Abstract
Mechanical loading may be required for proper tendon formation. However, it is not well understood how tendon formation is impacted by the development of weight-bearing locomotor activity in the neonate. This study assessed tendon mechanical properties, and concomitant changes in weight-bearing locomotion, in neonatal rats subjected to a low thoracic spinal cord transection or a sham surgery at postnatal day (P)1. On P10, spontaneous locomotion was evaluated in spinal cord transected and sham controls to determine impacts on weight-bearing hindlimb movement. The mechanical properties of P10 Achilles tendons (ATs), as representative energy-storing, weight-bearing tendons, and tail tendons (TTs), as representative positional, non-weight-bearing tendons were evaluated. Non- and partial weight-bearing hindlimb activity decreased in spinal cord transected rats compared to sham controls. No spinal cord transected rats showed full weight-bearing locomotion. ATs from spinal cord transected rats had increased elastic modulus, while cross-sectional area trended lower compared to sham rats. TTs from spinal cord transected rats had higher stiffness and cross-sectional area. Collagen structure of ATs and TTs did not appear impacted by surgery condition, and no significant differences were detected in the collagen crimp pattern. Our findings suggest that mechanical loading from weight-bearing locomotor activity during development regulates neonatal AT lateral expansion and maintains tendon compliance, and that TTs may be differentially regulated. The onset and gradual increase of weight-bearing movement in the neonate may provide the mechanical loading needed to direct functional postnatal tendon formation.
1 Introduction
Tendons are collagenous tissues that transfer mechanical forces from muscle to bone to enable movement and locomotion. Tendons are frequently injured and heal poorly [1], making them an active area of investigation for tissue engineering and regenerative medicine. A number of cellular [2–8], biochemical [2–5,9–12], and mechanical [13–27] cues have been found to influence tenogenesis (e.g., tenogenic differentiation and tendon formation) in vivo and in vitro, but there is a need to further understand how mechanical loading regulates functional tendon formation.
Recent studies have characterized the mechanical properties of tendon throughout embryonic, neonatal, and postnatal development to better understand their formation. Tendon mechanical properties increase throughout development in embryonic chick [21,28,29], postnatal mouse [30] and rat [31], and humans [32], but the factors driving these changes remain largely unknown. The impact of mechanical loading on the mechanical and biochemical properties of embryonic tendon has been investigated in both avian and murine developmental models. In vivo, mechanical loading originates from contractions of the adjacent, concurrently forming skeletal muscles [33], and increases as the embryo or neonate begins to move [31,34–36]. In chick embryos, both rigid and flaccid paralysis downregulate expression of the tenogenic markers, scleraxis, and tenomodulin, compared to nonparalyzed controls [3]. Rigid paralysis induced for 48 h (h) also significantly reduced calcaneal tendon elastic modulus, and reduced activity of lysyl oxidase, an enzyme that mediates the formation of collagen crosslinks [35]. Conversely, induced hypermotility led to increased elastic modulus in chick calcaneal tendons [35]. In Pax3 knockout mice (Pax3Spd/Spd), which lack skeletal muscle, tendon progenitor cells were identifiable before embryonic day (E)12.5, but were lost without mechanical stimulation from the developing muscles [33]. Though tenogenesis in embryonic chick and mouse tendon development may proceed via divergent cellular signaling pathways (e.g., fibroblast growth factor 4 appears to be tenogenic in chick, but not in mouse [3]), combined, these studies highlight the importance of mechanical loading for embryonic tendon development. However, few studies have assessed the role of mechanical loading during postnatal development.
Postnatal growth in rodents and humans is associated with the development of full weight-bearing locomotor behavior [31,37], suggesting that the developing tendons are exposed to increasingly high levels of mechanical loading postnatally, especially compared to embryonic ages. Though there are limited studies on how this locomotor development impacts postnatal tendon formation, mechanical loading has been explored in the postnatal maturation of the enthesis (the tendon-to-bone attachment). By postnatal day (P)56, mice receiving a botulinum toxin (Botox) injection in their supraspinatus muscle at birth show decreased muscle volume and bone mineralization, and delayed fibrocartilage development in the enthesis, compared to controls that received saline injections [36]. In a different study, Botox injection led to disrupted postnatal enthesis development at the cellular level [34], and decreased maximum force and stiffness of the supraspinatus attachment [38]. Supraspinatus tendon cross-sectional area, tensile strength, elastic modulus, collagen fibril alignment, and toughness were also decreased following a postnatal Botox injection [38]. In 10–12-week-old adult mice, loss of mechanical loading (via Botox injection) led to a reduction in stiffness and peak force of the Achilles tendons [26]. In a study using 16-week-old rats, Botox unloading led to increased stiffness and collagen deposition in the Achilles tendons [39]. Together, results of these studies suggest that both enthesis development and tendon maintenance at more mature stages require the mechanical loading associated with normal movement. However, not enough is known about the role loading plays in neonatal tendon formation.
We recently identified that increases in neonatal rat tendon mechanical properties coincide with the increased locomotor behavior that occurs during the first 10 postnatal days [31]. Interestingly, the observed increases in mechanical properties differed between the energy-storing, weight-bearing Achilles tendons (AT) and positional, non-weight-bearing tail tendons (TT), possibly due to the different mechanical loads these tendons experience as locomotion develops. Structural (size-dependent) and material (size-independent) properties increased at different rates in the different tendon types. Specifically, in energy-storing ATs, structural properties (maximum force, displacement at maximum force, and cross-sectional area) increased at P10, compared to P5 and P1. However, in positional TTs, both structural and material properties (maximum force, maximum stress, elastic modulus, and stiffness) increased from P5 to P10. These changes coincided with a significant increase in weight-bearing locomotion in P10 rats, compared to P1 and P5 [31]. Overall, our previous results suggest that increased mechanical loading from the postnatal development of weight-bearing locomotor behavior may differentially impact functionally distinct tendons.
Collectively, prior studies indicate that increased mechanical loading, possibly from the developing locomotor behavior, may be contributing to the changes observed in the postnatal tendons, and that tendons may respond to this loading differently based on their function. Disruption in the development of locomotor behavior may lead to changes in tendon mechanical properties, but how the onset of weight-bearing locomotion may affect developing neonatal tendon mechanical properties remains unknown. We hypothesized that altering locomotion, and hence mechanical loading, would impact neonatal tendon development. To test this hypothesis, we evaluated the collagen structure and measured the mechanical properties of energy-storing ATs and positional TTs in P10 rats, following a neonatal spinal cord transection or sham surgery at P1. Prior research has shown that a neonatal spinal cord transection significantly reduces, but does not eliminate, hindlimb locomotor behavior in rats during early postnatal development [40]. Thus, we also assessed changes in full, partial, and non-weight-bearing hindlimb movement during spontaneous open-field locomotion on P10. Disrupting locomotor development in neonates and assessing the impact on postnatal tendon mechanical properties provides novel insights into how the mechanical function of tendon emerges during development.
2 Methods
2.1 Animals and Spinal Cord Surgery.
Subjects were female offspring of Sprague-Dawley rats acquired from Simonsen laboratories (Santa Clara, CA). Adult animals were socially housed and mated. Pregnant females were individually housed and closely monitored during the week they were scheduled to deliver. On P1 (postnatal day 1; ∼24 h after birth), litters were culled to 8 pups to ensure all pups remained healthy and received a standard amount of maternal care, with roughly equal number of males and females. At that time, all pups remaining in the litter received a low thoracic spinal cord transection, or a sham surgery. All pups within a litter received the same surgical treatment. On P10, one female pup from a litter was randomly selected for behavioral testing. The animal colony room was maintained in accordance with the NIH, Institutes on Laboratory Animal Resources, and Idaho State University, Institutional Animal Care and Use Committee guidelines. The room was kept on a 12 h light:dark cycle, and access to food and water was ad libitum.
