Abstract
Epilepsy surgery is considered to reduce the risk of epilepsy-related mortality, including SUDEP, though data from existing surgical series are conflicting. We retrospectively examined all-cause mortality and SUDEP in a population of 590 epilepsy surgery patients and a comparison group of 122 patients with pharmacoresistant focal epilepsy who did not undergo surgery, treated at Columbia University Medical Center between 1977–2014.
There were 34 deaths in the surgery group, including 14 cases of SUDEP. SMR for the surgery group was 1.6 and SUDEP rate was 1.9 per 1,000 patient-years. There were 13 deaths in the comparison group, including 5 cases of SUDEP. SMR for the comparison group was 3.6 and SUDEP rate was 4.6 per 1,000 patient-years. Both were significantly greater than in the surgery group (p<0.05). All but one of the surgical SUDEP cases, and all of the comparison group SUDEP cases had a history of bilateral tonic-clonic seizures (BTCS). Of postoperative SUDEP cases, one was seizure-free and two were free of BTCS at last clinical follow-up. Time to SUDEP in the surgery group was longer than in the comparison group (10.1 vs 5.9 years, p=0.013), with 10 of the 14 cases occurring >10 years after surgery.
All-cause mortality was reduced after epilepsy surgery relative to the comparison group. There was an early benefit of surgery on the occurrence of SUDEP, which was reduced after 10 years. A larger, multicenter study is needed to further investigate the time course of post-surgical SUDEP.
Keywords: epilepsy surgery, epilepsy surgery mortality, SUDEP, standardized mortality ratio
1. Introduction
Sudden Unexpected Death in Epilepsy (SUDEP) is the most common cause of seizure-related mortality in individuals with epilepsy. Epilepsy surgery candidates are among those at highest risk [1]. It is widely assumed that surgery reduces the risk of epilepsy-related mortality, including SUDEP, particularly if patients are rendered seizure-free. Indeed, several surgical series have demonstrated reduced all-cause mortality post-operatively [2–4]. Data from existing surgical series with respect to SUDEP, however, are conflicting, and SUDEP has occurred in patients not known to have postoperative seizures [3,5]. A better understanding of the effect of epilepsy surgery on SUDEP risk is needed to answer crucial patient management questions, including electing surgery for seizure palliation and attempting medication withdrawal post-operatively. The present study aimed to assess the impact of epilepsy surgery on the occurrence of SUDEP by comparing SUDEP rates in individuals who have undergone epilepsy surgery to SUDEP rates in epilepsy surgery candidates who did not undergo resection.
2. Materials and Methods
2.1. Subjects
Surgical cases were identified through a retrospective surgical database maintained at Columbia University Medical Center (CUMC) that contains records dating back to July 1, 1977. All patients with surgical procedures involving cortical or subcortical resection prior to December 31, 2014 were included. Cases with active underlying processes such as autoimmune disease or high-grade tumors were excluded. A comparison population was identified by review of consecutive available surgical case conference records at CUMC from 1996 to December 31, 2014. Patients considered to have pharmacoresistant focal epilepsy and to be candidates for either invasive EEG monitoring, curative brain resection, or responsive neurostimulation implantation were included. Patients with acute presentations or active underlying processes were excluded, as were patients with generalized, combined generalized and focal, or unknown epilepsy syndromes. This was done in order to minimize differences between the surgery population and the comparison group, as such cases are rarely, if ever, referred for curative surgical procedures.
2.2. SUDEP case identification
Inpatient and outpatient clinical records for all patients in the cohort were reviewed to determine the time of last clinical contact, vital status and details surrounding death when available. Clinical vital status in the electronic medical record system is updated annually from National Death Index (NDI) reports. A list of patients identified as deceased through either record review or direct physician reporting was submitted to the NDI to confirm status and determine cause of death. Results included deaths through January 1, 2015. The Nashef, et al. unified definition was used for classification of deaths as SUDEP [6], with cases adjudicated by two board-certified epileptologists (LMB, CAS) on review of available clinical data, including medical records, death certificates and autopsy reports. Specifically, cases meeting criteria for definite, probable or possible SUDEP, or definite/probable SUDEP Plus, were counted as SUDEP cases. All-cause mortality, including the SUDEP deaths, was also determined.
