurons from cerebral ischemia/reperfusion injury via the Akt/JNK3/caspase-3 signaling pathway. Brain Research, 1657, 9–15. [DOI: 10.1016/j.brainres.2016.11.032] [PMID ] [DOI] [PubMed] [Google Scholar]
- Zhang Q. G., Raz L., Wang R., Han D., De Sevilla L., Yang F., et al. (2009). Estrogen attenuates ischemic oxidative damage via an estrogen receptor αmediated inhibition of NADPH oxidase activation. Journal of Neuroscience, 29(44), 13823–36. [DOI: 10.1523/JNEUROSCI.3574-09.2009] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. G., Wang R., Tang H., Dong Y., Chan A., Sareddy G. R., et al. (2014). Brain-derived estrogen exerts anti-inflammatory and neuroprotective actions in the rat hippocampus. Molecular and Cellular Endocrinology, 389(1–2), 84–91. [DOI: 10.1016/j.mce.2013.12.019] [PMID ] [PMCID ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J. T., Choi R. C., Chu G. K., Cheung A. W., Gao Q. T., Li J., et al. (2007). Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. Journal of Agricultural and Food Chemistry, 55(6), 2438–45. [DOI: 10.1021/jf063299z] [PMID ] [DOI] [PubMed] [Google Scholar]