Abstract
Pathological cardiac fibrosis is a common feature in multiple cardiovascular diseases that contributes to the occurrence of heart failure and life-threatening arrhythmias. Our previous study demonstrated that matrine could attenuate doxorubicin-induced oxidative stress and cardiomyocyte apoptosis. In this study, we investigated the effect of matrine on cardiac fibrosis. Mice received aortic banding (AB) operation or continuous injection of isoprenaline (ISO) to generate pathological cardiac fibrosis and then were exposed to matrine lavage (200 mg·kg−1·d−1) or an equal volume of vehicle as the control. We found that matrine lavage significantly attenuated AB or ISO-induced fibrotic remodeling and cardiac dysfunction. We also showed that matrine (200 μmol/L) significantly inhibited the proliferation, migration, collagen production, and phenotypic transdifferentiation of cardiac fibroblasts. Mechanistically, matrine suppressed p38 activation in vivo and in vitro, and overexpression of constitutively active p38 completely abolished the protective effects of matrine. We also demonstrated that ribosomal protein S5 (RPS5) upregulation was responsible for matrine-mediated inhibition on p38 and fibrogenesis. More importantly, matrine was capable of ameliorating preexisting cardiac fibrosis in mice. In conclusion, matrine treatment attenuates cardiac fibrosis by regulating RPS5/p38 signaling in mice, and it might be a promising therapeutic agent for treating pathological cardiac fibrosis.
Keywords: matrine, cardiac fibrosis, ribosomal protein S5, p38
Introduction
Cardiac fibrosis is a common feature in the evolution of various cardiovascular diseases and is characterized by the net accumulation of extracellular matrix (ECM) components in the cardiac interstitium and perivascular space [1, 2]. Excessive production and deposition of ECM increases myocardial stiffness, impairs the transmission of cardiomyocyte contractile force, and compromises normal electrical conduction, thereby contributing to the occurrence of systolic/diastolic dysfunction and life-threatening arrhythmias [3]. Hence, the discovery of pharmacological interventions that can restrain pathological cardiac fibrosis is of great significance.
Cardiac fibroblasts play critical roles in the pathophysiology of fibrogenesis, given their indispensable roles in ECM modulation and tissue remodeling [1–3]. In response to profibrotic stimulation, cardiac fibroblasts proliferate, migrate, and undergo phenotypic transition to myofibroblasts, resulting in abnormal cardiac fibroblast accumulation and ECM overproduction [4, 5]. Previous studies have found that the Smad-dependent pathway is required to mediate fibroblast activation and cardiac fibrosis and that the inhibition of canonical Smad signaling blunts fibrotic remodeling in mice [1]. In addition to the Smad-dependent pathway, the involvement of Smad-independent pathways in the pathogenesis of cardiac fibrosis, including protein kinase B/glycogen synthase kinase 3β (AKT/GSK3β) and mitogen-activated protein kinases (MAPKs), has been shown [1]. Accordingly, we previously found that pharmacological inhibitors of AKT suppressed myofibroblast transformation and prevented the induction of cardiac fibrosis [6, 7]. Moreover, increasing evidence has indicated that p38 kinase acts as a key regulator of fibrotic remodeling by regulating profibrotic transcription factors. The activation of p38 in fibroblasts was sufficient to drive myofibroblast formation and collagen synthesis, and in contrast, p38 inhibition resulted in the significant alleviation of fibroblast-to-myofibroblast transdifferentiation and fibrogenesis [8]. A recent study also determined that p38 played critical roles in controlling bromodomain-containing protein 4 loading on gene regulatory elements and was essential for the epigenetic activation of cardiac fibroblasts [9]. Therefore, exploring pharmacological agents that target these kinases or pathways is important for the treatment of cardiac fibrosis.
Matrine is one of the active components extracted from Sophora flavescens Aiton and is currently being used clinically for the treatment of viral hepatitis and hepatic tumors in the form of capsules or an injectable solution (China Food and Drug Administration approvals H20010242 or H20044669, China Food and Drug Administration 2014) with few adverse effects [10–12]. Previous studies have indicated that matrine and its synthesized derivatives inhibited AKT and MAPK pathways, thereby preventing bone loss in ovariectomized mice [13–15]. Matrine treatment also suppressed AKT phosphorylation in vitro and exhibited chemotherapeutic potential in cancer cells [16, 17]. More importantly, matrine was implicated in the attenuation of experimental pulmonary and hepatic fibrosis [18, 19]. In addition, our recent study demonstrated that matrine administration protected mice from doxorubicin-induced cardiac dysfunction by alleviating oxidative stress and cardiomyocyte apoptosis [20]. With these findings in mind, we speculated that matrine may be a promising agent for the treatment of pathological fibrosis and cardiac dysfunction.
Materials and methods
Reagents and antibodies
Matrine (M5319), transforming growth factor-β (TGF-β, T7039), isoprenaline (ISO, I5627), and angiotensin II (Ang II, A9525) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Antibodies against phosphorylated Smad3 (p-Smad3, 1:1000 dilution), total Smad3 (t-Smad3, 1:1000 dilution), p-AKT (1:1000 dilution), t-AKT (1:1000 dilution), p-c-Jun N-terminal protein kinase 1/2 (p-JNK1/2, 1:1000 dilution), t-JNK1/2 (1:1000 dilution), p-p38 (1:1000 dilution), t-p38 (1:1000 dilution), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1000 dilution) were obtained from Cell Signaling Technology (Danvers, MA, USA). The primary antibody against ribosomal protein S5 (RPS5, 1:200 dilution) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-α-smooth muscle actin (α-SMA, at a 1:100 dilution for staining and a 1:500 dilution for Western blot) and anti-connective tissue growth factor (CTGF, 1:500 dilution) were obtained from Abcam (Cambridge, UK), whereas anti-collagen 1 (Col1, 1:100 dilution) was purchased from Proteintech (Manchester, UK).
