Abstract
Rationale
Opioid abuse remains a serious public health problem. The pseudoirreversible mu opioid receptor antagonist methocinnamox (MCAM) might be useful for treating opioid abuse and overdose. Because endogenous opioid systems can modulate cognition and decision making, it is important to evaluate whether long-term blockade of mu opioid receptors by MCAM adversely impacts complex operant behavior involving memory.
Objective
This study tested the effects of MCAM in rhesus monkeys responding under a delayed matching-to-sample task, with correct responses reinforced by sucrose pellets. Because MCAM did not alter performance, antagonism of the rate-decreasing effects of morphine was used to confirm that an effective dose of MCAM was administered. Moreover, the muscarinic receptor antagonist scopolamine as well as the N-methyl-D-aspartate antagonist phencyclidine were studied as positive controls to demonstrate sensitivity of this procedure to memory disruption.
Results
Neither MCAM (0.32 mg/kg) nor morphine (1 – 5.6 mg/kg) impaired delayed matching-to-sample accuracy. Morphine dose-dependently decreased the number of trials completed before MCAM administration, and a single injection of MCAM blocked the behavioral suppressant effects of morphine for at least 7 days. Scopolamine (0.01 – 0.056 mg/kg) and phencyclidine (0.1 – 0.56 mg/kg) dose-dependently decreased delayed matching-to-sample accuracy and the number of trials completed.
Conclusions
MCAM did not impair memory (as measured by accuracy in a delayed-matching-sample task) and did not decrease responding for or consumption of sucrose pellets. This dose of MCAM attenuates self-administration of opioids and reverses as well as prevents opioid-induced respiratory depression. These results provide further support for a favorable safety profile for MCAM.
Keywords: opioid, antagonism, operant behavior, cognition, memory, rhesus monkey
INTRODUCTION
Opioid abuse remains a serious public health problem (Pergolizzi et al. 2019; Strand and Eukel 2019). Currently approved medications for opioid use disorder include methadone, buprenorphine, naltrexone, and lofexidine. Methadone and buprenorphine are mu opioid receptor agonists and are used as a replacement therapy in individuals with opioid use disorder. Because of their agonist properties at mu opioid receptors, buprenorphine and methadone share many effects, including adverse effects, with abused opioids such as heroin and fentanyl, including physical dependence, respiratory depression, overdose, and potentially dangerous interactions with other drugs such as alcohol and benzodiazepines (Jones et al. 2012; Kim et al. 2017; Carpenter et al. 2019). In contrast to agonist replacement therapy, mu opioid receptor antagonists are used to block the effects of opioids. Naltrexone and naloxone are mu opioid receptor antagonists approved for treating opioid use disorder and overdose, respectively. Naltrexone avoids the abuse liability and other adverse effects associated with agonist replacement therapies; however, it has limited effectiveness in that its duration of action is relatively short and its antagonist effects can be surmounted by taking large doses of an agonist (Young and Woods 1982; Steinmiller and Young 2008; Gerak et al. 2019a). Like naltrexone, naloxone also has a short duration of action, which limits its therapeutic utility. The antagonist effects of naloxone are shorter than the agonist effects of many abused opioids (e.g., heroin, fentanyl), which can lead to re-emergence of respiratory depression following rescue with naloxone (Dahan et al. 2010). These limitations, along with the fact that the opioid crisis persists despite the availability of these pharmacotherapies for more than 35 years, underscore the need for more and better treatments for opioid use disorder and overdose.
Methocinnamox (MCAM) is a mu opioid receptor antagonist with pharmacological properties that might make it especially well-suited for treating opioid use disorder and overdose. MCAM binds pseudoirreversibly to mu opioid receptors (Broadbear et al. 2000) and shifts the morphine dose-response function for antinociception (warm water tail withdrawal, acetic acid induced writhing) rightward up to 100-fold in rats and mice, with antagonism still evident 48 hrs after a single injection (Broadbear et al. 2000; Peckham et al. 2005). In nonhuman primates, MCAM attenuates intravenous self-administration of the mu opioid receptor agonists heroin and remifentanil without affecting responding for food or intravenous cocaine (Maguire et al. 2019). The effects of a single injection of MCAM on opioid self-administration persist for a week or longer, whereas naltrexone attenuates opioid self-administration only on the day it is administered (Maguire et al. 2019). Additionally, MCAM reverses and prevents the ventilatory depressant effects of heroin, with the duration of antagonism by MCAM lasting at least 4 days, significantly longer than the short-acting antagonist naloxone (Gerak et al. 2019a).
The endogenous opioid system is involved in various aspects of cognitive control (Steenbergen et al. 2019). Intravenous infusions of naloxone can impair memory as measured by a verbal learning task in humans (Cohen et al. 1983a, 1983b). The mu opioid receptor inverse agonist GSK1521498 moderately impairs attention including digit vigilance and reaction time (Nathan et al. 2012). In nonhuman subjects, naloxone dose-dependently impairs T-maze performance (Flood et al. 1987) and the irreversible mu opioid receptor antagonist β-funaltrexamine impairs memory formation in a passive-avoidance learning task (Freeman and Young 2000). Thus, one potential concern with using MCAM to treat opioid use disorder and overdose is that pseudoirreversible binding to mu opioid receptors might have adverse effects on cognition or memory. Moreover, mu opioid receptor antagonists can decrease consumption of palatable food (Yeomans and Grey 1997) and attention to food-paired stimuli (Chamberlain et al. 2012).
In the current study, monkeys were trained to respond under a delayed matching-to-sample task, and correct responses were reinforced with sucrose pellets. Delayed matching-to-sample is a well-established working memory task that is sensitive to both environmental and pharmacological manipulations (Blough 1959; White 1985; Hutsell and Banks 2015). MCAM was studied for its effects on delayed matching-to-sample accuracy and the number of trials completed. Because MCAM did not affect performance and because it is well established that MCAM antagonizes the effects of mu opioid receptor agonists for days or weeks, the effects of the mu opioid receptor agonist morphine on delayed matching-to-sample accuracy and the number of trials completed were determined before and after MCAM to test whether the dose of MCAM administered was behaviorally active under these conditions. Two other drugs were studied to confirm sensitivity of this procedure to memory-disrupting effects of drugs; both scopolamine, a drug with muscarinic receptor antagonist properties, and phencyclidine, a drug with N-methyl-D-aspartate receptor antagonist properties, impair memory under a broad range of conditions (McMillan 1981; Yamamoto et al. 2011).
