Abstract
Anthracyclines, such as doxorubicin (DOX), are well known for their high efficacy in treating multiple cancers, but their clinical usage is limited due to their potential to induce fatal cardiotoxicity. Such detrimental effects significantly impact the overall physical condition or even induce the morbidity and mortality of cancer survivors. Therefore, it is extremely important to understand the mechanisms of DOX-induced cardiotoxicity to develop methods for the early detection of cytotoxicity and therapeutic applications. Studies have shown that many molecular events are involved in DOX-induced cardiotoxicity. However, the precise mechanisms are still not completely understood. Recently, noncoding RNAs (ncRNAs) have been extensively studied in a diverse range of regulatory roles in cellular physiological and pathological processes. With respect to their roles in DOX-induced cardiotoxicity, microRNAs (miRNAs) are the most widely studied, and studies have focused on the regulatory roles of long noncoding RNAs (lncRNAs) and circular RNAs (circRNAs), which have been shown to have significant functions in the cardiovascular system. Recent discoveries on the roles of ncRNAs in DOX-induced cardiotoxicity have prompted extensive interest in exploring candidate ncRNAs for utilization as potential therapeutic targets and/or diagnostic biomarkers. This review presents the frontier studies on the roles of ncRNAs in DOX-induced cardiotoxicity, addresses the possibility and prospects of using ncRNAs as diagnostic biomarkers or therapeutic targets, and discusses the possible reasons for related discrepancies and limitations of their use.
Keywords: noncoding RNAs, doxorubicin, cardiotoxicity, biomarkers
Introduction
Doxorubicin (DOX), as a representative of the anthracycline family, is widely used in clinical settings for a variety of malignancies, such as breast cancer, lymphomas and leukemia [1]. DOX is also well known for its serious cardiotoxicity, manifesting as irreversible degenerative dilated cardiomyopathy (DCM) and the consequent congestive heart failure (CHF), which impacts long-term antitumor therapy outcomes [2]. In recent decades, researchers have extensively studied and addressed the mechanisms of DOX-induced cardiotoxicity, including DNA damage, excessive reactive oxygen species (ROS) generation, mitochondrial dysfunction, endoplasmic reticulum (ER)-mediated apoptosis, and disturbances to calcium homeostasis [3–7]. However, the whole picture is still far from being complete.
In recent years, the roles of noncoding RNAs (ncRNAs) in DOX-induced cardiotoxicity have attracted great attention and are considered a promising field to explore. NcRNAs have been reported to regulate gene expression and protein functions, thereby participating in cell proliferation, apoptosis, differentiation, metabolism and many other biological processes [8]. Unsurprisingly, with the depth of current research, ncRNAs have been shown to play key roles in DOX-induced cardiotoxicity, which is associated with multiple mechanisms [9, 10]. Thus, we summarize the updated research on DOX-induced cardiotoxicity related to ncRNAs and discuss their potential as diagnostic biomarkers and therapeutic targets. The goal of this review is to provide a new perspective for viewing cardiotoxicity prevention and intervention approaches during chemotherapy.
MicroRNAs and DOX-induced cardiotoxicity
Introduction to microRNAs
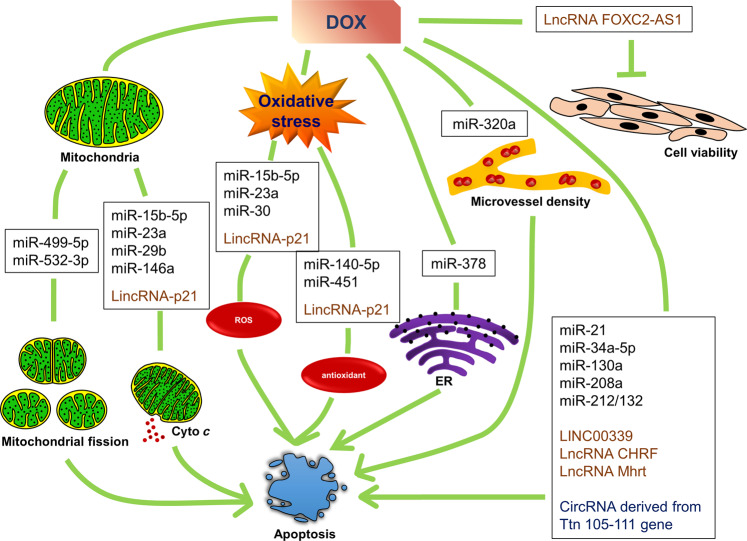
MicroRNAs (miRNAs) are a class of endogenous single-stranded ncRNA molecules that are ~20 nucleotides in length, and their sequences are highly conserved among different species [11]. MiRNA regulation is mainly realized through base pairing, with miRNA binding to specific sites of target gene messenger RNAs (mRNAs), to exert their function of negatively regulating gene expression [8, 12]. Clinical trials and animal experiments have shown that miRNAs take part in various cardiovascular diseases, such as coronary heart disease, ischemia-reperfusion injury, heart failure and DOX-induced cardiotoxicity [9, 13–15]. Regarding DOX-induced cardiotoxicity, many miRNAs have been reported to participate in multiple pathological processes (Fig. 1) [9, 10, 16]. These miRNAs target different protein mRNAs and damage heart cells by inducing apoptosis, mitochondrial dysfunction, ROS and ER stress (Table 1).
Fig. 1. NcRNAs involved in different mechanisms of DOX-induced cardiac cell apoptosis.
MiR-499-5p and miR-532-3p regulate DOX-induced mitochondrial fission; miR-15b-5p, miR-23a, miR-29b, miR-146a and LincRNA-p21 regulate the DOX-induced decline in mitochondrial membrane potential and cytochrome c release; miR-15b-5p, miR-23a, miR-30 and LincRNA-p21 regulate DOX-induced ROS production; miR-140-5p, miR-451 and LincRNA-p21 regulate DOX-induced change of antioxidant levels; miR-378 regulates DOX-induced ER stress; miR-320a regulates the DOX-induced impact on microvessel density; miR-21, miR-34a-5p, miR-130a, miR-208a, miR-212/132, Linc00339, LncRNA CHRF, LncRNA Mhrt and CircRNA derived from the Ttn 105-111 gene regulate DOX-induced apoptosis with no clearly indicated mechanisms; and LncRNA FOXC2-AS1 regulates DOX-induced reduction in cell viability.
Table 1.
NcRNAs in regulating DOX-induced cardiotoxicity.
| NcRNA | Regulation | Targets | Biological effects | Cell type | Reference |
|---|---|---|---|---|---|
| miRNAs | |||||
| miR-15b-5p | Up | Bmpr1a | Mitochondrial dysfunction, ROS & apoptosis | Cardiomyocyte | [33] |
| miR-21-5pa | Up | BTG2 | Apoptosis | Cardiomyocyte | [44] |
| miR-23a-3p | Up | PGC-1α | Mitochondrial dysfunction, ROS & apoptosis | Cardiomyocyte | [34] |
| miR-29b-3p | Down | Bax | Mitochondrial dysfunction & apoptosis | Cardiomyocyte | [27] |
| miR-30e-5p | Down | β1AR,β2AR,Gia-2 & BNIP3L | ROS & apoptosis | Cardiomyocyte | [35] |
| miR-34a-5p | Up | Sirt1/P66shc | Apoptosis | Cardiomyocyte | [45] |
| miR-130a-3p | Up | PPARγ | Apoptosis | mESC-derived cardiac cells | [46] |
| miR-140-5p | Up | Nrf2 & Sirt2 | Increase of ROS | Cardiomyocyte | [10] |
| miR-146a-5p | Up | ErbB4 | Mitochondrial dysfunction & apoptosis | Cardiomyocyte | [26] |
| miR-208a-3p | Up | GATA4 | Apoptosis | Cardiomyocyte | [43] |
| miR-212-3p/miR-132-3p | Down | Fitm2 | Apoptosis & atrophy | hiPSC-derived cardiomyocyte | [42] |
| miR-320a-3p | Up | VEGF-A | Reduce cardiac microvessel density & apoptosis | HUVEC | [48] |
| miR-378a-5p | Down | LDHA & PPIA | Energy metabolism disturbance & ER stress | Cardiomyocyte | [16] |
| miR-451-5p | Up | Cab39 | ROS & apoptosis | Cardiomyocyte | [39] |
| miR-499-5p | Down | p21 | Mitochondrial fission and apoptosis | Cardiomyocyte | [25] |
| miR-532-3p | Up | ARC | Mitochondrial fission & apoptosis | Cardiomyocyte | [9] |
| Let-7g-5p | Down | – | – | Cardiomyocyte | [47] |
| LncRNAs | |||||
| LINC00339 | Up | miR-484 | Apoptosis | Cardiomyocyte | [60] |
| lincRNA-p21 | Up | Wnt/β-catenin | Oxidative stress & cardiac senescence | Cardiomyocyte | [59] |
| LncRNA CHRF | Up | TGF-β/Smads TGF-β/p38 | Apoptosis | Cardiomyocyte | [61] |
| LncRNA FOXC2-AS1 | Down | WISP2 (intermediators in between) | Reduce viability | Cardiomyocyte | [63] |
| LncRNA Mhrt | Down | Nrf2 | Apoptosis | Cardiomyocyte | [62] |
| CircRNA | |||||
| CircRNA derived from Ttn 105-111 gene |
Upstream Qki5- an RNA-binding protein (RBP) |
Apoptosis & atrophy | Cardiomyocyte | [68] | |
aMiRNAs in Tables 1 and 2 were indicated with the -3p or -5p suffix according to their sequences in the latest version of miRBase (The miRBase Sequence Database - Release 22.1) to keep consistent with miRNA nomenclature. And the in-text miRNAs nomenclature is kept same with their original research articles.
