Abstract
Introduction:
Prevalence of fungi has been rising in the cystic fibrosis (CF) population. Scedosporium species (spp) is the second most common mold seen in the CF respiratory tract. However, the characteristics associated with Scedosporium isolation and its clinical implications are poorly understood. The goal of this study was to determine clinical factors associated with Scedosporium spp to better understand the mechanisms that may contribute to the emergence of filamentous fungi in CF.
Methods:
We conducted a retrospective cohort study of subjects followed in the CF Foundation Patient Registry between January 1, 2010 and December 31, 2012. Patients under 6 years of age, history of solid organ transplantation, and insufficient respiratory culture data were excluded. We used a multivariable logistic regression model to determine demographic data and baseline disease characteristics, medications and co-infections associated with Scedosporium spp recovery in CF sputum.
Results:
Among 19 023 subjects, prevalence of Scedosporium spp was 615 (3.2%). Older age (odds ratio [OR] 1.16, 95% confidence interval [CI] 1.07, 1.26) and white race (OR 1.69, 95% CI 1.09, 2.63) were the demographic factors associated with Scedosporium spp isolation. Inhaled antibiotic use had a significant association with Scedosporium isolation (OR 2.01, 95% CI 1.61, 2.52). For every additional course of intravenous antibiotics, the odds of Scedosporium isolation increased by 8% (OR 1.08, 95% CI 1.03, 1.14).
Conclusions:
The association between inhaled antibiotics and Scedosporium informs us that chronic inhaled antibiotics may be playing a role in Scedosporium isolation. Further investigation to better characterize this relationship is necessary.
Keywords: colonization, epidemiology, fungi, infection
1 |. INTRODUCTION
Cystic fibrosis (CF) is an autosomal recessive disease that affects approximately 30 000 in the United States alone.1 Over 2000 mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene cause reduced chloride transport affecting multiple organs.2,3 CFTR absence or dysfunction in the respiratory epithelium contributes the greatest morbidity and mortality in men and women with CF. Chronic airway infections are responsible for the development of bronchiectasis, progressive airflow obstruction, and eventually respiratory failure, the leading cause of death in the CF population.1 Chronic bacterial infections, such as Pseudomonas aeruginosa (Pa), Burkholderia cepacia complex, and Staphylococcus aureus, have traditionally been considered the culprits responsible for much of the comorbidity of CF; however, filamentous fungi have been increasingly observed in the CF airway within the past two decades.4–7
Scedosporium species (spp) is the second most common mold seen in the CF lung after Aspergillus spp.7–11 Scedosporium apiospermum complex (includes S. apiospermum sensu stricto, S. boydii, and S. auranticum) and Lomentospora prolificans (formerly known as Scedosporium prolificans) have been identified in CF hosts.12–16 Despite this, little is known about the characteristics associated with isolation of Scedosporium spp from CF sputum. Furthermore, there is poor understanding of the clinical implications of its detection in the airway, with the exception of its role after CF lung transplantation.17,18 Case reports and series have described improved outcomes with antifungal therapy in CF patients chronically colonized with S. apiospermum complex.10,13,19,20 However, systematic investigation of this topic is lacking. The objective of our study was to determine clinical factors associated with the isolation of Scedosporium spp to better understand the mechanisms that may be contributing to emergence of filamentous fungi in CF.
2 |. MET H ODS
2.1 |. Study design and participants
This was a retrospective cohort study of participants in the Cystic Fibrosis Foundation Patient Registry (CFFPR), a high-quality encounter-based database capturing demographic, clinical factors, and microbiology culture results of CF patients receiving care in the United States.21
We included subjects followed in the CFFPR between January 1, 2010 and December 31, 2012. We chose this start date as it was also the date that “Scedosporium species” was initially collected in the CFFPR. Patients under 6 years of age (less reliable respiratory specimen collection), history of solid organ transplantation, and insufficient respiratory culture data (defined as less than three cultures during study period) were excluded. Encounters after lung transplantation during the study period were excluded.
The primary outcome of interest was growth of Scedosporium spp in sputum culture during the study period. The secondary outcome was persistent isolation of Scedosporium, defined as two or more cultures which grew Scedosporium. This variable is defined at the genus level but does not differentiate the species level. Notably, a change in the taxonomy of Scedosporium spp, specifically Scedosporium prolificans to Lomentospora prolificans occurred in 2014,22 which should not affect this study sample.
The independent variables in the study included the following: age (in 10 year intervals), sex, race (white or non-white), F508del genotype (F508del homozygous or not), pancreatic insufficiency (determined by use of pancreatic enzymes in registry), body mass index (BMI) percentile (in 10 percent increments), BMI, CF related diabetes, forced expiratory volume in one second (FEV1) percent predicted (in 10 percent intervals), inhaled antibiotic use (determined by use of inhaled tobramycin, colistin, aztreonam, and/or other inhaled amino-glycoside), chronic macrolide use, number of intravenous (IV) antibiotic courses per year, inhaled corticosteroid use, Pa, methicillin-resistant Staphylococcus aureus (MRSA), Burkholderia cepacia complex (B. cepacia), and total number of encounters during the study period. With the exception of the total number of encounters during the study, all variables were used as coded at the patient’s first assessment in the time period of interest. Additional variables of interest in the CFFPR, such as oral antibiotic and oral corticosteroid, were considered, but not included in the analysis, due to inability to distinguish short-term (ie, treatment for a pulmonary exacerbation) or long-term (ie, chronic maintenance therapy) use.