To begin surgery on P1, rat pups were voided, weighed, and anesthetized via hypothermia. A small incision was made to expose the lower thoracic and lumbar spine, and a partial laminectomy was performed between T8 and T10. For animals in the transection group, the spinal cord was completely cut at one spinal level (between T8 and T10) and a collagen matrix was injected into the injury site to prevent regeneration. Rat pups in the sham surgery group underwent all procedures except the transection cut and collagen insertion. In both groups, animals received internal and external sutures and subcutaneous injections of 0.04 mg/kg of buprenorphine (50 μL) for analgesia and 9% saline to maintain internal fluid balance. Pups were placed in an infant incubator to recover before being returned to the home cage with the dam. The total time of separation from the dam was ∼40 min. Pups remained with the dam in their home cage until behavioral testing occurred on P10, and were monitored regularly to ensure recovery.
2.2 Evaluation of Locomotion.
On P10, a total of 16 female rat subjects (8 spinal cord transected + 8 sham) were tested in an open field for measurement of spontaneous locomotion, as previously described [31]. Briefly, individual rat pups were placed in an 8″ × 8″ × 8″ Plexiglas box that was located inside a 30 °C infant incubator, and allowed a 30-min acclimation period. Using a lateral camera angle, spontaneous locomotion was recorded on video for 20 min. Immediately after testing, CO2 inhalation was used to euthanize the subjects. Hindlimbs and tails were dissected at the proximal end and stored at -80 °C.
Locomotion was assessed from video records using the scoring program datavyu (version 1.3.4; Datavyu Team, New York, NY). The duration of hindlimb locomotor behavior was evaluated and categorized as full weight-bearing, partial weight-bearing, or non-weight-bearing [28]. Full weight-bearing behaviors include standing and walking (plantar contact with all four paws and belly off the ground) or full rearing (standing on hindlimbs with both forelimbs off the ground and head above the abdomen). Partial weight-bearing behaviors include hindlimb-active crawling (hindlimbs used to facilitate movement and belly in contact with the ground) and partial rearing (one forelimb off the ground and head above the abdomen). Non-weight-bearing behaviors include crawling (hindlimbs inactive), pivoting (torso and head moving while hindlimbs remain anchored), and hindlimb kicking. The categorization of these behaviors depended on the degree to which the hindlimbs supported the weight of the animal. The duration of each of these behaviors was scored and summed over the entire 20-min video with >90% intra- and interrater reliability.
2.3 Mechanical Evaluation of Achilles Tendons and Tail Tendons.
Mechanical evaluation was conducted as previously described [31]. Briefly, one TT (n = 10 sham, n = 10 spinal cord transected) and one AT per animal from P10 female (n = 11–14 sham, 9–11 spinal cord transected) rats were mechanically evaluated using our small-scale tensile load frame [41]. These subjects were in addition to those used for behavioral testing (n = 8). ATs were secured with cyanoacrylate in the grips, and a 500 g capacity load cell (Honeywell, Columbus, OH) was used to measure force. Force and displacement data were recorded using a custom LabVIEW program (National Instruments, Austin, TX). A digital camera (Thorcam DCC1645C, Thorlabs Inc., Newton, NJ) captured front- and side-view images of each tendon, which were analyzed (ImageJ, NIH, Bethesda, MD) to obtain tendon length and width, and measure gage length and cross-sectional area, respectively. A circular cross section was assumed for TTs, and an elliptical cross section was assumed for ATs, based on previous studies [42–44]. Ranges in n-numbers (P10 ATs) resulted from obtaining only structural properties for some samples due to missing images. A 0.05 N preload was applied to ATs. Next, ATs were preconditioned with 10 cycles of loading to 1% strain, and immediately pulled to failure at 0.1 mm/s [31]. To protect the TTs during mounting in the grips, they were first adhered to cardboard c-clamps using cyanoacrylate. The c-clamps were then cut prior to testing. A preload of 0.01 N was applied to TTs to remove the slack. As TTs are more fragile than ATs, they were not preconditioned and force was measured using a 150 g load cell (Honeywell) [31]. All tendons were kept hydrated with saline for the duration of testing. The force–displacement and cross-sectional area data were used to calculate maximum force, displacement at maximum force, maximum stress, and strain. The linear region stiffness and elastic modulus were determined from the slope of a line fit to the force–displacement and stress–strain curves, respectively, that had a R2 > 0.90 (average R2 = 0.961±0.017). A summary of all stress–strain curves from ATs and TTs of P10 sham and spinal cord transected subjects are shown in Fig. S1(a) and S1(b) available in the Supplemental Materials on the ASME Digital Collection. To assess differences in toe-region elastic moduli and toe-to-linear region transition strain between sham and spinal cord transected tendons, a bilinear fit (R2>0.90) was applied to the stress–strain curve [30,45].
2.4 Imaging and Evaluation of Collagen Structure.
To visualize collagen structure, P10 ATs (n = 4 sham, n = 3 spinal cord transected) and TTs (n = 5 sham and spinal cord transected) were imaged via second harmonic generation (SHG) [46] on an Olympus FluoView 1000 Multiphoton Confocal Microscope (Olympus, Tokyo, Japan). One TT and one AT, left or right, from each animal were dissected as described for mechanical testing, and fixed overnight at 4 °C in 10% formalin. Tendons were dissected and then fixed in a tension-free state to reduce the potential for variations in tensile loading or joint angle during fixation. Following fixation, and to image the AT, the myotendinous junction and calcaneus bone were trimmed off. Three images were taken for each tendon (left, right, and center positions). Distance between crests of the crimp pattern was measured using ImageJ (NIH) in 5 locations per image, and the average measurement was the crimp distance for that image. The average crimp distance of an image set was used to determine the average crimp distance of the whole tendon.
2.5 Statistical Analysis.
Differences in the duration of non-, partial, and full weight-bearing locomotion were evaluated using unpaired, two-tailed t-tests. Differences in mechanical properties of AT and TT, and collagen crimp distance in AT and TT between sham and spinal cord-transected conditions were evaluated using Mann–Whitney U tests. P < 0.05 was set to determine statistical significance.
3 Results
3.1 Weight-Bearing Locomotor Behavior Changes Following Spinal Cord Transection.
Overall durations of spontaneous non-, partial, and full weight-bearing locomotion during the open-field test for spinal cord transected and sham subjects are shown in Fig. 1(a). Spinal cord-transected rats showed significantly less non-weight-bearing (p = 0.017) and partial weight-bearing (p = 0.004) locomotion, compared to sham subjects. No spinal cord-transected subjects engaged in full weight-bearing behavior, while 6 of the 8 sham subjects did. The duration of full weight-bearing locomotion trended higher (p = 0.055) in sham rats (Fig. 1), though differences were not significant.
Fig. 1.
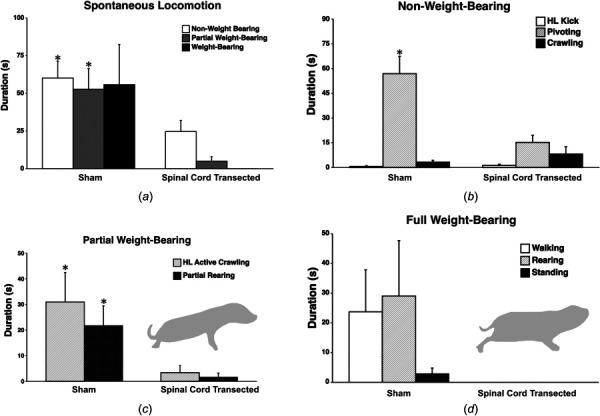
Duration of spontaneous locomotion in the open field by sham and spinal cord transected female P10 rats. (a) Spinal cord transected rats (n = 8) showed significantly less overall spontaneous non- and partial weight-bearing locomotion compared to sham rats (n = 8). (b) Within the category of non-weight-bearing behavior, spinal cord transected rats showed significantly less pivoting compared to shams. (c) For partial weight-bearing, both hindlimb (HL)-active crawling and rearing were significantly reduced in spinal cord transected rats. The cartoon illustrates an example of partial weight-bearing of the hindlimbs, in this case hindlimb-active crawling (belly is on the floor). (d) For full weight-bearing activity, only sham rats showed any behaviors in this category, including walking (six out of eight subjects). The cartoon shows an example of walking, whereby the hindlimbs are clearly underneath the pups and the belly is off the floor. Bars show group mean durations; vertical lines show SEM. *p < 0.05.