MiRNAs and DOX-induced mitochondrial-mediated apoptosis
Several studies have suggested that apoptosis is the predominant cellular event in DOX-induced cardiotoxicity, as evidenced by morphological changes and the results of TdT-mediated dUTP nick end-labeling (TUNEL) assays, as cardiomyocytes are terminally differentiated and cannot regenerate [17, 18]. Among the many pathways of apoptosis, mitochondrial-dependent apoptosis has been intensively studied. DOX-induced cardiotoxicity is closely associated with mitochondrial morphological changes and dysfunction, as mitochondria are enriched in cardiomyocytes [3, 4]. DOX binds to abundant mitochondrial cardiolipin and forms the DOX-cardiolipin complex. This complex disrupts the electron transport chain (ETC), and less free cardiolipin is available for anchoring cytochrome c [19]. In addition, DOX may directly intercalate into mitochondrial genome-mtDNA to form adducts. These adducts disturb normal mitochondrial functions and alter the functions of protein subunits within a mitochondrion [20]. Additionally, DOX induces cytochrome c release from a mitochondrion. Cytochrome c is involved in the activation of cytoplasmic apoptotic protease activation factor-1 (Apaf-1), ATP/dATP and caspase-9, resulting in the initiation of the caspase cascade, which ultimately leads to cardiomyocyte apoptosis [21].
Structurally, mitochondria are constantly undergoing fission and fusion to maintain their functions, and excessive fission has been shown to contribute to cardiac injury under certain conditions, such as ischemia and DOX treatment [22, 23]. Our previous studies focused on mitochondrial fission and DOX-induced cardiotoxicity, and we have identified the regulatory roles of miRNA-532-3p in DOX-induced cardiotoxicity [9]. Upon DOX treatment, miR-532-3p is upregulated and participates in DOX-induced mitochondrial fission and apoptosis by directly targeting ARC, an apoptosis suppressor. Our team reported that ARC was downregulated in pathological heart conditions, whereas the overexpression of ARC inhibited DOX-induced mitochondrial division, thereby attenuating myocardial apoptosis [9]. Another study by our team showed that miR-499-5p regulates mitochondrial fission during myocardial infarction [24]. Interestingly, in our most recent study, we found that miR-499-5p expression was reduced upon DOX treatment, which led to the upregulation of the target p21, a transcription factor involved in heart injury, subsequently causing mitochondrial fission and apoptosis in DOX-induced cardiotoxicity [25]. In addition, therapeutically enhanced miR-499-5p expression enhanced cardiac functions, suggesting that the downregulation of miR-499-5p may be the cause of DOX cardiotoxicity [25].
Functionally, DOX-related mitochondrial injury is characterized by the loss of mitochondrial membrane permeability and cytochrome c release [4, 21]. Heart-abundant miR-146a, which had previously been reported to be upregulated by NF-κB, showed an increase in neonatal rat cardiac myocytes after DOX exposure [26]. MiR-146a enhanced the DOX-induced decrease in mitochondrial membrane potential and cardiomyocyte apoptosis by targeting ErbB4, which belongs to the epidermal growth factor receptor (EGFR) family and is well known for its essential roles during cardiac and neuronal development [26]. However, this study did not explore whether this DOX-triggered miR-146a dysregulation was found in vivo. In addition, the downregulation of miR-29b negatively affected the proapoptotic protein Bax by directly binding the Bax 3’UTR region, which activates the mitochondria-mediated intrinsic apoptotic pathway [27]. MiR-29b agomir ameliorated DOX-induced cardiac injury, which showed that a decrease in miR-29b levels may be the causal factor in DOX-induced cardiotoxicity.
MiRNAs and DOX-induced ROS-related apoptosis
Mitochondria and oxidative stress are inextricably linked in DOX-induced cardiotoxicity, and their relationship creates a vicious circle. DOX metabolism produces semiquinone radicals, which then form superoxide and ROS in a series of reactions known as the redox cycle [3]. During the redox cycle, ROS are continuously formed, and they interact with some intracellular components and induce oxidative damage to biological macromolecules, eventually resulting in tissue injury [28, 29]. Mitochondria are the major sites at which ROS are produced in the heart, and the binding of DOX to mitochondrial cardiolipin leads to the hampers of the activity of complex I and disrupts the ETC, resulting in increased ROS production [30]. Elevated ROS levels can cause mtDNA damage, which can lead to the downregulation of ETC proteins encoded by mtDNA, exacerbating mitochondrial dysfunction [31]. In addition, excessive ROS alter the mitochondrial respiration rate and, together with Ca2+, augment mitochondrial permeability transition pore (mPTP) opening, resulting in the loss of mitochondrial membrane potential, which in turn causes the release of cytochrome c to trigger apoptotic factors [32].
The involvement of miR-15b-5p and miR-23a in DOX-induced cardiomyocyte apoptosis was investigated not only in the mitochondria-related pathway but also with respect to ROS-related mechanisms. MiR-15b-5p significantly enhanced the effects of DOX on mitochondrial membrane permeability impairment and ROS production [33]. Both the overexpression of miR-15b-5p or the inhibition of its target, Bmpr1a, exacerbates DOX-induced cardiomyocyte apoptosis by causing a decrease in the Bcl-2/Bax ratio (an antiapoptotic indicator) [33]. Similarly, miR-23a is upregulated upon DOX treatment, which can induce excessive mitochondrial fission, leading to enhanced phosphorylation of dynamin-related protein-1 (Drp1), suppression of mitofusin 2 (Mfn2) and mitochondria pathway-dependent cardiomyocyte apoptosis [34]. An miR-23a inhibitor demonstrated protective effects on DOX-treated cardiomyocytes by restoring the mitochondrial membrane potential and suppressing oxidative stress by targeting PGC-1α and repressing Drp1 phosphorylation [34]. Unfortunately, neither miR-15b-5p nor miR-23a has been studied for its role in regulating cardiac function.
MiR-30a, miR-30d, and miR-30e were found to be downregulated upon DOX treatment, and they share the same basal sequences, which is predicted to target the same genes. MiR-30e showed the greatest dysregulation among these miRNAs and has been shown to target β1AR, β2AR, Giα-2 and BNIP3L/NIX [35]. Additionally, activation of the BNIP3 gene led to mPTP opening, loss of mitochondrial membrane potential and cardiomyocyte death [36]. Hence, overexpression of miR-30 reduced DOX-triggered ROS, the Bax/Bcl-2 expression ratio, and caspase activity, which confirmed the benefit of high miR-30 levels in cardiomyocytes [35].
Moreover, DOX also markedly decreases endogenous antioxidants, which accelerates oxidative stress. Therefore, the overexpression of antioxidants shows beneficial effects [37, 38]. In vitro and in vivo studies revealed that DOX caused an elevation of miR-140-5p and a decrease in its targets Nrf2 and Sirt2, which control oxidative stress by binding with antioxidant response elements and activating FOXO3a, respectively [10]. Applying miR-140-5p agomir to DOX-treated mice resulted in a decrease in superoxide dismutase (SOD) activity, while an antagomir did not affect SOD activity or enhance cardiac functions, which indicated that upregulation of miR-140 may be critical for DOX-induced cardiotoxicity [10]. Recently, a study revealed that miR-451 inhibition restored antioxidant-SOD activity and protected against DOX-induced cardiomyocyte apoptosis by activating the AMPK signaling pathway. In addition, a miR-451 inhibitor also ameliorated DOX-induced cardiac dysfunction [39].
