Abstract
The neuropeptide oxytocin (OT) regulates important brain functions including feeding through activating OT receptors in multiple brain areas. Both OT fibers and OT receptors have been reported in the paraventricular thalamus (PVT), an area that was revealed to be important for the control of emotion, motivation, and food intake. However, the function and modulation of PVT OT signaling remain unknown. Here, we used a progressive ratio (PR) schedule of reinforcement to examine the role of PVT OT signaling in regulating the motivation for food and patch-clamp electrophysiology to study the modulation of OT on PVT neurons in brain slices. We demonstrate that PVT OT administration increases active lever presses to earn food rewards in both male and female mice under PR trials and OT receptor antagonist atosiban inhibits OT-induced increase in motivated lever presses. However, intra-PVT OT infusion does not affect food intake in normal conditions but attenuates hypophagia induced by stress and anxiety. Using patch-clamp recordings, we find OT induces long-lasting excitatory effects on neurons in all PVT regions, especially the middle to posterior PVT. OT not only evokes tonic inward currents but also increases the frequency of spontaneous excitatory postsynaptic currents on PVT neurons. The excitatory effect of OT on PVT neurons is mimicked by the specific OT receptor agonist [Thr4, Gly7]-oxytocin (TGOT) and blocked by OT receptor antagonist atosiban. Together, our study reveals a critical role of PVT OT signaling in promoting feeding motivation to attenuate stress-induced hypophagia through exciting PVT neurons.
Subject terms: Feeding behaviour, Motivation
Introduction
Oxytocin (OT), a neuropeptide synthesized by neurons in the paraventricular nucleus (PVN) and supraoptic nucleus of the hypothalamus, plays important roles in the regulation of stress response and food intake. Central OT signaling reduces food intake by activating OT receptors in multiple brain areas, including the ventral tegmental area (VTA) and the nucleus of the solitary tract (NTS) [1–6]. Although OT has been reported to primarily reduce food intake, the inhibitory effect of OT on food intake has been found to be conditional as it can be modified by factors such as social hierarchy, anxiety, and pregnancy of female [7–9]. For instance, intraperitoneal injection of the OT receptor agonist alleviates anxiety-induced hypophagia in a novel environment [10]. Although these findings point out that the regulation of OT on food intake is conditional, it remains unknown whether activation of central OT signaling in specific brain regions increases the motivation for food to promote food consumption in the condition of stress and anxiety.
Emerging evidence confirms that the paraventricular thalamus (PVT) plays an important role in controlling motivated behaviors including feeding [11–22]. To exert their control in food intake, PVT connects extensive feeding-related projections from the hypothalamus and zona incerta to the brain’s emotion and motivation centers [18, 22–27]. Although the majority of PVT neurons use glutamate as the neurotransmitter, findings from previous studies have suggested the diversity of PVT neurons across the anteroposterior axis in the control of the motivation for food rewards. For instance, optogenetic activation of some PVT glutamate neurons or their projections to the NAc inhibits food-seeking and consumption [18, 22, 28], while activation of specific glutamate neurons by orexin or decreased glucose level promotes food motivation [19, 26, 29]. In addition, manipulation of different PVT subregions produced differential effects on food-seeking and consumption [15, 25, 30, 31]. Indeed, the diversity of PVT neurons is supported by a recent study that neurons in the anterior PVT (aPVT) and the posterior PVT (pPVT) are genetically and functionally distinct [32]. Thus, further studies about the functional modulation of PVT neurons by various neural signals such as neuropeptides are important for understanding the diversity of PVT neurons in the regulation of food motivation.
Previous studies have identified both high-density OT fibers and OT receptors in the PVT [33–35]. A recent study also reported that OT receptor-expressing PVT neurons in prairie voles project to NAc [36], a brain area that was found to mediate the regulation of PVT neurons in the motivation for food rewards [26, 29]. Although both OT projections and OT receptor expression have been identified in the PVT, the function and modulation of PVT OT signaling remain largely unknown. Given the importance of the PVT in controlling both stress and the motivation for food rewards, we hypothesized that PVT OT signaling regulates feeding motivation in stress-associated conditions. The present study investigated the effect of PVT OT signaling on food motivation using a progressive ratio (PR) schedule of reinforcement, and hypophagia induced by acute stress and anxiety. Also, we investigated the modulatory effect of OT on the activity of PVT neurons using slice electrophysiology and compared the functional OT modulation in aPVT, middle PVT (mPVT), and pPVT of both male and female mice at different ages.
Materials and methods
Animals
Both C57BL/6J and Swiss Webster mice were purchased from the Jackson Laboratory. All mice were housed in a climate-controlled vivarium on a 12:12 h light/dark cycle and ad libitum access to food and water. All animals and experimental procedures in this study were approved by the Florida State University Institutional Animal Care and Use Committee.
Drugs
OT (051-01) and [Thr4, Gly7]-oxytocin (TGOT, 051-04) were purchased from Phoenix Pharmaceuticals Inc. Atosiban (Ato, 6332/10), D-2-amino-5-phosphonovalerate (AP5, 0106), bicuculline methiodide (Bic, 2503), and 6-Cyano-7-nitroquinoxaline-2,3-dione (CNQX, 0190) were purchased from Tocris Bioscience. For electrophysiological experiments, all drugs were dissolved in water and aliquoted as stock solutions that are stored at −80 °C. On the day of recording, the stock solutions were diluted to final concentrations in ACSF (at least 1:1000 dilution) for the experimental test. For behavioral tests, OT (1.0 and 2.0 μg/μl) and atosiban (2.0 μg/μl) were dissolved in saline for the direct intra-PVT infusion (0.5 μl) on the day with drug treatment [5, 37, 38].
Slice preparation and patch-clamp recording
Both C57BL/6J and Swiss Webster mice of 2–10 weeks old were used for preparing coronal brain slices (300 μm thick) containing the PVT (aPVT: −0.2 to −0.8 mm; mPVT: −1.0 to 1.8 mm; pPVT: −1.9 to −2.2 mm from bregma) as detailed in our previous studies [22, 39]. For the whole-cell recording of PVT neurons, fresh brain slices were transferred to a recording chamber mounted on a Zeiss upright microscope (Zeiss, Berlin, Germany) and perfused with a continuous flow of gassed ACSF solution containing (in mm) 124 NaCl, 3 KCl, 2 MgCl2, 2 CaCl2, 1.23 NaH2PO4, 26 NaHCO3, and 10 glucose (gassed with 95% O2/5% CO2; 300–305 mOsm). Pipettes used for whole‐cell recording had resistances ranging from 4 to 7 MΩ when filled with pipette solution containing (in mm) 145 potassium gluconate, 1 MgCl2, 10 HEPES, 1.1 EGTA, 2 Mg‐ATP, 0.5 Na2‐GTP, and 5 disodium phosphocreatine (pH 7.3 with KOH; 290–295 mOsm). The recording was performed at 33 ± 1 °C using a dual-channel heat controller (Warner Instruments, Holliston, MA, USA). EPC‐10 USB amplifier (HEKA Instruments, NY, USA) and PatchMaster 2 × 90.5 software (HEKA Elektronik, Lambrecht/Pfalz, Germany) were used to acquire and analyze the data. For voltage-clamp recording, the membrane potentials were held at −70 mV. Traces were processed using Igor Pro 6.37 (Wavemetrics, OR, USA). Spontaneous postsynaptic currents were analyzed with MiniAnalysis 6.03 (Synaptosoft Inc., GA, USA).
Stereotactic surgery for cannula implantation
Naïve male and female C57BL/6J mice of 8–10 weeks old were anesthetized with intraperitoneal injections of ketamine (100 mg/kg) and xylazine (10 mg/kg) and placed on a stereotaxic apparatus (David Kopf Instruments, CA, USA). After exposing the skull via a small incision and drilling a hole in the skull, a 26-gauge guide cannula (P1 Technologies, VA, USA) was inserted into the brain to target the mPVT (coordinates related to bregma: AP; −1.4 mm, DV: 2.9 mm, L: 0.05 mm). The guide cannula was then fixed by dental cement with surgical screws. Mice were returned to their single-housed cages for recovery of 10–14 days before they were used for operant conditioning training or food intake tests.
Progressive-ratio (PR) schedule of reinforcement
Before operant conditioning training in mouse operant chambers (Med Associates, VT, USA), all C57BL/6J mice were food-restricted (70% of their daily food intake) to facilitate the acquisition of lever-press responding until they learned to press the lever to obtain the food pellet in 3–5 days. Mice were provided their daily quota of food in the home cage after the termination of the training session. We used a program designed by our programming engineer for data acquisition. During the training, mice were initially trained under fixed-ratio 1 (FR1) sessions for 45 min daily. Animals had a choice between two press levers, an active lever press associated with a 3 s light cue, and a concomitant delivery of high-fat high-sucrose (HFHS) pellets (20 mg, 48.9% Kcal as fat, Bio Serv, NJ, USA) and an inactive lever press that remained inoperative and served as a control for general activity. Each active lever press triggered the delivery of one pellet during FR1 sessions. The lever remained inactive for 5 s after each food delivery so that mice were able to retrieve the pellet and supplementary presses during the inactive period did not drive food delivery. After a training period of about 7–10 days when three successive sessions of obtaining equal and more than 20 pellets during the FR1 session of 45 min, mice were then engaged in consecutive PR sessions of 45 min. For the PR session, the number of lever presses required for one food pellet delivery followed the order (calculated by the formula [5e (R*0.2)]−5 where R is equal to the number of food rewards already earned plus 1): 1, 2, 4, 6, 9, 12, 15, 20, and so on [40]. The maximal number of active lever presses performed to reach the final ratio was defined as the breakpoint, a value reflecting animals’ motivation to get the food reward.
