Abstract
The objective of this study was to determine the effects of yeast cultures (Candida zeylanoides and Debaryomyces hansenii) isolated from traditionally dry fermented Turkish sucuks, on some physicochemical and microbiological properties of the product. Eight different batches of the sucuks were produced by the inoculation of yeast and lactic acid bacteria (LAB) cultures (Lactobacillus curvatus, Lactobacillus plantarum and Lactobacillus sakei) in different combinations. The sucuks were ripened for 12 days and analyzed at 1st, 6th, and 12th days of ripening. Percent moisture content, pH, water activity (aw) and residual nitrite values of the sucuk inoculated with the yeast cultures were higher at the end of the ripening. The use of yeast cultures decreased hardness, gumminess, and chewiness values of the sucuk while increased adhesiveness values. Major volatile groups were aldehydes, terpenes, and sulphur compounds in the sucuk samples. The most noticeable results were for sensory properties of the sucuk that were positively improved by the yeast cultures.
Keywords: Turkish fermented sucuk, indigenous yeast, texture, volatile
Introduction
Turkish sucuk is a traditionally fermented dry-cured sausage, which is commonly consumed in Turkey. Sucuk is produced using meat (beef, water buffalo), sheep tail fat or tallow, garlic salt, sugar, nitrite, nitrate, and some spices including red pepper, black pepper, cumin, and pimento. In recent years, sucuk has been produced using either industrial method using heat treatment process by sucuk manufacturers. Ripening of sucuk takes a quite short time in the industrial method than that of the traditional process. Therefore, taste and aroma of sucuk are not well developed in case of industrial method (Gençcelep et al., 2007; Kaban and Kaya, 2009). Although industrial process is most widely used in sucuk production, some producers still use the traditional method. Fermented sucuk production can be performed including by spontaneous or commercial starter culture in the traditional process (Ozturk and Sagdic, 2014). In generally, commercial starter cultures are used to produce the standard-quality sucuk.
Lactic acid bacteria (Lactobacillus sakei, Lb. plantarum, Lb. curvatus, Pediococcus acidilactici, and P. pentosaceus) and coagulase negative cocci (CNC; Staphylococcus xylosus and S. carnosus and Kocuria varians) are the most popular bacteria in commercial starter culture used in fermented sausage production (Toldrá et al., 2001). Lactic acid bacteria (LAB) decrease the pH that is important in the formation of flavor and aroma and responsible for microbial safety of sucuk. CNC play an important role in the formation of desired biochemical reactions like proteolysis, lipolysis and color formation of the fermented sausage and sucuk (Toldrá et al., 2001).
Yeasts and molds can also be effective in the fermentation of sucuk and sausage. Yeasts may have lipolytic and proteolytic activity, and it could contribute to aroma and flavor formation as well as color stability of the fermented sausages (Durá et al., 2004; Flores et al., 2004; Toldrá et al., 2001). It has been known that Debaryomyces hansenii which is anamorph Candida famata is the most abundant species of yeast isolated from the fermented meat products (Cocolin et al., 2006; Mendonça et al., 2013). D. hansenii is an osmophilic yeast species and can grow at low aw and temperature levels. This species has poor ability to utilize lactose (Breuer and Harms, 2006), and D. hansenii can grow both in the interior part and on the surface of fermented meat products like sausage. Moreover, it is used as starter culture in the fermented sausages in some countries (Toldrá et al., 2001). It has been speculated that D. hansenii can prevent formation of oxidation products of lipids in the fermented sausages and leads to formation of some aroma components (Flores et al., 2004). It can also play significant role in the degradation of organic acids like lactic and acetic acids and produce ammonia (Demeyer and Stahnke, 2002). Also, there is no evidence about the toxic effects and the pathogenicity for the both yeast species (Durá et al., 2004). Therefore, the aim of this study was to determine the effects of D. hansenii and C. zeylanoides strains isolated from traditional Turkish fermented sausage (Sucuk), on some physicochemical, textural properties and volatile components of the sucuk, a traditional Turkish dry-fermented sausage, produced with LAB starter cultures (L. sakei, L. curvatus, and L. plantarum).
Materials and Methods
Materials
Fresh lean of beef from round region, tallow fat, spices (red pepper, black pepper, cumin, and pimento) and other ingredients (salt, fresh garlic, sugar, and sodium nitrite) were supplied from local suppliers in Kayseri, Turkey.
Production of sucuk
In order to produce about 1 kg of sucuk, main ingredients [(ground lean beef (800 g) and ground tallow fat (200 g)] and additives [salt (25 g), minced garlic (10 g), cumin (9 g), red pepper (7 g), black pepper (5 g), pimento (2.5 g), sugar (4 g) and sodium nitrite (NaNO2 150 mg)] were prepared and incorporated together. First, all the ingredients were homogenized by kneading in a kitchen bowl approximately 10 min and the mass was divided into 8 batches as following; S1: Control (without starter culture), S2: LAB (Lactobacillus curvatus, L. plantarum and L. sakei), S3: LAB+C. zeylanoides, S4: LAB+D. hansenii, S5: LAB+C. zeylanoides+D. hansenii, S6: C. zeylanoides, S7: D. hansenii and S8: C. zeylanoides+D. hansenii. The LAB and yeast cultures were added at levels of approximately 108 and 106 CFU/g, respectively. The mixtures were filled into standard artificial collagen casings (diameter 30–32 mm) by using a filling machine (Tefal Le Hachoir 1500, France). Production of sucuk was carried out at room temperature (~22°C). The ripening of sucuks was carried out according to the following program: First 3 days at 24±1°C and 90±2% relative humidity (RH), then following 4 days at 22±1°C and 85±2% RH and finally for 5 days at 18±1°C and 80±2% RH in fermentation cabinets (Nüve, TK 252, Ankara, Turkey). The sucuk samples were analyzed at 1st, 6th, and 12th days of ripening. All the batches were produced as triplicate.
Microbiological analysis of sucuks
The 25 g of the sample was weighed into the sterile stomacher bag and homogenized with 225 mL of sterile Maximum Recovery Diluent solution (MRD, Merck, Darmstadt, Germany) for 1.5 min using a homogenizer (Stomacher, IUL, Barcelona, Spain). Total mesophilic aerobic bacteria (TMAB) counts of the samples were determined after 48 h incubation at 30°C on Plate Count Agar (PCA, Merck). LAB counts of the samples were determined in pour plates of De Man Rogosa and Sharpe Agar (MRS, Merck), after incubation for 48 h at 37°C in anaerobic conditions. The number of Micrococcaceae on Mannitol Salt Phenol-Red agar (MSA, Merck) was determined after incubation for 48 h at 37°C. The Enterobacteriaceae counts were determined after incubation for 48 h at 35°C, in anaerobic conditions on Violet Red Bile Glucose Agar (VRBG, Merck). Yeast and mold counts were determined for 5 days at 25°C on Dichloran Rose Bengal Chloramphenicol Agar (DRBC, Merck). The microbiological analyses were carried out as triplicate.
Physicochemical analyses of sucuks
The moisture contents of the sucuk samples were determined by using oven air drying method in a drying oven (Nüve FN 120, Ankara, Turkey) (AOAC, 2000). Water activities (aw) of the samples were determined using aw meter (Aqua Lab 2.0, Pullman, WA, USA). To determine the pH values of the sucuk samples, 10 g of the sucuk sample was homogenized with 100 mL of distilled water using Ultraturrax (IKA T18 Basic, Staufen, Germany) and the values were measured by using pH meter (WTW, Inolab 720, Weilheim, Germany). Weight loss of the sucuk samples was determined by recording the weights of the samples at the first day and 12th day of ripening. It was calculated using the following equation:
where WB and WA are the weights of a certain sample before and after ripening, respectively.
Residual nitrite level was determined based on the method described by Taucmann (1987). They were calculated the residual nitrite level using a calibration curve and expressed as ppm of sodium nitrite (NaNO2). The physicochemical analyses of the sucuk samples were carried out as triplicate.
Color properties
Color properties of the sucuk samples were determined using an automatic colorimeter (Konica Minolta, model CM-5, Mississauga, ON, Canada) at an observer angle of 10° with the illuminant D65 and specular-component-excluded mode, according to The Commission Internationale de l’Eclairage (CIE)-Lab color scales. Artificial collagen casing was removed to measure color of the exterior part of the samples, and the cross-section of the sliced sucuk samples was used to measure the internal color. The color results were expressed as L*, a*, and b*. L* values measure the level of brightness (0–100), a* redness (+=red and −=green), and b* yellowness (+=yellow and −=blue). Color parameter values of the sucuk samples were measured in ten replicates.