METHODS
Subjects
Five adult rhesus monkeys (4 females, 1 male) served as subjects. The monkeys were housed individually under a 14/10-hr light/dark cycle. Monkeys had unrestricted access to water and were fed primate chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI), fresh fruit, and peanuts daily after experimental sessions to maintain a healthy body weight (range, 6.4 – 9.8 kg). Two monkeys (1 female, 1 male) were trained previously to press levers for food pellets and had received drug injections (Minervini and France, 2018); the other three female monkeys were trained previously to press a touchscreen (mounted in an operant chamber) for food pellets and had received drug injections. Monkeys had not received drug for at least 16 weeks prior to the current study. The experimental protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio and was in accordance with guidelines set forth by the Guide for the Care and Use of Laboratory Animals.
Apparatus
A custom-built stainless steel response panel was mounted to the homecage during sessions. The response panel contained an antiglare touchscreen monitor (30.4 cm x 22.8 cm; Model 1537L, Elo Touch Solutions Inc., Milpitas, CA) with Carroll Touch Infrared Technology (Model E461378, Elo Touch Solutions) to detect responses. A pellet dispenser (Model ENV-203, Med Associates) delivered 300-mg raspberry-flavored sucrose pellets (5TUT, TestDiet, Richmond, IN) to a receptacle centrally located 10 cm below the monitor. E-Prime Professional 2.0 (Psychology Software Tools Inc., Sharpsburg, PA) software controlled the presentation of visual stimuli and recorded data.
Procedure
Delayed Matching-to-Sample Task
An array of two 9.1 × 6.8 cm (approximately 30% of the total screen size) colored rectangles with distinct interior shapes served as the stimuli as well as the response manipulanda. The relative intensity of red (R), green (G), and blue (B) displayed was specified with RGB color values ranging from 0 to 255 bits. One rectangle was blue (0, 0, 255) with a white (255, 255, 255) circle 5.5 cm in diameter in the center, and the other rectangle was black (0, 0, 0) with a yellow (255, 255, 0) vertical stipe 1.5 cm in width and a yellow horizontal stripe 1.5 cm in height, both extending to the edges of the rectangle and centered to form a cross.
Sessions included up to 100 trials in 90 min, with no programmed omission criterion (i.e., limited hold to respond) per trial. Each trial comprised a sample component followed by a comparison component (i.e., opportunity to select the matching stimulus from the array). During the sample component, a stimulus was presented in the center of an otherwise blank (white) screen. Pressing the stimulus 10 times terminated the sample stimulus followed, either immediately or after a delay, by presentation of both stimuli for comparison. The comparison stimuli appeared at the bottom of the screen, one 7.6 cm from the left edge and the other 7.6 cm from the right edge. The position was not predictive of a correct response (i.e., the matching stimulus appeared an equal number of times on the left and right sides of the screen). A single response to the matching stimulus delivered one food pellet paired with visual feedback (pink [255, 0, 255] screen) for 1 sec, followed by a 5-sec inter-trial interval during which the screen was grey (200, 200, 200). A response to the incorrect (non-matching) stimulus immediately initiated a 35-sec inter-trial interval. During no-delay trials, the sample stimulus was terminated and the comparison stimuli were presented immediately upon completion of the fixed ratio (i.e., 10 presses) requirement in the sample component; these trials were included to assess disruptions in discrimination in the absence of delay. During training, all 100 trials were no-delay trials until accuracy was at least 90% correct for at least three consecutive sessions. Then delays were gradually faded in (starting with 1 sec and increasing by ½-log units) by replacing a set of 20 existing trials with the next delay in the series each time a monkey satisfied the following criteria for three consecutive sessions: accuracy in the no-delay trials was at least 90% correct and accuracy in delay trials was either at least 90% correct or stable (± 20% of the 3-session mean). The terminal delay series was 0, 1, 3.2, 10, and 32 sec, encompassing a delay at which mean baseline performance was maintained at 95 ± 5% accuracy (short delay) up to a delay that maintained no better than 65 ± 5% accuracy (long delay). Because performance in one monkey under the 32-sec delay exceeded 70% accuracy by the end of the MCAM and morphine experiments, the delays were adjusted to 0, 3.2, 10, 32, and 100 sec for the remainder of the study for that monkey only.
Each of the five delays was presented 20 times per session. A trial could also vary along dimensions such as sample stimulus color (i.e., blue/white or black/yellow) and comparison position (i.e., left or right), thereby yielding 20 possible trial configurations (5 delays x 2 stimuli x 2 positions). Trials were presented in quasi-random order with the constraints that the same delay was not presented for more than two consecutive trials and that each trial configuration was presented in five blocks of 20, selected randomly without replacement.
Determination of Drug Effects
At the beginning of this study, it was not known whether MCAM would affect performance under the delayed matching-to-sample task. However, because MCAM was shown to have long-term antagonist properties at mu opioid receptors, the effects of the mu opioid receptor agonist morphine on delayed matching-to-sample accuracy and the number of trials completed were determined before and after MCAM administration. First, the effects of morphine alone were determined up to a dose that decreased the number of trials completed to fewer than 25 for individual monkeys. Doses were given in an irregular order that varied among subjects. At least three consecutive baseline sessions in which percent correct responses across each of five delays did not vary by more than ± 20% preceded each test. The effects of a dose of morphine that decreased the number of trials completed to fewer than 25 were then re-determined, with saline administered in the preceding session. Following morphine tests, there were at least three consecutive baseline sessions before 0.32 mg/kg MCAM was administered. Then, the dose of morphine that decreased the number of trials completed to fewer than 25 (which varied among individual monkeys) was re-tested 1 and 7 days after MCAM and weekly thereafter until the number of trials completed was fewer than 25 (i.e., morphine was again effective in decreasing the number of trials completed). This dose of MCAM was selected because it effectively reverses and prevents the ventilatory depressant effects of heroin and attenuates intravenous self-administration of opioids in rhesus monkeys for a week or longer (Gerak et al. 2019; Maguire et al. 2019).
Neither MCAM nor morphine significantly impaired accuracy in this task. To confirm the sensitivity of this procedure to drug-induced memory impairment, scopolamine (a drug with muscarinic antagonist properties) and phencyclidine (a drug with N-methyl-D-aspartate receptor antagonist properties) were tested; both drugs have well-characterized memory impairing effects (McMillan 1981; Thompson et al. 1986; Danysz et al. 1988; Yamamoto et al. 2011; Mathews et al. 2018). Full dose-response functions were doubly determined, first for scopolamine then for phencyclidine, from a dose that did not affect accuracy or the number of trials completed up to a dose that significantly impaired accuracy or decreased the number of trials completed to fewer than 25 for individual monkeys. At least three consecutive baseline sessions in which percent correct responses across each of five delays did not vary by more than ± 20% preceded each test.