The study was approved by the Institutional Review Board at CUMC, which waived the need for written informed consent due to the study’s retrospective character.
2.3. Statistical analysis
Standardized mortality ratios (SMR) for all deaths in each population were calculated. The number of observed deaths was compared to age and gender-specific mortality data for expected deaths in New York State as reported for 2014. SUDEP rates were described as deaths per 1,000 person-years.
Statistical comparisons between SUDEP rates in the surgery and comparison groups were made using chi-square, Fisher’s exact test, or Wilcoxon non-parametric rank sum test. The number of patient-years of follow-up was calculated from the date of surgery for the surgical case group and from the date of the surgical case conference in the comparison group, as the delay to surgery from case conference date in the surgical group was < 1 year. Surgical outcomes were assessed using the Engel outcome scale [7].
3. Results
3.1. Epilepsy surgery population
There were 590 patients (322 female, 268 male) identified in the epilepsy surgery group (Table 1). The average age of epilepsy onset was 12.9+/−10.9 years. Average age at the date of surgery was 32.5 +/− 14.6 years. The time from surgery to the study endpoint, which was December 31, 2014 or the date of death, whichever came first, ranged from 4 months to 25 years, with an average of 12.9 years.
Table 1.
Patient demographics
| Surgical group | Comparison group | p value (significant findings) | |
|---|---|---|---|
| N (females) | 590 (322) | 122 (66) | |
| Mean/SD age at date of service* (years) | 32.5 +/− 14.6 | 36.2 +/− 14.5 | |
| Mean age of epilepsy onset (years) | 12.9 | 16.9 | < 0.01 |
| Mean follow-up (years) | 12.9 | 8.9 | < 0.001 |
| Total patient-years | 7371.7 | 1096 | |
| Total deaths | 34 | 13 | |
| SUDEP deaths | 14 | 5 | |
| SUDEP rate (per 1000 patient-years) | 1.9 | 4.6 | 0.04 |
Date of service is defined as date of surgery in the surgical group and date of surgical case conference in the comparison group.
There were 34 deaths in the surgery group, including 14 cases of definite, probable, or possible SUDEP. Causes of death are given in Table 2. The overall SMR was 1.6 and the SUDEP rate was 1.9 per 1,000 patient-years (95% confidence interval: 1.04–3.19). The average age of epilepsy onset for SUDEP cases was 12.6 years (range 1–30 years); with duration of epilepsy of 27.4 years (range 2–50). Surgical outcomes of the SUDEP cases by the Engel outcome scale and known postoperative follow-up time for seizure status are shown in Table 3. One patient was classified as Engel 1C at the last known follow up at 8 years, and six patients were classified as Engel 2. All but Patient 12 had a history of bilateral tonic-clonic seizures (BTCS) prior to surgery. Patients 9 and 11 were reported as being free of BTCS after surgery.
Table 2.
All causes of death in surgery and comparison groups
| Cause of Death | Surgery Group | Comparison Group |
|---|---|---|
| SUDEP | 14 | 5 |
| Tumor/cancer | 8 | 2 |
| Cardiovascular disease | 5 | 1 |
| Suicide/accidental | 3 | 2 |
| Systemic sclerosis | - | 1 |
| COPD | - | 1 |
| Status epilepticus | 1 | - |
| Diabetes | 1 | - |
| ALS | 1 | - |
| Lupus | 1 | 1 |
| TOTAL | 34 | 13 |
Table 3.