2.2 |. Statistical analysis
Baseline demographic, clinical variables, and microbiological data were compared between subjects with isolated Scedosporium to those without using t-tests for continuous variables and chi-square for categorical variables. Unadjusted relationships between individual factors and Scedosporium isolation were investigated using simple logistic regression. We used a multivariable logistic regression model and forced in total number of encounters during the study period. The covariates included in the final model were chosen based on clinical knowledge and univariate relationship with P-value <0.20. Subgroup analyses for children (baseline age <18 years) and adults (baseline age > = 18 years) incorporated body mass index (BMI) percentile for children and BMI for adults in the multivariable models. A sensitivity analysis omitting subjects 36 years or older at baseline was conducted. Pearson goodness-of-fit test was performed for model checking with P-value >0.05. Variables were examined for collinearity using chi-square, including Pa, inhaled antibiotic use, and macrolides. Results of univariate and multivariable logistic regression models are presented as odds ratios (OR) with corresponding 95% confidence intervals (CI). Statistical significance was defined as a P-value <0.05. Missing values of the independent variables were noted in 2.8% of the population. Subjects with missing data were omitted and complete case analysis was performed. Analyses were performed by STATA version 11.0 (StataCorp, College Station, TX). The study was reviewed and approved by the institutional review boards of the Johns Hopkins School of Medicine (NA_00088963) and University of Pennsylvania Perelman School of Medicine (829312).
3 |. RESULTS
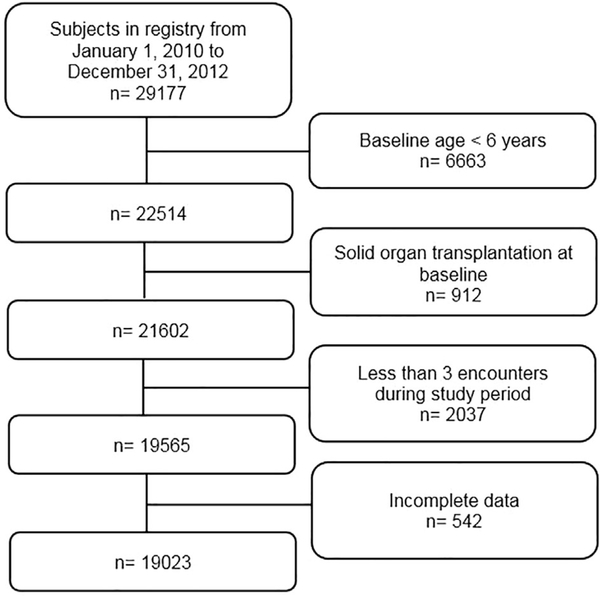
In 2010, 29 177 subjects were registered in the CFFPR. A total of 9612 (32.9%) individuals were excluded based on eligibility criteria and 542 (2.8%) subjects with incomplete data were omitted, leaving 19 023 in our study sample (Figure 1). Subjects entered the cohort at any point during the study period and remained in the study until solid organ transplantation, death, or December 31, 2012. During the study period, 344 subjects underwent organ transplant, 359 subjects died, and 615 subjects isolated Scedosporium at least once. Prevalence of Scedosporium isolation was 3.2%. Persistent Scedosporium isolation was found in 322 subjects (1.7%). Among the persistent cases, Scedosporium was isolated an average of 4.30 times (standard deviation 3.01) with a range of 2–22 occurrences during the study period.
FIGURE 1.
Flow diagram for study population
Baseline characteristics were compared between subjects with Scedosporium isolation and without Scedosporium isolation (Table 1). Female sex, white race, lower BMI/BMI percentile, lower FEV1 percent predicted, pancreatic insufficiency, CF related diabetes mellitus, inhaled antibiotics, chronic macrolides, higher number IV antibiotic courses, and Pa co-infection were observed to a greater degree in the Scedosporium group compared to individuals without Scedosporium isolation. Weak correlation was seen between Pa status and inhaled antibiotics by chi-square testing.
TABLE 1.
