Abstract
Δ9-tetrahydrocannabinol (THC) is the intoxicating constituent of cannabis and is responsible for the drug’s reinforcing effects. Retrospective human studies suggest that cannabis use during adolescence is linked to long-term negative psychological outcomes, but in such studies it is difficult to distinguish the effects of THC from those of coexisting factors. Therefore, translationally relevant animal models are required to properly investigate THC effects in adolescents. However, though the relevance of these studies depends upon human-relevant dosing, surprisingly little is known about THC pharmacology and its effects on behavior and brain activity in adolescent rodents—especially in females. Here, we conducted a systematic investigation of THC pharmacokinetics, metabolism and distribution in blood and brain, and of THC effects upon behavior and neural activity in adolescent Long Evans rats of both sexes. We administered THC during an early-middle adolescent window (postnatal days 27–45) in which the brain may be particularly sensitive to developmental perturbation by THC. We determined the pharmacokinetic profile of THC and its main first-pass metabolites (11-hydroxy-THC and 11-nor-9-carboxy-THC) in blood and brain following acute injection (0.5 or 5 mg/kg, intraperitoneal). We also evaluated THC effects on behavioral assays of anxiety, locomotion, and place conditioning, as well as c-Fos expression in 14 brain regions. Confirming previous work, we find marked sex differences in THC metabolism, including a female-specific elevation in the bioactive metabolite 11-hydroxy-THC. Furthermore, we find dose-dependent and sex-dependent effects on behavior, neural activity, and functional connectivity across multiple nodes of brain stress and reward networks. Our findings are relevant for interpreting results of rat adolescent THC exposure studies, and may lend new insights into how THC impacts the brain in a sex-dependent manner.
Subject terms: Experimental organisms, Translational research, Behavioural methods, Addiction, Reward
Introduction
Cannabis is among the psychoactive drugs most commonly used by teenagers, and retrospective studies suggest that early exposure to the drug is associated with negative psychological outcomes [1–5]. However, such studies do not allow differentiation between outcomes caused by the drug itself (and particularly by its intoxicating constituent Δ9-tetrahydrocannabinol (THC)) from those resulting from other coexisting factors such as stress, genetic risk, or use of other drugs. Since cannabis availability continues to expand in North America and Europe, there is a pressing need for information about how THC impacts brain function, especially during the sensitive adolescent period [2, 6, 7].
Interest in the effects of cannabinoid drugs on adolescent brain development has grown substantially in recent years. Many laboratories have developed rodent models of adolescent cannabinoid exposure, and have shown that THC or synthetic cannabinoids can persistently affect brain function and behavior. Domains impacted by these drugs include memory [8–10], cognition [7, 11, 12], emotionality [13–15], and intake of other psychoactive drugs [16–21]. Brain regions involved include, among others, the nucleus accumbens, prefrontal cortex, and hippocampus [9, 11, 12, 17]. Moreover, there is evidence that some persistent effects of adolescent cannabinoid receptor stimulation are sex-dependent [13, 14, 22–32]. Clearly, these findings are of great significance for public health, if they are relevant to teenage cannabis use.
Unfortunately, the available preclinical studies vary widely in cannabinoid agent administered, dosage, dosing protocol, washout period, and sex and developmental stage of test animals, making comparisons across datasets difficult. Further complicating matters, though clear sex differences in THC effects have emerged [23, 33–37], most studies have been conducted in male animals only. Age and sex modulate the biotransformation of THC, which primarily involves the oxidation to 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-THC (11-COOH-THC), catalyzed by cytochrome P450 enzymes in the liver [24, 38]. Yet, a systematic analysis of THC PK, and effects on behavior and neural activity in adolescent rodents has not been conducted, leaving unclear the translational value of these preclinical findings. Other unresolved issues include potential sex differences in the PK, metabolic, and pharmacodynamic profile of THC in adolescents, the brain regions recruited by THC, and the drug’s impact on functional connectivity within reward-processing and stress-processing networks.
This study provides a systematic evaluation of these questions, using male and female adolescent Long Evans rats at postnatal day (PD) 27–45, a peripubertal period that may be especially sensitive to neurodevelopmental disruption by THC [39, 40]. We investigated the PK of THC and its metabolic conversion to 11-OH-THC and 11-COOH-THC in blood and brain following administration of low (0.5 mg/kg) or moderate (5 mg/kg) doses of the drug. We also tested the behavioral effects of the same doses using conventional assays of anxiety-like behavior, locomotion, and conditioned place preference/aversion. Finally, we determined how THC dose-dependently and sex-dependently affects c-Fos expression in reward-related and stress-related regions of the brain, and analyzed THC effects on functional connectivity across these neural network nodes.
Materials and methods
All procedures were approved by the Institutional Animal Care and Use Committee at the University of California, Irvine, and carried out in strict accordance with the National Institutes of Health guidelines for the care and use of animals.
Subjects
Adolescent male and female Long-Evans rats (Charles River) were weaned at PD 21 and arrived in our vivarium at PD 21–23 (behavioral groups) or PD 27 (PK groups), and were allowed to acclimate for at least 5 days before commencing experiments. They were housed in groups of 4 in individually ventilated, 32 × 32 × 22 cm cages with corncob bedding and shredded paper enrichment. Males and females were housed in the same colony room, though air exchange was minimal due to cage ventilation. Housing rooms were maintained on a 12 h light/12 h dark cycle (lights off at 6:30 p.m. for pharmacokinetic experiments; 8:00 a.m. for behavioral experiments) under controlled conditions of temperature (20 ± 2 °C) and relative humidity (55–60%). Food and water were available ad libitum.
General protocol
Pharmacokinetic analyses
Rats received a single acute THC dose (0.5 or 5 mg/kg; i.p.; n = 4 per sex at each timepoint) on PD 33. This day was chosen to minimize any possible age-related variability for this acute PK experiment. Blood and brain samples were collected for THC and metabolite quantification. Animals were anesthetized with isoflurane at various time points after THC administration (15, 30, 60, 120, 240, and 480 min) and blood (3 mL) was collected by cardiac puncture and transferred into 4 mL polypropylene tubes containing spray-coated potassium-EDTA. Plasma was prepared by centrifugation at 1450 × g at 4 °C for 15 min. Brains were quickly removed and bisected mid-sagittally on an ice-cold glass plate. Samples were frozen on dry ice and stored at −80 °C until analyses. Liquid Chromatography/Mass Spectrometry (LC-MS/MS) analyses were carried out as described in ref. [41] (see Supplemental Materials).
Behavior and c-Fos expression
The acute behavioral effects of THC were also examined in a separate group of adolescent rats (male n = 52; female n = 53). Acute THC effects were measured starting between PD 27–31 and finishing by PD 45. Rats were handled for 5 min on the 2 days prior to the first behavioral test. Forty-eight hours after the last behavioral test, a subset of animals received acute THC (0, 0.5, or 5 mg/kg) for c-Fos analysis. Testing order was as follows, with ≥48 h elapsing between tests, and THC administered 30 min prior to: (1) elevated plus maze—1 day (n = 96), (2) novel environment activity—1 day (n = 104), or 10 min prior to: (3) conditioned place preference—1 day baseline, 8 days training, 1 day test (n = 47), (4) THC-induced c-Fos (n = 57).
Drugs
THC was provided by the NIDA Drug Supply Program or Cayman Chemicals (Ann Arbor, MI). Doses were chosen based upon those which typically produce rewarding and anxiolytic effects (<1 mg/kg), and those which typically produce anxiogenic and aversive effects (>5 mg/kg) in adult male rodents [42]. The drug was prepared daily by evaporating vehicle under N2 and dissolving to dose in 5% Tween 80 in saline (1 ml/kg) prior to intraperitoneal injections [29].
Behavioral testing
Elevated plus maze (EPM)
Rats were placed in the EPM apparatus for 5 min, starting 30 min after THC injection, and percent time spent on the open arms, and open arm entries were quantified.
Activity in novel open field
Time in center/periphery during initial exploration
Forty-eight hours after the EPM test, rats underwent a 1 h test in a novel testing chamber without bedding, food, or water, starting 30 min after THC injection. For analysis of anxiety-like avoidance of the open center of a novel chamber, we examined activity during the first 10 min of the session [43], and calculated percentage of time spent in the center.
Locomotor activity and rearing behavior
Distance traveled and rearing data from the 1 h open field session were analyzed in 15 min bins, starting 30 min and ending 90 min after THC administration.