Animals and treatment
All animal experimental protocols were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University and were in accordance with the instructions in the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, revised 2011). Healthy male C57/B6 mice (8–10 weeks old, 23.5–27.5 g) were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Beijing, China), and were subjected to adaptive feeding for at least 1 week prior to the study. All mice were kept in standard barrier conditions at a controlled temperature (20–25 °C) in suitable humidity (50% ± 5%) and were allowed free access to food and water. Mice were then randomly assigned to receive an aortic banding (AB) operation to generate pressure overload-induced cardiac fibrosis or a sham operation as a control, and the mice designated for matrine protection were intragastrically treated with matrine (200 mg·kg−1·d−1) beginning 1 day after surgery [20]. The AB surgery was performed according to our previous studies [6, 21]. The mice were first anesthetized with 3% pentobarbital sodium (50 mg/kg, ip). Then, the left chest was opened at a second intercostal space by performing a ministernotomy. Then, the thoracic aorta was surgically dissected and ligated with a 27-G needle using a 7–0 silk suture. Subsequently, the needle was removed, and the thoracic cavity was closed, thereby producing severe aortic constriction. The animals assigned to the Sham-operation control group underwent the same procedures without actual ligation of the aorta. All the mice were placed on a heating pad to maintain warmth during the surgical process. Four weeks after the surgery, the mice were sacrificed by an overdose injection of sodium pentobarbital (200 mg/kg, ip), and the heart was collected for further examination. To determine the effect of matrine on agonist-induced cardiac fibrosis, the mice received a continuous subcutaneous injection of ISO (50 mg/kg) or an equal volume of normal saline (NS) for 14 days [6]. To investigate whether matrine can reverse preexisting cardiac fibrosis, the mice were subjected to AB surgery and, 4 weeks later, were administered matrine or vehicle orally for an additional 4 weeks [7]. Eight weeks post-AB surgery, these animals were subjected to echocardiography and hemodynamic measurements and then were sacrificed for further molecular examination.
To determine the involvement of p38 in matrine-mediated protection against cardiac fibrosis, we constructed constitutively active p38 adenoviral vectors (Ad-ca.p38) with D176A and F327S mutations, and the p38 kinase activity in cardiac fibroblasts was determined as previously described [22, 23]. During the AB operation, the mice received an intramyocardial injection of Ad-ca.p38 (Hanbio Biotechnology Co.; Shanghai, China) to overexpress p38 in the myocardium or Ad-Gfp as a control, according to our previous studies [6, 7]. RPS5 knockdown was established via an intramyocardial injection of adenovirus carrying small hairpin RNA against RPS5 (shRps5, DesignGene Biotechnology; Shanghai, China), whereas the mice in the control groups were injected with scramble RNA (shRNA).
Echocardiography and hemodynamics
Left ventricular echocardiography was performed to monitor the mouse heart function as previously described [6, 7]. Under anesthesia with 1.5% inhaled isoflurane, the mice received transthoracic echocardiography with a MyLab 30CV ultrasound machine (Esaote SpA; Genoa, Italy) equipped with a 10 MHz phased array transducer to determine the morphological and functional parameters of the heart. Invasive hemodynamic monitoring was conducted with a 1.4-French Millar catheter transducer (SPR-839; Millar Instruments, Houston, TX, USA) to further evaluate heart function, as we previously described, and the hemodynamic parameters were analyzed by PVAN data analysis software (LabChart 7, ADInstruments; Sydney, Australia) [24].
Picrosirius red (PSR) and immunohistochemistry (IHC) staining
Collagen accumulation was assessed by PSR staining according to our previous studies [6, 7]. The average collagen volume was determined by an Image-Pro Plus 6.0 analysis system (IPP 6.0, Media Cybernetics; MD, USA), and more than 60 fields per group were assessed. IHC staining was performed to further clarify the effect of matrine on cardiac fibrosis. Briefly, after being exposed to 3% hydrogen peroxide for 20 min and 10% goat serum for 45 min to eliminate endogenous peroxidase and nonspecific binding of the antibody, cardiac slices were incubated with anti-Col1 overnight at 4 °C. Then, the slices were stained with the anti-rabbit/mouse EnVisionTM/HRP reagent for 1 h at 37 °C and with diaminobenzidine (DAB) for 2 min at room temperature. All slices were captured by light microscopy (Nikon H550L; Tokyo, Japan) and examined in a blinded fashion.