Demographics of surgical SUDEP cases, in order of survival time after surgery.
| Patient | Gender | Age of epilepsy onset | Time from surgery to SUDEP (years) | Age at SUDEP (years) | Seizure onset zone/site of surgery | Time to seizure recurrence post-surgery (years) | Last post-surgical follow up (years) | Engel Outcome at last follow-up |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 1 | 1.1 | 3.2 | Left temporal | <1 | 0.6 | 3A |
| 2 | M | 18 | 4.7 | 39.7 | Left temporal | 0.25 | 5.1 | 2D |
| 3 | F | 18 | 6.5 | 34.4 | Right temporal | 0.33 | 6.3 | 4A |
| 4 | F | 15 | 8.9 | 29.2 | Left temporal | 4 | 8.2 | 2D |
| 5 | M | 30 | 10.4 | 52.0 | Left temporal | 3 | 9.9 | 3A |
| 6 | M | 17 | 10.9 | 44.5 | Left temporal | >2 | 1.0 | 4B |
| 7 | M | 9 | 11.3 | 31.7 | Right mesial temporal | >2 | 6.7 | 2D |
| 8 | M | 17 | 11.3 | 47.8 | Left temporal | 2 | 10.5 | 2D |
| 9 | M | 1 | 11.4 | 50.6 | Right temporal | 10 | 11.2 | 2A |
| 10 | F | 12 | 11.7 | 46.8 | Left temporal | <1 | 10.5 | 4A |
| 11 | M | 6 | 11.7 | 47.3 | Right temporal | 1 | 7.9 | 1C |
| 12 | F | 23 | 13.0 | 46.0 | Left temporal | <1 | 9.8 | 4B |
| 13 | M | 7 | 13.4 | 51.4 | Right temporal | 11 | 12.5 | 2B |
| 14 | M | 2 | 15.8 | 35.2 | Right frontal and temporal | <1 | 1.0 | 4A |
3.2. Comparison population
There were 122 patients (66 female, 56 male) identified in the comparison group of epilepsy surgery candidates (Table 1). The gender distribution was not significantly different between the two groups (chi-square, p=0.92). Age at epilepsy onset was 16.9+/−12.9 years, which was greater than that of the surgery group (t test, p<0.01). Average age at the surgical case conference date was 36.2+/−14.5 years. This was not significantly different from the age at surgery date in the surgical group. Of these patients, 17 had intracranial EEG implants with no subsequent resection, 86 were recommended for targeted interventions (intracranial EEG monitoring for 62, surgical resection without invasive EEG monitoring for 20, RNS for four), seven were recommended for vagal nerve stimulation or callosotomy, eight for further testing and medication trials, and four were deemed not to be surgical candidates due to failure to define a single seizure focus. The average time from the surgical case conference date to study endpoint was 8.9 years, with the longest at 19 years. The shortest duration was only ten days, reflecting a drowning death soon after the case conference. Follow up duration was significantly shorter for the comparison group than in the surgery cases (Wilcoxon rank-sum test, p<.001).
There were 13 deaths in the comparison group including five cases of definite, probable or possible SUDEP. The overall SMR was 3.6, which was significantly greater than the surgical group (Wilcoxon rank-sum test, p=0.049). The SUDEP rate was 4.6 per 1000 patient-years (95% confidence interval: 1.48–10.65). This was significantly greater than in the surgery group (cases: 7371.7 patient-years and 14 SUDEP deaths, comparison group 1096 patient-years and 5 SUDEP deaths, Fisher’s exact test, p=0.04). The age of epilepsy onset of SUDEP cases in the comparison group was greater than that for the surgical SUDEP cases (mean 24.2 vs 16.9 years), but the difference was not significant (t-test, p=0.18). Epilepsy duration also did not differ significantly between SUDEP cases in the two populations (27.0 vs 27.4 years, t-test, p=0.75). All five SUDEPs occurred in patients known to have BTCS. The demographic information for the comparison group SUDEP cases is shown in Table 4.
Table 4.