Durations of non-weight-bearing locomotor activities are seen in Fig. 1(b). There were no significant differences in duration of hindlimb kicks (p = 0.456) or crawling (p = 0.296) between sham and spinal cord-transected subjects. However, spinal cord-transected pups showed significantly less pivoting compared to shams (p = 0.003). Within partial weight-bearing behaviors (Fig. 1(c)), spinal-cord transected pups showed significantly less hindlimb-active crawling (p = 0.035), and less partial rearing (p = 0.022) than shams. Within the full weight-bearing category (Fig. 1(d)), there were no differences in walking (p = 0.115), standing (p = 0.149), or rearing (p = 0.141) between sham and spinal cord-transected subjects, although full weight-bearing was only shown by shams.
3.2 Sham and Spinal Cord-Transected Achilles Tendon Mechanical Properties.
Average material and structural properties and sample gage lengths of sham and spinal cord transected ATs and TTs are listed in Table S1 available in the Supplemental Materials on the ASME Digital Collection. Representative force–displacement and stress–strain curves for sham and spinal cord transected P10 ATs are shown in Figs. 2(a) and 2(b). ATs from spinal cord transected rats had increased linear region elastic modulus (p = 0.031), compared to the sham condition (Fig. 3(g)), while cross-sectional area trended lower (p = 0.069) (Fig. 3(c)). Maximum force (p = 0.85), displacement at maximum force (p = 0.53), linear region stiffness (p = 0.25), toe region elastic modulus (p = 0.21), maximum stress (p = 0.23), strain at maximum stress (p = 0.72), and transition strain (p = 0.54) were consistent between the sham and spinal cord transected subjects (Figs. 3(a), 3(b), 3(d), 3(e), 3(f), and 3(h)).
Fig. 2.
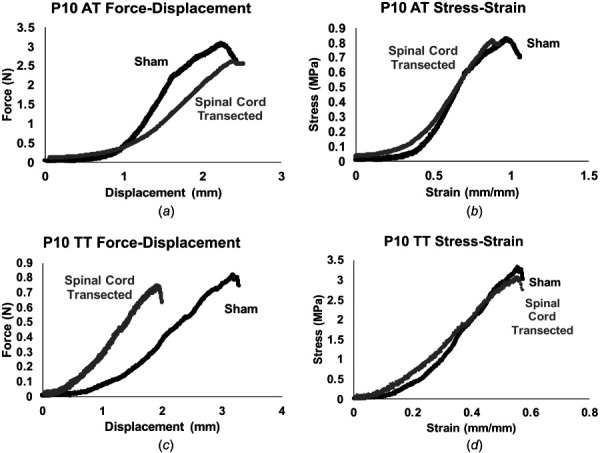
Representative force–displacement and stress–strain curves. (a) and (c) Representative force–displacement and (b) and (d) stress–strain curves for ATs and TTs from sham and spinal cord transected P10 rats.
Fig. 3.
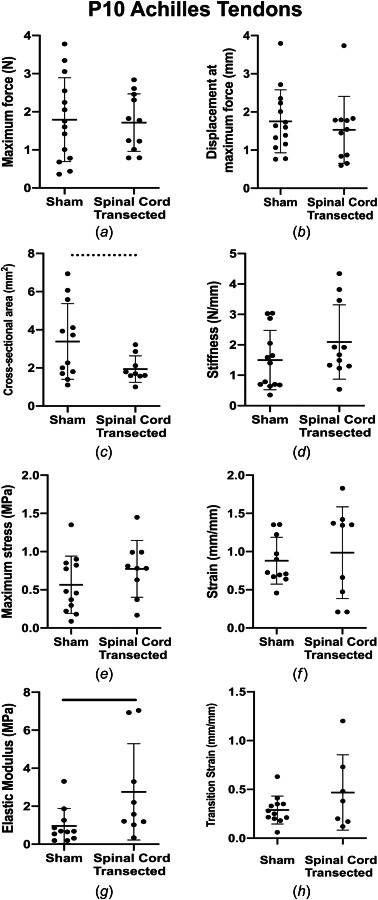
AT mechanical properties. (a) Maximum force, (b) displacement at maximum force, (c) cross-sectional area, (d) stiffness, (e) maximum stress, (f) strain at maximum stress, (g) elastic modulus, and (h) transition strain for sham and spinal cord transected conditions. ATs from spinal cord transected rats had significantly higher linear region elastic modulus and cross-sectional area trended lower, compared to sham controls. Solid lines denote significant differences between groups and dashed lines denote trends. Bars represent mean±standard deviation.
3.3 Sham and Spinal Cord-Transected Tail Tendon Mechanical Properties.
Representative force–displacement and stress–strain curves for sham and spinal cord transected P10 TTs are shown in Figs. 2(c) and 2(d). TTs from spinal cord-transected rats had higher linear region stiffness (p = 0.014; Fig. 4(d)) and cross-sectional area (p = 0.0081) (Fig. 4(c)). Maximum force (p = 0.19), displacement at maximum force (p = 0.14), toe (p = 0.48) and linear region elastic modulus (p = 0.31), maximum stress (p = 0.27), strain at maximum stress (p = 0.68), and transition strain (p = 0.74) were consistent between sham and spinal cord-transected subjects (Figs. 4(a), 4(b), 4(e), 4(f), 4(g), and 4(h)).
Fig. 4.
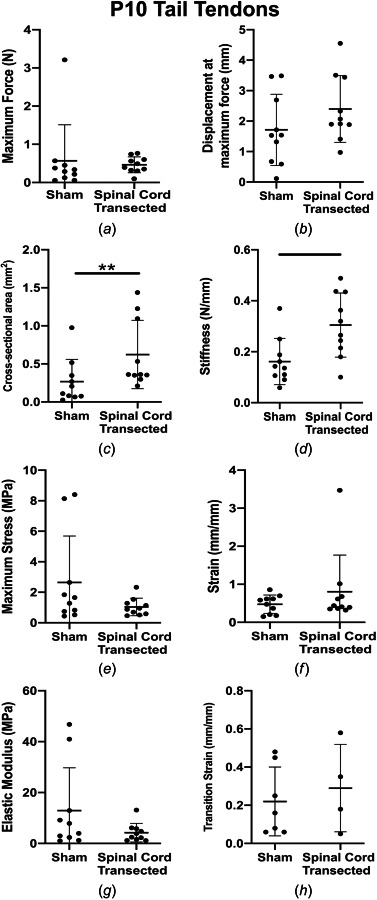
TT mechanical properties. (a) Maximum force, (b) displacement at maximum force, (c) cross-sectional area, (d) stiffness, (e) maximum stress, (f) strain at maximum stress, (g) elastic modulus, and (h) transition strain for sham and spinal cord transected conditions. TTs from spinal cord transected rats had significantly higher linear region stiffness and cross-sectional area trended higher, compared to sham controls. Solid lines denote significant differences between groups. Bars represent mean±standard deviation.
3.4 Collagen Structure.
Collagen structure of ATs and TTs did not appear to be impacted by the surgery condition (Figs. 5(a)–5(d)). Statistical analysis showed that there were no significant differences in crimp distance between sham and spinal cord transected conditions in ATs (p = 0.86) and TTs (p > 0.99) (Figs. 5(e) and 5(f)).
Fig. 5.
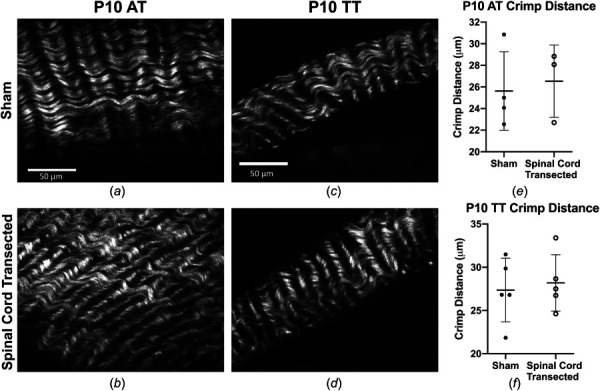
SHG images of collagen in neonatal tendons. Representative SHG images of collagen structure in ATs of (a) sham and (b) spinal cord transected rats, and in TTs of (c) sham and (d) spinal cord transected rats. No differences were detected in (e) and (f) crimp distance between sham and spinal cord transected conditions in ATs or TTs. Scale bar = 50 μm. Bars represent mean±standard deviation.