Western blot and quantitative real-time PCR analyses
Tissue and cell proteins were extracted according to our previous studies [25–27]. Then, the protein extract was separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to PVDF membranes (EMD Millipore; MA, USA). After being blocked with 5% skim milk for 1 h at room temperature, the membranes were incubated overnight with the indicated primary antibodies at 4 °C, followed by incubation with the secondary antibodies for an additional 1 h at room temperature. The membranes were subsequently visualized using ChemiDoc™ XRS+ System (Bio-Rad Laboratories, Inc.), and the images were analyzed by Image Lab Software (Bio-Rad Laboratories, Inc.). Total RNA was extracted by TRIzol reagent and reverse transcribed to complementary DNA (cDNA) with a Maxima First-Strand cDNA synthesis kit (Roche; Basel, Switzerland). The relative mRNA levels were normalized to Gapdh. All primer sequences are provided in Supplementary Table S1.
Cell culture and treatment
Neonatal rat cardiac fibroblasts were isolated as previously described, and their purity was determined by morphologic recognition and immunofluorescence staining of α-actinin together with vimentin [6, 7, 28]. Considering that untreated cardiac fibroblasts spontaneously transdifferentiate into myofibroblasts under normal culture conditions in late passage, we conducted our studies using fibroblasts prior to the second passage. All cardiac fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 containing 10% fetal bovine serum (FBS) and were allowed to grow to 70%–80% confluence, and then, they were serum deprived for 16 h to achieve synchronization before being subjected to stimuli. To evaluate the effect of matrine in vitro, cardiac fibroblasts were treated with matrine (200 μmol/L) or an equal volume of vehicle in the presence or absence of TGF-β (10 ng/mL) or Ang II (1 μmol/L) for 24 h [7, 20]. The dose of matrine (200 μmol/L) was selected according to previous studies performed by our laboratory and others. Ma et al. used a concentration of 50 μg/mL to treat fibroblasts, which is roughly equivalent to 200 μmol/L [29]. Guo et al. used this concentration (200 μmol/L) to protect cardiomyocytes from hypoxia/reoxygenation injury [30]. Previously, we had consistently found that this dose of matrine (200 μmol/L) was sufficient to protect H9C2 cardiomyocytes against doxorubicin-induced injury; thus, we selected this concentration in our present study [20]. To investigate the role of p38, the cells were pre-infected with adenoviral vectors carrying Ad-ca.p38 to overexpress constitutively active p38 in vitro or Ad-Gfp as the negative control. To knockdown RPS5 in vitro, the cells were preincubated with small interfering RNA against RPS5 (siRps5, 50 nmol/L) for 4 h using Lipo6000TM transfection reagent according to the manufacturer’s protocol (RiboBio Co., Ltd, Guangzhou, China) and then cultured in normal medium for 24 h before the next treatment. To enhance the clinical effect of our work, we also explored the antifibrotic effect of matrine in human cardiac fibroblasts. Human cardiac fibroblasts were isolated and cultured as previously described [6]. Briefly, ventricular samples were minced and digested in 0.125% trypsin and collagenase in a shaking water bath at 37 °C, and then, the isolated cells were cultured in DMEM/F12 to separate the fibroblasts from the cardiomyocytes. The experiments were performed on early passage cells (2–5 passages).
Wound scratch and cell proliferation assays
A wound scratch assay was performed to investigate the migration capacity of the cardiac fibroblasts according to previous studies with minor modifications [21, 31]. Briefly, confluent monolayer cells were scratched with a 200 μL sterilized micropipette tip and then gently washed with phosphate-buffered saline for three times to remove the suspended cells. To exclude the influence of cell proliferation, all the cells were cultured in serum-free medium. Data were captured using an inverted microscope (IX51, Olympus, Tokyo, Japan). The proliferation of cardiac fibroblasts was evaluated by a bromodeoxyuridine (BrdU) cell proliferation ELISA kit (ab126556, Abcam, Cambridge, UK) according to the manufacturer’s instructions [32, 33]. In brief, the cells were cultured in DMEM/F12 containing 1% or 10% FBS in the presence or absence of matrine for the indicated times, and then, diluted BrdU (1×, 20 μL) was added, followed by a fixing solution (200 μL) to fix the cells and denature the DNA. After washing, the cells were incubated with an anti-BrdU monoclonal detector antibody (100 μL), peroxidase goat anti-mouse IgG conjugate (1×, 100 μL), and TMB peroxidase substrate (100 μL), and then, the plate was read and quantified by a microplate reader (BioTek Instruments, Inc.).
Immunofluorescence staining
Immunofluorescence staining was performed according to previous studies by our laboratory and others [6, 7, 34, 35]. Briefly, cell coverslips were incubated overnight with anti-α-SMA at 4 °C after being fixed with 4% paraformaldehyde for 15 min and permeabilized in 0.2% Triton X-100 for 10 min. Then, the cells were stained with Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody (1:200 dilution) for 1 h at 37 °C. Images were captured with an OLYMPUS DX51 fluorescence microscope (Tokyo, Japan) after the cell nuclei were stained with 4ʼ,6-diamidino-2-phenylindole (DAPI).
Biochemical analysis
The serum levels of mouse N-terminal pro-brain natriuretic peptide (NT-proBNP) and matrix metalloproteinase-2 (MMP-2) were measured using commercially available enzyme-linked immunosorbent assay (ELISA) kits from MyBioSource according to the manufacturer’s instructions (San Diego, CA, USA). The serum concentrations of the liver enzymes alanine transaminase and aspartate transaminase were measured by an automatic biochemical analyzer (ADVIA® 2400 Siemens Ltd, Tarrytown, NY, USA) as previously described [7, 36].