Demographics of SUDEP cases in the comparison group, in order of survival time after case conference presentation.
| Patient | Gender | Age of onset | Time from case conference to SUDEP (years) | Age at SUDEP (years) | Seizure onset zone | Last post-conference follow up (years) |
|---|---|---|---|---|---|---|
| 1 | M | 20 | 0.7 | 34.8 | Bilateral temporal | N/A* |
| 2 | F | 14 | 1.0 | 33.3 | Bilateral occipital and bilateral temporal regions | 1.0 |
| 3 | M | 12 | 6.9 | 67.0 | Left temporal and frontal | 0.02 |
| 4 | F | 61 | 8.5 | 71.3 | Bilateral hippocampal | 6.3 |
| 5 | M | 15 | 12.5 | 51.5 | Right temporal and occipital | 4.5 |
No clinical follow up records were available for this patient.
3.3. Cause of death and time course of SUDEP
The leading cause of death for both groups was SUDEP (Table 2). Other causes included tumor, suicide or accidental death, status epilepticus, and other medical or neurological conditions.
A closer evaluation of the data revealed that all but four of the SUDEP deaths in the surgery group occurred 10 years or more after the surgery date, with an average of 10.1 years (range 4.6–15.8 years). For the comparison group, the average time to SUDEP from the case conference date was 5.9 years (range 8 months to 12.5 years); only one SUDEP occurred more than 10 years after the case conference date. This difference was significant (Wilcoxon rank sum test, p=<0.0125). Figure 1 shows SUDEP rates during four epochs after the beginning of follow up for the surgical and comparison groups, divided at two, five, and ten years, demonstrating a narrowing gap in SUDEP rates over time, especially after 10 years.
Figure 1:
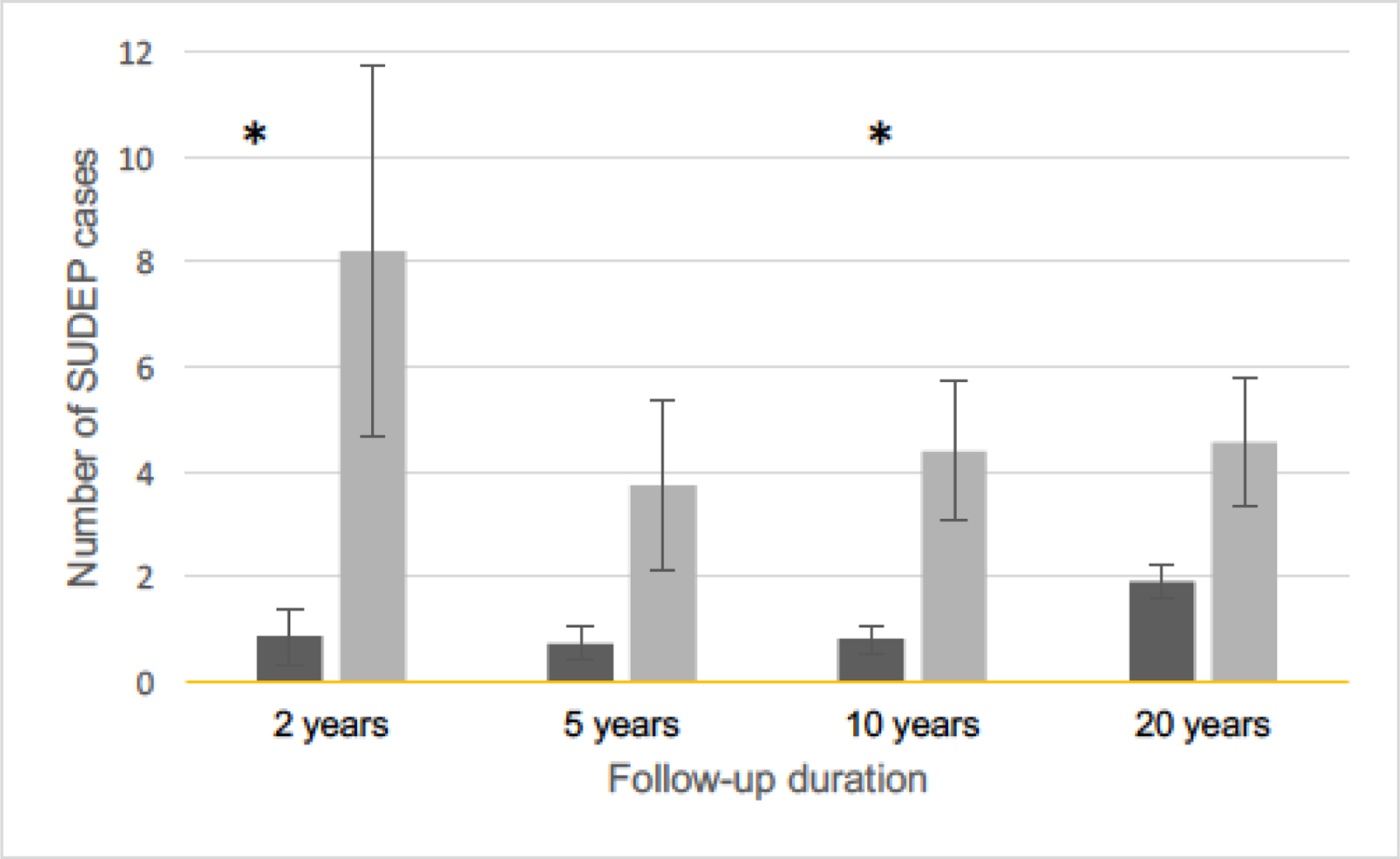
Comparison of SUDEP rates between groups over time. SUDEP rates=deaths/1000 patient-years and 95% confidence intervals over time in the surgery (dark grey) and comparison (light grey) groups. The rate in the surgery group is notably low and stable through 10 years of follow up, then rises after the 10-year mark. The SUDEP rate in the comparison group is immediately higher and remains relatively stable across the entire follow up period. The difference between SUDEP rates in the surgery and comparison groups are significant at 0–2 years (cases: 1156.6 patient-years and 3 SUDEP deaths, comparison group 237.7 patient-years and 2 SUDEP deaths, chi-square test, p=0.01) and 5–10 years (cases: 7371.7 patient-years and 14 SUDEP deaths, comparison group 1096 patient-years and 5 SUDEP deaths, chi-square test, p=0.04) after Bonferroni correction (asterisks).