4 Discussion
There is a need to enhance our understanding of the relationship between locomotor behavior and tendon development, as the limited information on the role of mechanical loading in tendon formation challenges efforts to engineer functional tendon replacements. In this study, we examined the effects of disrupting weight-bearing locomotion in early postnatal tendon development for two functionally distinct tendon types (e.g., the energy-storing AT and the positional TT). In spinal cord-transected rats, the amount of non- and partial weight-bearing hindlimb locomotion was lower when compared to sham controls, and no spinal cord transected rats showed full weight-bearing locomotion during the study period (Fig. 1), providing an in vivo system to study mechanical regulators of tendon formation. Characterization of this in vivo model is useful, as investigating the impacts of loading on tendon formation during postnatal development in other systems is a challenge. Tendon cells harvested postnatally for in vitro studies may have altered phenotypes, different characteristics depending on the postnatal isolation day, or de-differentiate when isolated from tendons that are already formed [47]. Additionally, shifts in cell behavior in response to load in vitro may not result in readily apparent changes in tissue function at the tendon level. Therefore, using spinal cord transection surgery as a model system to disrupt locomotor development in neonates provides insights into how the mechanical function of tendon emerges during development, possibly driven by the typical increases in weight-bearing locomotor behavior.
In P10 ATs, we observed significantly increased elastic modulus and a trend toward lower cross-sectional area (p = 0.069) with spinal cord transection (Figs. 3(g) and 3(c)). Previously, we found that the cross-sectional area of ATs increased as a function of age and with the onset of locomotor behavior [31]. Taken together, weight-bearing locomotor activity may regulate the lateral expansion of developing neonatal ATs. The increase in elastic modulus of P10 ATs with decreased loading is consistent with a prior study that identified increased elastic modulus in ATs with Botox unloading in 4-month-old rats [39]. These findings suggest that decreased mechanical stimulation leads to smaller energy-storing tendons that are less compliant and more rigid. It is also possible that the reduced loading causes the ATs to resemble positional tendons, as positional tendons typically having higher elastic moduli [48–54]. Interestingly, SHG imaging did not identify changes in the collagen crimp distance of ATs between sham and spinal cord transected conditions (Figs. 5(a) and 5(b)). Though unexpected, this finding is consistent with a prior study in embryonic chick calcaneal tendon that found neither paralysis nor hypermotility visibly affected collagen structure [35]. The changes in mechanical properties that follow alterations in the mechanical loading environment during early postnatal development may be due to cellular or biochemical factors, such as enzymatic crosslinking of the collagen fibers [55], rather than changes to the overall collagen structure.
Similarly, P10 TT mechanical properties remained mostly unaltered with spinal cord transection, with the exception of increased stiffness and a larger cross-sectional area in spinal cord transected rats (Figs. 4(c) and 4(d)). Future studies will need to investigate if the TTs are experiencing increased loading demands in the spinal cord transected condition as the animals attempt to maintain balance without normal use of their hindlimbs. If this is the case, then TTs may increase in size and stiffness to compensate for the additional mechanical loading, similar to the AT during normal development of weight-bearing locomotion [31]. As with the ATs, SHG imaging showed no changes in the collagen crimp distance of the TTs between sham and spinal cord transected conditions (Figs. 5(c) and 5(d)).
Taken together, the spinal cord transection surgery and reduced mechanical stimuli influenced both AT and TT postnatal development, but the impact was unique between the two tendon types. With spinal cord surgery, elastic modulus and cross-sectional area were altered in ATs, whereas stiffness and cross-sectional area increased in TTs. While this study did not investigate the mechanisms resulting in these outcomes, it is possible that the differential impact of unloading on ATs compared to TTs resulted from the physiological function of each tendon. ATs are weight-bearing, energy-storing tendons that must withstand large mechanical loads during daily activity. In contrast, TTs are generally regarded as non-weight-bearing, positional tendons, whose main function in rodents is tail positioning [56,57]. Future work will determine if the increase in stiffness of TTs from spinal cord transected subjects could be driven by the neonatal rats using their tail to supplement their impaired locomotion, and hence exposing their tails to increased loads, compared to normal development. Additionally, neonatal ATs may mature more slowly than TTs [31], and thus be more plastic and susceptible to manipulations of the loading environment at P10. Other studies demonstrated that neonatal ATs regenerate following a transection and subsequent unloading [58]. While we did not induce a tendon injury, an unidentified regenerative mechanism in neonates may be preventing more pronounced changes in mechanical properties following tendon unloading due to the spinal cord injury-induced decrease in movement. As weight-bearing tendons that may require exposure to mechanical stimulation to develop mechanical function, ATs may depend on the onset of normal locomotion, and hence normal loading. Our previous findings suggest that increases in hindlimb weight-bearing locomotion behavior and tendon mechanical properties are coordinated during postnatal development, and first occur between P5 and P10 [31]. Since mechanical loading impacts the expression of tenogenic markers, such as scleraxis [22], and the production of the later stage tenogenic marker tenomodulin [59,60], postnatal weight-bearing locomotor behavior may regulate both cellular maturation and functional tendon development, but future studies are needed to determine how ATs and TTs are differentially regulated at the cellular level.
Mechanical loading has been shown to influence mechanical properties of cell-seeded scaffolds in vitro [13–17,18–24]. Tendon cells respond to mechanical stimulation by upregulating genes for collagen (Col) I and III [14,15], synthesizing collagen fibrils and aligning them along the axis of tension [16], and remodeling their extracellular matrix [61]. During embryonic and postnatal development, tendons initially display high cellularity and relatively low collagen content [28,30,46,62,63]. As they mature, they contain 60–85% collagen by dry weight, 95% of which is Col I, and are relatively hypocellular [64]. Cells in mature tendon are sensitive to mechanical loading and respond by remodeling their extracellular matrix or secreting inflammatory cytokines [65–68], both of which ultimately lead to alterations in the tissue mechanical properties. Unloading also impacts healing in mature tendons. In adult rats, immobilization following a supraspinatus tendon injury led to upregulation of chondrogenic genes [69], and immobilization after an Achilles tendon transection led to increased laxity of healed tendons, compared to controls [70]. Collectively, these results illustrate that cells and tendons respond to their mechanical environment to direct tendon formation and maintenance via upregulation of tenogenic genes and secretion of biochemicals, but mechanically regulated cellular mechanisms and the possibility for unique developmental pathways in functionally distinct postnatal tendons deserve further study.
This study has some limitations. The neonatal spinal cord transection model used here does not lead to elimination of hindlimb movement. During the early neonatal period, rats that have undergone a mid- or low-thoracic spinal cord transection show less myoclonic twitching [71] and spontaneous locomotion in the hindlimbs [40]. However, when the transection occurs early in postnatal life (i.e., before P14 in rats [72]), spinal cord-injured animals still go on to develop hindlimb locomotor behavior, albeit not as coordinated as in normal controls [73], and remain able to adapt their locomotor behavior to sensory stimulation [74]. Findings from this study of decreased locomotor activity in spinal cord transected female P10 rats are consistent with a recent study showing similar decreases in spinal cord transected male P10 rats, compared to shams [40]. Thus, because the neonatal rat transection procedure leads to alterations (i.e., decreases) in weight-bearing locomotion, and not paralysis, it provides a useful model to examine activity-based developmental processes such as tendon development. In future studies, it will be important to extend the age range out to older animals, as P10/P11 is the typical age that quadrupedal walking is regularly shown in rats [37]. Thus, examining tendon properties at a later time point from surgery will likely reveal more drastic and activity-dependent effects between sham and spinal cord-transected animals. Two-tailed unpaired t-tests showed that P10 ATs and TTs from rats subjected to the sham surgery did not differ significantly in their mechanical properties from P10 ATs and TTs from rats allowed to develop normal locomotor behavior, as reported in our prior study (data not shown) [31]. Thus, the sham surgery, on its own, did not appear to alter mechanical properties of developing tendons. It also would be important to examine changes in muscle force and relations between muscle load and respective tendon type following a neonatal spinal cord transection, as studies with adults have revealed time-dependent alterations in motor unit force and muscle contractile properties and spasticity [75], following low thoracic spinal cord transection [76,77]. Additionally, there are inherent challenges to testing neonatal tissues (small size, fragility, damage during dissection, and slipping during testing; a detailed summary of testing limitations has been previously described [31]). To minimize errors, any data with apparent damage during tissue isolation or slipping during testing were excluded from the analysis, and special care was taken to ensure the cyanoacrylate gel did not diffuse into the tendons during testing. Finally, although sex differences in tendons have been detected at later ages [78,79], at ages below 4 weeks, body mass, collagen content, elastic modulus, maximum stress [78], and early locomotor development [37] do not differ between male and female mice. Therefore, we do not believe it is likely that using only female rats affected our results.
