To the editor.
Novel coronavirus disease (COVID-19) or SARS-CoV-2 was discovered in December 2019 in Wuhan City, China. The infection became a global pandemic over few months.1 Duhok is a big city in the Kurdistan Region of Iraq with a population of 1.3 million. The city experienced a large epidemic with one of the highest COVID-19 infection and case fatality rates in Iraq.2 The centralized COVID-19 database registration system and the intensity of infection spread in the city provided an excellent opportunity to evaluate the risk of reinfection in the city. It is worth mentioning that reinfection may be possible after recent reports showing recovered patients testing positive after a symptom-free period.3–5 This study aimed to evaluate the risk of reinfection in a cohort of 445,660 COVID-19 RT-PCR-tested cases.
Methods
The database for COVID-19 testing, management, and clinical outcomes at Duhok city, Kurdistan Region of Iraq, was analyzed. This database covers all COVID-19 confirmed cases in the city and shows RT-PCR testing results between March 1, 2020, and January 24, 2021. Besides, the database contains the number of tests performed, number of suspected cases, cure rate, case fatality rates, and clinical outcomes. We analyzed the centralized and standardized national SARS-CoV-2 testing and hospitalization. In addition, data from all COVID-19 centers and clinics were matched and analyzed. All COVID-19 confirmed cases, with at least one RT-PCR positive result after ≥45 days after the first positive swab, were considered suspected cases. Previous studies showed that a cutoff of 45 days could be used as a mark for the end of prolonged PCR positivity.6 Suspected cases of reinfection were classified as showing either strong or weak evidence of reinfection. Strong evidence of reinfection included positive RT-PCR cases, the appearance of signs and symptoms, resolution of signs and symptoms, negative RT-PCR confirming cure (two negative RT-PCR results on sequential samples taken at least 24 hours apart), the re-appearance of symptoms, and positive RT-PCR after ≥45 days. Any case that missed one of these conditions was considered reinfection with weak evidence and was excluded from further analysis. The risk of reinfection was calculated by quantifying the proportion of cases with strong evidence for reinfection out of all confirmed COVID-19 cases. Apart from the patient with cancer, our patients were immunocompetent, and they had no disorders that may facilitate reinfection.
Confirmed cases were defined as patients with laboratory confirmation of COVID-19. Mild cases were defined as confirmed cases of COVID-19 without evidence of viral pneumonia or hypoxia, whereas moderate cases were defined as patients with confirmed COVID-19 with radiological findings of pneumonia, SpO2 ≥ 90% on room air but no signs of severe pneumonia.7 Severe cases were defined as patients with confirmed COVID-19 with radiological findings of pneumonia plus one of the following: respiratory rate > 30 breaths/min; severe respiratory distress; or SpO2 < 90% on room air.7
RT-PCR testing
Each RT-PCR testing was composed of two reactions using two different kits. First, LightMix Modular SARS Wuhan CoV E-gene was used to target a 76 bp long fragment from a conserved region in the E gene. Second, LightMix Modular Wuhan CoV RdRP-gene was used to target a 100 bp long fragment from a conserved region of the RNA-dependent RNA polymerase (RdRP) gene. If the results of both reactions were positive, then the test was considered positive. The test was considered negative when the results of both reactions were negative. If one reaction was positive and the other is negative, the test result was considered indeterminate.
Results
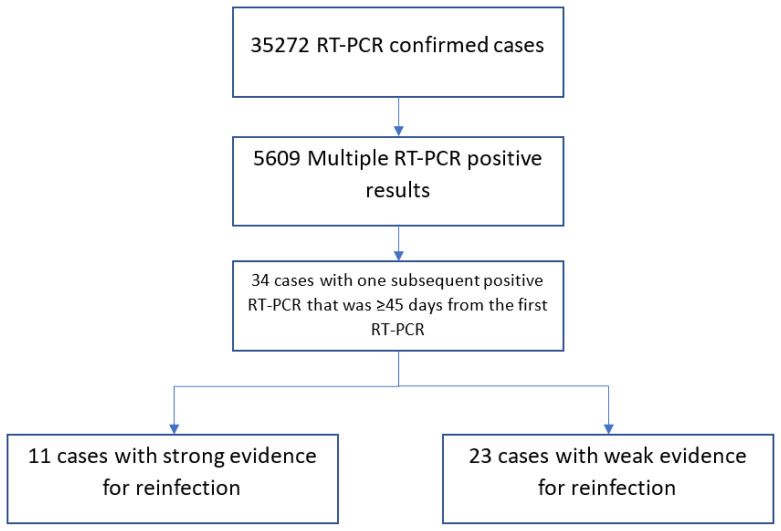
For the period between March 1, 2020, and January 24, 2021, the database showed that the RT-PCR test was performed on 445,660 cases. Among those, 35272 tests were positive. Amongst RT-PCR-positive cases, 30486 (86.4%; 95% CI: 86.1–86.8%) were cured and 703 (1.99%; 95% CI: 1.84–2.14%) died, while the rest were under medical observation. Out of 35272 confirmed COVID-19 cases, 29663 (84%; 95% CI: 83.7–84.5%) had only one positive RT-PCR result and therefore were excluded from the study. Of the remaining 5609 cases with more than one positive swab, only 34 (0.6%; 95% CI: 0.42–0.84%) cases had at least one subsequent positive swab that was ≥45 days from the first positive swab and thus qualified for inclusion in analysis. Detailed history taking with individual investigations of the 34 cases yielded 11 cases with strong evidence for reinfection. This gave reinfection risk rate of 0.031% (95% CI:0.012–0.049%) (Figure 1). Among our cases, 6/11 (54.54%; 95% CI:23–83%) were males. All patients were not related, apart from patients 8 and 9, who were twins (Table 1). The average age was 39.5±14.6 years (range: 26–79), and the average time between the first swab and the reinfection swab was 79.9±38.3 days (range: 49–174). The infection in 4/11 (36.36%; 95% CI:10–69%) patients was more severe than the first round of infection. The infection was severe in both rounds in one patient.