Intra-PVT drug infusion
When FR1 or PR performance was maintained stable for at least three successive FR1 or PR sessions (the variation of the averaged daily active lever presses was less than 10% for FR1 sessions and less than 20% for PR sessions), mice were ready for testing the effect of intra-PVT drug infusion on the FR1 or PR. To do the intra-PVT infusion, an infusion cannula (2 mm longer than guide cannula, P1 Technologies, VA, USA) was connected to a Hamilton syringe (10 μl) using a clear plastic tubing. The syringe and the tubing were backfilled with mineral oil and the drug solution was sucked through the infusion injector into the tubing. Forty-five minutes before FR1 or PR sessions on test days of drug treatments, mice were shortly anesthetized by isoflurane, and the infusion injector was then quickly inserted into guide cannula for intra-PVT injection of 0.5 μl drug solutions at a rate of 0.1 μl per min controlled by a syringe pump. Injectors were left in place for an additional 2–3 min to allow for a complete drug infusion. Treatments were counterbalanced based on the experimental design.
Regular food intake test
Naïve male C57BL/6J mice with cannula targeting PVT were singly housed for the experiments. Regular chow intake over 4 h was measured daily from 11:00 a.m. to 3:00 p.m. in their home cages. When the daily regular chow intake over 4 h was relatively stable for at least three successive days, mice were ready for food intake tests with drug treatment. On test days, regular chow intake was measured 45 min after intra-PVT drug infusion.
Stress-induced hypophagia
Naïve male C57BL/6J mice with cannula targeting PVT were singly housed for the experiments. Mice had ad libitum access to regular mouse chow except during the test with HFHS food intake over 3 h in their home cages. When the daily HFHS food intake over 3 h was relatively stable for at least three successive days, mice were ready for food intake tests following acute restraint stress [41, 42]. On test days with drug treatment, mice were randomly assigned into four groups. Mice in group 1 received intra-PVT saline infusion 45 min before the HFHS food intake test. Mice in group 2 received intra-PVT saline infusion 45 min before the HFHS food intake test. 15 min after saline infusion, mice were kept immobilized in transparent plastic tubes with breathing holes for 30 min. Mice in group 3 received intra-PVT OT (1.0 μg) infusion 45 min before the HFHS food intake test. Mice in group 4 received OT (1.0 μg) infusion 45 min before the HFHS food intake test. Fifteen minutes after OT infusion, mice then received restraint stress in plastic tubes for 30 min. HFHS Food intake over 3 h was measured in their home cage immediately after restraint stress.
Light/dark box test with HFHS food
C57BL/6J mice were exposed to HFHS food pellets 2 h daily, for at least 3 days, before food intake was tested in the light/dark box where HFHS pellets were placed in a plastic dish in one corner of the light compartment. On the experimental test day, mice received an intra-PVT infusion of either saline or OT (1.0 μg) 45 min before the 30-min test. At the beginning of the test, mice were placed in the light compartment, facing the entry to the dark compartment. A digital camera over the light/dark box was used to track and record the activity of mice in the light compartment. Latency to first exit, total entries to the light compartment, percentage of time spent in the light compartment, food approaches, and food intake over the 30 min test was recorded for testing the effect of intra-PVT OT infusion on the exploratory behaviors of mice and food consumption in an anxiety-like condition. In addition, we observed mouse behavior in the absence of food in the food zone.
Statistical analysis
Data are expressed as mean ± SEM. Statistical significance was assessed using a two-sided Student’s t-test and Chi-squared test for comparison of two groups, and one-way, two-way, or three-way ANOVA followed by a Bonferroni post hoc test for three or more groups. Prism 8 (GraphPad, CA, USA) was used for statistical analysis and figure making.
Results
Intra-PVT OT infusion promotes the motivation for food rewards through activating OT receptors
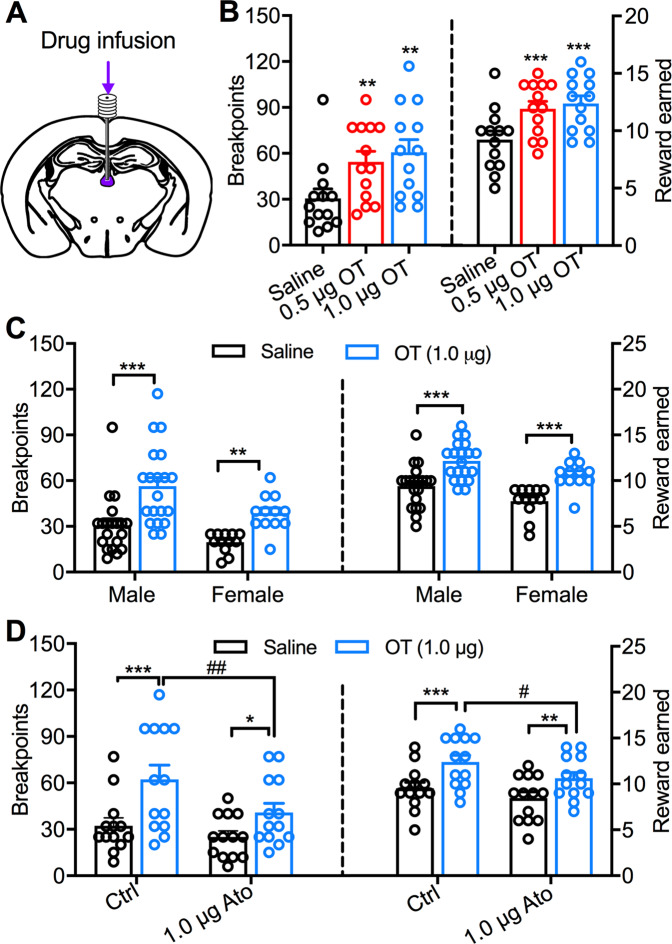
To study whether OT controls the motivation for food through activating OT receptors in the PVT, we used a PR schedule of reinforcement to test the behavioral response of mice to HFHS food reward. Intra-PVT infusion of both doses of OT (0.5 and 1.0 μg) promoted breakpoints (F(1.710,20.52) = 11.45, p = 0.0007, one-way ANOVA repeated measures, Fig. 1b) to increase reward earned (F(1.917,23.25) = 19.17, p < 0.0001, one-way ANOVA repeated measures, Fig. 1b) in male mice during 45-min PR trials. We next compared the effect of intra-PVT OT infusion between male and female mice. Generally, male mice had significantly higher levels of both breakpoints (F(1,30) = 5.99, p = 0.020, two-way ANOVA repeated measures, Fig. 1c) and rewarded earned (F(1,30) = 5.79, p = 0.023, two-way ANOVA repeated measures, Fig. 1c). However, we did not observe significant sex difference in OT-induced increase of either breakpoint (Sex × OT interaction: F(1,30) = 0.80, p = 0.38, two-way ANOVA repeated measures, Fig. 1c) or reward earned (Sex × OT interaction: F(1,30) = 0.07, p = 0.79, two-way ANOVA repeated measures, Fig. 1c). Intra-PVT OT (1.0 μg) infusion significantly promoted breakpoints (Male: p < 0.0001, female: p = 0.0042, Bonferroni post hoc test, Fig. 1c) to increase reward earned (Male: p < 0.0001, female: p < 0.0001, Bonferroni post hoc test Fig. 1c) in both male and female mice. In addition, OT infusion did not affect inactive lever presses in both male and female mice (p > 0.9999, Bonferroni post hoc tests), suggesting increased active lever presses are specifically associated with the motivation for food rewards. These data together suggest that activation of PVT OT signaling promotes the motivation for food in both male and female mice.
Fig. 1. Intra-PVT OT infusion increases motivation for HFHS food through activating PVT OT receptors.
a Schematics of the PVT location targeted by drug infusion. b Effects of intra-PVT OT (0.5 and 1.0 μg) infusion on breakpoints and reward earned in male mice (n = 13). One-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons. 0.5 and 1.0 μg OT vs. saline infusion: **p < 0.01, ***p < 0.001. c Effects of intra-PVT OT (1.0 μg) on breakpoints and reward earned in both male (n = 20) and female (n = 12) mice. Two-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons. OT vs. saline infusion: **p < 0.01, ***p < 0.001. d Effects of OT (1.0 μg) infusion on breakpoints and reward earned in the absence (n = 13 male mice) and presence (n = 13 male mice) of OT receptor antagonist Ato (1.0 μg). Two-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons. OT vs. saline infusion in the absence and presence of Ato: *p < 0.05, **p < 0.01, ***p < 0.001; OT plus Ato vs. OT infusion: #p < 0.05, ##p < 0.01.