In this study, we demonstrated that disrupting the development of normal locomotion of postnatal rats via spinal cord transection at P1 results in unique changes to AT and TT mechanical properties, without disrupting the collagen structure. Future investigations will identify the cellular mechanisms affected by manipulating the postnatal mechanical environment, with particular focus on how these mechanisms may be impacted differently in the energy-storing and weight-bearing (e.g., AT) and the positional and non-weight-bearing (e.g., TTs) tendons. These results provide new insights into how mechanical stimuli, particularly the onset of weight-bearing locomotion during neonatal development, impact tendon formation.
Supplementary Material
Supplementary Table PDF
Acknowledgment
This work was supported by funding from the INBRE program, P20 GM103408 (National Institutes of General Medical Sciences), Burroughs Wellcome Fund, John F. Keegan Fellowship (to SKT), and Beckman Scholars Award from the Arnold and Mabel Beckman Foundation (to NMP).
Funding Data
Arnold and Mabel Beckman Foundation (Grant No. Beckman Scholars Award; Funder ID: 10.13039/100000997).
Burroughs Wellcome Fund (Grant No. Collaborative Research Travel Grant; Funder ID: 10.13039/100000861).
National Institute of General Medical Sciences (Grant No. P20 GM103408; Funder ID: 10.13039/100000057).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- [1]. Lin, T. W. , Cardenas, L. , and Soslowsky, L. J. , 2004, “ Biomechanics of Tendon Injury and Repair,” J Biomech., 37(6), pp. 865–877. 10.1016/j.jbiomech.2003.11.005 [DOI] [PubMed] [Google Scholar]
- [2]. Guerquin, M.-J. , Charvet, B. , Nourissat, G. , Havis, E. , Ronsin, O. , Bonnin, M.-A. , Ruggiu, M. , Olivera-Martinez, I. , Robert, N. , Lu, Y. , Kadler, K. E. , Baumberger, T. , Doursounian, L. , Berenbaum, F. , and Duprez, D. ,. 2013, “ Transcription Factor EGR1 Directs Tendon Differentiation and Promotes Tendon Repair,” J. Clin. Invest., 123(8), pp. 3564–3576. 10.1172/JCI67521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Havis, E. , Bonnin, M. A. , de Lima, J. E. , Charvet, B. , Milet, C. , and Duprez, D. , 2016, “ TGFβ and FGF Promote Tendon Progenitor Fate and Act Downstream of Muscle Contraction to Regulate Tendon Differentiation During Chick Limb Development,” Development, 143(20), pp. 3839–3851. 10.1242/dev.136242 [DOI] [PubMed] [Google Scholar]
- [4]. Havis, E. , Bonnin, M.-A. , Olivera-Martinez, I. , Nazaret, N. , Ruggiu, M. , Weibel, J. , Durand, C. , Guerquin, M.-J. , Bonod-Bidaud, C. , Ruggiero, F. , Schweitzer, R. , and Duprez, D. , 2014, “ Transcriptomic Analysis of Mouse Limb Tendon Cells During Development,” Development, 141(19), pp. 3683–3696. 10.1242/dev.108654 [DOI] [PubMed] [Google Scholar]
- [5]. Lejard, V. , Blais, F. , Guerquin, M.-J. , Bonnet, A. , Bonnin, M.-A. , Havis, E. , Malbouyres, M. , Bidaud, C. B. , Maro, G. , Gilardi-Hebenstreit, P. , Rossert, J. , Ruggiero, F. , and Duprez, D. ,. 2011, “ EGR1 and EGR2 Involvement in Vertebrate Tendon Differentiation,” J. Biol. Chem., 286(7), pp. 5855–5867. 10.1074/jbc.M110.153106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Theodossiou, S. K. , Murray, J. B. , and Schiele, N. R. , 2020, “ Cell-Cell Junctions in Developing and Adult Tendons,” Tissue Barriers, 8(1), p. 1695491. 10.1080/21688370.2019.1695491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Berthet, A. B. , Chen, C. , Butcher, K. B. , Schneider, R. A. , Alliston, T. , and Amirtharajah, M. , 2013, “ Smad3 Binds Scleraxis and Mohawk and Regulates Tendon Development,” J. Orthop. Res., 31(9), pp. 1475–1483. 10.1002/jor.22382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Ito, Y. , Toriuchi, N. , Yoshitaka, T. , Ueno-Kudoh, H. , Sato, T. , Yokoyama, S. , Nishida, K. , Akimoto, T. , Takahashi, M. , Miyaki, S. , and Asahara, H. , 2010, “ The Mohawk Homeobox Gene is a Critical Regulator of Tendon Differentiation,” Proc. Natl. Acad. Sci. U. S. A., 107(23), pp. 10538–10542. 10.1073/pnas.1000525107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Liu, H. , Zhang, C. , Zhu, S. , Lu, P. , Zhu, T. , Gong, X. , Zhang, Z. , Hu, J. , Yin, Z. , Heng, B. C. , Chen, X. , and Wei Ouyang, H. ,. 2015, “ Mohawk Promotes the Tenogenesis of Mesenchymal Stem Cells Through Activation of the TGFβ Signaling Pathway,” Stem Cells, 33(2), pp. 443–455. 10.1002/stem.1866 [DOI] [PubMed] [Google Scholar]
- [10]. Miyabara, S. , Yuda, Y. , Kasashima, Y. , Kuwano, A. , and Arai, K. , 2014, “ Regulation of Tenomodulin Expression Via Wnt/Beta-Catenin Signaling in Equine Bone Marrow-Derived Mesenchymal Stem Cells,” J. Equine Sci./Jpn Soc. Equine Sci., 25(1), pp. 7–13. 10.1294/jes.25.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Otabe, K. , Nakahara, H. , Hasegawa, A. , Matsukawa, T. , Ayabe, F. , Onizuka, N. , Inui, M. , Takada, S. , Ito, Y. , Sekiya, I. , Muneta, T. , Lotz, M. , and Asahara, H. ,. 2015, “ The Transcription Factor Mohawk Controls Tenogenic Differentiation of Bone Marrow Mesenchymal Stem Cells In Vitro and In Vivo,” J. Orthop. Res., 33(1), pp. 1–8. 10.1002/jor.22750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Tan, G.-K. , Pryce, B. A. , Stabio, A. , Brigande, J. V. , Wang, CJie. , Xia, Z. , Tufa, S. F. , Keene, D. R. , and Schweitzer, R. ,. 2020, “ TGFβ Signaling is Critical for Maintenance of the Tendon Cell Fate,” eLife, 9, p. e52695. 10.7554/eLife.52695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Butler, D. L. , Juncosa-Melvin, N. , Boivin, G. P. , Galloway, M. T. , Shearn, J. T. , Gooch, C. , and Awad, H. ,. 2008, “ Functional Tissue Engineering for Tendon Repair: A Multidisciplinary Strategy Using Mesenchymal Stem Cells, Bioscaffolds, and Mechanical Stimulation,” J. Orthop. Res., 26(1), pp. 1–9. 10.1002/jor.20456 [DOI] [PubMed] [Google Scholar]
- [14]. Chokalingam, K. , Juncosa-Melvin, N. , Hunter, S. A. , Gooch, C. , Frede, C. , Florert, J. , Bradica, G. , Wenstrup, R. , and Butler, D. L. , 2009, “ Tensile Stimulation of Murine Stem Cell-Collagen Sponge Constructs Increases Collagen Type I Gene Expression and Linear Stiffness,” Tissue Eng. Part A, 15(9), pp. 2561–2570. 10.1089/ten.tea.2008.0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Juncosa-Melvin, N. , Matlin, K. S. , Holdcraft, R. W. , Nirmalanandhan, V. S. , and Butler, D. L. , 2007, “ Mechanical Stimulation Increases Collagen Type I and Collagen Type III Gene Expression of Stem Cell-Collagen Sponge Constructs for Patellar Tendon Repair,” Tissue Eng., 13(6), pp. 1219–1226. 10.1089/ten.2006.0339 [DOI] [PubMed] [Google Scholar]