Volatile compounds
Volatile compounds profile of the sucuk samples produced with different yeast and/or LAB starter cultures was determined using a Gas Chromatographic-Mass Spectrometry (GC–MS) (Agilent 7890A GC system, Agilent, Santa Clara, CA, USA) equipped with a mass selective detector (Agilent Technologies, Agilent) and HP5-MS capillary column (60 m×0.250 mm i.d.; film thickness 0.25 μm) (Agilent). The 5 g of the sucuk sample was weighed into GC-MS vial (Agilent). and the vial was sealed with PTFE-faced silicone septum (Supelco, Bellefonte, PA, USA). Then vial was kept in a termoblock (IKA, RCT basic, Germany) at 40°C for 1 h. Then SPME fiber (75 μm, 23 ga, carboxen/polydimethylsiloxane (CAR/PDMS)) (Supelco) was subjected to the headspace while maintaining the sample at 40°C for 1 h. The volatile compounds adsorbed by the fibers were desorbed from the injection port for 20 min at 50°C and injected to GC–MS in the splitless mode. The compounds were identified by comparison with spectra from the libraries of Flavor 2, Nist05 and Wiley7n. GC–MS conditions were adjusted according to Kaban and Kaya (2009). The volatile compound analyses were run as triplicate.
Texture profile analysis (TPA)
To determine textural properties of the sucuk samples, TPA test was conducted using a texture analyzer (TA.XT Plus Texture Analyzer, Texture Technologies, Scarsdale, NY/Stable Micro System, Surrey, UK). The sucuk samples ripened were cut into small pieces having a 20 mm diameter and 20±0.5 mm thickness. Firstly, the parameters of measurement were set to be following: pre test speed 2 mm/s, test speed 1 mm/s, post test speed 1 mm/s and compression (strain) level 25%. For the determination of TPA parameters, a spherical probe (SMS/1S) and 30 kg load cell were used. Hardness (g), adhesiveness (g s), springiness (mm), cohesiveness, gumminess (g), chewiness (g mm) and resilience values of the sucuk samples were measured by calculation using TPA curves (Bozkurt and Bayram, 2006). Texture parameters of the sucuk samples were measured in ten replicates.
Statistical analysis
All the data were means of triplicate data with their standard deviations. Analysis of data was performed by using one-way ANOVA and/or two-way ANOVA. Duncan’s Multiple Range Test was also applied to determine significant differences between means at the p<0.05 significance level using SAS 8.0 statistical software (SAS, 2000).
Results and Discussion
Physicochemical properties of sucuk samples
Some physicochemical properties of the sucuk samples prepared with C. zeylanoides (CZ), D. hansenii (DH) and LAB cultures are given in Table 1. Moisture contents of the sucuk samples varied from 54% to 57% in the first day of the ripening. Then, it decreased during ripening as expected depending on the dehydration of sucuks. Moisture contents of the sucuk samples (S2-S4) with LAB cultures were lower than that of the samples with CZ and DH at end of the ripening, and these results were also insignificant (p>0.05). Water activity (aw) levels of the sucuk samples correlated with their moisture contents. Again, aw values of the samples containing LAB were lower when compared to other samples at the 6th and 12th days. The pH levels of the samples may be the reason for low moisture and aw values of the samples containing LAB. Low pH causes to decrease in water-holding capacity of the meat proteins. This promotes the drying process of sucuk or fermented sausages (Lücke, 1998; Ordóñez et al., 1999; Toldrá et al., 2001). Additionally, higher pH value of meat is considered as a problem in sucuk or fermented sausage production. When a meat having high pH is used in sucuk or fermented sausage production, sufficient drying cannot be achieved due to high water retention capacity (Toldrá, 2007). Therefore, pH values of meats which will be used in sucuk or fermented sausage should be in between 5.4 and 5.8 (Öztan, 2005). The pH values of the sucuks were approximately 5.8 at the first day of the ripening, and the pH values of sucuks produced with LAB cultures were lower than that of the control and samples inoculated yeast culture at the end of the ripening. However, pH results were not (p>0.05) different at first and 12th days, but they were significantly different (p<0.05) at 6th days of ripening. Andrade et al. (2010) investigated effects of three D. hansenii strains on microbiological, physiochemical properties and volatile compounds of salchichón, a dry fermented sausage. They found aw and pH values to be high in the samples with D. hansenii as compared to the control sample at the end of ripening (54 days). In the same study, however, aw and pH values of fermented sausage changed depending on the D. hansenii strains. Kaban and Kaya (2009) reported that pH and aw values of sucuk samples produced with L. plantarum and Staphylococcus xylosus were lower than that of the control sucuks. LAB are the bacteria group that mainly responsible for pH decrease in fermented sausage and sucuks, and it affects moisture and aw values of fermented meat products during the ripening. In another study, dry matter, aw and pH values of dry fermented sausage produced with different yeast strains (C. famata, Yarrowia lipolytica, D. hansenii and Trichosporon mucoides) were 66%–68%, 0.81%–0.82% and 4.6%–4.7%, respectively, after 21 days of ripening (Selgas et al., 2003). These results are not in accordance with our findings probably due to fermentation time and conditions, process applied and materials used such as starter culture, meat, spices and other additives. The Turkish Food Codex (2000) states that ripened sucuk which is high quality should have pH between 5.2 and 5.4. Again pH levels of the sucuk samples produced in the current study are not in line with Turkish Food Codex.
Table 1. Physicochemical properties of the sucuk samples during ripening period.
| Batches | Ripening period (d) | ||
|---|---|---|---|
| 1st | 6th | 12th | |
| Moisture (%) | |||
| S1 | 55.63Abc±1.42 | 45.18Ba±3.23 | 36.74Ca±1.70 |
| S2 | 57.13Aab±0.54 | 46.56Ba±0.99 | 35.40Ca±2.67 |
| S3 | 57.77Aa±1.88 | 45.54Ba±2.66 | 35.46Ca±1.46 |
| S4 | 57.41Aab±0.77 | 46.40Ba±1.81 | 35.82Ca±0.66 |
| S5 | 57.76Aa±0.70 | 46.74Ba±4.03 | 37.73Ca±2.45 |
| S6 | 56.97Aabc±1.35 | 46.56Ba±1.69 | 37.48Ca±0.70 |
| S7 | 54.99Ac±0.75 | 46.84Ba±1.49 | 38.45Ca±0.12 |
| S8 | 56.76Aabc±0.44 | 47.18Ba±1.45 | 37.44Ca±1.76 |
| pH | |||
| S1 | 5.84Aa±0.05 | 5.01Ba±0.16 | 5.08Ba±0.10 |
| S2 | 5.86Aa±0.03 | 4.74Bb±0.02 | 4.90Ba±0.17 |
| S3 | 5.86Aa±0.02 | 4.75Bb±0.05 | 4.90Ba±0.17 |
| S4 | 5.85Aa±0.03 | 4.76Cb±0.04 | 4.92Ba±0.07 |
| S5 | 5.84Aa±0.04 | 4.80Bb±0.01 | 4.98Ba±0.13 |
| S6 | 5.87Aa±0.01 | 4.98Ba±0.02 | 5.13Ba±0.17 |
| S7 | 5.88Aa±0.01 | 5.01Ba±0.04 | 5.06Ba±0.11 |
| S8 | 5.88Aa±0.01 | 4.99Ba±0.05 | 5.08Ba±0.16 |
| aw | |||
| S1 | 0.962Aa±0.003 | 0.937Bab±0.003 | 0.891Cb±0.015 |
| S2 | 0.961Aa±0.003 | 0.920Bc±0.005 | 0.876Cc±0.010 |
| S3 | 0.964Aa±0.001 | 0.932Bb±0.003 | 0.891Cb±0.004 |
| S4 | 0.964Aa±0.001 | 0.938Bab±0.002 | 0.895Cab±0.003 |
| S5 | 0.963Aa±0.002 | 0.938Bab±0.006 | 0.900Cab±0.006 |
| S6 | 0.965Aa±0.003 | 0.943Ba±0.004 | 0.904Cab±0.007 |
| S7 | 0.961Aa±0.002 | 0.942Ba±0.004 | 0.903Cab±0.005 |
| S8 | 0.963Aa±0.002 | 0.943Ba±0.005 | 0.907Ca±0.003 |
| Residual nitrite (ppm) | |||
| S1 | 80.6Aa±3.8 | 15.6Ba±1.7 | 14.6Bab±0.5 |
| S2 | 54.7Ac±5.0 | 9.4Bb±2.2 | 8.1Bc±0.8 |
| S3 | 53.3Ac±5.5 | 9.1Bb±2.2 | 8.8Bc±1.1 |
| S4 | 71.0Ab±3.1 | 9.0Bb±1.6 | 9.8Bc±0.2 |
| S5 | 68.6Ab±3.2 | 10.1Bb±1.9 | 8.2Bc±1.1 |
| S6 | 78.2Aa±2.3 | 14.9Ba±2.2 | 14.9Bab±3.4 |
| S7 | 79.4Aa±1.6 | 16.1Ba±0.2 | 15.5Ba±0.4 |
| S8 | 80.9Aa±4.6 | 14.5Ba±1.4 | 13.5Bb±1.1 |
S1, Control; S2, LAB; S3, LAB+C. zeylanoides; S4, LAB+D. hansenii; S5, LAB+C. zeylanoides+D. hansenii; S6, C. zeylanoides; S7, D. hansenii; S8, C. zeylanoides+D. hansenii.
The uppercase within the same line show that the results are not significantly different (p>0.05).
The lowercase within the same column show that the results are not significantly different (p>0.05) for physicochemical properties of sucuk samples.
Weight loss values of the sucuk samples are seen in Fig. 1. The highest weight loss values were determined in the sucuks produced with LAB cultures, and the differences were significant (p<0.05). However, use of the yeast cultures decreased weight loss of the sucuks. Weight loss is an important parameter for the economical reasons for the meat processors, it means saving money for the manufacturers.