Baseline characteristics, comparing between who developed Scedosporium isolation versus no Scedosporium isolation (n = 19 023)
|
Scedosporium (n = 615) |
No Scedosporium (n = 18 408) |
P-value | |
|---|---|---|---|
| Demographics | |||
| Age (years ± sd) | 22.9 ± 12.3 | 21.1 ± 11.8 | 0.001 |
| Female | 333 (54.2) | 8907 (48.4) | 0.01 |
| White race | 594 (96.6) | 17254 (93.7) | 0.004 |
| F508del homozygous | 300 (48.8) | 8553 (46.5) | 0.16 |
| BMI for adults (kg/m2 ± sd) | 21.6 ± 3.62 | 22.6 ± 3.98 | <0.001 |
| BMI percentile for children (% ± sd) | 43.4 ± 24.5 | 49.6 ± 26.1 | <0.001 |
| Disease characteristics | |||
| Baseline FEV1 % predicted (% ± sd) | 71.4 ± 22.9 | 77.2 ± 25.7 | <0.001 |
| Pancreatic insufficiency | 557 (90.6) | 16131 (87.6) | 0.03 |
| CFRD | 149 (24.2) | 3683 (20.0) | 0.01 |
| ABPA | 63 (10.2) | 1083 (5.9) | <0.001 |
| Medications | |||
| Inhaled antibiotics | 490 (79.7) | 11304 (61.4) | <0.001 |
| Macrolide | 404 (65.7) | 10434 (56.7) | <0.001 |
| Inhaled corticosteroid | 376 (61.1) | 10 765 (58.5) | 0.19 |
| IV antibiotic courses during study period (number ± sd) | 4.13 ± 4.17 | 2.64 ± 3.53 | <0.001 |
| IV antibiotic courses during baseline year (number ± sd) | 1.36 ± 1.63 | 0.90 ± 1.41 | <0.001 |
| Microorganisms | |||
| Pseudomonas aeruginosa | 424 (68.9) | 10 776 (58.5) | <0.001 |
| MRSA | 163 (26.5) | 5236 (28.4) | 0.29 |
| Burkholderia cepacia complex | 20 (3.3) | 567 (3.1) | 0.81 |
BMI, body mass index; FEV1, forced expiratory volume in 1 second; CFRD, Cystic fibrosis related diabetes; ABPA, allergic bronchopulmonary aspergillosis; IV, intravenous; MRSA, methicillin resistant Staphylococcus aureus; sd, standard deviation.
The unadjusted and adjusted associations between individual factors and Scedosporium isolation are represented in Table 2. Older baseline age, by 10 year intervals (OR 1.16, 95% CI 1.07, 1.26, P < 0.001) and white race vs non-white (OR 1.69. 95% CI 1.09, 2.63, P = 0.02) were the demographic factors associated with Scedosporium isolation. Inhaled antibiotic use had a significant association with Scedosporium isolation (OR 2.01, 95% CI 1.61, 2.52, P < 0.001). For every additional course of IV antibiotics, the odds of Scedosporium isolation increased by 8% (OR 1.08, 95% CI 1.03, 1.14, P = 0.04). The associations between host factors and persistent Scedosporium isolation are shown in Table 3. In the adjusted model, similar associations were observed for age (OR 1.15, 95% CI 1.03, 1.28, P = 0.01), inhaled antibiotics (OR 2.10, 95% CI 1.52, 2.90, P < 0.001), and number of IV antibiotic courses (OR 1.13, 95% CI 1.06, 1.21, P < 0.001).
TABLE 2.
Clinical factors associated with Scedosporium species isolation, unadjusted and adjusteda (n = 19 023)
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Demographics | ||||||
| Baseline age, 10 year increments | 1.13 | 1.06, 1.20 | <0.001 | 1.16 | 1.07, 1.26 | <0.001 |
| Female | 1.26 | 1.07, 1.48 | 0.01 | 1.18 | 1.00, 1.39 | 0.05 |
| White vs non-white | 1.89 | 1.22, 2.94 | 0.004 | 1.69 | 1.09, 2.63 | 0.02 |
| F508del | 1.07 | 0.95, 1.21 | 0.27 | |||
| Disease characteristics | ||||||
| Pancreatic insufficiency | 1.36 | 1.03, 1.78 | 0.03 | 1.09 | 0.82, 1.47 | 0.54 |
| Baseline FEV1 % predicted, 10% increments | 0.92 | 0.89, 0.95 | <0.001 | 0.99 | 0.96, 1.04 | 0.88 |
| CFRD | 1.28 | 1.06, 1.54 | 0.01 | 0.91 | 0.75, 1.12 | 0.38 |
| Treatments | ||||||
| Inhaled antibiotics | 2.46 | 2.01, 3.00 | <0.001 | 2.01 | 1.61, 2.52 | <0.001 |
| Macrolide | 1.46 | 1.24, 1.73 | <0.001 | 1.00 | 0.83, 1.20 | 0.98 |
| IV antibiotic courses, number in baseline year | 1.18 | 1.13, 1.23 | <0.001 | 1.08 | 1.03, 1.14 | 0.04 |
| Inhaled corticosteroid | 1.12 | 0.95, 1.32 | 0.19 | 0.85 | 0.72, 1.00 | 0.06 |
| Infections | ||||||
| Pseudomonas aeruginosa | 1.57 | 1.32, 1.87 | <0.001 | 1.07 | 0.88, 1.29 | 0.51 |
| MRSA | 0.91 | 0.76, 1.09 | 0.29 | |||
| Burkholderia cepacia complex | 1.06 | 0.67, 1.66 | 0.81 | |||
FEV1, Forced expiratory volume in 1 second; CFRD, Cystic fibrosis related diabetes; IV, intravenous; MRSA, methicillin resistant Staphylococcus aureus. Bold effect estimates are the ones that are statistically significant.
