Abstract
Leuconostoc mesenteroides H40 (H40) was isolated from kimchi, and its probiotic properties and neuroprotective effect was evaluated in oxidatively stressed SH-SY5Y cells. H40 was stable in artificial gastric conditions and can be attached in HT-29 cells. In addition, H40 did not produce β-glucuronidase and showed resistant to several antibiotics. The conditioned medium (CM) was made using HT-29 cells refined with heat-killed probiotics (Probiotics-CM) and heated yogurts (Y-CM) to investigate the neuroprotective effect. Treatment with H40-CM not only increased cell viability but also significantly improved brain derived neurotropic factor (BDNF) expression and reduced the Bax/Bcl-2 ratio in oxidatively stress-induced SH-SY5Y cells. Besides, probiotic Y-CM significantly increased BDNF mRNA expression and decreased Bax/Bcl-2 ratio. The physicochemical properties of probiotic yogurt with H40 was not significantly different from the control yogurt. The viable cell counts of lactic acid bacteria in control and probiotic yogurt with H40 was 8.66 Log CFU/mL and 8.96 Log CFU/mL, respectively. Therefore, these results indicate that H40 can be used as prophylactic functional dairy food having neuroprotective effects.
Keywords: probiotics, Leuconostoc mesenteroides, neuroprotective effect, probiotic yogurt, oxidative stress
Introduction
Probiotics are living microbes that deliver health benefits to the host when ingested in adequate amounts by the FAO/WHO (Chambers et al., 2019). The common probiotic strains mainly belong to the Lactobacillus, Leuconostoc, Pediococcus, and Bifidobacterium species and are widely used in many probiotic products (O’Toole et al., 2017). Probiotics have many reported health benefits such as improvement of cognitive function (Ton et al., 2020), antioxidant (Jang et al., 2018; Yu et al., 2019b), anti-inflammatory (Yu et al., 2019c), antihypertensive (Klippel et al., 2016), or cholesterol lowering (Ishimwe et al., 2015) activities. To utilize this functionality, probiotics are also used as medical or food additives.
The brain, which is rich in phospholipids, is an organ with high oxygen demand and is vulnerable to the effects of reactive oxygen species (ROS) (Dussert et al., 2006). ROS is an essential byproduct of aerobic metabolism (Wang and Michaelis, 2010). However, excessive ROS levels cause cell damage by oxidizing cellular biomolecules, including nucleic acids, proteins, and lipids (Lobo et al., 2010). ROS can contribute to pathologies, such as cancer (Lee et al., 2014), cardiovascular disease (Elahi et al., 2009), diabetes, and aging (Pamplona and Barja, 2006).
The bidirectional signaling connecting the brain and the gastrointestinal tract is crucial for maintaining homeostasis and is regulated the neural, hormonal, and immunological levels (Ghaisas et al., 2016; Wang and Kasper, 2014). Probiotics have recently become a target as live bacterial cell biotherapies for neurodegenerative disease (Quigley, 2017; Wang et al., 2016). Clostridium butyricum can exert neuroprotective effects against ischemia/reperfusion injury mice through antioxidant and anti-apoptosis mechanisms (Sun et al., 2016). Lactobacillus buchneri KU200793 showed neuroprotective effect using SH-SY5Y cells induced with 1‐methyl‐4‐phenylpyridinium (MPP+) (Cheon et al., 2020).
Brain derived neurotropic factor (BDNF) expression occurs in the brain, and low secretion of BDNF influences human memory and hippocampal functions (Egan et al., 2003). BDNF is medicated by extracellular signal-regulated kinase (ERK) 1/2, ERK5, and phosphatidylinositol-3 kinase (PI3k) pathways in cortical neurons to promote neuronal survival (Liu et al., 2003). Oxidative stress may induce mitochondrial dysfunction and deficiency in protein aggregation and ultimately cause nerve cell death (Lobo et al., 2010). The mitochondrial apoptotic pathways are mediated through the Bcl-2 family proteins, which include Bax that promotes pro-apoptotic mitochondrial permeability and anti-apoptotic Bcl-2 that inhibits apoptotic effects (Azmi et al., 2013). The Bax/Bcl-2 ratio is a determining factor in the regulation of apoptotic cell death.
Leuconostoc mesenteroides is bacteria sometimes related to fermentation under salinity and low temperature in fermented foods (Yoon et al., 2018). L. mesenteroides is an obligate heterofermentative lactic acid bacterium that is mostly used in dairy fermentation. L. mesenteroides has been studied as a probiotic strain that facilitates the removal of Pb (II) toxicity (Yi et al., 2017) and inhibits biofilm formation against Listeria monocytogenes (Shao et al., 2019). However, the neuroprotective effects of L. mesenteroides have not been studied. Therefore, the aims of this study were to demonstrate the probiotic properties and neuroprotective effect of L. mesenteroides H40 isolated from kimchi and confirm this effect in yogurt fermented using L. mesenteroides H40.
Materials and Methods
Bacterial strains and culture condition
Lactobacillus fermentum KU200060, Lactobacillus brevis KU200080, and Leuconostoc mesenteroides H40 were isolated from kimchi with salted water, mustard leaf (Brassica juncea) kimchi, and Chinese cabbage kimchi using lactobacilli MRS medium (MRS; BD Biosciences, Franklin Lakes, NJ, USA) and identified by 16S rRNA analysis (Bionics, Seoul, Korea). Lactobacillus rhamnosus GG (Cell Biotech., Gimpo, Korea) was used as a control strain. Bacteria were propagated and maintained in MRS medium at 37°C for 24 h.
