Abstract
Ventromedial hypothalamic nucleus (VMN) glycogen metabolism affects local glucoregulatory signaling. The hindbrain metabolic-sensitive catecholamine (CA) neurotransmitter norepinephrine controls VMN glycogen phosphorylase (GP)-muscle (GPmm) and -brain (GPbb) type expression in male rats. Present studies addressed the premise that CA regulation of hypoglycemic patterns of VMN glycogen metabolic enzyme protein expression is sex-dimorphic, and that this signal is responsible for sex differences in acclimation of these profiles to recurrent insulin-induced hypoglycemia (RIIH). VMN tissue was acquired by micropunch-dissection from male and female rats pretreated by caudal fourth ventricular administration of the CA neurotoxin 6-hydroxydopamine (6OHDA) before single or serial insulin injection. 6-OHDA averted acute hypoglycemic inhibition of VMN glycogen synthase (GS) and augmentation of GPmm and GPbb protein expression in males, and prevented GPmm and -bb down-regulation in females. Males recovered from antecedent hypoglycemia (AH) exhibited neurotoxin-preventable diminution of baseline GS profiles, whereas acclimated GPmm and -bb expression in females occurred irrespective of pretreatment. RIIH did not alter VMN GS, GPmm, and GPbb expression in vehicle- or 6-OHDA-pretreated animals of either sex. VMN glycogen content was correspondingly unchanged or increased in males versus females following AH; 6-OHDA augmented glycogen mass in AH-exposed animals of both sexes. RIIH did not alter VMN glycogen accumulation in vehicle-pretreated rats of either sex, but diminished glycogen in neurotoxin-pretreated animals. AH suppresses baseline GS (CA-dependent) or GPmm/GPbb (CA-independent) expression in male and female rats, respectively, which corresponds with unaltered or augmented VMN glycogen content in those sexes. AH-associated loss of sex-distinctive CA-mediated enzyme protein sensitivity to hypoglycemia (male: GS, GPmm, GPbb; female: GPmm, Gpbb) may reflect, in part, VMN target desensitization to noradrenergic input.
Keywords: Ventromedial hypothalamic nucleus, Recurrent insulin-induced hypoglycemia, 6-hydroxydopamine, Glycogen synthase, Glycogen phosphorylase, Sex differences
Introduction
Brain neuro-glial metabolic coupling involves cell-type compartmentation of glucose catabolism and astrocyte provision of the oxidizable glycolytic end-product L-lactate to support nerve cell aerobic respiration. Astrocytes maintain the multi-branched glucose polysaccharide glycogen as a principal energy fuel alternative to blood-derived glucose. The brain glycogen depot, despite limitations in total mass, is dynamic during normal brain activity and metabolic stability (Bélanger et al., 2011, Stobart and Anderson, 2013, DiNuzzo et al., 2019), and is an important source of lactate equivalents during states of heightened activity or hypoglycemia (Gruetter, 2003, Brown, 2004). Glycogen metabolism is controlled by opposing glycogen synthase (GS) and glycogen phosphorylase (GP) enzyme actions that correspondingly catalyze glycogen synthesis or breakdown. The ventromedial hypothalamic nucleus (VMN) processes sensory information on oxidizable fuels and peripheral (e.g. adipose tissue) energy stores to regulate glucose homeostasis. VMN glycogen mass or turnover status is a plausible monitored variable since pharmacological inhibition of glycogen breakdown stimulates local AMP-activated protein kinase (AMPK) energy sensor activity and respectively up- or down-regulates neuronal nitric oxide synthase (nNOS) and glutamate decarboxylate65/67 (GAD), which are respective marker proteins for VMN gluco-stimulatory (nitric oxide; NO) and -inhibitory (γ-aminobutyric acid; GABA) neurotransmitters [6; Alshamrani and Briski, personal communication].
The catecholamine neurotransmitter norepinephrine (NE) links hindbrain dorsal vagal complex (DVC) metabolic-sensory A2 noradrenergic neurons with the VMN and other hypothalamic glucoregulatory structures. A2 neurons are a plausible source of sensory information on nerve cell energetic status as hypoglycemic intensification of AMPK activity in this cell group and augmentation of hypothalamic NE accumulation are both reversed by exogenous lactate delivery to the DVC (Shrestha et al., 2014). VMN astrocytes are a presumptive NE target as they express alpha1, alpha2, and beta1 adrenergic receptor (AR) proteins (Ibrahim et al., 2019). Distinctive GP-muscle- (GPmm) and brain- (GPbb) type variants are expressed in the brain, and differ regarding cell-type localization and regulation by phosphorylation versus AMP (Nadeau et al., 2018). Both variants are contained in astrocytes, whereas only GPbb occurs in neurons. Phosphorylation causes corresponding complete or partial activation of GPmm versus GPbb, while GPbb exhibits relatively greater affinity for and sensitivity to AMP activation and requires AMP binding for optimal enzyme function and Km. In vitro research points to GPmm and -bb involvement in stimulus-specific, i.e. NE versus energy deficit regulation of cortical astrocyte glycogen mobilization (Müller et al., 2015). In vivo studies utilizing hindbrain lactoprivation as a model for hindbrain metabolic deficit signal input to the male rat VMN document divergent, e.g. stimulatory versus inhibitory noradrenergic control of VMN GPmm and GPbb expression during energy homeostasis, as well as hindbrain lactoprivic suppression of the former VMN protein profile alongside up-regulation of the latter (Briski and Mandal, 2020). Inverse hindbrain lactoprivic regulation of VMN GPmm and GPbb expression is presumed to impel energy deficit- as opposed to neurotransmitter-mediated control of glycogen breakdown in that hypothalamic site as a reaction to this discrete metabolic stimulus.
Recurrent insulin-induced hypoglycemia (RIIH) can cause mal-adaptive attenuation of glucose counter-regulatory outflow, which, in turn, exacerbates hypoglycemia (Cryer, 2010; Cryer et al., 2003). In male rats, the VMN exhibits desensitization to this metabolic stress as RIIH correlates with diminished VMN neuronal nitric oxide synthase (nNOS) and glutamate decarboxylase65/67 (GAD) sensitivity to hypoglycemia alongside diminution of the tissue phosphoAMPK/AMPK ratio (Mandal et al., 2017). Glycogen supercompensation, which refers to adaptive augmentation of male rat brain glycogen accumulation after hypoglycemic exposure, is hypothesized to impair neural detection and/or signaling of subsequent neuro-glucopenic episodes (Choi et al., 2003). Elevated brain tissue glycogen reportedly occurs after hypoglycemia in distinct brain regions of the male rat brain, such as striatum and cerebellum (Duarte et al., 2017), but not others, e.g. hippocampus or hypothalamus (Herzog et al., 2008). The absence of acclimated enhancement of glycogen content in the latter region is interpreted as proof that supercompensation does not contribute to habituated glucose counter-regulation in that sex. The possibility that residual effects of prior, e.g. antecedent hypoglycemia (AH) on brain tissue glycogen content are sex-dimorphic has not been investigated. The aim of current research was to determine if hindbrain CA signaling imposes sex-specific control of VMN glycogen metabolism and tissue glycogen mass during acute hypoglycemia, and is responsible for potential sex-dimorphic habituation of these responses over the course of RIIH. VMN tissue samples were obtained by micropunch dissection from adult 6-hydroxydopamine (6-OHDA)-pretreated testes-intact male and estradiol-replaced ovariectomized female rats following subcutaneous injection of one or four doses of the intermediate-release insulin formulation neutral protamine Hagedorn, one dose per day, for Western blot and ELISA analyses of VMN glycogen metabolic enzyme protein expression. The likelihood that averaged glycogen values for whole hypothalamic tissue may obscure small, but significant alterations in post-AH of accumulation in distinct hypothalamic loci, including the VMN, cannot be overlooked. Here, uHPLC-electrospray ionization-mass spectrometric (LC-ESI-MS) methodology was used to quantify VMN glycogen content in male and female rats exposed to single versus serial bouts of hypoglycemia.