Statistical analysis
The results are expressed as the means ± standard error of the mean (SEM) in our study. Differences between two groups were compared by unpaired Student’s t test, and comparisons among three or more groups were performed by one-way analysis of variance followed by Tukey’s post hoc test. A P value < 0.05 was considered significant.
Results
Matrine attenuated pathological cardiac fibrosis and cardiac dysfunction in the mice
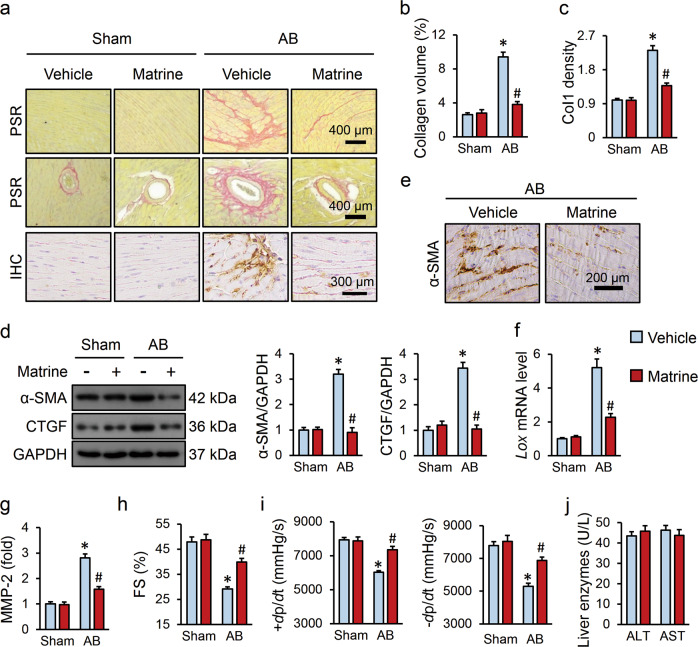
The mice were first subjected to an AB operation for 4 weeks to generate pressure overload-induced cardiac fibrosis. As shown in Fig. 1a, b, AB surgery significantly increased myocardial collagen deposition in the mice, and the deposition was reduced in the matrine-treated mice. Immunohistochemistry staining of Col1 further confirmed the beneficial effect of matrine on fibrotic remodeling in the mice (Fig. 1c). Consistently, the mRNA levels of fibrotic markers, namely, Col1, Col3, Ctgf, and α-Sma, were markedly decreased in the hearts of the matrine-treated mice compared with those in mice treated with an equal volume of vehicle after AB surgery (Supplementary Fig. S1a). Pressure overload-triggered upregulation of the α-SMA and CTGF proteins was also deceased by matrine treatment (Fig. 1d). In the presence of profibrotic factors, cardiac fibroblasts are activated and transdifferentiate into ECM-secreting myofibroblasts expressing α-SMA. We therefore labeled myofibroblasts with α-SMA and found that matrine administration reduced the myofibroblast density in fibrotic hearts (Fig. 1e). Collagen cross-linking is a key determinant of fibrogenesis and myocardial stiffness, which correlates with the records of hospitalizations and cardiovascular deaths of patients with heart failure [37]. Herein, we observed that matrine lavage suppressed the expression of lysyl oxidase (LOX), an enzyme critical for collagen cross-linking (Fig. 1f) [37]. MMP has been proven to contribute to the development of cardiac fibrosis by modulating matrix turnover. As shown in Fig. 1g, the mice subjected to AB surgery had increased serum MMP-2 levels, which were remarkably reduced by matrine treatment, consistent with the previously described results [38, 39]. In addition, we observed that the AB-induced increase in the heart weight/tibia length (HW/TL) ratio was dramatically inhibited by matrine (Supplementary Fig. S1b). The pressure overload-elicited increase in the interventricular septal thickness at systole (IVSs) was also reduced by matrine; however, the interventricular septal thickness at diastole (IVSd) was not altered (Supplementary Fig. S1c). It has been established that pathological cardiac fibrosis increases myocardial stiffness and compromises the transmission of cardiomyocyte contractile force, which subsequently results in systolic/diastolic dysfunction of the heart [3]. In line with this, the cardiac function was impaired in mice that underwent AB surgery, as evidenced by the decreased fractional shortening (FS), ±dp/dt and increased left ventricular end diastolic/systolic dimension (LVIDd/LVIDs), which was overtly attenuated by matrine administration (Fig. 1h, i and Supplementary Fig. S1d). Accordingly, serum NT-proBNP levels were reduced in matrine-treated mice after AB surgery (Supplementary Fig. S1e). However, matrine treatment had no effect on the heart rate and blood pressure (BP) either under basal conditions or after AB surgery (Supplementary Fig. S1f). More importantly, the dose of 200 mg·kg−1·d−1 was well tolerated by the mice and did not affect serum liver enzyme concentrations (Fig. 1j).
Fig. 1. Matrine attenuated pathological cardiac fibrosis and cardiac dysfunction in the mice.
a Representative images of picrosirius red (PSR) and immunohistochemistry (IHC) staining (n = 6). b Statistical results of average collagen volume for PSR staining (n = 6). c Statistical results of collagen 1 (Col1) density (n = 6). d Representative images of western blots and quantitative data (n = 6). e Representative images of IHC staining (n = 6). f The relative mRNA level of lysyl oxidase (LOX) in the murine hearts in the presence or absence of matrine (n = 6). g Serum level of matrix metalloproteinase-2 (MMP-2) in the mice (n = 8). h, i Echocardiography and hemodynamic analysis for fractional shortening (FS), ±dp/dt (n = 8). j Serum concentrations of liver enzymes (n = 8). Values represent the means ± SEM. *P < 0.05 versus sham + vehicle, #P < 0.05 versus AB + vehicle.
