Abstract
Collagen is a ubiquitous biomaterial in vertebrate animals. Although each of its 28 subtypes contributes to the functions of many different tissues in the body, most studies on collagen or collagenous tissues have focussed on only one or two subtypes. With recent developments in analytical chemistry, especially mass spectrometry, significant advances have been made toward quantifying the different collagen subtypes in various tissues; however, high-throughput and low-cost methods for collagen subtype quantification do not yet exist. In this Review, we introduce the roles of collagen subtypes and crosslinks, and describe modern assays that enable a deep understanding of tissue physiology and disease states. Using cartilage as a model tissue, we describe the roles of major and minor collagen subtypes in detail; discuss known and unknown structure–function relationships; and show how tissue engineers may harness the functional characteristics of collagen to engineer robust neotissues.
Introduction
Collagens — the most abundant proteins in the body by weight — are the main structural proteins in the extracellular matrix (ECM) of various tissues, including cartilage, bone, blood vessels, skin, and other connective tissues (Figure 1). Collagen has been studied since the 1800s1. Since then, it (and its crosslinks) been shown to be implicated in tissue biomechanics2-4, and disease states, such as cancer5,6, arthritis7,8, and over 40 hereditary diseases9,10. Collagen is categorized into 28 subtypes, with types I, II, and III, making up 80–90% of the collagen in the human body11. The so-called ‘minor collagens,’ which do not have a set definition, are present in very low amounts but have vital functional roles12. Despite this, most studies in collagen and collagenous tissues have only focused on general collagen (that is, total collagen without subtype specificity) or just one or two collagen subtypes.
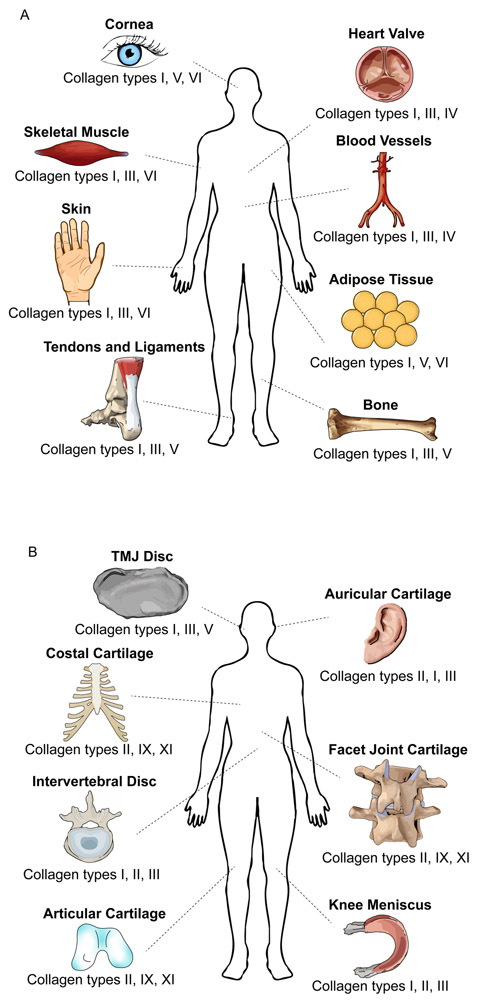
Figure 1. Collagen types of the human body.
Different collagen types are found in various tissues all over the body. Three of the most abundant collagen types are displayed for (a) non-cartilage tissues and (b) cartilage tissues. TMJ, temporomandibular joint.
In this Review, we first describe the collagen superfamily. We then discuss recent advances in mass spectrometry that allow sensitive quantification of individual collagen subtypes and determination of their role in disease states and tissue biomechanics. Our attention is then turned to the subtypes of cartilage, which have been neglected in the literature. Finally, we describe the need for new studies, in particular those that leverage high-throughput technologies. We urge tissue engineers to take advantage of bottom-up proteomics techniques to better understand the ECM of engineered tissue, potentially unveiling how to engineer stiffer, stronger, and more durable neotissues. A similar approach focusing on all collagen subtypes would lead to a deeper understanding of the roles of minor collagens, and may explain the gap in functionality between engineered implants and native tissues. Outside the scope of this Review are collagen assembly, molecular mechanics, or in-depth cellular interactions, which have been reviewed in depth elsewhere13-16.
Collagens and collagen crosslinks
All 28 subtypes of collagen contain specific amino acid sequences that encode one or more triple-helical domains.17 Triple-helical domains consist of a signature repetition of amino acids G-X-Y, with G always representing glycine, X usually representing proline, and Y usually representing hydroxyproline. This repeated sequence creates favourable hydrogen bonding, allowing for three polypeptides, called alpha-chains, to be assembled into a triple-helical collagen protein. The 28 types of collagen are divided into groups based on the location, size, and distribution of triple-helical domains; these groups are summarized in Table 1. Collagens may consist of three of the same alpha-chain or of different alpha-chains. For example, collagen type I usually consists of two α1 chains and one α2 chain18, collagen type II consists of three α1 chains19, and collagen type IX consists of α1, α2, and α3 chains20, Even though collagen types I and II are the most abundant, minor collagens, which account for less than 10% of the total collagen content, have important roles in collagenous tissues, as described later.
Table 1 ∣. Classically defined groups of collagen subtypes.
| Group name | Key features | Collagen types |
|---|---|---|
| Fibril-forming collagens | Long triple-helical domains for fibril formation | I, II, III, V, XI, XXIV, XXVII |
| Fibril-associated collagens with interrupted triple helices (FACITs) | Do not form fibrils, but associate with fibril surfaces | IX, XII, XIV, XVI, XIX, XX, XXI, XXII |
| Network-forming collagens | Form repeating patterns | IV, VIII, X |
| Membrane collagens | Span the cell membrane | XIII, XXIII, XXV |
| Multiplexins | Have many non-collagenous domains, but are not FACITs | XV, XVIII |
| Others | Do not belong to any of the above categories | VI, VII, XXVIII |
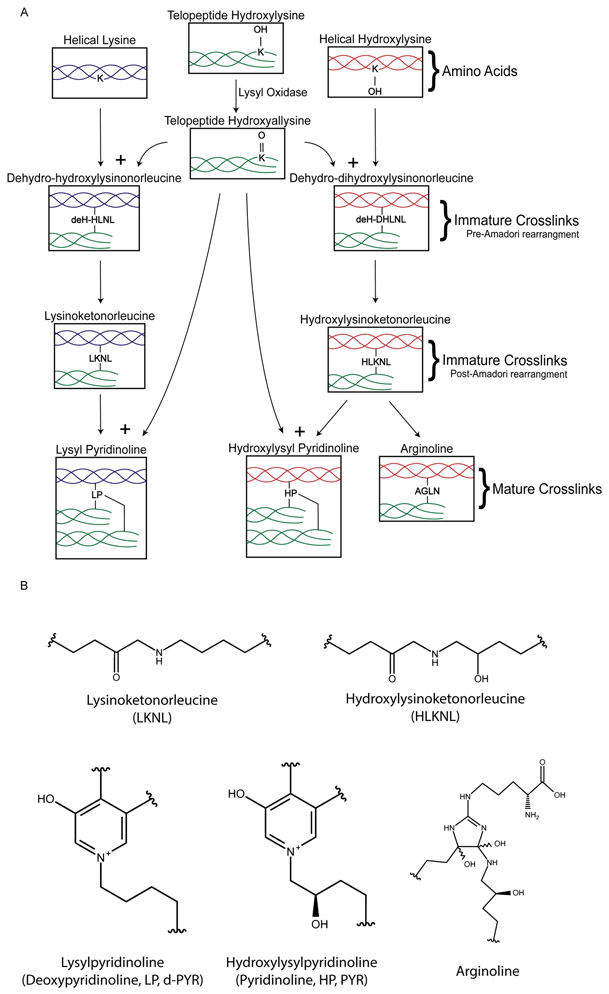
Collagen types I, II, III, V, XI, and IX form covalent enzymatic collagen crosslinks, which strengthen and mature the collagen network21. The formation pathway of these collagen crosslinks is displayed in Figure 2. The first step is catalysed by lysyl oxidase (LOX), and involves deamination of specific hydroxylysines in the collagen amino acid sequence to form hydroxyallysine22. Hydroxyallysine reacts with lysine or hydroxylysine in another collagen alpha-chain to form the divalent crosslinks dehydro-dihydroxylysinonorleucine (deH-DHLNL) and dehydro-hydroxylysinonorleucine (deH-HLNL). These crosslinks undergo a spontaneous Amadori rearrangement to the more stable hydroxylysinoketonorleucine (HLKNL) and lysinoketonorleucine (LKNL), respectively23,24. These molecules are also known as immature, reducible, or intermediate crosslinks. Because HLKNL and LKNL are destroyed upon hydrolysis, they are analysed in their borohydride-reduced forms, dihydroxylysinonorleucine (DHLNL) and hydroxylysinonorleucine (HLNL).25. HKLNL and LKNL react with another hydroxyallysine to connect to a third alpha-chain of collagen, forming the trivalent crosslinks hydroxylysylpyridinoline (HP) and lysylpyridinoline (LP), respectively26. HP and LP, which are also called pyridinoline and deoxypyridinoline, are known as mature or non-reducible crosslinks. More recently, another ketoiminie maturation, arginoline, was found to form when ketoimines undergo oxidation and free arginine addition; arginoline exists in approximately equimolar amounts to HP in articular cartilage27. Pyrrole crosslinks, which are created by telopeptide lysines, are discussed in detail elsewhere21. There are also non-enzymatic crosslinks, by which lysines or hydroxylysines in helical collagen react with sugars like glucose or ribose to form glucosepane and pentosidine through the Maillard reaction. These crosslinks are known as advanced glycation end-products (AGEs) and are associated with aging and disease states28.
Figure 2. Lysyl oxidase pathway.
a ∣ The enzymatic pathway for the formation of pyridinoline and arginoline crosslinks. The initial process of hydroxylysine to hydroxyallysine is catalysed by lysyl oxidase, which is necessary for the cascade of reactions shown in the pathway. b ∣ Structural formulas of the immature and mature crosslinks.
HP and LP are important for the tensile properties of collagenous tissues, and the amount of HP varies greatly in connective tissues. In the knee, the cruciate ligaments have 3–5 times as much HP per collagen mass than the collateral ligaments, and articular cartilage has about twice the relative HP content than the knee meniscus3. In bovine sternomandibularis muscle and nuchal ligament, the relative HP content was shown to increase with animal age29; however, the HP content in human intervertebral discs was shown to decrease with age30. Moreover, HP and LP are excreted during bone resorption and collagen degradation, and may be used as urinary biomarkers for diseases such as osteoporosis31 andosteogenesis imperfecta32, and for cancer metastasis33.
Identification and quantification
Methods for identification and quantification of collagen and its subtypes have been in iterative development for over a century34,35. Collagen analysis is relevant to fields such as tissue engineering, tissue characterization, drug development and delivery, and biomechanics. Although several methods currently exist for collagen identification and quantification, all lack in sensitivity, specificity, and/or cost effectivenessNext-generation high-throughput assays may enable the highly sensitive parallel processing of several samples in a short time, with individual subtype specificity. We provide an overview of existing and next-generation methods in this section, covering traditional and antibody-based assays, imaging, mass spectrometry, and promising proteomics approaches. A summary of the quantitative assays for collagen subtypes and crosslinks is displayed in Figure 3.
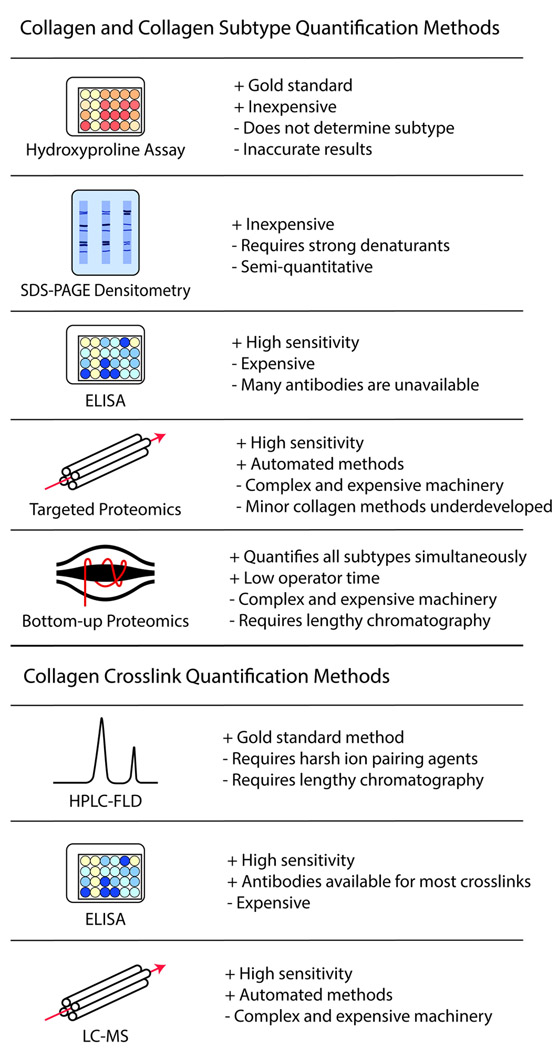
Figure 3. Examples of quantitative assays for collagen and collagen crosslinks.
Several different available assays for quantification of collagen subtypes and crosslinks are displayed. Pros and cons for each assay are denoted.
Traditional assays
Because hydroxyproline content is highly correlated to the collagen content, the hydroxyproline assay has been frequently used to quantify collagen in biological samples. Since its establishment in the mid-20th century, this procedure has been improved and simplified through many iterations36-38. This method has been cited as the ‘gold standard’ collagen quantification assay39,40. Although this assay is relatively simple and low cost, it does not discriminate between collagen types or other non-collagen molecules that contain hydroxyproline, such as elastin, which is present in many collagenous tissues41. Moreover, the hydroxyproline content varies among different collagen subtypes; for example, collagen type VI contains less hydroxyproline per chain than fibrillar types42. Therefore, although the hydroxyproline assay may be sufficient for estimating overall collagen, additional analysis is needed for highly sensitive or type-specific collagen quantification.