Adjusted model includes baseline age, female, white race, pancreatic insufficiency, FEV1 percent predicted, CFRD, inhaled antibiotics, macrolide, IV antibiotic courses per year, Pseudomonas aeruginosa co-infection, and total number of encounters during study period.
TABLE 3.
Clinical factors associated with persistent Scedosporium isolation, unadjusted, and adjusteda
| Unadjusted | Adjusteda | |||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | P-value | Odds ratio | 95% CI | P-value | |
| Demographics | ||||||
| Baseline age, 10 year increments | 1.15 | 1.06, 1.25 | 0.001 | 1.15 | 1.03, 1.28 | 0.01 |
| Female | 1.38 | 1.11, 1.73 | 0.01 | 1.23 | 0.98, 1.54 | 0.07 |
| White vs non-white | 1.26 | 0.76, 2.10 | 0.37 | |||
| F508del | 1.08 | 0.91, 1.28 | 0.38 | |||
| Disease characteristics | ||||||
| Pancreatic insufficiency | 1.15 | 0.81, 1.64 | 0.44 | |||
| Baseline FEV1 % predicted, 10% increments | 0.87 | 0.83, 0.90 | <0.001 | 0.96 | 0.90, 1.01 | 0.11 |
| CFRD | 1.53 | 1.19, 1.95 | 0.001 | 0.95 | 0.73, 1.23 | 0.68 |
| Treatments | ||||||
| Inhaled antibiotics | 3.09 | 2.30, 4.14 | <0.001 | 2.10 | 1.52, 2.90 | <0.001 |
| Macrolide | 1.94 | 1.52, 2.47 | <0.001 | 1.20 | 0.92, 1.56 | 0.18 |
| IV antibiotic courses, number in baseline year | 1.27 | 1.21, 1.34 | <0.001 | 1.13 | 1.06, 1.21 | <0.001 |
| Inhaled corticosteroid | 1.30 | 1.03, 1.63 | 0.03 | 0.88 | 0.70, 1.12 | 0.31 |
| Infections | ||||||
| Pseudomonas aeruginosa | 1.73 | 1.36, 2.21 | <0.001 | 1.01 | 0.77, 1.32 | 0.95 |
| MRSA | 1.04 | 0.82, 1.33 | 0.75 | |||
| Burkholderia cepacia | 1.22 | 0.68, 2.19 | 0.50 | |||
FEV1, Forced expiratory volume in 1 second; CFRD, Cystic fibrosis related diabetes; IV, intravenous; MRSA, methicillin resistant Staphylococcus aureus. Bold effect estimates are the ones that are statistically significant.
Adjusted model includes baseline age, female, FEV1 percent predicted, CFRD, inhaled antibiotics, macrolide, IV antibiotic courses per year, inhaled corticosteroids, Pseudomonas aeruginosa co-infection, and total number of encounters during study period.
In the age-stratified analyses, higher BMI in adults was associated with a lower probability of Scedosporium isolation; however, this relationship between BMI percentile and Scedosporium isolation was not seen in children (Table S1). Consistent with the primary models, inhaled antibiotic use was the only additional risk factor for Scedosporium isolation in both children and adults. Pa had a positive association with Scedosporium isolation in children (OR 1.48, 95% CI 1.11, 1.97, P = 0.007), but negative relationship in adults (OR 0.72, 95% CI 0.56, 0.92, P = 0.01). In children, pancreatic insufficiency was an independent risk factor for Scedosporium isolation (OR 2.52, 95% CI 1.10, 5.74, P = 0.03). In a sensitivity analysis of subjects age 6 to 35 years (n = 16 808), similar relationships between inhaled antibiotics and Scedosporium isolation (OR 1.95, 95% CI 1.53, 2.49, P < 0.001) and IV antibiotic courses (OR 1.07, 95% CI 1.01, 1.13, P = 00.2) and Scedosporium isolation were seen (data not shown). Female sex (OR 1.17, P = 0.08) and pancreatic insufficiency (OR 1.43, P = 0.07) in this younger cohort trended toward increased Scedosporium isolation, but did not meet statistical significance. Post hoc analysis was conducted to explore the differences of Pa status in children and adults and demonstrated an interaction between Pa and age (P for interaction <0.001, Table S2). Additionally, a subgroup analysis by Pa status was conducted to further evaluate the relationship between inhaled antibiotic use and Scedosporium isolation. In the multivariable model in those without Pa (n = 7823), the association between inhaled antibiotics and Scedosporium remained strong, OR 2.20, 95% CI 1.57, 3.08, P < 0.0001. Similarly, in subjects with Pa (n = 11 200), inhaled antibiotics was associated with Scedosporium isolation; however, the association was attenuated, OR 1.72, 95% CI 1.28, 2.31, P < 0.001 (data not shown).