- [16]. Kalson, N. S. , Holmes, D. F. , Herchenhan, A. , Lu, Y. , Starborg, T. , and Kadler, K. E. , 2011, “ Slow Stretching That Mimics Embryonic Growth Rate Stimulates Structural and Mechanical Development of Tendon-Like Tissue In Vitro,” Dev. Dyn., 240(11), pp. 2520–2528. 10.1002/dvdy.22760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Kuo, C. K. , and Tuan, R. S. , 2008, “ Mechanoactive Tenogenic Differentiation of Human Mesenchymal Stem Cells,” Tissue Eng. Part A, 14(10), pp. 1615–1627. 10.1089/ten.tea.2006.0415 [DOI] [PubMed] [Google Scholar]
- [18]. Mubyana, K. , and Corr, D. T. , 2018, “ Cyclic Uniaxial Tensile Strain Enhances the Mechanical Properties of Engineered, Scaffold-Free Tendon Fibers,” Tissue Eng. Part A, 24(23–24), pp. 1807–1817. 10.1089/ten.TEA.2018.0028 [DOI] [PubMed] [Google Scholar]
- [19]. Qin, T.-W. , Sun, Y.-L. , Thoreson, A. R. , Steinmann, S. P. , Amadio, P. C. , An, K.-N. , and Zhao, C. ,. 2015, “ Effect of Mechanical Stimulation on Bone Marrow Stromal Celleseeded Tendon Slice Constructs: A Potential Engineered Tendon Patch for Rotator Cuff Repair,” Biomaterials, 51, pp. 43–50. 10.1016/j.biomaterials.2015.01.070 [DOI] [PubMed] [Google Scholar]
- [20]. Nirmalanandhan, V. S. , Dressler, M. R. , Shearn, J. T. , Juncosa-Melvin, N. , Rao, M. , Gooch, C. , Bradica, G. , and Butler, D. L. , 2007, “ Mechanical Stimulation of Tissue Engineered Tendon Constructs: Effect of Scaffold Materials,” ASME J. Biomech. Eng., 129(6), pp. 919–923. 10.1115/1.2800828 [DOI] [PubMed] [Google Scholar]
- [21]. Schiele, N. R. , Marturano, J. E. , and Kuo, C. K. , 2013, “ Mechanical Factors in Embryonic Tendon Development: Potential Cues for Stem Cell Tenogenesis,” Curr. Opin. Biotechnol., 24(5), pp. 834–840. 10.1016/j.copbio.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Scott, A. , Danielson, P. , Abraham, T. , Fong, G. , Sampaio, A. V. , and Underhill, T. M. , 2011, “ Mechanical Force Modulates Scleraxis Expression in Bioartificial Tendons,” J. Musculoskelet. Neuronal. Interact., 11(2), pp. 124–132.https://pubmed.ncbi.nlm.nih.gov/21625049/ [PubMed] [Google Scholar]
- [23]. Shearn, J. T. , Juncosa-Melvin, N. , Boivin, G. P. , Galloway, M. T. , Goodwin, W. , Gooch, C. , Dunn, M. G. , and Butler, D. L. , 2007, “ Mechanical Stimulation of Tendon Tissue Engineered Constructs: Effects on Construct Stiffness, Repair Biomechanics, and Their Correlation,” ASME J. Biomech. Eng., 129(6), pp. 848–854. 10.1115/1.2800769 [DOI] [PubMed] [Google Scholar]
- [24]. Subramony, S. D. , Dargis, B. R. , Castillo, M. , Azeloglu, E. U. , Tracey, M. S. , Su, A. , and Lu, H. H. , 2013, “ The Guidance of Stem Cell Differentiation by Substrate Alignment and Mechanical Stimulation,” Biomaterials, 34(8), pp. 1942–1953. 10.1016/j.biomaterials.2012.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Herchenhan, A. , Kalson, N. S. , Holmes, D. F. , Hill, P. , Kadler, K. E. , and Margetts, L. , 2012, “ Tenocyte Contraction Induces Crimp Formation in Tendon-Like Tissue,” Biomech. Model Mechanobiol., 11(3–4), pp. 449–459. 10.1007/s10237-011-0324-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Maeda, T. , Sakabe, T. , Sunaga, A. , Sakai, K. , Rivera, A. L. , Keene, D. R. , Sasaki, T. , Stavnezer, E. , Iannotti, J. , Schweitzer, R. , Ilic, D. , Baskaran, H. , and Sakai, T. ,. 2011, “ Conversion of Mechanical Force Into TGF-Beta-Mediated Biochemical Signals,” Curr. Biol., 21(11), pp. 933–941. 10.1016/j.cub.2011.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Schweitzer, R. , Zelzer, E. , and Volk, T. , 2010, “ Connecting Muscles to Tendons: Tendons and Musculoskeletal Development in Flies and Vertebrates,” Development, 137(17), pp. 2807–2817. 10.1242/dev.047498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Marturano, J. E. , Arena, J. D. , Schiller, Z. A. , Georgakoudi, I. , and Kuo, C. K. , 2013, “ Characterization of Mechanical and Biochemical Properties of Developing Embryonic Tendon,” Proc. Natl. Acad. Sci. U. S. A., 110(16), pp. 6370–6375. 10.1073/pnas.1300135110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. McBride, D. J. , Trelstad, R. L. , and Silver, F. H. , 1988, “ Structural and Mechanical Assessment of Developing Chick Tendon,” Int. J. Biol. Macromol., 10(4), pp. 194–200. 10.1016/0141-8130(88)90048-7 [DOI] [Google Scholar]
- [30]. Ansorge, H. L. , Adams, S. , Birk, D. E. , and Soslowsky, L. J. , 2011, “ Mechanical, Compositional, and Structural Properties of the Post-Natal Mouse Achilles Tendon,” Ann. Biomed. Eng., 39(7), pp. 1904–1913. 10.1007/s10439-011-0299-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Theodossiou, S. K. , Bozeman, A. L. , Burgett, N. , Brumley, M. R. , Swann, H. E. , Raveling, A. R. , Becker, J. J. , and Schiele, N. R. , 2019, “ Onset of Neonatal Locomotor Behavior and the Mechanical Development of Achilles and Tail Tendons,” J. Biomech., 96, p. 109354. 10.1016/j.jbiomech.2019.109354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Waugh, C. M. , Blazevich, A. J. , Fath, F. , and Korff, T. , 2012, “ Age-Related Changes in Mechanical Properties of the Achilles Tendon,” J. Anat., 220(2), pp. 144–155. 10.1111/j.1469-7580.2011.01461.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Huang, A. H. , Riordan, T. J. , Pryce, B. , Weibel, J. L. , Watson, S. S. , Long, F. , Lefebvre, V. , Harfe, B. D. , Stadler, H. S. , Akiyama, H. , Tufa, S. F. , Keene, D. R. , and Schweitzer, R. , 2015, “ Musculoskeletal Integration at the Wrist Underlies the Modular Development of Limb Tendons,” Development, 142(14), pp. 2431–2441. 10.1242/dev.122374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Schwartz, A. G. , Long, F. , and Thomopoulos, S. , 2015, “ Enthesis Fibrocartilage Cells Originate From a Population of Hedgehog-Responsive Cells Modulated by the Loading Environment,” Development, 142(1), pp. 196–206. 10.1242/dev.112714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Pan, X. S. , Li, J. , Brown, E. B. , and Kuo, C. K. , 2018, “ Embryo Movements Regulate Tendon Mechanical Property Development,” Philos. Trans. R. Soc. B, 373(1759), p. 20170325. 10.1098/rstb.2017.0325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Thomopoulos, S. , Kim, H. M. , Rothermich, S. Y. , Biederstadt, C. , Das, R. , and Galatz, L. M. , 2007, “ Decreased Muscle Loading Delays Maturation of the Tendon Enthesis During Postnatal Development,” J. Orthop. Res., 25(9), pp. 1154–1163. 10.1002/jor.20418 [DOI] [PubMed] [Google Scholar]