4. Discussion
Our retrospective study found that patients with pharmacoresistant focal epilepsy syndromes who underwent epilepsy surgery had reduced rates of both overall mortality and SUDEP compared to patients who were evaluated for surgery but either were not deemed surgical candidates or declined surgery. These findings are compatible with the conclusions of a decision analysis model predicting a 5-year increase in survival following temporal lobe resection in typical surgically eligible patients [8], and other surgical series demonstrating reductions in all-cause mortality following epilepsy surgery, with potential specific benefits for SUDEP [3,9,10].
Prior studies have demonstrated conflicting results as to specific SUDEP risk reduction following epilepsy surgery. Bell et al. found an approximately 4.5-fold reduction in SUDEP rates, while Nilsson et al. demonstrated a 2.6-fold reduction [9,10]. Others, however, did not demonstrate SUDEP-specific risk reduction, even when there were documented benefits in all-cause mortality [2,5]. Further, one study with an average of 25 years of post-surgical follow-up demonstrated no beneficial effect of epilepsy surgery on survival compared with medically treated patients [11]. However, there were differences in comparison populations that may have influenced results. Two studies, like ours, used comparison groups drawn from patients considered to be surgical candidates [9,10]. Others compared the epilepsy surgery group to a general epilepsy population [2–4] or did not clearly specify a comparison group [5,12]. Our comparison group did have notable differences from the surgical group, including later age of epilepsy onset and a shorter duration of follow-up. However, if anything, this should have biased the results toward higher mortality, and potentially higher SUDEP rates in the surgery group, given that younger age of epilepsy onset is an independent risk factor for SUDEP [13].