Drugs
Morphine sulfate (National Institute on Drug Abuse Drug Supply Program, Bethesda, MD, USA) was dissolved in sterile water. Phencyclidine hydrochloride and scopolamine hydrobromide (Sigma Aldrich, St. Louis, MO, USA) were dissolved in sterile saline. MCAM was synthesized as previously described (Broadbear et al. 2000) and dissolved in a 10% w/v solution of 2-hydroxypropyl-β-cyclodextrin (Accela ChemBio, Inc., San Diego, CA, USA) in sterile saline. Drugs were administered subcutaneously in a volume of 0.2 – 0.8 ml with a 15 min pretreatment time.
Data Analysis
Percent correct matches (the number of correct comparison responses divided by the number of trials completed [maximum of 20] at each delay) quantified delayed matching-to-sample accuracy, and the number of trials completed (out of a maximum of 100) quantified behavioral suppression. A one-way repeated measures ANOVA with Bonferroni’s post-hoc test was used to analyze drug effects on delayed matching-to-sample accuracy and trials completed. The effects of each dose were compared to the effects of saline. Results obtained with doses of morphine, scopolamine, or phencyclidine that decreased the number of trials completed to fewer than 25 for a given monkey were excluded from the statistical analysis of delayed matching-to-sample accuracy. The fewest number of trials completed was used as a conservative estimate of the number of trials completed at larger doses. At least three monkeys had to contribute to the data for a given dose to be included in the statistical analysis for delayed matching-to-sample accuracy. Analyses were conducted using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA) with significance set to p<0.05.
RESULTS
Under baseline conditions (no injection; data not shown) and after injection of saline (Fig 1a, shaded region), accuracy was greater than 90% in trials with no delay and decreased systematically, approaching chance performance, with increasing delay between the sample stimulus and the comparison stimuli. Monkeys always completed all 100 trials under baseline conditions and after saline injections (not shown). Performance after injection of MCAM (t12=0.28, p>0.99) or morphine (t12=0.86, p>0.99 for 0.32 mg/kg; and t12=0.71, p>0.99 for 1 mg/kg) was not significantly different from performance after injection of saline (Fig 1a). A dose of 0.32 mg/kg MCAM (t20=0.26, p>0.99) did not decrease the number of trials completed (Fig 1b), whereas morphine (t20=0.85, p>0.99 for 0.32 mg/kg; t20=3.7, p=0.007 for 1 mg/kg; t20=7.1, p<0.001 for 3.2 mg/kg; and t20=8.1, p<0.001 for 5.6 mg/kg) decreased the number of trials completed in a dose-dependent manner (Fig 1b).
Fig 1.
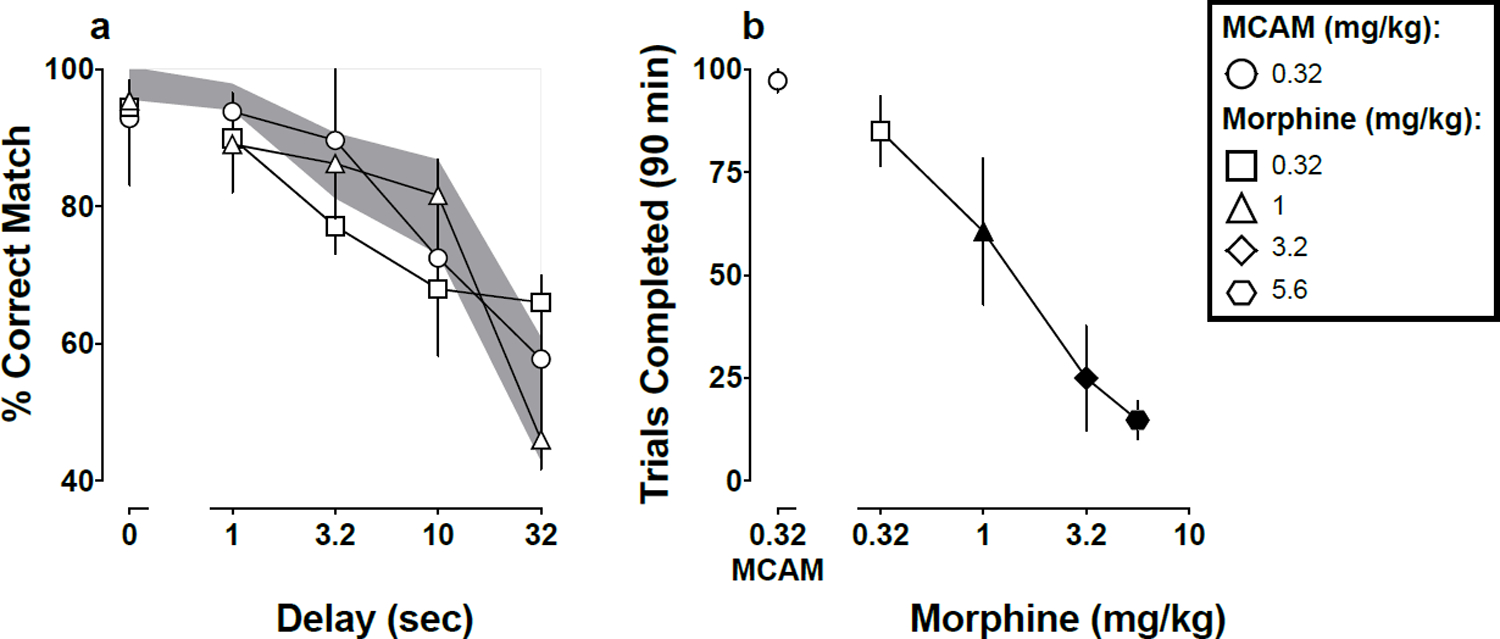
Mean (±1 SEM) accuracy expressed as percent correct matches after MCAM or morphine (1a) and mean (±1 SEM) number of trials completed out of 100 possible after MCAM (0.32 mg/kg) or morphine (0.32–5.6 mg/kg; 1b) for five monkeys responding under a delayed matching-to-sample task. Abscissae: delay in seconds between the sample and comparison components (1a) and dose in mg/kg body weight (1b). Filled symbols denote statistical significance (p<0.05) compared with saline. The shaded region denotes the 95% CI for saline.