To further investigate the antifibrotic effect of matrine, the mice were exposed to continuous subcutaneous injection of ISO for 14 days [6]. Consistent with the beneficial effects of matrine in pressure overload-induced fibrotic remodeling, the matrine treatment also inhibited collagen accumulation in the hearts of the ISO-treated mice (Supplementary Fig. S2a). In addition, the ISO infusion-triggered increases in HW/TL and fibrotic markers were suppressed in the presence of matrine (Supplementary Fig. S2b–d). Subsequently, the impaired cardiac function was distinctly attenuated by matrine treatment (Supplementary Fig. S2e, f). In addition, matrine lavage significantly decreased the IVSs but not the IVSd in the fibrotic murine hearts (Supplementary Fig. S2g). Collectively, these data suggested that matrine administration effectively and safely attenuated pathological cardiac fibrosis and cardiac dysfunction in the mice.
Matrine administration inhibited p38 activation in vivo
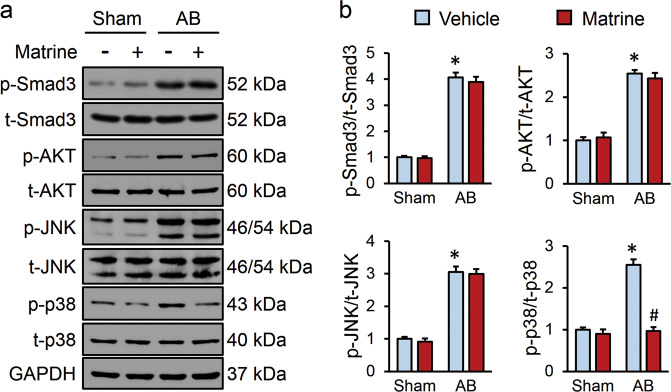
Smad-dependent and Smad-independent pathways have been proven to be involved in the pathogenesis of cardiac fibrosis, and emerging studies indicated that matrine treatment can inhibit the activation of AKT and MAPK signaling; therefore, we sought to determine whether these pathways were critical for the antifibrotic effect of matrine [13, 14]. As shown in Fig. 2a, b, matrine lavage did not affect the phosphorylation of Smad3 or AKT. Recent studies verified that cardiac fibrosis can also be triggered through a noncanonical pathway mediated by MAPK effectors [8, 40]. We therefore determined the alterations of the MAPK pathway and found that matrine evidently suppressed p38 activation with a negligible influence on JNK1/2 (Fig. 2a, b). These data implied that the antifibrotic effect of matrine might be attributable to p38 inhibition.
Fig. 2. Matrine administration inhibited p38 activation in vivo.
a Representative images of Western blots (n = 6). b Quantitative results of Western blots (n = 6). Values represent the means ± SEM. *P < 0.05 versus sham + vehicle, #P < 0.05 versus AB + vehicle.
Matrine regulated the proliferation, migration, and transdifferentiation of cardiac fibroblasts via p38 in vitro
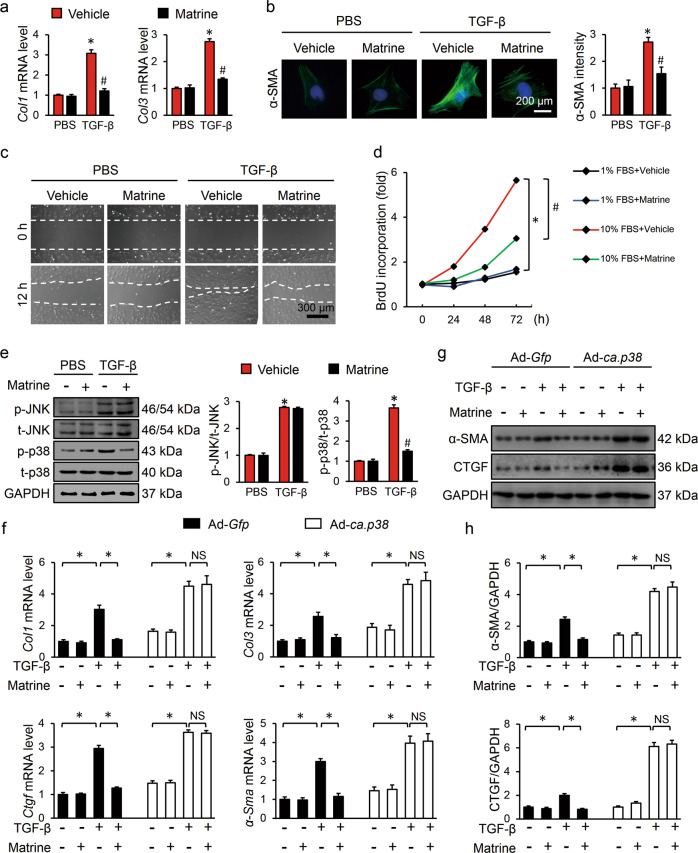
Neonatal rat cardiac fibroblasts were separated to explore the effect of matrine in vitro. In line with the results in vivo, matrine incubation dramatically decreased the mRNA levels of fibrotic markers in the cultured cardiac fibroblasts in response to TGF-β stimulation (Fig. 3a). Enhanced α-SMA expression is a hallmark of myofibroblast transdifferentiation. We performed an immunostaining assay to verify whether matrine could repress α-SMA upregulation. Our results showed that TGF-β incubation significantly induced fibroblast-to-myofibroblast transdifferentiation, and coapplication of matrine markedly attenuated this alteration (Fig. 3b). Moreover, we treated cultured cardiac fibroblasts with Ang II to further assess the effect of matrine in vitro, and we found that matrine attenuated Ang II-mediated upregulation of α-SMA and collagen synthesis (Supplementary Fig. S3a, b). In addition, we also investigated whether the cell migration capacity was regulated by matrine by performing a wound scratch assay, and the results showed that matrine could block TGF-β-induced migration of cardiac fibroblasts (Fig. 3c). Previous studies demonstrated that the abnormal proliferation of cardiac fibroblasts was