Fig. 1. Weight loss (%) of the ripened sucuk samples.
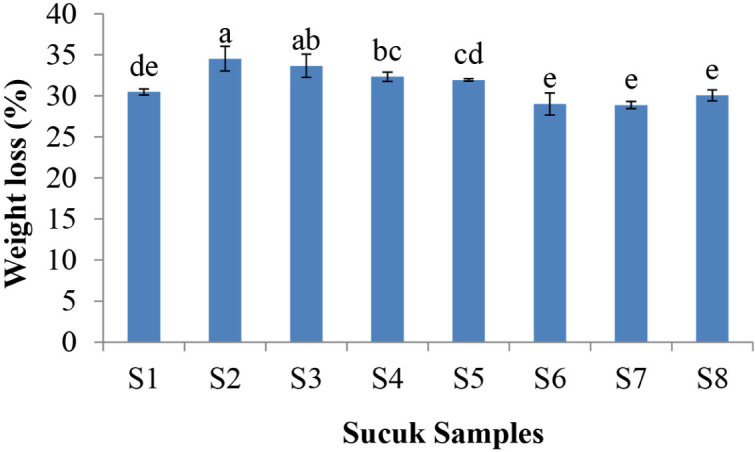
S1, Control; S2, LAB; S3, LAB+C. zeylanoides; S4, LAB+D. hansenii; S5, LAB+C. zeylanoides+D. hansenii; S6, C. zeylanoides; S7, D. hansenii; S8, C. zeylanoides+D. hansenii. a–e Different lowercase indicate the statistical difference (p<0.05) for weight loss of sucuk samples. LAB, lactic acid bacteria.
Residual nitrite values of the sucuk samples are shown in Table 1. At the first day of ripening, nitrite values of the sucuks varied from 53.3 to 80.9 ppm, and it was the lowest in the sucuks produced with LAB cultures. The nitrite level significantly (p<0.05) decreased with the ripening time. Again, the lowest residual nitrite values were in the sucuks produced with LAB culture and ranged between 8.1–9.8 ppm. Our residual nitrite results were in agreement with the results of Gençcelep et al. (2007) who reported that residual nitrite values of sucuks with starter culture were lower than those of the control group (without starter culture) sucuks. Nitrite is used to improve color and oxidative properties of the product, and it also inhibits Clostridium botulinum as well (Oh et al., 2004). However, it is at higher levels in foods a potential carcinogen and toxic agent for human (Cammack et al., 1999). According to the Turkish Food Codex (2000), residual nitrite levels of the fermented Turkish sucuks must be lower than 150 mg/kg. It has been reported that nitrite levels can be reduced by LAB having a nitrite reductase enzyme system (Fournaud and Mocquot, 1966). The nitrite values of the cured meat products decrease spontaneously during the storage period (Oh et al., 2004). Several studies have shown that certain LAB strains are able to degrade nitrite in the fermented meat products (Dodds and Collins-Thompson, 1984; Oh et al., 2004; Yan et al., 2008).
Microbiological properties
Microbiological properties of the sucuk samples are given in Table 2. TMAB counts of the samples were approximately 9.0 Log CFU/g at the beginning of ripening. Then, it increased at 6th days of the ripening and decreased at the end of ripening. TMAB and LAB counts of samples were 9.85–10.21 Log CFU/g and 9.89–10.17 Log CFU/g, respectively and the differences were not significant (p>0.05) in the 12th days of ripening. However, Micrococcaceae population of the sucuk samples was different (p<0.05) at the 6th days and 12th days of ripening. Enterobacteriaceae numbers decreased during the ripening (Table 2). Yeast and mold counts of the sucuk samples ranged from 4.12 to 6.10 Log CFU/g at the beginning of the ripening but they were closer to each other at the 6th days of ripening. Additionally, use of CZ and DH in the sucuk production affected (p<0.05) the yeast and mold counts as expected and Micrococcaceae and Enterobacteriaceae counts as well, while the TMAB and LAB counts of sucuk samples were not affected (p>0.05) from the presence of these yeasts at the end of ripening. It was reported that LAB, Micrococcaceae and yeast counts of fermented sausage produced with different D. hansenii strains were approximately 107, 103, and 104–105 CFU/g at the end of ripening, respectively (Andrade et al., 2010). In another study, Bolumar et al. (2006) used D. hansenii and L. sakei to improve sensory properties of fermented dry sausage, and yeast and LAB counts of ripened fermented sausage were determined as approximately 3.5 and 8.5 Log CFU/g, respectively. At the same time, yeast count of fermented sausage produced with only D. hansenii increased at the end of ripening while the number of LAB did not change considerably during ripening. Durá et al. (2004) reported that yeast number decreased during ripening of fermented sausage produced with two different D. hansenii strains while the yeast count decreased in the control sample during ripening. Additionally, LAB counts of the samples were approximately 7 Log CFU/g at the beginning of ripening and reached to approximately 9.5 Log CFU/g within the ripening. These results were in accordance with our findings related to yeast and LAB counts (Table 2). Kaban and Kaya (2009) reported that Enterobacteriaceae was not detected in the control and the sucuk samples produced with starter culture (L. plantarum and S. xylosus) at the 7th and 3rd days of ripening, respectively. In this study, Enterobacteriaceae was present in the all sucuk samples after ripening, but their counts were low. It is known that Enterobacteriaceae is sensitive against low acidity and water activity (Kaban and Kaya, 2009). In this study, the pH and aw values of the sucuks were higher than pH and aw results of the sucuk samples produced by Kaban and Kaya (2009). Microbiological characteristics of fermented meat products are generally influenced from natural meat microbiota and starter cultures used.
Table 2. Microbiological results of the sucuk samples during ripening period (Log CFU/g).
| Batches | Ripening period (d) | ||
|---|---|---|---|
| 1st | 6th | 12th | |
| TMAB | |||
| S1 | 9.13Babc±0.68 | 10.17Aa±0.26 | 9.92ABa±0.19 |
| S2 | 9.60Bab±0.38 | 10.60Aa±0.31 | 9.85ABa±0.44 |
| S3 | 9.62Bab±0.13 | 10.53Aa±0.43 | 10.01ABa±0.21 |
| S4 | 9.71Bab±0.26 | 10.47Aa±0.14 | 10.15ABa±0.28 |
| S5 | 9.83Ba±0.21 | 10.33Aa±0.16 | 10.18ABa±0.20 |
| S6 | 9.03Bbc±0.30 | 10.32Aa±0.25 | 10.21Aa±0.26 |
| S7 | 8.95Bbc±0.49 | 10.29Aa±0.06 | 9.96Aa±0.50 |
| S8 | 8.66Bc±0.52 | 10.26Aa±0.14 | 10.11Aa±0.18 |
| LAB | |||
| S1 | 8.81Bb±0.51 | 10.07Aa±0.15 | 9.94Aa±0.10 |
| S2 | 9.61Ba±0.34 | 10.64Aa±0.28 | 9.89Ba±0.30 |
| S3 | 9.59Ba±0.16 | 10.34Aa±0.50 | 9.97ABa±0.24 |
| S4 | 9.20Bab±0.24 | 10.67Aa±0.56 | 10.14Aa±0.28 |
| S5 | 9.70Ba±0.27 | 10.51Aa±0.36 | 10.17ABa±0.14 |
| S6 | 8.74Bb±0.51 | 9.91Aa±0.09 | 10.17Aa±0.29 |
| S7 | 8.64Bb±0.44 | 10.62Aa±0.68 | 9.92Aa±0.45 |
| S8 | 8.83Bb±0.61 | 10.19Aa±0.18 | 10.08Aa±0.34 |
| Micrococcaceae | |||
| S1 | 6.33Aa±0.55 | 7.01Aa±0.14 | 7.08Aa±0.45 |
| S2 | 5.61Aa±1.20 | 5.95Acd±0.31 | 5.99Abc±0.42 |
| S3 | 5.61Aa±1.14 | 5.79Ad±0.72 | 5.70Ac±0.67 |
| S4 | 5.72Aa±1.07 | 5.99Abcd±0.39 | 5.72Ac±0.46 |
| S5 | 5.63Aa±1.29 | 5.92Acd±0.78 | 6.55Aabc±0.86 |
| S6 | 6.49Aa±0.32 | 6.84Aa±0.18 | 7.22Aa±0.65 |
| S7 | 6.27Ba±0.40 | 6.77ABab±0.30 | 6.97Aab±0.13 |
| S8 | 6.33Aa±0.38 | 6.73Aabc±0.10 | 7.22Aa±0.66 |
| Enterobacteriaceae | |||
| S1 | 5.36Aa±0.35 | 3.79Ba±0.98 | 2.32Cab±0.15 |
| S2 | 4.03Ab±0.71 | 2.90Bb±0.53 | 2.13Bb±0.12 |
| S3 | 4.65Aab±0.36 | 2.74Bb±0.18 | 2.15Cab±0.15 |
| S4 | 4.48Aab±0.68 | 2.64Bb±0.29 | 2.10Bb±0.09 |
| S5 | 3.81Ab±0.94 | 2.82ABb±0.43 | 2.12Bb±0.20 |
| S6 | 5.35Aa±0.17 | 3.17Bab±0.39 | 2.31Cab±0.28 |
| S7 | 5.48Aa±0.44 | 2.83Bb±0.39 | 2.52Ba±0.26 |
| S8 | 5.24Aa±0.75 | 2.87Bb±0.22 | 2.21Bab±0.24 |
| Yeast and Mold | |||
| S1 | 4.59Abc±0.31 | 5.02Ab±0.55 | 4.78Ab±0.42 |
| S2 | 4.12Bc±0.85 | 5.61Aab±0.07 | 5.37Ab±0.58 |
| S3 | 5.56Aab±0.47 | 5.76Aab±0.46 | 5.60Ab±1.07 |
| S4 | 5.13Aabc±0.65 | 5.51Aab±0.31 | 5.24Ab±0.82 |
| S5 | 5.35Bab±1.07 | 5.50Bab±0.60 | 7.01Aa±0.24 |
| S6 | 6.10Aa±0.20 | 5.91Aa±0.42 | 5.91Ab±0.55 |
| S7 | 5.81Aa±0.20 | 5.39Aab±0.16 | 5.04Ab±0.23 |
| S8 | 6.05Aa±0.13 | 5.39Aab±0.09 | 5.46Ab±0.61 |
S1, Control; S2, LAB; S3, LAB+C. zeylanoides; S4, LAB+D. hansenii; S5, LAB+C. zeylanoides+D. hansenii; S6, C. zeylanoides; S7, D. hansenii; S8, C. zeylanoides+D. hansenii.