We then examined the effect of intra-PVT OT (1.0 μg) infusion in the absence and presence of OT receptor antagonist atosiban (1.0 μg). Intra-PVT OT infusion increased both breakpoints (F(1,12) = 17.49, p = 0.0013, two-way ANOVA repeated measures, Fig. 1d) and reward earned (F(1,12) = 22.67, p = 0.0005, two-way ANOVA repeated measures, Fig. 1d). However, intra-PVT atosiban infusion decreased breakpoints (F(1,12) = 19.87, p = 0.0008, two-way ANOVA repeated measures, Fig. 1d) and reward earned (F(1,12) = 26.50, p = 0.0002, two-way ANOVA repeated measures, Fig. 1d). In addition, intra-PVT atosiban infusion attenuated OT-induced increase in breakpoints (Ato × OT interaction: F(1,12) = 5.58, p = 0.036, two-way ANOVA repeated measures, Fig. 1d). We further analyzed the effect of atosiban in the absence and presence of OT infusion. In the absence of OT, intra-PVT atosiban infusion had no significant effect on breakpoints (p = 0.75, Bonferroni post hoc test, Fig. 1d) or reward earned (p = 0.20, Bonferroni post hoc test, Fig. 1d). However, intra-PVT atosiban infusion decreased both breakpoints (p = 0.0019, Bonferroni post hoc test, Fig. 1d) and reward earned (p = 0.012, Bonferroni post hoc test, Fig. 1d) in the presence of OT. Together, these data indicate that the activation of PVT OT receptors contributes to an OT-induced increase in food motivation.
Intra-PVT OT infusion attenuates hypophagia in the condition of stress and anxiety
Based on the effect of intra-PVT OT signaling on food motivation, we asked whether activation of PVT OT signaling regulates food intake. We first applied the FR1 task in operant chambers to test if the intra-PVT infusion of OT increases food intake following a simple operant task. OT administration selectively increased active lever presses (p < 0.0001, Bonferroni post hoc test, Fig. 2a). However, the pellet consumption was not significantly different from that with saline infusion (t(16) = 1.93, p = 0.072, paired t-test, Fig. 2b) since some pellets were delivered to the receptacle but not consumed by mice with OT administration, suggesting activation of PVT OT signaling increases the motivation to obtain food but not food consumption when food is easily available. To further confirm this possibility, we then tested regular chow intake of mice in their home cage and found that intra-PVT OT infusion produced no obvious effect on normal food consumption over 4 h (F(1,48) = 1.31, p = 0.258, one-way ANOWA, Fig. 2c).
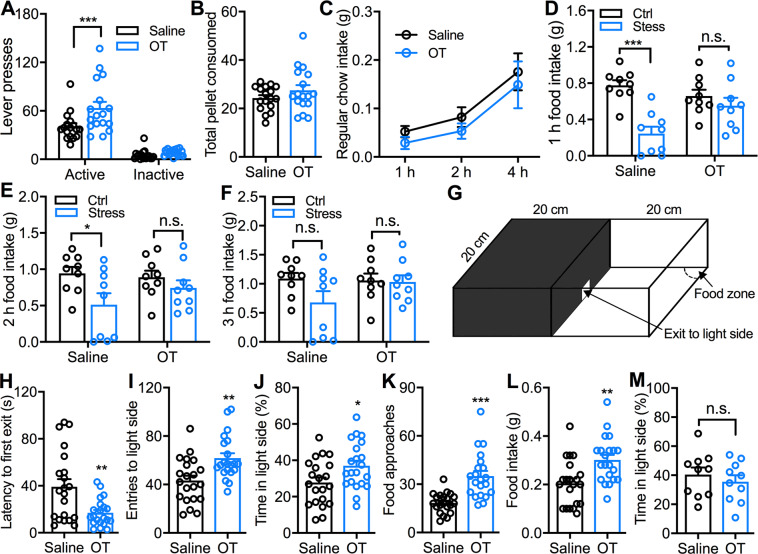
Fig. 2. Intra-PVT OT infusion attenuates acute stress-induced hypophagia and increases food intake of mice in light/dark conflict environment.
a Effects of intra-PVT OT (1.0 μg) infusion on both active and inactive lever presses during an FR1 task. n = 17 mice for each group. Two-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. OT vs. saline infusion on active lever presses: ***p < 0.001. b Effect of intra-PVT OT (1.0 μg) infusion on HFHS pellet consumed during an FR1 task. n = 17 mice. Paired t-test, p = 0.07. c Effect of intra-PVT OT (1 μg) infusion on regular chow intake. n = 9 mice for each group. Two-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. d Effect of intra-PVT OT (1.0 μg) infusion on 1 h palatable HFHS food intake in normal mice and mice after restraint stress of 30 min. n = 9 mice for each group. Two-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. OT vs. saline infusion in normal mice: ***p < 0.001; OT vs. saline infusion in stressed mice: n.s. no significant difference. e Effect of intra-PVT OT infusion on 2 h palatable food intake in both normal and stressed mice. n = 9 mice for each group. Two-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. OT vs. saline infusion in normal mice: *p < 0.05; OT vs. saline infusion in stressed mice: n.s., no significant difference. f Effect of intra-PVT OT infusion on 3 h palatable food intake in both normal and stressed mice. n = 9 mice for each group. Two-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. OT vs. saline infusion in both normal mice and stressed mice: n.s. no significant difference. g A diagram of the light/dark conflict box that was used for testing. h–l Effects of intra-PVT OT (1.0 μg) infusion on latency to the first exit from the dark compartment (h), total entries to the light compartment (i), percentage of time spent in the light compartment (j), total food approaches (k), and food intake (l) when food was placed in the food zone. n = 21 mice for each group. Unpaired t-test, OT vs. saline: *p < 0.05, **p < 0.01, ***p < 0.001. m Effect of intra-PVT (1.0 μg) infusion on the percentage of time spent in the light compartment in the absence of food in the food zone. n = 10 mice for each group. Unpaired t-test, n.s. no significant difference.
To test whether the PVT OT-evoked increase in food motivation affects food intake in the condition of stress, we measured the HFHS food intake of mice following acute restraint stress of 30 min. Restraint stress decreased cumulative food intake in mice with intra-PVT saline infusion (1 h: p < 0.0001; 2 h: p = 0.025; 3 h: p = 0.082, Bonferroni post hoc test, Fig. 2d–f), suggesting acute restraint stress produced hypophagia as reported by previous studies [41, 42]. However, no stress-induced decrease in food intake was observed in mice with intra-PVT OT injection (1 h: p = 0.610; 2 h: p = 0.746; 3 h: p > 0.999, Bonferroni post hoc test, Fig. 2d–f). Together, these data indicate intra-PVT OT infusion attenuates acute stress-induced hypophagia.
The light/dark test has been used to induce anxiety in mice based on the innate aversion of rodents to brightly illuminated areas [43]. The light/dark box was thus used to test food-seeking and consumption of mice in the condition of anxiety [22, 44, 45]. Specifically, we used the light/dark box to study the effect of PVT OT administration on HFHS food intake of mice in an anxiety-like condition (Fig. 2g). Intra-PVT OT infusion decreased the latency to the first exit from the dark compartment to the light side (t(40) = 3.20, p = 0.0027, unpaired t-test, Fig. 2h) and increased total entries to the light compartment (t(40) = 3.31, p = 0.002, unpaired t-test, Fig. 2i) as well as total time spent in the light compartment (t(40) = 2.36, p = 0.023, unpaired t-test, Fig. 2j). Furthermore, intra-PVT infusion increased both food approaches (t(40) = 4.92, p < 0.0001, unpaired t-test, Fig. 2k) and food intake (t(40) = 3.35, p = 0.0018, Fig. 2l) during the 30-min test in the light/dark box. However, intra-PVT OT infusion did not affect the percentage of time spent on the light side (t18 = 0.75, p = 0.46, unpaired t-test, Fig. 2m) when no food was placed in the light compartment. These data together suggest that activation of PVT OT signaling promotes the motivation for food consumption in mice in a light/dark conflict environment.