MiRNAs and DOX-induced ER stress-related apoptosis
In response to DOX treatment, increased ROS levels and calcium overload trigger myocardial ER stress, and in severe and persistent conditions, excessive calcium can affect the mitochondrial membrane potential and activate a range of distinct cell apoptotic signaling pathways [40, 41]. In a study by Wang et al., miR-378 played a cardioprotective role by affecting energy metabolism and ER stress, changing the mitochondrial membrane potential, which correspondingly repressed mitochondria-related gene-lactate dehydrogenase A (LDHA) and ER stress-related gene-cyclophilin A (PPIA) expression. As a result, miR-378 promoted cardiomyocyte viability and decreased the apoptosis rate [16].
MiRNAs and other DOX-induced mechanisms
In addition, the pro-hypertrophic miR-212/132 family attenuated DOX-induced apoptosis and atrophy in primary rodent- and human-induced pluripotent stem cell (hs-iPSC)-derived cardiomyocytes [42]. DOX-treated experimental animals demonstrated that the overexpression of the miR-212/132 cluster improved the ejection fraction. In addition, a DOX-triggered decrease in the wall thickness of the myocardium, which is a parameter for atrophy, was alleviated by enhancing the miR-212/132 level. These findings revealed that lower miR-212/132 levels may be related to DOX-induced cardiotoxicity. At the cellular level, the overexpression of the miR-212/132 cluster led to a decreased apoptosis rate by directly enhancing its downstream target Fitm2, which is localized in the endoplasmic reticulum and is involved in lipid droplet accumulation [42]. Moreover, miR-21, miR-34a-5p, miR-130a, and miR-208a were also shown to be involved in the regulation of DOX-induced cardiomyocyte apoptosis, but no specific mechanisms were investigated (Table 1) [43–46]. Additionally, the downregulation of let-7g upon DOX treatment had an effect on DOX-induced cardiac injury [47].
In addition to the majority of studies being focused on cardiomyocyte apoptosis, Yin and colleagues investigated DOX-induced cardiotoxicity in endothelial cells and its impact on vascular homeostasis. MiR-320a was first reported to contribute to atherogenesis and was markedly increased in human umbilical vein endothelial cells (HUVECs) than it was in H9c2 cells upon DOX stimulation [48]. DOX increased the apoptosis rate and hampered the proliferation of HUVECs and impaired endothelial cell function. The inhibition of miR-320a attenuated DOX-induced endothelial cell damage and enhanced microvessel density by targeting vascular endothelial growth factor (VEGF)-A, an important factor in vascular homeostasis, especially in regulating new vessel formation [48]. Endothelial cells promote cardiomyocyte survival via the paracrine secretion of vascular bioactive molecules [49]. For instance, a decrease in endothelial cell-derived nitric oxide (NO) was observed in DOX-treated HUVECs and mouse hearts, and restoration of cardiac NO levels preserved cardiac function in doxorubicin-treated mice [48, 50]. Moreover, endothelial cells act as a barrier to prevent the exposure of cardiomyocytes to harmful substances; however, the damage of DOX to the endothelium affects tight junctions, resulting in an increase in microvascular permeability, and in vivo studies have shown that increased permeability significantly decreases contractility and cardiac function [51, 52]. Hence, microvascular injury may be a preceding and contributory event to DOX-induced cardiotoxicity. In addition, miR-320a suppression also improved cardiac function, suggesting that DOX-induced upregulation of miR-320a may be the cause of cardiotoxicity.
LncRNAs and DOX-induced cardiotoxicity
Introduction to lncRNAs
Long ncRNAs (lncRNAs) differ from miRNAs in that they are composed of more than 200 nucleotides, have mRNA-like structures, with some having poly-A tails [53]. LncRNAs can exert their biological functions through epigenetic modification, transcriptional regulation, and posttranscriptional regulation, and they can modulate the localization and function of proteins [54, 55]. Moreover, lncRNAs also serve as “miRNA sponges/decoys” and interact with miRNAs as competitive endogenous RNAs (ceRNAs) [56]. Recently, an increasing number of studies have focused on lncRNA regulation in cardiac development and remodeling [57, 58]. Additionally, lncRNA roles in DOX-induced cardiotoxicity have gained attention (Fig. 1) (Table 1).
LncRNAs in regulating DOX-induced cardiotoxicity
Similar to those of miRNAs, the mechanisms of lncRNAs in regulating DOX-induced cardiotoxicity are also involved in mitochondrial dysfunction and ROS generation. LincRNA-p21 plays a regulatory role in DOX-induced cardiomyopathy, and suppressing lincRNA-p21 attenuates the DOX-induced loss of mitochondrial membrane potential, oxidative stress and cardiomyocyte senescence by negatively modulating the β-catenin pathway, which has been shown to play an important role in age-related heart conditions [59].
Apoptosis remains the main focus of lncRNA- and DOX-induced cardiotoxicity. LncRNA LINC00339 was upregulated in response to DOX treatment and enhanced DOX-induced apoptosis by competitively sponging heart-enriched miR-484, which has been shown to inhibit mitochondrial fission and apoptosis [60]. LncRNA cardiac hypertrophy-related factor (CHRF), known to be involved in regulating cardiac hypertrophy, was also elevated upon DOX treatment. The inhibition of lncRNA CHRF ameliorated DOX-induced cardiomyocyte apoptosis via the TGF-β/Smads and TGF-β/p38 pathways [61]. The lncRNA Mhrt level was decreased upon DOX treatment, a finding that was also observed in other heart conditions. The overexpression of Mhrt facilitated the binding of H3 histone to the Nrf2 promoter, thereby positively regulating Nrf2 expression and consequently abrogating DOX-induced cardiomyocyte apoptosis [62]. In addition, lncRNA FOXC2-AS1 and WNT1-inducible signaling pathway protein-1 (WISP1) levels were both decreased and positively correlated in DOX-induced cardiotoxicity [63]. The overexpression of FOXC2-AS1 promoted cardiomyocyte viability by increasing WISP1 upon DOX treatment. However, the study did not further explore whether there are intermediators involved during the FOXC2-AS1- and WISP1-mediated regulation of DOX cardiotoxicity [63].
CircRNAs and DOX-induced cardiotoxicity
Introduction to circRNAs
Circular RNAs (CircRNAs) have a specific structure with exons and/or introns back-splicing formed loops, without 5’ end caps or 3’ end poly-A tails, they are mostly present in the cytoplasm and derived from exons [64]. Some circRNAs can be translated into proteins, but the majority are noncoding RNAs [65]. In addition to interacting with various proteins, similar to other ncRNAs, circRNAs play regulatory roles similar to ceRNAs, thereby abolishing the effect of miRNAs on their target genes [66, 67]. Although studies on circRNAs are still in the early phase, the results of some studies have suggested that circRNAs are closely related to cardiovascular development and disease, and they drawing attention because their extensive expression and sustained stability are highly suitable for use as biomarkers [66, 68, 69].
CircRNAs regulating DOX-induced cardiotoxicity
Qki5, an RNA-binding protein (RBP), is the most abundant Quaking family member in the heart, and it has been reported to regulate the formation of numerous circRNAs during the epithelial to mesenchymal transition and to suppress cardiomyocyte apoptosis in an ischemia-reperfusion model [70, 71]. DOX-induced downregulation of Qki5 suppressed circRNAs derived from Ttn, Fhod3 and Strn3 in mouse cardiomyocytes, which increased the DOX-induced apoptosis rate and extent of cell atrophy [68] (Fig. 1). On the other hand, simultaneous lentiviral-mediated Qki5 overexpression and knockdown of Ttn 105-111 did not reverse DOX-induced caspase activation. These findings indicate that Qki5-derived circRNAs may be downstream protective mediators in Qki5-regulated DOX cardiotoxicity. Further in vivo studies revealed that the nuclear localization and moderate overexpression of Qki5 may be a requirement for its protective role in DOX-induced cardiotoxicity in mice (Table 1) [68].