Materials and methods
Experimental design
Adult Sprague Dawley rats were housed 2–3 per cage, by sex, under a 14 hr light:10 hr dark cycle (lights on at 05.00 h). Animals had ad-libitum access to standard laboratory rat chow and water, and were handled daily to facilitate familiarization with investigators. Experimental protocols were carried out in compliance with NIH guidelines for care and use of laboratory animals, under ULM Institutional Animal Care and Use Committee approval. Twelve days before the study, animals were anesthetized with ketamine/xylazine (0.1 mL/100 g bw; 90 mg ketamine:10 mg xylazine/mL; Henry Schein Inc., Melville, NY), and implanted with a PE-20 cannula aimed at the caudal fourth ventricle (CV4) (Singh and Briski, 2004); females were also bilaterally ovariectomized (OVX) while anesthetized. After surgery, rats were injected subcutaneously (sc) with ketoprofen (1 mg/kg bw) and intramuscularly with enrofloxacin (10 mg/0.1 mL), treated by topical application of 0.25% bupivacaine to closed incisions, then transferred to individual cages. Plasma estradiol levels were standardized by exogenous hormone replacement to OVX female subjects in order to avoid potential variability owing differential endogenous steroid release at individual stages of the estrous cycle. Five days prior to experimentation, female rats were implanted under isoflurane anesthesia (5% - induction; 2.5% - maintenance) with a sc silastic capsule (i.d. 0.062 in./o.d. 0.125 in.; 10 mm/100 g bw) filled with 30 ug 17 β estradiol-3-benzoate/mL safflower oil. This hormone replacement regimen yields approximate plasma estradiol concentrations of 22 pg/ml (Briski et al., 2001), which replicate circulating hormone levels characteristic of metestrus in 4-day cycling animals (Butcher et al., 1974). Animals of each sex were randomly assigned to treatment groups (Fig. 1). On study days 1 and 2, rats of each sex were injected into the CV4 with 6-OHDA (75 μg/1.0 μL/day; n = 16 males, n = 16 females; (Selvage et al., 2004)) or vehicle (V; sterile apyrogenic water containing 0.2% ascorbic acid ; n = 16 males, n = 16 females), as described (Gujar et al., 2014, Tamrakar et al., 2015, Alhamami et al., 2018). This treatment paradigm causes significant loss of DVC CA (but not neuropeptide) neurons (Selvage et al., 2004). At 9.00 hr (time zero, to) on days 3–6, groups of 6-OHDA- and V-pretreated animals were injected sc with neutral protamine Hagedorn insulin (I; 10.0 U/kg bw; Henry Schein, Inc.) or the vehicle sterile diluent (V), as summarized in Fig. 1: 1) VVVV groups: sc V injection on days 3–6; n = 4 6-OHDA- and n = 4 V-pretreated males; n = 4 6-OHDA- and n = 4 V-pretreated females; 2) VVVI groups: sc V injection on days 3–5, followed by sc I injection on day 6; n = 4 6-OHDA- and n = 4 V-pretreated males; n = 4 6-OHDA- and n = 4 V-pretreated females; 3) IIIV groups: sc I injection on days 3–5, followed by sc V injection on day 6; n = 4 6-OHDA- and n = 4 V-pretreated males; n = 4 6-OHDA- and n = 4 V-pretreated females; 4) IIII groups: sc I injection on days 3–6; n = 4 6-OHDA- and n = 4 V-pretreated males; n = 4 6-OHDA- and n = 4 V-pretreated females. On day 17, animals were sacrificed one hour after injections (10.00 hr) by microwave fixation (1.45 sec exposure; In Vivo Microwave Fixation System, 5kW; Stoelting Co., Wood Dale, IL).
Fig. 1.
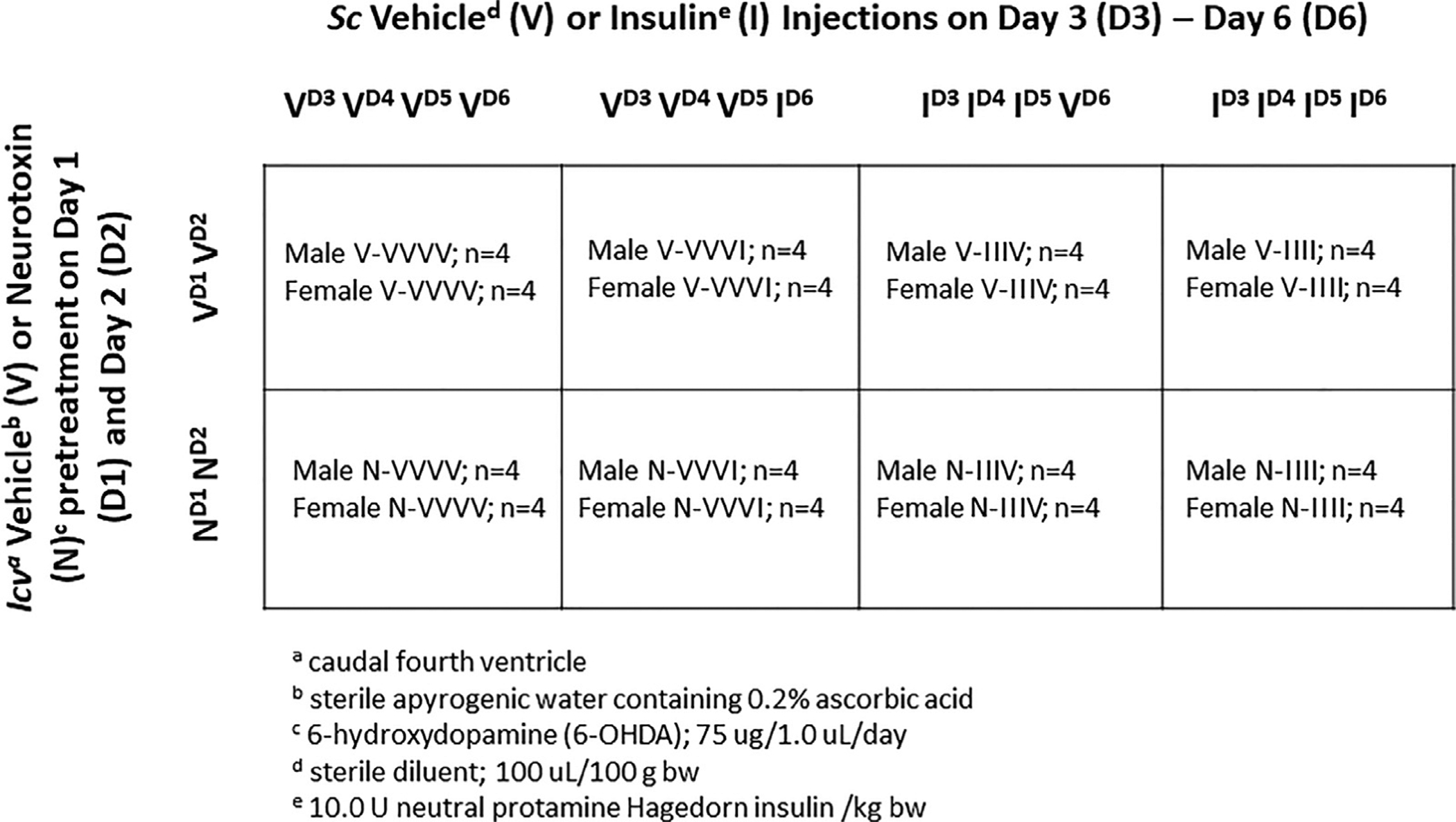
Experimental Design. Rats of each sex were randomly assigned to groups that were pretreated by neurotoxin (N) versus vehicle (V) injection into the caudal fourth ventricle on study days 1 and 2. On days 3–6, groups were injected subcutaneously with either insulin (I) or vehicle (V) to expose animals to single or serial hypoglycemia.