Post-operative seizure freedom and the occurrence of generalized (GTCS) or BTCS have been a focus of prior SUDEP outcome studies. GTCS/BTCS are the seizure type most strongly associated with SUDEP risk [13], but SUDEP is known to occur without GTCS/BTCS, both in witnessed and EEG-monitored cases [14–16]. While none of our SUDEP cases were known to be completely seizure free at the time of death, one had an Engel 1C outcome at the last known follow up 3.7 years prior to the SUDEP death. Six had enjoyed several years of post-operative seizure freedom before relapsing, and two were not known to have BTCS at any time following surgery.
It may be that some portion of SUDEP risk is attributable to overall seizure burden, rather than the occurrence of a specific seizure type alone. In our study, a reduction in SUDEP rate in the epilepsy surgery group was noted in the first 10 years after surgery, but this group’s advantage began to lessen after the 10-year mark. It is known that epilepsy surgery outcomes worsen with time, particularly 10 years or more after surgery [17–20]. Reductions in SUDEP rates have been documented even in patients treated palliatively to reduce seizure burden, including with anticonvulsant medication trials, [21] vagus nerve stimulation (VNS) [22–23], and possibly responsive neurostimulation [24]. Further, the time frame of SUDEP risk benefit appears to parallel the timing of the seizure benefit of these interventions. SUDEP rates have shown to be reduced on a short-term basis during add-on medication trials [21]. SUDEP risk reduction with VNS therapy may occur after a delay of 2 or more years, consistent with the time required to achieve maximal improvement in seizure frequency with this device [22,23]. In our series, the benefit of resective surgery appeared immediately and was sustained for several years before declining. This is consistent with the known temporal profile of seizure control after epilepsy surgery [17–20]. Thus, the timing of SUDEP benefit for these different therapies appears to correlate well with their respective impacts on seizure burden.
Prior outcome studies have not examined the time-dependent evolution in SUDEP risk following surgery. Several of these may have been able to detect a comparable early benefit, with mean follow up times ranging from 5–25 years [4,9,11,12]. This underscores a need to continue long-term follow up of epilepsy surgery patients, even if seizure-free, and to revisit surgical options in patients who experience seizure recurrence.
4.1. Limitations
Although our study yielded statistically supported findings, the overall number of SUDEP cases in both groups is small, and the number of comparison population cases in our series, while comparable to other studies, is limited. Additionally, while our selection of the comparison group was designed to minimize differences with the surgical group and is comparable or more stringent than the criteria used in previous studies, it is possible that the epilepsy syndromes of the two groups have differing risk for SUDEP. However, the age of onset being greater in the comparison group, as well as the inclusion criteria of presentation for consideration of epilepsy surgery, generally reserved for likely surgical candidates at our center, mitigates the possibility of different populations in the surgery and comparison groups.
The rates of SUDEP and all-cause mortality were lower in our comparison group than have been reported in similar populations [1,9,10,11]. This could reflect the improved survival benefit demonstrated for individuals receiving care in a comprehensive epilepsy program (CEP) [25]. Our finding of a further risk reduction following surgery over optimal CEP care without surgical intervention emphasizes the potential additional benefit of epilepsy surgery on mortality and SUDEP in individuals with pharmacoresistant focal epilepsy who may be surgical candidates.
Our finding of a narrowing gap in SUDEP rates over time between surgical cases and a comparison group from a similar population who did not undergo surgery was conducted as a post hoc analysis and not as a primary outcome. A larger multicenter study is needed to confirm our observation of the temporal pattern of SUDEP following epilepsy surgery.