NcRNAs are promising diagnostic biomarkers and therapeutic targets
MiRNAs as diagnostic biomarkers
The current diagnostic methods for DOX-induced cardiotoxicity are mainly based on echocardiography, which can only detect heart dysfunction or tissue damage after it has occurred. Circulating biomarkers such as troponin I, atrial-type and brain-type natriuretic peptides (ANP and BNP) are also used to detect early signs of cardiac abnormalities [72, 73]. However, these diagnostic methods are neither specific nor sufficiently sensitive to diagnose preclinical DOX-related cardiomyopathy [72]. The more precise diagnostic method, endomyocardial biopsy, is invasive and costly and thus rarely used [74]. MiRNAs are drawing great attention as biomarkers of cardiac diseases, especially for early detection and for the possible prediction of DOX-induced myocardial injuries (Table 2). The initial studies on miRNAs as DOX-induced cardiotoxicity biomarkers were based on tissue miRNAs. A study conducted on different cumulative doses in DOX-treated mice showed that cardiac miR-34a was upregulated with cumulative doses of 6 mg/kg, and its expression level was positively related to DOX dose. This elevation of miR-34a occurred earlier than that of cardiac troponin T (cTnT), which was upregulated at a cumulative dose of 18 mg/kg [75]. In addition, miR-150 was markedly decreased at a dose of 12 mg/kg and was decreased further at higher doses, which might suggest sensitivity to DOX cardiotoxicity [75]. These findings provide guidance for developing miRNAs as biomarkers to detect DOX-induced cardiotoxicity prior to tissue damage. However, human tissue testing requires invasive procedures that make it impractical to use in clinical settings.
Table 2.
MiRNAs as potential biomarkers and their clinical significance.
| MiRNA | Heart tissue | Circulating blood | Clinical significance |
|---|---|---|---|
| miR-34a-5p | Upregulated in mice | Not tested |
✧ Superior to cTnT ✧ Positively related to DOX dose |
| miR-150-5p | Downregulated in mice | Not tested | |
| miR-133a-3p/miR-133b-3p | Not tested | Upregulated in mice & patients |
✧ Possible due to skeletal muscle injury ✧ No difference between cardiotoxic and non-cardiotoxic patients |
| miR-1-3p | Not tested | Upregulated in patients downregulated in mice |
✧ Superior to cTnI ✧ Correlated with LVEF changes |
| miR-29b-3p | Downregulated in mice | Upregulated in patients | ✧ Higher in patients with acute elevated cTnT |
| miR-29c-3p | Not tested | Upregulated in patients | ✧ Initiation of anthracycline |
| miR-499-5p | Downregulated in mice | Upregulated in patients downregulated in mice |
✧ Higher in patients with acute elevated cTnT ✧ Positively correlated to decline in LVEF from initiation to completion of anthracycline |
| miR-208a-3p | Downregulated in mice | No changes in patients | |
| miR-208b-3p | Upregulated in mice | No changes in patients |
Subsequently, researchers have shifted their focus to circulating miRNAs, as several advantages make microRNAs suitable biomarkers: high stability, presence in almost all body fluids (blood, urine, saliva, serum, etc.), tissue-specific expression, and advanced measuring techniques [76, 77]. In the extracellular space and body fluids, miRNAs are resistant to degradation because they are formed and transported in complexes with protein Argonaute 2 (AGO2) or high density lipoproteins (HDLs) [78, 79]. On the other hand, circulating miRNAs are packed into extracellular vesicles (EVs), predominantly exosomes [80]. These major extracellular carriers make miRNAs highly stable and readily detected in blood samples. In a rat model, the elevated serum miRNA level post-acute myocardial infarction (AMI) was downregulated in heart tissue, indicating that miRNAs were released into the bloodstream during cardiac injury [81]. In view of the variety of myocardial cells that may be affected during heart injury, a recent study revealed that the expression of plasma miRNAs is not cell-specific, but cardiomyocytes remain the main source of cells during the response to injury and stress [82]. Hence, plasma miRNAs as biomarkers for DOX-induced cardiotoxicity may be a promising research area.
Circulating miR-133a (heart- and skeletal muscle-specific) is increased earlier than cTnT in myocardial infarction patients [83]. In DOX-treated rats, both plasma miR-133a and miR-133b were also upregulated 24 h after a single dose was administered, which makes them possible biomarkers for DOX-induced cardiomyopathy [84]. However, this increase was possibly due to skeletal muscle injury, as indicated by heart-specific miR-208 levels remaining unchanged under the same conditions [85]. In 2016, 56 female breast cancer patients were enrolled in a clinical study, and the results demonstrated an increase in circulating miR-133b, but no obvious differences were observed for patients with induced cardiotoxicity and those without induced cardiotoxicity, which suggests that miR-133 is not a sensitive diagnostic marker [86].
Circulating miR-1 levels consistently increase in DOX-treated breast cancer patients with induced cardiotoxicity beginning during the second cycle of chemotherapy. In addition, this upregulation correlated with left ventricular ejection fraction (LVEF) changes, and the receiver operating characteristic (ROC) curve revealed that circulating miR-1 was more reliable than cardiac troponin I (cTnI) in identifying cardiotoxicity in terms of its sensitivity and specificity [86]. With results consistent to these outcomes, Leger et al. demonstrated that plasma miR-1 levels were elevated as early as 6 h post-anthracycline administration in children and young adult patients [87]. These findings make miR-1 a potential sensitive biomarker in the detection of DOX-induced cardiotoxicity. However, a recent study was conducted on mice treated with intraperitoneal injections of DOX (4 mg/kg) 3 times per week for 2 weeks (a total of 24 mg/kg) to screen differentially expressed circulating miRNAs in DOX-induced cardiac impaired mice with respect to those in mice unaffected by DOX. Surprisingly, the plasma miR-1-3p level was significantly decreased in the DOX-treated mice with measurable heart impairment [73]. Hence, more studies should consider these contradictory results to develop precise biomarkers.
In children and young adult patients receiving anthracyclines, plasma miR-29b and miR-499 are both increased. Moreover, patients with acute cardiac injury had higher plasma miR-29b and miR-499 levels compared with those with chronically elevated or normal cTnT levels, which suggests that the increases in miR-29b and miR-499 may be indicators of anthracycline-induced acute cardiac injury [87]. However, this study did not provide an assessment of LVEF. Another study conducted on pediatric patients receiving anthracycline chemotherapy revealed that serum miR-29c-3p and miR-499a-5p were upregulated during the initiation and completion of an anthracycline course, respectively. Additionally, the change in miR-499a-5p levels from initiation to completion of the anthracycline regimen was positively correlated with a decline in LVEF [88]. These findings may suggest that miR-499a-5p can serve as a biomarker of LVEF decline in pediatric patients receiving anthracycline. Interestingly, in mice treated with a total DOX dose of 24 mg/kg, similar to miR-1, the plasma miR-499-5p level was found to be decreased in DOX-induced cardiac impaired mice [73]. Additionally, as described above in this review, both miR-29b and miR-499-5p expression in cardiac tissue is downregulated in DOX-treated mouse models [25, 27].
MiR-208a and miR-208b show different expression trends in DOX-treated mice that are similar to those of the host transcripts (Myh6 and Myh7, respectively) [89]. However, neither miR-208a nor miR-208b was detectable in an experiment with breast cancer patients receiving DOX treatment [86]. Taken together, these data suggest that the potential for using miRNAs as biomarkers showed contrasting results in different studies, especially between species. The precise reasons for the differential expression of these circulating miRNAs are unclear. Species heterogeneity, animal model representativeness, and patients’ basic medical condition may partially explain the discrepancies. Whether different signals are triggered following a few doses of DOX over 2 weeks in a mouse model versus cumulative doses over months in patients requires further study. It is also uncertain whether the time frames evaluated in most animal studies are comparable to those evaluated in clinical studies. Hence, there is a clear need for increased consistency in the research model used. Moreover, miRNA (e.g., miR-133b mentioned previously) perturbation was also observed during DOX-induced cardiac impairment and nonimpairment, suggesting that miRNA regulatory roles in different phases of DOX cardiotoxicity still need to be explored. Additionally, whether patients’ other medical conditions interfere with the mechanisms of DOX cardiotoxicity, resulting in inaccurate findings, also needs further investigation. Patients’ preexisting medical conditions may contribute to the alteration of miRNA expression. For example, serum miR-29a is significantly upregulated in type 2 diabetes mellitus; miR-29b positively correlates with nonalcoholic fatty liver disease in a Chinese population; miR-29a, miR-29b, and miR-29c show higher expression levels in hypertensive patients compared with healthy control individuals [90–92]. In these cases, an elevation of miR-29 may not reflect the effectiveness of DOX in the patients receiving DOX treatment. All these findings affect the validity and reliability of the studies on miRNAs as biomarkers.
In addition, the current definition of cancer treatment-associated cardiovascular toxicity is characterized by LVEF reduction of more than 10% and less than 50% based on echocardiography, while changes in plasma troponins can be used only as adjunctive measurements to identify patients at risk for long-term cardiotoxicity [93]. Thus, studies comparing the sensitivity of miRNAs to troponins can address only the early detection of subclinical cardiac diseases. Further studies should focus more on the clinical relevance of using miRNAs as biomarkers and the extent of LVEF decline. Notably, studies have revealed that chronic progressive cardiac dysfunction is still observed in pediatric patient survivors years after anthracycline treatment, and in the one year after anthracycline treatment, 17% of pediatric patients still showed declined LVEF [94, 95]. Thus, exploring the specific and sensitive biomarkers for DOX-induced myocardial injury in this population needs more attention.