Cell culture condition
The HT-29 (human colon adenocarcinoma, KCLB 30038) and SH-SY5Y (human neuroblastoma, KCLB 22266) cells were used for this study. The cells were grown in Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco, Grand Island, NY, USA) and Dulbecco’s Modified Eagle’s Medium (HyClone Laboratories, Logan, UT, USA), respectively. All media were accompanied with 10% (v/v) fetal bovine serum (Gibco) and 1% (v/v) penicillin/streptomycin (Gibco). The cells were maintained at 37°C in 5% CO2. The cultured cells were maintained to monolayer.
Tolerance to artificial gastric conditions
To measure the stability against gastric conditions, artificial gastric juice and bile salts were followed the methods by Yang et al. (2019). The tested strains were incubated in MRS broth at 37°C for 18 h. Initial cells were inoculated at the concentration of 7 Log CFU/mL. Artificial gastric conditions were dealt on 0.3% pepsin (Sigma-Aldrich, St. Louis, MO, USA) adjusted to pH 2.5 at 37°C for 3 h. Artificial bile conditions were used 0.3% oxgall (BD Biosciences) at 37°C for 24 h. After incubation, the survival rate was determined by calculating viable cells on MRS plates.
Adhesion ability to HT-29 cells
The adhesion ability of isolated strains was examined using HT-29. HT-29 cells (1×105 cells/mL) was planted in a 24-well cell culture plate and incubated at 37°C (Lee et al., 2015). After 24 h, isolated strains (1×107 CFU/mL) were inoculated and incubated in HT-29 cells at 37°C for 2 h. Non-adherent bacteria were washed three times using PBS buffer (Gibco), 1% Triton X-100 (Sigma-Aldrich) solution was used for separate the adherent bacteria. The number of adherent bacteria was determined by dilution and plating on MRS plates.
Enzyme production
To measure of enzyme production, the API ZYM kit (BioMerieux, Lyon, France) were used as manufacture’s guideline. Each strain at 6 Log CFU/mL was put in each cupule and incubated at 37°C for 4 h. After incubation, zym A and B reagents put in each cupule, and represented as production concentration (between 0 and ≥40 nM).
Antibiotic resistance
Antibiotic resistance was followed Clinical and Laboratory Standards Institute guideline (CLSI, 2012). One hundred microliters of each lactic acid bacteria (LAB) strains (7 Log CFU/mL) was inoculated onto MRS agar and paper disc were put on agar plate. Used antibiotics were ampicillin (10 μg), gentamycin (10 μg), kanamycin (30 μg), ciprofloxacin (5 μg), chloramphenicol (30 μg), streptomycin (10 μg), tetracycline (30 μg), and doxycycline (30 μg). After incubation at 37°C for 24 h, the inhibitory diameter zone was calculated and compared to the cut-off value (>20 mm, susceptible; 15–19 mm, intermediate; ≤14, resistant) by represented in CLSI.
Conditioned medium (CM) from HT-29 cells
The CM was prepared using HT-29 cells following the method of Park et al. (2017) with minor modifications. For CM preparation, each sample of LAB strains and yogurt was heated at 121°C for 15 min and stored at –80°C upto use. HT-29 cells were inoculated into 6-well plates to 1.0×106 cells/well and incubated to a confluent monolayer. After incubation, cells were handled with heat-killed LAB (8 Log CFU/mL) or heated yogurt for 24 h. CM treated PBS (Gibco) instead to samples were used as control. The mixture was centrifuged (12,000×g, 10 min) and the supernatant was assembled using a syringe filter (0.45 μm pore size, Millipore Sigma, Burlington, MA, USA).
Protective effect on oxidative stress-induced apoptosis
To confirm the protective effect on oxidative stress-induced apoptosis, oxidative stress was induced utilizing H2O2 (Junsei Chemical, Tokyo, Japan) or NaAsO2 (Sigma-Aldrich). The SH-SY5Y cells (100 μL, 1.0×105 cells/well) were inoculated in 96-well plate with of 50 μM H2O2 (20 μL) or 10 μM NaAsO2 (20 μL) for 20 h after pretreatment with 80 μL of sample (CM) for 4 h. After incubation, the media were eliminated, and the cells were incubated with 5 mg/mL MTT solutions (100 μL) for 1 h. After incubation, the liquid was removed and DMSO (100 μL) was added to each well. Absorbance was gauged at 540 nm utilizing microplate reader. The cell viability (%) was calculated as follows:
Yogurt production, physicochemical composition, and viable cell counts of LAB
Yogurt was prepare from whole milk (Seoul Milk, Seoul, Korea) purchased from a local market. The milk was heated at 90°C for 10 min and cooled to 40°C using water bath. An overnight culture of L. mesenteroides H40 was centrifuged (14,000×g, 10 min, 4°C) and the cells were washed twice with PBS (Gibco). Then, the pasteurized milk was inoculated with ABT-B commercial yogurt starter culture containing Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Bifidobacterium longum, and Streptococcus thermophilus (Samik Dairy, Gimje, Korea) or a mixed culture (1:1) of L. mesenteroides H40 and ABT-B commercial yogurt starter culture. The inoculated mixture was incubated at 40°C to pH 4.5. Then, the yogurt samples fortified with L. mesenteroides H40 were ripened for 24 h in the refrigerator, and its physicochemical properties were analyzed. Composition and pH of yogurt was analyzed using Milko Scan Minor (Foss, Hillerod, Denmark) and a pH-meter (WTW inoLab 7110, Weilheim, Germany), respectively. Titratable acidity was assessed according to AOAC International (1999) by titration with sodium hydroxide using phenolphthalein. Measurements of viscosity were performed with Brookfield DV-E Viscometer (Brookfield Eng. Lab, Middleboro, MA, USA) using spindle No. 3. Viable cell counts of LAB in yogurt samples was confirmed using decimal dilutions, spread-plated on MRS medium, and incubation at 37°C for 48 h.