4 |. DISCUSSION
This retrospective analysis sheds light on the clinical and host characteristics associated with Scedosporium isolation in a United States CF population. To our knowledge, this is largest study of CF patients addressing the epidemiology of Scedosporium spp. Existing data have focused on Aspergillus fumigatus and non-fumigatus spp in the CF lungs; however, there has been increasing interest in the recovery and role of Scedosporium/Lomentospora spp in the CF respiratory tract. Much of the published literature on epidemiology of Scedosporium spp and other filamentous fungi in CF has largely originated from Europe and Australia, with the exception of our previous work.7,23,24 The prevalence of Scedosporium/Lomentospora spp found in our study is similar to numbers published in existing US studies, ranging from 1.3% and 2.3%.7,8,23 Of note, the CF Foundation infection control guidelines strongly recommend quarterly bacterial respiratory culture evaluation and annual mycobacterial sampling.25,26 However, there are no guidelines for fungal culture evaluation. The reported prevalence of Scedosporium/Lomentospora spp is greater in European and Australian studies (3.3–10.6%), most of which employed a Scedosporium-selective culture media.9,11,27–29 As clinicians’ practices and microbiology laboratory protocols for mycological evaluation vary (eg, utilization of selective fungal culture media, frequency of fungal culture testing), the observed differences in prevalence of Scedosporium isolation between the United States and other countries are not surprising.
The source and pathogenesis of Scedosporium/Lomentospora spp isolation in the human respiratory tract are unclear. Scedosporium spp have been associated with soil and livestock feces, and nearly exclusively limited to the outdoor environment.30 However, an environmental study investigating samples in homes of CF patients, including air, garden soil, pet litter, bathroom water, identified only potted plants as a potential source of Scedosporium contamination.31 Within our registry analysis, environmental factors, such as possession of potted plants, cannot be ascertained, but conceptually plausible.
Our study demonstrated use of inhaled antibiotics was associated with a greater risk of Scedosporium isolation and persistence (OR 2.01, P < 0.001 and 2.10, P < 0.001 respectively). This relationship held in both children and adults in the age-stratified analysis as well. Chotirmall et al suggests that prolonged antibiotic use may facilitate growth of fungi in CF patients.32 Inhaled antibiotic exposure has been identified as a potential risk factor for Aspergillus spp in several studies.6,24,33–35 In the most compelling study, Burns et al conducted a post hoc analysis of the clinical trials data comparing chronic intermittent inhaled tobramycin with placebo in CF patients and found that tobramycin increased the isolation of Aspergillus spp compared to that in the placebo group (18% vs 8%, P = 0.001).36 A clear independent association between inhaled antibiotics and Scedosporium isolation has not been previously described; albeit only pursued in one single-center study.37 An alternative mechanism to explain this relationship could be fungal contamination of nebulizer equipment permitting an avenue for conidial inhalation. However, Scedosporium spp was not isolated on nebulizer surfaces in the only published study on this topic, making this less likely.38 Parize et al did not find inhaled antibiotics to be associated with Scedosporium apiospermum complex seropositivity14; however, there was no report on the relationship between inhaled antibiotics and Scedosporium status by culture. Pa may inhibit the growth of Scedosporium and Lomentospora spp in vitro,39 which could explain the positive association between antipseudomonal inhaled antibiotics and Scedosporium isolation; however, a negative relationship between Pa isolation itself was not observed in our data. Furthermore, the positive association between inhaled antibiotics and Scedosporium remained in the Pa-negative individuals in the subgroup analysis, suggesting inhaled antibiotics may independently play a role in Scedosporium isolation and recovery. (However, “Pa-negative” represents their Pa status during the baseline year and does not exclude previous Pa isolation prior to the study period.) We also observed a positive relationship between the number of IV antibiotic courses for pulmonary exacerbations during the baseline year and Scedosporium isolation (OR 1.08, P = 0.04). Interestingly, the effect estimates of inhaled antibiotic usage and IV antibiotic exposure were slightly greater in the persistent Scedosporium isolation model, which strengthens these independent relationships. Our findings highlight the potential relationship between chronic or recurrent antibiotic exposure and its effects on the CF airway microenvironment. A probable mechanism is the antibiotic exposure results in decreased bacterial density permitting the condition for fungi to thrive. The other plausible explanation is that sicker patients require more antibiotics and Scedosporium isolation may represent the more diseased phenotype. As these findings require further exploration, our data should not suggest that inhaled and IV antibiotic use, which are important proven treatment modalities that incur clinical benefit in the CF population, should be tempered.
Older age was independently associated with both isolation and persistence of Scedosporium. The mean age of Scedosporium positive subjects was 22.9 years (Table 1). Older age has been highlighted in several studies as a potential risk factor for filamentous fungi.7,34,40 The average age of first isolation of Scedosporium spp in CF has been described as approximately 14.5 years.10 In contrast, younger age was associated with Scedosporium/Lomentospora colonization in a German CF cohort.15 The detection of fungi in older individuals may be due sampling bias, given less reliable microbiological data in younger children unable to expectorate sputum. Further study of this relationship is necessary.