MiRNAs as therapeutic targets
Although some plasma miRNAs are not consistently expressed, others are stable and are considered therapeutic targets for DOX-induced cardiotoxicity because they have normalized dysregulated miRNAs in mouse models. MiRNA agomirs and antagomirs have been used in animal studies to achieve short-term gain- or loss-of-function [96]. The antagomir was injected into the experimental mice to therapeutically silence miR-208a, which resulted in cardiac function improvements following DOX treatment [43]. Rats that were pretreated with miR-29b agomir displayed improved cardiac functions and lower mortality post-DOX treatment [27]. Treatment with miR-140-5p antagomir prior to DOX treatment markedly alleviated the abnormal histopathology of the myocardium and aberrant ECG induced by DOX [10]. Regarding the study of delivering miRNA to humans, miravirsen is a leading nucleic acid-modified DNA phosphorothioate antisense oligonucleotide that inhibits miR-122 for the treatment of hepatitis C that has completed a multicenter phase 2a trial [97]. However, there are restrictions to using antisense oligonucleotides. For example, the targeted miRNAs must be exquisitely designed for tissue specificity, as ubiquitously expressed miRNAs can be internalized by several organs (mainly the liver and kidney) upon systemic administration; however, studies insufficiently measure the effects on nontargeted tissues, ignoring off-target effects [97, 98]. In addition, high doses of synthetic oligonucleotides are required for systemic administration, which may elicit an immune response that could compromise safety [99].
Adeno-associated virus (AAV) vectors enhanced miR-499-5p expression in mouse hearts and attenuated DOX cardiotoxicity [25]. AAV-mediated miR-212/132 administration improved cardiac function and prevented cardiac apoptosis and atrophy in a DOX-induced cardiotoxic mouse model [42]. A combination of AAV vectors with miRNA decoys (tough decoys) achieved long-term miR-320a inhibition in mice and attenuated cardiac dysfunction [48]. However, AAV can be possibly transduced in other organs, and prolonged expression of miRNAs may last a lifetime, which may cause harm [100]. These concerns about AAVs limit their applications. A new method for delivering miRNA mimics through direct intracardiac injection was proposed for use in myocardial infarcted mice, and this transient miRNA delivery was sufficient to drive a significant cardiomyocyte regenerative response [100]. Recently, CRISPR/Cas9 technology was used to edit miRNAs and was shown to be efficient and stable in the control of cross-organelle off-target effects [101]. New delivery methods are emerging, including negatively charged calcium phosphate nanoparticles, localized injection of miRNA-enriched extracellular vesicles, and ultrasound-based and microbubble-targeted delivery [102–104]. Overall, miRNA-based therapeutics for cardiovascular disorders are still in the preclinical phase. Hopefully, exploring tissue/cell-specific delivery techniques will provide more efficient and harmless methods for use in miRNA therapeutic interventions.
Future perspectives and conclusions
Cancer therapies have continuously improved malignant patient outcomes and prognosis. However, DOX-induced irreversible cardiomyopathy is still a main concern and obstacle for clinical applications, which have gained increasing awareness. Despite years of investigations, the exact mechanisms of DOX-induced cytotoxicity are not fully elucidated. Moreover, advances in treatments have increased the number of survivors with late-occurring, treatment-related cardiovascular complications, especially children or young patients, making it extremely difficult to detect and prevent. However, the majority of the current in vitro studies provide only short-term stimuli and investigate these acute changes, which might involve different mechanisms and signaling pathways than are involved in people who received DOX years ago. In addition, whether cardiomyocytes can represent all patient age groups is an issue to be considered. These issues may be the reasons that several potential biomarker miRNAs show different expression trends in mice and humans. In addition, in vivo studies lack definitive methods for detecting the early signs of cardiomyopathy in addition to the formation of tissue damage. Another concern is that the tumor itself may cause diverse expression of ncRNAs involved in the regulation of DOX cardiotoxicity. For instance, a study to identify the circulating miRNA signatures in breast cancer patients (without indicating their treatment status) showed that miR-1, miR-92a, miR-133a and miR-133b were upregulated in patient serum [105]. However, miR-1 and miR-133b showed the same trend in study of breast cancer patients who received DOX treatment introduced above. Therefore, although plasma miRNAs are easily accessible, measurable and possibly sensitive, thus showing great potential as diagnostic biomarkers, more specific studies are needed in the future. The protection of the heart without reducing the effect of DOX on tumors while exploring the use of ncRNAs as therapeutic targets is another problem to be solved. In addition, other anticancer agents have also been reported to cause cardiac events and dysregulation of ncRNAs, especially emerging targeted therapies and immune checkpoint inhibitors [106–109]. However, research in this field is still in its infancy, and further research is needed to distinguish the dysregulated ncRNAs caused by specific drugs. Finally, effective methods for therapeutically manipulating dysregulated miRNAs to reverse or prevent DOX-induced cardiotoxicity need to be explored.
Among the three types of ncRNAs, miRNAs are the most extensively studied, while the understanding the roles of lncRNAs and circRNAs in DOX-induced cardiotoxicity is still at an initial stage. However, mounting evidence suggests that both lncRNAs and circRNAs are associated with many cardiovascular diseases, such as the lncRNA HOX transcript antisense RNA (HOTAIR) and the circRNA transcribed from the sodium/calcium exchanger 1 (ncx1) gene (circNCX1), both of which play regulatory roles in myocardial infarction [110, 111], and the lncRNA taurine upregulated gene 1 (TUG1) and the heart-related circRNA (HRCR), which participate in the regulation of cardiac hypertrophy [69, 112]. Hence, the above mentioned ncRNAs possibly play functional roles in DOX cardiotoxicity, which requires further study. Increasing research suggests that lncRNAs and circRNAs are promising diagnostic/therapeutic targets because they are mostly tissue-specific and stably expressed. The LncRNA cardiac hypertrophy-associated transcript (CHAST), myocardial infarction-associated circular RNA (MICRA) and hsa_circ_0124644 have also been tested for their potential roles as biomarkers for cardiovascular conditions [113–115]. These studies indicated that certain lncRNAs and circRNAs can be employed as biomarkers for heart diseases. Nevertheless, to determine whether they show the identical indications or can be identified as diagnostic tools in DOX-induced cardiotoxicity, further investigation is needed. In summary, future studies on ncRNAs in early-stage detection and preservation of cardiac structure and function upon DOX treatment while maintaining their roles in tumor cells will broaden the prospects for the clinical utilization of DOX.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (Grant no. JQ201815), National Natural Science Foundation of China (Grant no. 81770232), and a grant from Fu Wai Hospital (No. 2019kf-03).
Competing interest
The authors declare no competing interests.