BDNF, Bax, and Bcl-2 expression on oxidative stress-induced apoptosis in SH-SY5Y cells
SH-SY5Y cells (1.0×106 cells/well) were seeded on 6-well plate and incubated to form a confluent monolayer. After incubation, the cells were treated with 800 μL of CM for 4 h. To induce oxidative stress, 200 μL of H2O2 (50 μM) or NaAsO2 (10 μM) was added for 20 h. Total RNA was isolated using the RNeasy Mini total RNA isolation kit (Cheon et al., 2020; Park et al., 2017).
Real-time polymerase chain reaction
The RNA quality was quantified using microplate reader (MultiscanTM Go, Thermo Fisher Scientific, Waltham, MA, USA). cDNA was manufactured using cDNA synthesis kit (Thermo Fisher Scientific). Semi-quantitative real-time PCR was performed according to the PikoReal 96 system (Thermo Fisher Scientific). The reactants contained SYBR Green master mix, primer (Table 1), cDNA, and RNase free water. Further, 20 μL of the mixture was amplified as 95°C for 2 min as initial denaturation; 40 cycles of 95°C for 5 s as denaturation; 60°C for 15 s as annealing and extension. The results were analyzed by △△Ct method using the melt curve analysis method.
Table 1. Primer sequence for neuroprotective effect used in semi-quantitative real-time PCR.
| Gene | Primer sequence | References | |
|---|---|---|---|
| GAPDH | Forward | 5' GAGTCAACGGATTTGGTCGT 3' | Park et al., 2017 |
| Reverse | 5' GACAAGCTTCCCGTTCTCAG 3' | ||
| BDNF | Forward | 5' CAAACATCCGAGGACAAGGTGG 3' | |
| Reverse | 5' CTCATGGACATGTTTGCAGCATCT 3' | ||
| Bax | Forward | 5' GTGGTTGCCCTCTTCTACTTTGC 3' | |
| Reverse | 5' GAGGACTCCAGCCACAAAGATG 3' | ||
| Bcl-2 | Forward | 5' CGGCTGAAGTCTCCATTAGC 3' | |
| Reverse | 5' CGGCTGAAGTCTCCATTAGC 3' |
BDNF, brain-derived neurotrophic factor; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma.
Statistical analysis
All tested data are represented as mean±SD by three replicates. One-way analysis of variance (ANOVA) was utilized to verify significant differences. The mean values were used for the Duncan’s multiple range test to perform post-hoc verification (p<0.05).
Results and Discussion
Tolerance to artificial gastric conditions and adhesion to HT-29 cells
L. fermentum KU200060, L. brevis KU200080, and L. mesenteroides H40 was isolated from various kimchi for probiotic use. L. rhamnosus GG, L. fermentum KU200060, L. brevis KU200080, and L. mesenteroides H40 was confirmed probiotic properties (Table 2; p<0.05). These strains showed high tolerance to artificial gastric conditions. L. rhamnosus GG and L. mesenteroides H40 decreased to 8.51 Log CFU/mL and 7.17 Log CFU/mL in acidic conditions, however increased to 8.58 Log CFU/mL and 8.26 Log CFU/mL in bile conditions, respectively. L. fermentum KU200060 and L. brevis KU200080 showed strong acid tolerance having 8.29 Log CFU/mL and 7.91 Log CFU/mL, however decreased to 7.41 Log CFU/mL and 7.75 Log CFU/mL in bile conditions, respectively. L. plantarum Ln1 and KCTC 3108 showed similar trends having decrease in acidic conditions and remaining in bile conditions (Jang et al., 2018).
Table 2. Probiotic properties of isolated strains.
| Treatment | LGG1) | 200060 | 200080 | H40 |
|---|---|---|---|---|
| Tolerance to artificial gastric conditions [Viable cell number (Log CFU/mL)] | ||||
| Initial cell number | 8.55±0.01a | 8.26±0.01b | 7.79±0.01c | 8.17±0.03b |
| 0.3% (w/v) pepsin, pH 2.5, 3 h | 8.51±0.05a | 8.29±0.01ab | 7.91±0.00b | 7.17±0.01c |
| 0.3% (w/v) oxgall, 24 h | 8.58±0.03a | 7.41±0.01bc | 7.75±0.02b | 8.26±0.01a |
| Adhesion rate (%)2) | 2.34±0.26c | 1.18±0.08d | 3.42±0.49a | 2.86±0.16b |
| β-Glucuronidase (nM) | 0 | 0 | 0 | 0 |
| Antibiotic resistance | Gentamycin, kanamycin, streptomycin, ciprofloxacin | Gentamycin, kanamycin, ciprofloxacin | Gentamycin, kanamycin, streptomycin, ciprofloxacin | Gentamycin, kanamycin, ciprofloxacin |
Data are represented as the mean±SD of triplicate experiments. Means within a row with same superscript differ (p<0.05).
LGG, L. rhamnosus GG; 200060, L. fermentum KU200060; 200080, L. brevis KU200080; H40, L. mesenterodies H40.
Adhesion rate=(adhered bacteria to HT-29 cells after 2 h)/(initial bacteria)×100.
L. rhamnosus GG, L. fermentum KU200060, L. brevis KU200080, and L. mesenteroides H40 showed 2.34%, 1.18%, 3.42%, and 2.86% adhesion rate to HT-29 cells. Especially, L. brevis KU200080 and L. mesenteroides H40 showed a higher adhesion rate than L. rhamnosus GG. Jang et al. (2018) showed lower 2.19% adhesion rate of L. plantarum KCTC 3108. Adhered probiotic strains may be temporary colonization and influence host health trough adjustment of intestinal microflora (Jang et al., 2019; Yu et al., 2019a).