Western blot analyis of VMN micropunch tissue
Consecutive frozen 100 μm-thick sections were cut through the VMN of each animal. Bilateral micropunch samples were obtained, using calibrated Brain Punch tools (prod. no. 57401; Stoelting, Wood Dale, IL), from sections collected between −1.80 to −2.20 mm and −2.70 to −3.10 mm posterior to bregma and pooled in lysis buffer [2.0% sodium dodecyl sulfate, 0.05 M dithiothreitol, 10.0% glycerol, 1.0 mM EDTA, 60.0 mM Tris-HCl, pH 7.2] for heat-denaturation [95°C]. For each treatment group, tissue aliquots from individual subjects were combined to create separate sample pools for analysis of each protein of interest, prior to separation in BioRad TGX 10% Stain-Free gels (prod. no. 1610183, Bio-Rad Laboratories Inc., Hercules, CA) (Ibrahim et al., 2019). Each protein as analyzed in triplicate at minimum. After gel activation (UV light; 1 min) in a Bio-Rad ChemiDoc TM Touch Imaging System, proteins were transblotted overnight (4–5°C) to 0.45-μm PVDF-Plus membranes (prod. no. PV4HY00010; ThermoFisherScientific, Waltham, MA)
After blocking (2 hr) with Tris-buffered saline, pH 7.4, containing 0.1 % Tween-20 and 2% bovine serum albumin, membranes were incubated (24–48 hr; 4–5°C) with rabbit primary antisera against GS (1:2,000; prod. no. 3893S; Cell Signaling Technology, Danvers, MA), GPmm (1:2,000; prod. no. NBP2–16689; Novus Biologicals, LLC; Centennial, CO), or GPbb (1:2,000; prod. no. NBP1–32799; Novus Biol.). Membranes were exposed to horseradish peroxidase-labeled goat anti-rabbit secondary antibodies (prod. no. NEF812001EA, 1:5,000; PerkinElmer, Waltham, MA; 1 hr) prior to SuperSignal West Femto maximum sensitivity chemiluminescent substrate (prod. no. 34096; ThermoFisherSci.). Membrane buffer washes and antibody incubations were performed by Freedom Rocker™ Blotbot® automation (Next Advance, Inc., Troy NY). Chemiluminescence band optical density (O.D.) values measured in the ChemiDoc MP system were normalized to total in-lane protein using Imagelab software (Image Lab™ 6.0.0; Bio-Rad). Precision plus protein molecular weight dual color standards (prod. no. 161-0374; Bio-Rad) were included in each Western blot analysis.
LC-ESI-MS analysis of VMN glycogen tissue content
For each animal, micropunched VMN tissue was pooled, heat-denatured, homogenized by ultra-sonification, stored at −80°C, and analyzed for glycogen concentration, as described (Bheemanapally et al., 2020). Supernatant aliquots were hydrolyzed by incubation with amyloglucosidase and 0.1 M sodium acetate, pH 6.0. Hydrolyzed and non-hydrolyzed samples were derivatized with 1-phenyl-3-methyl-5-pyrazolone (PMP) reagent and NaOH, then acidified, extracted with chloroform, vacuum-concentrated, and lyophilized. Lyophilized samples were diluted with 10 mM ammonium acetate, vortexed (30s), and centrifuged. Supernatant aliquots were transferred to 350 μL inserts, which were placed into 2 mL Surestop vials in an autosampler tray. D-(+)-Glucose-PMP derivative was resolved through a chromatographic column (Shodex™ Asahipak™ NH2P-40 3E) with mobile phase (75:25 v/v) acetonitrile:10 mM ammonium acetate (0.2 mL/min) in a ThermoFisherScientific Vanquish UHPLC+ System equipped with Thermo Scientific™ Dionex™ Chromeleon™ 7 Chromatography Data System software and coupled to an ISO ISQ EC mass spectrometer (ThermoFisherScientific). Analysis of D-(+)-Glucose-PMP was performed in negative ionization mode. D-(+)-Glucose-PMP ion chromatograms were extracted from Total Ion Current (TIC) at m/z 510.2 to generate area-under-the-curve data. Parameters for sheath gas pressure (25 psig), auxiliary gas pressure (4.6 psig), sweep gas pressure (0.5 psig), vaporizer temperature (150°C), ion transfer tube temperature (150°C), source voltage (−2000V), foreline pressure (1.76 Torr; auto-set by instrument- and variable), nitrogen source gas, and mass peak area detection algorithm (ICIS/Genesis) were maintained at optimum.
Plasma glucose analyses
Circulating glucose levels was measured with an ACCU-CHECK Aviva Plus glucometer (Roche Diagnostics USA, Indianapolis, IN), as described [Kale et al., 2006]
Statistical analyses
Mean normalized tissue Western blot protein O.D., tissue glycogen, and plasma glucose measures were evaluated among treatment groups within each sex by three-way analysis of variance and Student-Newman-Keuls post-hoc test. Differences of p<0.05 were considered significant. In each figure, statistical differences between specific pairs of treatment groups are denoted with the following symbols: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Results
Fig. 2 illustrates effects of intra-CV4 6-OHDA pretreatment on VMN GS protein expression during single versus serial induction of insulin-induced hypoglycemia (IIH) in male [Panel A; F(7,16): 11.5296; p<0.0001] versus female [Panel B; F(7,16): 4.96; p = 0.004] rats. Data show that neurotoxin administration correspondingly suppressed or elevated VMN GS expression in male and female animals (Table 1 ; N/VVVV versus V/VVVV). Acute IIH reduced GS profiles in male (V/VVVI versus V/VVVV), but not female rats; this sex-specific inhibitory response was averted by 6-OHDA (N/VVVI versus N/VVVV). Animals recovered from prior exposure to hypoglycemia on days 3–5 exhibited on day 6 either diminished (males) or no change (females) in baseline VMN GS content (V/IIIV versus V/VVVV). Adaptive decrements in GS expression following AH were not observed in neurotoxin-pretreated male rats (N/IIIV versus N/VVVV). IIII groups of male and female animals showed no difference in VMN GS levels compared to basal V/IIIV values.
Fig. 2.