References
- 1.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–25. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–14. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 3.Davies KJ, Doroshow JH. Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by nadh dehydrogenase. J Biol Chem. 1986;261:3060–7. [PubMed] [Google Scholar]
- 4.Green PS, Leeuwenburgh C. Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochim Biophys Acta. 2002;1588:94–101. doi: 10.1016/s0925-4439(02)00144-8. [DOI] [PubMed] [Google Scholar]
- 5.Arai M, Tomaru K, Takizawa T, Sekiguchi K, Yokoyama T, Suzuki T, et al. Sarcoplasmic reticulum genes are selectively down-regulated in cardiomyopathy produced by doxorubicin in rabbits. J Mol Cell Cardiol. 1998;30:243–54. doi: 10.1006/jmcc.1997.0588. [DOI] [PubMed] [Google Scholar]
- 6.Arai M, Yoguchi A, Takizawa T, Yokoyama T, Kanda T, Kurabayashi M, et al. Mechanism of doxorubicin-induced inhibition of sarcoplasmic reticulum Ca2+-ATPase gene transcription. Circ Res. 2000;86:8–14. doi: 10.1161/01.res.86.1.8. [DOI] [PubMed] [Google Scholar]
- 7.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–42. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 8.Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Wang JX, Zhang XJ, Feng C, Sun T, Wang K, Wang Y, et al. Microrna-532-3p regulates mitochondrial fission through targeting apoptosis repressor with caspase recruitment domain in doxorubicin cardiotoxicity. Cell Death Dis. 2015;6:e1677. doi: 10.1038/cddis.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L, Qi Y, Xu L, Tao X, Han X, Yin L, et al. Microrna-140-5p aggravates doxorubicin-induced cardiotoxicity by promoting myocardial oxidative stress via targeting nrf2 and sirt2. Redox Biol. 2018;15:284–96. doi: 10.1016/j.redox.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ambros V. Micrornas: tiny regulators with great potential. Cell. 2001;107:823–6. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 12.Hutvagner G, Zamore PD. A microrna in a multiple-turnover rnai enzyme complex. Science. 2002;297:2056–60.. doi: 10.1126/science.1073827. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking mirna-1-2. Cell. 2007;129:303–17. doi: 10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 14.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of micrornas after myocardial infarction reveals a role of mir-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–32. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elia L, Contu R, Quintavalle M, Varrone F, Chimenti C, Russo MA, et al. Reciprocal regulation of microrna-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120:2377–85. doi: 10.1161/CIRCULATIONAHA.109.879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Zhang Q, Wei C, Zhao L, Guo X, Cui X, et al. Mir-378 modulates energy imbalance and apoptosis of mitochondria induced by doxorubicin. Am J Transl Res. 2018;10:3600–9. [PMC free article] [PubMed] [Google Scholar]
- 17.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio-Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Res. 2000;60:1789–92. [PubMed] [Google Scholar]
- 18.Delpy E, Hatem SN, Andrieu N, de Vaumas C, Henaff M, Rucker-Martin C, et al. Doxorubicin induces slow ceramide accumulation and late apoptosis in cultured adult rat ventricular myocytes. Cardiovasc Res. 1999;43:398–407. doi: 10.1016/s0008-6363(99)00142-x. [DOI] [PubMed] [Google Scholar]
- 19.Goormaghtigh E, Chatelain P, Caspers J, Ruysschaert JM. Evidence of a complex between adriamycin derivatives and cardiolipin: possible role in cardiotoxicity. Biochem Pharmacol. 1980;29:3003–10. doi: 10.1016/0006-2952(80)90050-7. [DOI] [PubMed] [Google Scholar]
- 20.Ashley N, Poulton J. Mitochondrial DNA is a direct target of anti-cancer anthracycline drugs. Biochem Biophys Res Commun. 2009;378:450–5. doi: 10.1016/j.bbrc.2008.11.059. [DOI] [PubMed] [Google Scholar]
- 21.Childs AC, Phaneuf SL, Dirks AJ, Phillips T, Leeuwenburgh C. Doxorubicin treatment in vivo causes cytochrome c release and cardiomyocyte apoptosis, as well as increased mitochondrial efficiency, superoxide dismutase activity, and bcl-2:Bax ratio. Cancer Res. 2002;62:4592–8. [PubMed] [Google Scholar]
- 22.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–22. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 23.Sardao VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB. Morphological alterations induced by doxorubicin on h9c2 myoblasts: Nuclear, mitochondrial, and cytoskeletal targets. Cell Biol Toxicol. 2009;25:227–43. doi: 10.1007/s10565-008-9070-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, et al. Mir-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–8. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 25.Wan Q, Xu T, Ding W, Zhang X, Ji X, Yu T, et al. Mir-499-5p attenuates mitochondrial fission and cell apoptosis via p21 in doxorubicin cardiotoxicity. Front Genet. 2018;9:734. doi: 10.3389/fgene.2018.00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horie T, Ono K, Nishi H, Nagao K, Kinoshita M, Watanabe S, et al. Acute doxorubicin cardiotoxicity is associated with mir-146a-induced inhibition of the neuregulin-erbb pathway. Cardiovasc Res. 2010;87:656–64. doi: 10.1093/cvr/cvq148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jing X, Yang J, Jiang L, Chen J, Wang H. Microrna-29b regulates the mitochondria-dependent apoptotic pathway by targeting bax in doxorubicin cardiotoxicity. Cell Physiol Biochem. 2018;48:692–704. doi: 10.1159/000491896. [DOI] [PubMed] [Google Scholar]
- 28.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin ii-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–96. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 29.Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009;296:H1466–83. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. Ii. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–74. [PubMed] [Google Scholar]
- 31.Lebrecht D, Setzer B, Ketelsen UP, Haberstroh J, Walker UA. Time-dependent and tissue-specific accumulation of mtdna and respiratory chain defects in chronic doxorubicin cardiomyopathy. Circulation. 2003;108:2423–9. doi: 10.1161/01.CIR.0000093196.59829.DF. [DOI] [PubMed] [Google Scholar]
- 32.Wallace KB, Sardao VA, Oliveira PJ. Mitochondrial determinants of doxorubicin-induced cardiomyopathy. Circ Res. 2020;126:926–41. doi: 10.1161/CIRCRESAHA.119.314681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wan GX, Cheng L, Qin HL, Zhang YZ, Wang LY, Zhang YG. Mir-15b-5p is involved in doxorubicin-induced cardiotoxicity via inhibiting bmpr1a signal in h9c2 cardiomyocyte. Cardiovasc Toxicol. 2019;19:264–75. doi: 10.1007/s12012-018-9495-6. [DOI] [PubMed] [Google Scholar]
- 34.Du J, Hang P, Pan Y, Feng B, Zheng Y, Chen T, et al. Inhibition of mir-23a attenuates doxorubicin-induced mitochondria-dependent cardiomyocyte apoptosis by targeting the pgc-1alpha/drp1 pathway. Toxicol Appl Pharmacol. 2019;369:73–81. doi: 10.1016/j.taap.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Roca-Alonso L, Castellano L, Mills A, Dabrowska AF, Sikkel MB, Pellegrino L, et al. Myocardial mir-30 downregulation triggered by doxorubicin drives alterations in beta-adrenergic signaling and enhances apoptosis. Cell Death Dis. 2015;6:e1754. doi: 10.1038/cddis.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regula KM, Ens K, Kirshenbaum LA. Inducible expression of bnip3 provokes mitochondrial defects and hypoxia-mediated cell death of ventricular myocytes. Circ Res. 2002;91:226–31. doi: 10.1161/01.res.0000029232.42227.16. [DOI] [PubMed] [Google Scholar]
- 37.Siveski-Iliskovic N, Kaul N, Singal PK. Probucol promotes endogenous antioxidants and provides protection against adriamycin-induced cardiomyopathy in rats. Circulation. 1994;89:2829–35. doi: 10.1161/01.cir.89.6.2829. [DOI] [PubMed] [Google Scholar]
- 38.Yen HC, Oberley TD, Vichitbandha S, Ho YS, St Clair DK. The protective role of manganese superoxide dismutase against adriamycin-induced acute cardiac toxicity in transgenic mice. J Clin Invest. 1996;98:1253–60. doi: 10.1172/JCI118909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Wan W, Chen T, Tong S, Jiang X, Liu W. Mir-451 silencing inhibited doxorubicin exposure-induced cardiotoxicity in mice. Biomed Res Int. 2019;2019:1528278. doi: 10.1155/2019/1528278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, et al. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene. 2008;27:285–99. doi: 10.1038/sj.onc.1210638. [DOI] [PubMed] [Google Scholar]