Enzyme production
β-Glucuronidase can be produced by the human intestine microbiota and liberate toxin and mutagen in liver (Dabek et al., 2008). Therefore, isolated strains were confirmed nonproduction of β-glucuronidase using API ZYM kit (Table 2). L. rhamnosus GG produced 30 nM of leucine aryamidase, 30 nM of valine arylamidase, 20 nM of naphthol-AS-BI-phosphohydrase, 20 nM of β-galactosidase, and 30 nM of β-glucosidase. L. fermentum KU200060 produced 30 nM of α-galactosidase and ≥40 nM of β-galactosidase. L. brevis KU200080 produced 20 nM of β-galactosidase, 30 nM of β-glucosidase, and 30 nM of leucine arylamidase. L. mesenteroides H40 produced 20 nM of α-glucosidase and 30 nM of β-glucosidase. α-Galactosidase and β-galactosidase can act the use of indigestible carbohydrates of raffinose family oligosaccharides and milk products, respectively. In addition, β-glucosidase may influence bioavailability by the cleavage of glycosidic bonds in ginsenoside, isoflavone, and phenolic compounds (Son et al., 2018). Produced enzyme by these isolated strains may be useful for carbohydrate digestion.
Antibiotic resistance
L. fermentum KU200060 and L. mesenteroides H40 are resistant to gentamycin, kanamycin, and ciprofloxacin. L. rhamnosus GG and L. brevis KU200080 are resistant to gentamycin, kanamycin, streptomycin, and ciprofloxacin. Among tested antibiotics, most Lactobacillus sp. are intrinsically resistant to aminoglycoside (gentamycin, kanamycin, and streptomycin), inhibitors of nucleic acid synthesis (ciprofloxacin) (Campedelli et al., 2015). Therefore, isolated strains showed a potential of safe probiotic strains in a view of antibiotic resistance.
Protective effects of probiotics-CM on oxidative stress-induced apoptosis in SH-SY5Y cells
H2O2 and NaAsO2 converts to a highly reactive toxic hydroxyl radical (Pardillo-Díaz et al., 2016), causing damage by reducing antioxidant enzymes in brain (Herrera et al., 2013). Additionally, gut microbiota influence the neurophysicals at the base of the gut-brain axis (Park et al., 2017). The modulatory effect of probiotics in intestinal microbiota was demonstrated by increased a ratio of Firmicutes to Bacteriodes and it can relieve inflammation by cytokine expression (Martin et al., 2018). Therefore, the CM using HT-29 cells with probiotics was used for neuroprotective effects.
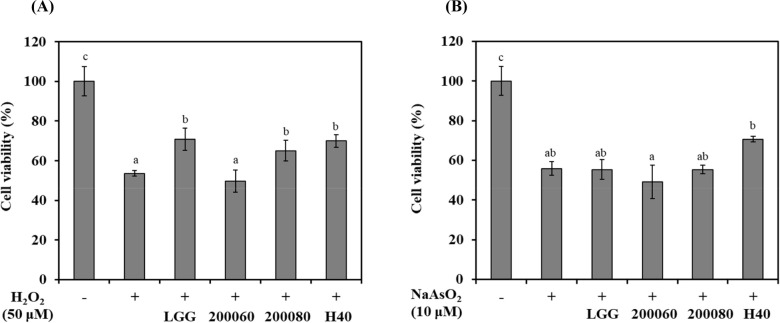
Oxidative stress was induced in SH-SY5Y cells using H2O2 or NaAsO2, and cell viability was confirmed by MTT assay (Fig. 1). During the induction of oxidative stress by H2O2, the cell viability of SH-SY5Y cells was 53.5% (Fig. 1A; p<0.05). The cell viability of the probiotics-CM for L. rhamnosus GG, L. fermentum KU200060, L. brevis KU200080, and L. mesenteroides H40 was 70.7%, 49.7%, 65.0%, and 69.9%, respectively. L. rhamnosus GG, L. brevis KU200080, and L. mesenteroides H40 showed a protective effect compared to H2O2 treated cells (53.5%).
Fig. 1. Cell viability of conditioned medium using lactic acid bacteria (LAB-CM) in SH-SY5Y cells with oxidative stress induced by (A) H2O2 (50 μM) and (B) NaAsO2 (10 μM).
LGG, L. rhamnosus GG; 200060, L. fermentum KU200060; 200080, L. brevis KU200080; H40, L. mesenteroides H40. Error bars indicate standard deviation from three independent experiments. Different letters on each bar represent significantly different (p<0.05). LAB, lactic acid bacteria; CM, conditioned medium.
During induction of oxidative stress by NaAsO2, the cell viability of SH-SY5Y cells was 55.8% (Fig. 1B; p<0.05). The cell viability of the probiotics-CM of L. rhamnosus GG, L. fermentum KU200060, L. brevis KU200080, and L. mesenteroides H40 was 55.3%, 49.2%, 55.3%, and 70.7%, respectively. Only L. mesenteroides H40 showed a protective effect compared to NaAsO2 treated cells (55.8%).
Among these strains, L. mesenteroides H40 has highest cell viability in SH-SY5Y cells using both H2O2 and NaAsO2. Cheon et al. (2020) showed the cell viability of L. rhamnosus GG (72.0%), L. fermentum KU200060 (60.2%), Lactobacillus delbrueckii KU2000171 (66.8%), and L. buchneri KU200793 (73.4%) with MPP+ as Parkinson-inducing toxin having oxidative phosphorylation. Therefore, L. rhamnosus GG and L. mesenteroides H40 was demonstrated neuroprotective effects against oxidative stress.