- [37]. Swann, H. E. , and Brumley, M. R. , 2018, “ Locomotion and Posture Development in Immature Male and Female Rats (Rattus norvegicus): Comparison of Sensory-Enriched Versus Sensory-Deprived Testing Environments,” J. Comp. Psychol., 133(2), pp. 183–196. 10.1037/com0000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Schwartz, A. G. , Lipner, J. H. , Pasteris, J. D. , Genin, G. M. , and Thomopoulos, S. , 2013, “ Muscle Loading is Necessary for the Formation of a Functional Tendon Enthesis,” Bone, 55(1), pp. 44–51. 10.1016/j.bone.2013.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Khayyeri, H. , Blomgran, P. , Hammerman, M. , Turunen, M. J. , Löwgren, A. , Guizar-Sicairos, M. , Aspenberg, P. , and Isaksson, H. ,. 2017, “ Achilles Tendon Compositional and Structural Properties Are Altered After Unloading by Botox,” Sci. Rep., 7(1), p. 13067. 10.1038/s41598-017-13107-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Doherty, T. M. , Bozeman, A. L. , Roth, T. L. , and Brumley, M. R. , 2019, “ DNA Methylation and Behavioral Changes Induced by Neonatal Spinal Transection,” Infant Behav. Dev., 57, p. 101381. 10.1016/j.infbeh.2019.101381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Raveling, A. R. , Theodossiou, S. K. , and Schiele, N. R. , 2018, “ A 3D Printed Mechanical Bioreactor for Investigating Mechanobiology and Soft Tissue Mechanics,” MethodsX, 5, pp. 924–932. 10.1016/j.mex.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Bruneau, A. , Champagne, N. , Cousineau-Pelletier, P. , Parent, G. , and Langelier, E. , 2010, “ Preparation of Rat Tail Tendons for Biomechanical and Mechanobiological Studies,” J. Visual. Exp. JoVE, (41), p. 2176. 10.3791/2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Parent, G. , Cyr, M. , Desbiens-Blais, F. , and Langelier, E. , 2010, “ Bias and Precision of Algorithms in Estimating the Cross-Sectional Area of Rat Tail Tendons,” Meas. Sci. Technol., 21(12), p. 125802. 10.1088/0957-0233/21/12/125802 [DOI] [Google Scholar]
- [44]. Lee, S.-Y. , Chieh, H.-F. , Lin, C.-J. , Jou, I.-M. , Sun, Y.-N. , Kuo, L.-C. , Wu, P.-T. , and Su, F.-C. , 2017, “ Characteristics of Sonography in a Rat Achilles Tendinopathy Model: Possible Non-Invasive Predictors of Biomechics,” Sci. Rep., 7(1), p. 5100. 10.1038/s41598-017-05466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Javidi, M. , McGowan, C. P. , Schiele, N. R. , and Lin, D. C. , 2019, “ Tendons From Kangaroo Rats Are Exceptionally Strong and Tough,” Sci. Rep., 9(1), p. 8196. 10.1038/s41598-019-44671-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Schiele, N. R. , von Flotow, F., Tochka, Z. L., Hockaday, L. A., Marturano, J. E., Thibodeau, J. J., Kuo, C. K., 2015, “Actin Cytoskeleton Contributes to the Elastic Modulus of Embryonic Tendon During Early Development,” J. Orthop. Res., 33(6), pp. 874–881. 10.1002/jor.22880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Chen, J. , Zhang, W. , Liu, Z. , Zhu, T. , Shen, W. , Ran, J. , Tang, Q. , Gong, X. , Backman, L. J. , Chen, X. , Chen, X. , Wen, F. , and Ouyang, H. , 2016, “ Characterization and Comparison of Post-Natal Rat Achilles Tendon-Derived Stem Cells at Different Development Stages,” Sci. Rep., 6(1), p. 22946. 10.1038/srep22946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Shearer, T. , Thorpe, C. T. , and Screen, H. R. C. , 2017, “ The Relative Compliance of Energy-Storing Tendons May Be Due to the Helical Fibril Arrangement of Their Fascicles,” J. R. Soc. Interface, 14(133), p. 20170261. 10.1098/rsif.2017.0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Spiesz, E. M. , Thorpe, C. T. , Thurner, P. J. , and Screen, H. R. C. , 2018, “ Structure and Collagen Crimp Patterns of Functionally Distinct Equine Tendons, Revealed by Quantitative Polarised Light Microscopy (qPLM),” Acta Biomater., 70, pp. 281–292. 10.1016/j.actbio.2018.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Thorpe, C. T. , Klemt, C. , Riley, G. P. , Birch, H. L. , Clegg, P. D. , and Screen, H. R. C. , 2013, “ Helical Sub-Structures in Energy-Storing Tendons Provide a Possible Mechanism for Efficient Energy Storage and Return,” Acta Biomater., 9(8), pp. 7948–7956. 10.1016/j.actbio.2013.05.004 [DOI] [PubMed] [Google Scholar]
- [51]. Choi, R. K. , Smith, M. M. , Smith, S. , Little, C. B. , and Clarke, E. C. , 2019, “ Functionally Distinct Tendons Have Different Biomechanical, Biochemical and Histological Responses to In Vitro Unloading,” J. Biomech., 95, p. 109321. 10.1016/j.jbiomech.2019.109321 [DOI] [PubMed] [Google Scholar]
- [52]. Eekhoff, J. D. , Fang, F. , Kahan, L. G. , Espinosa, G. , Cocciolone, A. J. , Wagenseil, J. E. , Mecham, R. P., and Lake, S. P., 2017, “ Functionally Distinct Tendons From Elastin Haploinsufficient Mice Exhibit Mild Stiffening and Tendon-Specific Structural Alteration,” ASME J. Biomech. Eng., 139(11), p. 111003. 10.1115/1.4037932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Herod, T. W. , Chambers, N. C. , and Veres, S. P. , 2016, “ Collagen Fibrils in Functionally Distinct Tendons Have Differing Structural Responses to Tendon Rupture and Fatigue Loading,” Acta Biomater., 42, pp. 296–307. 10.1016/j.actbio.2016.06.017 [DOI] [PubMed] [Google Scholar]
- [54]. Quigley, A. S. , Bancelin, S. , Deska-Gauthier, D. , Legare, F. , Kreplak, L. , and Veres, S. P. , 2018, “ In Tendons, Differing Physiological Requirements Lead to Functionally Distinct Nanostructures,” Sci. Rep., 8(1), p. 4409. 10.1038/s41598-018-22741-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55]. Marturano, J. E. , Xylas, J. F. , Sridharan, G. V. , Georgakoudi, I. , and Kuo, C. K. , 2014, “ Lysyl Oxidase-Mediated Collagen Crosslinks May Be Assessed as Markers of Functional Properties of Tendon Tissue Formation,” Acta Biomater., 10(3), pp. 1370–1379. 10.1016/j.actbio.2013.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56]. Kondratko-Mittnacht, J. , Duenwald-Kuehl, S. , Lakes, R. , and Vanderby, R., Jr. , 2015, “ Shear Load Transfer in High and Low Stress Tendons,” J. Mech. Behav. Biomed. Mater., 45, pp. 109–120. 10.1016/j.jmbbm.2015.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Screen, H. R. C. , Toorani, S. , and Shelton, J. C. , 2013, “ Microstructural Stress Relaxation Mechanics in Functionally Different Tendons,” Med. Eng. Phys., 35(1), pp. 96–102. 10.1016/j.medengphy.2012.04.004 [DOI] [PubMed] [Google Scholar]