Another assay used to identify collagens is sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), which separates molecules by molecular weight and charge. Because the molecular weights of many collagen alpha-chains are documented, they can be identified using a prepared ladder of proteins43. Nevertheless, separating the alpha-chains is challenging and requires strong denaturants44. Semi-quantification of protein in gel bands from SDS-PAGE is possible via densitometric analysis45. Although SDS-PAGE alone does not confirm the identity of proteins, they can be identified through antibody binding in a Western Blot.
High-performance liquid chromatography (HPLC) with fluorescence detection (HPLC-FLD) has been used for the identification and quantification of HP and LP since the mid-1980s. This method uses reverse-phase chromatography with ion-pairing agents46. This was extended to quantify the AGE pentosidine and, using post-column derivatization, quantify immature crosslinks HLKNL and LKNL47. While HPLC-FLD has been a gold standard assay for collagen crosslink quantification for many years, its lengthy chromatography and usage of harsh ion-pairing agents are significant disadvantages.
Quantitative antibody-based assays
Enzyme-linked immunosorbent assay (ELISA) is a plate-based assay to quantify target molecules based on antigen recognition and thus can be used to measure individual collagen subtypes or crosslinks. Commercial ELISA kits exist for several collagen subtypes, and ELISA has been developed for crosslinks such as HP48, LP49, and pentosidine50. ELISA’s drawbacks include its high cost and requirement for separate runs for different molecules (for example, different collagen types, different collagen crosslinks, or collagen from different biological species). Antibodies specific to minor collagen types for many species are not readily available. Identifying or quantifying different collagen types in the same sample is not possible, and because different collagen types have a high degree of sequence homology, careful attention is needed when choosing custom epitopes for collagen subtypes.
Protein microarrays are emerging technologies that can characterize proteins in a parallel and high-throughput manner. This technique consists of several parallel, miniaturized assays on a small plate — a concept originally used in the DNA microarray51. ECM protein microarrays can be used to profile cell adhesion to different collagen subtypes52 but have not been used for collagen quantification. Similar immunoassays may also be multiplexed with magnetic bead-based technologies, which may allow for parallel quantification of different collagen types53. Although magnetic bead-based assays for human collagen types I, II, IV, and VI assays are available, poor concurrence of quantitative values between bead-based assays and ELISAs were demonstrated, and results from different vendors were not consistent54. The DNA microarray, in combination with SOMAmers (small off-rate modified aptamers), can be used to quantify proteins in the SOMAscan assay55. This assay can target collagen subtype fragments in human blood56 and can be multiplexed, but is not yet fully developed for tissue homogenate.
Collagen imaging and spectroscopy
Visible light, fluorescence, and many other microscopy techniques allow for visualization of bulk collagen, collagen types, and organization parameters, such as fibre or fibril size, and spacing. For light microscopy, histochemical techniques, such as Van Gieson’s stain (which was developed in the late 19th century), are still commonly used. Collagens are acidophilic and can be stained with eosin. Other stains include picrosirius red, methyl blue, and water blue. Although these stains give appealing visualizations of fibrillar collagen, they have been noted to give inaccurate results based on non-random sampling or sample inhomogeneity. Furthermore, these stains are not collagen-specific57; for example, they have been noted to stain other matrix components, such as tenascin58 and amyloid59. Moreover, fibrillar collagen stains do not differentiate among collagen subtypes60,61, necessitating the use of additional methods.
Immunohistochemistry (IHC) and immunofluorescence (IF) enable targeted labelling of individual collagen types through antibody-binding. As with ELISA, the availability of antibodies for minor collagens or for particular species can pose a challenge, and collagen sequence homology may induce cross-reactivity. Some examples of these techniques are IHC to show the location of collagen type X in avian tissues62 and IF to show co-distribution of collagen types XII and XIV with cartilage oligomeric matrix protein (COMP)63.
Imaging techniques are particularly useful for examining collagen architecture and organization. Several imaging modalities may be used for quantifying the alignment of fibrillar collagens. These include transmission polarized light microscopy64, second-harmonic generation65, and liquid crystal-based polarization microscopy66. Diffusion tensor imaging, which can measure the differences in water diffusion across collagen fibres, has been used to evaluate the orientation of collagen fibrils in engineered cardiovascular tissues67. Electron microscopy (EM) techniques, including scanning electron microscopy (SEM), transmission electron microscopy (TEM), and environmental scanning electron microscopy (ESEM) may be used for visualization of collagen fibres and fibrils. For example, TEM has been used to visualize fibril diameter and spacing68, as well as size and organization69; SEM was used to measure the diameter of collagen fibres in normal and degraded articular cartilage70; and ESEM has been used to study the morphology and wetting behaviour of sponges made from collagen type I71.
Some imaging techniques are also useful for discerning among collagen types. For example, immunoelectron microscopy, which uses electron microscopy to label specific proteins, has been used to visualize the location of collagen subtypes, such as types XIII72, XV73, and XXVII74. Moreover, fluorescence lifetime imaging microscopy (FLIM) can be used to differentiate between collagen types I and III75. FLIM has also been used to correlate collagen content to fluorescence lifetime in native cartilage with varying amounts of collagen depletion76. This same technique was used in a study of tissue-engineered cartilage to correlate fluorescence lifetime to ultimate tensile strength77. A similar FLIM technique was used to quantify crosslinking in collagen hydrogels78. Because these FLIM systems can be used in sterile conditions without damaging engineered tissue, they may be used for quality control or release criteria for engineered implants, without the need to manufacture extra implants for destructive analysis.
Spectroscopic techniques, including Fourier-transform infrared (FTIR) spectroscopy, Raman spectroscopy, and time-resolved laser-induced fluorescence spectroscopy (TR-LIFS) may be used for analysing collagen or collagen subtypes. In one study, the spectral characteristics of FTIR, such as the amide I band profile and area, were compared among different types of tendon, and correlated to pyridinoline crosslinking and fibre organization79. In another example of FTIR spectroscopy, highly discriminant absorption bands for individual collagen subtypes were determined80. Raman spectroscopy is emerging as a technique to characterize ECM, including the collagen of engineered and native cartilages, both in localized regions and throughout the entire tissue depth81,82. Raman spectroscopy has also recently been used to show changes in collagen secondary structure after damage from burning83, and to discriminate between collagen types I and IV in skin84. TR-LIFS has been used to characterize collagen types I, II, III, IV, and V in vitro85, and was used to assess collagen type I in arterial plaques to diagnose atherosclerosis86.
Quantification with mass spectrometry
Mass spectrometry (MS) techniques have been developed for the identification and quantification of proteins, including collagen subtypes. To identify or quantify collagen subtypes with MS, collagen proteins or peptides must be ionized and then analysed in a mass spectrometer. Ionization techniques include matrix-assisted laser desorption/ionization (MALDI) or electrospray ionization (ESI). Liquid chromatography (LC) is frequently used in conjunction with ESI to separate analytes out of complex mixtures. After ionization, analytes are separated by their mass-to-charge ratio in time-of-flight (TOF), quadrupole, or ion-trap mass spectrometers. MS techniques can be used to quantify overall collagen and collagen crosslinks, individual collagen subtypes with targeted methods, or many collagen subtypes at once in bottom-up proteomics methods.
Quantification of overall collagen and collagen crosslinks.
LC and multiple reaction monitoring (MRM) — an MS technique that quantifies fragmentation product ions from parent ions — can be used to quantify hydroxyproline in hydrolysed tissues as an estimation of total collagen content87, similar to the photometric hydroxyproline assay described above, but with higher sensitivity and specificity. MALDI-TOF can also be used to quantify the amount of glycine-proline-hydroxyproline tripeptide as an estimation of total collagen content88. In addition to estimating total collagen, LC-MS has been used for measuring collagen crosslinks. In one example, LC-MS MRM was used to quantify HP, LP, DHLNL, HLNL, and pentosidine in mouse cervical tissue during pregnancy using a reverse-phase chromatography column and ion-pairing agent89. Another technique uses selected ion monitoring rather than MRM, and foregoes ion pairing by using a silica hydride column; this method quantified HP and LP in skin and urine samples90. LC-MS is more sensitive and accurate than HPLC-FLD for several compounds91-93, although a thorough comparison between LC-MS and HPLC-FLD for collagen crosslink quantification has not been performed.
Quantification of targeted collagen subtypes.
Aside from measuring small molecules, such as hydroxyproline, mass spectrometry methods can be used to detect intact proteins or specific marker peptides. For example, in an early study, whole fibrils of collagen types I, III, and V were identified with MALDI-TOF; however, because enzymatic digestion was not carried out, they formed large polymeric structures, making reproducible quantification difficult94. More recently, MRM has been used to quantify peptides resulting from cyanogen bromide and trypsin cleavage of collagen types I, II, III, IV, and V in placenta and cartilage samples95. MRM has also been used to measure the release of collagen type II and III in human articular cartilage after mechanical injury and cytokine treatment96, and to identify collagen type I in trypsin digests of leather to determine the animal source97. Within mass spectrometry systems, a number of quantitative approaches exist, including isobaric tags for relative and absolute quantitation (iTRAQ)98, stable isotope labelling by amino acids in cell culture (SILAC)99, tandem mass tags (TMT)100, oxygen-18 stable isotope labelling101, and label-free methods based on peak intensity or spectral count102. Prior to targeted analysis, operators must determine variables, such as retention time, ionization voltages, and the masses of ions, and careful attention must be paid to ensure complete and reproducible enzymatic digestion and chromatography. Although such techniques are promising for quantifying individual collagen subtypes, methods for most minor collagen subtypes are yet to be developed; collagen is part of the insoluble ECM, does not digest easily in trypsin103, and the low quantity of minor collagens relative to other tissue components render them difficult for targeted analysis.
Bottom-up proteomics approaches.
The resolving power of modern ion-trap and Orbitrap mass spectrometers allows for quantification of many trypsinized proteins within a single run. After trypsin digestion, bottom-up proteomics analysis, also called shotgun proteomics, may be used for identification and quantification of many different proteins, including collagen subtypes. These proteins may be quantified with the aforementioned tagging methods or with label-free methods, such as intensity-based absolute quantification (iBAQ)104 or MaxLFQ105.
Bottom-up approaches are useful for characterizing proteins relevant to disease states, such as cancer, or quantifying ECM components in native tissues. For example, bottom-up proteomics was used to quantify collagen types I, II, III, V, VI, IX, X, XI, and XII relative to a reference sample in different types of cartilage106 and compare collagen types I, II, III, VI, XI, and XII in osteoarthritic and healthy cartilage107. Bottom-up approaches also revealed the peptide sequences of collagen types I, III, IV, V, and IX in renal cell carcinoma108 and increased deposition of collagen types X, XII, XIV, and XV in colorectal cancer and colorectal liver metastasis compared to healthy tissue,109 which may serve as potential biomarkers. By quantifying native tissue compositions and determining variations in disease states, new understandings of ECM remodelling through disease progression may be achieved toward developing new treatments. However, although bottom-up proteomics datasets give a great amount of information, they are more costly and slower than most targeted approaches.
Modern assays to quantify collagen subtypes are applicable to all collagen-containing tissues in the body. In the remainder of this Review, cartilage will be used as a model tissue. We describe the role of each collagen subtype in cartilage and outline future directions for collagen subtype quantification.
Collagens in cartilage
Cartilage is a family of collagenous tissues with major structural and mechanical roles in the body. Depending on the anatomical location, cartilage’s functions include bearing load, providing shape, cushioning, and lubricating diarthrodial joints. Cartilage contains an ECM rich in glycosaminoglycans (GAGs) and up to 15 types of collagen. Several of these are displayed in Figure 4, which shows these collagen subtypes’ structure, location, and roles in cartilage formation. The combination of collagens and other ECM molecules gives cartilage biomechanical properties in compression, shear, and tension that are unlike any other soft tissues. However, because cartilage is largely avascular and aneural, its lack of inherent healing poses significant medical problems when it is injured or diseased110. In this section, we briefly describe the different classifications of cartilage, its degeneration and promising therapies, and the role of each collagen type in cartilage.
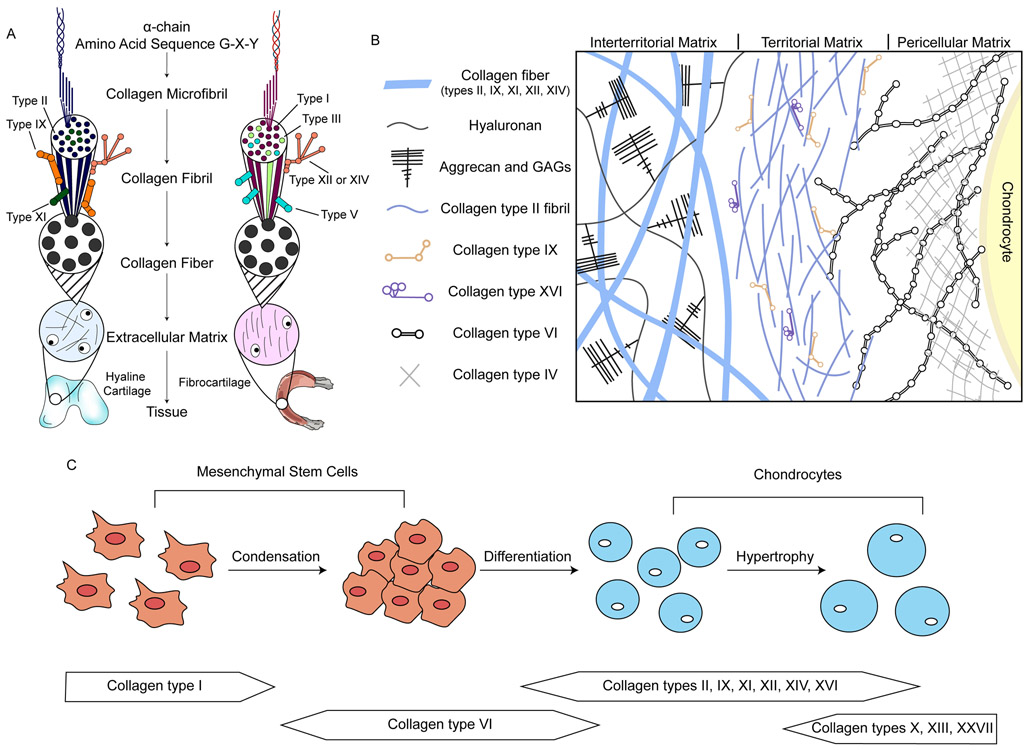
Figure 4. Collagen structure and location in cartilage, and its role in cartilage development.
a ∣ Heterofibrils of different collagen types compose the structure of the cartilage extracellular matrix (ECM). Hyaline and fibrocartilage ECMs are shown as examples. b ∣ The location of different collagen types in hyaline cartilage ECM. Many other ECM molecules are present but not shown. c ∣ Different collagens are involved in the process of cartilage formation during embryonic development. Collagen type I is expressed by mesenchymal stem cells. Collagen type VI accumulates during condensation, and is then replaced by collagen type II and minor collagens as the cells differentiate and deposit chondrogenic ECM. GAGs, glycosaminoglycans.