BDNF mRNA expression and anti-apoptotic effects of probiotics-CM on oxidative stress-induced apoptosis in SH-SY5Y cells
The gut-brain axis (GBA) is bi-directional communication network encompassing the autonomic nervous system (ANS), the central nervous system (CNS), and the enteric nervous system (ENS). These complex network was influenced by gastrointestinal tract (Kennedy et al., 2016; Ranuh et al., 2019). Among serum response factor, BDNF have known as regulator of the synaptic protein and precursors for appropriated neuronal function, survival, and apoptosis (Numakawa et al., 2010). Decreased BDNF mRNA expression confirms brain related diseases such as Alzheimer’s disease, Parkinson’s disease, and depression. Increased ratio of Bax/Bcl-2 induced apoptosis.
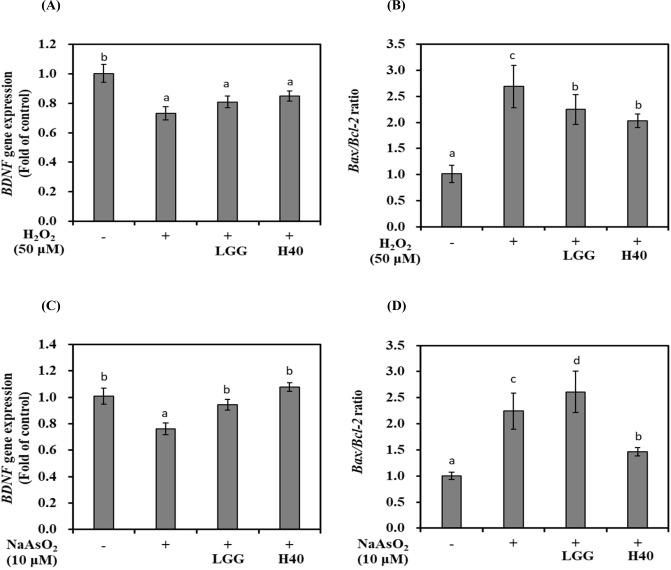
BDNF mRNA expression and Bax/Bcl-2 ratio is shown in Fig. 2. Treatment with H2O2 reduced BDNF mRNA expression by 0.73-fold compared with that in H2O2 nontreated cells (Fig. 2A; p<0.05). L. rhamnosus GG and L. mesenteroides H40 showed 0.80- and 0.85-fold BDNF mRNA expression, respectively. The Bax/Bcl-2 ratio in H2O2 nontreated cells was 1.00-fold, whereas H2O2 increased the ratio of 2.69-fold (Fig. 2B; p<0.05). Treatment with L. rhamnosus GG and L. mesenteroides H40 reduced the Bax/Bcl-2 ratio to 2.24- and 2.03-fold, respectively.
Fig. 2. mRNA expression levels of BDNF and apoptosis-related genes on oxidatively stressed SH-SY5Y cells treated with conditioned medium using lactic acid bacteria (LAB-CM).
(A) BDNF mRNA expression and (B) Bax/Bcl-2 ratio in oxidative stress-induced SH-SY5Y cells induced by H2O2 (50 μM). (C) BDNF mRNA expression and (D) Bax/Bcl-2 ratio in oxidatively stress-induced SH-SY5Y cells induced by NaAsO2 (10 μM). LGG, L. rhamnosus GG; H40, L. mesenteroides H40. Error bars indicate standard deviation from three independent experiments. Different letters on each bar represent significantly different (p<0.05). LAB, lactic acid bacteria; CM, conditioned medium.
Treatment with NaAsO2 reduced 0.76-fold BDNF mRNA expression compared with that in the control without NaAsO2 treatment (Fig. 2C; p<0.05). L. rhamnosus GG and L. mesenteroides H40 represented 0.95- and 1.08-fold BDNF mRNA expression, respectively. The Bax/Bcl-2 ratio in NaAsO2 nontreated cells was 1.00-fold, while NaAsO2 increased 2.24-fold in NaAsO2 treated cells. Treatment with L. rhamnosus GG increased 2.61-fold, while treatment with L. mesenteroides H40 reduced 1.46-fold (Fig. 2D; p<0.05).
L. mesenteroides H40 can increase BDNF mRNA expression and reduce apoptosis of SH-SY5Y cells oxidatively stressed using both H2O2 and NaAsO2. The difference of neuroprotective effect of L. rhamnosus GG and L. mesenteroides H40 depends on strain and oxidant.
Physicochemical property and LAB cell counts of control and probiotic yogurt
Yogurt is a major probiotic carrier to consumers without side-effect. Each yogurt was manufactured using the following: 1) ABT-B commercial starter culture (control yogurt) and 2) ABT-B commercial starter mixed with L. mesenteroides H40 (probiotic yogurt). The fat, protein, lactose, total solids, and acidity content are shown in Table 3 (p<0.05). Probiotic yogurt made using L. mesenteroides H40 had 2.96% fat, 3.23% protein, 6.16% lactose, and 27.33% total solids. In addition, probiotic yogurt was not significantly different from control yogurt. However, probiotic yogurt exhibited significantly higher viscosity than control yogurt. Texture of stirred yogurt is the result of both acid aggregation of casein micelles by ropy strains during incubation (Zhao et al., 2016). The viable cell counts of lactic acid bacteria in control and probiotic yogurt with H40 was 8.66 Log CFU/mL and 8.96 Log CFU/mL, respectively (data not shown).