The uppercase within the same line show that the results are not significantly different (p>0.05).
The lowercase within the same column show that the results are not significantly different (p>0.05) for microbiological properties of sucuk samples.
TMAB, total mesophilic aerobic bacteria; LAB, lactic acid bacteria.
Color
Color properties of the sucuk samples were determined in exterior and interior sections as shown in Table 3. The L* values of sucuks were found in the range 43.26–43.80 and 29.99–32.95 in the interior and exterior section, respectively. Use of LAB, CZ and DH starter cultures did not affect (p>0.05) the L* values of the sucuks in the neither interior nor exterior section. The a* values of sucuks were higher (p>0.05) in interior section. The highest a* value was in the S8 (CZ+DH), while the lowest value was determined in S2 sample (only LAB). However, the a* value of sucuks was lower (p>0.05) in the exterior part. Again, the highest and lowest a* values were in the S8 (CZ+DH) and S2 (only LAB) in the exterior part of sucuks, respectively (Table 3). The a* value is one of the most important color parameters for the quality of sucuk. The a* value is generally low at the beginning of ripening of dry-cured fermented meat products. However, it increases during the ripening due to the formation of nitrosomyoglobin which is associated with the red color of fermented meat products and moisture loss in the fermented meat products (Pérez-Alvarez et al., 1999). The reason of low a* values may be acidity in the sucuks produced with LAB cultures. Because, lactic acid produced by LAB might denature (partly or totally) the myoglobin during ripening (Pérez-Alvarez et al., 1999). The b* values of sucuk samples were variable (p<0.05) in both interior and exterior section. As L* and a* values of sucuks, the b* values were high in the interior section of sucuk samples.
Table 3. Color parameters of the ripened sucuk samples.
| Batches | L* | a* | b* |
|---|---|---|---|
| Internal section | |||
| S1 | 42.26A±1.12 | 17.18A±1.26 | 5.13BC±0.99 |
| S2 | 42.57A±1.48 | 16.77A±1.34 | 4.45C±0.36 |
| S3 | 43.80A±1.38 | 17.05A±1.35 | 5.87ABC±0.95 |
| S4 | 42.74A±1.85 | 17.70A±2.07 | 6.01ABC±1.40 |
| S5 | 42.71A±1.55 | 17.69A±1.96 | 5.49ABC±0.97 |
| S6 | 42.92A±1.65 | 17.96A±1.40 | 7.11A±0.84 |
| S7 | 42.66A±1.50 | 17.70A±0.43 | 6.83AB±0.93 |
| S8 | 42.66A±1.14 | 18.39A±0.96 | 7.05A±0.76 |
| Exterior part | |||
| S1 | 32.63A±3.48 | 14.24A±2.05 | 1.75C±0.25 |
| S2 | 29.99A±2.84 | 12.93A±2.25 | 1.03D±0.09 |
| S3 | 31.45A±1.88 | 13.37A±3.56 | 2.07BC±0.26 |
| S4 | 31.05A±3.69 | 13.43A±4.87 | 1.61C±0.25 |
| S5 | 32.47A±2.81 | 11.74A±2.68 | 2.55A±0.39 |
| S6 | 32.39A±2.20 | 15.47A±2.60 | 2.47AB±0.08 |
| S7 | 32.95A±2.46 | 13.95A±3.59 | 1.89C±0.34 |
| S8 | 30.59A±3.89 | 15.80A±4.08 | 1.78C±0.25 |
S1, Control; S2, LAB; S3, LAB+C. zeylanoides; S4, LAB+D. hansenii; S5, LAB+C. zeylanoides+D. hansenii; S6, C. zeylanoides; S7, D. hansenii; S8, C. zeylanoides+D. hansenii.
The uppercase within the same column show that the results are not significantly different (p>0.05) for color properties of sucuk samples.
Texture profile
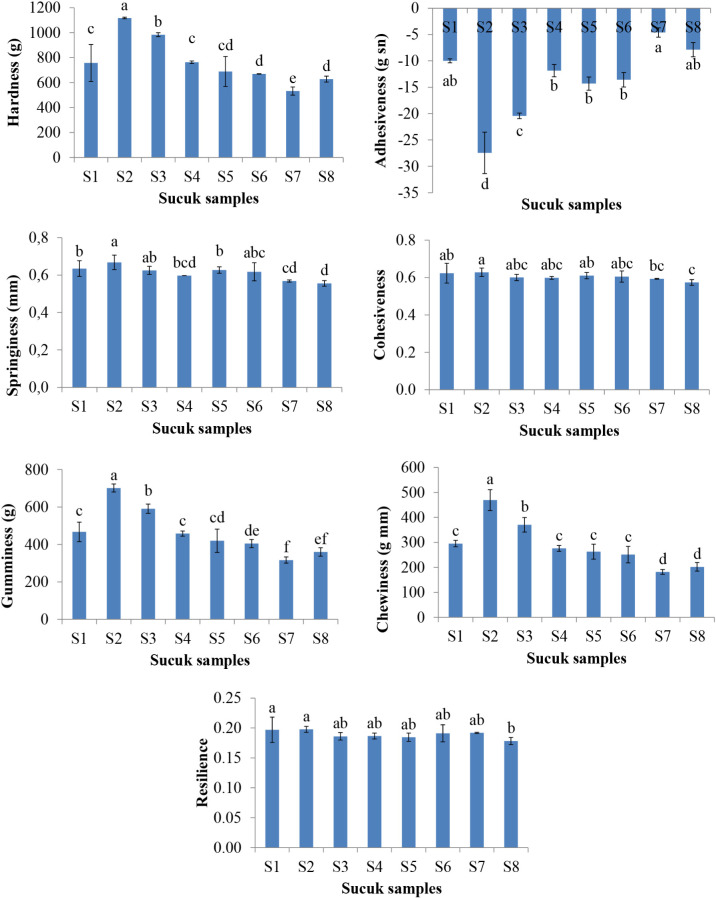
Textural properties including hardness, adhesiveness, springiness, cohesiveness, gumminess, chewiness and resilience parameters were determined in the ripened sucuk samples (at the 12th days of ripening), and these results are shown in Fig. 2. Hardness values of the sucuks with LAB cultures were higher than those of the sucuks without LAB cultures and control group. In general, use of LAB and yeast cultures affected (p<0.05) the hardness of the sucuk samples. The highest hardness value was found in the sucuk sample produced by only LAB cultures (S2) while the lowest hardness was determined for the sucuk with DH culture. Again, in general, the presence of CZ and DH cultures decreased hardness values of sucuk samples. This result might be due to low pH value and higher moisture loss of the sucuk samples. Adhesiveness values of sucuk were different (p<0.05) from each other, and it was higher in sucuk samples with yeast cultures compared to the sample produced with only LAB culture (S2). Adhesiveness values were –27.46 (g.sn) and –20.42 (g.sn) for S2 and S3 sucuks, respectively, and it was –9.99 (g.sn) in control sample (S1). However, it ranged from –4.60 to –13.58 (g.sn) and was higher for the sucuk sample produced without LAB culture. Springiness and cohesiveness values of the sucuk produced using yeast culture were lower than those of the sample with LAB culture. Use of only yeast culture on sucuk significantly (p<0.05) decreased both springiness and cohesiveness values (Fig. 2). Especially, it was lower in sucuks only with DH (S7) or CZ+DH (S8) samples, and it was 0.57 and 0.59 for in S7, respectively. However, springiness and cohesiveness values were higher in S4 and S5 samples (yeast+LAB cultures) than that of the S7 and S8 sucuks (yeast cultures). Although use of LAB cultures increased gumminess values of sucuk, use of only yeast cultures in formulation decreased that parameter. Gumminess values of the samples ranged from 316.7 to 701.7. The lowest gumminess values were determined as 316.7 and 360.4 in S7 and S8 samples, respectively. Chewiness values were similar to gumminess and ranged between 181.6 and 469.5 (Fig. 2). Again, both parameters were lower in sucuk samples (S5–S8) produced with only used yeast cultures. Both gumminess and chewiness values of the sucuk samples were significantly (p<0.05) different. Resilience values of the sucuk samples varied from 0.18 to 0.20, and it was lower in the samples with CZ and DH.