OT produces long-lasting excitatory effects on PVT neurons
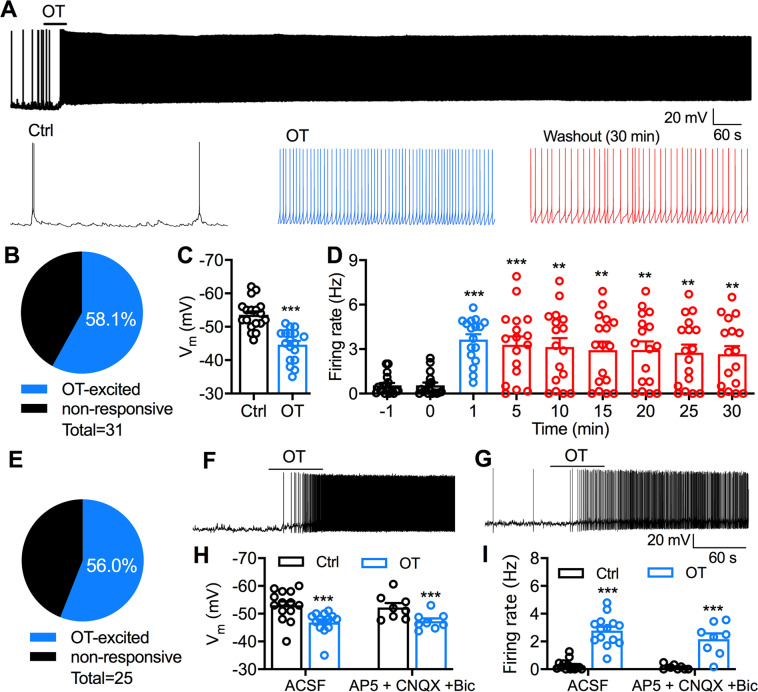
We next used whole-cell patch-clamp recordings to examine the effect of OT on the activity of PVT neurons in slices of mice. In current-clamp mode, 58.1% (18 of 31) of recorded PVT neurons responded to OT with excitation (Fig. 3a, b) in C57BL/6J mice. OT application (1 μM, 1 min) depolarized the membrane potential of OT-responsive PVT neurons from −53.6 ± 1.1 mV to −44.6 ± 1.1 mV (t(17)= 12.91, p < 0.0001, paired t-test, Fig. 3c). The firing rate of OT-responsive PVT neurons was significantly increased from 0.56 ± 0.19 Hz to 3.63 ± 0.36 Hz during OT application (1 μM, 1 min) and 2.65 ± 0.55 Hz after 30-min washout (F(1.59,25.37) = 17.82, p < 0.0001, one-way ANOVA repeated measures, Fig. 3d). We also examined the effect of OT on PVT neurons in Swiss Webster mice. 56.0% (14 of 25) of recorded neurons responded to OT with excitation (Fig. 3e, f). Similar to C57BL/6J mice, OT application (1 μM, 1 min) depolarized the membrane potential of PVT neurons in Swiss Webster mice from −53.0 ± 1.5 mV to −46.9 ± 1.1 mV (p < 0.0001, Bonferroni post hoc test, Fig. 3h) and increased the firing rate from 0.27 ± 0.10 Hz to 2.78 ± 0.28 Hz (OT effect: p < 0.0001, Bonferroni post hoc test, Fig. 3i) in normal ACSF. In the presence of AP5 (50 μM), CNQX (10 μM), and Bic (30 μM) to block synaptic transmission (Fig. 3g), OT still depolarized the membrane potential (p = 0.0001, Bonferroni post hoc test, Fig. 3h,) and increased the firing rate of PVT neurons (OT effect: p < 0.0001, Bonferroni post hoc test, Fig. 3i). Overall, these data indicate that short-time OT application produces long-lasting excitatory effects on PVT neurons in both C57BL/6J and Swiss Webster mice.
Fig. 3. OT excites PVT neurons in both C57BL/6J and Swiss Webster mice.
a A representative current-clamp trace shows the membrane potential and action potentials of a PVT neuron in a C57BL/6J mouse before, during, and after OT (1 μM) application of 1 min. Expanded sections (10 s) are shown underneath the trace. b Percentage of recorded PVT neurons (n = 31) in slices of 15 C57BL/6J mice. If the depolarization was more than 1.5 mV and/or the firing rate was increased at least 20% by OT treatment, we considered those neurons as OT-excited or OT-responsive neurons. c Effect of OT on the resting membrane potential of responsive PVT neurons in C57BL/6J mice. n = 18 cells from nine mice. Paired t-test, ***p < 0.0001 compared with control before OT application. d Long-lasting effect on firing rate of responsive PVT neurons in C57BL/6J mice. n = 17 cells from nine mice. Firing rates of 1 min were measured at each time point for the recording of at least 30 min. One-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons: **p < 0.01, ***p < 0.001 compared with control at the time point before OT application. e Percentage of recorded PVT neurons (n = 25) in slices of 12 Swiss Webster mice. f A representative trace shows the membrane potential and action potentials of a PVT neuron in a Swiss Webster mouse before, during, and after OT (1 μM) application of 1 min. g A representative trace shows the membrane potential and action potentials of a PVT neuron before, during, and after OT (1 μM) application of 1 min in the presence of AP5 (50 μM), CNQX (10 μM), and Bic (30 μM). h A bar graph shows the effect of OT on the membrane potential of PVT neurons in Swiss Webster mice in the absence (n = 14 cells from nine mice) and presence of AP5, CNQX, and Bic (n = 8 cells from four mice). Two-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons: ***p < 0.001 compared with control before OT application. i OT effect on the firing rate of PVT neurons in Swiss Webster mice in the absence (n = 14 cells from nine mice) and presence of AP5, CNQX, and Bic (n = 8 cells from four mice). Two-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons: ***p < 0.001 compared with control before OT application.
OT activates tonic inward currents and potentiates excitatory glutamate transmission on PVT neurons
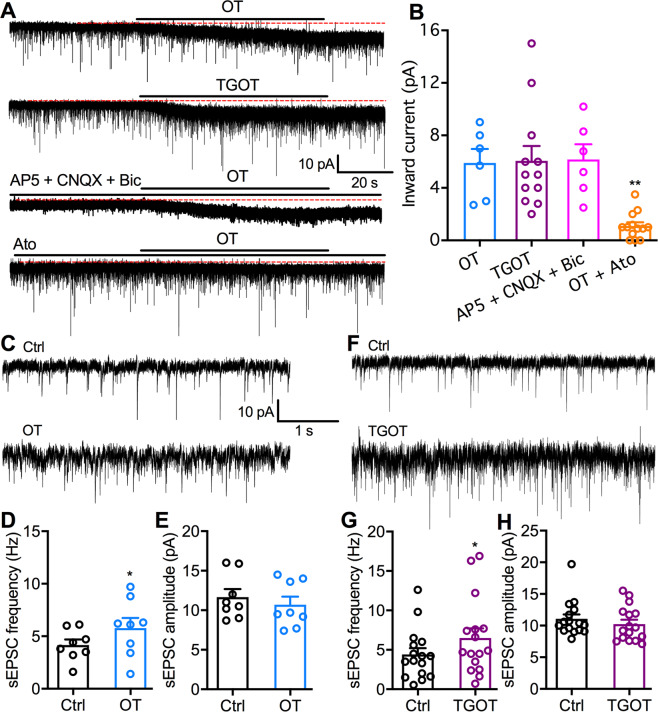
To determine whether OT directly evokes inward current on PVT neurons to exert excitatory modulation, we used voltage-clamp recordings to examine the effect of OT on membrane currents of PVT neurons in Swiss Webster mice. OT application (1 μM, 1 min) evoked an inward current of 6.1 ± 1.1 pA (Fig. 4b) when the membrane potential was held at −70 mV. Similarly, OT receptor-selective agonist TGOT (1 μM, 1 min) evoked an inward current of 5.9 ± 1.1 pA on (Fig. 4b). However, OT (1 μM, 1 min) failed to evoke obvious inward current (p = 0.0078 compared to OT alone, Bonferroni post hoc test, Fig. 4b) in the presence of OT receptor antagonist Ato (1 μM). In addition, OT evoked an inward current of 6.2 ± 1.1 pA in the presence of AP5 (50 μM), CNQX (10 μM), and Bic (30 μM), suggesting the tonic outward current did not result directly or indirectly from ionotropic glutamatergic or GABAergic transmission. Together, these data indicate that OT activates OT receptors on PVT neurons to produce inward currents for the depolarizing effect.
Fig. 4. OT receptor is responsible for the OT excitation of PVT neurons.
a Representative voltage-clamp traces show the inward currents evoked by the application of OT (1 μM, top), selective OT receptor agonist TGOT (1 μM, second), OT (1 μM) in the presence of AP5 (50 μM), and CNQX (10 μM, third trace), and OT (1 μM) in the presence of OT receptor antagonist atosiban (1 μM, bottom). b The amplitudes of inward currents evoked by OT, TGOT, OT plus Ato, and OT in the presence of AP5 (50 μM), CNQX (10 μM), and Bic (30 μM) on all sampled PVT neurons. OT group: n = 6 cells from two mice, TGOT group: n = 12 cells from four mice, OT in the presence of AP5, CNQX, and Bic: n = 6 cells from two mice, OT + Ato group: n = 13 cells from three mice. One-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons: **p < 0.01 compared with OT application only. c Representative traces show sEPSCs in the absence (top) and the presence of OT (1 μM, bottom). d OT increases the sEPSC frequency of all sampled PVT neurons (n = 8 from two mice). Paired t-test, *p < 0.05 compared with control before OT application. e OT has no obvious effect on the sEPSC amplitude of all sampled PVT neurons (n = 8 from two mice). Paired t-test, p = 0.10. f Representative traces show sEPSC in the absence (top) and the presence of TGOT (1 μM, bottom). g TGOT increases the sEPSC frequency of all sampled PVT neurons (n = 16 from eight mice). Paired t-test, *p < 0.05 compared with control before OT application. h TGOT has no obvious effect on the sEPSC amplitude of all sampled PVT neurons (n = 16 from eight mice). Paired t-test, p = 0.09.