Table 3. Physicochemical properties of control and probiotic yogurt.
| Physicochemical properties | Yogurt type | |
|---|---|---|
| Control yogurt1) | Probiotic yogurt2) | |
| Fat (%) | 2.96±0.05a | 2.96±0.20a |
| Protein (%) | 3.16±0.20a | 3.23±0.11a |
| Lactose (%) | 6.23±0.11a | 6.16±0.15a |
| Total solid (%) | 27.66±0.15a | 27.33±0.28a |
| Titratable acidity | 0.82±0.01a | 0.81±0.01a |
| pH | 4.33±0.04a | 4.26±0.03a |
| Viscosity (cP) | 1,724.20±15.60a | 2,048.30±7.30b |
Data are represented as the mean±SD of triplicate experiments. Means within a row with same superscript differ (p<0.05).
Control yogurt, yogurt manufactured by ABT-B starter.
Probiotic yogurt, yogurt manufactured by ABT-B starter and L. mesenteroides H40.
Protective effects of Y-CM oxidative stress-induced apoptosis in SH-SY5Y neuroblastoma cells
Y-CM was manufactured with HT-29 cells and yogurt, and its neuroprotective effect was assessed in SH-SY5Y cells (Table 4; p<0.05). The treatment of H2O2 reduced cell viability of SH-SY5Y cells to 55.5%. However, cell viability of control yogurt CM (CY-CM) and probiotic yogurt CM (PY-CM) was 72.2% and 114.8%, respectively. Under treatment with NaAsO2, cell viability of positive control was 51.4% and that of CY-CM and PY-CM was 49.9% and 109.5%, respectively. The PY-CM using L. mesenteroides H40 showed high cell viability in oxidatively stressed SH-SY5Y cells in both H2O2 and NaAsO2 treatment. When compare Fig. 1 and Table 4, PY-CM showed higher cell viability than L. mesenteroides H40. These results showed that the PY-CM effectively protected the cells from oxidative damage caused by H2O2 and NaAsO2.
Table 4. Neuroprotective effect of control and probiotic yogurt.
| Characteristics | Oxidant | |
|---|---|---|
| H2O2 (50 μM) | NaAsO2 (10 μM) | |
| Cell viability (%) | ||
| Non-treatment | 100±5.43c | 100±11b |
| Control | 55.45±0.78a | 51.37±3.5a |
| CY-CM1) | 72.21±1.55b | 49.92±1.50a |
| PY-CM2) | 114.76±9.30d | 109.5±11.5b |
| BDNF expression (fold of control) | ||
| Non-treatment | 1.00±0.06b | 1.00±0.07b |
| Control | 0.73±0.02a | 0.76±0.03a |
| CY-CM1) | 0.78±0.12a | 1.03±0.02b |
| PY-CM2) | 1.05±0.05b | 1.18±0.02b |
| Bax/Bcl-2 ratio | ||
| Non-treatment | 1.00±0.08a | 1.00±0.07a |
| Control | 2.69±0.08c | 2.25±0.04b |
| CY-CM1) | 2.05±0.07b | 1.88±0.21b |
| PY-CM2) | 1.24±0.13a | 1.32±0.17a |
Data are represented as the mean±SD of triplicate experiments.
Means within same characteristics a row with same superscript differ (p<0.05).
CY-CM, conditioned medium using yogurt manufactured by ABT-B starter.
PY-CM, conditioned medium using yogurt manufactured by ABT-B starter and L. mesenteroides H40.
H2O2 treatment resulted in a 0.73-fold increase in BDNF mRNA expression compared with that in the H2O2 nontreated cells (Table 4; p<0.05). CY-CM and PY-CM increased BDNF mRNA expression by 0.78- and 1.05-fold, respectively. The ratio of Bax/Bcl-2 ratio in H2O2 nontreated cells was 1.00, while H2O2 increased to 2.69-fold. The treatment with CY-CM and PY-CM reduced the Bax/Bcl-2 ratio to 2.05- and 1.24-fold, respectively.
The treatment with NaAsO2 reduced BDNF mRNA expression by 0.76-fold compared to that in NaAsO2 nontreated cells (Table 4; p<0.05). CY-CM and PY-CM treatment resulted in a 1.03- and 1.18-fold BDNF mRNA expression, respectively. The ratio of Bax/Bcl-2 ratio in NaAsO2 nontreated cells was 1.00-fold, while NaAsO2 treatment increased this to 2.25-fold in NaAsO2 treated cells. The treatment with CY-CM and PY-CM reduced to 1.88- and 1.32-fold, respectively. Thus, PY-CM can reduce apoptosis of H2O2 or NaAsO2 stressed SH-SY5Y cells.
The treatment with PY-CM significantly increased BDNF mRNA expression and reduced apoptosis on SH-SY5Y mRNA. In addition, mRNA expression was markedly higher based on the yogurt type than with the strain alone or with the yogurt and probiotic strain. These synergistic effects originate from whey protein containing methionine, lysine, and proline, which are associated with apoptosis (Lee and Hur, 2019). Therefore, PY-CM using L. mesenteroides H40 has potent neuroprotective effect in preventing the oxidative stress induced by H2O2 and NaAsO2.
Conclusion
L. mesenteroides H40 was isolated from kimchi and its probiotic property was demonstrated through stability in gastric conditions, adhesion to intestinal cells, enzyme production, and safe antibiotic resistance. For neuroprotective effect, L. mesenteroides H40-CM confirmed an increase of cell viability with increase of BDNF mRNA expression and decrease of the Bax/Bcl-2 ratio in oxidatively stress-induced SH-SY5Y cells. Compared to probiotic strain and yogurt type, the probiotic yogurt showed a higher neuroprotective effect than the strain alone. Therefore, these results suggested a potential of prophylactic therapy as probiotics and functional dairy products. In addition, the cognitive function-related experiments will need to be performed in humans for efficacy verification.
Conflicts of Interest
The authors declare no potential conflict of interest.