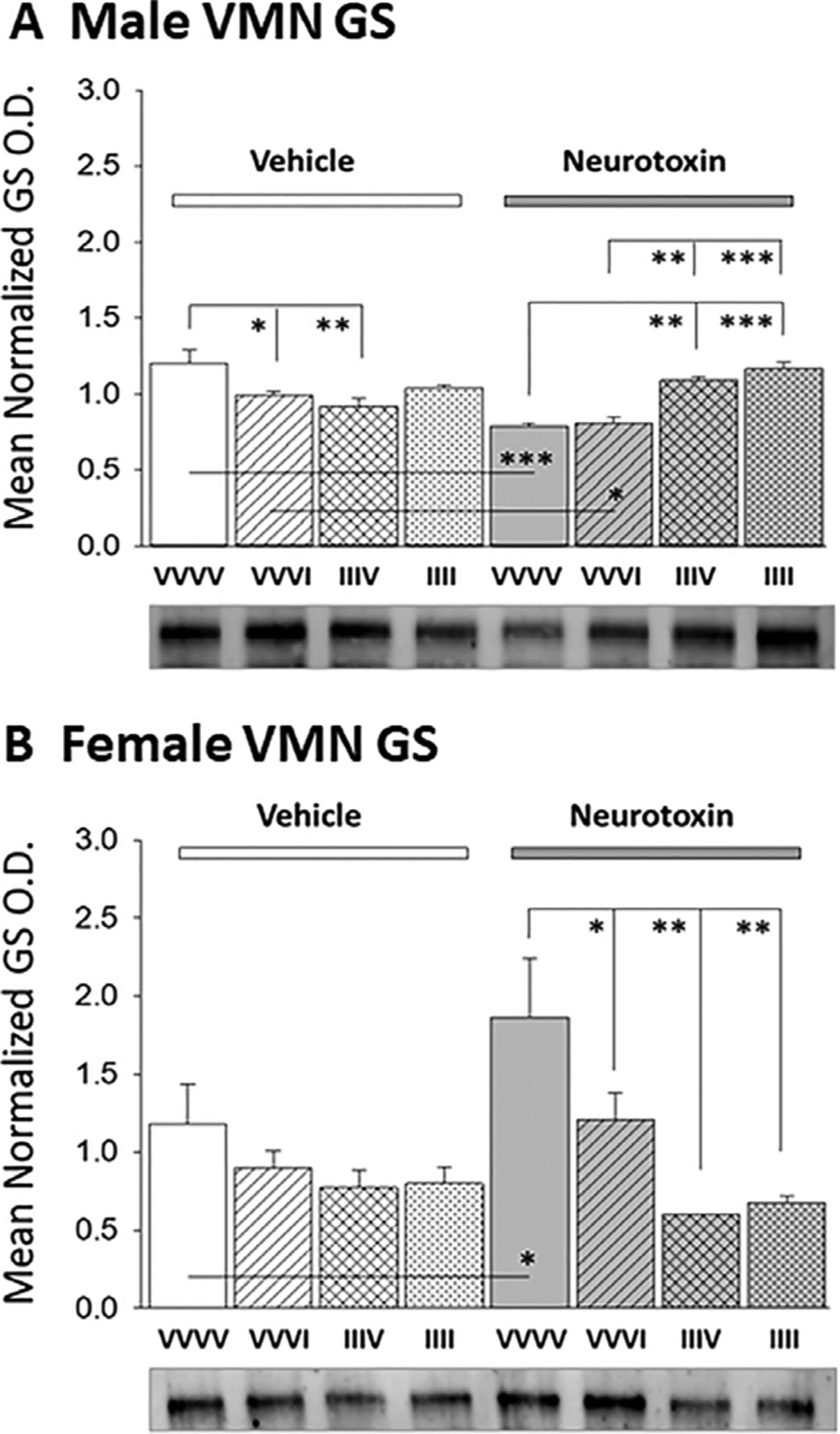
Effects of Caudal Fourth Ventricular (CV4) Administration of the Catecholamine Neurotoxin 6-Hydroxydopamine (6-OHDA) on Ventromedial Hypothalamic Nucleus (VMN) Glycogen Synthase (GS) Protein Expression during Single versus Serial Exposure to Insulin-Induced Hypoglycemia (IIH) in Male versus Female Rats. Groups of testes-intact and ovariectomized, estradiol-implanted female rats were pretreated by intra-CV4 administration on study days 1 and 2 of 6-OHDA (N) or vehicle (V; sterile apyrogenic water) prior to subcutaneous (sc) insulin (I; 10.0 U/kg bw) injection on one or four consecutive days (study days 3–6) according to the following treatment paradigm: sc vehicle (V; sterile diluent) injection on 3–6 (VVVV); sc V injection on study days 3–5, I treatment on day 6 (VVVI); sc I injection on days 3–5, V treatment on day 6 (IIIV); sc I injection on study days 3–6 (IIII). Data depict mean normalized GS protein optical density (O.D.) values + S.E.M. for micropunch-dissected VMN tissue acquired from groups of VVVV, VVVI, IIIV, and IIII male (Panel A) or female (Panel B) rats. In each Panel, groups given V or 6-OHDA pretreatment are depicted at left versus right, respectively. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Table 1.
Summary Ventromedial Hypothalamic Nucleus Glycogen Metabolic Enzyme Protein Responses to Acute versus Recurring Insulin-Induced Hypoglycemia in Male and Female Rats: Impact of Hindbrain Catecholamine Lesioning.
| MALE | FEMALE | |||||||
|---|---|---|---|---|---|---|---|---|
| VVVVa (Ne vs Vf) | VVVIb (N vs V) | IIIVC (N vs V) | IIIId (N vs V) | VVVV (N vs V) | VVVI (N vs V) | IIIV (N vs V) | IIII (N vs V) | |
| GSg | (↓) | ↓ vs VVVV | ↓ vs VVVV | N.D.J vs IIIV | (↑) | N.D. vs VVVV | N.D. vs VVVV | N.D. vs IIIV |
| (response loss) | (response loss) | (N.D.) | (↓ vs VVVV) | (↓ vs VVVV) | (N.D.) | |||
| GPmmh | (N.D.) | ↑vs VVVV | N.D. vs VVVV | N.D. vs IIIV | (N.D.) | ↓ vs VVVV | ↓ vs VVVV | N.D. vs IIIV |
| (response loss) | (↓ vs VVVV) | (N.D.) | (response loss) | (N.D.) | (N.D.) | |||
| GPbbi | (↓) | ↑ vs VVVV | N.D. vs VVVV | N.D. vs IIIV | (↓) | ↓ vs VVVV | ↓ vs VVVV | N.D. vs IIIV |
| (response loss) | (↑ vs VVVV) | (N.D.) | (loss response) | (N.D.) | (response gain) |
subcutaneous (sc) injection of vehicle (V; sterile diluent) on days 14—17; n = 4 male, n = 4 females.
sc V injection on days 14–16, followed by sc injection of neutral protamine Hagedorn insulin (I; 10.0 U/kg bw) on day 17; n = 4 males, n = 4 females.
sc I injection on days 14–16, followed by sc V injection on day 17; n = 4 males, n = 4 females.
sc I injection on days 14–17; n = 4 males, n = 4 females.
pretreatment by intra-caudal fourth ventricle (CV4) administration of 6-hydroxydopamine (75 ug/1.0 uL) on days 12 and 13.
pretreatment by CV4 administration of sterile apyrogenic water containing 0.2% ascorbic acid on days 12 and 13.
glycogen synthase.
glycogen phosphorylase-muscle type.
glycogen phosphorylase-brain type.
not different.
Data in Fig. 3 depict VMN GPmm protein expression profiles after injection of one versus four insulin doses to neurotoxin- versus vehicle-pretreated rats of each sex. As shown in Panel A [F(7,24) : 6.92; p<0.0001] and Panel B [GPmm: F(7,24): 18.44; p<0.0001], 6-OHDA had no impact on basal VMN GPmm protein content in male or female animals. A single insulin injection increased or decreased GPmm expression in male and female rats, respectively. These sex-contingent GPmm responses were each prevented by neurotoxin pretreatment. On day 6, AH-exposed female, but not male rats exhibited diminished VMN GPmm protein content; this negative acclimation was not prevented by 6-OHDA. RIIH was associated with loss of hypoglycemic stimulation or inhibition of VMN GPmm expression in the male and female, respectively. In each sex, GPmm habituation to RIIH occurred despite neurotoxin administration.
Fig. 3.