Figure 1.
Flow chart showing the selection process of COVID-19 eligible cases and summarizing the results of their reinfection status.
Table 1.
Characteristics of patients showing strong evidence for reinfection
| Patients | Age | Sex | First infection | Clinical features | Time between first and second RT-PCR (Days) | Second infection | Clinical features | Outcome | Chronic disease |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 32 | M | Mild | Myalgia, fever | 82 | Mild | Myalgia | Cure | No |
| Patient 2 | 40 | M | Severe | Fever, loss of smell, Myalgia, dyspnea | 50 | Mild | Fever, Sore throat | Cure | No |
| Patient 3 | 46 | M | Mild | Fever, dry cough | 74 | Moderate | Fever, Sore throat, loss of taste and smell | Cure | No |
| Patient 4 | 39 | M | Severe | Fever, dry cough, dyspnea | 122 | Mild | Fever, sore throat | Cure | No |
| Patient 5 | 32 | F | Mild | Fever, dry cough, loss of smell, sore throat | 174 | Mild | Fever, sore throat, myalgia | Cure | No |
| Patient 6 | 44 | M | Mild | Fever, myalgia | 51 | Mild | myalgia | Cure | Colon Cancer |
| Patient 7 | 26 | F | Mild | Headache, sweating, loss of taste | 84 | Mild | Headache, Myalgia | Cure | No |
| Patient 8 | 26 | F | Mild | Headache, loss of taste | 84 | Moderate | Myalgia, cough, dyspnea | Cure | No |
| Patient 9 | 36 | F | Mild | Sore throat, fever | 51 | Severe | Fever, myalgia, cough, dyspnea | Cure | Diabetes |
| Patient 10 | 34 | M | Mild | Headache, fever | 49 | Severe | Myalgia, fever, headache, anorexia | Cure | No |
| Patient 11 | 79 | F | Severe | dyspnea, fever | 58 | Severe | Cough, anorexia fever | Cure | Heart failure and hypertension |
Discussion
In the Duhok City, in the Kurdistan Region of Iraq, strict measures were put to combat COVID-19 infection spread. Such measures started March 1, 2020, and continued until May 21, 2020.8,9 Then, political necessity and mounting economic pressure on the local government demanded the start of a reopening process at a progressively rapid pace.8 After reopening, the number of COVID-19 cases increased sharply, with a concurrent increase in the number of symptomatic patients and a two-fold increase in the case-fatality rate.2 In this study, we found that our cases’ cure and case fatality rates were 86.4% and 1.99%, respectively. In a study conducted in China, the cure and case fatality rates were estimated to be 85.97% and 14.03%, respectively.10 In a study conducted in Iran and Turkey, neighboring countries to Iraq, the case fatality rates were 6.35% and 2.6%, respectively.11 The variation in cure and case fatality rates among different geographical regions can be explained in part by the variation in preventive measures, the health system’s efficiency, the genomic makeup of the virus, and host genetics. International cooperation and more studies are needed to explore this.