When the smallest dose morphine that decreased the number of trials completed to fewer than 25 before administration of MCAM was re-tested one and seven days after administration of MCAM, performance on delayed matching-to-sample accuracy was not different from performance after saline (Fig 2a; t8=0.84, p=0.85 for day 1; and t8=0.77, p=0.93 for day 7). The effects of morphine are also shown for individual subjects one day (Fig 2b) and seven days (Fig 2c) after MCAM, and these effects at the individual subject level generally were represented well by the analysis at the level of the group (mean) performance (Fig 2a).
Fig 2.
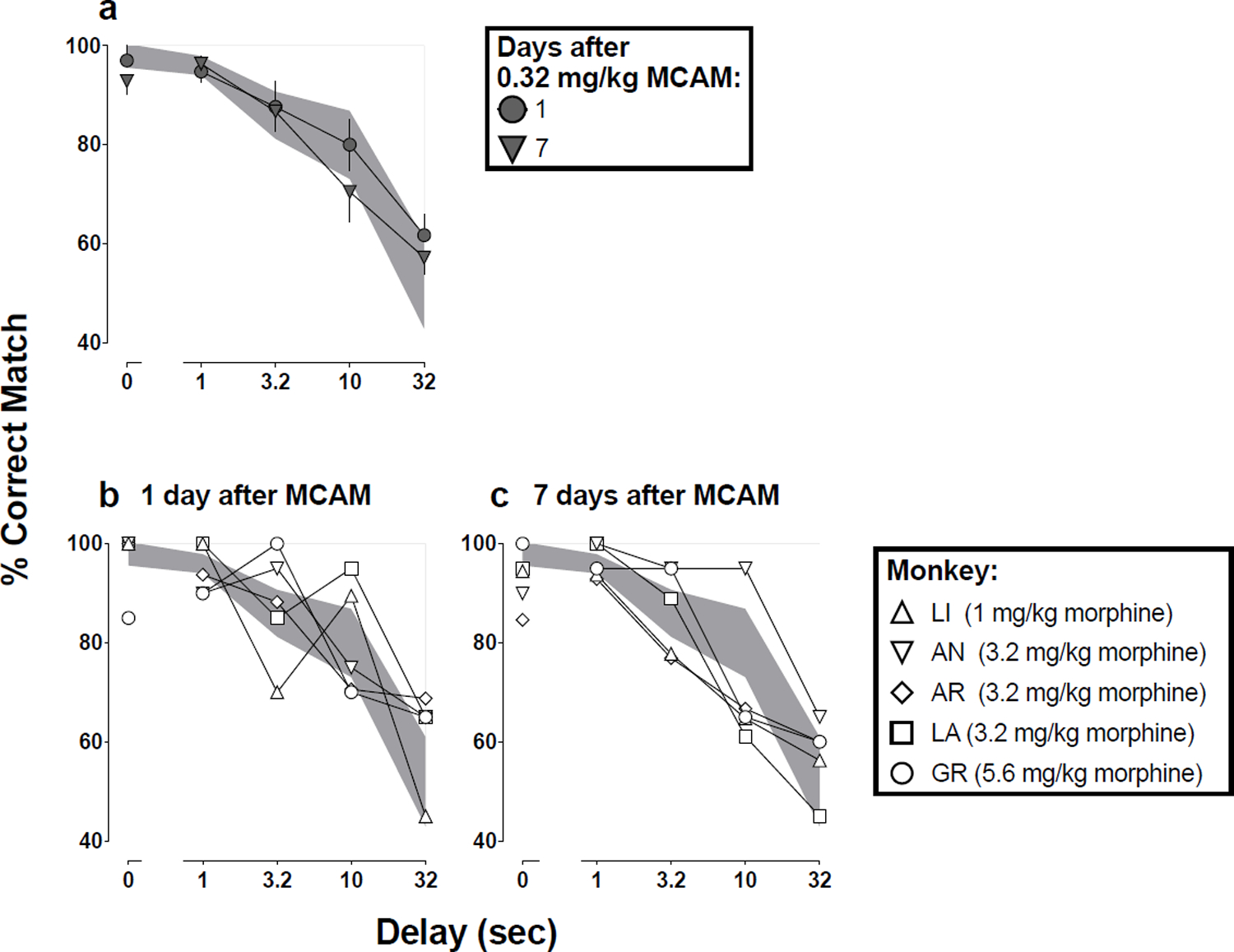
Mean (±1 SEM) accuracy expressed as percent correct matches when morphine was tested one and seven days after MCAM in a group of five monkeys (group data, 2a). The same data, for individual monkeys, are plotted in Fig 2b (one day after MCAM) and Fig 2c (seven days after MCAM). Abscissae: delay in seconds between the sample and comparison components. The shaded regions denote the 95% CI for saline.
Monkeys differed with respect to sensitivity to the effects of morphine as well as the time course of MCAM. The smallest dose of morphine that decreased the number of trials completed to fewer than 25 (dashed line in Fig 3) differed among individual subjects. Monkey LI was the most sensitive to the behavioral suppressant effects of morphine, with 1 mg/kg decreasing the number of trials completed to fewer than 25, whereas Monkey GR was the least sensitive, with a larger dose or morphine (5.6 mg/kg) needed to decrease the number of trials completed to fewer than 25 (Fig 3). In each monkey, the dose of morphine that markedly decreased the number of trials completed before MCAM treatment, had little or no effect on the number of trials completed one day after MCAM (Fig 3). The single injection of MCAM continued to antagonize the behavioral suppressant effects of morphine although there were differences among individual subjects with respect to the time course of MCAM. Morphine was again effective at decreasing the number of trials completed to fewer than 25 (dashed line in Fig 3) 14 days after MCAM for Monkeys LI and AR, 21 days after MCAM for Monkey GR, 42 days after MCAM for Monkey AN, and 63 days after MCAM for Monkey LA (Fig 3).
Fig 3.