5. Conclusions
In conclusion, our study has demonstrated a reduction in all-cause mortality and SUDEP after epilepsy surgery, relative to a comparison group of epilepsy surgery candidates who did not undergo resection. There was an early benefit of surgery on the occurrence of SUDEP, which was reduced after 10 years. A larger, multicenter study is needed to further investigate the time course of post-surgical SUDEP, and perhaps could be used to identify specific predictors of SUDEP in the epilepsy surgery population.
Highlights:
All-cause mortality is reduced after surgery in patients with pharmacoresistant focal epilepsy.
SUDEP rate is reduced after surgery in patients with pharmacoresistant focal epilepsy.
The benefit of epilepsy surgery on SUDEP occurrence is seen early, but is reduced after 10 years.
Acknowledgements
This study was supported by NIH/NINDS (R01-NS084142 to CAS) and Citizens United for Research in Epilepsy (CURE), Henry Lapham Memorial Award (to LMB, CAS).
Footnotes
Declarations of Interest
None.
References
- [1].Téllez-Zenteno JF, Ronquillo LH, Wiebe S. Sudden unexpected death in epilepsy: Evidence-based analysis of incidence and risk factors. Epilepsy Res 2005;65:101–15. doi: 10.1016/J.EPLEPSYRES.2005.05.004. [DOI] [PubMed] [Google Scholar]
- [2].Salanova V, Markand O, Worth R. Temporal lobe epilepsy surgery: Outcome, complications, and late mortality rate in 215 patients. Epilepsia 2002;43:170–4. doi: 10.1046/j.1528-1157.2002.33800.x. [DOI] [PubMed] [Google Scholar]
- [3].Seymour N, Granbichler CA, Polkey CE, Nashef L. Mortality after temporal lobe epilepsy surgery. Epilepsia 2012. doi: 10.1111/j.1528-1167.2011.03343.x. [DOI] [PubMed] [Google Scholar]
- [4].Sperling Michael R, Harris Adam, Maromi Nei, Liporace Joyce, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia 2005;46:49–53. doi: 10.1007/978-3-319-17783-0_9. [DOI] [PubMed] [Google Scholar]
- [5].Garcia de Almeida A, Nunes ML, Palmini ALF, Costa da Costa J. Incidence of SUDEP in a cohort of patients with refractory epilepsy: the role of surgery and lesion localization. Arq Neuropsiquiatr 2010;68:898–902. doi: 10.1590/S0004-282X2010000600013. [DOI] [PubMed] [Google Scholar]
- [6].Nashef L, So EL, Ryvlin P, Tomson T. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–33. doi: 10.1111/j.1528-1167.2011.03358.x. [DOI] [PubMed] [Google Scholar]
- [7].Engel JJ. Outcome with respect to epileptic seizures. Surg. Treat. Epilepsies, New York: Raven Press; 1987, p. 535–571. [Google Scholar]
- [8].Choi H, Sell RL, Lenert L, Muennig P, Goodman RR, Gilliam FG, et al. Epilepsy Surgery for Pharmacoresistant Temporal Lobe Epilepsy. JAMA 2008;300:2497. doi: 10.1001/jama.2008.771. [DOI] [PubMed] [Google Scholar]
- [9].Bell GS, Sinha S, De Tisi J, Stephani C, Scott CA, Harkness WF, et al. Premature mortality in refractory partial epilepsy: does surgical treatment make a difference? n.d. doi: 10.1136/jnnp.2008.170837. [DOI] [PubMed]
- [10].Nilsson L, Ahlbom A, Farahmand BY, Tomson T. Mortality in a population-based cohort of epilepsy surgery patients. Epilepsia 2003;44:575–81. doi: 10.1046/j.1528-1157.2003.03302.x. [DOI] [PubMed] [Google Scholar]
- [11].Stavem K, Guldvog B. Long-term survival after epilepsy surgery compared with matched epilepsy controls and the general population. Epilepsy Res 2005;63:67–75. doi: 10.1016/J.EPLEPSYRES.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [12].Sperling MR, Barshow S, Nei M, Asadi-Pooya AA. A reappraisal of mortality after epilepsy surgery. Neurology 2016;86:1938–44. doi: 10.1212/WNL.0000000000002700. [DOI] [PubMed] [Google Scholar]