involved in the pathophysiologic process of fibrotic remodeling; hence, we evaluated the proliferative rates of cardiac fibroblasts with or without matrine treatment. As shown in Fig. 3d, cardiac fibroblasts cultured in DMEM/F12 containing 10% FBS had a significantly higher proliferative rate than those cultured with 1% FBS, and proliferation was significantly reduced by matrine incubation. Consistent with the in vivo data, matrine blocked p38 activation in the presence of TGF-β or Ang II without affecting Smad3 or JNK1/2 phosphorylation (Fig. 3e, Supplementary Fig. S3c, d). To confirm the involvement of p38, we preinfected cardiac fibroblasts with adenoviral vectors carrying Ad-ca.p38 to overexpress constitutively active p38 in vitro or Ad-Gfp as the negative control. Notably, the protective effects of matrine were abolished in the presence of p38 activation, as indicated by the mRNA levels of Col1, Col3, Ctgf, and α-Sma (Fig. 3f). Consistently, matrine incubation markedly decreased α-SMA and CTGF protein levels in cardiac fibroblasts infected with Ad-Gfp but failed to have this effect in cells infected with Ad-ca.p38 (Fig. 3g, h). Taking these findings together, we concluded that matrine suppressed the proliferation, migration, and transdifferentiation of cardiac fibroblasts in vitro via p38.
Fig. 3. Matrine regulated the proliferation, migration, and transdifferentiation of cardiac fibroblasts via p38 in vitro.
a The mRNA levels of Col1 and Col3 in neonatal rat cardiac fibroblasts among groups (n = 6). b Immunofluorescence staining of α-SMA and statistical results in neonatal rat cardiac fibroblasts (n = 6). c Representative images of the wound scratch assay at 0 and 12 h (n = 4). d Cell proliferation as detected with a bromodeoxyuridine (BrdU) cell proliferation ELISA kit. e, f Representative Western blots and quantitative results (n = 6). g The relative mRNA levels of Col1, Col3, Ctgf, and α-Sma (n = 6). h Representative images of Western blots and the quantitative data (n = 6). Values represent the means ± SEM. *P < 0.05 versus PBS + vehicle, #P < 0.05 versus TGF-β + vehicle. d *P < 0.05 versus 1% FBS + vehicle, #P < 0.05 versus 10% FBS + vehicle. g, h *P < 0.05 versus the matched group, NS indicates no significance.
Matrine prevented fibrotic remodeling and cardiac dysfunction by suppressing p38 in the mice
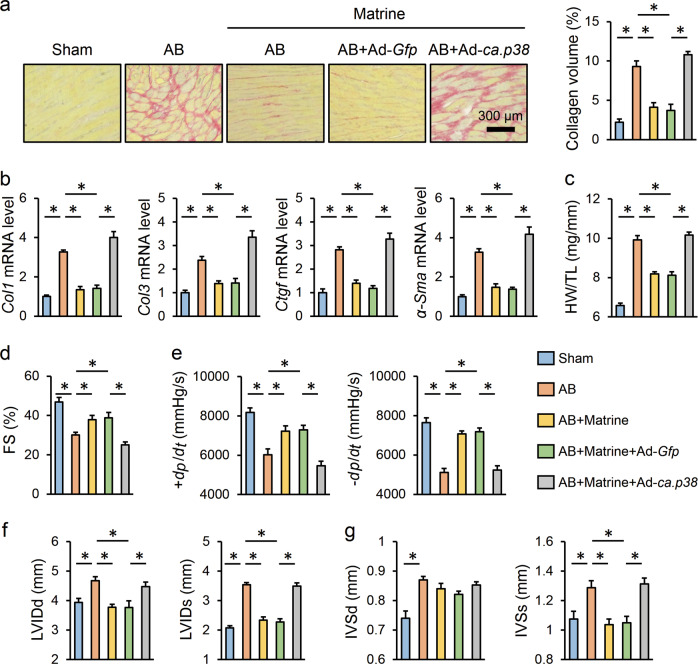
To validate whether p38 inhibition contributed to the protective effects of matrine on fibrotic remodeling and cardiac dysfunction in vivo, we treated the mice with Ad-ca.p38 or Ad-Gfp, respectively. Then, adult mouse cardiac fibroblasts were isolated to determine the p38 kinase activity and thus verify its overexpression according to previous studies (Supplementary Fig. S3e) [6, 22]. In accordance with previous data, matrine evidently attenuated AB-induced cardiac fibrosis but failed to have an effect in the presence of p38 overexpression, as indicated by the collagen volume and mRNA levels of the fibrotic markers (Fig. 4a, b). In addition, the matrine-mediated inhibition of HW/TL was also abolished when p38 was constitutively activated (Fig. 4c). Correspondingly, matrine supplementation attenuated the impaired cardiac function and deformed structure induced by AB surgery but had no effects in p38-overexpressing murine hearts (Fig. 4d–g). These data supported the notion that matrine exerted beneficial effects on fibrotic remodeling and cardiac dysfunction by suppressing p38 in the mice.