Classification of cartilage
Cartilage is categorized into hyaline cartilage, fibrocartilage, and elastic cartilage (collectively termed cartilages). This classification is based on the presence of major collagens or elastin in the ECM. For example, the ECM of hyaline cartilage contains mostly collagen type II, whereas the fibrocartilage ECM contains mostly collagen type I. Hyaline cartilage is the most abundant type of cartilage in the body and can be further classified as articular or non-articular, based on whether the cartilage is found on the surface of articulating bones. Articular cartilage distributes forces generated in musculoskeletal movement and provides a low-friction surface to facilitate movement in diarthrodial joints111,112. Non-articular hyaline cartilage is found in locations such as the ends of ribs, nose, larynx, and in tracheal rings.
Fibrocartilage is distinct from hyaline cartilage owing to the presence of collagen type I and the lower GAG content. It is also 5–20 times stiffer than hyaline cartilage in tension and at least five times less stiff in compression113,114. Fibrocartilage is found in the knee meniscus, the temporomandibular joint, intervertebral discs, and the pubic symphysis. Some cartilages in articulating joints, such as the mandibular condyle cartilage, are classified as fibrocartilage115,116. Fibrocartilage can also be created as repair tissue when hyaline articular cartilage is damaged; however, this repair mechanism is ineffective for long-term replacement117.
Elastic cartilage is distinct from other cartilages as it contains elastin — a highly crosslinked protein that makes this type of cartilage flexible. Elastic cartilage is primarily found in the ear and epiglottis. Although septal cartilage of the nose contains elastin, this is categorized as hyaline cartilage118. Elastic cartilage is more cellular and has different mechanical properties to hyaline cartilage and fibrocartilage119. The main collagen types in elastic cartilage are types I and II120,121.
Cartilage degeneration and therapies
Arthritides are degenerative diseases of cartilage that affect over 30 million US adults according to the US Centers for Disease Control and Prevention. Osteoarthritis, which is often associated with aging or injury, leads to cartilage that has different mechanical properties than its healthy counterpart: for example, the tensile modulus can decrease by as much as 90% and the compressive modulus also decreases122. Osteoarthritis transforms the collagen in articular cartilage from mostly type II to a mixture of types I, II, and III; osteoarthritis can induce a 100-fold upregulation in collagen type I123, fivefold downregulation of collagen type II124, and sixfold increased deposition of collagen type III125. The collagen type II in articular cartilage has negligible turnover, and the low regenerative capacity of cartilage may partly come from this inability to repair and replace collagen126.
For treatment of cartilage pathologies, arthroscopic debridement, the surgical removal of damaged cartilage, used to be commonly performed127 but was shown to not relieve symptoms128. Osteochondral autografts and allograft plugs may be used for small chondral defects, although matching the surface shape from the implant to the defect remains a challenge129. The dense collagenous matrix in cartilage can impede tissue–tissue integration, hindering the effectiveness of graft approaches130. Surgical interventions for cartilage defects also include microfracture and matrix-assisted autologous chondrocyte implantation (MACI), although both may lead to fibrocartilage repair tissue and long-term degeneration131,132. Microfracture specifically leads to repair tissue that contains collagen type I, unlike healthy articular cartilage133. Minor collagens in repair tissue from microfracture or MACI have not been identified or quantified.
Fibrocartilage disorders, such as those of the knee meniscus, temporomandibular joint (TMJ) disc, and intervertebral disc can originate from a variety of aetiologies. Tears in the knee meniscus are common injuries among athletes, and often occur alongside knee-ligament injuries, which can severely limit mobility134. TMJ pain afflicts approximately 10% of the adult population135, with a disproportionately high occurrence in premenopausal women136. The intervertebral disc shows age-related degeneration earlier than any other connective tissue in the body137, and is strongly associated with back pain, which has a lifetime prevalence rate of 49–80%138,139. Surgical removal procedures, such as meniscectomy and discectomy, offer short-term relief, but lead to worsening of symptoms and eventual fibrocartilage degeneration140-143.
There are currently no therapies to repair or regenerate either hyaline cartilage or fibrocartilage that are effective in the long-term144. Because cartilages lack intrinsic healing, tissue-engineering techniques are potential therapies145. Tissue-engineered cartilage, or neocartilage, has the potential to fill defects or regenerate damaged tissues to restore function146,147. To do this, neocartilage implants must meet the mechanical demands imposed upon the native tissue148. A systematic approach to designing neocartilage starts from delineating quantitative design criteria, such as the specific biomechanical and biochemical properties of the native tissue to serve as gold standards149. Biomechanical properties include compressive and tensile moduli, and biochemical properties include the quantity of collagen and collagen crosslinks. Although a major effort in the field of cartilage tissue engineering is to mimic the structure and mechanics of native cartilage, most current attempts to create mimetic neocartilage fall short. Quantification of minor collagens would form the foundation of a systematic approach toward engineering biomimetic cartilages.
Cartilage tissue engineers frequently use scaffolds, such as collagen or synthetic materials, for cell seeding150-152. Collagen scaffolds are naturally occurring biomaterials that support cell adhesion, are degradable, and can have a low or pro-healing immune response153,154. Collagen can also be blended with natural (for example, silk, hyaluronic acid) or synthetic polymers (for example, poly(lactic acid), poly(L-lactide-co-glycolic acid)) to improve the mechanical properties and support a chondrogenic phenotype in both primary and stem cells155. 3D woven composite scaffolds can recapitulate certain biomechanical properties of native cartilage, such as anisotropy, viscoelasticity, and tension–compression nonlinearity156. Although these attributes may help neocartilage formation, collagen and synthetic scaffolds do not recapitulate the variety and distribution of different collagen subtypes and crosslinks of native tissues. It remains unknown whether scaffolds are sufficiently biomimetic to effectively regenerate damaged and diseased tissues.
Collagen subtypes in cartilage
For this Review, we define types I and II as major collagens in cartilages because they account for a majority of the collagen mass (for example, mature articular cartilage collagen is about 90% type II157, and knee meniscus collagen is about 90% type I158). The remaining collagen types (III, IV, V, VI, IX, X, XI, XII, XIII, XIV, XVI, XXII, and XXVII) make up the minor collagens in cartilage. It may appear daunting at first for cartilage researchers to consider these 15 collagen types simultaneously. Thus, we recommend organizing collagens not by the classical categorization, but by their functions in cartilage, as described in Figure 5a. For example, in tissue engineering, this system may make it easier to design criteria without an extensive background in collagen biochemistry.
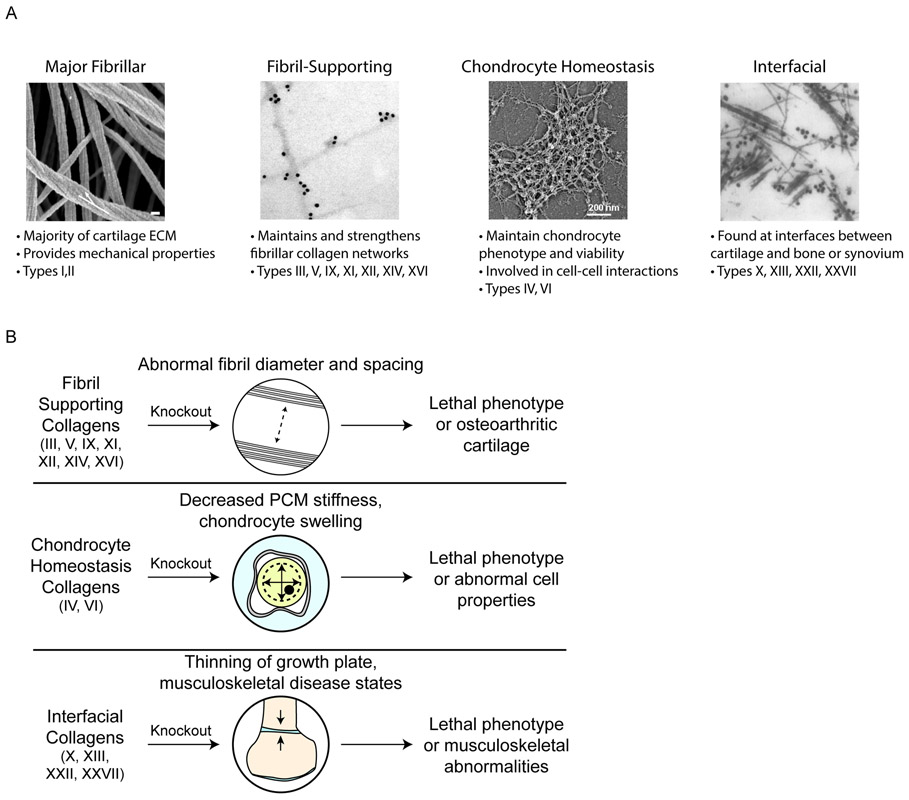
Figure 5. Functional groups of collagen subtypes in cartilage.
a ∣ (left to right) SEM image of collagen fibres in knee articular cartilage (white arrow: twisting of fibrils in the axial direction; scale bar, 100 nm); immunogold EM image of labelled collagen IX; rotary shadowing EM showing a collagen type VI network; and immunogold EM image of labelled collagen type X. b ∣ Knocking out different categories of minor collagens can cause lethal or abnormal phenotypes in cartilage and other musculoskeletal tissues. SEM, scanning electron microscopy; EM, electron microscopy. PCM, pericellular matrix. In panel a, the images (left to right) are reproduced with permission from ref. 168, Public Library of Science; ref. 249, American Society for Microbiology; ref. 250, American Society for Biochemistry and Molecular Biology; and ref. 251. Elsevier.
Although the main collagens in cartilage are frequently identified and quantified with histological stains or ELISA methods, minor collagens are rarely identified in engineered cartilage, likely because of their low quantity and lesser known roles; however, these minor collagens are necessary for the function of cartilage159. For example, knockouts of many minor collagens result in lethal phenotypes (Figure 5b). In this section, we describe the function of each major and minor subtype (for example, for supporting collagen fibrils, maintaining chondrocytes, and forming interfaces between cartilages and other tissues) while noting its importance for cartilage tissue mechanics. A summary of all collagen subtypes in cartilages, including the alpha-chain isoforms, location, description, and structure, is provided in Table 2.
Table 2. The collagen subtypes of cartilage.
E, Elastic; F, fibrocartilage; H, hyaline; ECM, extracellular matrix; PCM, pericellular matrix; TM, territorial matrix. Refer to the subsections under ‘Collagen subtypes in cartilage’ for information about the isoforms.
| Collagen subtype |
Cartilage location |
Description | Structure | Refs |
|---|---|---|---|---|
| I | ECM (E,F) | Main fibrillar type of fibrocartilage and elastic cartilages | T3 1 | 111, 114,121 |
| II | ECM (E,F,H) | Main fibrillar type of hyaline, fibrocartilage, and elastic cartilages | T3 1 | 3, 111,112 |
| III | ECM (E,F,H) |
|
T3 1 | 124,179,180 |
| IV | PCM (H) |
|
T3 2 | 201-204 |
| V | ECM (E,F) | Fibrillar type co-assembling with collagen type I | T3 3 | 181-183 |
| VI | PCM (E,F,H) | Regulation of PCM properties and mechanotransduction | T3 4 | 205-207 |
| IX | ECM (E,F,H) |
|
T3 5 | 184,185 |
| X | Deep zone (H) |
|
T3 6 | 62,208 |
| XI | ECM (E,F,H) |
|
T3 7 | 157,189 |
| XII | ECM (E,F,H) |
|
T3 8 | 192-194 |
| XIII | Deep zone (H) |
|
T3 9 | 210,211 |
| XIV | ECM (E,F,H) |
|
T3 8 | 196,197 |
| XVI | TM (H) |
|
T3 10 | 198-200 |
| XXII | Synovial junction (H) |
|
Not yet determined | 212, 213 |
| XXVII | Deep zone (H) |
|
Not yet determined | 215-217 |
Major fibrillar collagens (I, II).
Collagen type I comprises a large portion of the ECM of many connective tissues, lending stiffness to skin, tendon, ligament, bone, and fibrocartilage. For example, collagen type I accounts for about 85% of dry weight of the TMJ disc160. Its spatial distribution can vary among different cartilages. For example, the collagen type I content in the outer annulus of the intervertebral disc is over 80% by dry weight, but drops to about 70% in the inner annulus161. Similarly, in the knee meniscus, the outer red-red zone of the tissue is approximately 80% collagen type I by dry weight; however, in the inner white-white zone, this proportion drops by over half114. Collagen type I is also found in elastic cartilage, giving structure and mechanical properties to the tissue alongside collagen type II and elastin121. Collagen type I in hyaline articular cartilage can indicate damage or pathology, and it can be found when ineffective fibrocartilage repair tissue fills a defect, eventually leading to osteoarthritis110. Collagen type I is usually found in the heterotrimer α12α2 (a triple helix of two α1 chains and one α2 chain), but is also found in the α13 homotrimer (three α1 chains) in fetal tissues, fibrosis, and cancer; this isomer is not found in healthy non-fetal tissues162.