- [13].Hesdorffer DC, Tomson T, Benn E, Sander JW, Nilsson L, Langan Y, et al. Combined analysis of risk factors for SUDEP. Epilepsia 2011;52:1150–9. doi: 10.1111/j.1528-1167.2010.02952.x. [DOI] [PubMed] [Google Scholar]
- [14].Lhatoo SD, Nei M, Raghavan M, Sperling M, Zonjy B, Lacuey N, et al. Nonseizure SUDEP: Sudden unexpected death in epilepsy without preceding epileptic seizures. Epilepsia 2016;57:1161–8. doi: 10.1111/epi.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Donner EJ, Smith CR, Snead OC 3rd. Sudden unexplained death in children with epilepsy. Neurology 2001;57:430–4. doi: 10.1212/WNL.57.3.430. [DOI] [PubMed] [Google Scholar]
- [16].Ba-Armah DM, Donner EJ, Ochi A, Go C, McCoy B, Snead C, et al. “Saved by the Bell”: Near SUDEP during intracranial EEG monitoring. Epilepsia Open 2018;3:98–102. doi: 10.1002/epi4.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dunlea O, Doherty CP, Farrell M, Fitzsimons M, O’Brien D, Murphy K, et al. The Irish epilepsy surgery experience: Long-term follow-up. Seizure 2010;19:247–52. doi: 10.1016/j.seizure.2010.03.001. [DOI] [PubMed] [Google Scholar]
- [18].Salanova V, Markand O, Worth R. Longitudinal follow-up in 145 patients with medically refractory temporal lobe epilepsy treated surgically between 1984 and 1995. Epilepsia 1999;40:1417–23. doi: 10.1111/j.1528-1157.1999.tb02014.x. [DOI] [PubMed] [Google Scholar]
- [19].Yoon HH, Kwon HL, Mattson RH, Spencer DD, Spencer SS. Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology 2003;61:445–50. doi: 10.1212/01.WNL.0000081226.51886.5B. [DOI] [PubMed] [Google Scholar]
- [20].McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GCA, Briellmann RS, Berkovic SF. Temporal lobectomy: Long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 2004;127:2018–30. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- [21].Ryvlin P, Cucherat M, Rheims S. Articles Risk of sudden unexpected death in epilepsy in patients given adjunctive antiepileptic treatment for refractory seizures: a meta-analysis of placebo-controlled randomised trials. Lancet Neurol 2011;10:961–8. doi: 10.1016/S1474. [DOI] [PubMed] [Google Scholar]
- [22].Ryvlin P, So EL, Gordon CM, Hesdorffer DC, Sperling MR, Devinsky O, et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia 2018;59:562–72. doi: 10.1111/epi.14002. [DOI] [PubMed] [Google Scholar]
- [23].Annegers JF, Coan SP, Hauser WA, Leestma J, Duffell W, Tarver B. Epilepsy, vagal nerve stimulation by the NCP system, mortality, and sudden, unexpected, unexplained death. Epilepsia 1998;39:206–12. doi: 10.1111/j.1528-1157.1998.tb01360.x. [DOI] [PubMed] [Google Scholar]
- [24].Devinsky O, Friedman D, Duckrow RB, Fountain NB, Gwinn RP, Leiphart JW, et al. Sudden unexpected death in epilepsy in patients treated with brain-responsive neurostimulation. Epilepsia 2018;59:555–61. doi: 10.1111/epi.13998. [DOI] [PubMed] [Google Scholar]
- [25].Lowerison MW, Josephson CB, Jette N, Sajobi TT, Patten S, Williamson T, et al. Association of levels of specialized care with risk of premature mortality in patients with epilepsy. JAMA Neurol 2019;76;1352–58. doi: 10.1001/jamaneurol.2019.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]