Fig. 4. Matrine prevented fibrotic remodeling and cardiac dysfunction by suppressing p38 in the mice.
a Representative images of PSR staining and the statistical results (n = 6). b The relative mRNA levels of Col1, Col3, Ctgf, and α-Sma among groups (n = 6). c Statistical result of HW/TL (n = 8). d–g Echocardiography and hemodynamic analyses for FS, ±dp/dt, left ventricular end diastolic/systolic dimension (LVIDd/LVIDs) and interventricular septal thickness at diastole or systole (IVSd/IVSs) (n = 8). Values represent the means ± SEM. *P < 0.05 versus the matched group.
Matrine repressed the p38 pathway by upregulating RPS5 expression in vivo and in vitro
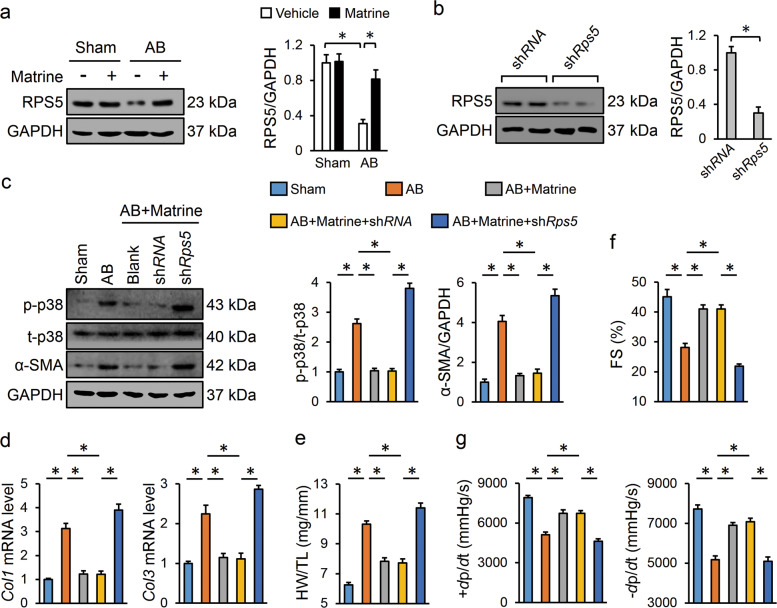
Next, we explored the possible mechanism by which matrine decreased p38 phosphorylation. Previous studies had identified RPS5 as a molecular target of MASM (M19), a synthesized derivative of matrine, and more importantly, the compound binds directly to RPS5 and therefore prevents its degradation [10, 13]. Recent studies also indicated that matrine and its derivative-M54 can target RPS5 to maintain its protein level; thus, we speculated that RPS5 upregulation might be the cause of the matrine-mediated inhibition of p38 and cardiac fibrosis [15]. As expected, we observed that matrine treatment restored RPS5 expression after AB insult, and RPS5 knockdown abrogated matrine-mediated suppression of p38 phosphorylation (Fig. 5a–c). Consistently, the inhibitory effects of matrine on α-SMA expression and collagen synthesis were also abolished in the Rps5-deficient mice (Fig. 5c, d). AB operation-triggered HW/TL increases and cardiac dysfunction were both prevented in the matrine-treated mice but not in the Rps5-deficient mice treated with matrine (Fig. 5e–g).
Fig. 5. Matrine repressed the p38 pathway by upregulating RPS5 expression in vivo.
a Representative images of western blots and statistical results (n = 6). b Protein levels of RPS5 in the murine hearts infected with shRps5 or shRNA (n = 6). c Representative images of Western blots and statistical results (n = 6). d The relative mRNA levels of Col1 and Col3 among groups (n = 6). e Statistical result of HW/TL (n = 8). f Echocardiography analysis for FS (n = 6). g Hemodynamic analysis for ±dp/dt (n = 8). Values represent the means ± SEM. *P < 0.05 versus the matched group.