Fig. 2. Textural properties of the sucuks produced with yeast cultures.
S1, Control; S2, LAB; S3, LAB+C. zeylanoides; S4, LAB+D. hansenii; S5, LAB+C. zeylanoides+D. hansenii; S6, C. zeylanoides; S7, D. hansenii; S8, C. zeylanoides+D. hansenii. a–f Different lowercase for each parameters indicate the statistical difference (p<0.05) for textural properties of sucuk samples. LAB, lactic acid bacteria.
Limited numbers of studies are available related to effects of yeast starter cultures (D. hansenii) on textural properties of fermented sausages, and there is no report for the sucuk in the literature. In a study performed on Portuguese traditional sausage, use of Lactobacillus spp., Micrococcaceae and yeasts in production of fermented sausage slightly improved the cohesiveness properties of sausage, and other textural properties including hardness, adhesiveness, springiness, gumminess, chewiness and resilience were not significantly affected (Elias et al., 2014). In another study, D. hansenii affected the hardness and chewiness values, while no effect was observed on springiness and cohesiveness properties of sausage (Corral et al., 2014). In the current study, use of yeast culture significantly (p<0.05) affected the textural properties of sucuk (Fig. 2).
In general, microbial growth decreases pH level of sausages during the ripening. It leads to drying of sausage, and the denaturation and gelation properties of meat proteins. This affects hardness values of sausage (Bozkurt and Bayram 2006; Wu et al., 2010). Lower moisture levels in sausage also decrease the adhesiveness values, improving cutting ability of the product (Bozkurt and Bayram, 2006).
Volatile profile
Volatile composition of the sucuk samples is given in Table 4. In this study, total 85 volatile compounds were identified in the sucuk samples, and volatile compounds were in the following groups: 5 aldehydes, 2 alkanes, 2 alkines, 7 acids, 8 alcohols, 5 esters, 19 sulphur compounds, 31 terpenes, 3 aromatic hydrocarbons and 3 other components. Cuminaldehyde, di-2-propenyl disulfide and p-cymene were the major volatile aldehydes, sulphur compounds and terpenes, respectively. Again, eugenol, carvacrol and naphthalene were the aromatic hydrocarbons observed in the sucuk samples.
Table 4. Volatile compounds of the sucuk samples during ripening period (peak area %).
| Volatile Compounds | Ripening period (d) | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 6th | 12th | ||||||||||||||||||||||
| S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 | |
| Aldehydes | ||||||||||||||||||||||||
| Benzaldehyde | 0.23 | 0.24 | 0.23 | 0.32 | 0.47 | 0.44 | 0.49 | 0.43 | 0.5 | 0.25 | 0.31 | 0.37 | 0.3 | 0.49 | 0.37 | 0.48 | 0.43 | 0.37 | 0.42 | 0.26 | - | 0.34 | 0.42 | 0.39 |
| Nonanal | - | 0.17 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Benzeneacetaldehyde | - | - | - | - | - | 0.45 | - | - | 0.28 | - | - | - | - | - | 0.63 | 0.34 | 0.96 | - | - | - | - | 0.38 | 0.3 | 0.3 |
| Phellandral | - | - | - | 0.27 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Cuminaldehyde | 25.1 | 47.3 | 36.9 | 36.8 | 12.5 | 33.2 | 44 | 37.2 | 30.7 | 24.9 | 18.1 | 22 | 18.6 | 41.5 | 32.9 | 33.4 | 34.5 | 16 | 4.44 | 6.94 | 9.69 | 35.4 | 31.1 | 33.9 |
| Alkanes | ||||||||||||||||||||||||
| n-Eicosane | - | - | 0.48 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Pentadecane | 0.53 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Alkines | ||||||||||||||||||||||||
| 1-Decyne | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.57 | - | 0.25 | - | - | - | - | - |
| 1-Undecyne | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.36 | - | - | - | - | 0.55 |
| Acids | ||||||||||||||||||||||||
| Hexanoic acid | 0.17 | 0.19 | - | 0.27 | - | - | 0.17 | - | 0.18 | - | - | 0.26 | - | - | 0.17 | 0.13 | 0.17 | 0.19 | 0.32 | 0.19 | - | - | 0.19 | 0.18 |
| Butyric acid | 0.17 | 0.28 | 0.24 | - | 0.27 | - | 0.21 | 0.17 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Isovalericacid | - | - | - | - | - | - | - | - | 0.48 | - | - | - | - | - | 0.21 | - | 0.54 | - | 0.37 | 0.38 | 0.38 | 0.7 | 0.62 | 0.59 |
| Stearic acid | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.69 |
| Palmitic acid | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.89 | - |
| 9-Octadecenoic acid | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.3 | - |
| Propionic (Propanoic) acid | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.31 | - | 0.32 |
| Alcohols | - | |||||||||||||||||||||||
| Amyl alcohol | - | 0.92 | - | - | 1.33 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1-Hexanol | 0.17 | 0.21 | - | - | 0.23 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Benzyl alcohol | - | 0.22 | 0.23 | 0.33 | 0.59 | 0.58 | 0.44 | - | 0.49 | - | 0.47 | 0.74 | 1.04 | - | - | 0.83 | 0.44 | - | - | - | - | 0.38 | 0.64 | 0.68 |
| Phenethyl alcohol | 0.09 | - | 0.34 | - | 0.48 | 0.57 | 0.17 | 0.37 | 0.49 | 0.21 | 0.42 | 0.63 | 0.25 | 0.16 | 0.35 | 0.58 | 0.53 | 0.16 | 0.44 | 0.35 | 0.52 | 0.75 | 0.7 | 0.97 |
| α-Methylbenzyl-alcohol | 0.29 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.3 | 0.34 | 0.41 | - | - | - |
| 1-Phenyl-1-butanol | - | - | - | - | - | - | - | - | - | - | 0.78 | - | 0.71 | - | - | - | - | - | - | - | - | - | - | |
| Farnesol (E,E-) | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.15 | - | - | - | - | - |
| 1,3-Cyclohexadiene-1-methanol,4-(1-methylethyl)- | - | - | - | - | - | - | - | - | - | 0.14 | 0.14 | 0.16 | - | - | - | - | - | 0.13 | 0.14 | 0.14 | 0.19 | - | - | - |
| Esters | ||||||||||||||||||||||||
| Anisylformate | 0.2 | 0.19 | 0.22 | 0.35 | 0.63 | 0.28 | 0.3 | 0.23 | 0.19 | - | - | - | - | 0.34 | 0.19 | 0.18 | - | - | - | - | - | - | - | 0.21 |
| α,α-Dimethyl phenethyl acetate | 0.18 | - | 0.26 | - | 0.22 | - | - | - | - | - | - | 0.46 | - | - | - | - | - | 0.41 | - | - | - | - | - | - |
| Linalyl butyrate | - | - | 0.13 | - | - | 0.24 | - | 0.19 | - | - | 0.24 | - | - | - | - | 0.24 | - | - | - | 0.26 | 0.34 | 0.23 | - | - |
| Terpinyl acetate | - | - | - | 0.59 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.7 | - | 0.36 | 0.46 | - |
| Ethyl valerate | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.35 | 0.9 | - | - | - | - |
| Sulphur compounds | ||||||||||||||||||||||||
| Methylthiirane | 1.94 | 0.35 | 1.03 | 0.79 | - | 0.65 | - | 0.91 | 0.83 | - | - | - | 1.16 | 1.05 | - | - | 1.86 | 0.7 | - | - | - | 0.39 | 0.53 | 0.57 |
| Thietane | 2.99 | 0.46 | 0.45 | - | 0.55 | 0.75 | 1.08 | 3.38 | 2.65 | 2.42 | 1.66 | 1.53 | 3.73 | 3.03 | - | 2.14 | 1.49 | 3.12 | 3.3 | 3.65 | 4.79 | 0.5 | - | 1.28 |
| Methyl 2-propenyl disulfide | 1.26 | 1.59 | 1.47 | 1.48 | 1.3 | 0.75 | 0.95 | 1.03 | 1.13 | 1.36 | 1.05 | 1.14 | 1.69 | 1.19 | 1 | 0.87 | 0.75 | 1.2 | 1.21 | 1.12 | 1.35 | 0.76 | 0.84 | 0.64 |