We further recorded spontaneous excitatory postsynaptic currents (sEPSCs) of PVT neurons to test whether OT excites PVT neurons partially through potentiating glutamate neurotransmission. OT (1 μM, 1 min) increased sEPSC frequency from 4.17 ± 0.53 Hz to 5.78 ± 0.96 Hz (t(7) = 2.59, p = 0.036, paired t-test, Fig. 4d) but not sEPSC amplitude (t(7) = 1.91, p = 0.10, paired t-test, Fig. 4e). Similarly, selective OT receptor agonist TGOT (1 μM, 1 min) increased sEPSC frequency from 4.41 ± 0.80 Hz to 6.82 ± 1.39 Hz (t(15) = 2.97, p = 0.010, paired t-test, Fig. 4g). These data thus indicate that OT potentiates excitatory neurotransmission through increasing synaptic glutamate release onto PVT neurons.
Age and subregional differences in PVT OT modulation
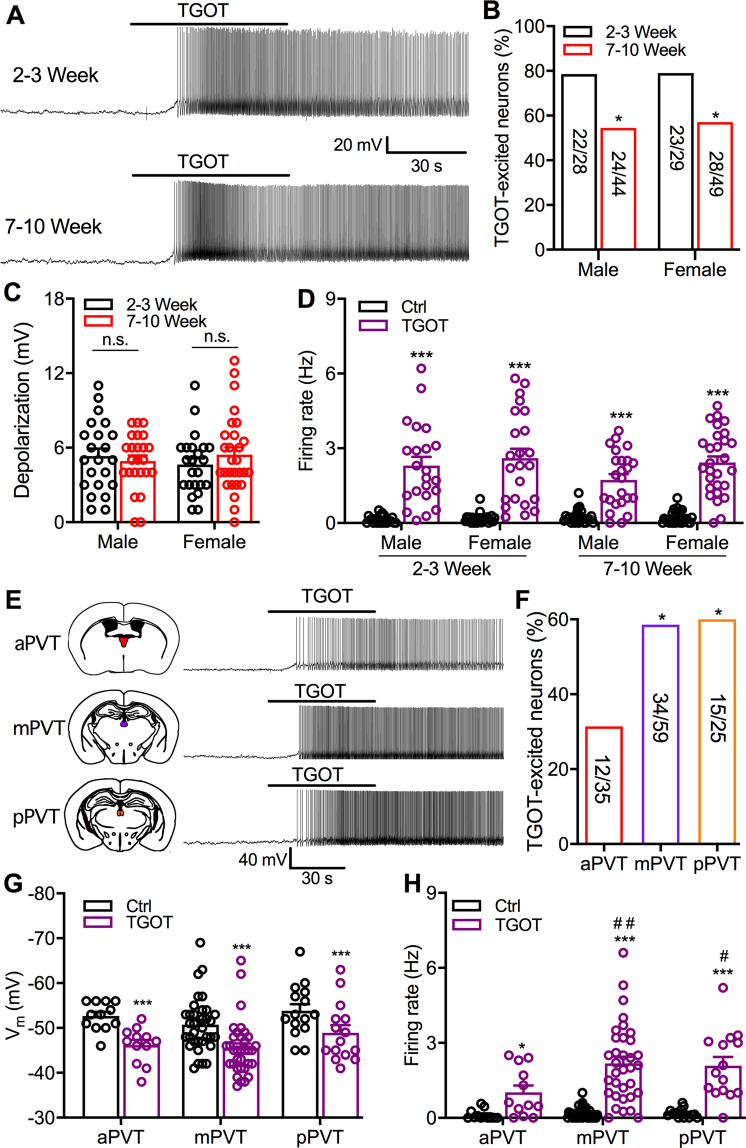
In mice, the PVT OT receptor density was reported to be higher in juveniles (21–22 day-old) than adults (60–65 day-old) [46]. To explore whether functional PVT OT receptor signaling is developmentally changed, we tested the effect of TGOT on the activity of PVT neurons in Swiss Webster mice at ages of 2–3 and 7–10 weeks. In male mice, TGOT-excited PVT neurons were decreased from 78.6% (22 of 28 cells) at age of 2–3 weeks to 54.5% (24 of 44 cells) at age of 7–10 weeks (p = 0.039, Chi-square test, Fig. 5b). Similarly, TGOT-excited PVT neurons in female mice were decreased from 79.3% (23 of 29 cells) at age of 2–3 weeks to 57.1% (28 of 49 cells) at age of 7–10 weeks (p = 0.046, Chi-square test, Fig. 5b). TGOT (1 μM, 1 min) produced depolarization (Fig. 5c) and increased firing rate (F(1,93) = 199.2, p < 0.0001, Three-way ANOVA repeated measures, Fig. 5d) of TGOT-responsive neurons in mice of both sexes at different ages. However, both the mouse age and sex did not cause a significant difference in TGOT-induced depolarization (Age: F(1,93) = 0.13, p = 0.72; sex: F(1,93) = 0.02, p = 0.90; Two-way ANOVA, Fig. 5c) or excitation of TGOT-responsive neurons (Age × TGOT interaction: F(1,93) = 2.33, p = 0.13; sex × TGOT interaction: F(1,93) = 2.73, p = 0.10; Three-way ANOVA repeated measures, Fig. 5d). These data together suggest that more PVT neurons in mice of 2–3 weeks are excited by OT than those of 7–10 weeks of age.
Fig. 5. Age and subregional differences in TGOT modulation of PVT neuron.
a Representative traces show TGOT (1 μM) excites PVT neurons of mice at postnatal 2–3 and 7–10 weeks. b Percentage of TGOT-responsive PVT neurons in both male and female mice at postnatal 2–3 and 7–10 weeks. Chi-square test, *p < 0.05 compared with postnatal 2–3 weeks. c TGOT evoked depolarization on TGOT-responsive neurons in both male and female mice at postnatal 2–3 weeks (n = 22 cell from ten male mice, n = 23 cells from ten female mice) and 7–10 weeks (n = 24 cells from 22 male mice, n = 28 cells from 20 female mice). Two-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. 2–3 vs. 7–10 weeks in both male and female mice: n.s., no significant difference. d Effect of TGOT on firing rate of TGOT-responsive PVT neurons in both male and female mice at postnatal 2–3 weeks (n = 22 cell from ten male mice, n = 23 cells from ten female mice) and 7–10 weeks (n = 24 cell from 22 male mice, n = 28 cells from 20 female mice). Three-way ANOVA followed by Bonferroni post hoc tests for multiple comparisons. TGOT vs. Ctrl: ***p < 0.001. e Representative traces show TGOT (1 μM) excites neurons in aPVT, mPVT, and pPVT of mice at postnatal 7–10 weeks. f Percentage of TGOT-excited neurons in aPVT, mPVT, and pPVT of mice at postnatal 7–10 weeks. Chi-square test, *p < 0.05 compared with aPVT area. g Effect of TGOT on the membrane potential of TGOT-responsive neurons in aPVT (n = 12 cells from eight mice), mPVT (n = 34 cells from 18 mice), and pPVT (n = 15 cells from 12 mice) of mice at postnatal 7–10 weeks. Two-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons. TGOT vs. Ctrl: ***p < 0.001. h Effect of TGOT on firing rate of TGOT-responsive neurons in aPVT (n = 12 cells), mPVT (n = 34 cells), and pPVT (n = 15 cells) of mice at postnatal 7–10 weeks. Two-way ANOVA repeated measures followed by Bonferroni post hoc tests for multiple comparisons. TGOT vs. Ctrl in various PVT subregions: *p < 0.05, ***p < 0.001. mPVT and pPVT neurons with TGOT treatment vs. aPVT neurons with TGOT treatment. #p < 0.05, ##p < 0.01.
We further tested the effect of TGOT on the activity of neurons located in different PVT subregions of Swiss Webster mice at age of 7–10 weeks. TGOT excited 34.3% of aPVT neurons, 57.6% of mPVT neurons, and 60.0% of pPVT neurons (aPVT vs. mPVT: p = 0.029; aPVT vs. mPVT: p = 0.048, Chi-square test, Fig. 5f). However, no subregional difference was observed in TGOT depolarization of TGOT-responsive PVT neurons (F(2,58) = 1.85, p = 0.17, two-way ANOVA repeated measures, Fig. 5g). TGOT also increased the firing rate of TGOT-responsive PVT neurons in all subregions (F(1,58) = 67.3, p < 0.0001, two-way ANOVA repeated measures, Fig. 5h). In the absence of TGOT treatment, the firing rate of aPVT neurons was not different from those of mPVT and pPVT neurons (p > 0.999, Bonferroni post hoc test, Fig. 5h). However, in the presence of TGOT, the firing rate of TGOT-responsive aPVT neurons was lower than those of TGOT-responsive mPVT (p = 0.0025, Bonferroni post hoc test, Fig. 5h) and pPVT (p = 0.021, Bonferroni post hoc test, Fig. 5h) neurons. Together, these data indicate that more neurons in both the mPVT and the pPVT respond to TGOT with a stronger excitatory effect than neurons of the aPVT.