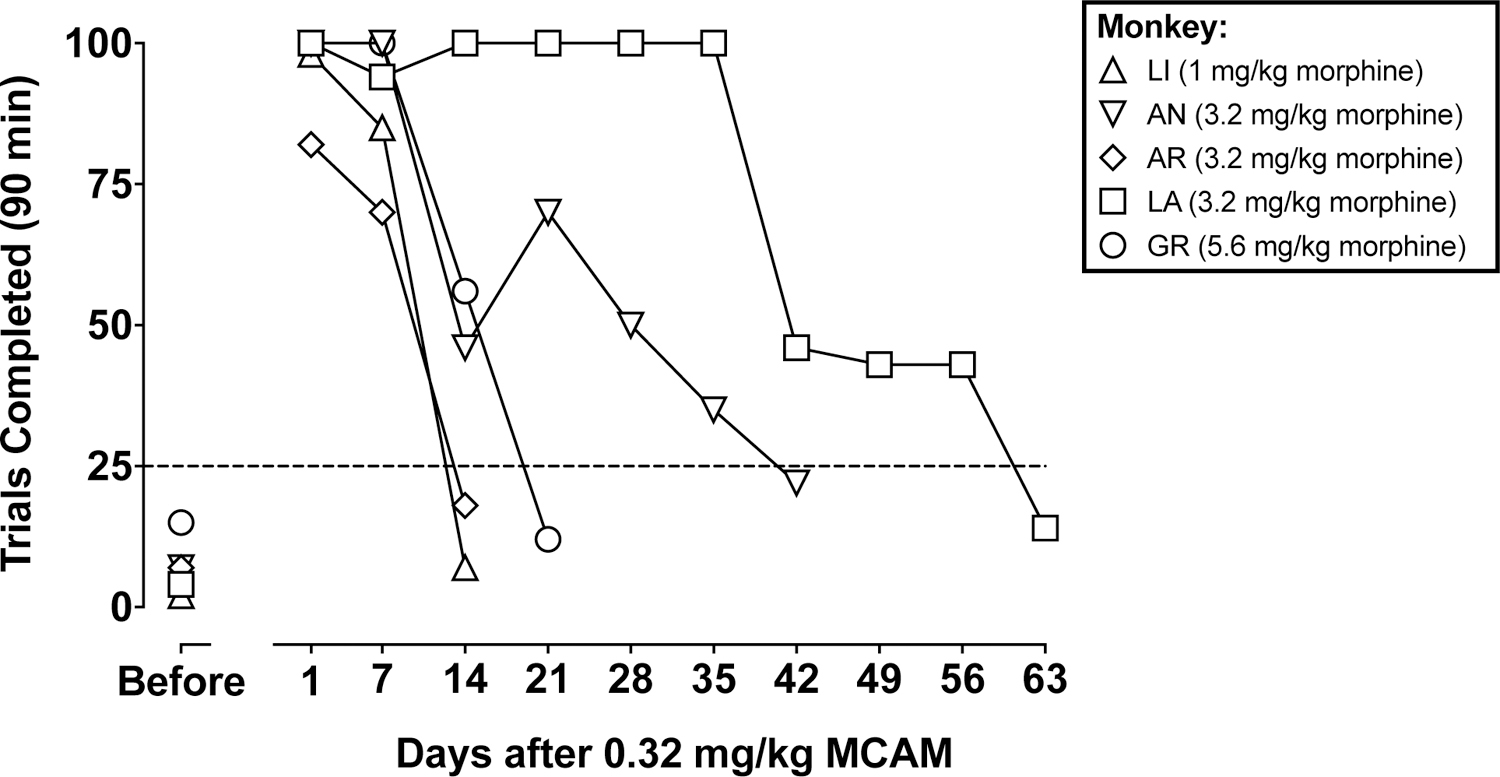
Number of trials completed out of 100 possible during morphine tests for individual monkeys, each represented by a different symbol. Data above “Before” indicate the effects of morphine one day after a saline injection (i.e., before MCAM). The horizontal dashed line is at 25 trials; morphine tests were discontinued when a monkey completed fewer than 25 trials. Abscissae: days after administration of 0.32 mg/kg MCAM.
In contrast to the effects obtained with MCAM and morphine, scopolamine and phencyclidine decreased both delayed matching-to-sample accuracy and the number of trials completed in a dose-dependent manner (Fig 4). A dose of 0.032 mg/kg scopolamine significantly decreased accuracy, compared with saline (Fig 4a; t8=4.8, p=0.003), and this dose as well as 0.056 mg/kg decreased significantly the number of trials completed (Fig 4b; t12=8.1, p<0.001 for 0.032 mg/kg; and t12=7.5, p<0.001 for 0.056 mg/kg). A dose of 0.32 mg/kg phencyclidine decreased significantly accuracy, compared with saline (Fig 4c; t8=3.9, p=0.009), and this dose as well as 0.56 mg/kg decreased significantly the number of trials completed (Fig 4d; t12=3.2, p=0.024 for 0.32 mg/kg; and t12=7.2, p<0.001 for 0.56 mg/kg).
Fig 4.