Collagen type II represents 90–95% of the collagen in hyaline cartilage112. It is found in the isoform α13, and in hyaline cartilage, is interwoven with proteoglycan aggregates consisting of hyaluronic acid, aggrecan core protein, and GAGs. The collagen network formed by collagen type II is a crosslinked polymer that also contains collagen types IX, XI, XII, and XIV. This collagen network and its crosslinks are highly correlated with tensile stiffness and strength3, but also have roles in in articular cartilage compression163. Collagen type II is also present in fibrocartilage and elastic cartilage ECM, making up the majority of the inner white-white zone of the knee meniscus, as well as a portion of the TMJ disc, intervertebral disc, and auricular cartilage114,121,161,164.
The major fibrillar collagens form a hierarchical structure. Collagen molecules, which are about 300-nm long and about 1.5 nm in diameter, are axially staggered by a multiple of approximately 67 nm, which creates a characteristic ‘D-period’ or ‘D-band’165. D-periodic molecular segments pack together in a quasi-hexagonal lattice to form collagen microfibrils166. In hyaline cartilage, collagen II microfibrils surround collagen type XI microfibrils to form thin fibrils, with a diameter of about 20 nm (Ref. 167). These fibrils grow laterally, organizing like ropes threaded with thin fibrils, to form fibril bundles or fibres with diameters from 40 to 200 nm (Ref.168). In fibrocartilage, collagen type I can form fibrils by itself, but it can also form heterofibrils with collagens III and V169,170. The fibril size in fibrocartilage can vary greatly by location, from 35-nm fibrils at the meniscus surface to 120-nm fibrils below171. Collagen fibrils with a diameter 100–150 nm have been observed in the intervertebral disc172, and thinner fibrils (20–40-nm diameter) were found in human fetal intervertebral discs173.
Why collagen type II, not type I, makes up most of the hyaline cartilage ECM, and is almost absent from other parts of the body, remains an important question. There is ample evidence that fibrocartilage, which is abundant in collagen type I, is ineffective at replacing hyaline cartilage as a repair tissue174,175. The repaired fibrocartilage is mechanically weaker and more permeable than articular cartilage, and degrades over two years110,176. When articular cartilage is compressed, osmotic swelling pressure from GAGs provides compressive resistance, and this swelling is restrained by the collagen type II network177. Collagen type II has a larger molecular spacing than collagen type I due to glycosylated hydroxylysine residues, allowing it to contain 50–100% more water than collagen type I178. The ability of collagen type II to hold more water may be significant for restraining osmotic swelling and dissipating compressive forces, and, thus, explain why this collagen type is uniquely present in hyaline cartilage178.
Fibril-supporting collagens (III, V, IX, XI, XII, XIV, XVI).
Collagen type III is found in the ECM of various musculoskeletal tissues, organs, and skin, and has the isoform α13. It is essential for normal collagen type I fibrillogenesis, organizing and regulating the diameter of type I fibrils179. Collagen type III is found alongside collagen type I in fibrocartilages, although it accounts for less than 10% of the overall collagen content158. In articular cartilage, collagen type III is also crosslinked to collagen type II, which superimposes it onto collagen type II-IX-XI copolymers180. Moreover, collagen type III can add additional cohesion or strength when a collagen type II network is damaged. For example, one study found a sixfold upregulation of collagen type III in osteoarthritic hip cartilage compared to control125, although this may be due to co-polymerization with collagen type I, which is also present in osteoarthritic cartilage124. Although global knockout of collagen type III results in perinatal lethality from rupture of major blood vessels179, cartilage mechanics have not been studied in knockouts.
Collagen type V is found in the ECM of bone, cartilage, and corneal stroma. The major isoform of collagen type V is α12α2, which co-assembles with collagen type I181, where type I fibrils are co-polymerized on a template of collagen type V182. Global collagen type V mouse knockouts are embryonic lethal, and a conditional knockout in the cornea showed that collagen type V is necessary for fibrillogenesis; the knockout mice produced normal amounts of collagen type I, but with increased fibril and abnormal structure183. This implies that collagen type V increases the mechanical strength in tissues containing collagen type I (for example, fibrocartilage, tendon, and skin). However, potentially because of the neonatal lethality of global collagen type V knockouts, the relationship between collagen type V and tissue mechanics has not been determined.
Collagen type IX, which has the isoform α1α2α3, is found in articular cartilage, where it forms LOX-mediated crosslinks with collagen type II fibrils to stabilize and strengthen the ECM. Collagen type IX, alongside collagen type XI, is colocalized with collagen type II fibrils184. All three alpha-chains of collagen type IX contain intermolecular crosslinking sites, where they form covalent bonds with other collagen type IX molecules, or with collagen type II185. The quantity of collagen type IX decreases with cartilage age, starting at over 10% of the overall collagen content in fetal articular cartilage and dropping to about 1% in adult articular cartilage157. This collagen type is also necessary for the maturation of cartilage during fracture repair, as shown in a global knockout study186. Knockouts lead to degenerative joint disease resembling osteoarthritis187; however, the mechanics of collagen type IX-deficient cartilage have not been tested.
Collagen type XI has the isoform α1α2α3 and is broadly distributed in many tissues. In articular cartilage, it crosslinks with itself in fibrils that also contain collagen types II and IX, and is involved with fibrillogenesis by maintaining the spacing and diameter of collagen type II fibrils157. The α3 chain of collagen type XI has the same primary structure as the α1 chain of collagen type II.188 In articular cartilage, collagen type XI was shown to trimerize with collagen type V in the isoforms α1(XI)α1(V)α3(XI) and α1(XI)α2(V)2189. This means it would be more accurate to describe collagen types V and XI as a single type, collagen V/XI190. A collagen type XI α1 global knockout mouse model was neonatal lethal, with thick banded collagen fibrils in cartilage ECM191. Like collagen type IX, collagen type XI decreases with age, accounting for over 10% of overall collagen in fetal articular cartilage and about 3% in adult articular cartilage157.
Collagen type XII is codistributed with collagen type I in bone, ligament, tendon, fibrocartilage, muscle, and skin, and codistributed with collagen type II in articular cartilage192. It has the isoform α13 and is associated with fibril formation, cell adhesion, fibrosis, and osteogenesis. One study on passaged chondrocytes showed that collagen type XII is present in cartilage-forming chondrocytes, but not in the other dedifferentiated chondrocytes, indicating that this collagen subtype is necessary to support the formation of hyaline cartilage193. It may contribute to fibril organization, alignment, and stabilization, helping the matrix bear load194. Collagen type XII expression is localized to the growth plate and the surface of articular cartilage, and has been found in juvenile but not embryonic rib hyaline cartilage194. Children with collagen type XII mutations show muscle weakness and joint hyperlaxity195, but cartilage effects have not been reported.
Collagen type XIV has the isoform α13. A global knockout mouse model of collagen type XIV resulted in deteriorated mechanical properties of skin and tendon, and showed that this collagen type has a role in fibrillogenesis and regulating fibril diameter196. In this study, collagen fibrils were thicker, and mature fibrils did not form; however, the resulting effects on cartilage mechanics were not tested. Collagen type XIV has also been identified as a binding partner of COMP63, and mutations of ECM proteins matrilin-3 and COMP decreased the relative amount of collagen type XIV in cartilage, indicating that these proteins stabilize collagen type XIV197.
Collagen type XVI has the isoform α13 and is found in the territorial matrix of hyaline cartilage in a population of thin collagen type II fibrils, which also contains collagen type IX (but without colocalization of types IX and XVI)198. The role of collagen type XVI is not well understood, but it is hypothesized to stabilize ECM by connecting and organizing large fibrillar networks199. It binds to integrins, fibronectin, and fibrillin-1 and −2, participating in intermolecular interactions, organization, and maintenance200. Knockout studies of collagen type XVI have not been performed.
Chondrocyte-homeostasis collagens (IV, VI).
Collagen type IV is the main component of the basement membrane in articular cartilage and has α12α2 isoform. It forms a tetrameric network in the pericellular matrix (PCM) of articular cartilage, where it is involved in maintaining the viability and phenotype of articular chondrocytes201. Collagen type IV expression is decreased in damaged cartilage and clinically failed repair tissue202. It contains anti-angiogenic domains, suggesting that it may have an important role in cartilage homeostasis by arresting tumour growth in vivo203. Owing to its role in the basement membrane, global knockout mouse models are embryonic lethal204. Although collagen type IV seems to have an important role in cartilage PCM, the specific mechanisms must be further investigated to understand how this collagen type maintains healthy chondrocyte properties.
Collagen type VI makes up about 1% of total collagen in adult articular cartilage, where it is mainly found in the PCM157. It has been hypothesized to have important roles in mediating cell–cell and intermolecular interactions205. It was originally thought to have a single isoform of α1α2α3, but the α3 chain can be switched for an α4, α5, or α6 chain206. In a global knockout model, a lack of collagen type VI resulted in swelling cells and decreased stiffness of the PCM, indicating that it regulates PCM and cellular properties207.
Interfacial collagens (X, XIII, XXII, XXVII).
Collagen type X, which has the isoform α13, creates a hexagonal network that is limited locally to hypertrophic cartilage and close to the calcified zone of the cartilage. Because collagen type X is only found at this boundary between cartilage and bone, we classify it as an interfacial collagen. Although it is not produced by most chondrocytes, about 45% of hypertrophic chondrocyte collagen production is collagen type X62. It is highly correlated spatially and temporally with endochondral ossification, making it a biomarker of cartilage hypertrophy208. A collagen type X global knockout study showed that without collagen type X, mice exhibited 14% lethality, whereas the rest had dwarfism and growth-plate compression209.
Collagen type XIII, which has the isoform α13, is the only collagen in cartilage that spans the cell membrane. It is observed in the perichondrium, hypertrophic and proliferative cartilage, and in many other tissues210. A transgenic mouse overexpressing collagen type XIII showed abnormally high bone mass; collagen type XIII was strongly expressed before mineralization started, suggesting that this collagen type has important roles in endochondral ossification211.
Collagen type XXII, which has the isoform α13, is expressed at junctions between many different types of tissues, including the muscle attachment sites of ribs and the junction of articular cartilage and synovial fluid, where it associates with microfibrils, such as fibrillins, or collagen type VI212. It has been shown to bind to collagen-binding integrins in the myotendinous junction, especially α2β1 and α11β1213; however, its function in articular cartilage remains largely understudied and unknown. Although knockouts in mammals have not been performed, global knockdown of collagen type XXII in zebrafish resulted in muscular dystrophy214.
Collagen type XXVII, like collagen type X, is found near the osteochondral junction and is associated with endochondral ossification215. It has the isoform α13 and has a structural role in the PCM of growth-plate cartilage216. Collagen type XXVII is much less abundant than the other types of fibril-forming collagens, and its function is not well understood217. Mutations in the collagen type XXVII gene lead to Steel syndrome — a disease involving skeletal abnormalities, such as dislocations, short stature, and scoliosis218.
Collagen and mechanical properties
The structure of collagen subtypes and crosslinks affects the function of the cartilage tissue. For the purpose of this Review, the ‘structure’ of matrix components refers to measurable physical properties, such as the quantity of collagen, quantity of crosslinks, and the spatial distribution and homogeneity of the collagen network. The function of cartilage refers to the measurable material characteristics of the overall tissue, such as the tensile stiffness, viscoelastic measures (such as the aggregate modulus), and the lubricity of the cartilage surface.
Some structure–function relationships in cartilages have been well characterized, particularly those between the quantity of GAGs and compressive stiffness219, and between the quantity of collagen crosslinks and tensile stiffness3,220. Relationships that correlate collagen content to compressive properties and Poisson’s ratio in articular cartilage have also been described14. Structure–function relationships of specific collagen subtypes are yet to be explicitly defined, but some may be inferred from previous work; because most of the collagen of fibrocartilage is collagen type I, and most of the collagen of hyaline cartilage is collagen type II, it can be assumed that non subtype-specific structure–function relationships are mostly due to these two collagen types. Collagen type VI knockout models showed a structure–function relationship between the amount of collagen type VI and PCM stiffness207. Because full knockouts of collagen types are often neonatal lethal, and collagens form heterofibrils of more than one collagen type, it may be difficult to determine the influence of individual collagen subtypes on the mechanical properties. Nonetheless, the vital roles of minor collagens (for example, in fibril assembly) suggest that their relative contribution to cartilage biomechanics is far greater than their relative proportion of mass in cartilage ECM.
Because the goal of cartilage tissue engineering is functional restoration of diseased or damaged cartilage, the engineered tissue must have similar functional properties to native cartilage. With defined structure–function relationships, certain structural measurements can serve as quality-control measures or design criteria for engineering cartilage. For example, specific GAG or measurements of collagen subtype can be used to assess tissue quality. If these structural analyses are nondestructive, they could be used as preliminary benchmarks prior to implantation. For example, native-like amounts of collagen types IV and VI can be indicative of healthy chondrocytes and PCM suitable for implantation. The presence of collagen type II at 50–60% by dry weight and with crosslinks similar to those of native tissue, would indicate a tissue with native-like tensile properties. Understanding the structure–function relationships of different collagen subtypes would give insight to further develop the cartilage tissue-engineering process, and may hold the key to further strengthening neocartilages. For example, quantifying interfacial collagens may indicate how well an osteochondral implant can maintain healthy subchondral bone, and quantification of fibril-supporting collagens can inform tissue engineers whether the ECM is mature and robust.