| di-2-Propenyl disulfide | 11.4 | 4.68 | 8.19 | 5.1 | 8.3 | 5.75 | 10.9 | 8.23 | 6.83 | 6.5 | 8.62 | 11.3 | - | 10.6 | 4.79 | 5.13 | 7.88 | - | 6.68 | 6.57 | 7.48 | 4.67 | 5.66 | 4.87 |
| N-Ethyl-1,3-dithioisoindoline | 0.54 | 0.34 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| N.N’-Dimethylthiourea | 0.39 | 0.24 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Methyl 2-propenyl trisulfide | - | - | - | - | 0.37 | - | - | - | - | - | - | - | - | - | - | 0.23 | - | - | 0.16 | - | - | - | - | - |
| Diallyldisulphide | 0.36 | - | 0.29 | - | 0.23 | - | - | 0.3 | - | - | - | - | - | - | - | 0.37 | 0.35 | - | 0.3 | - | - | - | 0.26 | - |
| 3-(Methylthio)-1-propene | - | - | - | 2.46 | - | 1.02 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | |
| Allyl methyl sulfide | - | - | - | - | - | 0.49 | 0.4 | 0.64 | 0.54 0.43 | 1.2 | - | 0.32 | 0.55 | 0.56 | 0.42 | - | - | - | - | 0.81 | - | - | - | - |
| 3,3’-Thiobis-1-propene | 2.17 | 2.65 | 2.63 | - | 1.8 | - | 1.9 | 1.8 | 1.92 | 2.38 | 1.7 | 2.32 | 2.63 | 2.74 | 1.73 | 2.07 | 1.65 | - | 3.4 | - | - | 1.9 | 2.26 | - |
| 2,5-Dimethylthiazole | - | 0.16 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 3,4-Dihydro-3-vinyl-1,2-dithiin | - | 0.35 | - | 0.28 | - | - | 0.42 | 0.29 | 0.26 | - | - | - | - | - | - | 0.25 | - | - | - | - | - | - | - | - |
| 3-Vinyl-1,2-dithiacyclohex-4-ene | 0.44 | 0.33 | 0.3 | - | - | - | - | - | 0.29 | - | - | - | - | 0.36 | - | - | - | - | - | - | - | - | - | - |
| 2-Vinyl-1,3-dithiane | 4.09 | - | 6.06 | 5.95 | 3.2 | 3.31 | 5.1 | 4.32 | 5.83 | 5.09 | 4.25 | 4.97 | 7.66 | 4.5 | 4.72 | 4.39 | 5.46 | 3.97 | 3.4 | 3.51 | - | 3.64 | 2.67 | 3.39 |
| 2-Vinyl-4H-1,3-dithiin | - | - | - | 0.35 | 0.27 | - | 0.29 | - | - | - | - | - | - | - | 0.26 | - | - | - | - | - | - | - | - | - |
| 1-Oxa-4,6-diazacyclooctane-5-thione | 1.04 | - | - | - | 1.16 | 1.29 | 0.66 | 0.89 | 0.78 | 1.87 | - | 1.16 | 1.25 | 1.01 | 1.07 | - | 0.97 | 0.62 | 0.59 | 0.58 | 0.85 | 1.31 | 1.18 | - |
| 5-Methyl-1,2,3,4-tetrathia-cyclohexane | 0.32 | - | - | - | - | - | - | - | - | - | - | - | - | 0.32 | - | - | - | - | - | - | - | - | - | - |
| 3,4-Dimethoxy-1,2,5-thiadiazole | - | - | - | - | - | - | - | - | - | - | 0.75 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Terpenes | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| β-Pinene | 3.13 | 1.63 | 5.15 | - | 5.77 | 0.41 | - | - | 0.83 | 1.07 | 0.78 | - | - | - | 0.73 | 0.71 | - | 0.87 | 1.25 | 1.8 | 1.15 | - | - | 0.36 |
| Limonene | 2.05 | 2.49 | 2.38 | 0.65 | 2.4 | 1.89 | 1.12 | 1.53 | 2.51 | 2.48 | 2.61 | 2.86 | 3.05 | 1.59 | 2.52 | 2.33 | 1.49 | 3.68 | 4.85 | 4.77 | 3.95 | 2.53 | 2.68 | 2.18 |
| γ-Terpinene | 4.07 | 4.41 | 2.03 | 2.88 | 6.01 | 10.5 | 3.68 | 7.48 | 8.55 | 3.95 | 11.8 | 3.4 | 12.4 | 3.98 | 11.1 | 9.14 | 8.48 | 15.9 | 22 | 13 | 18.7 | 9.07 | 9.8 | 8.94 |
| p-Cymene | 5.46 | 7.93 | 9.15 | 3.55 | 5.7 | 12 | 5.22 | 7.81 | 8.93 | 12.2 | 14.1 | 9.36 | 11.3 | 7.26 | 10.1 | 9.87 | 9.14 | 17.2 | 16.1 | 13.8 | 15.7 | 11.8 | 12.1 | 10.5 |
| α-Terpinene | 0.35 | - | 0.36 | - | - | 0.19 | - | 0.18 | 0.21 | - | 0.23 | 1.35 | 0.81 | 0.24 | 0.89 | 0.21 | - | 0.32 | 0.32 | 0.31 | 0.63 | 0.35 | - | 0.21 |
| α-Amorphene | - | - | 0.33 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Sabinene | - | - | 0.58 | 0.34 | - | 0.6 | - | - | - | - | - | 1.14 | 1.09 | - | - | 0.48 | - | - | 1.55 | 0.82 | 0.71 | - | - | - |
| Myrcene | 0.25 | 0.22 | 0.16 | - | - | 1.64 | 0.95 | 1.18 | 1.85 | 1.86 | 2.14 | 1.55 | 1.59 | 1.01 | 1.83 | 1.74 | 1.31 | 2.37 | 0.32 | 0.99 | 3.04 | 1.95 | 1.97 | 1.73 |
| delta-3-Carene | 4.23 | 8.19 | 4.79 | 7.26 | 6.38 | 1.74 | - | - | 1.89 | - | 1.61 | - | - | - | 1.84 | 1.63 | - | 2.42 | 2.82 | 3.12 | 2.85 | 1.98 | 1.7 | 1.55 |
| α-Phellandrene | 0.48 | 0.88 | 1.06 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 1.66 | 3.44 | 2.96 | - | - | - |
| Terpinolene | 0.23 | 0.22 | 0.45 | 0.31 | 0.49 | 0.25 | - | 0.5 | 0.5 | - | 1.11 | 0.26 | 0.93 | 0.2 | 0.21 | 0.69 | 0.34 | 0.3 | 0.49 | 0.86 | 0.36 | 0.26 | 0.23 | 0.26 |
| Linalol | 0.57 | 0.92 | - | 0.29 | 0.58 | 0.91 | 0.54 | 0.78 | 0.94 | 0.98 | 1.02 | 0.84 | 1.1 | 0.67 | 0.99 | 0.94 | 0.63 | 1.26 | 1.45 | 1.4 | 1.18 | 0.85 | 0.94 | 0.83 |
| Copaene | 0.41 | 0.28 | - | - | 0.41 | 0.66 | 0.33 | 0.44 | 0.66 | - | 0.6 | - | 0.61 | 0.4 | 0.6 | 0.6 | 0.51 | 0.77 | 0.78 | 0.74 | 0.84 | 0.6 | 0.65 | 0.65 |
| α-Terpineol | 0.16 | 0.22 | 0.21 | - | - | - | 0.19 | - | - | - | - | - | - | - | 0.25 | - | - | - | - | 0.28 | - | - | 0.26 | 0.27 |
| Valencene | 0.24 | 0.79 | - | - | 0.61 | 0.39 | 0.36 | - | 0.36 | 0.5 | 0.39 | 0.44 | 0.4 | - | 0.38 | 0.33 | 0.3 | 0.35 | 0.32 | 0.59 | 0.39 | |||
| β-Caryophyllene | 1.82 | 2.12 | 1.33 | 1.05 | 1.56 | 3.15 | 1.94 | 2.57 | 3.29 | 2.72 | 3.4 | 4.29 | 3.2 | 2.15 | 3.24 | 3.65 | 2.57 | 3.75 | 4.78 | 4.47 | 3.95 | 3.08 | 2.88 | 3.44 |
| p-Cymenene | - | - | - | 0.34 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Camphene | - | - | - | - | - | 0.25 | - | 0.45 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| α-Selinene | - | - | - | - | - | 0.15 | 0.14 | 0.15 | 0.15 | 0.15 | 0.17 | 0.22 | 0.16 | 0.19 | 0.15 | 0.17 | 0.12 | 0.2 | 0.15 | 0.17 | 0.17 | 0.17 | 0.16 | 0.17 |
| Styrene | - | - | - | - | - | - | 0.73 | 0.84 | - | - | - | - | - | - | - | - | - | - | - | - | 0.89 | - | - | - |
| Pulegone | 1.26 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.38 | - | - | - | - | - | - | - | - | - |
| α-Copaene | 0.39 | - | - | - | - | - | - | - | - | 0.6 | 0.56 | - | 0.71 | - | 0.37 | 0.49 | - | - | 0.55 | - | - | - | 0.74 | - |
| α-Pinene | 0.6 | - | - | - | 0.81 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| β-Selinene | 0.29 | 0.29 | 0.3 | - | - | 0.41 | 0.35 | 0.4 | - | - | - | - | - | 0.37 | 0.33 | 0.44 | 0.29 | - | - | - | 0.4 | 0.3 | 0.36 | |
| 1,2-Dimethoxy-4-benzene | 0.21 | 0.26 | 0.24 | 0.29 | 0.25 | 0.27 | 0.19 | 0.22 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1,2-Dimethoxy-4-(2-propenyl)-benzene | 0.24 | 0.2 | 0.28 | 0.26 | 0.38 | 0.22 | 0.27 | 0.24 | 0.21 | 0.26 | 0.24 | 0.29 | 0.25 | 0.27 | 0.19 | 0.22 | 0.2 | 0.24 | 0.17 | 0.18 | 0.25 | 0.3 | 0.24 | 0.29 |
| Safranal | 5.66 | 5.7 | 5.96 | 6.64 | 6.97 | 6.51 | 6.32 | 6.12 | 5.12 | 4.86 | 4.28 | 3.26 | 3.33 | 5.76 | 6.34 | 6.22 | 4.93 | 3.53 | 1.58 | 1.88 | 2.12 | 5.58 | 4.13 | 6.37 |
| β-Elemene | - | - | - | - | - | - | - | - | - | - | 0.17 | - | - | - | - | 0.16 | - | 0.13 | 0.14 | 0.12 | - | - | - | - |
| trans-β-Farnesene | - | - | - | - | - | - | - | - | - | - | 0.14 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 1,3-bis-(1,1-Dimethylethyl)-benzene | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.29 | 0.23 | 0.33 | - | - | - | - |
| Bisabolene | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.17 | - | - | - | - | - | - | - |
| Aromatic hydrocarbons | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Eugenol | 0.26 | 0.53 | 0.62 | 6.77 | 0.52 | 1 | 0.7 | 0.78 | 1.52 | 0.83 | 1.04 | 0.68 | 1.07 | 0.54 | 0.64 | 0.66 | 0.76 | 0.54 | 0.55 | 0.58 | 0.88 | 0.88 | 0.96 | 1.03 |