Discussion
Our data provide clear evidence that activation of PVT OT signaling increases the motivation for food rewards and attenuates stress-induced hypophagia. Using a PR schedule of reinforcement, we found that intra-PVT OT infusion increased motivated lever presses to earn food rewards. The effect of OT on the motivated lever presses was significantly inhibited by OT receptor antagonist atosiban, suggesting activation of PVT OT receptors contribute to the OT-induced increase in food motivation. PVT OT administration did not affect normal food consumption in mice with readily available food in their home cage. However, PVT OT infusion attenuated hypophagia induced by acute restraint stress and increased food consumption of mice in a light/dark conflict environment. Using slice patch-clamp recordings, we revealed that short-time application of both OT and selective OT receptor agonist TGOT produced long-lasting excitatory effects on PVT neurons.
PVT OT signaling regulates food motivation
Central OT administration was reported to reduce food intake and the motivation for food through activating OT receptors in multiple brain regions such as VTA and NTS [5, 6]. Surprisingly, our present findings indicate that PVT OT administration significantly increased active lever presses, breakpoints, and food rewards earned and consumed during PR schedule of reinforcement trials of 45 min, suggesting activation of PVT OT signaling promotes feeding motivation. OT produced similar effects in both male and female mice, suggesting no sex difference in the OT regulation. We also tested the effect of OT receptor antagonist atosiban and found that intra-PVT atosiban administration alone had no significant effect on active lever presses to obtain palatable food. However, intra-PVT infusion of atosiban significantly inhibited the enhancement of OT on active lever presses and reward earned. These findings thus suggest that OT promotes food motivation through exciting PVT neurons following the activation of OT receptors. According to previous studies, OT primarily reduces the motivation for food and inhibits food consumption by targeting other brain areas [1–6]. Two recent studies reported that intracerebroventricular injection of OT reduced food motivation and food-seeking [5, 6], arguing against the possibility that OT increases the motivation for food through targeting other brain areas after leaking out into the brain ventricle following PVT OT infusion. Therefore, our findings reveal a different but novel role of OT in the regulation of food motivation by specifically activating PVT neurons. PVT has been found to play an important role in controlling food motivation and food consumption [15, 18, 19, 22, 47]. Activation of PVT neurons and their projections to NAc by both neuropeptide orexin and changed glucose levels increases the motivation for food [26, 29]. Inhibition of NAc-projecting PVT neurons by glucagon-like peptide (GLP-1) signaling reduces food motivation and inhibits food intake [20]. In addition, OT receptor-expressing PVT neurons have been recently reported to project to NAc in prairie voles [36]. Therefore, OT may share a similar neural target with orexin, glucose, and GLP-1 receptor signaling to promote food motivation by activating NAc-projecting PVT neurons [20, 26, 29].
Our results also indicate that PVT OT administration produced no obvious effect on either regular chow or palatable HFHS food intake tested in normal conditions. These findings suggest that the increased motivation for food will promote the efforts to earn food rewards but will not necessarily increase normal food consumption. This is consistent with previous findings that activation of mesolimbic dopamine projections increases motivated food reward-seeking but not reward retrieval and consumption [48–51]. Chemogenetic activation of VTA DA neurons increased active lever presses to earn food rewards during PR schedule of reinforcement trials, while activation of DA neurons and their projections to NAc produced no significant effect on normal food consumption [50, 51]. Similarly, activation of VTA DA neurons by a melanocortin-3 receptor (MC3R) agonist increased the motivation for food rewards but did not change normal food intake [49]. In addition, previous studies already indicated that PVT glutamate neurons regulate motivated behaviors through increasing VTA DA neuronal activity and DA release in the NAc by targeting presynaptic DA axonal terminals [52, 53]. In addition, orexin activation of PVT neurons increases DA levels in the NAc for the regulation of hedonic feeding [21, 54]. Therefore, PVT OT signaling may play a similar role as orexin to increase the motivation for food through activating mesolimbic DA release in the NAc.
In our present study, PVT OT administration increased active lever presses and breakpoints to earn food rewards and reward consumption during the PR schedule of reinforcement trials, where obtaining rewards progressively increases in difficulty. To ease the operant task for mice to earn food rewards, we also used FR1 trials in which each active lever press evoked the delivery of one pellet reward. Despite the PVT OT administration promoting the motivation to earn food rewards during FR1 trials, it did not increase reward consumption. Compared to PR trials, control mice consumed more than twice the food pellets during FR1 trials (FR1: about 24 pellets; PR: about 9 pellets). It is possible that increased basal food consumption in mice during FR1 trials produces negative feedback to terminate further food intake though they continuously work to obtain food rewards.
Stress and anxiety are important factors that cause hypophagia in patients with anorexia nervosa, a common eating disorder characterized by severe hypophagia, high anxiety, and social deficits [55–57]. Brain circuitry dysfunction in patients with anorexia reduces the patients’ sensitivity to food rewards and homeostatic hunger thus does not produce a strong motivation to eat due to stress, anxiety, and cognitive changes [58–60]. OT dysfunction has been reported to be involved in anorexia nervosa. In patients with anorexia nervosa, OT levels in both cerebrospinal fluid and blood plasma are lower than control subjects [61, 62]. Also, OT has been tested to treat anorexia nervosa in clinical trials [63–67]. Although our study indicates that PVT OT signaling has no obvious effect on normal food intake, it increases food consumption in stressed mice and mice in a novel light/dark conflict environment. However, intra-PVT infusion did not affect the exploratory behaviors of mice in the light compartment in the absence of food rewards during the light/dark conflict test. These findings thus suggest that activation of PVT OT signaling increases feeding motivation to attenuate hypophagia induced by stress and anxiety. Therefore, our present study suggests a potential central target for future studies to reveal the role of OT signaling in the control of anorexia nervosa.
In rodents, PVT neurons are activated following a wide variety of stressors including conditioned restraint, foot shock, and sleep deprivation [12]. Due to the heavy projections of PVT glutamate neurons to the prefrontal cortex (PFC), NAc, and amygdala, stress-induced activation of PVT neurons may participate in the expression of both positive and negative emotion and motivation [12]. Therefore, it is possible that OT activation of PVT neurons after acute stress contributes to increased feeding motivation. In future studies, it should be interesting to know if acute stress activates hypothalamic OT neurons to increase PVT OT signaling for the regulation of feeding motivation and food intake.
Functional modulation of OT on the activity of PVT neurons
OT exerts its excitatory effect on neurons in many regions through activating OT receptors [39, 68–70], the Gq-type G-protein coupled receptors. Following the activation of OT receptors and the intracellular second messengers, it depolarizes the neurons through opening transient receptor potential C (TRPC) channels and Na+/Ca2+ exchangers [68, 69]. Using patch-clamp recording in the present study, we found that local OT application for 1 min produced a long-lasting excitatory effect on PVT neurons in brain slices of both C57BL/6J and Swiss Webster mice. In most responsive PVT neurons, OT-evoked increase in firing rate remained at least 30 min following application of only 1 min. The excitatory response to OT is mediated primarily by activation of OT receptors since the OT-evoked inward currents were blocked by OT receptor antagonist atosiban. Selective OT receptor agonist TGOT induced similar inward currents and excitatory effect on PVT neurons as OT. Both OT and TGOT not only evoked tonic inward currents but also increased sEPSC frequency of PVT neurons, suggesting that OT excites PVT neurons through activating OT receptors in these neurons for a direct depolarization and also OT receptors in presynaptic glutamate terminals that innervate these neurons for an indirect excitation. However, it is unclear whether the OT receptor-expressing axonal terminals are from local PVT neurons or neurons in other brain regions such as PFC that were found to send excitatory projections to PVT [18, 22, 32, 71].
Neurons in different PVT subregions are genetically and functionally distinct based on the protein expression and neural circuit connections [32]. Our findings indicate that selective OT receptor agonist TGOT activated neurons in all PVT subregions. Compared to aPVT, both mPVT and pPVT have almost double the percentage of neurons that respond to TGOT with excitatory responses. In addition, TGOT produced a significantly stronger increase in the firing rate of both mPVT and pPVT neurons than those of aPVT neurons. These results together suggest that more neurons in the mPVT and pPVT express OT receptors with a higher density compared to the aPVT. This is consistent with a recent study that found more OT receptor-expressing neurons in the pPVT than the aPVT identified by in situ hybridization [35]. In addition to subregional differences, OT receptor expression was found to be higher in juvenile mice [46]. Indeed, the present study provides direct evidence that more PVT neurons in mice of 2–3 weeks of age excited by TGOT compared to mice aged 7–10 weeks. However, no sex difference in OT modulation of PVT neurons was observed in mice of any age. Together, these findings suggest that PVT OT signaling may play a significant role during early postnatal development. However, the potential role of PVT OT signaling during development should be investigated in future studies.
In summary, our present findings provide evidence that activation of PVT OT signaling promotes the motivation for food rewards and attenuates stress and anxiety-induced hypophagia through exciting PVT neurons. Moreover, more mPVT and pPVT neurons respond to OT with excitatory responses, suggesting OT signaling may play a different role in functions mediated by aPVT and pPVT neurons. Given the importance of the PVT in controlling emotion- and motivation-related behaviors, it should be important to know if PVT OT signaling also regulates fear and drug addiction in future studies.