Outlook
Although bottom-up proteomics allows the simultaneous analysis of many collagen types, and targeted MRM techniques offer fast analysis of individual collagen types, no methods exist for high-throughput quantification of both major and minor collagen subtypes. The development of such a method would be an important milestone for understanding the distributions and roles in all tissue types of minor collagens. Ideally, this would be low-cost and allow for processing of many samples quickly, while keeping operator time to a minimum. Because collagen has roles throughout the body, characterization studies on a multitude of tissues will not only deepen our understanding of ECM composition, tissue disease states, and structure–function relationships, but also lead to design criteria for engineered tissues (Figure 6).
Figure 6. Broad applications for high-throughput, low cost collagen quantification.
The development of next generation assays for collagen subtype quantification will impact many different fields of biomedical engineering. Examples are shown for healthy and diseased tissues, structure-function relationships, tissue engineering release criteria, and tissue engineering strategies.
Characterization in disease states
Although shotgun proteomics has increased our understanding of the collagen profile of cartilages and other musculoskeletal tissues221-223, sufficiently powered studies to characterize the collagen subtypes of these native tissues must be performed. If highly sensitive targeted or bottom-up LC-MS methods for all minor collagens can be performed in a short (for example, 5- or 10-minute) run, this would allow the analysis of collagens in tissues from different ages, sexes, and species to provide a more comprehensive understanding of the biochemical makeup of healthy native tissues. Once these values are defined, screening efforts can show how cellular phenotype and tissue proteome change through disease or injury. For example, MRM was recently used to show that collagen types II, III, and VI were deposited in greater amounts in osteoarthritic articular cartilage than in the control articular cartilage224, and label-free, bottom-up proteomics was used in an in vitro lung fibrosis model to quantify the relative amounts of collagen types I–VIII, XII, XV, XVI, and XVIII225. In recent years, machine learning has become increasingly important for analysing large datasets in biomedicine226-228. Proteomic datasets with thousands of quantified peptides are excellent candidates for machine-learning algorithms, which may lead to new discoveries of biomarkers for prediction or early detection of many different diseases. In the coming years, quantitative assessments of collagen subtypes in healthy and diseased tissues will be of the utmost importance in understanding the ECM of native tissue and how it remodels through degeneration and disease.
Biomechanical relationships
Several structure–function relationships between biochemical and biomechanical properties are yet to be defined for cartilages, particularly those of minor collagens. Knockout or mutation mouse studies have been completed for collagen types IX229, XI230, and XIV196, but only one study has tested the resultant effects on cartilage mechanics; this knockout study of collagen type VI found a reduced Young’s modulus of the PCM but not of the bulk cartilage231. The quantity, spatial distribution, homogeneity, and assembly of other minor collagens have yet to be tied to the functional properties of cartilage.
Defined structure–function relationships are largely limited to compressive and tensile moduli, which do not constitute the full picture. Cartilages are viscoelastic and lubricious, making it necessary to understand correlations with functional properties such as instantaneous and relaxation moduli, permeability, and tribological properties. Once high-throughput quantification methods for minor collagens are developed, it may be possible to draw correlations between specific collagen subtypes and these and other additional functional properties. For example, well-defined correlations between tensile stiffness and major fibrillar collagens exist but are not yet complemented by studies on fibril-supporting collagens. Collagen type VI has been shown to correlate to PCM stiffness, but collagen type IV has not. It is not known if the presence of interfacial collagens could be correlated to integration strength, or if collagen type XXII at the interface with synovial fluid could have a role in cartilage lubricity. These relationships, if determined experimentally, would further help to elucidate the roles of minor collagens in different tissues..
Screening for engineered therapeutics
Minor collagens and collagen subtype quantification are absent from tissue engineering, perhaps owing to the lack of robust and high-throughput methods for collagen subtype quantification. Although the hydroxyproline assay is frequently used to estimate overall collagen, individual collagen subtypes are rarely quantified, and relatively little attention has been paid to minor collagens in engineered tissues. For example, collagen type XIV is only mentioned in two tissue-engineering studies: one identified mRNA232 and the other identified the protein with LC-MS233. Collagen subtype identification is present in some tissue-engineering studies. For example, IF has been used to identify collagen types I and III in cultured fibroblasts for engineering ligaments234; IHC has been used to visualize collagen types I and II in mesenchymal stem cell-seeded scaffolds for engineering of intervertebral discs235; collagen type III mRNA has been identified in engineered arterial grafts236; and TR-LIFS has been used to assess the relative expression of collagen types I, III, IV, and V in osteogenic ECM from adipose-derived stem cells237. However, the lack of subtype specificity for collagen histological stains and the non-quantitative nature of collagen IHC and IF techniques inhibit a deep understanding of the role of minor collagens in engineered tissues. Ideally, engineered tissues are fully biomimetic, exhibiting the same quantities of all collagen subtypes in native tissues. With the advent of new high-throughput collagen quantification technologies, all tissue engineers will be able to measure the biomimicry of their tissues on a collagen subtype level; the quality of engineered cartilages, bones, heart valves, ligaments, tendons, blood vessels, and skin will particularly depend on their major and minor collagen content.
Collagens for engineering neocartilages
Tissue engineers have put much effort in harnessing major fibrillar collagens to improve the mechanical properties of neocartilage, for example, by using tensile stimulation to increase the alignment of collagen fibrils238. Spectroscopic techniques are promising for quantitative and nondestructive measurement of collagen in cartilage tissue engineering. For example, TR-LIFS was used to measure collagen type II239 and diffuse fibre-optic Raman spectroscopy with hydroxyproline assay was used assess collagen deposition240 in engineered cartilage.
Compared with the tissue-engineering research on total or major collagens, relatively little has focusses specifically on minor collagens. Some tissue-engineering studies using primary chondrocytes or stem cells quantified collagen type IX with ELISA241, and identified collagen type XI242, and types VI, IX, XI, XII, and XIV with mass spectrometry233. However, no studies have determined if specific collagen types in engineered cartilage ECM are similar to those in native tissues. Similarly, there has been no attempt to promote deposition of minor collagens for engineered cartilages. We suggest that, because different collagen types are expressed by mesenchymal stem cells and differentiated chondrocytes during fetal development, tissue engineers could use these collagen types as markers of neocartilage development. For example, the self-assembling process in cartilage tissue engineering is reminiscent of mesenchymal condensation243, and follows the pattern of collagen type VI being remodelled and replaced by collagen type II244.
Collagen crosslinks have been the subject of some cartilage tissue-engineering studies, and could be used to better characterize engineered tissues. For example, the deposition of HP crosslinks in engineered cartilage has been enhanced via hypoxia245, endogeneous LOX246, and mechanical stimulation with centrifugation247. HP is the only crosslink identified in engineered cartilages. A study on the presence of HLKNL, arginoline, or AGEs would better describe the quantity of different crosslinks in engineered cartilages.
The need for different collagen subtypes in engineered cartilages is indicated by their functional roles. The fibril-supporting collagens maintain and regulate fibrils of collagen types I and II, and, thus, should be used to assemble fibrils with correct spacing and diameter. Although chondrocyte-homeostasis collagens do not directly affect the strength of cartilage ECM, they interact with chondrocytes and maintain healthy tissue. Interfacial collagens are particularly important for osteochondral implants. Endochondral ossification continues until about age 18 for females and 21 for males;248 thus, these collagens are needed to produce implants that ossify and grow with the patient. A lack of minor collagens accounts for neonatal lethality or inferior cartilage tissue. Therefore, native-like amounts of all minor collagens are necessary in engineered cartilages. A better understanding and new methods for identifying novel biochemical and biophysical stimuli to enhance the deposition of collagen crosslinks and specific collagen subtypes are the next key steps toward strong, durable, and biomimetic cartilages.
Acknowledgements
This work was funded by NIH Grant No. R01DE015038, NIH Grant No. R01AR071457, and NIH Grant No. R01AR067821.
Footnotes
Competing Interests
None.
References
- 1.Whitslar WH A Study of the Chemical Composition of the Dental Pulp. Am J Dent Sci 23, 350–355 (1889). [PMC free article] [PubMed] [Google Scholar]
- 2.Lin S & Gu L Influence of Crosslink Density and Stiffness on Mechanical Properties of Type I Collagen Gel. Materials 8, 551–560 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eleswarapu SV, Responte DJ & Athanasiou KA Tensile Properties, Collagen Content, and Crosslinks in Connective Tissues of the Immature Knee Joint. PLoS One 6, e26178 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brüel A, Ortoft G & Oxlund H Inhibition of cross-links in collagen is associated with reduced stiffness of the aorta in young rats. Atherosclerosis 140, 135–145 (1998). [DOI] [PubMed] [Google Scholar]
- 5.Fang M, Yuan J, Peng C & Li Y Collagen as a double-edged sword in tumor progression. Tumour Biol. 35, 2871–2882 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu S et al. The role of collagen in cancer: from bench to bedside. J. Transl. Med 17, 309 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poole AR et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann. Rheum. Dis 61 Suppl 2, ii78–81 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landewé RBM et al. Arthritis instantaneously causes collagen type I and type II degradation in patients with early rheumatoid arthritis: a longitudinal analysis. Ann. Rheum. Dis 65, 40–44 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuivaniemi H, Tromp G & Prockop DJ Mutations in collagen genes: causes of rare and some common diseases in humans. The FASEB Journal vol. 5 2052–2060 (1991). [DOI] [PubMed] [Google Scholar]
- 10.Arseni L, Lombardi A & Orioli D From Structure to Phenotype: Impact of Collagen Alterations on Human Health. Int. J. Mol. Sci 19, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorushanova A et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater 31, e1801651 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Kadler KE, Hill A & Canty-Laird EG Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators. Curr. Opin. Cell Biol 20, 495–501 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shoulders MD & Raines RT Collagen structure and stability. Annu. Rev. Biochem 78, 929–958 (2009).This review paper describes the structure and characteristics of collagen triple helices.
- 14.Responte DJ, Natoli RM & Athanasiou KA Collagens of articular cartilage: structure, function, and importance in tissue engineering. Crit. Rev. Biomed. Eng 35, 363–411 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Lin K et al. Advanced Collagen-Based Biomaterials for Regenerative Biomedicine. Advanced Functional Materials vol. 29 1804943 (2019). [Google Scholar]
- 16.Chang S-W & Buehler MJ Molecular biomechanics of collagen molecules. Materials Today vol. 17 70–76 (2014). [Google Scholar]
- 17.Ricard-Blum S The collagen family. Cold Spring Harb. Perspect. Biol 3, a004978 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma U et al. Structural basis of homo- and heterotrimerization of collagen I. Nat. Commun 8, 14671 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An B et al. Definition of the native and denatured type II collagen binding site for fibronectin using a recombinant collagen system. J. Biol. Chem 289, 4941–4951 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pihlajamaa T et al. Characterization of recombinant human type IX collagen. Association of alpha chains into homotrimeric and heterotrimeric molecules. J. Biol. Chem 274, 22464–22468 (1999). [DOI] [PubMed] [Google Scholar]
- 21.Eyre DR, Weis MA & Wu J-J Advances in collagen cross-link analysis. Methods 45, 65–74 (2008).This paper highlights methods for analysing crosslinked collagen peptides with LC-MS/MS, and shows intermolecular crosslinks between collagen types IX and II.
- 22.Siegel RC Biosynthesis of collagen crosslinks: increased activity of purified lysyl oxidase with reconstituted collagen fibrils. Proc. Natl. Acad. Sci. U. S. A 71, 4826–4830 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnard K, Light ND, Sims TJ & Bailey AJ Chemistry of the collagen cross-links. Origin and partial characterization of a putative mature cross-link of collagen. Biochem. J 244, 303–309 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robins S Fibrillogenesis and Maturation of Collagens. Dynamics of Bone and Cartilage Metabolism 41–53 (2006) doi: 10.1016/b9-78-012088-5/62650-0030. [DOI] [Google Scholar]
- 25.Avery NC, Sims TJ & Bailey AJ Quantitative Determination of Collagen Cross-links. Methods in Molecular Biology 103–121 (2009) doi: 10.1007/978-1-59745-413-1_6. [DOI] [PubMed] [Google Scholar]
- 26.Saito M & Marumo K Effects of Collagen Crosslinking on Bone Material Properties in Health and Disease. Calcif. Tissue Int 97, 242–261 (2015). [DOI] [PubMed] [Google Scholar]
- 27.Eyre DR, Weis MA & Wu J-J Maturation of collagen Ketoimine cross-links by an alternative mechanism to pyridinoline formation in cartilage. J. Biol. Chem 285, 16675–16682 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett TL, Kandel R, De Croos JNA, Avery NC & Grynpas MD Enhanced levels of non-enzymatic glycation and pentosidine crosslinking in spontaneous osteoarthritis progression. Osteoarthritis Cartilage 20, 736–744 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Steinhart H, Bosselmann A & Moeller C Determination of Pyridinolines in Bovine Collagenous Tissues. Journal of Agricultural and Food Chemistry vol. 42 1943–1947 (1994). [Google Scholar]
- 30.Tan CI, Kent GN, Randall AG, Edmondston SJ & Singer KP Age-related changes in collagen, pyridinoline, and deoxypyridinoline in normal human thoracic intervertebral discs. J. Gerontol. A Biol. Sci. Med. Sci 58, B387–93 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Delmas PD, Schlemmer A, Gineyts E, Riis B & Christiansen C Urinary excretion of pyridinoline crosslinks correlates with bone turnover measured on iliac crest biopsy in patients with vertebral osteoporosis. J. Bone Miner. Res 6, 639–644 (1991). [DOI] [PubMed] [Google Scholar]
- 32.Lindert U et al. Urinary pyridinoline cross-links as biomarkers of osteogenesis imperfecta. Orphanet J. Rare Dis 10, 104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeuchi S, Arai K, Saitoh H, Yoshida K & Miura M Urinary pyridinoline and deoxypyridinoline as potential markers of bone metastasis in patients with prostate cancer. J. Urol 156, 1691–1695 (1996). [PubMed] [Google Scholar]
- 34.Siegfried M Reticulin and collagen. The Journal of Physiology vol. 28 319–324 (1902). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tebb MC & Christine Tebb M Reticulin and collagen. The Journal of Physiology vol. 27 463–472 (1902). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neuman RE & Logan MA The determination of hydroxyproline. J. Biol. Chem 184, 299–306 (1950). [PubMed] [Google Scholar]
- 37.Reddy GK & Enwemeka CS A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem 29, 225–229 (1996). [DOI] [PubMed] [Google Scholar]
- 38.Cissell DD, Link JM, Hu JC & Athanasiou KA A Modified Hydroxyproline Assay Based on Hydrochloric Acid in Ehrlich’s Solution Accurately Measures Tissue Collagen Content. Tissue Eng. Part C Methods 23, 243–250 (2017).This paper describes the most recent edition of the photometric hydroxyproline assay, which uses safer and less expensive materials without sacrificing assay sensitivity.