- [58]. Howell, K. , Chien, C. , Bell, R. , Laudier, D. , Tufa, S. F. , Keene, D. R. , Andarawis-Puri, N. , and Huang, A. H. , 2017, “ Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing,” Sci. Rep., 7(1), p. srep45238. 10.1038/srep45238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Popov, C. , Burggraf, M. , Kreja, L. , Ignatius, A. , Schieker, M. , and Docheva, D. , 2015, “ Mechanical Stimulation of Human Tendon Stem/Progenitor Cells Results in Upregulation of Matrix Proteins, Integrins and MMPs, and Activation of p38 and ERK1/2 Kinases,” BMC Mol. Biol., 16, p. 6. 10.1186/s12867-015-0036-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Yang, G. , Rothrauff, B. B. , Lin, H. , Gottardi, R. , Alexander, P. G. , and Tuan, R. S. , 2013, “ Enhancement of Tenogenic Differentiation of Human Adipose Stem Cells by Tendon-Derived Extracellular Matrix,” Biomaterials, 34(37), pp. 9295–306. 10.1016/j.biomaterials.2013.08.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Kalson, N. S. , Holmes, D. F. , Kapacee, Z. , Otermin, I. , Lu, Y. , Ennos, R. A. , Canty-Laird, E. G. , and Kadler, K. E. , 2010, “ An Experimental Model for Studying the Biomechanics of Embryonic Tendon: Evidence That the Development of Mechanical Properties Depends on the Actinomyosin Machinery,” Matrix Biol., 29(8), pp. 678–689. 10.1016/j.matbio.2010.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Liu, C. F. , Aschbacher-Smith, L. , Barthelery, N. J. , Dyment, N. , Butler, D. , and Wylie, C. , 2012, “ Spatial and Temporal Expression of Molecular Markers and Cell Signals During Normal Development of the Mouse Patellar Tendon,” Tissue Eng. Part A, 18(5–6), pp. 598–608. 10.1089/ten.tea.2011.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Chaplin, D. M. , and Greenlee, T. K., Jr. , 1975, “ The Development of Human Digital Tendons,” J. Anat., 120, pp. 253–274. https://pubmed.ncbi.nlm.nih.gov/1201962/ [PMC free article] [PubMed] [Google Scholar]
- [64]. Kastelic, J. , Galeski, A. , and Baer, E. , 1978, “ The Multicomposite Structure of Tendon,” Connect Tissue Res., 6(1), pp. 11–23. 10.3109/03008207809152283 [DOI] [PubMed] [Google Scholar]
- [65]. Sun, H. B. , Li, Y. , Fung, D. T. , Majeska, R. J. , Schaffler, M. B. , and Flatow, E. L. , 2008, “ Coordinate Regulation of IL-1beta and MMP-13 in Rat Tendons Following Subrupture Fatigue Damage,” Clin. Orthop. Relat. Res., 466(7), pp. 1555–1561. 10.1007/s11999-008-0278-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Spiesz, E. M. , Thorpe, C. T. , Chaudhry, S. , Riley, G. P. , Birch, H. L. , Clegg, P. D. , and Screen, H. R. C. , 2015, “ Tendon Extracellular Matrix Damage, Degradation and Inflammation in Response to In Vitro Overload Exercise,” J. Orthop. Res., 33(6), pp. 889–897. 10.1002/jor.22879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67]. Thorpe, C. T. , Chaudhry, S. , Lei, I. I. , Varone, A. , Riley, G. P. , Birch, H. L. , Clegg, P. D. , and Screen, H. R. C. , 2015, “ Tendon Overload Results in Alterations in Cell Shape and Increased Markers of Inflammation and Matrix Degradation,” Scand. J. Med. Sci. Sports, 25(4), pp. e381–e91. 10.1111/sms.12333 [DOI] [PubMed] [Google Scholar]
- [68]. Yang, G. , Im, H. J. , and Wang, J. H. , 2005, “ Repetitive Mechanical Stretching Modulates IL-1beta Induced COX-2, MMP-1 Expression, and PGE2 Production in Human Patellar Tendon Fibroblasts,” Gene, 363, pp. 166–172. 10.1016/j.gene.2005.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69]. Thomopoulos, S. , Williams, G. R. , and Soslowsky, L. J. , 2003, “ Tendon to Bone Healing: Differences in Biomechanical, Structural, and Compositional Properties Due to a Range of Activity Levels,” ASME J. Biomech. Eng., 125(1), pp. 106–113. 10.1115/1.1536660 [DOI] [PubMed] [Google Scholar]
- [70]. Freedman, B. R. , Fryhofer, G. W. , Salka, N. S. , Raja, H. A. , Hillin, C. D. , Nuss, C. A. , Farber, D. C. , and Soslowsky, L. J. , 2017, “ Mechanical, Histological, and Functional Properties Remain Inferior in Conservatively Treated Achilles Tendons in Rodents: Long Term Evaluation,” J. Biomech., 56, pp. 55–60. 10.1016/j.jbiomech.2017.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71]. Blumberg, M. S. , and Lucas, D. E. , 1994, “ Dual Mechanisms of Twitching During Sleep in Neonatal Rats,” Behav. Neuro, 108(6), pp. 1196–1202. 10.1037/0735-7044.108.6.1196 [DOI] [PubMed] [Google Scholar]
- [72]. Weber, E. D. , and Stelzner, D. J. , 1977, “ Behavioral Effects of Spinal Cord Transection in the Developing Rat,” Brain Res., 125(2), pp. 241–255. 10.1016/0006-8993(77)90618-7 [DOI] [PubMed] [Google Scholar]
- [73]. Yuan, Q. , Su, H. , Chiu, K. , Wu, W. , and Lin, Z. , 2013, “ Contrasting Neuropathology and Functional Recovery After Spinal Cord Injury in Developing and Adult Rats,” Neurosci. Bull., 29(4), pp. 509–516. 10.1007/s12264-013-1356-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74]. Strain, M. M. , Kauer, S. D. , Kao, T. , and Brumley, M. R. , 2014, “ Inter- and Intralimb Adaptations to a Sensory Perturbation During Activation of the Serotonin System After a Low Spinal Cord Transection in Neonatal Rats,” Front. Neural Circuits, 8, p. 80. 10.3389/fncir.2014.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75]. Thompson, F. J. , Parmer, R. , and Reier, P. J. , 1998, “ Alteration in Rate Modulation of Reflexes to Lumbar Motorneurons After Midthoracic Spinal Cord Injury in the Rat,” J. Neurotrauma, 15(7), pp. 495–508. 10.1089/neu.1998.15.495 [DOI] [PubMed] [Google Scholar]
- [76]. Celichowski, J. , Mrówczyński, W. , Krutki, P. , Górska, T. , Majczyński, H. , and Sławińska, U. , 2006, “ Changes in Motor Units Contractile Properties of the Rat Medial Gastrocnemius Muscle After Spinal Cord Transection,” Exp. Physiol., 91(5), pp. 887–95. 10.1113/expphysiol.2005.033076 [DOI] [PubMed] [Google Scholar]
- [77]. Mrówczyński, W. , Celichowski, J. , Krutki, P. , Cabaj, A. , Sławińska, U. , and Majczyński, H. , 2011, “ Changes of the Force-Frequency Relationship in the Rat Medial Gastrocnemius Muscle After Total Transection and Hemisection of the Spinal Cord,” J. Neurophysiol., 105(6), pp. 2943–2950. 10.1152/jn.00687.2010 [DOI] [PubMed] [Google Scholar]
- [78]. Mikic, B. , Amadei, E. , Rossmeier, K. , and Bierwert, L. , 2010, “ Sex Matters in the Establishment of Murine Tendon Composition and Material Properties During Growth,” J Orthop Res, 28(5), pp. 631–8. 10.1002/jor.21034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79]. Pardes, A. M. , Beach, Z. M. , Raja, H. , Rodriguez, A. B. , Freedman, B. R. , and Soslowsky, L. J. , 2017, “ Aging Leads to Inferior Achilles Tendon Mechanics and Altered Ankle Function in Rodents,” J. Biomech., 60, pp. 30–38. 10.1016/j.jbiomech.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table PDF