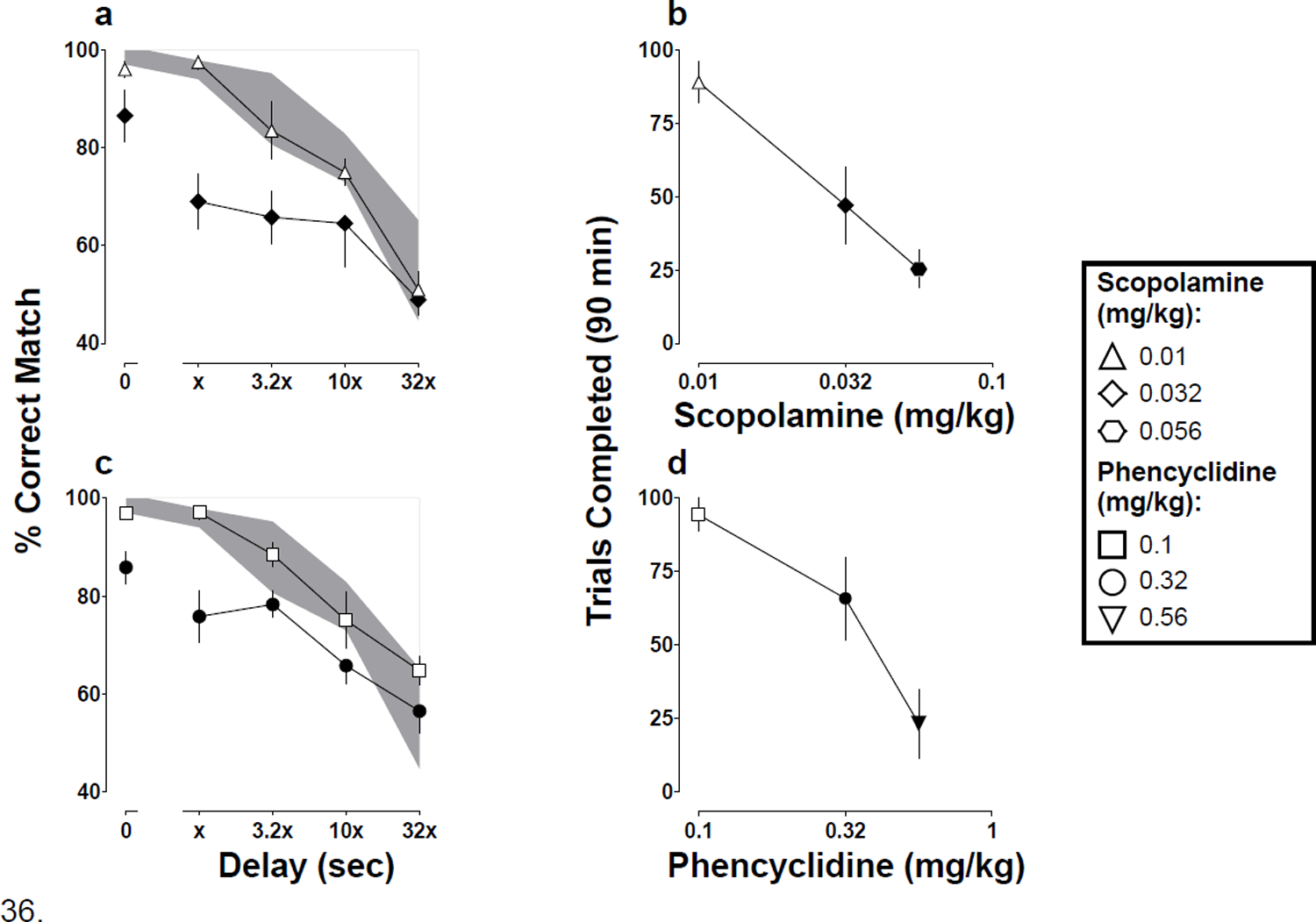
Mean (±1 SEM) accuracy expressed as percent correct matches after scopolamine (4a) or phencyclidine (4c) and mean (±1 SEM) number of trials completed out of 100 possible after scopolamine (4b) or phencyclidine (4d) for five monkeys responding under a delayed matching-to-sample task. Abscissae: delay in seconds between the sample and comparison components, where x = 1 sec for all monkeys except Monkey AN where x = 3.2 sec (4a, 4c) and dose in mg/kg (4b, 4d). Filled symbols denote statistical significance (p<0.05) compared with saline. The shaded regions denote the 95% CI for saline.
DISCUSSION
Abuse of and overdose from opioids remain significant public health challenges (Compton and Jones 2019), despite the availability of medications (methadone, buprenorphine, naltrexone, and naloxone) that are effective in many patients. The pseudoirreversible mu opioid receptor antagonist MCAM might offer significant advantages over the currently approved treatments for opioid use disorder and overdose because of its long duration of antagonist action and because antagonism by MCAM is not surmounted by larger doses of opioid agonist (Broadbear et al. 2000; Gerak et al. 2019b; Peckham et al. 2005). In nonhuman primates, MCAM produces a significant and prolonged suppression of opioid self-administration (Maguire et al. 2019) and it rescues as well as protects against opioid-induced ventilatory depression (Gerak et al. 2019a). The present study tested a dose of MCAM that blocks the effects of opioid agonists under other conditions for its effects on cognition and memory in monkeys responding under a delayed matching-to-sample task.
The primary finding of the present experiment is that a dose of MCAM (0.32 mg/kg) that attenuates opioid self-administration and rescues and protects against heroin-induced ventilatory depression for a week or longer does not impair delayed matching-to-sample performance; that is, accuracy on the delayed matching-to-sample task following MCAM treatment did not differ from accuracy on this task during baseline (no injection) sessions or saline tests. Although the endogenous opioid system can be involved in various aspects of cognitive control (Steenbergen et al. 2019), long-term blockade of mu receptors does not appear to have adverse effects on cognition and memory. Morphine was studied to test whether this dose of MCAM in these subjects had mu opioid receptor antagonist properties. Following an acute injection of MCAM, the behavioral suppressant effects of morphine were blocked completely or markedly attenuated in all monkeys for at least seven days (and much longer in some subjects [Fig. 3]) indicating marked and long-lasting antagonist effects of MCAM in these subjects. Moreover, this same dose of MCAM significantly decreases the positive reinforcing effects of the mu opioid receptor agonists remifentanil and heroin (Maguire et al. 2019) and protects against the ventilatory-depressant effects of heroin (Gerak et al. 2019a) for a week or longer. Taken together, these findings suggest that MCAM could have therapeutic value at doses that are without adverse effects on cognition and memory as determined by performance in the delayed-matching-to-sample task.
MCAM did not decrease the number of trials completed in the delayed-matching-to-sample task on the day it was administered (Fig 1b) or in subsequent sessions (not shown). This was a noteworthy finding because endogenous opioids are thought to mediate motivation of food intake. For example, compared with wild-type controls, genetic knockout of mu opioid receptors in mice decreases responding for and consumption of grain-based and sucrose-based pellets (Papaleo et al. 2007). Similarly, blockade of mu opioid receptor signaling with the irreversible mu opioid receptor antagonist beta-funaltrexamine (β-FNA) reduces consumption of high-caloric, palatable food such as high-fat chow and chocolate-flavored Ensure without reducing consumption of standard chow (Lenard et al. 2010); however, β-FNA also has agonist properties at kappa opioid receptors (Qi et al. 1990) and activation of kappa opioid receptors can decrease intake of palatable food (Cooper et al. 1985). In contrast to β-FNA, MCAM does not have agonist properties at kappa opioid receptors and, in fact, has very short-lasting antagonist properties at kappa receptors (Broadbear et al. 2000; Gerak et al. 2019b). In the present experiment, any pellets earned during the session were consumed relatively quickly (within 90 min). A previous study found that 3.2 mg/kg MCAM (a dose 10-fold larger than the dose used in the present experiment) had no effect in monkeys responding for sucrose pellets under a simple fixed-ratio 10 schedule of reinforcement (Maguire et al. 2019). The current data add to those results and suggest a favorable safety profile for MCAM in that there was no effect on accuracy or the number of trials completed in the delayed-matching-to-sample task. Longer term studies that administer MCAM repeatedly are needed to model conditions relevant to using MCAM to treat opioid use disorder.