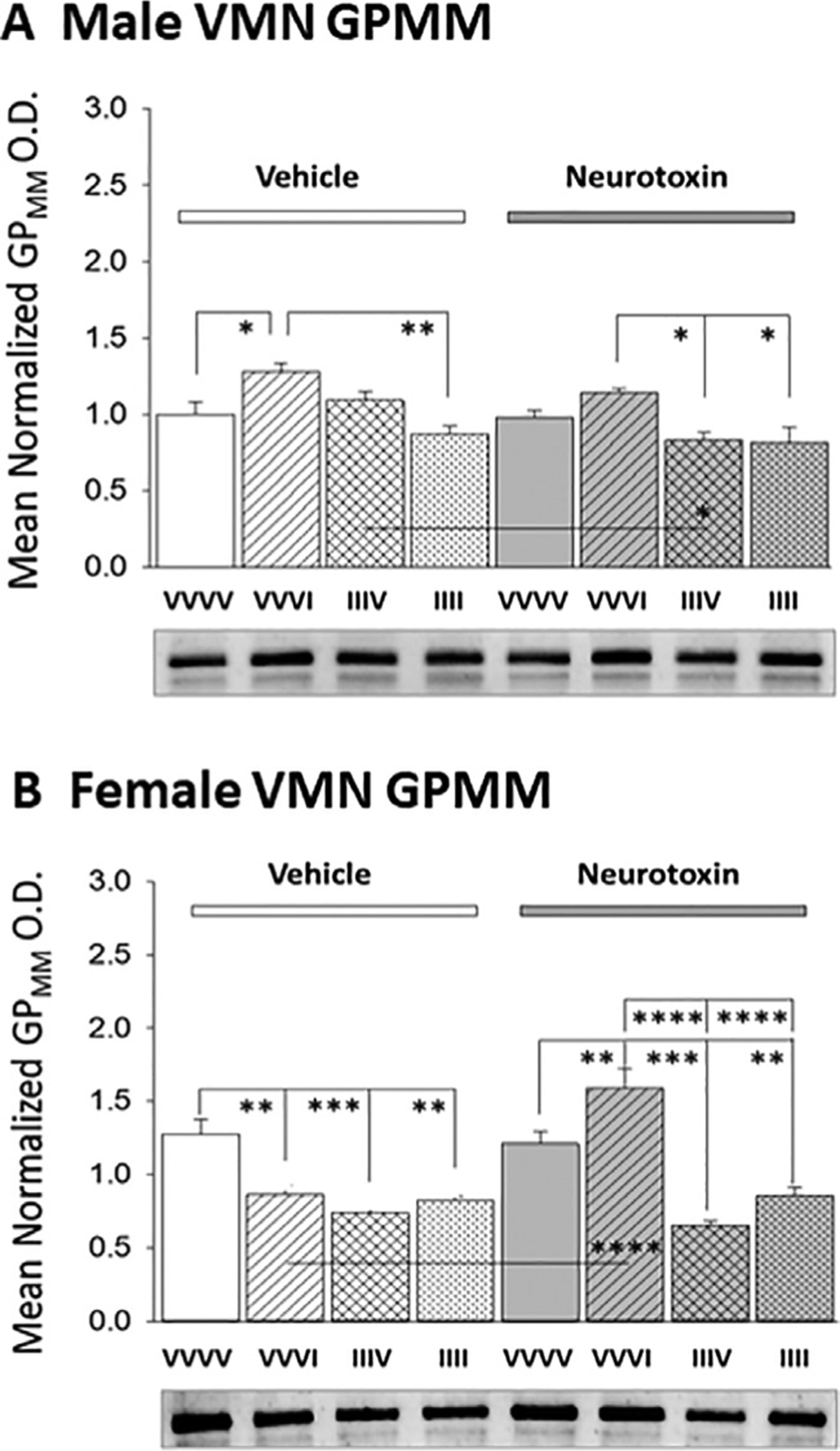
Effects of Antecedent Hypoglycemia Exposure on Baseline and Hypoglycemic Patterns of VMN Glycogen Phosphorylase-Muscle Type (GPmm) Protein Expression in Male and Female Rats: Impact of Hindbrain 6-OHDA Pretreatment. Panels A (male rats) and B (female rats) depict mean normalized VMN GPmm O.D. values + S.E.M. for groups of rats pretreated with V (left-hand side) versus N (right-hand side) on days 1 and 2 prior to subcutaneous VVVV, VVVI, IIIV, or IIII injection regimens on days 3–6. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Fig. 4 presents outcomes of 6-OHDA pretreatment on VMN GPbb expression profiles in male [F(7,16): 9.71; p<0.0001] and female [F(7,16): 16.17; p<0.0001] rats after induction of single versus renewed exposure to hypoglycemia. Neurotoxin administration decreased GPbb protein content in both sexes (N/VVVV versus V/VVVV). Animals injected once with insulin exhibited augmented (males) or diminished (females) VMN GPbb protein profiles. These sex-specific responses were abolished by 6-OHDA. Following recovery from AH, female, but not male rats exhibited a decline in baseline VMN GPbb levels (V/IIIV versus V/VVVV); this inhibitory acclimation occurred despite neurotoxin administration. IIII groups of male and female rats showed no difference in GPbb measures compared to V/IIIV baseline values. In each sex, habituation of GPbb reactivity to hypoglycemia was not prevented by 6-OHDA pretreatment.
Fig. 4.
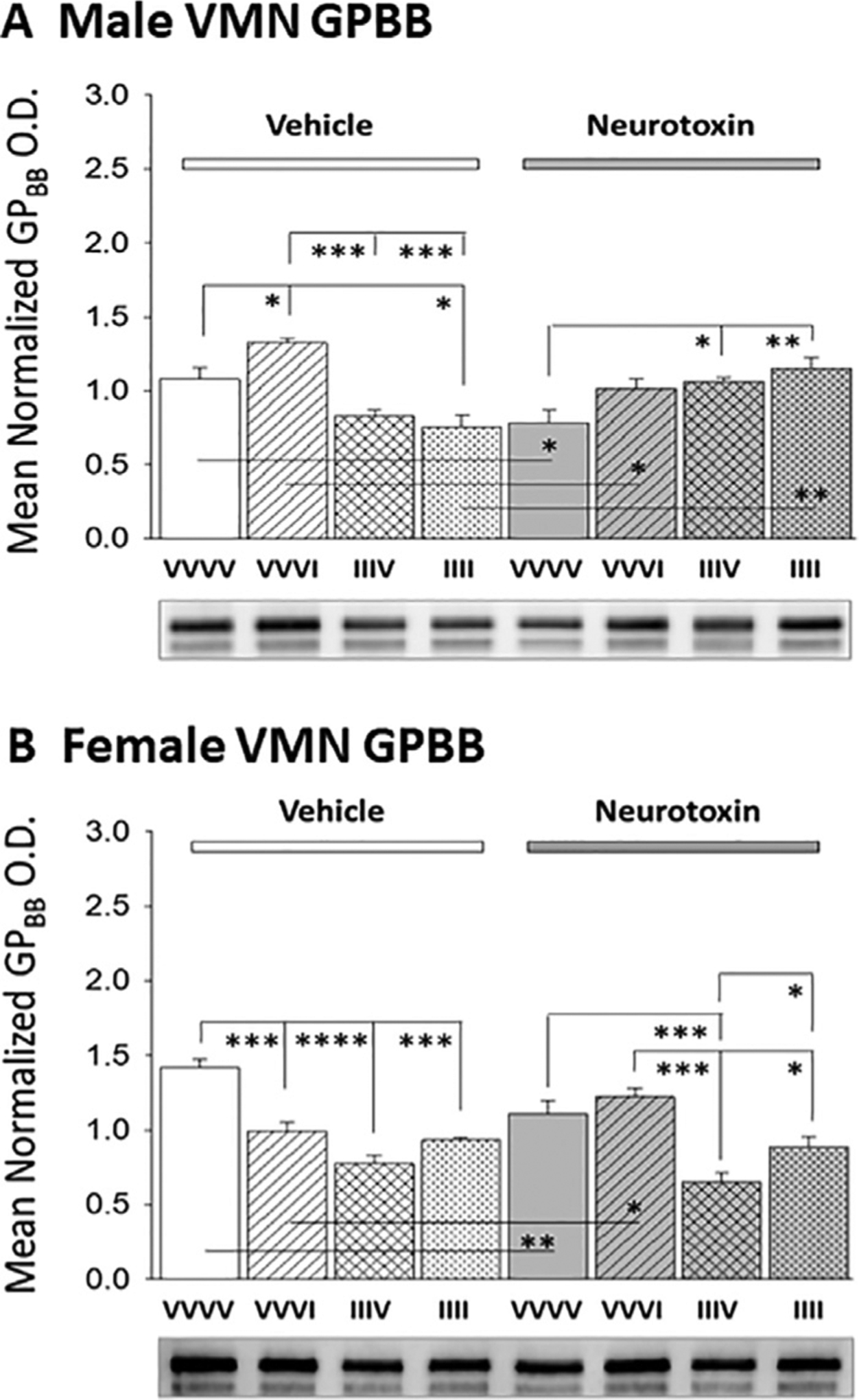
Hindbrain Neurotoxin Pretreatment Effects on VMN Glycogen Phosphorylase-Brain Type (GPbb) Protein Responses to Single versus Serial IIH Exposure in Male and Female Rats. Data depict mean normalized VMN GPbb O.D. values + S.E.M. for groups of testes-intact male (Panel A) or ovariectomized, estradiol-implanted female (Panel B) rats pretreated with V (left-hand side) versus N (right-hand side) prior to subcutaneous VVVV, VVVI, IIIV, or IIII injection regimens. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Data presented in Fig. 5 illustrate effects of intra-CV4 neurotoxin administration on VMN glycogen tissue content in male (Panel A [F(7,24): 8.49; p<0.0001]) and female (Panel B[F(7,24): 23.10; p<0.0001]) rats exposed to one or the fourth of four episodes of hypoglycemia. Data obtained by LC-ESI-MS analysis show that 6-OHDA did not modify basal glycogen content in either sex. A single insulin injection did not significantly alter VMN glycogen levels in male and female rats pretreated with vehicle or neurotoxin. After AH exposure on days 3–5, female, but not male rats showed augmented baseline glycogen mass on day 6. VMN glycogen content was significantly diminished in V/IIIV versus N/IIIV groups of male and female rats. In each sex, RIIH caused a statistically insignificant reduction in VMN glycogen relative to basal V/IIIV values.