| Carvacrol | 0.81 | 2.09 | 2.41 | 2.87 | 1.05 | 4.22 | 3.40 | 3.49 | 9.00 | 8.00 | 8.80 | 11.40 | 4.39 | 2.91 | 3.55 | 4.01 | 8.15 | 6.52 | 7.11 | 6.83 | 3.71 | 3.48 | 3.70 | 4.78 |
| Naphthalene | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.25 | - | - | - | - | - | 0.26 |
| Others | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| trans-Anethole | - | 0.13 | 0.16 | 0.19 | - | - | 0.15 | 0.14 | 0.13 | 0.16 | 0.12 | - | 0.15 | 0.17 | - | 0.12 | - | 0.15 | - | - | - | - | 0.14 | |
| α-Phellandrene epoxide | - | 0.13 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.14 | - | - | - | - | - | - | - | - |
| 1,4-Dioxan-2-yl hydroperoxide | 0.43 | - | - | - | - | - | 0.48 | - | - | - | 0.21 | - | - | 0.36 | - | - | - | - | - | - | - | - | - | - |
S1, Control; S2, LAB; S3, LAB+C. zeylanoides; S4, LAB+D. hansenii; S5, LAB+C. zeylanoides+D. hansenii; S6, C. zeylanoides; S7, D. hansenii; S8, C. zeylanoides+D. hansenii.
LAB, lactic acid bacteria.
Cuminaldehyde level in the volatile compounds decreased gradually during the ripening, and it was lower in the sucuk samples produced with LAB cultures at the end of ripening. However, the p-cymene, γ-terpinene, β-caryophyllene and limonene levels were higher in the samples produced with LAB cultures at the end of the ripening. Safranal was one of the major terpenes and was higher level in the control group and the sample produced with only yeast cultures. Propionic (propanoic) acid was detected in the sucuk samples produced only with yeast cultures at the end of ripening, and its level was 0.31% and 0.32% in S6 and S8, respectively. These samples were prepared with CZ yeast culture. Butyric acid was not detected at 6th and 12th days of ripening while it was observed at the beginning of the ripening. However, stearic acid, palmitic acid and 9-octadecenoic acid (oleic) which are fatty acids were determined in sucuk samples produced only with yeast cultures at the end of ripening. It was speculated that the degradation of free fatty acids to volatile compounds may have caused to these results (Flores and Olivares, 2014). Also the yeast species having lipolytic activity are able to hydrolyze fatty acids during the ripening, because CZ and DH cultures used in this study have lipolytic activity too (Ozturk and Sagdic, 2014).
The flavor properties of fermented sausages are associated with breakdown of carbohydrates, lipids and proteins by enzymes which are microbial origin and/or endogenous meat enzymes (Ansorena et al., 2001; Flores and Olivares, 2014; Kaban and Kaya 2009). Volatile compounds of fermented sausages can be also affected from meat origin, type of starter culture, process conditions and the spices used in production (Kaban and Kaya 2009; Leroy et al., 2006; Toldrá et al., 2001). Terpenes and sulphur compounds are important volatile compound groups in ripened meat products. Terpenes generally originate from spices used in production of fermented sausages as well as arise from the meat which based on animal nutrition with some terpenes (Ansorena et al., 2001). Again, cuminaldehyde, limonene, carvacrol and safranal can be resulted from cumin used in production of sucuk (Ağaoğlu et al., 2007, Li and Jiang 2004). While benzaldehyde, benzeneacetaldehyde and phenethyl alcohol compounds determined in the samples are the yields of degradation of amino acids (bacterial metabolism), 1-hexanol, propanoic acid and hexanoic acid comprise by lipid autooxidation in fermented sausages (Corral et al., 2013; Olivares et al., 2011). Kaban and Kaya (2009) found that major volatile compounds were 2-methyl-3-phenyl propanal, o-cymene, γ-terpinene and di-2-propenyl disulfide in sucuks produced with L. plantarum and S. xylosus. In another study, major volatile compounds of traditional sucuk samples were terpenes (o-cymene, γ-terpinene), acids (acetic acid), aldehydes (propanal,2-methyl-3-phenyl) and sulphur compounds (1-Propene,3,3’-thiobis) (Kaban, 2010). Küçüktaş (2012) isolated 38 volatile compounds from sucuk samples produced with yeast cultures (Y. lipolytica and D. hansenii) during ripening and major volatile compounds of sucuk were terpenes and sulphur compounds at the 9th day of ripening. However, some volatile compounds such as acetic acid, 2-methylpentanoic acid, 2-hydroxypropanoic acid, hexanoic acid ethanol, 1-pentanol, 1-hexanol and benzyl alcohol were reported as yields of lipid autooxidation, carbohydrate fermentation and amino acid degradation in that study. When considering this volatile composition of fermented sausages, some differences are available between the findings of the current study and literature. Analysis of volatile compounds of fermented sausage and other similar fermented meat products could be affected from several other factors such as solid phase microextraction (SPME), column and method conditions as well as meat origin, type of starter culture, process conditions and the spices used.
Conclusion
In this research, the effects of yeast cultures (Candida zeylanoides and Debaryomyces hansenii) isolated from traditionally dry fermented Turkish sucuks, on some properties of the sucuk samples were determined. In the results, use of the yeast cultures (C. zeylanoides and D. hansenii) in the sucuk affected aw and residual nitrite levels, moisture content and pH values as well. Again, it was noted that weight loss of the sucuks were decreased by use of the yeast cultures. Propionic (propanoic) acid, stearic acid, palmitic acid and 9-octadecenoic acid compounds were found only in the sucuk samples produced with the yeast cultures. Textural properties of the sucuks were also affected by the use of yeast cultures. It might be concluded that further studies are necessary to improve technological, sensory, textural and aromatic properties of the sucuk, dry fermented sausage by using different strains of D. hansenii and C. zeylanoides or other beneficial yeast species originate from the fermented sausages. Again, more research is needed on how yeasts prevent weight loss in fermented sausages that noted in this study.
Acknowledgements
This study was supported by Erciyes University, Scientific Research Projects Coordination Unit (FBA-10-3330 and FBD-10-3347).
Conflicts of Interests
The authors declare no potential conflicts of interest.
Author Contributions
Conceptualization: Ozturk I, Sagdic O. Data curation: Ozturk I. Formal analysis: Ozturk I. Methodology: Ozturk I. Validation: Ozturk I. Investigation: Ozturk I, Sagdic O. Writing - original draft: Ozturk I, Sagdic O, Yetim H. Writing - review & editing: Ozturk I, Sagdic O, Yetim H.
Ethics Approval
This article does not require IRB/IACUC approval because there are no human and animal participants.