Conditioned place preference
A three-chamber place preference box (Med Associates, San Diego, CA) with infrared beam detection was used to examine rewarding or aversive effects of THC. Rats were allowed to explore all 3 chambers for 15 min on 3 consecutive days, and average time spent in each zone served as a baseline measure of preference in order to increase stability of baseline preference measurement, though we note that such preconditioning can also decrease effect size of place conditioning for other reinforcing drugs [44]. Sides were assigned for pairing vehicle/THC pairing randomly, and no significant side bias was observed (F1,43 = 3.9, p = 0.055), nor were there interactions of side with sex (F1,43 = 2.7, p = 0.108), or subsequent THC dose (F1,43 = 1.3, p = 0.486). Vehicle and THC pairings (administered 10 min prior to CPP training sessions [45]) alternated over the next 8 days, for a total of 4 vehicle and 4 THC pairings. Forty-eight hours after the last pairing, rats were again allowed to explore all 3 chambers for 15 min. Data were analyzed by subtracting time spent in each of the 3 chambers at baseline, from time spent in the chambers on the post-pairing test. THC preference/aversion score was computed by subtracting baseline-normalized vehicle side time from baseline-normalized THC side time.
c-Fos induction and immunohistochemistry
Sample collection
Forty-eight hours after the final behavioral test, a subset of rats was given vehicle or THC (vehicle: male n = 7, female n = 12; 0.5 mg/kg: male n = 8, female n = 11; 5 mg/kg: male n = 9, female n = 10) and was then returned to the home cages for 2 h, since translation of c-Fos mRNA to protein is maximal between 60–120 min [46]. The animals were then transcardially perfused with sterile saline (0.9%) and paraformaldehyde (4%), and processed for DAB-based staining of Fos, or Fos + orexinA.
c-Fos quantification
Samples were imaged at ×5 magnification, and quantified using StereoInvestigator software (Microbrightfield) by a trained observer blind to experimental groups. Fos data were averaged for all 4 samples from each rat (2 slices, 2 hemispheres) for analyses. Fos density (Fos/mm2) was computed for the following brain regions [47]: medial prefrontal cortex (prelimbic; PLC and infralimbic; ILC), nucleus accumbens (core: NAcC, shell: NAcSh), ventral pallidum (rostral half: RVP, caudal half: CVP [48, 49]), extended amygdala (basolateral: BLA, central nucleus: CeA, bed nucleus of the stria terminalis: BNST), epithalamus (habenula lateral: LHb and medial: MHb portions), and tail of the ventral tegmental area (tVTA) [50, 51]. Dorsal hippocampus samples were also quantified, though total Fos/hemisphere was computed rather than Fos density, since layer-specific Fos expression in dentate gyrus (DG), and cornus Ammoni (CA) regions 1–3 is not well captured with density analysis. Finally, the number of hypothalamic orexin neurons, and percentage of these expressing Fos was also quantified, as orexin mediates some THC effects in adult rodents [52, 53].
c-Fos network analyses
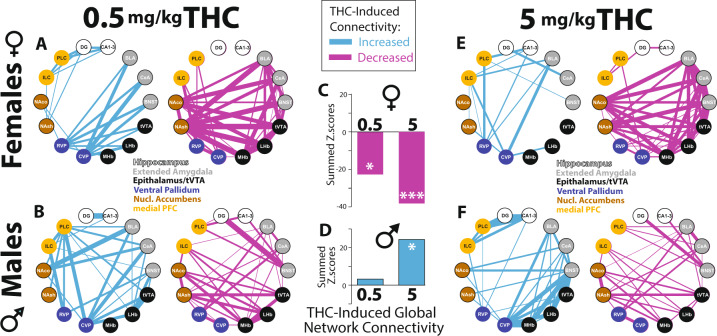
We compared Fos expression in 14 brain regions. Network analyses were conducted by first scaling Fos data to normalize within each sex/dose group, then computing correlations between activity in each structure within each group. Fos expression across all samples was scaled, and Spearman correlation values between brain-regions were calculated for each experimental group. Both positive correlations and negative correlations between activity in pairs of nodes were quantified. To determine effects of THC (0.5, 5 mg/kg) on functional connectivity, correlation p values were converted to Z-scores using Fisher’s transformation, which enabled us to compare Z-scores across experimental groups, and use them to display vehicle-relative THC effects for each sex. We represent the THC-induced changes in correlated activity (Z-scores), where line weight represents the relative strength of THC-induced global network connectivity, or global decoupling. We also statistically examined effects of THC, relative to vehicle, on global network connectivity within the 14 measured regions by summing positive and negative Z-scores for all connections.
Statistical analyses
PK analyses were performed as described [54]. Effects of THC dose and sex on behavioral assays and regional Fos were analyzed using one-way or two-way ANOVAs with Tukey HSD post hoc tests, or Bonferroni-corrected t-test, as appropriate. Greenhouse–Geisser degrees of freedom correction was used when homogeneity of variance assumption was violated (i.e., significant Mauchly’s sphericity test). Repeated measures or mixed model ANOVAs were used for time series data.
Results
Pharmacokinetic profile of THC
Blood plasma
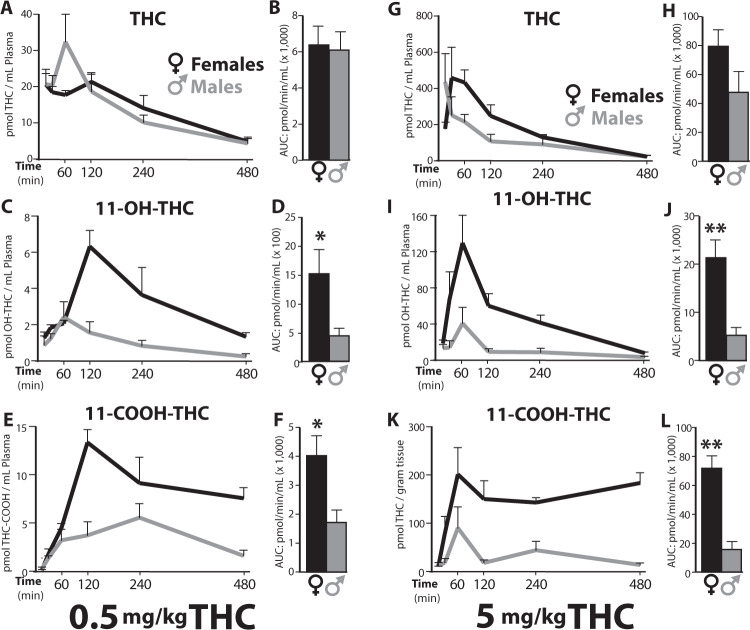
Figure 1 shows the plasma PK profile for THC and its two main first-pass metabolites, 11-OH-THC and 11-COOH-THC, after IP administration of 0.5 or 5 mg/kg THC in adolescent male and female rats. Table S1 reports peak concentration (Cmax) in plasma, time at which Cmax was attained (Tmax), half-life time (t1/2) of elimination for THC, and total exposure (area under curve, AUC) for each compound. The plasma PK profile of THC was similar in the two sexes. At both 0.5 and 5 mg/kg, the Cmax and AUC values were comparable (Table S1) though a non-significant trend toward higher levels was observed in females treated with the 5 mg/kg dose (AUC: 79,718 ± 11,214 vs. 47,445 ± 14,649 pmol/min/mL; mean ± S.E.M.; p = 0.13). By contrast, plasma concentrations of THC metabolites were strikingly higher in female than male rats. For example, at the 5 mg/kg dose, the Cmax for bioactive 11-OH-THC was 3.2 times higher (130 ± 30 vs. 41 ± 17 pmol/mL; p = 0.04) and the AUC 4 times higher (21,311 ± 3593 vs. 5127 ± 1705 pmol/min/mL; p = 0.007) in females than males (Fig. 1c, f; Table S1). At the same dose, 11-COOH-THC concentrations were also substantially greater in female animals (p = 0.001) (Fig. 1e–l).
Fig. 1. Plasma concentrations of THC and its first-pass metabolites.
Concentration profile of THC, 11-OH-THC, and 11-COOH-THC in plasma after IP injection of THC, 0.5 mg/kg (left panel: a–e) or 5 mg/kg (right panel: g–l), in female (black) or male (gray) adolescent rats. Lines represent the mean ± SEM, n = 4/sex and dose group at each timepoint (15, 30, 60, 120, 240, 480 min post THC). Adjacent bar graphs represent total exposure (area under the curve, AUC, pmol/min/mL) to THC, 11-OH-THC and 11-COOH-THC, respectively, in females or males after 0.5 mg/kg (b, d, f) or 5 mg/kg THC (h, j, l). Bars represent the mean ± SEM, *p < 0.05, **p < 0.01.