Fig. 5.
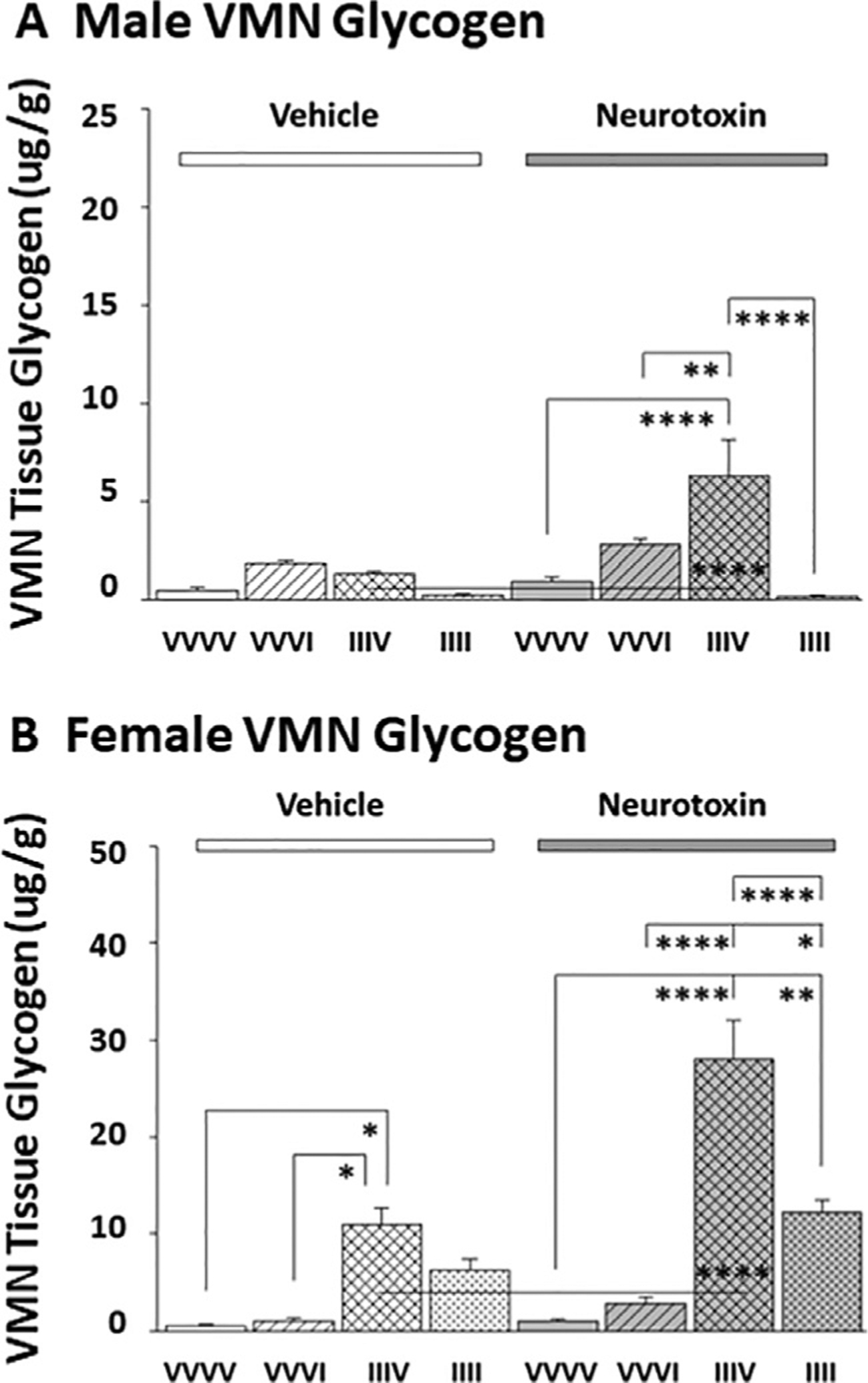
Effects of Acute or Recurring IIH on VMN Glycogen Content in Male versus Female Rats: Impact of 6-OHDA Pretreatment. Panels A (male rats) and B (female rats) depict mean VMN glycogen concentrations + S.E.M. for groups of rats pretreated with V (left-hand side) versus N (right-hand side) on days 1 and 2 prior to subcutaneous VVVV, VVVI, IIIV, or IIII injection regimens on days 3–6. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
Data presented in Table 2 illustrate effects of single versus serial insulin dosing on plasma glucose levels in V- versus N-pretreated male and female rats. In each sex, V-pretreated animals exhibited a similar magnitude of decline in circulating glucose levels measured after one versus four insulin injections (V/IIII versus V/VVVI). In contrast, N-treated male rats showed lesser glucose reductions after one compared to final insulin treatment.
Table 2.
Effects of Single versus Serial Insulin Dosing on Plasma Glucose Levels in Male and Female Rats; Effects of Caudal Fourth Ventricular (CV4) Neurotoxin Administration.
| Treatment Groups | ||||
|---|---|---|---|---|
| Sex/CV4 Pretreatment | VVVVa | VVVlb | lllvc | lllld |
| Male/vehiclee | 167.8 ± 7.5 | 63.3 ± 4.0* | 152.8 ± 3.6 | 76.2 ± 5.9* |
| Male/neurotoxinf | 132.8 ± 8.9** | 96.0 ± 6.4*, ** | 153.8 ± 6.6 | 65.5 ± 7.9* |
| Female/vehicle | 157.8 ± 2.2 | 76.7 ± 1.6* | 154.5 ± 8.3 | 78.0 ± 2.4* |
| Female/neurotoxin | 147.8 ± 3.1 | 75.3 ± 2.3* | 155.8 ± 3.9 | 87.7 ± 2.5* |
subcutaneous (sc) vehicle (V; sterile diluent) injection days 14–17.
sc V injection days 14–16; sc neutral protamine Hagedorn insulin (I; 10.0 U/kg bw) injection day 17.
sc I injection days 14–16; sc V injection day 17.
sc I injection days 14–17.
sterile apyrogenic water containing 0.2% ascorbic acid .