Author Contributions
Conceptualization: Lee NK, Lim SM, Paik HD. Data curation: Lee NK, Lim SM, Cheon MJ, Paik HD. Formal analysis: Lim SM, Cheon MJ. Methodology: Cheon MJ, Lim SM. Software: Lee NK, Lim SM. Validation: Lee NK, Lim SM, Paik HD. Investigation: Lee NK, Lim SM. Writing - original draft: Lee NK, Lim SM. Writing - review & editing: Lee NK, Lim SM, Cheon MJ, Paik HD.
Ethics Approval
This article does not require IRB/IACUC approval because there are no human and animal participants.
References
- AOAC International . Caseins and casein micelle structure. 16th ed. AOAC International; Washington, DC, USA: 1999. [Google Scholar]
- Azmi NH, Ismail N, Imam MU, Ismail M. Ethyl acetate extract of germinated brown rice attenuates hydrogen peroxide-induced oxidative stress in human SH-SY5Y neuroblastoma cells: Role of anti-apoptotic, pro-survival and antioxidant genes. BMC Complemt Altern Med. 2013;13:177. doi: 10.1186/1472-6882-13-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campedelli I, Mathur H, Salvetti E, Clarke S, Rea MC, Torriani S, Ross RP, Hill C, O’Toole PW. Genus-wide assessment of antibiotic resistance in Lactobacillus spp. Appl Environ Microbiol. 2019;85:e01738-18. doi: 10.1128/AEM.01738-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers L, Avery A, Dalrymple J, Farrell L, Gibson G, Harrington J, Rikjers G, Rowland I, Spiro A, Varela-Moreiras G, Vokes L, Younge L, Whelan K, Stanner S. Translating probiotic science into practice. Nutr Bull. 2019;44:165–173. doi: 10.1111/nbu.12385. [DOI] [Google Scholar]
- Cheon MJ, Lim SM, Lee NK, Paik HD. Probiotic properties and neuroprotective effects of Lactobacillus buchneri KU200793 isolated from Korean fermented foods. Int J Mol Sci. 2020;21:1227. doi: 10.3390/ijms21041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute . Performance standards for antimicrobial disk susceptibility testing; approved standard. 11th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2012. CLSI Document M02-A11. [Google Scholar]
- Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P. Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol. 2008;66:487–495. doi: 10.1111/j.1574-6941.2008.00520.x. [DOI] [PubMed] [Google Scholar]
- Dussert S, Davey MW, Laffargue A, Doulbeau S, Swennen R, Etienne H. Oxidative stress, phospholipid loss and lipid hydrolysis during drying and storage of intermediate seeds. Physiol Plant. 2006;127:192–204. doi: 10.1111/j.1399-3054.2006.00666.x. [DOI] [Google Scholar]
- Elahi MM, Kong YX, Matata BM. Oxidative stress as a mediator of cardiovascular disease. Oxid Med Cell Longev. 2009;2:920580. doi: 10.4161/oxim.2.5.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger D. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/S0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- Ghaisas S, Maher J, Kanthasamy A. Gut microbiome in health and disease: Linking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol Ther. 2016;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera A, Pineda J, Antonio MT. Toxic effects of perinatal arsenic exposure on the brain of developing rats and the beneficial role of natural antioxidants. Environ Toxicol Pharmacol. 2013;36:73–79. doi: 10.1016/j.etap.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Ishimwe N, Daliri EB, Lee BH, Fang F, Du G. The perspective on cholesterol‐lowering mechanisms of probiotics. Mol Nutr Food Res. 2015;59:94–105. doi: 10.1002/mnfr.201400548. [DOI] [PubMed] [Google Scholar]
- Jang HJ, Lee NK, Paik HD. Probiotic characterization of Lactobacillus brevis KU15153 showing antimicrobial and antioxidant effect isolated from kimchi. Food Sci Biotechnol. 2019;28:1521–1528. doi: 10.1007/s10068-019-00576-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang HJ, Song MW, Lee NK, Paik HD. Antioxidant effects of live and heat-killed probiotic Lactobacillus plantarum Ln1 isolated from kimchi. J Food Sci Technol. 2018;55:3174–3180. doi: 10.1007/s13197-018-3245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Murphy AB, Cryan JF, Ross PR, Dinan TG, Stanton C. Microbiome in brain function and mental health. Trends Food Sci Technol. 2016;57:289–301. doi: 10.1016/j.tifs.2016.05.001. [DOI] [Google Scholar]
- Klippel BF, Duemke LB, Leal MA, Friques AGF, Dantas EM, Dalvi RF, Gava AL, Pereira TMC, Andrade TU, Meyrelles SS, Campagnaro BP, Vasquez EC. Effects of kefir on the cardiac autonomic tones and baroreflez sensitivity in spontaneously hypertensive rats. Front Physiol. 2016;7:211. doi: 10.3389/fphys.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Kim SY, Han KJ, Eom SJ, Paik HD. Probiotic potential of Lactobacillus strains with anti-allergic effects from kimchi for yogurt starters. LWT-Food Sci Technol. 2014;58:130–134. doi: 10.1016/j.lwt.2014.02.028. [DOI] [Google Scholar]
- Lee NK, Han KJ, Son SH, Eom SJ, Lee SK, Paik HD. Multifunctional effect of probiotic Lactococcus lactis KC24 isolated from kimchi. LWT-Food Sci Technol. 2015;64:1036–1041. doi: 10.1016/j.lwt.2015.07.019. [DOI] [Google Scholar]
- Lee SY, Hur SJ. Mechanisms of neuroprotective effects of peptides derived from natural materials and their production and assessment. Compr Rev Food Sci Food Saf. 2019;18:923–935. doi: 10.1111/1541-4337.12451. [DOI] [PubMed] [Google Scholar]