Brain
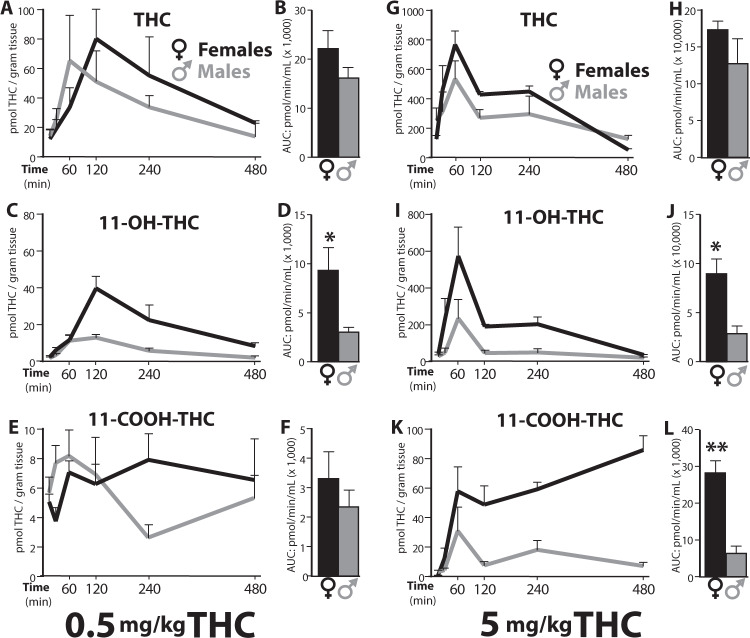
The brain PK profile for THC and its metabolites are illustrated in Fig. 2. Key PK parameters are reported in Table S2, and values for brain-to-plasma ratio in Table S3. The overall exposure to THC was comparable between sexes, though non-significant trends toward higher Cmax and AUC were noted in females. For example, the AUC for 0.5 mg/kg THC was 22,012 ± 3772 pmol/min/g in females vs. 15,999 ± 2285 pmol/min/g in males (p = 0.2). By contrast, as seen in plasma, brain exposure to the bioactive THC metabolite 11-OH-THC was substantially greater in female than male rats, with AUC values > 3 times higher at both THC doses: 9324 ± 2326 vs. 3065 ± 460 pmol/min/g at 0.5 mg/kg, and 89,634 ± 14,950 vs. 27,764 ± 8,731 pmol/min/g at 5 mg/kg (p = 0.01). A similar difference in AUC was seen for 11-COOH-THC at 5 mg/kg THC (28,232 ± 3289 vs. 6309 ± 2006 pmol/min/g, p = 0.001), whereas the lower THC dose produced similar 11-COOH-THC concentration in both sexes (p = 0.4). Lastly, the brain-to-plasma ratios for THC and its metabolites were similar in females and males (Table S3). Collectively, the findings indicate that first-pass THC metabolism, and most notably the conversion of THC into bioactive 11-OH-THC, is substantially greater in female than male adolescent rats.
Fig. 2. Brain concentrations of THC and its first-pass metabolites.
Concentration profile of THC, 11-OH-THC, and 11-COOH-THC in brain tissue after IP injection of THC, 0.5 mg/kg (left panel, a–e) or 5 mg/kg (right panel, g–l), in female (black) or male (gray) adolescent rats, n = 4/group. Adjacent bar graphs represent total exposure (area under the curve, AUC, pmol/min/mL) to THC, 11-OH-THC and 11-COOH-THC, respectively, for females or males after 0.5 mg/kg (b, d, f) or 5 mg/kg THC (h, j, l). *p < 0.05, **p < 0.01.
Behavioral effects of THC
EPM behavior
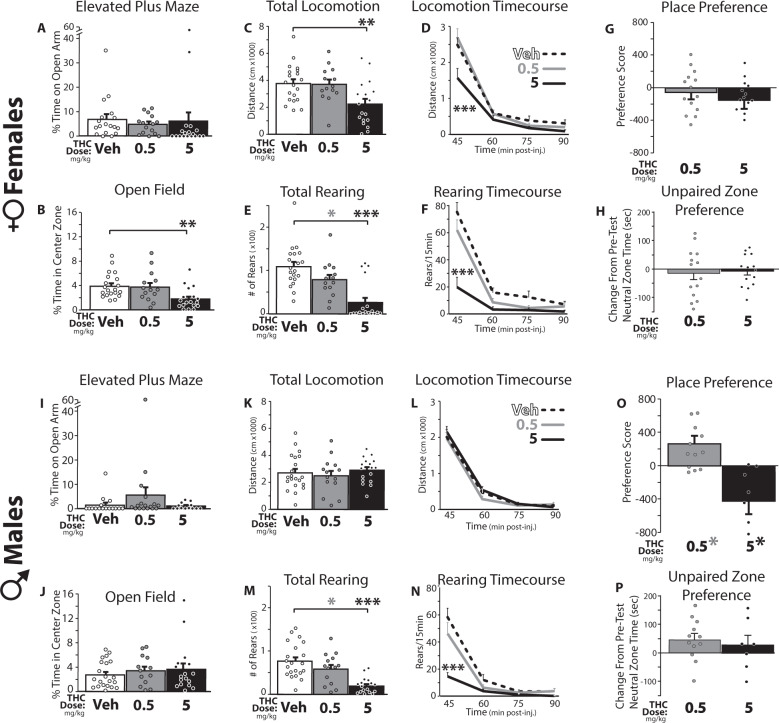
Adolescent female rats spent more time than males in the open arms of the apparatus (main effect of sex on percentage of the session spent in the open arms: F1,90 = 9.215, p = 0.003), but neither sex showed significant THC effects on open-arm time (no main effect of dose: F2,90 = 0.263, p = 0.769; or sex × dose interaction: F2,90 = 0.299, p = 0.742; Fig. 3a, i). However, since vehicle-day open arm exploration was very low in these adolescent rats, it is possible that a floor effect limited the ability to detect THC-induced anxiety-like behavior in this assay. At 5 mg/kg, THC had sedative-like effects in females, in that the number of open arm entries was suppressed (sex × dose interaction: F2,90 = 3.692, p = 0.029; main effect of dose in females: F2,48 = 3.954, p = 0.026, vehicle vs. 5 mg/kg THC: p = 0.024; no dose effect in males: F2,42 = 1.009, p = 0.373; Fig. S1A, B).
Fig. 3. THC effects on adolescent male and female anxiety, activity, and reward/aversion-related behaviors.
Effects of vehicle (Veh; White bars/dashed lines) or THC (0.5 mg/kg: gray bars and lines; 5 mg/kg: black bars and lines) on behaviors are shown. In adolescent female rats (a–h), (a) THC did not affect the percentage of time spent on the open arms of the elevated plus maze (conducted 30–35 min post-THC), but b) in the open field test (30–40 min post-THC), 5 mg/kg THC decreased time spent in the center of the novel environment, which is considered to be an anxiety-like phenotype. c Locomotor activity was also suppressed over the 60 min session by 5 mg/kg THC, and (d) this effect was especially prominent from 30–45 min after i.p. injection (the first 15 min of the session). e Rearing onto the hind legs was also strongly suppressed by 5 mg/kg THC, (f) again most prominently in the first 15 min of the test. g When injected 10 min prior to 30 min training sessions, neither THC dose induced either a conditioned place preference or aversion in females, (h) nor did THC affect time spent in the unpaired neutral zone in the 3-chamber apparatus. In adolescent male rats (i–p), neither THC dose affected (i) percent time on the open arms of the plus maze, (j) percent time in the center of the open field, or (k) locomotor activity in the whole session, or (l) at any timepoint. As in females, (m) rearing was suppressed by 5 mg/kg THC, (n) especially in the first 15 min of the test. o 0.5 mg/kg THC induced a place preference, while 5 mg/kg THC induced a place aversion. p Neither dose impacted time in the central neutral zone. *p < 0.05, **p < 0.01, ***p < 0.001.
Initial exploration of an open field
As expected, rats of both sexes generally avoided the center of the testing chamber (no effect of sex on percent time in center: F1,98 = 0.006, p = 0.938). THC (5 mg/kg) further decreased center zone time in females but not males (sex × dose interaction: F2,98 = 3.6, p = 0.031; main effect of dose in females: F2,49 = 5.89, p = 0.005; veh vs. 5 mg/kg: p = 0.007; but not males: F2,48 = 0.47, p = 0.63; Fig. 3b, j), indicating a female-specific anxiogenic effect.
General locomotor activity
To further evaluate the motor effects of THC, we examined locomotion in a novel chamber for 60 min, corresponding to 30–90 min after THC injection (including the 10 min initial center/surround period described above). THC suppressed locomotion in a dose-dependent and sex-dependent manner (sex × dose interaction: F2,98 = 5.06, p = 0.008; Fig. 3c, k), and concurrently increased time spent immobile (sex × dose interaction: F2,98 = 5.5, p = 0.005; Fig. S1B, C). In females, THC (5 mg/kg) suppressed session-long locomotion (dose effect: F2,49 = 6.02, p = 0.005), but this effect was particularly strong in the first 15 min spent in the novel chamber (30–45 min post-THC; dose × time interaction: F6,147 = 3.84, p = 0.001; Fig. 3d). Reciprocal effects were seen on immobility time in females (dose main effect: F2,49 = 11.83, p < 0.001; dose × time interaction: F6,147 = 2.9, p = 0.011; Fig. S1C, E). In striking contrast, neither locomotion nor immobility were affected by THC in males at either dose (locomotion: F2,49 = 0.446, p = 0.64, Fig. 3k, l; immobility: F2,49 = 0.41, p = 0.666; Fig. S1D, F). Unlike locomotor activity, rearing behavior was strongly suppressed by both THC doses in both sexes (Dose effect: F2,98 = 29.43, p < 0.001; vehicle vs. 0.5 mg/kg: p = 0.043; vehicle vs. 5 mg/kg: p < 0.001; 0.5 vs. 5 mg/kg: p < 0.001; no sex × dose interaction: F2,98 = 0.934, p = 0.40; Fig. 3e, m). This suppression of vertical exploration was most prominent at the start of the session (dose × time interaction: F2,98 = 24.63, p < 0.001; Fig. 3f, n), when rearing was most prevalent in vehicle-treated rats.