The duration of action of MCAM was confirmed with administration of the mu opioid receptor agonist morphine at various times after a single injection of MCAM. In the present experiment, the dose of morphine used was individually tailored for each subject so that the dose was functionally equivalent among subjects (i.e., the smallest dose of morphine that decreased the number of trials completed to fewer than 25). Full morphine dose-response functions were not obtained after MCAM treatment; the delayed-matching-to-sample task is not well-suited for within-session cumulative-dosing procedures because it requires presentation of many trials to quantify accuracy across a series of delays. In all subjects, MCAM antagonized the effects of morphine for at least 7 days, for most subject for 14 days, and for some subjects every longer. The apparent duration of action of MCAM was substantially longer in Monkey LA compared with the other subjects (Fig 3).
There are a number of reasons why Monkey LA appeared different from the others with regard to the return to sensitivity to morphine. Variability in the effects of a single dose of morphine might contribute to this effect. That is, if a larger morphine dose had been chosen for this monkey (e.g., 5.6 mg/kg or 10 mg/kg), then it is possible that the duration of antagonism by MCAM would have more closely approximated the effects obtained in other subjects. There were 3 consecutive tests (days 42, 49, and 56 after MCAM) when the number of trials completed was reduced significantly (although still greater than the pre-defined threshold of < 25), suggesting a return of sensitivity to the behavioral suppressant effects of morphine. Maguire et al. (2019) also reported individual subject variability with respect to the time course of MCAM in monkeys self-administering remifentanil or heroin; for 0.32 mg/kg MCAM administered intravenously the shortest and longest durations of action of were 4 days and 22 days, respectively. Differences in the time course of MCAM among individual subjects, here and in previous work, might represent differences in the total number of mu receptors, the fraction of available mu receptors, or both. Imaging studies might be helpful in determining whether receptor differences are related to variations among individuals.
Morphine had no significant effect on performance under the delayed-matching-to-sample task. These results are consistent with prior studies in the effects of morphine in monkeys (e.g. Bain et al. 2003). Even when morphine was administered in doses that significantly decreased significantly the number of trials completed, delayed-matching-to-sample accuracy was not changed significantly, compared with control performance. Because the task involved just two stimuli and the subjects were well-trained on the task prior to testing drugs, it is possible that the task was not sufficiently difficult to detect any disruptions in performance. Therefore, drugs that are known to disrupt learning and memory under a broad range of conditions were also studied to test for the sensitivity of this procedure and these monkeys for detecting drug-induced impairments in performance. Scopolamine and phencyclidine were selected because they have well-characterized memory impairing effects (McMillan 1981; Thompson et al. 1986; Danysz et al. 1988; Yamamoto et al. 2011; Mathews et al. 2018). Both scopolamine and phencyclidine decreased delayed matching-to-sample accuracy in a dose-related manner (Fig 4a, 4c), demonstrating that this task and these monkeys were sensitive to memory impairing effects of drugs. Doses of scopolamine or phencyclidine that decreased accuracy also decreased the number of trials completed (Fig 4b, 4d); however, these apparent behavioral suppressant effects might not reflect a direct effect of the drugs on behavioral output since a decrease in accuracy necessarily decreases the number of trials that result in delivery of a reinforcer. Moreover, successive trials in which a reinforcer is not delivered could increase the likelihood of extinction of responding.
The pseudo-irreversible mu opioid receptor antagonist MCAM might be useful for treating opioid use disorder and opioid overdose (Gerak et al. 2019a, 2019b; Maguire et al. 2019). One potential concern is that the endogenous opioid system is involved in various aspects of cognition and that long-term blockade of mu opioid receptors might adversely impact complex operant behavior involving memory (Cohen et al. 1983a, 1983b; Flood et al. 1987; Freeman and Young 2000; Nathan et al. 2012; Steenberger et al. 2019). The primary findings from the current study are that a single injection of MCAM (0.32 mg/kg) provided long-lasting (at least 7 days) antagonism of mu agonist effects without impairing delayed matching-to-sample accuracy maintained by delivery of sucrose pellets. These data are consistent with the duration of action of MCAM in previous reports (Broadbear et al. 2005; Gerak et al. 2019a, 2019b; Maguire et al. 2019) and add to a growing body of evidence supporting the safety profile of MCAM.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) National Institute on Drug Abuse (Grants R01DA005018 [to CPF], R01DA048417 [to CPF], and R01DA07315 [to SMH]) and the Welch Foundation (Grant AQ-0039 [to CPF]). The content expressed here is solely the responsibly of the authors and does not necessarily represent the views of the NIH.
The authors thank Lisa Gerak and David Maguire for helpful comments on the manuscript.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
CPF is a coholder of a US provisional patent for MCAM.