Funding and disclosure
This research was supported by Florida State University startup funding and the First Year Assistant Professor (FYAP) award to XZ. The authors have no conflict of interest.
Supplementary information
Acknowledgements
We thank Te Tang and Fred Fletcher for developing the program for the operant conditioning chambers.
Author contributions
XZ designed the study. LRB and XZ conducted electrophysiological recordings and stereotactic surgery. JN and XZ performed behavioral experiments. XZ analyzed the data, wrote the manuscript. All authors reviewed, revised, and approved the final manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-021-00961-3).
References
- 1.Maejima Y, Yokota S, Nishimori K, Shimomura K. The anorexigenic neural pathways of oxytocin and their clinical implication. Neuroendocrinology. 2018;107:91–104. doi: 10.1159/000489263. [DOI] [PubMed] [Google Scholar]
- 2.Onaka T, Takayanagi Y. Role of oxytocin in the control of stress and food intake. J Neuroendocrinol. 2019;31:e12700. doi: 10.1111/jne.12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mullis K, Kay K, Williams DL. Oxytocin action in the ventral tegmental area affects sucrose intake. Brain Res. 2013;1513:85–91. doi: 10.1016/j.brainres.2013.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ong ZY, Alhadeff AL, Grill HJ. Medial nucleus tractus solitarius oxytocin receptor signaling and food intake control: the role of gastrointestinal satiation signal processing. Am J Physiol Regul Integr Comp Physiol. 2015;308:R800–6. doi: 10.1152/ajpregu.00534.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wald HS, Chandra A, Kalluri A, Ong ZY, Hayes MR, Grill HJ. NTS and VTA oxytocin reduces food motivation and food seeking. Am J Physiol Regul Integr Comp Physiol. 2020:R673–83. [DOI] [PMC free article] [PubMed]
- 6.Liu CM, Hsu TM, Suarez AN, Subramanian KS, Fatemi RA, Cortella AM, et al. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm Behav. 2020;126:104855. doi: 10.1016/j.yhbeh.2020.104855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olszewski PK, Klockars A, Levine AS. Oxytocin: a conditional anorexigen whose effects on appetite depend on the physiological, behavioural and social contexts. J Neuroendocrinol. 2016;28:1–9. doi: 10.1111/jne.12376. [DOI] [PubMed] [Google Scholar]
- 8.Olszewski PK, Allen K, Levine AS. Effect of oxytocin receptor blockade on appetite for sugar is modified by social context. Appetite. 2015;86:81–7. doi: 10.1016/j.appet.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Douglas AJ, Johnstone LE, Leng G. Neuroendocrine mechanisms of change in food intake during pregnancy: a potential role for brain oxytocin. Physiol Behav. 2007;91:352–65. doi: 10.1016/j.physbeh.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 10.Olszewski PK, Ulrich C, Ling N, Allen K, Levine AS. A non-peptide oxytocin receptor agonist, WAY-267,464, alleviates novelty-induced hypophagia in mice: insights into changes in c-Fos immunoreactivity. Pharm Biochem Behav. 2014;124:367–72. doi: 10.1016/j.pbb.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Beas BS, Wright BJ, Skirzewski M, Leng Y, Hyun JH, Koita O, et al. The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat Neurosci. 2018;21:963–73. doi: 10.1038/s41593-018-0167-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsu DT, Kirouac GJ, Zubieta JK, Bhatnagar S. Contributions of the paraventricular thalamic nucleus in the regulation of stress, motivation, and mood. Front Behav Neurosci. 2014;8:73. doi: 10.3389/fnbeh.2014.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong X, Li Y, Kirouac GJ. Blocking of orexin receptors in the paraventricular nucleus of the thalamus has no effect on the expression of conditioned fear in rats. Front Behav Neurosci. 2015;9:161. doi: 10.3389/fnbeh.2015.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Penzo MA, Robert V, Tucciarone J, De Bundel D, Wang M, Van Aelst L, et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature. 2015;519:455–9. doi: 10.1038/nature13978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Do-Monte FH, Minier-Toribio A, Quinones-Laracuente K, Medina-Colon EM, Quirk GJ. Thalamic regulation of sucrose seeking during unexpected reward omission. Neuron. 2017;94:388–400 e4. doi: 10.1016/j.neuron.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu Y, Wienecke CF, Nachtrab G, Chen X. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–22. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keyes PC, Adams EL, Chen Z, Bi L, Nachtrab G, Wang VJ, et al. Orchestrating opiate-associated memories in thalamic circuits. Neuron. 2020;107:1113–23 e4. doi: 10.1016/j.neuron.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otis JM, Zhu M, Namboodiri VMK, Cook CA, Kosyk O, Matan AM, et al. Paraventricular thalamus projection neurons integrate cortical and hypothalamic signals for cue-reward processing. Neuron. 2019;103:423–31 e4. doi: 10.1016/j.neuron.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng J, Wang J, Ma X, Ullah R, Shen Y, Zhou YD. Anterior paraventricular thalamus to nucleus accumbens projection is involved in feeding behavior in a novel environment. Front Mol Neurosci. 2018;11:202. doi: 10.3389/fnmol.2018.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong ZY, Liu JJ, Pang ZP, Grill HJ. Paraventricular thalamic control of food intake and reward: role of glucagon-like peptide-1 receptor signaling. Neuropsychopharmacology. 2017;42:2387–97. doi: 10.1038/npp.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi DL, Davis JF, Magrisso IJ, Fitzgerald ME, Lipton JW, Benoit SC. Orexin signaling in the paraventricular thalamic nucleus modulates mesolimbic dopamine and hedonic feeding in the rat. Neuroscience. 2012;210:243–8. doi: 10.1016/j.neuroscience.2012.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, van den Pol AN. Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science. 2017;356:853–59. doi: 10.1126/science.aam7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JS, Lee EY, Lee HS. Hypothalamic, feeding/arousal-related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Res. 2015;1598:97–113. doi: 10.1016/j.brainres.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 24.Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017;546:611–16. doi: 10.1038/nature22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stratford TR, Wirtshafter D. Injections of muscimol into the paraventricular thalamic nucleus, but not mediodorsal thalamic nuclei, induce feeding in rats. Brain Res. 2013;1490:128–33. doi: 10.1016/j.brainres.2012.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labouebe G, Boutrel B, Tarussio D, Thorens B. Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat Neurosci. 2016;19:999–1002. doi: 10.1038/nn.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirouac GJ. Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev. 2015;56:315–29. doi: 10.1016/j.neubiorev.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Lafferty CK, Yang AK, Mendoza JA, Britt JP. Nucleus accumbens cell type- and input-specific suppression of unproductive reward seeking. Cell Rep. 2020;30:3729–42 e3. doi: 10.1016/j.celrep.2020.02.095. [DOI] [PubMed] [Google Scholar]
- 29.Meffre J, Sicre M, Diarra M, Marchessaux F, Paleressompoulle D, Ambroggi F. Orexin in the posterior paraventricular thalamus mediates hunger-related signals in the nucleus accumbens core. Curr Biol. 2019;29:3298–306 e4. doi: 10.1016/j.cub.2019.07.069. [DOI] [PubMed] [Google Scholar]
- 30.Bhatnagar S, Dallman MF. The paraventricular nucleus of the thalamus alters rhythms in core temperature and energy balance in a state-dependent manner. Brain Res. 1999;851:66–75. doi: 10.1016/s0006-8993(99)02108-3. [DOI] [PubMed] [Google Scholar]
- 31.Nakahara K, Fukui K, Murakami N. Involvement of thalamic paraventricular nucleus in the anticipatory reaction under food restriction in the rat. J Vet Med Sci. 2004;66:1297–300. doi: 10.1292/jvms.66.1297. [DOI] [PubMed] [Google Scholar]
- 32.Gao C, Leng Y, Ma J, Rooke V, Rodriguez-Gonzalez S, Ramakrishnan C, et al. Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci. 2020;23:217–28. doi: 10.1038/s41593-019-0572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–66. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 34.Newmaster KT, Nolan ZT, Chon U, Vanselow DJ, Weit AR, Tabbaa M, et al. Quantitative cellular-resolution map of the oxytocin receptor in postnatally developing mouse brains. Nat Commun. 2020;11:1885. doi: 10.1038/s41467-020-15659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watarai A, Tsutaki S, Nishimori K, Okuyama T, Mogi K, Kikusui T. The blockade of oxytocin receptors in the paraventricular thalamus reduces maternal crouching behavior over pups in lactating mice. Neurosci Lett. 2020;720:134761. doi: 10.1016/j.neulet.2020.134761. [DOI] [PubMed] [Google Scholar]