Conditioned place preference
THC elicited dose-dependent and sex-dependent conditioned place preference and aversion in adolescent rats (sex × dose interaction for preference/aversion score: F1,43 = 7.40, p = 0.001; Fig. 3g, o). In females, THC caused neither preference nor aversion at either dose (F1,26 = 0.46, p = 0.51), nor did it alter time spent in the center neutral zone (F1,26 = 0.10, p = 0.75, Fig. 3g, h). However, in male rats THC had markedly dose-dependent effects (F1,17 = 16.68, p = 0.001). A conditioned place preference for 0.5 mg/kg THC was formed (t11 = 2.87, p = 0.015), whereas place aversion was seen at the 5 mg/kg dose (t6 = 2.73, p = 0.034; Fig. 3o). No effect of THC on neutral zone time was observed in males (F1,17 = 0.27, p = 0.61; Fig. 3p). Raw time spent in the vehicle-, THC-, and unpaired-chambers on test day is shown in Fig. S2.
Brain activity elicited by THC
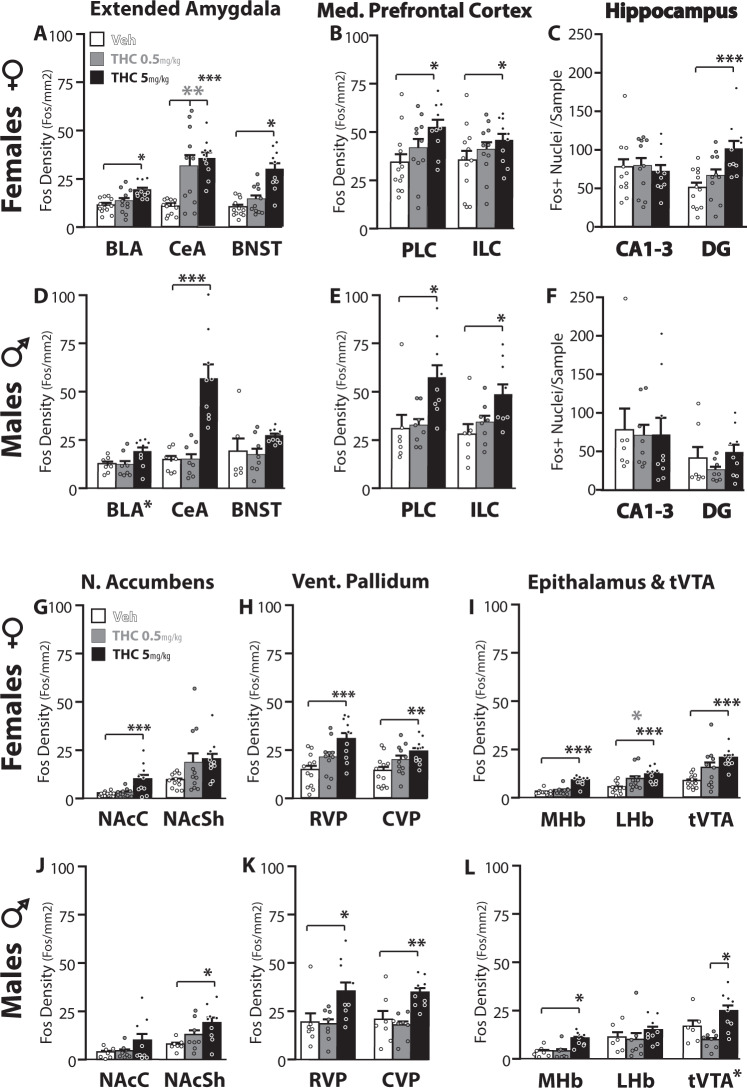
THC had pronounced effects on brain activity in most of the 14 reward-related and aversion-related brain regions surveyed in this study. THC effects were sex-specific, dose-specific, and region-specific (Fos density; 3-way interaction: F7.35,183.77 = 2.19, p = 0.035). Regardless of dose, females had more Fos in dentate gyrus than males (main effect of sex: F1,46 = 16.19, p < 0.001), but no main effects of sex were seen in other regions. Main effects of THC dose were seen in all measured regions (Fs > 4.205, ps < 0.022), with the exception of LHb and CA1-3. Statistically significant interactions between sex and THC dose were seen in CeA (F2,46 = 6.96, p = 0.003), CVP (F2,46 = 3.98, p = 0.027), and tVTA (F2,46 = 4.0, p = 0.026). Per-region Fos expression is shown in Fig. 4, presented in groupings of regions based on anatomical and functional similarities [55].
Fig. 4. THC effects on c-Fos expression.
In females (a–c; g–i), THC dose-dependently induced Fos immunoreactivity in several structures, as indicated by *symbol under bars that significantly differ from vehicle in that structure. Vehicle-treated rats = white bars, 0.5 mg/kg THC rats = gray bars, 5 mg/kg THC rats = black. Individual rat data is represented with dots. In males (d–f; j–l), data are represented using the same scheme. *p < 0.05, **p < 0.01, ***p < 0.001 as indicated, or adjacent to structure name in Dose main effect without significant post-hoc. BLA basolateral amygdala, CeA central amygdala, BNST bed nucleus of the stria terminalis, PLC prelimbic medial prefrontal cortex, ILC infralimbic mPFC, CA1-3 cornus ammoni regions 1-3, DG dentate gyrus, NAcC/NAcSh nucleus accumbens core/shell, RVP/CVP rostral/caudal ventral pallidum, MHb/LHb medial/lateral habenula, tVTA tail of the ventral tegmental area.
THC-Induced c-Fos expression in females
In females (Fig. 4a–c, g–h), 5 mg/kg THC induced Fos relative to vehicle in the following structures: PLC (F2,30 = 3.86, p = 0.032; 5 mg/kg: p = 0.025), NAcC (F2,30 = 8.89, p = 0.001; 5 mg/kg: p = 0.002), RVP (F2,30 = 7.67, p = 0.002; 5 mg/kg: p = 0.001), CVP F2,30 = 5.01, p = 0.013; 5 mg/kg: p = 0.01), BLA (F2,30 = 7.75, p = 0.002; 5 mg/kg: p = 0.002), CeA (F2,30 = 12.21, p < 0.001; 5 mg/kg: p < 0.001), BNST (F2,30 = 19.407, p < 0.001; 5 mg/kg: p < 0.001), DG (F2,30 = 7.82, p = 0.002; 5 mg/kg: p = 0.001), LHb (F2,30 = 7.23, p = 0.003; 5 mg/kg: p = 0.002), MHb (F2,30 = 30.4, p < 0.001; 5 mg/kg: p < 0.001), and tVTA (F2,30 = 8.76, p = 0.001; 5 mg/kg: p = 0.001). Moreover, in females the lower 0.5 mg/kg THC dose also elicited Fos in CeA (p = 0.002) and LHb (p = 0.047), with a similar trend in tVTA (p = 0.056). Neither the number of orexin neurons (F2,22 = 0.42, p = 0.66), nor the percentage of these neurons expressing Fos (F2,22 = 1.6, p = 0.23) was significantly altered by THC in females, though females did have a greater number of orexin neurons than males (main effect of Sex: F1,41 = 6.96, p = 0.012; Fig. S3).
THC-Induced c-Fos expression in males
5 mg/kg THC induced Fos in some of the same regions as in females (Fig. 4d–f, j–l), including BLA (F2,21 = 3.47, p = 0.05; though posthoc tests for neither dose reached significance), CeA (F2,21 = 20.77, p < 0.001; 5 mg/kg: p < 0.001), PLC (F2,21 = 5.73, p = 0.01; 5 mg/kg: p = 0.017), RVP (F2,21 = 4.97, p = 0.017; 5 mg/kg: p = 0.047), CVP (F2,21 = 8.36, p = 0.002; 5 mg/kg p = 0.003), MHb (F2,21 = 10.91, p = 0.001; 5 mg/kg: p = 0.002), and tVTA (F2,20 = 7.91, p = 0.003; neither dose differed from vehicle in posthoc analysis, though 0.5 and 5 mg/kg differed from each other: p = 0.002). Unlike in females, THC in males failed to alter Fos in NAcC, BNST, DG, or LHb, but instead 5 mg/kg THC induced Fos in NAcSh (F2,21 = 4.38, p = 0.026; 5 mg/kg: p = 0.021) and ILC (F2,21 = 4.7, p = 0.021; 5 mg/kg: p = 0.025). 0.5 mg/kg THC in males failed to significantly impact Fos in any of the regions included in the survey. Neither the number of orexin neurons (F2,18 = 0.76, p = 0.48), nor the percentage of these neurons expressing Fos (F2,18 = 0.042, p = 0.96) was significantly altered by THC (Fig. S3).
THC-Induced changes in network connectivity
Male and female rats displayed distinct patterns of correlated activity after injection of vehicle and THC. The activities of pairs of network nodes were either positively (Fig. S4) or negatively correlated (Fig. S5), and these patterns appeared to differ in a sex-dependent and dose-dependent manner. To examine this statistically, we calculated Z-scores to compare correlation strengths in THC-injected and vehicle-injected rats of the same sex. THC altered patterns of co-activation between individual network nodes, either increasing or decreasing co-activation (Fig. 5a, b, e, f; blue lines show regions with increased correlation strength due to THC on the left, magenta lines show decreases in correlated activity on the right). Furthermore, global connectivity patterns across regions were altered in a sex-dependent manner, with predominant decoupling of the overall network in females (0.5 mg/kg: p = 0.01; 5 mg/kg: p = 1.14e–05, Fig. 5c), and increased functional connectivity in males after 5 mg/kg THC (p = 0.01; 0.5 mg/kg: p = 0.69, Fig. 5d), including increased coupling of CA1-3 with PLC (Z = 2.02, p = 0.044), and non-significant trends toward enhanced co-activation of BNST with LHb (Z = 1.83, p = 0.067) and MHb (Z = 1.68, p = 0.09). These results indicate that THC has markedly sex-dependent effects on overall functional connectivity within reward-processing and stress-processing networks of the adolescent rat brain, with predominant decoupling in females and increased functional connectivity in males.