REFERENCES
- Bain JN, Prendergast MA, Terry AV, Arneric SP, Smith MA, Buccafusco JJ (2003) Enhanced attention in rhesus monkeys as a common factor for the cognitive effects of drugs with abuse potential. Psychopharmacology 169: 150–160. [DOI] [PubMed] [Google Scholar]
- Blough DS (1959). Delayed matching in the pigeon. Journal of the Experimental Analysis of Behavior 2: 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbear JH, Sumpter TL, Burke TF, Husbands SM, Lewis JW, Woods JH, Traynor JR (2000) Methocinnamox is a potent, long-lasting, and selective antagonist of morphine-mediated antinoniception in the mouse: comparison with clocinnamox, β-funaltrexamine, and β-chlornaltrexamine. J Pharmacol Exp Ther 294: 933–940. [PubMed] [Google Scholar]
- Carpenter M, Berry H, Pelletier AL (2019) Clinically relevant drug-drug interactions in primary care. Am Fam Physician 99: 558–564. [PubMed] [Google Scholar]
- Chamberlain SR, Mogg K, Bradley BP, Koch A, Dodds CM, Tao WX, Maltby K, Sarai B, Napolitano A, Richards DB, Bullmore ET, Nathan PJ (2012) Effects of mu opioid receptor antagonism on cognition in obese binge-eating individuals. Psychopharmacology 224: 501–509. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Cohen MR, Weingartner H, Pickar D, Murphy DL (1983b) High-dose naloxone affects task performance in normal subjects. Psychiatry Res 8: 127–136. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Cohen RM, Pickar D, Weingartner H, Murphy DL (1983a) High-dose naloxone infusions in normal: dose-dependent behavioral, hormonal, and physiological responses. Arch Gen Psychiatry 40: 613–619. [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM (2019) Epidemiology of the U.S. opioid crisis: the importance of the vector. Ann NY Acad Sci. DOI: 10.1111/nyas.14209 [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- Cooper SJ, Moores WR, Jackson A, Barber DJ (1985) Effects of tifuadom on food consumption compared with chlordiazepoxide and kappa agonists in the rat. Neuropharmacology 24: 877–883. [DOI] [PubMed] [Google Scholar]
- Dahan A, Aarts L, Smith TW (2010) Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology 112: 226–238. [DOI] [PubMed] [Google Scholar]
- Danysz W, Wroblewski JT, Costa E (1988) Learning impairment in rats by N-methyl-D-aspartate receptor antagonists. Neuropharmacology 27: 653–656. [DOI] [PubMed] [Google Scholar]
- Effects of environmental and pharmacological manipulations on a novel delayed nonmatching-to-sample “working memory” procedure in unrestrained rhesus monkeys. J Neurosci Methods 251: 62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Cherkin A, Morley JE (1987) Antagonism of endogenous opioids modulates memory processing. Brain Research 422: 218–234. [DOI] [PubMed] [Google Scholar]
- Freeman FM, Young IG (2000) Identification of the opioid receptors involved in passive-avoidance learning in the day-old chick during the second wave of neuronal activity. Brain Research 864: 230–239. [DOI] [PubMed] [Google Scholar]
- Gerak LR, Maguire DR, Woods JH, Husbands SM, Disney A, France CP (2019a) Reversal and prevention of the respiratory-depressant effects of heroin by the novel mu-opioid receptor antagonist methocinnamox in rhesus monkeys. J Pharmacol Exp Ther 368: 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, Minervini V, Latham E, Ghodrati S, Lillis KV, Wooden J, Disney A, Husbands SM, France CP (2019b) Methocinnamox (MCAM) produces long-lasting antagonism of the behavioral effects of µ opioid receptor agonists but not prolonged precipitated withdrawal in rats. J Pharmacol Exp Ther. DOI: 10.1124/jpet.119.260331 [e-pub ahead of print] [DOI] [PMC free article] [PubMed]
- Jones JD, Mogali S, Comer SD (2012) Polydrug abuse: a review of opioid and benzodiazepine combination use. Drug Alcohol Dependence 125: 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, McCarthy DM, Courtney DM, Lank PM, Lambert BL (2017) Benzodiazepine-opioid co-prescribing in a national probability sample of ED encounters. American Journal of Emergency Medicine 35: 458–464. [DOI] [PubMed] [Google Scholar]
- Lenard NR, Zheng H, Bethoud HR (2010) Chronic suppression of mu-opioid receptor signaling in the nucleus accumbens attenuates development of diet-induced obesity in rat. Int J Obes (Lond) 34: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, Woods JH, Husbands SM, Disney A, France CP (2019) Long-lasting effects of methocinnamox on opioid self-administration in rhesus monkeys. J Pharmacol Exp Ther 368: 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews MJ, Mead RN, Galizio M (2018) Effects of N-Methyl-D-aspartate (NMDA) antagonists ketamine, methoxetamine, and phencyclidine on the odor span test of working memory in rats. Exp Clin Psychopharmacol 26: 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan DE (1981) Effects of chemicals on delayed matching behavior in pigeons I: acute effects of drugs. Neurotoxicology 2: 485–498. [PubMed] [Google Scholar]
- Nathan PJ, Bush MA, Tao WX, Koch A, Davies KM, Maltby K, O’Neil BV, Napolitano A, Skeggs AL, Brooke AC, Richards DB, Williams PM, Bullmore ET. Multiple-dose safety, pharmacokinetics, and pharmacodynamics of the mu-opioid receptor inverse agonist GSK1521498. J Clin Pharmacol 52: 1456–1467. [DOI] [PubMed] [Google Scholar]
- Papaleo F, Kieffer BL, Tabarin A, Contarino A (2007) Decreased motivation to eat in mu-opioid receptor-deficient mice. Eur J Neurosci 25: 3398–3405. [DOI] [PubMed] [Google Scholar]
- Peckham EM, Barkley LM, Divin MF, Cicero TJ, Traynor JR (2005) Comparison of the antinociceptive effect of acute morphine in female and male Sprague-Dawley rats using the long-lasting mu-antagonist methocinnamox. Brain Research 1058: 137–147. [DOI] [PubMed] [Google Scholar]
- Pergolizzi JV, Rosenblatt M, LeQuang JA (2019) Three years down the road: the aftermath of the CDC guideline for prescribing opioids for chronic pain. Adv Ther 36: 1235–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JA, Heyman JS, Sheldon RJ, Koslo RJ, Porreca F (1990) Mu antagonist and kappa agonist properties of beta-funaltrexamine (beta-FNA) in vivo: long-lasting spinal analgesia in mice. J Pharmacol Exp Ther 252: 1006–1011. [PubMed] [Google Scholar]
- Steinmiller CL, Young AM (2008) Pharmacological selectivity of CTAP in a warm water tail-withdrawal antinociception assay in rats. Psychopharmacology 195: 497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand MA, Eukel H (2019) A primary prevention approach to the opioid epidemic. American Journal of Public Health 109: 861–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DM, Mastropaolo J, Winsauer PJ, Moerschbaecher JM (1986) Repeated acquisition and delayed performance as a baseline to assess drug effects on retention in monkeys. Pharmacol Biochem Behav 25: 201–207. [DOI] [PubMed] [Google Scholar]
- Van Steenbergen H, Eikemo M, Leknes S (2019) The role of the opioid system in decision making and cognitive control: a review. Cognitive, Affective, & Behavioral Neuroscience (in press). 10.3758/s13415-019-00710-6. [DOI] [PMC free article] [PubMed]
- White GK (1985) Characteristics of forgetting functions in delayed matching to sample. Journal of the Experimental Analysis of Behavior 44: 15–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Nishiyama S, Kawamata M, Ohba H, Wakuda T, Takei N, Tsukada H, Domino EF (2011) Muscarinic receptor occupancy and cognitive impairment: a PET study with [11C](+)3-MPB and scopolamine in conscious monkeys. Neuropsychopharmacology 36: 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeomans MR, Gray RW (1997) Effects of naltrexone on food intake and changes in subjective appetite during eating: evidence for opioid involvement in the appetizer effect. Physiol Behav 62: 15–21. [DOI] [PubMed] [Google Scholar]
- Young AM, Woods JH (1982) Limitations on the antagonistic actions of opioid antagonists. Fed Proc 41: 2333–2338. [PubMed] [Google Scholar]