- 39.Caetano GF, Fronza M, Leite MN, Gomes A & Frade MAC Comparison of collagen content in skin wounds evaluated by biochemical assay and by computer-aided histomorphometric analysis. Pharm. Biol 54, 2555–2559 (2016). [DOI] [PubMed] [Google Scholar]
- 40.Kliment CR, Englert JM, Crum LP & Oury TD A novel method for accurate collagen and biochemical assessment of pulmonary tissue utilizing one animal. Int. J. Clin. Exp. Pathol 4, 349–355 (2011). [PMC free article] [PubMed] [Google Scholar]
- 41.Stoilov I, Starcher BC, Mecham RP & Broekelmann TJ Measurement of elastin, collagen, and total protein levels in tissues. in Methods in Cell Biology 133–146 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Khan T et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol 29, 1575–1591 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J et al. Extraction and isolation of type I, III and V collagens and their SDS-PAGE analyses. Trans. Tianjin Univ 17, 111–117 (2011). [Google Scholar]
- 44.Hayashi T & Nagai Y Separation of the α Chains of Type I and III Collagens by SDS-Polyacrylamide Gel Electrophoresis. J. Biochem 86, 453–459 (1979). [DOI] [PubMed] [Google Scholar]
- 45.Vincent SG, Cunningham PR, Stephens NL, Halayko AJ & Fisher JT Quantitative densitometry of proteins stained with coomassie blue using a Hewlett Packard scanjet scanner and Scanplot software. Electrophoresis 18, 67–71 (1997). [DOI] [PubMed] [Google Scholar]
- 46.Eyre DR, Koob TJ & Van Ness KP Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal. Biochem 137, 380–388 (1984). [DOI] [PubMed] [Google Scholar]
- 47.Saito M, Marumo K, Fujii K & Ishioka N Single-column high-performance liquid chromatographic-fluorescence detection of immature, mature, and senescent cross-links of collagen. Anal. Biochem 253, 26–32 (1997). [DOI] [PubMed] [Google Scholar]
- 48.Robins SP, Stewart P, Astbury C & Bird HA Measurement of the cross linking compound, pyridinoline, in urine as an index of collagen degradation in joint disease. Ann. Rheum. Dis 45, 969–973 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robins SP et al. Direct, enzyme-linked immunoassay for urinary deoxypyridinoline as a specific marker for measuring bone resorption. J. Bone Miner. Res 9, 1643–1649 (1994). [DOI] [PubMed] [Google Scholar]
- 50.Taneda S & Monnier VM ELISA of pentosidine, an advanced glycation end product, in biological specimens. Clin. Chem 40, 1766–1773 (1994). [PubMed] [Google Scholar]
- 51.Sutandy FXR, Reymond Sutandy FX, Qian J, Chen C-S & Zhu H Overview of Protein Microarrays. Current Protocols in Protein Science vol. 72 27.1.1–27.1.16 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuschel C et al. Cell adhesion profiling using extracellular matrix protein microarrays. BioTechniques vol. 40 523–531 (2006). [DOI] [PubMed] [Google Scholar]
- 53.Baker HN, Murphy R, Lopez E & Garcia C Conversion of a capture ELISA to a Luminex xMAP assay using a multiplex antibody screening method. J. Vis. Exp (2012) doi: 10.3791/4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elshal MF & McCoy JP Multiplex bead array assays: performance evaluation and comparison of sensitivity to ELISA. Methods 38, 317–323 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim CH et al. Stability and reproducibility of proteomic profiles measured with an aptamer-based platform. Sci. Rep 8, 8382 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coghlan RF et al. A degradation fragment of type X collagen is a real-time marker for bone growth velocity. Sci. Transl. Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koolmees PA & Bijker PG Histometric and chemical methods for determining collagen in meats. Vet. Q 7, 84–90 (1985). [DOI] [PubMed] [Google Scholar]
- 58.Grimm PC et al. Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J. Am. Soc. Nephrol 14, 1662–1668 (2003). [DOI] [PubMed] [Google Scholar]
- 59.Brigger D & Muckle RJ Comparison of Sirius red and Congo red as stains for amyloid in animal tissues. J. Histochem. Cytochem 23, 84–88 (1975). [DOI] [PubMed] [Google Scholar]
- 60.Coelho PGB, Souza M. V. de, Conceição LG, Viloria MIV & Bedoya SAO Evaluation of dermal collagen stained with picrosirius red and examined under polarized light microscopy. An. Bras. Dermatol 93, 415–418 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai M et al. Alteration of cartilage surface collagen fibers differs locally after immobilization of knee joints in rats. J. Anat 226, 447–457 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmid TM & Linsenmayer TF Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J. Cell Biol 100, 598–605 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agarwal P et al. Collagen XII and XIV, new partners of cartilage oligomeric matrix protein in the skin extracellular matrix suprastructure. J. Biol. Chem 287, 22549–22559 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yakovlev DD et al. Quantitative mapping of collagen fiber alignment in thick tissue samples using transmission polarized-light microscopy. J. Biomed. Opt 21, 71111 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Nadiarynkh O, Plotnikov S & Campagnola PJ Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc 7, 654–669 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keikhosravi A et al. Quantification of collagen organization in histopathology samples using liquid crystal based polarization microscopy. Biomed. Opt. Express 8, 4243–4256 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghazanfari S, Driessen-Mol A, Strijkers GJ, Baaijens FPT & Bouten CVC The evolution of collagen fiber orientation in engineered cardiovascular tissues visualized by diffusion tensor imaging. PLoS One 10, e0127847 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Starborg T, Lu Y, Kadler KE & Holmes DF Chapter 17 Electron Microscopy of Collagen Fibril Structure In Vitro and In Vivo Including Three-Dimensional Reconstruction. Methods in Cell Biology 319–345 (2008) doi: 10.1016/s0091-679x(08)00417-2. [DOI] [PubMed] [Google Scholar]
- 69.Starborg T et al. Using transmission electron microscopy and 3View to determine collagen fibril size and three-dimensional organization. Nat. Protoc 8, 1433–1448 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Changoor A et al. Structural characteristics of the collagen network in human normal, degraded and repair articular cartilages observed in polarized light and scanning electron microscopies. Osteoarthritis Cartilage 19, 1458–1468 (2011). [DOI] [PubMed] [Google Scholar]
- 71.Ruozi B et al. Intact collagen and atelocollagen sponges: Characterization and ESEM observation. Materials Science and Engineering: C vol. 27 802–810 (2007). [Google Scholar]
- 72.Snellman A et al. A short sequence in the N-terminal region is required for the trimerization of type XIII collagen and is conserved in other collagenous transmembrane proteins. The EMBO Journal vol. 19 5051–5059 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manferdini C et al. Immunoelectron microscopic localization of Collagen type XV during human mesenchymal stem cells mineralization. Connect. Tissue Res 59, 42–45 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Plumb DA et al. Collagen XXVII is developmentally regulated and forms thin fibrillar structures distinct from those of classical vertebrate fibrillar collagens. J. Biol. Chem 282, 12791–12795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ranjit S et al. Imaging Fibrosis and Separating Collagens using Second Harmonic Generation and Phasor Approach to Fluorescence Lifetime Imaging. Sci. Rep 5, 13378 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Haudenschild AK et al. Nondestructive fluorescence lifetime imaging and time-resolved fluorescence spectroscopy detect cartilage matrix depletion and correlate with mechanical properties. Eur. Cell. Mater 36, 30–43 (2018). [DOI] [PubMed] [Google Scholar]
- 77.Haudenschild AK et al. Non-destructive detection of matrix stabilization correlates with enhanced mechanical properties of self-assembled articular cartilage. Journal of Tissue Engineering and Regenerative Medicine vol. 13 637–648 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sherlock BE et al. Nondestructive assessment of collagen hydrogel cross-linking using time-resolved autofluorescence imaging. J. Biomed. Opt 23, 1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de C. Vidal B & Mello MLS Collagen type I amide I band infrared spectroscopy. Micron 42, 283–289 (2011). [DOI] [PubMed] [Google Scholar]
- 80.Belbachir K, Noreen R, Gouspillou G & Petibois C Collagen types analysis and differentiation by FTIR spectroscopy. Anal. Bioanal. Chem 395, 829–837 (2009). [DOI] [PubMed] [Google Scholar]
- 81.Bergholt MS, Serio A & Albro MB Raman Spectroscopy: Guiding Light for the Extracellular Matrix. Front Bioeng Biotechnol 7, 303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Albro MB et al. Raman spectroscopic imaging for quantification of depth-dependent and local heterogeneities in native and engineered cartilage. NPJ Regen Med 3, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ye H et al. Burn-related Collagen Conformational Changes in ex vivo Porcine Skin using Raman Spectroscopy. Sci. Rep 9, 19138 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen TT et al. Characterization of Type I and IV Collagens by Raman Microspectroscopy: Identification of Spectral Markers of the Dermo-Epidermal Junction. Spectroscopy: An International Journal vol. 27 421–427 (2012). [Google Scholar]
- 85.Marcu L, Cohen D, Maarek J-MI & Grundfest WS Characterization of type I, II, III, IV, and V collagens by time-resolved laser-induced fluorescence spectroscopy. Optical Biopsy III (2000) doi: 10.1117/12.382720. [DOI] [Google Scholar]
- 86.Sun Y et al. Development of a dual-modal tissue diagnostic system combining time-resolved fluorescence spectroscopy and ultrasonic backscatter microscopy. Rev. Sci. Instrum 80, 065104 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Colgrave ML, Allingham PG, Tyrrell K & Jones A Multiple Reaction Monitoring for the Accurate Quantification of Amino Acids: Using Hydroxyproline to Estimate Collagen Content. Methods Mol. Biol 2030, 33–45 (2019). [DOI] [PubMed] [Google Scholar]
- 88.Nimptsch A et al. Quantitative analysis of denatured collagen by collagenase digestion and subsequent MALDI-TOF mass spectrometry. Cell Tissue Res. 343, 605–617 (2011).This paper uses MALDI-TOF mass spectrometry to quantify glycine-proline-hydroxyproline tripeptides as a more sensitive method than the hydroxyproline assay.
- 89.Yoshida K et al. Quantitative evaluation of collagen crosslinks and corresponding tensile mechanical properties in mouse cervical tissue during normal pregnancy. PLoS One 9, e112391 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Naffa R et al. Rapid analysis of pyridinoline and deoxypyridinoline in biological samples by liquid chromatography with mass spectrometry and a silica hydride column. Journal of Separation Science vol. 42 1482–1488 (2019). [DOI] [PubMed] [Google Scholar]
- 91.Kovacevic I, Pokrajac M, Miljkovic B, Jovanovic D & Prostran M Comparison of liquid chromatography with fluorescence detection to liquid chromatography-mass spectrometry for the determination of fluoxetine and norfluoxetine in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 830, 372–376 (2006). [DOI] [PubMed] [Google Scholar]
- 92.Lin H, Goodin S, Strair RK, DiPaola RS & Gounder MK Comparison of LC-MS Assay and HPLC Assay of Busulfan in Clinical Pharmacokinetics Studies. ISRN Analytical Chemistry vol. 2012 1–5 (2012). [Google Scholar]
- 93.Santa T Recent advances in analysis of glutathione in biological samples by high-performance liquid chromatography: a brief overview. Drug Discov. Ther 7, 172–177 (2013). [PubMed] [Google Scholar]
- 94.Dreisewerd K, Rohlfing A, Spottke B, Urbanke C & Henkel W Characterization of whole fibril-forming collagen proteins of types I, III, and V from fetal calf skin by infrared matrix-assisted laser desorption ionization mass spectrometry. Anal. Chem 76, 3482–3491 (2004). [DOI] [PubMed] [Google Scholar]
- 95.Pataridis S, Eckhardt A, Mikulikova K, Sedlakova P & Miksik I Determination and Quantification of Collagen Types in Tissues Using HPLC-MS/MS. Curr. Anal. Chem 5, 316–323 (2009). [Google Scholar]
- 96.Wang Y et al. Quantitative proteomics analysis of cartilage response to mechanical injury and cytokine treatment. Matrix Biol. 63, 11–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kumazawa Y, Taga Y, Iwai K & Koyama Y-I A Rapid and Simple LC-MS Method Using Collagen Marker Peptides for Identification of the Animal Source of Leather. J. Agric. Food Chem 64, 6051–6057 (2016). [DOI] [PubMed] [Google Scholar]
- 98.Wiese S, Reidegeld KA, Meyer HE & Warscheid B Protein labeling by iTRAQ: A new tool for quantitative mass spectrometry in proteome research. PROTEOMICS vol. 7 1004–1004 (2007). [DOI] [PubMed] [Google Scholar]
- 99.Lanucara F & Eyers CE Mass spectrometric-based quantitative proteomics using SILAC. Methods Enzymol. 500, 133–150 (2011). [DOI] [PubMed] [Google Scholar]
- 100.Zhang L & Elias JE Relative Protein Quantification Using Tandem Mass Tag Mass Spectrometry. Methods in Molecular Biology 185–198 (2017) doi: 10.1007/978-1-4939-6747-6_14. [DOI] [PubMed] [Google Scholar]
- 101.Ye X, Luke B, Andresson T & Blonder J 18O stable isotope labeling in MS-based proteomics. Brief. Funct. Genomic. Proteomic 8, 136–144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu W, Smith JW & Huang C-M Mass spectrometry-based label-free quantitative proteomics. J. Biomed. Biotechnol 2010, 840518 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dresner E & Schubert M THE COMPARATIVE SUSCEPTIBILITY TO COLLAGENASE AND TRYPSIN OF COLLAGEN, SOLUBLE COLLAGENS AND RENAL BASEMENT MEMBRANE. Journal of Histochemistry & Cytochemistry vol. 3 360–368 (1955). [DOI] [PubMed] [Google Scholar]
- 104.Krey JF et al. Accurate label-free protein quantitation with high- and low-resolution mass spectrometers. J. Proteome Res 13, 1034–1044 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cox J et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol. Cell. Proteomics 13, 2513–2526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Önnerfjord P, Khabut A, Reinholt FP, Svensson O & Heinegård D Quantitative proteomic analysis of eight cartilaginous tissues reveals characteristic differences as well as similarities between subgroups. J. Biol. Chem 287, 18913–18924 (2012).This paper shows a wide proteomics analyses on several types of cartilage. iTRAQ and LC-MS/MS technologies were used for relative quantification of many ECM molecules.