Fig. 5. THC effects on functional connectivity within reward and stress networks.
Effects of 0.5 mg/kg THC on functional coupling (left; blue lines representing strength of Fos co-activation, relative to vehicle-injected rats), or functional de-coupling (right; magenta lines representing strength of Fos de-coupling, relative to vehicle rats) is shown in females (a), and males (b). Effects of 5 mg/kg THC, relative to vehicle, are represented using the same logic in females (e), and males (f). Effects of THC doses on global network connectivity (THC-induced change in overall regional Fos cross-correlation) are shown in females (c) and males (d). *p = 0.01, ***p < 0.0001.
Discussion
We report a systematic analysis of the PK properties of THC in female and male adolescent rats, and characterize the effects of low and moderate doses of the drug on behavior and neural network activity. We found that such effects are markedly modulated by sex. There were major sex differences in THC metabolism, especially with regard to the bioactive product 11-OH-THC in blood and brain. In addition, we observed signs of female-specific anxiogenesis and locomotor suppression with 5 mg/kg THC, as well as dose-dependent induction of conditioned place preference (at low dose) or avoidance (at moderate dose) in males. We also show pronounced effects of THC on c-Fos expression within stress and reward circuit nodes, leading to sex-dependent impacts on functional connectivity within wider neural networks. These results lay groundwork for comparison of THC effects in adolescent rodents vs. humans, and provide a framework for future studies determining how sex modulates THC effects across developmental stages.
Sex differences in THC metabolism
THC metabolism is markedly different in male and female adolescent rats. Although levels of the parent drug did not significantly differ in blood or brain at any measured timepoint, we found greatly increased levels of 11-OH-THC in both compartments of females, relative to males. This metabolite has significant activity at cannabinoid receptors [56–58], and produces cannabinoid-typical behavioral effects in adult rats [59–61]. Higher levels of 11-OH-THC in adolescent females emerged 60–120 min after THC injection, and persisted for at least 4–8 h, consistent with prior reports of preferential 11-OH-THC metabolism in adult and adolescent females after higher doses of THC [24, 38, 62, 63]. Although we did not establish a causal link between the observed sex-dependent difference in 11-OH-THC production and other sexually dimorphic effects of THC, this seems plausible—especially since direct administration of THC and 11-OH-THC generally produce stronger behavioral effects in females than in males, some of which are attenuated when cytochrome P450-dependent THC metabolism is inhibited pharmacologically [60, 61]. We also saw higher levels of the inactive metabolite 11-COOH-THC [64, 65] in female than male adolescents, further verifying strongly sex-dependent THC metabolism. We note that this female-specific accumulation of a bioactive THC metabolite may have significant implications for designing adolescent THC exposure studies in rodents, if equipotent and equieffective THC doses are desired in both sexes. Importantly, similar female-specific 11-OH-THC increases were also seen in adult humans after oral THC [66], suggesting that this metabolic difference may be important more generally for sex differences in THC effects, and cannabis usage [32, 35, 37, 67].
Sex differences in THC behavioral effects
We also found distinct behavioral effects of THC in adolescents of each sex. The lower THC dose (0.5 mg/kg) had minimal impact on anxiety and locomotor assays, though it did suppress vertical exploration of a novel chamber in both sexes. In the conditioned place preference task, 0.5 mg/kg THC induced place preference in males but not females, indicating that this dose has sex-dependent rewarding effects. This is consistent with prior findings that low (<1 mg/kg) THC doses are more likely than higher doses (≥5 mg/kg) to elicit place preference in adult male rodents [42]. In females, 0.5 mg/kg THC caused neither place preference nor avoidance.
At the 5 mg/kg dose, THC had more pronounced and robustly sexually dimorphic effects on adolescent rat behavior. In females, THC increased avoidance of the open center of a novel environment, consistent with anxiety-like behavior. No such anxiogenic profile was apparent in the elevated plus maze assay in either sex however, though THC did suppress open arm entries in females at the higher dose. At 5 mg/kg THC also suppressed locomotion in a novel open field, most prominently at the beginning of the session (30–45 min after THC). Rats explore the novel chamber for the first time in this period, and 5 mg/kg THC markedly suppressed exploring in females, replacing it with time spent immobile—a behavioral profile that could indicate fear-like huddling or freezing (or potentially, general sedation). Interestingly, rearing behavior was suppressed by both THC doses in both sexes; especially in the initial 15 min in the novel chamber (30–45 min post-THC). Suppressed rearing under these conditions could represent reduced escape motivation, or general sedation/intoxication, and it is notable that this was a highly sensitive assay of low-dose, as well as moderate-dose THC effects. We also found that 5 mg/kg THC induced robust conditioned place aversion in male adolescents, as is typical in adults [42]. Despite its anxiogenic/sedative effects in adolescent females, 5 mg/kg THC did not have consistent effects on conditioned place preference in rats of this sex. Together, these results demonstrate that THC has distinct behavioral effects in adolescent male and female rats, with evidence of THC-induced anxiogenesis in females, and dose-dependent U-shaped rewarding or aversive effects in males. These findings add to a growing literature showing across species that THC effects are sex-dependent, with generally stronger effects in females regardless of age [24, 30, 34, 37, 38, 60, 61, 68, 69]. In humans, these sex-dependent THC effects may be compensated for behaviorally—whether cannabinoid drug self-administration is similarly titrated in rodents based on sex and age should be determined.
Sex differences in region-specific c-Fos induced by THC
THC had overlapping, but distinct effects on region-specific Fos expression in adolescent male and females. Relative to vehicle, 5 mg/kg THC robustly and sex-dependently induced Fos in both stress-related and reward-related brain regions. Some regions were activated similarly in both sexes, including the stress-linked and aversion-linked CeA, MHb, and tVTA. Both dorsal (PLC) and ventral (ILC) medial prefrontal cortex were likewise recruited in both sexes, which may be relevant to the pronounced impact of adolescent THC exposure on mPFC development, especially the maturation of dopamine and GABA interneuron circuits linked to schizophrenia [70–72]. We also found that the entire VP, a structure that mediates both appetitive and aversive motivation [48, 73–77], was activated in both sexes by 5 mg/kg THC. In addition, neither dose of THC affected Fos in lateral hypothalamic orexin neurons, despite prior reports of orexin involvement in THC effects [52, 53]—it is unclear whether this lack of THC effect is specific to adolescent rats.
Several brain regions were recruited by 5 mg/kg THC in females only. These include the stress-linked and aversive learning-linked BLA, BNST, and LHb [78–80], an observation that might relate to the observed female-specific anxiogenesis seen at this dose. CeA and LHb were also more sensitive to THC in females, in that the lower 0.5 mg/kg dose also elicited a robust Fos response (though we note that no anxiety-like behavior was observed with this dose). In males, no surveyed brain region was significantly recruited by 0.5 mg/kg THC, but since this dose reduced rearing and induced place preference, it is likely that other brain regions might be involved, or that neural network-level effects might be more important than node-specific changes. We also found female-specific activation of the hippocampal DG, which could help explain why adolescent THC may have long-lasting impacts on learning and cognition in rats [7, 11, 12] and in humans who start using cannabis at an early age [4, 81]. Finally, 5 mg/kg THC also impacted Fos in the NAc in a sex-dependent manner, with NAcC activation in females, and NAcSh activation in males. The NAc has been linked to both reward and stress/aversion [82–89], so the neural subpopulations there most affected by THC are worth further examination.
Sex differences in network connectivity
THC had pronounced, sex-dependent effects on Fos expression in numerous brain regions within stress-processing and reward-processing circuits, so we asked whether THC might alter the propensity of these network nodes to co-activate (i.e., display functional connectivity), or instead to become less correlated in their activity (i.e., decouple), as determined with Fos [43, 90–92]. Therefore, we examined correlation strength of scaled Fos data amongst 14 reward-related and stress-related brain regions, and examined how THC impacted this parameter relative to vehicle in each sex. In females, THC caused marked decoupling of the measured network overall, which was especially prominent at the 5 mg/kg THC dose. Notably, these effects are consistent with fMRI findings in humans [93–96], where acute and chronic THC use is associated with decreased structural and functional connectivity within an overlapping set of limbic forebrain regions, though impacts of sex or age on network integrity in humans are unknown. In contrast, 5 mg/kg THC had a clearly distinct effect on network co-activation in males, increasing overall global connectivity—the opposite of females. This was driven, in part, by increased co-activation of PLC and the hippocampal CA1–3, as well as by trends toward increased interconnectivity of stress nodes in epithalamus and extended amygdala. It is important to note that correlated/de-correlated Fos expression does not imply direct neural connections between the structures in question—indeed several correlated regions here do not directly innervate one another. Instead, these results should be construed as hypothesis-generating in nature, motivating future functional connectivity analyses using assays with better time resolution, e.g., electrophysiology or functional imaging.