- 36.Horie K, Inoue K, Nishimori K, Young LJ. Investigation of oxtr-expressing neurons projecting to nucleus accumbens using oxtr-ires-cre knock-in prairie voles (Microtus ochrogaster) Neuroscience. 2020;448:312–24. doi: 10.1016/j.neuroscience.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herisson FM, Waas JR, Fredriksson R, Schioth HB, Levine AS, Olszewski PK. Oxytocin acting in the nucleus accumbens core decreases food intake. J Neuroendocrinol. 2016;28. [DOI] [PubMed]
- 38.Han WY, Du P, Fu SY, Wang F, Song M, Wu CF, et al. Oxytocin via its receptor affects restraint stress-induced methamphetamine CPP reinstatement in mice: involvement of the medial prefrontal cortex and dorsal hippocampus glutamatergic system. Pharm Biochem Behav. 2014;119:80–7. doi: 10.1016/j.pbb.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, van den Pol AN. Dopamine/tyrosine hydroxylase neurons of the hypothalamic arcuate nucleus release gaba, communicate with dopaminergic and other arcuate neurons, and respond to dynorphin, met-enkephalin, and oxytocin. J Neurosci. 2015;35:14966–82. doi: 10.1523/JNEUROSCI.0293-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma S, Hryhorczuk C, Fulton S. Progressive-ratio responding for palatable high-fat and high-sugar food in mice. J Vis Exp. 2012;63:e3754. doi: 10.3791/3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holt MK, Richards JE, Cook DR, Brierley DI, Williams DL, Reimann F, et al. Preproglucagon neurons in the nucleus of the solitary tract are the main source of brain GLP-1, mediate stress-induced hypophagia, and limit unusually large intakes of food. Diabetes. 2019;68:21–33. doi: 10.2337/db18-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terrill SJ, Maske CB, Williams DL. Endogenous GLP-1 in lateral septum contributes to stress-induced hypophagia. Physiol Behav. 2018;192:17–22. doi: 10.1016/j.physbeh.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourin M, Hascoet M. The mouse light/dark box test. Eur J Pharm. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 44.Lockie SH, McAuley CV, Rawlinson S, Guiney N, Andrews ZB. Food seeking in a risky environment: a method for evaluating risk and reward value in food seeking and consumption in mice. Front Neurosci. 2017;11:24. doi: 10.3389/fnins.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Globa AK, Mills F, Naef L, Qiao M, Bamji SX, et al. Consumption of palatable food primes food approach behavior by rapidly increasing synaptic density in the VTA. Proc Natl Acad Sci USA. 2016;113:2520–5. doi: 10.1073/pnas.1515724113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olazabal DE, Alsina-Llanes M. Are age and sex differences in brain oxytocin receptors related to maternal and infanticidal behavior in naive mice? Horm Behav. 2016;77:132–40. doi: 10.1016/j.yhbeh.2015.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Millan EZ, Ong Z, McNally GP. Paraventricular thalamus: gateway to feeding, appetitive motivation, and drug addiction. Prog Brain Res. 2017;235:113–37. doi: 10.1016/bs.pbr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Halbout B, Marshall AT, Azimi A, Liljeholm M, Mahler SV, Wassum KM, et al. Mesolimbic dopamine projections mediate cue-motivated reward seeking but not reward retrieval in rats. Elife. 2019;8:e43551. doi: 10.7554/eLife.43551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pandit R, Omrani A, Luijendijk MC, de Vrind VA, Van Rozen AJ, Ophuis RJ, et al. Melanocortin 3 receptor signaling in midbrain dopamine neurons increases the motivation for food reward. Neuropsychopharmacology. 2016;41:2241–51. doi: 10.1038/npp.2016.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boekhoudt L, Roelofs TJM, de Jong JW, de Leeuw AE, Luijendijk MCM, Wolterink-Donselaar IG, et al. Does activation of midbrain dopamine neurons promote or reduce feeding? Int J Obes. 2017;41:1131–40. doi: 10.1038/ijo.2017.74. [DOI] [PubMed] [Google Scholar]
- 51.Boekhoudt L, Wijbrans EC, Man JHK, Luijendijk MCM, de Jong JW, van der Plasse G, et al. Enhancing excitability of dopamine neurons promotes motivational behaviour through increased action initiation. Eur Neuropsychopharmacol. 2018;28:171–84. doi: 10.1016/j.euroneuro.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Parsons MP, Li S, Kirouac GJ. Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol. 2007;500:1050–63. doi: 10.1002/cne.21224. [DOI] [PubMed] [Google Scholar]
- 53.Perez SM, Lodge DJ. Convergent inputs from the hippocampus and thalamus to the nucleus accumbens regulate dopamine neuron activity. J Neurosci. 2018;38:10607–18. doi: 10.1523/JNEUROSCI.2629-16.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ishibashi M, Takano S, Yanagida H, Takatsuna M, Nakajima K, Oomura Y, et al. Effects of orexins/hypocretins on neuronal activity in the paraventricular nucleus of the thalamus in rats in vitro. Peptides. 2005;26:471–81. doi: 10.1016/j.peptides.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 55.Marucci S, Ragione LD, De Iaco G, Mococci T, Vicini M, Guastamacchia E, et al. Anorexia nervosa and comorbid psychopathology. Endocr Metab Immune Disord Drug Targets. 2018;18:316–24. doi: 10.2174/1871530318666180213111637. [DOI] [PubMed] [Google Scholar]
- 56.Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, Moran TH. Anorexia nervosa as a motivated behavior: Relevance of anxiety, stress, fear and learning. Physiol Behav. 2015;152:466–72. doi: 10.1016/j.physbeh.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Oldershaw A, Treasure J, Hambrook D, Tchanturia K, Schmidt U. Is anorexia nervosa a version of autism spectrum disorders? Eur Eat Disord Rev. 2011;19:462–74. doi: 10.1002/erv.1069. [DOI] [PubMed] [Google Scholar]
- 58.Holsen LM, Lawson EA, Blum J, Ko E, Makris N, Fazeli PK, et al. Food motivation circuitry hypoactivation related to hedonic and nonhedonic aspects of hunger and satiety in women with active anorexia nervosa and weight-restored women with anorexia nervosa. J Psychiatry Neurosci. 2012;37:322–32. doi: 10.1503/jpn.110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaye WH, Wierenga CE, Bailer UF, Simmons AN, Bischoff-Grethe A. Nothing tastes as good as skinny feels: the neurobiology of anorexia nervosa. Trends Neurosci. 2013;36:110–20. doi: 10.1016/j.tins.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Strober M. Pathologic fear conditioning and anorexia nervosa: on the search for novel paradigms. Int J Eat Disord. 2004;35:504–8. doi: 10.1002/eat.20029. [DOI] [PubMed] [Google Scholar]
- 61.Demitrack MA, Lesem MD, Listwak SJ, Brandt HA, Jimerson DC, Gold PW. CSF oxytocin in anorexia nervosa and bulimia nervosa: clinical and pathophysiologic considerations. Am J Psychiatry. 1990;147:882–6. doi: 10.1176/ajp.147.7.882. [DOI] [PubMed] [Google Scholar]
- 62.Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, et al. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry. 2011;72:1546–51. doi: 10.4088/JCP.10m06617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maguire S, O’Dell A, Touyz L, Russell J. Oxytocin and anorexia nervosa: a review of the emerging literature. Eur Eat Disord Rev. 2013;21:475–8. doi: 10.1002/erv.2252. [DOI] [PubMed] [Google Scholar]
- 64.Russell J, Maguire S, Hunt GE, Kesby A, Suraev A, Stuart J, et al. Intranasal oxytocin in the treatment of anorexia nervosa: randomized controlled trial during re-feeding. Psychoneuroendocrinology. 2018;87:83–92. doi: 10.1016/j.psyneuen.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 65.Kim YR, Kim CH, Cardi V, Eom JS, Seong Y, Treasure J. Intranasal oxytocin attenuates attentional bias for eating and fat shape stimuli in patients with anorexia nervosa. Psychoneuroendocrinology. 2014;44:133–42. doi: 10.1016/j.psyneuen.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 66.Kim YR, Kim CH, Park JH, Pyo J, Treasure J. The impact of intranasal oxytocin on attention to social emotional stimuli in patients with anorexia nervosa: a double blind within-subject cross-over experiment. PLoS ONE. 2014;9:e90721. doi: 10.1371/journal.pone.0090721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leppanen J, Cardi V, Ng KW, Paloyelis Y, Stein D, Tchanturia K, et al. The effects of intranasal oxytocin on smoothie intake, cortisol and attentional bias in anorexia nervosa. Psychoneuroendocrinology. 2017;79:167–74. doi: 10.1016/j.psyneuen.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 68.Yao Y, Fu LY, Zhang X, van den Pol AN. Vasopressin and oxytocin excite MCH neurons, but not other lateral hypothalamic GABA neurons. Am J Physiol Regul Integr Comput Physiol. 2012;302:R815–24. doi: 10.1152/ajpregu.00452.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Briffaud V, Williams P, Courty J, Broberger C. Excitation of tuberoinfundibular dopamine neurons by oxytocin: crosstalk in the control of lactation. J Neurosci. 2015;35:4229–37. doi: 10.1523/JNEUROSCI.2633-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- 71.Yamamuro K, Bicks LK, Leventhal MB, Kato D, Im S, Flanigan ME, et al. A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice. Nat Neurosci. 2020;23:1240–52. doi: 10.1038/s41593-020-0695-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.