- 41.Dodd DA, Atkinson JB, Olson RD, Buck S, Cusack BJ, Fleischer S, et al. Doxorubicin cardiomyopathy is associated with a decrease in calcium release channel of the sarcoplasmic reticulum in a chronic rabbit model. J Clin Invest. 1993;91:1697–705. doi: 10.1172/JCI116379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta SK, Garg A, Avramopoulos P, Engelhardt S, Streckfuss-Bomeke K, Batkai S, et al. Mir-212/132 cluster modulation prevents doxorubicin-mediated atrophy and cardiotoxicity. Mol Ther. 2019;27:17–28. doi: 10.1016/j.ymthe.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tony H, Yu K, Qiutang Z. Microrna-208a silencing attenuates doxorubicin induced myocyte apoptosis and cardiac dysfunction. Oxid Med Cell Longev. 2015;2015:597032. doi: 10.1155/2015/597032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tong Z, Jiang B, Wu Y, Liu Y, Li Y, Gao M, et al. Mir-21 protected cardiomyocytes against doxorubicin-induced apoptosis by targeting btg2. Int J Mol Sci. 2015;16:14511–25. doi: 10.3390/ijms160714511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu JN, Fu YH, Hu ZQ, Li WY, Tang CM, Fei HW, et al. Activation of mir-34a-5p/sirt1/p66shc pathway contributes to doxorubicin-induced cardiotoxicity. Sci Rep. 2017;7:11879. doi: 10.1038/s41598-017-12192-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pakravan G, Foroughmand AM, Peymani M, Ghaedi K, Hashemi M-S, Hajjari M, et al. Downregulation of mir-130a, antagonized doxorubicin-induced cardiotoxicity via increasing the pparγ expression in mescs-derived cardiac cells. Cell Death Dis. 2018;9:758. doi: 10.1038/s41419-018-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu J, Peng C, Wang W, Jin H, Tang Q, Wei X. Let-7 g is involved in doxorubicin induced myocardial injury. Environ Toxicol Pharmacol. 2012;33:312–7. doi: 10.1016/j.etap.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Yin Z, Zhao Y, Li H, Yan M, Zhou L, Chen C, et al. Mir-320a mediates doxorubicin-induced cardiotoxicity by targeting vegf signal pathway. Aging (Albany NY) 2016;8:192–207. doi: 10.18632/aging.100876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luu AZ, Chowdhury B, Al-Omran M, Teoh H, Hess DA, Verma S. Role of endothelium in doxorubicin-induced cardiomyopathy. JACC Basic Transl Sci. 2018;3:861–70. doi: 10.1016/j.jacbts.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Octavia Y, Kararigas G, de Boer M, Chrifi I, Kietadisorn R, Swinnen M, et al. Folic acid reduces doxorubicin-induced cardiomyopathy by modulating endothelial nitric oxide synthase. J Cell Mol Med. 2017;21:3277–87. doi: 10.1111/jcmm.13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fernandez-Fernandez A, Carvajal DA, Lei T, McGoron AJ. Chemotherapy-induced changes in cardiac capillary permeability measured by fluorescent multiple indicator dilution. Ann Biomed Eng. 2014;42:2405–15. doi: 10.1007/s10439-014-1110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilkinson EL, Sidaway JE, Cross MJ. Cardiotoxic drugs herceptin and doxorubicin inhibit cardiac microvascular endothelial cell barrier formation resulting in increased drug permeability. Biol Open. 2016;5:1362–70. doi: 10.1242/bio.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Batista PJ, Chang HY. Long noncoding rnas: cellular address codes in development and disease. Cell. 2013;152:1298–307.. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mercer TR, Dinger ME, Mattick JS. Long non-coding rnas: insights into functions. Nat Rev Genet. 2009;10:155–9. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 55.Rinn JL, Kertesz M, Wang JK, Squazzo SL, Xu X, Brugmann SA, et al. Functional demarcation of active and silent chromatin domains in human hox loci by noncoding rnas. Cell. 2007;129:1311–23. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cesana M, Cacchiarelli D, Legnini I, Santini T, Sthandier O, Chinappi M, et al. A long noncoding rna controls muscle differentiation by functioning as a competing endogenous rna. Cell. 2011;147:358–69. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Han P, Li W, Lin CH, Yang J, Shang C, Nuernberg ST, et al. A long noncoding rna protects the heart from pathological hypertrophy. Nature. 2014;514:102–6. doi: 10.1038/nature13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thum T, Condorelli G. Long noncoding rnas and micrornas in cardiovascular pathophysiology. Circ Res. 2015;116:751–62. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 59.Xie Z, Xia W, Hou M. Long intergenic noncoding rnap21 mediates cardiac senescence via the wnt/betacatenin signaling pathway in doxorubicin-induced cardiotoxicity. Mol Med Rep. 2018;17:2695–704. doi: 10.3892/mmr.2017.8169. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Li L, Li X, Wu S. Long noncoding rna linc00339 aggravates doxorubicin-induced cardiomyocyte apoptosis by targeting mir-484. Biochem Biophys Res Commun. 2018;503:3038–43. doi: 10.1016/j.bbrc.2018.08.090. [DOI] [PubMed] [Google Scholar]
- 61.Chen L, Yan KP, Liu XC, Wang W, Li C, Li M, et al. Valsartan regulates tgf-beta/smads and tgf-beta/p38 pathways through lncrna chrf to improve doxorubicin-induced heart failure. Arch Pharmacol Res. 2018;41:101–9. doi: 10.1007/s12272-017-0980-4. [DOI] [PubMed] [Google Scholar]
- 62.Li HQ, Wu YB, Yin CS, Chen L, Zhang Q, Hu LQ. Obestatin attenuated doxorubicin-induced cardiomyopathy via enhancing long noncoding mhrt rna expression. Biomed Pharmacother. 2016;81:474–81. doi: 10.1016/j.biopha.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 63.Zhang S, Yuan Y, Zhang Z, Guo J, Li J, Zhao K, et al. Lncrna foxc2-as1 protects cardiomyocytes from doxorubicin-induced cardiotoxicity through activation of wnt1-inducible signaling pathway protein-1. Biosci Biotechnol Biochem. 2019;83:653–8. doi: 10.1080/09168451.2018.1553606. [DOI] [PubMed] [Google Scholar]
- 64.Nigro JM, Cho KR, Fearon ER, Kern SE, Ruppert JM, Oliner JD, et al. Scrambled exons. Cell. 1991;64:607–13. doi: 10.1016/0092-8674(91)90244-s. [DOI] [PubMed] [Google Scholar]
- 65.Chen CY, Sarnow P. Initiation of protein synthesis by the eukaryotic translational apparatus on circular rnas. Science. 1995;268:415–7. doi: 10.1126/science.7536344. [DOI] [PubMed] [Google Scholar]
- 66.Li M, Ding W, Sun T, Tariq MA, Xu T, Li P, et al. Biogenesis of circular rnas and their roles in cardiovascular development and pathology. FEBS J. 2018;285:220–32. doi: 10.1111/febs.14191. [DOI] [PubMed] [Google Scholar]
- 67.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural rna circles function as efficient microrna sponges. Nature. 2013;495:384–8. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 68.Gupta SK, Garg A, Bar C, Chatterjee S, Foinquinos A, Milting H, et al. Quaking inhibits doxorubicin-mediated cardiotoxicity through regulation of cardiac circular rna expression. Circ Res. 2018;122:246–54. doi: 10.1161/CIRCRESAHA.117.311335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, et al. A circular rna protects the heart from pathological hypertrophy and heart failure by targeting mir-223. Eur Heart J. 2016;37:2602–11. doi: 10.1093/eurheartj/ehv713. [DOI] [PubMed] [Google Scholar]
- 70.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, et al. The rna binding protein quaking regulates formation of circrnas. Cell. 2015;160:1125–34. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 71.Guo W, Jiang T, Lian C, Wang H, Zheng Q, Ma H. Qki deficiency promotes foxo1 mediated nitrosative stress and endoplasmic reticulum stress contributing to increased vulnerability to ischemic injury in diabetic heart. J Mol Cell Cardiol. 2014;75:131–40. doi: 10.1016/j.yjmcc.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 72.Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–31. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 73.Ruggeri C, Gioffre S, Chiesa M, Buzzetti M, Milano G, Scopece A, et al. A specific circulating microrna cluster is associated to late differential cardiac response to doxorubicin-induced cardiotoxicity in vivo. Dis Markers. 2018;2018:8395651. doi: 10.1155/2018/8395651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Torti FM, Bristow MM, Lum BL, Carter SK, Howes AE, Aston DA, et al. Cardiotoxicity of epirubicin and doxorubicin: assessment by endomyocardial biopsy. Cancer Res. 1986;46:3722–7. [PubMed] [Google Scholar]
- 75.Desai VG, J CK, Vijay V, Moland CL, Herman EH, Lee T, et al. Early biomarkers of doxorubicin-induced heart injury in a mouse model. Toxicol Appl Pharmacol. 2014;281:221–9. doi: 10.1016/j.taap.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microrna spectrum in 12 body fluids. Clin Chem. 2010;56:1733–41. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, et al. Circulating micrornas as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–8. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating micrornas independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–8. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. Micrornas are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–33. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mrnas and micrornas is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–9. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 81.Cheng Y, Tan N, Yang J, Liu X, Cao X, He P, et al. A translational study of circulating cell-free microrna-1 in acute myocardial infarction. Clin Sci (Lond) 2010;119:87–95. doi: 10.1042/CS20090645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Danielson KM, Shah R, Yeri A, Liu X, Camacho Garcia F, Silverman M, et al. Plasma circulating extracellular rnas in left ventricular remodeling post-myocardial infarction. EBioMedicine. 2018;32:172–81. doi: 10.1016/j.ebiom.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, et al. Increased microrna-1 and microrna-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet. 2011;4:446–54. doi: 10.1161/CIRCGENETICS.110.958975. [DOI] [PubMed] [Google Scholar]