Relevance to humans
This study employs a rat model of adolescent THC exposure, but there are several factors which may lend insight into human cannabis effects. Although the PK profile of inhaled (smoked, vaped) THC is more rapid than with injection dosing, we show here that 5 mg/kg THC approximates the total drug exposure (AUC) seen in human blood after voluntary cannabis inhalation [97, 98]. 5 mg/kg THC also caused anxiety-like or stress-like behaviors in females and conditioned place aversion in males, suggesting that this dose might be useful for interrogating the sex-dependent anxiogenic/aversive effects of THC. Intriguingly, a 10-fold lower dose caused place preference in males (though not females), suggesting that low-dose THC can have rewarding effects in adolescent males, as it does in adults [42]. In females, THC did not induce either rewarding or aversive effects in the place preference assay, and it is not presently clear whether THC induces place preference in adolescent females at any dose (though interestingly, adult female Long Evans rats self-administer a synthetic cannabinoid agonist to a greater extent than males [99]). It is also notable that in a prior study [12] 1 mg/kg THC caused place aversion in adult males, while failing to cause either preference or aversion in adolescent males. Here, adolescent males showed highly dose-dependent THC effects, with reward at a low 0.5 mg/kg dose, and aversion at a moderate 5 mg/kg dose. In contrast, these doses caused neither preference nor aversion in adolescent females. We are not aware of prior THC place preference studies in females of any age with which to compare these results. Clearly, more work on sex-modulation and age-modulation of THC effects is sorely needed.
We conclude that no particular dosage of THC is a perfect model of the drug’s effects on the adolescent brain, and that each dose has advantages and disadvantages. Though these findings identify several metabolic and behavioral considerations necessary for interpreting outcomes of adolescent THC exposure in rats, further improvement of THC dosing and protocols is still required. Moving forward, multi-dose designs, inhalation or ingestion dosing methods, exposure to THC along with other phytocannabinoids found in cannabis, and even adolescent THC self-administration will provide additional valuable insight into the most human-relevant THC effects [100–102]. Other outstanding questions involve the effects of intra-adolescent age, puberty timing (females begin puberty PD ~ 33, males start around PD ~ 39) [39, 103, 104], tolerance from repeated injections [105], and gonadal hormones [106, 107] on THC effects—the impact of such factors on the present data remain to be determined. Potential impacts of circadian factors on THC kinetics should also be considered [108], and analogous work should be conducted in other species and strains as well to verify cross-species relevance.
Summary
In sum, the present findings drive home the point that sex is a major factor modulating THC effects in adolescent rats. Along with markedly sexually dimorphic THC metabolism, THC had stronger anxiogenic and sedative effects in females than males, and had dose-dependent rewarding or aversive effects in males only. We contend that our approach is a promising one for evaluating models of adolescent cannabis exposure, and caution against ignoring the impact of sex in such analyses.
Funding and disclosures
This study was funded by NIH grants: P50 DA0441118, R00 DA035251, the Hellman Fellowship Program, and the American Foundation for Aging Research. The authors declare no conflict of interest.
Supplementary information
Acknowledgements
We thank Brandon McNeil for assistance with tissue collection, and NIDA Drug Supply Program for providing THC.
Author contributions
CMR: conceived of and collected behavioral data and wrote manuscript. AT: conceived of and collected pharmacokinetic data and wrote manuscript. EC: collected and analyzed Fos expression data. CRP: collected and analyzed behavioral data. JC: collected and analyzed behavioral data. VI: collected and analyzed Fos staining data. EH: collected and analyzed behavioral data. DJ: analyzed behavioral data. MAH: conceived of, and advised development of project. VS: conducted network analyses of Fos data. DP: conceived of and led pharmacokinetic studies and wrote manuscript. SVM: conceived of and led behavior and Fos studies and wrote manuscript.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Christina M. Ruiz, Alexa Torrens
Contributor Information
Daniele Piomelli, Email: piomelli@uci.edu.
Stephen V. Mahler, Email: mahlers@uci.edu
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-020-00839-w).
References
- 1.Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharm. 2010;160:511–22. doi: 10.1111/j.1476-5381.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chadwick B, Miller ML, Hurd YL. Cannabis use during adolescent development: susceptibility to psychiatric illness. Front Psychiatry. 2013;4:129. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubino T, Zamberletti E, Parolaro D. Adolescent exposure to cannabis as a risk factor for psychiatric disorders. J Psychopharmacol. 2012;26:177–88. doi: 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- 4.Volkow ND, Swanson JM, Evins AE, DeLisi LE, Meier MH, Gonzalez R, et al. Effects of cannabis use on human behavior, including cognition, motivation, and psychosis: a review. JAMA Psychiatry. 2016;73:292–7. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- 5.Ehrenreich H, Rinn T, Kunert HJ, Moeller MR, Poser W, Schilling L, et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology. 1999;142:295–301. doi: 10.1007/s002130050892. [DOI] [PubMed] [Google Scholar]
- 6.Spear LP. Consequences of adolescent use of alcohol and other drugs: Studies using rodent models. Neurosci Biobehav Rev. 2016;70:228–43. doi: 10.1016/j.neubiorev.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes FV, Guimarães FS, Grace AA. Effects of pubertal cannabinoid administration on attentional set-shifting and dopaminergic hyper-responsivity in a developmental disruption model of schizophrenia. Int J Neuropsychopharmacol. 2014;13:18:pyu018. 10.1093/ijnp/pyu018. PMID: 25522381; PMCID: PMC4368886. [DOI] [PMC free article] [PubMed]
- 8.Abela AR, Rahbarnia A, Wood S, Le AD, Fletcher PJ. Adolescent exposure to Delta9-tetrahydrocannabinol delays acquisition of paired-associates learning in adulthood. Psychopharmacology. 2019;236:1875–86. doi: 10.1007/s00213-019-5171-1. [DOI] [PubMed] [Google Scholar]
- 9.Rubino T, Realini N, Braida D, Guidi S, Capurro V, Vigano D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–72. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- 10.O’Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol. 2004;18:502–8. doi: 10.1177/026988110401800407. [DOI] [PubMed] [Google Scholar]
- 11.Rubino T, Realini N, Braida D, Alberio T, Capurro V, Vigano D, et al. The depressive phenotype induced in adult female rats by adolescent exposure to THC is associated with cognitive impairment and altered neuroplasticity in the prefrontal cortex. Neurotox Res. 2009;15:291–302. doi: 10.1007/s12640-009-9031-3. [DOI] [PubMed] [Google Scholar]
- 12.Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N, et al. Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology. 2008;33:1113–26. doi: 10.1038/sj.npp.1301475. [DOI] [PubMed] [Google Scholar]
- 13.Rubino T, Vigano D, Realini N, Guidali C, Braida D, Capurro V, et al. Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology. 2008;33:2760–71. doi: 10.1038/sj.npp.1301664. [DOI] [PubMed] [Google Scholar]
- 14.Keeley RJ, Trow J, Bye C, McDonald RJ. Part II: Strain- and sex-specific effects of adolescent exposure to THC on adult brain and behaviour: Variants of learning, anxiety and volumetric estimates. Behav Brain Res. 2015;288:132–52. doi: 10.1016/j.bbr.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Pushkin Anna N., Eugene Angeline J., Lallai Valeria, Torres-Mendoza Alan, Fowler J. P., Chen Edison, Fowler Christie D., Homberg Judith. Cannabinoid and nicotine exposure during adolescence induces sex-specific effects on anxiety- and reward-related behaviors during adulthood. PLOS ONE. 2019;14:e0211346. doi: 10.1371/journal.pone.0211346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecca D, Scifo A, Pisanu A, Valentini V, Piras G, Sil A, et al. Adolescent cannabis exposure increases heroin reinforcement in rats genetically vulnerable to addiction. Neuropharmacology. 2020;166:107974. doi: 10.1016/j.neuropharm.2020.107974. [DOI] [PubMed] [Google Scholar]
- 17.Ellgren M, Spano SM, Hurd YL. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology. 2007;32:607–15. doi: 10.1038/sj.npp.1301127. [DOI] [PubMed] [Google Scholar]
- 18.Friedman AL, Meurice C, Jutkiewicz EM. Effects of adolescent Delta9-tetrahydrocannabinol exposure on the behavioral effects of cocaine in adult Sprague-Dawley rats. Exp Clin Psychopharmacol. 2019;27:326–37. doi: 10.1037/pha0000276. [DOI] [PubMed] [Google Scholar]