- 107.Lourido L et al. Quantitative proteomic profiling of human articular cartilage degradation in osteoarthritis. J. Proteome Res 13, 6096–6106 (2014). [DOI] [PubMed] [Google Scholar]
- 108.Frantzi M et al. Discovery and validation of urinary biomarkers for detection of renal cell carcinoma. J. Proteomics 98, 44–58 (2014). [DOI] [PubMed] [Google Scholar]
- 109.van Huizen NA et al. Up-regulation of collagen proteins in colorectal liver metastasis compared with normal liver tissue. J. Biol. Chem 294, 281–289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Huey DJ, Hu JC & Athanasiou KA Unlike Bone, Cartilage Regeneration Remains Elusive. Science 338, 917–921 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Athanasiou KA, Darling EM, Hu JC, DuRaine GD & Hari Reddi A Articular Cartilage. (CRC Press, 2017). [Google Scholar]
- 112.Sophia Fox AJ, Bedi A & Rodeo SA The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health: A Multidisciplinary Approach 1, 461–468 (2009).This review paper provides a thorough overview of articular cartilage, its zonal variations, and its biomechanical functions.
- 113.Almarza AJ & Athanasiou KA Design Characteristics for the Tissue Engineering of Cartilaginous Tissues. Ann. Biomed. Eng 32, 2–17 (2004). [DOI] [PubMed] [Google Scholar]
- 114.Makris EA, Hadidi P & Athanasiou KA The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32, 7411 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kuroda S et al. Biomechanical and biochemical characteristics of the mandibular condylar cartilage. Osteoarthritis Cartilage 17, 1408–1415 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Singh M & Detamore MS Biomechanical properties of the mandibular condylar cartilage and their relevance to the TMJ disc. J. Biomech 42, 405–417 (2009). [DOI] [PubMed] [Google Scholar]
- 117.Mithoefer K, McAdams T, Williams RJ, Kreuz PC & Mandelbaum BR Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am. J. Sports Med 37, 2053–2063 (2009). [DOI] [PubMed] [Google Scholar]
- 118.Bos EJ et al. Structural and Mechanical Comparison of Human Ear, Alar, and Septal Cartilage. Plast Reconstr Surg Glob Open 6, e1610 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Griffin MF, Premakumar Y, Seifalian AM, Szarko M & Butler PEM Biomechanical Characterisation of the Human Auricular Cartilages; Implications for Tissue Engineering. Ann. Biomed. Eng 44, 3460–3467 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Naumann A et al. Immunochemical and mechanical characterization of cartilage subtypes in rabbit. J. Histochem. Cytochem 50, 1049–1058 (2002). [DOI] [PubMed] [Google Scholar]
- 121.Madsen K, von der Mark K, van Menxel M & Friberg U Analysis of collagen types synthesized by rabbit ear cartilage chondrocytes in vivo and in vitro. Biochem. J 221, 189–196 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Setton LA, Elliott DM & Mow VC Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage 7, 2–14 (1999). [DOI] [PubMed] [Google Scholar]
- 123.Miosge N, Hartmann M, Maelicke C & Herken R Expression of collagen type I and type II in consecutive stages of human osteoarthritis. Histochem. Cell Biol 122, 229–236 (2004). [DOI] [PubMed] [Google Scholar]
- 124.Lahm A et al. Changes in content and synthesis of collagen types and proteoglycans in osteoarthritis of the knee joint and comparison of quantitative analysis with Photoshop-based image analysis. Arch. Orthop. Trauma. Surg 130, 557–564 (2010). [DOI] [PubMed] [Google Scholar]
- 125.Hosseininia S et al. Evidence for enhanced collagen type III deposition focally in the territorial matrix of osteoarthritic hip articular cartilage. Osteoarthritis Cartilage 24, 1029 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Heinemeier KM Type II Collagen; Designed to Last a Lifetime? Osteoarthritis and Cartilage vol. 25 S5 (2017). [Google Scholar]
- 127.Owings MF & Kozak LJ Ambulatory and inpatient procedures in the United States, 1996. Vital Health Stat. 13 1–119 (1998). [PubMed] [Google Scholar]
- 128.Moseley JB et al. A Controlled Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. N. Engl. J. Med 347, 81–88 (2002). [DOI] [PubMed] [Google Scholar]
- 129.Koh JL, Wirsing K, Lautenschlager E & Zhang L-O The effect of graft height mismatch on contact pressure following osteochondral grafting: a biomechanical study. Am. J. Sports Med 32, 317–320 (2004). [DOI] [PubMed] [Google Scholar]
- 130.Khan IM, Gilbert SJ, Singhrao SK, Duance VC & Archer CW Cartilage integration: evaluation of the reasons for failure of integration during cartilage repair. A review. Eur. Cell. Mater 16, 26–39 (2008). [DOI] [PubMed] [Google Scholar]
- 131.Makris EA, Gomoll AH, Malizos KN, Hu JC & Athanasiou KA Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol 11, 21–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang Z et al. Matrix-induced autologous chondrocyte implantation for the treatment of chondral defects of the knees in Chinese patients. Drug Des. Devel. Ther 8, 2439–2448 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frisbie DD et al. Early events in cartilage repair after subchondral bone microfracture. Clin. Orthop. Relat. Res 215–227 (2003). [DOI] [PubMed] [Google Scholar]
- 134.Barber FA What is the terrible triad? Arthroscopy 8, 19–22 (1992). [DOI] [PubMed] [Google Scholar]
- 135.LeResche L Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit. Rev. Oral Biol. Med 8, 291–305 (1997). [DOI] [PubMed] [Google Scholar]
- 136.Murphy MK, MacBarb RF, Wong ME & Athanasiou KA Temporomandibular disorders: a review of etiology, clinical management, and tissue engineering strategies. Int. J. Oral Maxillofac. Implants 28, e393–414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Urban JPG & Roberts S Degeneration of the intervertebral disc. Arthritis Res. Ther 5, 120–130 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Luoma K et al. Low Back Pain in Relation to Lumbar Disc Degeneration. Spine vol. 25 487–492 (2000). [DOI] [PubMed] [Google Scholar]
- 139.Maniadakis N & Gray A The economic burden of back pain in the UK. Pain vol. 84 95–103 (2000). [DOI] [PubMed] [Google Scholar]
- 140.Ha AY, Shalvoy RM, Voisinet A, Racine J & Aaron RK Controversial role of arthroscopic meniscectomy of the knee: A review. World J. Orthop 7, 287–292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Miloro M & Henriksen B Discectomy as the primary surgical option for internal derangement of the temporomandibular joint. J. Oral Maxillofac. Surg 68, 782–789 (2010). [DOI] [PubMed] [Google Scholar]
- 142.Thorlund JB et al. Patient reported outcomes in patients undergoing arthroscopic partial meniscectomy for traumatic or degenerative meniscal tears: comparative prospective cohort study. BMJ 356, j356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Illien-Jünger S et al. Detrimental effects of discectomy on intervertebral disc biology can be decelerated by growth factor treatment during surgery: a large animal organ culture model. Spine J. 14, 2724–2732 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kwon H et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol 15, 550–570 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Makris EA, Gomoll AH, Malizos KN, Hu JC & Athanasiou KA Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol 11, 21–34 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Higashioka MM, Chen JA, Hu JC & Athanasiou KA Building an anisotropic meniscus with zonal variations. Tissue Eng. Part A 20, 294–302 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cao Y, Vacanti JP, Paige KT, Upton J & Vacanti CA Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast. Reconstr. Surg 100, 297–302; discussion 303–4 (1997). [DOI] [PubMed] [Google Scholar]
- 148.Little CJ, Bawolin NK & Chen X Mechanical properties of natural cartilage and tissue-engineered constructs. Tissue Eng. Part B Rev 17, 213–227 (2011). [DOI] [PubMed] [Google Scholar]
- 149.Salinas EY, Hu JC & Athanasiou K A Guide for Using Mechanical Stimulation to Enhance Tissue-Engineered Articular Cartilage Properties. Tissue Eng. Part B Rev 24, 345–358 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pulkkinen HJ et al. Repair of osteochondral defects with recombinant human type II collagen gel and autologous chondrocytes in rabbit. Osteoarthritis and Cartilage vol. 21 481–490 (2013). [DOI] [PubMed] [Google Scholar]
- 151.Matsiko A, Levingstone TJ, O’Brien FJ & Gleeson JP Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. Journal of the Mechanical Behavior of Biomedical Materials vol. 11 41–52 (2012). [DOI] [PubMed] [Google Scholar]
- 152.Munir N & Callanan A Novel phase separated polycaprolactone/collagen scaffolds for cartilage tissue engineering. Biomed. Mater 13, 051001 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Corradetti B et al. Immune tuning scaffold for the local induction of a pro-regenerative environment. Sci. Rep 7, 17030 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Boehler RM, Graham JG & Shea LD Tissue engineering tools for modulation of the immune response. Biotechniques 51, 239–40, 242, 244 passim (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Irawan V, Sung T-C, Higuchi A & Ikoma T Collagen Scaffolds in Cartilage Tissue Engineering and Relevant Approaches for Future Development. Tissue Eng Regen Med 15, 673–697 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Moutos FT & Guilak F Composite scaffolds for cartilage tissue engineering. Biorheology vol. 45 501–512 (2008). [PMC free article] [PubMed] [Google Scholar]
- 157.Eyre D Collagen of articular cartilage. Arthritis Res. 4, 30–35 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Eyre DR & Wu JJ Collagen of fibrocartilage: a distinctive molecular phenotype in bovine meniscus. FEBS Lett. 158, 265–270 (1983). [DOI] [PubMed] [Google Scholar]
- 159.Luo Y et al. The minor collagens in articular cartilage. Protein Cell 8, 560–572 (2017).This review paper describes the minor collagens found in articular cartilage, including the molecules to which they bind, and which collagens may be used as biomarkers of joint diseases.