- Liu L, Cavanaugh JE, Wang Y, Sakagami H, Mao Z, Xia Z. ERK5 activation of MEF2-mediated gene expression plays a critical role in BDNF-promoted survival of developing but not mature cortical neurons. Proc Natl Acad Sci USA. 2003;100:8532–8537. doi: 10.1073/pnas.1332804100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain-gut-microbiome axis. CMGH Cell Mol Gastroenterol Hepatol. 2018;6:133–148. doi: 10.1016/j.jcmgh.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Suzuki S, Kumamaru E, Adachi N, Richards M, Kunugi H. BDNF function and intracellular signaling in neurons. Histol Histopathol. 2010;25:237–258. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- O’Toole PW, Marchesi JR, Hill C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat Microbiol. 2017;2:17057. doi: 10.1038/nmicrobiol.2017.57. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: The protein and methionine connection. Biochim Biophys Acta. 2006;1757:496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Park J, Lee J, Yeom Z, Heo D, Lim YH. Neuroprotective effect of Ruminococcus albus on oxidatively stressed SH-SY5Y cells and animals. Sci Rep. 2017;7:14520. doi: 10.1038/s41598-017-15163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardillo-Díaz R, Carrascal L, Muñoz MF, Ayala A, Nunez-Abades P. Time and dose dependent effects of oxidative stress induced by cumene hydroperoxide in neuronal excitability of rat motor cortex neurons. Neurotoxicology. 2016;53:201–214. doi: 10.1016/j.neuro.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Quigley EM. Microbiota-brain-gut axis and neurodegenerative diseases. Curr Neurol Neurosci Rep. 2017;17:94. doi: 10.1007/s11910-017-0802-6. [DOI] [PubMed] [Google Scholar]
- Ranuh R, Athiyyah AF, Darma A, Risky VP, Riawan W, Surono IS, Sudarmo SM. Effect of the probiotic Lactobacillus plantarum IS-10506 on BDNF and 5HT stimulation: Role of intestinal microbiota on the gut-brain axis. Iran J Microbiol. 2019;11:145–150. doi: 10.18502/ijm.v11i2.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Fang K, Medina D, Wan J, Lee JL, Hong SH. The probiotic, Leuconostoc mesenteroides, inhibits Listeria monocytogenes biofilm formation. J Food Saf. 20192019:e12750 [Google Scholar]
- Son SH, Jeon H, Yang SJ, Sim MH, Kim YJ, Lee NK, Paik HD. Probiotic lactic acid bacteria isolated from traditional Korean fermented foods based on β-glucosidase activity. Food Sci Biotechnol. 2018;27:123–129. doi: 10.1007/s10068-017-0212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Ling Z, Wang F, Chen W, Li H, Jin J, Zhang H, Pang M, Yu J, Liu J. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett. 2016;613:30–35. doi: 10.1016/j.neulet.2015.12.047. [DOI] [PubMed] [Google Scholar]
- Ton AMM, Campagnaro BP, Alves GA, Aires R, Côco LZ, Arpini CM, Oliveira TG, Campos-Toimil M, Meyrelles SS, Pereira TMC, Vasquez EC. Oxidative stress and dementia in Alzheimer’s patients: Effects of synbiotic supplementation. Oxid Med Cell Longev. 2020;2020:2638703. doi: 10.1155/2020/2638703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lee IS, Braun C, Enck P. Effect of probiotics on central nervous system functions in animals and humans: A systematic review. J Neurogastroenterol Motil. 2016;22:589–605. doi: 10.5056/jnm16018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Michaelis EK. Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci. 2010;2:12. doi: 10.3389/fnagi.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1–12. doi: 10.1016/j.bbi.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Lee JE, Lim SM, Kim YJ, Lee NK, Paik HD. Antioxidant and immune-enhancing effects of probiotic Lactobacillus plantarum 200655 isolated from kimchi. Food Sci Biotechnol. 2019;28:491–499. doi: 10.1007/s10068-018-0473-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi YJ, Lim JM, Lim JM, Gu S, Lee WK, Oh E, Lee SM, Oh BT. Potential use of lactic acid bacteria Leuconostoc mesenteroides as a probiotic for the removal of Pb (II) toxicity. J Microbiol. 2017;55:296–303. doi: 10.1007/s12275-017-6642-x. [DOI] [PubMed] [Google Scholar]
- Yoon JY, Kim D, Kim EB, Lee SK, Lee M, Jang A. Quality and lactic acid bacteria diversity of pork salami containing kimchi powder. Korean J Food Sci Anim Resour. 2018;38:912–926. doi: 10.5851/kosfa.2018.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Jang HJ, Lee NK, Paik HD. Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi. LWT-Food Sci Technol. 2019a;112:108229. doi: 10.1016/j.lwt.2019.05.127. [DOI] [Google Scholar]
- Yu HS, Lee NK, Choi AJ, Choe JS, Bae CH, Paik HD. Antagonistic and antioxidant effect of probiotic Weissella cibaria JW15. Food Sci Biotechnol. 2019b;28:851–855. doi: 10.1007/s10068-018-0519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Lee NK, Choi AJ, Choe JS, Bae CH, Paik HD. Anti-inflammatory potential of probiotic strain Weissella cibaria JW15 isolated from kimchi through regulation of NF-κB and MAPKs pathways in LPS-induced RAW 264.7 cells. J Microbiol Biotechnol. 2019c;29:1022–1032. doi: 10.4014/jmb.1903.03014. [DOI] [PubMed] [Google Scholar]
- Zhao LL, Wang XL, Tian Q, Mao XY. Effect of casein to whey protein ratios on the protein interactions and coagulation properties of low-fat yogurt. J Dairy Sci. 2016;99:7768–7775. doi: 10.3168/jds.2015-10794. [DOI] [PubMed] [Google Scholar]