Although baseline characteristics depicted in Table 1 suggested that sicker individuals (pancreatic insufficiency, CF related diabetes, lower FEV1 percent predicted, Pa positivity, and greater number of IV antibiotic courses) isolated Scedosporium spp, clinical markers of disease severity, such as FEV1 percent predicted, pancreatic insufficiency, and co-infections were not independently associated with Scedosporium positivity in the adjusted model (Tables 2 and 3). However, the age-stratified analysis suggests that this notion may hold true in younger aged children. Pancreatic insufficiency (OR 2.52, P = 0.03) and Pa status (OR 1.48, P = 0.007) were positively associated with Scedosporium isolation in children. In contrast, in adults, the effect estimates between pancreatic insufficiency, FEV1 percent predicted, and Pa were in the opposite direction (odds ratios less than one). This may be impacted by the heterogeneity of CF adults spanning 18–81 years (ie, milder phenotypes with residual function mutations) and potential survival bias where sicker individuals would receive lung transplantation or die prior to adult age. A sensitivity analysis omitting subjects aged 36 years or greater demonstrated a trend for pancreatic insufficiency and Pa status at baseline to be associated with increased Scedosporium isolation (OR 1.43, P = 0.07 and OR 1.19, P = 0.10 respectively); supporting this hypothesis (data not shown). In this analysis of subjects age 6 through 35 years, inhaled antibiotics (OR 1.95, P < 0.001) and IV antibiotic episodes during baseline year (OR 1.07, P = 0.02) were consistently associated with Scedosporium isolation. Also, the significant interaction between age and Pa colonization further reveals that Pa may be independently associated with Scedosporium isolation in younger individuals with CF, representing the more classic CF phenotype (Table S2). An unexpected finding was the negative relationship between MRSA status and Scedosporium status in children (OR 0.57, P < 0.001), which has not been described previously (Table S1). Although the mechanism is unclear, one could hypothesize that MRSA may competitively inhibit Scedosporium growth on culture media.
Our study does have limitations, particularly due to the nature of our retrospective registry-based study. However, the accuracy of the CFFPR and clear advantages of evaluating the impact of therapies are well-established.21,41 It is important to emphasize that the United States does not employ a standardized approach for fungal detection or surveillance in the CF population. In turn, the heterogeneous practices likely affect the recovery and report of Scedosporium spp in the registry. Furthermore, Scedosporium/Lomentospora spp are challenging to isolate on traditional bacteria culture and standard fungal culture media due to the presence of competing organisms. Scedosporium-selective agars have demonstrated better detection of Scedosporium spp; however, these methods are not the clinical standard and this practice is absent in the United States.42 The most evident limitation is our study’s inability to delineate a temporal or causal relationship between the identified risk factors and Scedosporium isolation and persistence. It is plausible that Scedosporium isolation is merely a marker for disease severity; distinguishing this would be impossible in a retrospective study. We attempted to address this by utilizing baseline status of variables. The short study period of 3 years limited our ability to evaluate the temporal relationships further. Despite this limitation, we achieved our objective to identify potential independent relationships for future studies. Due to the retrospective nature of the analysis, risk for information bias, and residual confounding cannot be avoided. However, our multivariable analysis adjusted for pertinent host characteristics and number of total encounters during the study period. Missing or incomplete data was present in approximately 2% of the eligible population, which is unlikely to result in significant bias.43 Finally, the CFFPR captures only culture-based results alone. Despite suggestion of greater detection, serological evaluation, and molecular detection of Scedosporium/Lomentospora spp does not routinely occur in clinical centers and therefore, not collected in the CFFPR.44
The impact of fungi in the CF airway, specifically Scedosporium/Lomentospora, is not yet known and requires rigorous investigation.18,45 Parize et al did not detect an association between Scedosporium apiospermum complex serologic status with lung function; however, the association between culture positivity was not evaluated.14 Although several case reports suggest that persistent isolation of Scedosporium/Lomentospora may represent infection and negatively impact CF lung health, this question remains unanswered. For CF patients with end-stage lung disease, greater understanding of the development, detection, and treatment of Scedosporium/Lomentospora is imperative given the potential implications of the multidrug resistant organisms, particularly Lomentospora prolificans, on lung transplant candidacy.46,47
In conclusion, our study discovered a positive relationship between inhaled and IV antibiotics and Scedosporium isolation in the CF respiratory tract, in addition to other demographic factors. Although our analysis cannot confirm a causal relationship, it informs us that chronic inhaled antibiotic use may be affecting the microbial environment of the airway or individuals with Scedosporium/Lomentospora isolation and persistence may reflect illness severity and increased treatment burden of inhaled antibiotics. Both potential explanations for our findings require further investigation with time-dependent analyses as data in the CFFPR continues to grow; with the consideration that the taxonomic change in Scedosporium spp in 2014 may affect the interpretation.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the Cystic Fibrosis Foundation for the use of CF Foundation Patient Registry data to conduct this study. Additionally, we would like to thank the patients, care providers, and clinic coordinators at CF Centers throughout the United States for their contributions to the CF Foundation Patient Registry. G.H. receives funding from the Cystic Fibrosis Foundation (HONGA160).
Funding information
Cystic Fibrosis Foundation, Grant number: HONG16A0
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.2016 Annual Data Report. 2017.