- 160.Johns DE & Athanasiou KA Design characteristics for temporomandibular joint disc tissue engineering: learning from tendon and articular cartilage. Proc. Inst. Mech. Eng. H 221, 509–526 (2007). [DOI] [PubMed] [Google Scholar]
- 161.Eyre DR & Muir H Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem. J 157, 267–270 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Han S et al. Molecular mechanism of type I collagen homotrimer resistance to mammalian collagenases. J. Biol. Chem 285, 22276–22281 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li LP, Herzog W, Korhonen RK & Jurvelin JS The role of viscoelasticity of collagen fibers in articular cartilage: axial tension versus compression. Med. Eng. Phys 27, 51–57 (2005). [DOI] [PubMed] [Google Scholar]
- 164.Aryaei A, Vapniarsky N, Hu JC & Athanasiou KA Recent tissue engineering advances for the treatment of temporomandibular joint disorders. Curr. Osteoporos. Rep 14, 269 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bozec L, van der Heijden G & Horton M Collagen fibrils: nanoscale ropes. Biophys. J 92, 70–75 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Orgel JPRO, San Antonio JD & Antipova O Molecular and structural mapping of collagen fibril interactions. Connect. Tissue Res 52, 2–17 (2011). [DOI] [PubMed] [Google Scholar]
- 167.Holmes DF & Kadler KE The 10+4 microfibril structure of thin cartilage fibrils. Proc. Natl. Acad. Sci. U. S. A 103, 17249–17254 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gottardi R et al. Supramolecular Organization of Collagen Fibrils in Healthy and Osteoarthritic Human Knee and Hip Joint Cartilage. PLoS One 11, e0163552 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Eryilmaz E, Teizer W & Hwang W In Vitro Analysis of the Co-Assembly of Type-I and Type-III Collagen. Cellular and Molecular Bioengineering vol. 10 41–53 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gelse K Collagens—structure, function, and biosynthesis. Advanced Drug Delivery Reviews vol. 55 1531–1546 (2003). [DOI] [PubMed] [Google Scholar]
- 171.Petersen W & Tillmann B Collagenous fibril texture of the human knee joint menisci. Anat. Embryol 197, 317–324 (1998). [DOI] [PubMed] [Google Scholar]
- 172.Inoue H & Takeda T Three-dimensional observation of collagen framework of lumbar intervertebral discs. Acta Orthop. Scand 46, 949–956 (1975). [DOI] [PubMed] [Google Scholar]
- 173.Hickey DS & Hukins DW Collagen fibril diameters and elastic fibres in the annulus fibrosus of human fetal intervertebral disc. J. Anat 133, 351–357 (1981). [PMC free article] [PubMed] [Google Scholar]
- 174.Armiento AR, Alini M & Stoddart MJ Articular fibrocartilage - Why does hyaline cartilage fail to repair? Adv. Drug Deliv. Rev 146, 289–305 (2019). [DOI] [PubMed] [Google Scholar]
- 175.Karuppal R Current concepts in the articular cartilage repair and regeneration. J Orthop 14, A1–A3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang L, Hu J & Athanasiou KA The Role of Tissue Engineering in Articular Cartilage Repair and Regeneration. Critical Reviews™ in Biomedical Engineering vol. 37 1–57 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Han E, Chen SS, Klisch SM & Sah RL Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys. J 101, 916–924 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Grynpas MD, Eyre DR & Kirschner DA Collagen type II differs from type I in native molecular packing. Biochim. Biophys. Acta 626, 346–355 (1980). [DOI] [PubMed] [Google Scholar]
- 179.Liu X, Wu H, Byrne M, Krane S & Jaenisch R Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc. Natl. Acad. Sci. U. S. A 94, 1852 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wu J-J, Weis MA, Kim LS & Eyre DR Type III collagen, a fibril network modifier in articular cartilage. J. Biol. Chem 285, 18537–18544 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Birk DE, Fitch JM, Babiarz JP & Linsenmayer TF Collagen type I and type V are present in the same fibril in the avian corneal stroma. J. Cell Biol 106, 999–1008 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wenstrup RJ et al. Type V collagen controls the initiation of collagen fibril assembly. J. Biol. Chem 279, 53331–53337 (2004). [DOI] [PubMed] [Google Scholar]
- 183.Sun M et al. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J. Cell Sci 124, 4096–4105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Olsen BR Collagen IX. Int. J. Biochem. Cell Biol 29, 555–558 (1997). [DOI] [PubMed] [Google Scholar]
- 185.Eyre DR, Pietka T, Weis MA & Wu J-J Covalent cross-linking of the NC1 domain of collagen type IX to collagen type II in cartilage. J. Biol. Chem 279, 2568–2574 (2004). [DOI] [PubMed] [Google Scholar]
- 186.Opolka A et al. Collagen IX is indispensable for timely maturation of cartilage during fracture repair in mice. Matrix Biology vol. 26 85–95 (2007). [DOI] [PubMed] [Google Scholar]
- 187.Fässler R et al. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. Proc. Natl. Acad. Sci. U. S. A 91, 5070–5074 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oxford JT, Doege KJ, Horton WE Jr, & Morris NP Characterization of type II and type XI collagen synthesis by an immortalized rat chondrocyte cell line (IRC) having a low level of type II collagen mRNA expression. Exp. Cell Res 213, 28–36 (1994). [DOI] [PubMed] [Google Scholar]
- 189.Wu J-J, Weis MA, Kim LS, Carter BG & Eyre DR Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue. J. Biol. Chem 284, 5539–5545 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fichard A, Kleman J-P & Ruggiero F Another look at collagen V and XI molecules. Matrix Biol. 14, 515–531 (1995). [DOI] [PubMed] [Google Scholar]
- 191.Fernandes RJ, Weis M, Scott MA, Seegmiller RE & Eyre DR Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biol. 26, 597–603 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Chiquet M, Birk DE, Bönnemann CG & Koch M Collagen XII: Protecting bone and muscle integrity by organizing collagen fibrils. Int. J. Biochem. Cell Biol 53, 51–54 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Taylor DW et al. Collagen type XII and versican are present in the early stages of cartilage tissue formation by both redifferentating passaged and primary chondrocytes. Tissue Eng. Part A 21, 683–693 (2015). [DOI] [PubMed] [Google Scholar]
- 194.Gregory KE, Keene DR, Tufa SF, Lunstrum GP & Morris NP Developmental distribution of collagen type XII in cartilage: association with articular cartilage and the growth plate. J. Bone Miner. Res 16, 2005–2016 (2001). [DOI] [PubMed] [Google Scholar]
- 195.Zou Y et al. Recessive and dominant mutations in COL12A1 cause a novel EDS/myopathy overlap syndrome in humans and mice. Human Molecular Genetics vol. 23 2339–2352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ansorge HL et al. Type XIV Collagen Regulates Fibrillogenesis: PREMATURE COLLAGEN FIBRIL GROWTH AND TISSUE DYSFUNCTION IN NULL MICE. J. Biol. Chem 284, 8427–8438 (2009).This paper shows a structure–function relationship of collagen type XIV in skin and immature tendons in a mouse knockout. Cartilage mechanics were not tested but may display similar weakening.
- 197.Bell PA et al. Analysis of the cartilage proteome from three different mouse models of genetic skeletal diseases reveals common and discrete disease signatures. Biol. Open 2, 802–811 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kassner A et al. Discrete integration of collagen XVI into tissue-specific collagen fibrils or beaded microfibrils. Matrix Biol. 22, 131–143 (2003). [DOI] [PubMed] [Google Scholar]
- 199.Grässel S & Bauer RJ Collagen XVI in health and disease. Matrix Biol. 32, 64–73 (2013). [DOI] [PubMed] [Google Scholar]
- 200.Kassner A et al. Molecular structure and interaction of recombinant human type XVI collagen. J. Mol. Biol 339, 835–853 (2004). [DOI] [PubMed] [Google Scholar]
- 201.Kvist AJ et al. The major basement membrane components localize to the chondrocyte pericellular matrix--a cartilage basement membrane equivalent? Matrix Biol. 27, 22–33 (2008). [DOI] [PubMed] [Google Scholar]
- 202.Foldager CB et al. Collagen Type IV and Laminin Expressions during Cartilage Repair and in Late Clinically Failed Repair Tissues from Human Subjects. Cartilage 7, 52–61 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sudhakar A et al. Human alpha1 type IV collagen NC1 domain exhibits distinct antiangiogenic activity mediated by alpha1beta1 integrin. J. Clin. Invest 115, 2801–2810 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 204.Poschl E Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619–1628 (2004). [DOI] [PubMed] [Google Scholar]
- 205.Pfaff M et al. Integrin and Arg-Gly-Asp dependence of cell adhesion to the native and unfolded triple helix of collagen type VI. Exp. Cell Res 206, 167–176 (1993). [DOI] [PubMed] [Google Scholar]
- 206.Gara SK et al. Three novel collagen VI chains with high homology to the alpha3 chain. J. Biol. Chem 283, 10658–10670 (2008). [DOI] [PubMed] [Google Scholar]
- 207.Zelenski NA et al. Type VI Collagen Regulates Pericellular Matrix Properties, Chondrocyte Swelling, and Mechanotransduction in Mouse Articular Cartilage. Arthritis & Rheumatology 67, 1286–1294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shen G The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod. Craniofac. Res 8, 11–17 (2005). [DOI] [PubMed] [Google Scholar]
- 209.Gress CJ & Jacenko O Growth plate compressions and altered hematopoiesis in collagen X null mice. J. Cell Biol 149, 983–993 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Sund M et al. Distinct expression of type XIII collagen in neuronal structures and other tissues during mouse development. Matrix Biol. 20, 215–231 (2001). [DOI] [PubMed] [Google Scholar]
- 211.Ylönen R et al. Type XIII collagen strongly affects bone formation in transgenic mice. J. Bone Miner. Res. 20, 1381–1393 (2005). [DOI] [PubMed] [Google Scholar]
- 212.Koch M et al. A Novel Marker of Tissue Junctions, Collagen XXII. J. Biol. Chem. 279, 22514–22521 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zwolanek D et al. Collagen XXII binds to collagen-binding integrins via the novel motifs GLQGER and GFKGER. Biochem. J 459, 217–227 (2014). [DOI] [PubMed] [Google Scholar]
- 214.Charvet B et al. Knockdown of col22a1 gene in zebrafish induces a muscular dystrophy by disruption of the myotendinous junction. Development 140, 4602–4613 (2013). [DOI] [PubMed] [Google Scholar]
- 215.Hjorten R et al. Type XXVII collagen at the transition of cartilage to bone during skeletogenesis. Bone 41, 535–542 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Plumb DA et al. Collagen XXVII organises the pericellular matrix in the growth plate. PLoS One 6, e29422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Exposito J-Y, Valcourt U, Cluzel C & Lethias C The fibrillar collagen family. Int. J. Mol. Sci 11, 407–426 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gonzaga-Jauregui C et al. Mutations in COL27A1 cause Steel syndrome and suggest a founder mutation effect in the Puerto Rican population. Eur. J. Hum. Genet 23, 342–346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Pfeiffer E, Vickers SM, Frank E, Grodzinsky AJ & Spector M The effects of glycosaminoglycan content on the compressive modulus of cartilage engineered in type II collagen scaffolds. Osteoarthritis Cartilage 16, 1237–1244 (2008). [DOI] [PubMed] [Google Scholar]
- 220.Rieppo J et al. Structure-function relationships in enzymatically modified articular cartilage. Cells Tissues Organs 175, 121–132 (2003). [DOI] [PubMed] [Google Scholar]
- 221.Lee J-H & Cho J-Y Proteomics approaches for the studies of bone metabolism. BMB Rep. 47, 141–148 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sato N et al. Proteomic Analysis of Human Tendon and Ligament: Solubilization and Analysis of Insoluble Extracellular Matrix in Connective Tissues. J. Proteome Res 15, 4709–4721 (2016). [DOI] [PubMed] [Google Scholar]
- 223.Deshmukh AS et al. Deep proteomics of mouse skeletal muscle enables quantitation of protein isoforms, metabolic pathways, and transcription factors. Mol. Cell. Proteomics 14, 841–853 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hosseininia S, Önnerfjord P & Dahlberg LE Targeted proteomics of hip articular cartilage in OA and fracture patients. J. Orthop. Res 37, 131–135 (2019). [DOI] [PubMed] [Google Scholar]
- 225.Merl-Pham J et al. Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis. Matrix Biology Plus vol. 1 100005 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Ko J et al. Machine learning to detect signatures of disease in liquid biopsies – a user’s guide. Lab on a Chip vol. 18 395–405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Saha S et al. Automated detection and classification of early AMD biomarkers using deep learning. Sci. Rep 9, 10990 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Banaei N et al. Machine learning algorithms enhance the specificity of cancer biomarker detection using SERS-based immunoassays in microfluidic chips. RSC Advances vol. 9 1859–1868 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Van Camp G et al. A new autosomal recessive form of Stickler syndrome is caused by a mutation in the COL9A1 gene. Am. J. Hum. Genet 79, 449–457 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hafez A et al. Col11a1 Regulates Bone Microarchitecture during Embryonic Development. J Dev Biol 3, 158–176 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Alexopoulos LG, Youn I, Bonaldo P & Guilak F Developmental and osteoarthritic changes in Col6a1-knockout mice: biomechanics of type VI collagen in the cartilage pericellular matrix. Arthritis Rheum. 60, 771–779 (2009).This paper describes the structure–function relationship of collagen type VI and cartilage pericellular matrix stiffness.
- 232.Bayer ML et al. Release of tensile strain on engineered human tendon tissue disturbs cell adhesions, changes matrix architecture, and induces an inflammatory phenotype. PLoS One 9, e86078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Pu X & Oxford JT Proteomic Analysis of Engineered Cartilage. Methods Mol. Biol 1340, 263–278 (2015).This paper is the first and only proteomics study of engineered tissue that identifies collagen type XIV.
- 234.Fawzi-Grancher S, De Isla N, Faure G, Stoltz JF & Muller S Optimisation of biochemical condition and substrates in vitro for tissue engineering of ligament. Ann. Biomed. Eng 34, 1767–1777 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Richardson SM et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials 27, 4069–4078 (2006). [DOI] [PubMed] [Google Scholar]
- 236.Kurobe H et al. Development of small diameter nanofiber tissue engineered arterial grafts. PLoS One 10, e0120328 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Ashjian P et al. Noninvasive in situ evaluation of osteogenic differentiation by time-resolved laser-induced fluorescence spectroscopy. Tissue Eng. 10, 411–420 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Lee JK et al. Tension stimulation drives tissue formation in scaffold-free systems. Nat. Mater 16, 864–873 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kutsuna T et al. Noninvasive evaluation of tissue-engineered cartilage with time-resolved laser-induced fluorescence spectroscopy. Tissue Eng. Part C Methods 16, 365–373 (2010). [DOI] [PubMed] [Google Scholar]
- 240.Bergholt MS, Albro MB & Stevens MM Online quantitative monitoring of live cell engineered cartilage growth using diffuse fiber-optic Raman spectroscopy. Biomaterials 140, 128–137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Riesle J, Hollander AP, Langer R, Freed LE & Vunjak-Novakovic G Collagen in tissue-engineered cartilage: types, structure, and crosslinks. J. Cell. Biochem 71, 313–327 (1998). [DOI] [PubMed] [Google Scholar]
- 242.Murdoch AD, Hardingham TE, Eyre DR & Fernandes RJ The development of a mature collagen network in cartilage from human bone marrow stem cells in Transwell culture. Matrix Biol. 50, 16–26 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Lee JK, Link JM, Hu JCY & Athanasiou KA The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harb. Perspect. Med 7, pii: a025668 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Ofek G et al. Matrix development in self-assembly of articular cartilage. PLoS One 3, e2795 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Makris EA, Hu JC & Athanasiou KA Hypoxia-induced collagen crosslinking as a mechanism for enhancing mechanical properties of engineered articular cartilage. Osteoarthritis Cartilage 21, 634–641 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Makris EA, Responte DJ, Paschos NK, Hu JC & Athanasiou KA Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc. Natl. Acad. Sci. U. S. A 111, E4832–41 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yan D et al. The impact of low levels of collagen IX and pyridinoline on the mechanical properties of in vitro engineered cartilage. Biomaterials 30, 814–821 (2009). [DOI] [PubMed] [Google Scholar]
- 248.Setiawati R & Rahardjo P Bone Development and Growth. Osteogenesis and Bone Regeneration 13 (2018) doi: 10.5772/intechopen.82452. [DOI] [Google Scholar]
- 249.Budde B et al. Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol. Cell. Biol 25, 10465–10478 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Zhang R-Z et al. Recessive COL6A2 C-globular missense mutations in Ullrich congenital muscular dystrophy: role of the C2a splice variant. J. Biol. Chem 285, 10005–10015 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Schmid TM & Linsenmayer TF Immunoelectron microscopy of type X collagen: Supramolecular forms within embryonic chick cartilage. Developmental Biology vol. 138 53–62 (1990). [DOI] [PubMed] [Google Scholar]