- 84.Nishimura Y, Kondo C, Morikawa Y, Tonomura Y, Torii M, Yamate J, et al. Plasma mir-208 as a useful biomarker for drug-induced cardiotoxicity in rats. J Appl Toxicol. 2015;35:173–80. doi: 10.1002/jat.3044. [DOI] [PubMed] [Google Scholar]
- 85.Ruggeri C, Gioffre S, Achilli F, Colombo GI, D’Alessandra Y. Role of micrornas in doxorubicin-induced cardiotoxicity: An overview of preclinical models and cancer patients. Heart Fail Rev. 2018;23:109–22. doi: 10.1007/s10741-017-9653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rigaud VO, Ferreira LR, Ayub-Ferreira SM, Avila MS, Brandao SM, Cruz FD, et al. Circulating mir-1 as a potential biomarker of doxorubicin-induced cardiotoxicity in breast cancer patients. Oncotarget. 2017;8:6994–7002. doi: 10.18632/oncotarget.14355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leger KJ, Leonard D, Nielson D, de Lemos JA, Mammen PP, Winick NJ. Circulating micrornas: potential markers of cardiotoxicity in children and young adults treated with anthracycline chemotherapy. J Am Heart Assoc. 2017;6:e004653. doi: 10.1161/JAHA.116.004653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oatmen KE, Toro-Salazar OH, Hauser K, Zellars KN, Mason KC, Hor K, et al. Identification of a novel microrna profile in pediatric patients with cancer treated with anthracycline chemotherapy. Am J Physiol Heart Circ Physiol. 2018;315:H1443–52. doi: 10.1152/ajpheart.00252.2018. [DOI] [PubMed] [Google Scholar]
- 89.Vacchi-Suzzi C, Bauer Y, Berridge BR, Bongiovanni S, Gerrish K, Hamadeh HK, et al. Perturbation of micrornas in rat heart during chronic doxorubicin treatment. PLoS ONE. 2012;7:e40395. doi: 10.1371/journal.pone.0040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, et al. Significance of serum micrornas in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol. 2011;48:61–9. doi: 10.1007/s00592-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 91.He Z, Yang JJ, Zhang R, Li HT, Wu L, Jiang F, et al. Circulating mir-29b positively correlates with non-alcoholic fatty liver disease in a chinese population. J Dig Dis. 2019;20:189–95. doi: 10.1111/1751-2980.12716. [DOI] [PubMed] [Google Scholar]
- 92.Huang Y, Tang S, Huang C, Chen J, Li J, Cai A, et al. Circulating mirna29 family expression levels in patients with essential hypertension as potential markers for left ventricular hypertrophy. Clin Exp Hypertens. 2017;39:119–25. doi: 10.1080/10641963.2016.1226889. [DOI] [PubMed] [Google Scholar]
- 93.Zamorano JL, Lancellotti P, Rodriguez Munoz D, Aboyans V, Asteggiano R, Galderisi M, et al. 2016 esc position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the esc committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the european society of cardiology (esc) Eur Heart J. 2016;37:2768–801. doi: 10.1093/eurheartj/ehw211. [DOI] [PubMed] [Google Scholar]
- 94.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD, et al. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. doi: 10.1200/JCO.2005.12.121. [DOI] [PubMed] [Google Scholar]
- 95.Temming P, Qureshi A, Hardt J, Leiper AD, Levitt G, Ancliff PJ, et al. Prevalence and predictors of anthracycline cardiotoxicity in children treated for acute myeloid leukaemia: retrospective cohort study in a single centre in the united kingdom. Pediatr Blood Cancer. 2011;56:625–30. doi: 10.1002/pbc.22908. [DOI] [PubMed] [Google Scholar]
- 96.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of micrornas in vivo with ‘antagomirs’. Nature. 2005;438:685–9. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 97.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of hcv infection by targeting microrna. N. Engl J Med. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 98.van Rooij E, Olson EN. Microrna therapeutics for cardiovascular disease: opportunities and obstacles. Nat Rev Drug Disco. 2012;11:860–72. doi: 10.1038/nrd3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dalpke A, Helm M. Rna mediated toll-like receptor stimulation in health and disease. RNA Biol. 2012;9:828–42. doi: 10.4161/rna.20206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lesizza P, Prosdocimo G, Martinelli V, Sinagra G, Zacchigna S, Giacca M. Single-dose intracardiac injection of pro-regenerative micrornas improves cardiac function after myocardial infarction. Circ Res. 2017;120:1298–304. doi: 10.1161/CIRCRESAHA.116.309589. [DOI] [PubMed] [Google Scholar]
- 101.Chang H, Yi B, Ma R, Zhang X, Zhao H, Xi Y. CRISPR/cas9, a novel genomic tool to knock down microrna in vitro and in vivo. Sci Rep. 2016;6:22312. doi: 10.1038/srep22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Di Mauro V, Iafisco M, Salvarani N, Vacchiano M, Carullo P, Ramirez-Rodriguez GB, et al. Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of micrornas. Nanomed (Lond) 2016;11:891–906. doi: 10.2217/nnm.16.26. [DOI] [PubMed] [Google Scholar]
- 103.Kopechek JA, McTiernan CF, Chen X, Zhu J, Mburu M, Feroze R, et al. Ultrasound and microbubble-targeted delivery of a microrna inhibitor to the heart suppresses cardiac hypertrophy and preserves cardiac function. Theranostics. 2019;9:7088–98. doi: 10.7150/thno.34895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Song Y, Zhang C, Zhang J, Jiao Z, Dong N, Wang G, et al. Localized injection of mirna-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics. 2019;9:2346–60. doi: 10.7150/thno.29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chan M, Liaw CS, Ji SM, Tan HH, Wong CY, Thike AA, et al. Identification of circulating microrna signatures for breast cancer detection. Clin Cancer Res. 2013;19:4477–87. doi: 10.1158/1078-0432.CCR-12-3401. [DOI] [PubMed] [Google Scholar]
- 106.Fan J, Yin Z, Xu J, Wu F, Huang Q, Yang L, et al. Circulating micrornas predict the response to anti-pd-1 therapy in non-small cell lung cancer. Genomics. 2020;112:2063–71. doi: 10.1016/j.ygeno.2019.11.019. [DOI] [PubMed] [Google Scholar]
- 107.de Azambuja E, Ponde N, Procter M, Rastogi P, Cecchini RS, Lambertini M, et al. A pooled analysis of the cardiac events in the trastuzumab adjuvant trials. Breast Cancer Res Treat. 2020;179:161–71. doi: 10.1007/s10549-019-05453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhao Z, He J, Zhang J, Liu M, Yang S, Li N, et al. Dysregulated mir1254 and mir579 for cardiotoxicity in patients treated with bevacizumab in colorectal cancer. Tumour Biol. 2014;35:5227–35. doi: 10.1007/s13277-014-1679-5. [DOI] [PubMed] [Google Scholar]
- 109.Zhou F, Lu X, Zhang X. Serum mir-30c level predicted cardiotoxicity in non-small cell lung cancer patients treated with bevacizumab. Cardiovasc Toxicol. 2018;18:284–9. doi: 10.1007/s12012-018-9457-z. [DOI] [PubMed] [Google Scholar]
- 110.Li L, Zhang M, Chen W, Wang R, Ye Z, Wang Y, et al. Lncrna-hotair inhibition aggravates oxidative stress-induced h9c2 cells injury through suppression of mmp2 by mir-125. Acta Biochim Biophys Sin (Shanghai) 2018;50:996–1006. doi: 10.1093/abbs/gmy102. [DOI] [PubMed] [Google Scholar]
- 111.Li M, Ding W, Tariq MA, Chang W, Zhang X, Xu W, et al. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting mir-133a-3p. Theranostics. 2018;8:5855–69. doi: 10.7150/thno.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zou X, Wang J, Tang L, Wen Q. Lncrna tug1 contributes to cardiac hypertrophy via regulating mir-29b-3p. Vitr Cell Dev Biol Anim. 2019;55:482–90. doi: 10.1007/s11626-019-00368-x. [DOI] [PubMed] [Google Scholar]
- 113.Wang X, Wang L, Ma Z, Liang W, Li J, Li Y, et al. Early expressed circulating long noncoding rna chast is associated with cardiac contractile function in patients with acute myocardial infarction. Int J Cardiol. 2020;302:15–20. doi: 10.1016/j.ijcard.2019.12.058. [DOI] [PubMed] [Google Scholar]
- 114.Vausort M, Salgado-Somoza A, Zhang L, Leszek P, Scholz M, Teren A, et al. Myocardial infarction-associated circular rna predicting left ventricular dysfunction. J Am Coll Cardiol. 2016;68:1247–8. doi: 10.1016/j.jacc.2016.06.040. [DOI] [PubMed] [Google Scholar]
- 115.Zhao Z, Li X, Gao C, Jian D, Hao P, Rao L, et al. Peripheral blood circular rna hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci Rep. 2017;7:39918. doi: 10.1038/srep39918. [DOI] [PMC free article] [PubMed] [Google Scholar]