- 19.Panlilio LV, Solinas M, Matthews SA, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2007;32:646–57. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- 20.Stopponi S, Soverchia L, Ubaldi M, Cippitelli A, Serpelloni G, Ciccocioppo R. Chronic THC during adolescence increases the vulnerability to stress-induced relapse to heroin seeking in adult rats. Eur Neuropsychopharmacol. 2014;24:1037–45. doi: 10.1016/j.euroneuro.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 21.Panlilio LV, Zanettini C, Barnes C, Solinas M, Goldberg SR. Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology. 2013;38:1198–208. doi: 10.1038/npp.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burgdorf CE, Jing D, Yang R, Huang C, Hill MN, Mackie K, et al. Endocannabinoid genetic variation enhances vulnerability to THC reward in adolescent female mice. Sci Adv. 2020;6:eaay1502. doi: 10.1126/sciadv.aay1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci. 2011;5:64. doi: 10.3389/fnbeh.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craft RM, Britch SC, Buzitis NW, Clowers BH. Age-related differences in Delta(9)-tetrahydrocannabinol-induced antinociception in female and male rats. Exp Clin Psychopharmacol. 2019;27:338–47.. doi: 10.1037/pha0000257. [DOI] [PubMed] [Google Scholar]
- 25.Wagner EJ. Sex differences in cannabinoid-regulated biology: a focus on energy homeostasis. Front Neuroendocrinol. 2016;40:101–9. doi: 10.1016/j.yfrne.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen JD, Creehan KM, Kerr TM, Taffe MA. Lasting effects of repeated (9) -tetrahydrocannabinol vapour inhalation during adolescence in male and female rats. Br J Pharm. 2020;177:188–203. doi: 10.1111/bph.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva L, Harte-Hargrove L, Izenwasser S, Frank A, Wade D, Dow-Edwards D. Sex-specific alterations in hippocampal cannabinoid 1 receptor expression following adolescent delta-9-tetrahydrocannabinol treatment in the rat. Neurosci Lett. 2015;602:89–94. doi: 10.1016/j.neulet.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prini P, Rusconi F, Zamberletti E, Gabaglio M, Penna F, Fasano M, et al. Adolescent THC exposure in female rats leads to cognitive deficits through a mechanism involving chromatin modifications in the prefrontal cortex. J Psychiatry Neurosci. 2018;43:87–101. doi: 10.1503/jpn.170082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharm. 2010;161:103–12. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–81. doi: 10.1016/j.lfs.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weed PF, Filipeanu CM, Ketchum MJ, Winsauer PJ. Chronic delta9-tetrahydrocannabinol during adolescence differentially modulates striatal CB1 receptor expression and the acute and chronic effects on learning in adult rats. J Pharm Exp Ther. 2016;356:20–31. doi: 10.1124/jpet.115.227181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubino T, Parolaro D. Sex-dependent vulnerability to cannabis abuse in adolescence. Front Psychiatry. 2015;6:56. doi: 10.3389/fpsyt.2015.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorzalka BB, Hill MN, Chang SC. Male-female differences in the effects of cannabinoids on sexual behavior and gonadal hormone function. Horm Behav. 2010;58:91–9. doi: 10.1016/j.yhbeh.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 34.Craft RM. Sex differences in behavioral effects of cannabinoids. Life Sci. 2005;77:2471–8. doi: 10.1016/j.lfs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 35.Calakos KC, Bhatt S, Foster DW, Cosgrove KP. Mechanisms underlying sex differences in cannabis use. Curr Addict Rep. 2017;4:439–53. doi: 10.1007/s40429-017-0174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlienz NJ, Budney AJ, Lee DC, Vandrey R. Cannabis withdrawal: a review of neurobiological mechanisms and sex differences. Curr Addict Rep. 2017;4:75–81. doi: 10.1007/s40429-017-0143-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cooper ZD, Craft RM. Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology. 2018;43:34–51. doi: 10.1038/npp.2017.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiley JL, Burston JJ. Sex differences in Delta(9)-tetrahydrocannabinol metabolism and in vivo pharmacology following acute and repeated dosing in adolescent rats. Neurosci Lett. 2014;576:51–5. doi: 10.1016/j.neulet.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–63. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- 40.Silva L, Black R, Michaelides M, Hurd YL, Dow-Edwards D. Sex and age specific effects of delta-9-tetrahydrocannabinol during the periadolescent period in the rat: the unique susceptibility of the prepubescent animal. Neurotoxicol Teratol. 2016;58:88–100. [DOI] [PubMed]
- 41.Vozella V, Zibardi C, Ahmed F, Piomelli D. Fast and sensitive quantification of delta(9)-tetrahydrocannabinol and its main oxidative metabolites by liquid chromatography/tandem mass spectrometry. Cannabis Cannabinoid Res. 2019;4:110–23. doi: 10.1089/can.2018.0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kubilius RA, Kaplick PM, Wotjak CT. Highway to hell or magic smoke? The dose-dependence of Delta(9)-THC in place conditioning paradigms. Learn Mem. 2018;25:446–54. doi: 10.1101/lm.046870.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolton JL, Ruiz CM, Rismanchi N, Sanchez GA, Castillo E, Huang J, et al. Early-life adversity facilitates acquisition of cocaine self-administration and induces persistent anhedonia. Neurobiol Stress. 2018;8:57–67. doi: 10.1016/j.ynstr.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- 45.Braida D, Iosue S, Pegorini S, Sala M. Delta9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur J Pharm. 2004;506:63–9. doi: 10.1016/j.ejphar.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 46.Muller R, Bravo R, Burckhardt J, Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984;312:716–20. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edn. Amsterdam; Boston: Academic Press/Elsevier; 2006. [Google Scholar]
- 48.Mahler SV, Vazey EM, Beckley JT, Keistler CR, McGlinchey EM, Kaufling J, et al. Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nat Neurosci. 2014;17:577–85. doi: 10.1038/nn.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith KS, Berridge KC. The ventral pallidum and hedonic reward: neurochemical maps of sucrose “liking” and food intake. J Neuroscience. 2005;25:8637–49. doi: 10.1523/JNEUROSCI.1902-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jhou TC, Fields HL, Baxter MG, Saper CB, Holland PC. The rostromedial tegmental nucleus (RMTg), a GABAergic afferent to midbrain dopamine neurons, encodes aversive stimuli and inhibits motor responses. Neuron. 2009;61:786–800. doi: 10.1016/j.neuron.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufling J, Aston-Jones G. Persistent adaptations in afferents to ventral tegmental dopamine neurons after opiate withdrawal. J Neurosci. 2015;35:10290–303. doi: 10.1523/JNEUROSCI.0715-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Flores A, Maldonado R, Berrendero F. The hypocretin/orexin receptor-1 as a novel target to modulate cannabinoid reward. Biol Psychiatry. 2014;75:499–507. doi: 10.1016/j.biopsych.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 53.Flores A, Julia-Hernandez M, Maldonado R, Berrendero F. Involvement of the orexin/hypocretin system in the pharmacological effects induced by Delta(9) -tetrahydrocannabinol. Br J Pharm. 2016;173:1381–92.. doi: 10.1111/bph.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Torrens A, Vozella V, Huff H, McNeil B, Ahmed F, Ghidini A, et al. Comparative pharmacokinetics of Δ9-tetrahydrocannabinol in adolescent and adult male mice. J Pharmacol Exp Ther. 2020;374:151–160. [DOI] [PMC free article] [PubMed]
- 55.Mahler SV, Aston-Jones GS. Fos activation of selective afferents to ventral tegmental area during cue-induced reinstatement of cocaine seeking in rats. J Neurosci. 2012;32:13309–26. doi: 10.1523/JNEUROSCI.2277-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharm. 2006;147:S163–71. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Agurell S, Halldin M, Lindgren JE, Ohlsson A, Widman M, Gillespie H, et al. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharm Rev. 1986;38:21–43. [PubMed] [Google Scholar]
- 58.Yamamoto I, Watanabe K, Matsunaga T, Kimura T, Funahashi T, Yoshimura H. Pharmacology and toxicology of major constituents of marijuana—on the metabolic activation of cannabinoids and its mechanism. J Toxicol. 2003;22:577–89. [Google Scholar]
- 59.Browne RG, Weissman A. Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J Clin Pharm. 1981;21:227S–34S. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 60.Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharm. 2001;430:41–7. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- 61.Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 62.Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM. Cannabidiol-Delta(9)-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend. 2017;175:187–97. doi: 10.1016/j.drugalcdep.2017.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Narimatsu S, Watanabe K, Yamamoto I, Yoshimura H. Sex difference in the oxidative metabolism of delta 9-tetrahydrocannabinol in the rat. Biochem Pharm. 1991;41:1187–94. doi: 10.1016/0006-2952(91)90657-q. [DOI] [PubMed] [Google Scholar]
- 64.Compton DR, Rice KC, De Costa BR, Razdan RK, Melvin LS, Johnson MR, et al. Cannabinoid structure-activity relationships: correlation of receptor binding and in vivo activities. J Pharm Exp Ther. 1993;265:218–26. [PubMed] [Google Scholar]
- 65.Nye JS, Seltzman HH, Pitt CG, Snyder SH. High-affinity cannabinoid binding sites in brain membranes labeled with [3H]-5’-trimethylammonium delta 8-tetrahydrocannabinol. J Pharm Exp Ther. 1985;234:784–91. [PubMed] [Google Scholar]
- 66.Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, et al. Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract. Ther Drug Monit. 2005;27:799–810. doi: 10.1097/01.ftd.0000177223.19294.5c. [DOI] [PubMed] [Google Scholar]
- 67.Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–55. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Colizzi M, Weltens N, McGuire P, Van Oudenhove L, Bhattacharyya S. Descriptive psychopathology of the acute effects of intravenous delta-9-tetrahydrocannabinol administration in humans. Brain Sci. 2019;9:93. [DOI] [PMC free article] [PubMed]