- 2.Farrell PM, White TB, Ren CL, et al. Diagnosis of cystic fibrosis: consensus guidelines from the cystic fibrosis foundation. J. Pediatr. 2017;181s:S4–S15. e1. [DOI] [PubMed] [Google Scholar]
- 3.Elborn JS. Cystic fibrosis. Lancet (London, England). 2016;388: 2519–2531. [DOI] [PubMed] [Google Scholar]
- 4.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23:299–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Middleton PG, Chen SC, Meyer W. Fungal infections and treatment in cystic fibrosis. Curr Opin Pulm Med. 2013;19:670–675. [DOI] [PubMed] [Google Scholar]
- 6.Pihet M, Carrere J, Cimon B, et al. Occurrence and relevance of filamentous fungi in respiratory secretions of patients with cystic fibrosis-a review. Med Mycol. 2009;47:387–397. [DOI] [PubMed] [Google Scholar]
- 7.Sudfeld CR, Dasenbrook EC, Merz WG, Carroll KC, Boyle MP. Prevalence and risk factors for recovery of filamentous fungi in individuals with cystic fibrosis. J Cystic Fibrosis. 2010;9:110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.2012 Annual Data Report. 2013. [Google Scholar]
- 9.Blyth CC, Harun A, Middleton PG, et al. Detection of occult Scedosporium species in respiratory tract specimens from patients with cystic fibrosis by use of selective media. J Clin Microbiol. 2010;48:314–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cimon B, Carrere J, Vinatier JF, Chazalette JP, Chabasse D, Bouchara JP. Clinical significance of Scedosporium apiospermum in patients with cystic fibrosis. Eur J Clin Microbiol Infect Dis. 2000;19:53–56. [DOI] [PubMed] [Google Scholar]
- 11.Ziesing S, Suerbaum S, Sedlacek L. Fungal epidemiology and diversity in cystic fibrosis patients over a 5-year period in a national reference center. Med Mycol. 2016;54:781–786. [DOI] [PubMed] [Google Scholar]
- 12.Gilgado F, Cano J, Gene J, Guarro J. Molecular phylogeny of the Pseudallescheria boydii species complex: proposal of two new species. J Clin Microbiol. 2005;43:4930–4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noni M, Katelari A, Kapi A, Stathi A, Dimopoulos G, Doudounakis SE. Scedosporium apiospermum complex in cystic fibrosis; should we treat? Mycoses. 2017;60:594–599. [DOI] [PubMed] [Google Scholar]
- 14.Parize P, Billaud S, Bienvenu AL, et al. Impact of Scedosporium apiospermum complex seroprevalence in patients with cystic fibrosis. J Cystic Fibrosis. 2014;13:667–673. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz C, Brandt C, Antweiler E, et al. Prospective multicenter German study on pulmonary colonization with Scedosporium/Lomentospora species in cystic fibrosis: epidemiology and new association factors. PLoS ONE. 2017;12:e0171485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sedlacek L, Graf B, Schwarz C, et al. Prevalence of Scedosporium species and Lomentospora prolificans in patients with cystic fibrosis in a multicenter trial by use of a selective medium. J Cystic Fibrosis. 2015;14:237–241. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LS, Shields RK, Clancy CJ. Epidemiology, clinical manifestations, and outcomes of Scedosporium infections among solid organ transplant recipients. Transplant Infect Dis. 2014;16:578–587. [DOI] [PubMed] [Google Scholar]
- 18.Tracy MC, Moss RB. The myriad challenges of respiratory fungal infection in cystic fibrosis. Pediatr Pulmonol. 2018;53:S75–S85. [DOI] [PubMed] [Google Scholar]
- 19.Holle J, Leichsenring M, Meissner PE. Nebulized voriconazole in infections with Scedosporium apiospermum—case report and review of the literature. J Cystic Fibrosis. 2014;13:400–402. [DOI] [PubMed] [Google Scholar]
- 20.Rolfe NE, Haddad TJ, Wills TS. Management of Scedosporium apiospermum in a pre- and post-lung transplant patient with cystic fibrosis. Med Mycol Case Rep. 2013;2:37–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knapp EA, Fink AK, Goss CH, et al. The cystic fibrosis foundation patient registry. design and methods of a national observational disease registry. Annals Am Thorac Soc. 2016;13:1173–1179. [DOI] [PubMed] [Google Scholar]
- 22.Lackner M, de Hoog GS, Yang L, et al. Proposed nomenclature for Pseudallescheria, Scedosporium and related genera. Fungal Diversity. 2014;67:1–10. [Google Scholar]
- 23.Hong G, Miller HB, Allgood S, Lee R, Lechtzin N, Zhang SX. Use of selective fungal culture media increases rates of detection of fungi in the respiratory tract of cystic fibrosis patients. J Clin Microbiol. 2017;55:1122–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong G, Psoter KJ, Jennings MT, et al. Risk factors for persistent Aspergillus respiratory isolation in cystic fibrosis. J Cystic Fibrosis. 2018;17:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saiman L, Siegel J. Infection control recommendations for patients with cystic fibrosis: microbiology, important pathogens, and infection control practices to prevent patient-to-patient transmission. Am J Infect Control. 2003;31:S1–62. [PubMed] [Google Scholar]