References
- Ağaoğlu S, Dostbil N, Alemdar S. Antimicrobial activity of some spices used in the meat industry. Bull Vet Inst Pulawy. 2007;51:53–57. [Google Scholar]
- Andrade MJ, Córdoba JJ, Casado EM, Córdoba MG, Rodríguez M. Effect of selected strains of Debaryomyces hansenii on the volatile compound production of dry fermented sausage “salchichón”. Meat Sci. 2010;85:256–264. doi: 10.1016/j.meatsci.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Ansorena D, Gimeno O, Astiasarán I, Bello J. Analysis of volatile compounds by GC-MS of a dry fermented sausage: Chorizo de Pamplona. Food Res Int. 2001;34:67–75. doi: 10.1016/S0963-9969(00)00133-2. [DOI] [Google Scholar]
- AOAC . Official methods of analysis. 15th ed. Association of Official Analytical Chemists; Washington, DC USA: 2000. [Google Scholar]
- Bolumar T, Sanz Y, Flores M, Aristoy MC, Toldrá F, Flores J. Sensory improvement of dry-fermented sausages by the addition of cell-free extracts from Debaryomyces hansenii and Lactobacillus sakei. Meat Sci. 2006;72:457–466. doi: 10.1016/j.meatsci.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Bozkurt H, Bayram M. Colour and textural attributes of sucuk during ripening. Meat Sci. 2006;73:344–350. doi: 10.1016/j.meatsci.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Breuer U, Harms H. Debaryomyces hansenii: An extremophilic yeast with biotechnological potential. Yeast. 2006;23:415–437. doi: 10.1002/yea.1374. [DOI] [PubMed] [Google Scholar]
- Cammack R, Joannou CL, Cui XY, Torres Martinez C, Maraj SR, Hughes MN. Nitrite and nitrosyl compounds in food preservation. Biochim Biophys Acta. 1999;1411:475–488. doi: 10.1016/S0005-2728(99)00033-X. [DOI] [PubMed] [Google Scholar]
- Cocolin L, Urso R, Rantsiou K, Cantoni C, Comi G. Dynamics and characterization of yeasts during natural fermentation of Italian sausages. FEMS Yeast Res. 2006;6:692–701. doi: 10.1111/j.1567-1364.2006.00050.x. [DOI] [PubMed] [Google Scholar]
- Corral S, Salvador A, Belloch C, Flores M. Effect of fat and salt reduction on the sensory quality of slow fermented sausages inoculated with Debaryomyces hansenii yeast. Food Control. 2014;45:1–7. doi: 10.1016/j.foodcont.2014.04.013. [DOI] [Google Scholar]
- Corral S, Salvador A, Flores M. Salt reduction in slow fermented sausages affects the generation of aroma active compounds. Meat Sci. 2013;93:776–785. doi: 10.1016/j.meatsci.2012.11.040. [DOI] [PubMed] [Google Scholar]
- Demeyer D, Stahnke L. Quality control of fermented meat products. In: Kerry J, Kerry J, Ledward D, editors. In Meat processing improving quality. CRC Press; Boca Raton, FL, USA: 2002. pp. 359–393. (ed) [DOI] [Google Scholar]
- Dodds KL, Collins-Thompson DL. Nitrite tolerance and nitrite reduction in lactic acid bacteria associated with cured meat products. Int J Food Microbiol. 1984;1:163–170. doi: 10.1016/0168-1605(84)90007-2. [DOI] [Google Scholar]
- Durá MA, Flores M, Toldrá F. Effect of Debaryomyces spp. on the proteolysis of dry-fermented sausages. Meat Sci. 2004;68:319–328. doi: 10.1016/j.meatsci.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Elias M, Potes ME, Roseiro LC, Santos C, Gomes A, Agulheiro-Santos AC. The effect of starter cultures on the Portuguese traditional sausage “Paio do Alentejo” in terms of its sensory and textural characteristics and polycyclic aromatic hydrocarbons profile. J Food Res. 2014;3:45–56. doi: 10.5539/jfr.v3n3p45. [DOI] [Google Scholar]
- Flores M, Durá MA, Marco A, Toldrá F. Effect of Debaryomyces spp. on aroma formation and sensory quality of dry-fermented sausages. Meat Sci. 2004;68:439–446. doi: 10.1016/j.meatsci.2003.04.001. [DOI] [PubMed] [Google Scholar]
- Flores M, Olivares A. Flavor. In: Toldrá F, Hui YH, Astiasarán I, Sebranek JG, Talon R, editors. In Handbook of fermented meat and poultry. John Wiley and Sons; Hoboken, NJ, USA: 2014. pp. 217–225. (ed) [DOI] [Google Scholar]
- Fournaud J, Mocquot G. Study of the reduction of the nitrite ion by certain lactobacilli. C R Acad Hebd Seances Acad Sci D. 1966;262:230–2. [PubMed] [Google Scholar]
- Gençcelep H, Kaban G, Kaya M. Effects of starter cultures and nitrite levels on formation of biogenic amines in sucuk. Meat Sci. 2007;77:424–430. doi: 10.1016/j.meatsci.2007.04.018. [DOI] [PubMed] [Google Scholar]
- Kaban G. Volatile compounds of traditional Turkish dry fermented sausage (Sucuk) Int J Food Prop. 2010;13:525–534. doi: 10.1080/10942910802688184. [DOI] [Google Scholar]
- Kaban G, Kaya M. Effects of Lactobacillus plantarum and Staphylococcus xylosus on the quality characteristics of dry fermented sausage “sucuk”. J Food Sci. 2009;74:S58–S63. doi: 10.1111/j.1750-3841.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- Küçüktaş D. Effects of yeast species on quality characteristics of fermented Turkish sausages. Izmir Institute of Technology; Izmir, Turkish: 2012. Ph.D. dissertation. [Google Scholar]
- Leroy F, Verluyten J, De Vuyst L. Functional meat starter cultures for improved sausage fermentation. Int J Food Microbiol. 2006;106:270–285. doi: 10.1016/j.ijfoodmicro.2005.06.027. [DOI] [PubMed] [Google Scholar]
- Li R, Jiang ZT. Chemical composition of the essential oil of Cuminum cyminum L. from China. Flavour Frag J. 2004;19:311–313. doi: 10.1002/ffj.1302. [DOI] [Google Scholar]
- Lücke FK. Fermented sausages. In: Wood BJB, editor. In Microbiology of fermented foods. Blackie Academic & Professional; London, UK: 1998. pp. 441–483. (ed) [DOI] [Google Scholar]
- Mendonça RCS, Gouvêa DM, Hungaro HM, Sodré ADF, Querol-Simon A. Dynamics of the yeast flora in artisanal country style and industrial dry cured sausage (yeast in fermented sausage) Food Control. 2013;29:143–148. doi: 10.1016/j.foodcont.2012.05.057. [DOI] [Google Scholar]
- Oh CK, Oh MC, Kim SH. The depletion of sodium nitrite by lactic acid bacteria isolated from kimchi. J Med Food. 2004;7:38–44. doi: 10.1089/109662004322984680. [DOI] [PubMed] [Google Scholar]
- Olivares A, Navarro JL, Flores M. Effect of fat content on aroma generation during processing of dry fermented sausages. Meat Sci. 2011;87:264–273. doi: 10.1016/j.meatsci.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Ordóñez JA, Hierro EM, Bruna JM, de la Hoz L. Changes in the components of dry-fermented sausages during ripening. Crit Rev Food Sci Nutr. 1999;39:329–367. doi: 10.1080/10408699991279204. [DOI] [PubMed] [Google Scholar]
- Öztan A. Et Bilimi ve Teknolojisi. 4th ed. Union of Chambers of Turkish Engineers and Architects; Ankara, Turkey: 2005. p. 347. p. [Google Scholar]
- Ozturk I, Sagdic O. Biodiversity of yeast mycobiota in “sucuk,” a traditional turkish fermented dry sausage: Phenotypic and genotypic identification, functional and technological properties. J Food Sci. 2014;79:M2315–M2322. doi: 10.1111/1750-3841.12662. [DOI] [PubMed] [Google Scholar]
- Pérez-Alvarez JA, Sayas-Barberá ME, Fernández-López J, Aranda-Catalá V. Physicochemical characteristics of Spanish-type dry-cured sausage. Food Res Int. 1999;32:599–607. doi: 10.1016/S0963-9969(99)00104-0. [DOI] [Google Scholar]
- SAS . SAS/STAT user’s guide Version 8.2. SAS Institute; Cary, NC, USA: 2000. [Google Scholar]
- Selgas MD, Ros J, García ML. Effect of selected yeast strains on the sensory properties of dry fermented sausages. Eur Food Res Technol. 2003;217:475–480. doi: 10.1007/s00217-003-0778-0. [DOI] [Google Scholar]
- Taucmann F. Methoden der chemischen analytik von fleisch und fleischwaren. Bundensanstalt fur Fleischgforschung Klumbach 1987 [Google Scholar]
- Toldrá F. Biochemistry of meat and fat. In: Toldrá F, editor. In Handbook of fermented meat and poultry. Blackwell, Ames; IA, USA: 2007. pp. 51–58. (ed) [DOI] [Google Scholar]
- Toldrá F, Sanz Y, Flores M. Meat fermentation technology. In: Hui YH, Nip WK, Rogers RW, Young OA, editors. In Meat science and applications. Marcel Dekker; New York, NY, USA: 2001. (ed) [DOI] [Google Scholar]
- Turkish Food Codex Notification of Meat Products, Republic of Turkey Ministry of Agriculture and Rural Affairs. 2000. Official Gazette Date and Issue: 10.02.2000-23960, Ankara, Turkey.
- Wu Y, Zhao M, Yang BAO, Sun W, Cui C, Mu L. Microbial analysis and textural properties of cantonese sausage. J Food Process Eng. 2010;33:2–14. doi: 10.1111/j.1745-4530.2008.00255.x. [DOI] [Google Scholar]
- Yan PM, Xue WT, Tan SS, Zhang H, Chang XH. Effect of inoculating lactic acid bacteria starter cultures on the nitrite concentration of fermenting Chinese paocai. Food Control. 2008;19:50–55. doi: 10.1016/j.foodcont.2007.02.008. [DOI] [Google Scholar]