Additionally, the risk of reinfection was 0.031% in the city. The risk of reinfection in our study is comparable to what was found in Qatar as they found risk of reinfection at 0.04%.6 However, it seemed that reinfection is a rare phenomenon and may not pose a considerable threat. Previous case reports showed different severity in reinfection. While a case report from the USA showed increased symptoms in the reinfection that required giving antiviral treatment,12 reports from Hong Kong, Belgium and Netherlands demonstrated that the severity of reinfection cases was the same in both rounds of infection.4,5 In this study, four patients showed more severe symptoms that required giving antiviral treatment, and that can be explained by a very high dose of virus that led to a high viral load. Other possibility is that the reinfection might be caused by mutated version of the virus. Viral load studies and genetic comparison, both of which not available in Iraq, were needed to confirm this. The majority of the patients had mild or minimal symptoms and none of reinfection cases were critical or fatal. This may suggest that the vast majority of the patients developed immunity against the infection that may last for months and if reinfection occurs, it might be mild and none fatal. Long-term follow up study is suggested to determine the waning time of immunity. Our study has limitations. Firstly, this study provided epidemiological evidence for reinfections that needs confirmation by sequencing analysis of the paired viral samples. Secondly, we may have missed some cases that received treatment in nongovernmental clinics. However, we are confident that the vast majority of patients were covered in this study. To conclude, COVID-19 reinfection appeared to be possible but rare; most patients showed mild infection, and further research is needed to investigate the reasons behind that.
Footnotes
Competing interests: The authors declare no conflict of Interest.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W. A Novel Coronavirus from Patients with Pneumonia in China, 2019. The New England journal of medicine. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hussein NR, Naqid IA, Saleem ZSM, Almizori LA, Musa DH, Ibrahim N. A sharp increase in the number of COVID-19 cases and case fatality rates after lifting the lockdown in Kurdistan region of Iraq. Annals of medicine and surgery. 2020;57:140–142. doi: 10.1016/j.amsu.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tillett RL, Sevinsky JR, Hartley PD, Kerwin H, Crawford N, Gorzalski A, Laverdure C, Verma SC, Rossetto CC, Jackson D, Farrell MJ, Van Hooser S, Pandori M. Genomic evidence for reinfection with SARS-CoV-2: a case study. Lancet Infect Dis. 2020;21:52–58. doi: 10.1016/S1473-3099(20)30764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Mao R, Qiu Y, He J-S, Tan J-Y, Li X-H, Liang J, Shen J, Zhu L-R, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen M-H. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The Lancet Gastroenterology & Hepatology. 2020;5(7):667–678. doi: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abu-Raddad LJ, Chemaitelly H, Malek JA, Ahmed AA, Mohamoud YA, Younuskunju S, Ayoub HH, Al Kanaani Z, Al Khal A, Al Kuwari E, Butt AA, Coyle P, Jeremijenko A, Kaleeckal AH, Latif AN, Shaik RM, Rahim HFA, Yassine HM, Al Kuwari MG, Al Romaihi HE, Al-Thani MH, Bertollini R. Assessment of the risk of SARS-CoV-2 reinfection in an intense re-exposure setting. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020. [DOI] [PMC free article] [PubMed]
- 7.Hospital care for adolescents and adults. Geneva: World Health Organization; 2020. IMAI District Clinician Manual. [updated 2020; cited 05/01/2021]; Available from: https://apps.who.int/iris/bitstream/handle/10665/77751/9789241548290_Vol2_eng.pdf?sequence=3. [Google Scholar]
- 8.Hussein NR. The Role of Self-Responsible Response Versus Lockdown Approach in Controlling COVID-19 Pandemic in Kurdistan Region of Iraq. International Journal of Infection. 2020;7(4) doi: 10.5812/iji.107092. [DOI] [Google Scholar]
- 9.Hussein NR, Naqid IA, Saleem ZSM. A retrospective descriptive study characterizing coronavirus disease epidemiology among people in the Kurdistan Region, Iraq. Mediterranean Journal of Hematology and Infectious Diseases. 2020;12(1) doi: 10.4084/mjhid.2020.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diao Y, Liu X, Wang T, Zeng X, Dong C, Zhang Y, Zhou C, She X, Liu D, Hu Z. Estimating the cure rate and case fatality rate of the ongoing epidemic COVID-19. medRxiv. 2020. 2020.2002.2018.20024513. [DOI]
- 11.Oke J, Heneghan C. Global Covid-19 Case Fatality Rates - CEBM. The Centre for Evidence-Based Medicine; 2020. [updated 2020; cited 28/01/2021]; Available from: https://www.cebm.net/covid-19/global-covid-19-case-fatality-rates/ [Google Scholar]
- 12.Esme M, Koca M, Dikmeer A, Balci C, Ata N, Dogu BB, Cankurtaran M, Yilmaz M, Celik O, Unal GG, Ulgu MM, Birinci S. Older Adults With Coronavirus Disease 2019; A Nationwide Study in Turkey. The journals of gerontology Series A, Biological sciences and medical sciences. 2020. [DOI] [PMC free article] [PubMed]