- 69.Wiley JL, Lefever TW, Marusich JA, Craft RM. Comparison of the discriminative stimulus and response rate effects of (Delta9)-tetrahydrocannabinol and synthetic cannabinoids in female and male rats. Drug Alcohol Depend. 2017;172:51–59. doi: 10.1016/j.drugalcdep.2016.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Renard J, Szkudlarek HJ, Kramar CP, Jobson CEL, Moura K, Rushlow WJ, et al. Adolescent THC exposure causes enduring prefrontal cortical disruption of GABAergic inhibition and dysregulation of sub-cortical dopamine function. Sci Rep. 2017;7:11420. doi: 10.1038/s41598-017-11645-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Caballero A, Tseng KY. Association of cannabis use during adolescence, prefrontal CB1 receptor signaling, and schizophrenia. Front Pharmacol. 2012;3:101. doi: 10.3389/fphar.2012.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–85. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 73.Tooley J, Marconi L, Alipio JB, Matikainen-Ankney B, Georgiou P, Kravitz AV, et al. Glutamatergic ventral pallidal neurons modulate activity of the habenula-tegmental circuitry and constrain reward seeking. Biol Psychiatry. 2018;83:1012–23. doi: 10.1016/j.biopsych.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Faget L, Zell V, Souter E, McPherson A, Ressler R, Gutierrez-Reed N, et al. Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nat Commun. 2018;9:849. doi: 10.1038/s41467-018-03125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith KS, Tindell AJ, Aldridge JW, Berridge KC. Ventral pallidum roles in reward and motivation. Behav Brain Res. 2009;196:155–67. doi: 10.1016/j.bbr.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farrell MR, Ruiz CM, Castillo E, Faget L, Khanbijian C, Liu S, et al. Ventral pallidum is essential for cocaine relapse after voluntary abstinence in rats. Neuropsychopharmacology. 2019;44:2174–85. doi: 10.1038/s41386-019-0507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Farrell MR, Esteban JSD, Faget L, Floresco SB, Hnasko TS, Mahler SV. Mahler ventral pallidum gaba neurons mediate motivation underlying risky choice. bioRxiv 2020.08.04.221960; 10.1101/2020.08.04.221960. [DOI] [PMC free article] [PubMed]
- 78.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 79.Lecca S, Meye FJ, Mameli M. The lateral habenula in addiction and depression: an anatomical, synaptic and behavioral overview. Eur J Neurosci. 2014;39:1170–8. doi: 10.1111/ejn.12480. [DOI] [PubMed] [Google Scholar]
- 80.Bolton JL, Molet J, Regev L, Chen Y, Rismanchi N, Haddad E, et al. Anhedonia FOllowing Early-life Adversity Involves Aberrant Interaction of Reward and Anxiety Circuits and Is Reversed by Partial Silencing of Amygdala Corticotropin-releasing Hormone Gene. Biol Psychiatry. 2018;83:137–47. doi: 10.1016/j.biopsych.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Forti M, Morrison PD, Butt A, Murray RM. Cannabis use and psychiatric and cogitive disorders: the chicken or the egg? Curr Opin Psychiatry. 2007;20:228–34. doi: 10.1097/YCO.0b013e3280fa838e. [DOI] [PubMed] [Google Scholar]
- 82.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–5. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Al-Hasani R, McCall JG, Shin G, Gomez AM, Schmitz GP, Bernardi JM, et al. Distinct subpopulations of nucleus accumbens dynorphin neurons drive aversion and reward. Neuron. 2015;87:1063–77. doi: 10.1016/j.neuron.2015.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carlezon WA, Jr., Thomas MJ. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56:122–32. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volman SF, Lammel S, Margolis EB, Kim Y, Richard JM, Roitman MF, et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J Neurosci. 2013;33:17569–76. doi: 10.1523/JNEUROSCI.3250-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCutcheon JE, Ebner SR, Loriaux AL, Roitman MF. Encoding of aversion by dopamine and the nucleus accumbens. Front Neurosci. 2012;6:137. doi: 10.3389/fnins.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wenzel JM, Rauscher NA, Cheer JF, Oleson EB. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem Neurosci. 2015;6:16–26. doi: 10.1021/cn500255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Klawonn AM, Malenka RC. Nucleus accumbens modulation in reward and aversion. Cold Spring Harb Symp Quant Biol. 2018;83:119–29. doi: 10.1101/sqb.2018.83.037457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Floresco SB. The nucleus accumbens: an interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 90.Salvatore M, Wiersielis KR, Luz S, Waxler DE, Bhatnagar S, Bangasser DA. Sex differences in circuits activated by corticotropin releasing factor in rats. Horm Behav. 2018;97:145–53. doi: 10.1016/j.yhbeh.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McReynolds JR, Christianson JP, Blacktop JM, Mantsch JR. What does the Fos say? Using Fos-based approaches to understand the contribution of stress to substance use disorders. Neurobiol Stress. 2018;9:271–85. doi: 10.1016/j.ynstr.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, et al. Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci. 2018;21:404–14. doi: 10.1038/s41593-018-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim DJ, Skosnik PD, Cheng H, Pruce BJ, Brumbaugh MS, Vollmer JM, et al. Structural network topology revealed by white matter tractography in cannabis users: a graph theoretical analysis. Brain Connect. 2011;1:473–83. doi: 10.1089/brain.2011.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ramaekers JG, van Wel JH, Spronk D, Franke B, Kenis G, Toennes SW, et al. Cannabis and cocaine decrease cognitive impulse control and functional corticostriatal connectivity in drug users with low activity DBH genotypes. Brain Imaging Behav. 2016;10:1254–63. doi: 10.1007/s11682-015-9488-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wall MB, Pope R, Freeman TP, Kowalczyk OS, Demetriou L, Mokrysz C, et al. Dissociable effects of cannabis with and without cannabidiol on the human brain’s resting-state functional connectivity. J Psychopharmacol. 2019;33:822–30. doi: 10.1177/0269881119841568. [DOI] [PubMed] [Google Scholar]
- 96.Bloomfield MAP, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, et al. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharm Ther. 2019;195:132–61. doi: 10.1016/j.pharmthera.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cooper ZD, Haney M. Comparison of subjective, pharmacokinetic, and physiological effects of marijuana smoked as joints and blunts. Drug Alcohol Depend. 2009;103:107–13. doi: 10.1016/j.drugalcdep.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–82. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- 99.Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-administration in rats: sex differences and the influence of ovarian function. Br J Pharm. 2007;152:795–804. doi: 10.1038/sj.bjp.0707465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barrus DG, Capogrossi KL, Cates SC, Gourdet CK, Peiper NC, Novak SP, et al. Tasty THC: Promises and Challenges of Cannabis Edibles. Methods Rep. RTI Press. 2016;2016. 10.3768/rtipress.2016.op.0035.1611. PMID: 28127591; PMCID: PMC5260817. [DOI] [PMC free article] [PubMed]
- 101.Freels TG, Baxter-Potter LN, Lugo JM, Glodosky NC, Wright HR, Baglot SL, et al. Vaporized cannabis extracts have reinforcing properties and support conditioned drug-seeking behavior in rats. J Neurosci. 2020;40:1897–908. doi: 10.1523/JNEUROSCI.2416-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kirschmann EK, McCalley DM, Edwards CM, Torregrossa MM. Consequences of adolescent exposure to the cannabinoid receptor agonist WIN55,212-2 on working memory in female rats. Front Behav Neurosci. 2017;11:137. doi: 10.3389/fnbeh.2017.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Noriega NC, Howdeshell KL, Furr J, Lambright CR, Wilson VS, Gray LE., Jr Pubertal administration of DEHP delays puberty, suppresses testosterone production, and inhibits reproductive tract development in male Sprague-Dawley and Long-Evans rats. Toxicol Sci. 2009;111:163–78. doi: 10.1093/toxsci/kfp129. [DOI] [PubMed] [Google Scholar]
- 104.Rayner JL, Wood C, Fenton SE. Exposure parameters necessary for delayed puberty and mammary gland development in Long-Evans rats exposed in utero to atrazine. Toxicol Appl Pharm. 2004;195:23–34. doi: 10.1016/j.taap.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 105.Whitlow CT, Freedland CS, Porrino LJ. Functional consequences of the repeated administration of Delta9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2003;71:169–77. doi: 10.1016/s0376-8716(03)00135-2. [DOI] [PubMed] [Google Scholar]
- 106.Farquhar CE, Breivogel CS, Gamage TF, Gay EA, Thomas BF, Craft RM, et al. Sex, THC, and hormones: effects on density and sensitivity of CB1 cannabinoid receptors in rats. Drug Alcohol Depend. 2019;194:20–27. doi: 10.1016/j.drugalcdep.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Craft RM, Haas AE, Wiley JL, Yu Z, Clowers BH. Gonadal hormone modulation of (9)-tetrahydrocannabinol-induced antinociception and metabolism in female versus male rats. Pharm Biochem Behav. 2017;152:36–43. doi: 10.1016/j.pbb.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kervezee L, Hartman R, van den Berg DJ, Meijer JH, de Lange ECM. Diurnal variation in the pharmacokinetics and brain distribution of morphine and its major metabolite. Eur J Pharm Sci. 2017;109S:S132–S39. doi: 10.1016/j.ejps.2017.05.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.