6-hydroxydopamine (6-OHDA); 75 ug/1.0 uL per day, on days 12 and 13.
p<0.05 compared to control, e.g. VVVI versus VVVV, IIII versus IIIV.
p<0.05 compared to vehicle-pretreated animals treated by similar four-day sc injection protocol.
Discussion
Current research investigated effects of intra-CV4 CA neurotoxin administration on sex-specific changes in basal and hypoglycemic patterns of VMN glycogen metabolism owing to AH. Results implicate hindbrain CA signaling in IIH-associated suppression of VMN GS expression in males and corresponding up- or down-regulation of GPmm and GPbb profiles in male versus female rats. Acclimation of baseline VMN glycogen enzyme protein expression following AH was sex-dimorphic (6-OHDA-preventable inhibition of GS levels in males versus non-CA-dependent suppression of GPmm and GPbb content in females), and correlated with differential adjustment, e.g. no change versus augmentation of VMN glycogen content in AH-recovered males versus females, respectively. RIIH-associated CA-independent loss of glycogen enzyme reactivity to hypoglycemia coincided, in each sex, with an adaptive trend toward decreased VMN glycogen content. Outcomes support a need to elucidate local, e.g. VMN mechanisms that may underlie AH-mediated glycogen mass amplification in females and extinction, in each sex, of VMN glycogen metabolic enzyme sensitivity to hypoglycemia. Documented loss of CA impact on these protein profiles during RIIH infers that acquired desensitization of VMN targets to noradrenergic input may occur due to AH. It would also be informative to learn if and how habituated glycogen metabolism may affect VMN glucoregulatory neurotransmission in each sex between and during recurring episodes of hypoglycemia.
Present data provide unique evidence for sex-dimorphic effects of hindbrain CA signaling on baseline VMN GS protein profiles in male (stimulatory) versus female (inhibitory) rats, inferring that this neurochemical stimulus promotes or hampers local glycogen assembly, according to sex, during glucostasis. Outcomes also document up-regulated basal GPbb expression by CA input irrespective of sex, which emphasizes differential hindbrain regulation of GPbb (up-regulated) versus GPmm (refractory) variant proteins in male and female alike. Prior studies by our laboratory reported a decline in basal VMN GPmm expression alongside elevated GS and GPbb content in euglycemic male rats after CV4 neurotoxin administration (Briski and Mandal, 2020). Discrepant 6-OHDA effects on these profiles in earlier versus current work may reflect, in part, dissimilar tissue micropunch dissection paradigms involving collection of consecutive sections throughout the rostro-caudal VMN length contrasted with sample harvesting from pre-defined rostral and caudal segments of this nucleus, respectively. These divergent outcomes support the notion that region-based glycogen enzyme protein responses to CA input and other physiological cues may be obscured by averaged measures representative of whole VMN.
Observations here that VMN GS protein content was decreased in male, but not female rats during a single IIH episode indicate that acute effects of this metabolic stress on glycogen expansion are sex-specific. CA signaling is implicated in this male-only inhibitory response as GS expression in insulin-injected males was normalized by 6-OHDA pretreatment. Evidence for IIH suppression of VMN GS expression in neurotoxin-pretreated females points to non-CA regulation of this profile relative to 6-OHDA-augmented baseline levels of expression during glucoprivation. In each sex, IIH caused neurotoxin-reversible diminution of VMN GPmm and amplification of GPbb protein content, showing that divergent hindbrain CA regulation of NE- versus energy deficit-sensitive GP variant profiles is not sex-specific. These findings imply that hypoglycemic patterns of CA signaling are required for glucoprivic-mediated VMN glycogen mobilization. LC-ESI-MS analysis of VMN tissue glycogen mass showed that acute hypoglycemia had no significant impact on content, inferring that each sex the ratio of glycogen synthesis: break-down is unchanged. Nonetheless, it will be necessary for future research to determine if matched rates of glucose assimilation into and liberation from glycogen are up- or down-regulated, thereby resulting in increased or decreased glycogen turnover, during singular exposure to this metabolic stress. Perceived dissonance between VMN GP variant expression and glycogen content underscores the notable point that measures of total GP protein may not constitute an accurate readout of net enzyme activity, e.g. phosphorylation state. It would be important to learn if and how acute hypoglycemia may affect GPmm and GPbb protein phosphorylation, but such effort is currently impeded by lack of availability of antisera raised against phosphoGPmm or phosphoGPbb.
Current outcomes reveal sex-dimorphic effects of AH on baseline VMN glycogen metabolic enzyme protein expression. Subsequent to IIH induction on days 14–16, GS protein expression on day 17 was reduced in male, but not female rats compared to hypoglycemia-naïve controls; this male-specific adaptation was reversed by neurotoxin pretreatment. These data suggest that AH may obstruct GS-mediated glycogen buildup within the VMN in a sex-contingent manner. On the other hand, AH exposure led to suppression of basal GPmm and GPbb profiles in the female, a negative acclimation that was not prevented by 6-OHDA. These results infer, that in that sex, AH may act through CA-independent mechanisms to hinder VMN glycogen disassembly after recovery, as a means to amplify that energy reserve. Tissue glycogen measurements showed that VMN levels were not altered in AH-exposed males, but were significantly elevated in females. These data bolster the above premise that in the female, the VMN glycogen depot is expanded after recovery from AH. Previous studies reported no net effect of AH on whole-hypothalamus glycogen content in male rats [Herzog et al., 2006]. Current data affirm earlier observations in that sex, but at the same time delineate an important sex difference. Notably, in each sex, 6-OHDA augmented glycogen levels following AH exposure, implying that AH-driven hindbrain CA signals reduce the ratio of glycogen assembly versus disassembly, e.g. promote glucosyl unit mobilization versus incorporation. This input is apparently offset by as-yet-unidentified stimuli as, noted above, net glycogen mass was refractory to (males) or increased (females) after AH. As neurotoxin-associated augmentation of VMN glycogen was not accompanied by up-regulation of GS and/or down-regulation of GP expression (excepting decreased GPmm profiles in males), it can be speculated whether AH may alter GS and/or GP variant phosphorylation state in the absence of CA input.
Current outcomes provide novel proof that in each sex, distinctive VMN GS (↑ male), GPmm (↑ male; ↓ female), and GPbb (↑ male; ↓ female) responses to a single hypoglycemic bout are eliminated on the fourth consecutive day of insulin therapy. Data also implicate hindbrain CA-independent mechanisms in response cessation as these adaptations occur despite neurotoxin pretreatment. RIIH-associated loss of CA-sensitive glycogen enzyme protein reactivity (male: GS, GPmm, GPbb; female: GPmm, Gpbb) may involve, in part, desensitization of these protein profiles to hypoglycemic noradrenergic signaling owing to repetitive exposure. There remains a need to identify the mechanisms triggered by AH that underlie this response loss. RIIH did not significantly alter, but instead caused a trend-like reduction in VMN glycogen content in each sex compared to day 17 baseline measures in IIIV treatment groups, an outcome that correlates with above-noted glycogen metabolic enzyme protein refractoriness to repetitive insulin therapy. Intriguingly, recurring insulin injection of neurotoxin-pretreated animals caused a significant reduction in VMN glycogen in each sex alongside lack of GS or GP protein reactivity to RIIH. Thus, it may be surmised that in the absence of CA input, AH exposure may cause activity of one or both GP variants to be enhanced or GS activity to decline during re-exposure to hypoglycemia.