- 26.Saiman L, Siegel JD, LiPuma JJ, et al. Infection prevention and control guideline for cystic fibrosis: 2013 update. Infect Control Hosp Epidemiol. 2014;35:S1–s67. [DOI] [PubMed] [Google Scholar]
- 27.Coron N, Pihet M, Frealle E, et al. Toward the standardization of mycological examination of sputum samples in cystic fibrosis: results from a french multicenter prospective study. Mycopathologia. 2017;183:101–117. [DOI] [PubMed] [Google Scholar]
- 28.Nagano Y, Elborn JS, Millar BC, et al. Comparison of techniques to examine the diversity of fungi in adult patients with cystic fibrosis. Med Mycol. 2010;48:166–176. e1. [DOI] [PubMed] [Google Scholar]
- 29.Masoud-Landgraf L, Badura A, Eber E, Feierl G, Marth E, Buzina W. Modified culture method detects a high diversity of fungal species in cystic fibrosis patients. Med Mycol. 2014;52:179–186. [DOI] [PubMed] [Google Scholar]
- 30.Ramirez-Garcia A, Pellon A, Rementeria A, et al. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2018;56:102–125. [DOI] [PubMed] [Google Scholar]
- 31.Rougeron A, Giraud S, Alastruey-Izquierdo A, et al. Ecology of scedosporium species: present knowledge and future research. Mycopathologia. 2018;183:185–200. [DOI] [PubMed] [Google Scholar]
- 32.Chotirmall SH, McElvaney NG. Fungi in the cystic fibrosis lung: bystanders or pathogens? Int J Biochem Cell Biol. 2014;52:161–173. [DOI] [PubMed] [Google Scholar]
- 33.Bargon J, Dauletbaev N, Kohler B, Wolf M, Posselt HG, Wagner TO. Prophylactic antibiotic therapy is associated with an increased prevalence of Aspergillus colonization in adult cystic fibrosis patients. Respir Med. 1999;93:835–838. [DOI] [PubMed] [Google Scholar]
- 34.de Vrankrijker AM, van der Ent CK, van Berkhout FT, et al. Aspergillus fumigatus colonization in cystic fibrosis: implications for lung function? Clin Microbiol Infect. 2011;17:1381–1386. [DOI] [PubMed] [Google Scholar]
- 35.Liu JC, Modha DE, Gaillard EA. What is the clinical significance of filamentous fungi positive sputum cultures in patients with cystic fibrosis? J Cystic Fibrosis. 2013;12:187–193. [DOI] [PubMed] [Google Scholar]
- 36.Burns JL, Van Dalfsen JM, Shawar RM, et al. Effect of chronic intermittent administration of inhaled tobramycin on respiratory microbial flora in patients with cystic fibrosis. J Infect Dis. 1999;179: 1190–1196. [DOI] [PubMed] [Google Scholar]
- 37.Blyth CC, Middleton PG, Harun A, Sorrell TC, Meyer W, Chen SC. Clinical associations and prevalence of Scedosporium spp. in Australian cystic fibrosis patients: identification of novel risk factors? Med Mycol. 2010;48:S37–S44. [DOI] [PubMed] [Google Scholar]
- 38.Peckham D, Williams K, Wynne S, Denton M, Pollard K, Barton R. Fungal contamination of nebuliser devices used by people with cystic fibrosis. J Cystic Fibrosis. 2016;15:74–77. [DOI] [PubMed] [Google Scholar]
- 39.Chen SC, Patel S, Meyer W, et al. Pseudomonas aeruginosa inhibits the growth of scedosporium and lomentospora in vitro. Mycopathologia. 2018;183:251–261. [DOI] [PubMed] [Google Scholar]
- 40.Milla CE, Wielinski CL, Regelmann WE. Clinical significance of the recovery of Aspergillus species from the respiratory secretions of cystic fibrosis patients. Pediatr Pulmonol. 1996;21:6–10. [DOI] [PubMed] [Google Scholar]
- 41.Jackson AD, Goss CH. Epidemiology of CF: how registries can be used to advance our understanding of the CF population. J Cystic Fibrosis. 2017;17:297–305. [DOI] [PubMed] [Google Scholar]
- 42.Schwarz C, Bouchara JP, Buzina W, et al. Organization of patient management and fungal epidemiology in cystic fibrosis. Mycopathologia. 2017;183:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong Y, Peng C-YJ. Principled missing data methods for researchers. SpringerPlus. 2013;2:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen M, Kondori N, Deng S, et al. Direct detection of Exophiala and Scedosporium species in sputa of patients with cystic fibrosis. Med Mycol. 2017;56:695–702. [DOI] [PubMed] [Google Scholar]
- 45.Bouchara JP, Symoens F, Schwarz C, Chaturvedi V. Fungal respiratory infections in cystic fibrosis (CF): recent progress and future research agenda. Mycopathologia. 2018;183:1–5. [DOI] [PubMed] [Google Scholar]
- 46.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014—an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2015;34: 1–15. [DOI] [PubMed] [Google Scholar]
- 47.Limper AH, Knox KS, Sarosi GA, et al. An official American Thoracic Society statement: treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.