Recent studies show that VMN glycogen mass or turnover status may shape local glucoregulatory function as GP inhibition by 1,4-dideoxy-1,4-imino-d-arabinitol increased VMN AMPK activity and caused sex-specific adjustments in expression of marker proteins for the characterized gluco-stimulatory and -inhibitory transmitters NO and GABA in euglycemic rats [Alhamami et al., 2018 ; Alshamrani and Briski, personal communication]. Further research is warranted to investigate how glucoregulation may be affected by sex-dimorphic post-AH changes in baseline VMN glycogen content and by decrements (albeit non-significant) of sex-contingent magnitude in glycogen mass during RIIH.
In summary, current results uniquely document sex-specific and - nonspecific hindbrain CA regulation of eu- and hypoglycemic patterns of VMN GS and GP variant protein profiles in rats. Data show that CA signaling imposes divergent control of NE-sensitive GPmm and energy deficit-sensitive GPbb expression in both sexes during glucostasis, but up- (males) or down- (females) regulates both GP variants during hypoglycemia. AH affects baseline expression of different VMN glycogen metabolic enzyme proteins (GS in males; GPmm, GPbb in females) in each sex, involving CA-dependent (males) or -independent (females) mechanisms. Results also provide novel evidence that CA-driven glycogen metabolic protein responses to hypoglycemia are eradicated during RIIH without CA signal involvement. Additional effort is required to as-certain how sex-specific adjustments in VMN glycogen metabolism due to AH affect glucoregulation in each sex.
Funding
National Institutes of Health DK-109382.
Abbreviations:
- 6-OHDA
6-hydroxydopamine
- AH
antecedent hypoglycemia
- CV4
caudal fourth ventricle
- DVC
dorsal vagal complex
- GABA
γ-aminobutyric acid
- GAD65/67
glutamate decarboxylase65/67
- GS
glycogen synthase
- GPbb
glycogen phosphorylase-brain type
- GPmm
glycogen phosphorylase-muscle type
- IIH
insulin-induced hypoglycemia
- MBH
mediobasal hypothalamus
- NE
norepinephrine
- NO
nitric oxide
- RIIH
recurrent insulin-induced hypoglycemia
- VMN
ventromedial hypothalamic nucleus
Footnotes
Declaration of Competing Interest
The authors declare that they have no conflict of interest.
Ethics approval
All animal experimental was carried out in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, 8th Edition.
Availability of data and material
All data generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- Alhamami HN, Uddin MM, Mahmood ASMH, Briski KP, 2018. Lateral but not medial hypothalamic AMPK activation occurs at the hypoglycemic nadir in insulin-injected male rats: Impact of caudal dorsomedial hindbrain catecholamine signaling. Neuroscience 379, 103–114. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Allaman I, Magistretti PJ, 2011. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14, 724–738 . [DOI] [PubMed] [Google Scholar]
- Bheemanapally K, Ibrahim MMH, Briski KP, 2020. Combinatory high-resolution microdissection/ultra performance liquid chromatographic-mass spectrometry approach for small tissue volume analysis of rat brain glycogen. J. Pharmaceut. Biomed. Anal 178, 112884. doi: 10.1016/j.jpba.2019.112884 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briski KP, Mandal SK, 2020. Hindbrain metabolic deficiency regulates ventromedial hypothalamic nucleus glycogen metabolism and glucose-regulatory signaling. Acta Neurobiol. Exper 80, 57–65 . [PMC free article] [PubMed] [Google Scholar]
- Briski KP, Marshall ES, Sylvester PW, 2001. Effects of estradiol on glucoprivic trans-activation of catecholaminergic neurons in the female rat caudal brainstem. Neuroen-docrinology 73, 369–377 . [DOI] [PubMed] [Google Scholar]
- Brown AM, 2004. Brain glycogen re-awakened. J. Neurochem 89, 537–552. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW, 1974. Plasma concentrations of LH, FSH, progesterone, and estradiol-17beta throughout the 4-day estrous cycle of the rat. Endocrinology 94, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Choi IY, Seaquist ER, Gruetter R, 2003. Effect of hypoglycemia on brain glycogen metabolism in vivo. J. Neurosci Res 72, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer PE, Davis SN, Shamoon H, 2003. Hypoglycemia in diabetes. Diabetes Care 26, 1902–1912. [DOI] [PubMed] [Google Scholar]
- Cryer PE, 2010. Hypoglycemia in type 1 diabetes mellitus. Endocrinol. Metab. Clin. North Am 39, 641–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNuzzo M, Walls AB, Öz G, Seaquist ER, Waagepetersen HS, Bak LK, Nedergaard M, Schousboe A, 2019. State-Dependent Changes in Brain Glycogen Metabolism. Adv Neurobiol 23, 269–309. [DOI] [PubMed] [Google Scholar]
- Duarte JMN, Morgenthaler FD, Gruetter R, 2017. Glycogen supercompensation in the rat brain after acute hypoglycemia is independent of glucose levels during recovery. Neurochem. Res 42, 1629–1635. [DOI] [PubMed] [Google Scholar]
- Gruetter R, 2003. Glycogen: the forgotten cerebral energy store. J. Neurosci. Res 74, 179–183. [DOI] [PubMed] [Google Scholar]
- Gujar AD, Ibrahim BA, Tamrakar P, Koshy Cherian A, Briski KP, 2014. Hindbrain lactostasis regulates hypothalamic AMPK activity and hypothalamic metabolic neurotransmitter mRNA and protein responses to hypoglycemia. Amer. J. Physiol. Regul. Integr. Comp. Physiol 306, R457–RR69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RI, Chan O, Yu S, Dziura J, McNay EC, Sherwin RS, 2008. Effect of acute and recurrent hypoglycemia on changes in brain glycogen concentration. Endocrinology 149, 1499–1504 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MMH, Alhamami HN, Briski KP, 2019. Norepinephrine regulation of ventromedial hypothalamic nucleus metabolic transmitter biomarker and astrocyte enzyme and receptor expression: role of 5’-AMP-activated protein kinase. Brain Res. 1711, 48–57 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MS, Pedersen SE, Walls AB, Waagepetersen HS, Bak LK, 2015. Isoform-selective regulation of glycogen phosphorylase by energy deprivation and phosphorylation in astrocytes. Glia 63, 154–162. [DOI] [PubMed] [Google Scholar]
- Mandal SK, Shrestha PK, Alenazi FSH, Shakya M, Alhamami HN, Briski KP, 2017. Role of hindbrain adenosine 5’-monophosphate-activated protein kinase (AMPK) in hypothalamic AMPK and metabolic neuropeptide adaptation to recurring insulin-induced hypoglycemia in the male rat. Neuropeptides 66, 25–35. [DOI] [PubMed] [Google Scholar]
- Nadeau OW, Fontes JD, Carlson GM, 2018. The regulation of glycogenolysis in the brain. J. Biol. Chem 293, 7099–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvage DJ, Lee SY, Parsons LH, Seo DO, Rivier CL, 2004. A hypothalamic-testicular neural pathway is influenced by brain catecholamines, but not testicular blood flow. Endocrinology 145, 1750–1759. [DOI] [PubMed] [Google Scholar]
- Shrestha PK, Tamrakar P, Ibrahim BA, Briski KP, 2014. Hindbrain medulla catecholamine cell group involvement in lactate-sensitive hypoglycemia-associated patterns of hypothalamic norepinephrine and epinephrine activity. Neuroscience 278, 20–30.8. [DOI] [PubMed] [Google Scholar]
- Singh SR, Briski KP, 2004. Septopreoptic mu opioid receptor mediation of hindbrain gluco-privic inhibition of reproductive neuroendocrine function in the female rat. Endocrinology 145, 5322–5331. [DOI] [PubMed] [Google Scholar]
- Stobart JL, Anderson CM, 2013. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Cell. Neurosci 7, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamrakar P, Shrestha PK, Briski KP, 2015. Dorsomedial hindbrain catecholamine regulation of hypothalamic astrocyte glycogen metabolic enzyme protein expression: Impact of estradiol. Neuroscience 292, 34–45 . [DOI] [PubMed] [Google Scholar]