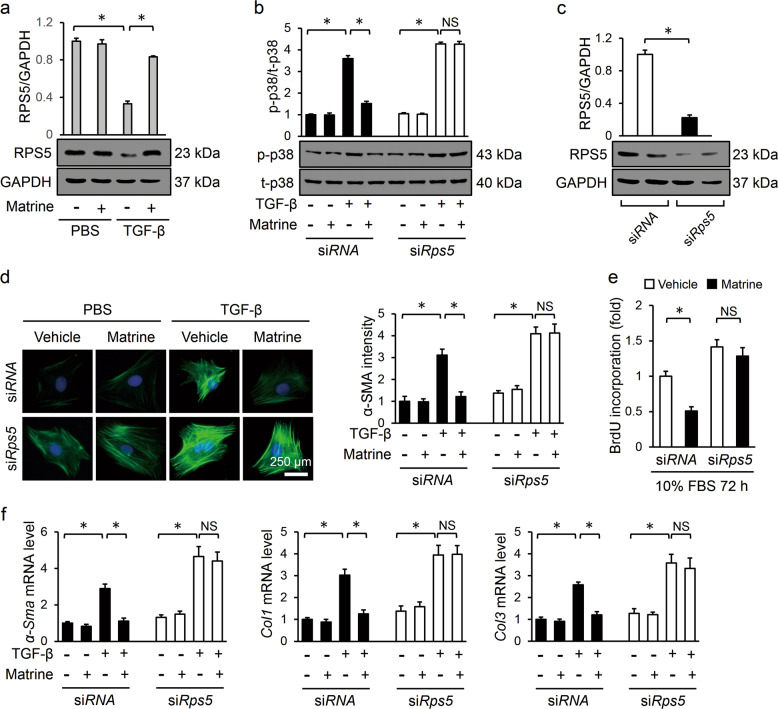
To confirm the contribution of RPS5, we treated cultured cardiac fibroblasts with siRps5 to knockdown RPS5 expression in vitro. In line with the data obtained in vivo, matrine incubation prevented the TGF-β-induced downregulation of RPS5 protein levels, and the knockdown of RPS5 significantly blunted matrine-mediated p38 inactivation (Fig. 6a–c). Consistent with these molecular alterations, RPS5 knockdown negated the inhibitory effects of matrine on α-SMA expression in TGF-β-treated cardiac fibroblasts and cell proliferation cultured in DMEM/F12 containing 10% FBS (Fig. 6d, e). The PCR data verified that the matrine treatment-triggered antifibrotic effect was counteracted in the absence of RPS5 (Fig. 6f). Thus, these results indicated that matrine repressed the p38 pathway by upregulating RPS5 expression in vivo and in vitro.
Fig. 6. Matrine repressed the p38 pathway by upregulating RPS5 expression in vitro.
a, b Representative images of Western blots and statistical results (n = 6). c Efficiency of siRps5 compared with siRNA in neonatal rat cardiac fibroblasts (n = 6). d Immunofluorescence staining of α-SMA and statistical results for the indicated groups (n = 6). e Cell proliferation detected with a BrdU cell proliferation ELISA kit (n = 5). f The relative mRNA levels of α-Sma, Col1, and Col3 by group (n = 6). Values represent the means ± SEM. *P < 0.05 versus the matched group, NS indicates no significance.
Matrine is a promising therapeutic agent for treating pathological cardiac fibrosis
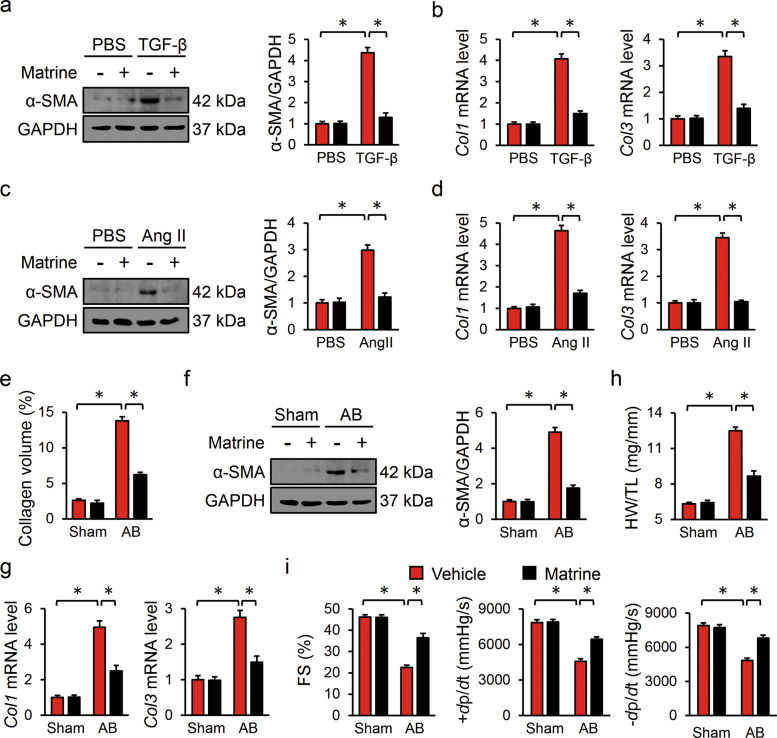
To enhance the clinical impact of our study, we assessed the antifibrotic effect of matrine in human cardiac fibroblasts. As shown in Fig. 7a–d, matrine incubation suppressed α-SMA protein levels as well as the mRNA levels of Col1 and Col3 in the presence of TGF-β or Ang II stimulation. Knowing that matrine possesses an antifibrotic effect, we finally investigated whether matrine treatment can reverse preexisting cardiac fibrosis, which is a more clinically relevant situation. The mice were subjected to AB surgery for 4 weeks to induce cardiac fibrosis and then received matrine lavage or vehicle for additional 4 weeks. As shown in Fig. 7e, matrine significantly reduced collagen accumulation in the mice subjected to AB surgery, and the protein level of α-SMA and the mRNA levels of Col1 and Col3 were also decreased in the mice given matrine lavage (Fig. 7f, g). In addition, matrine treatment reduced HW/TL in the mice with preestablished cardiac fibrosis (Fig. 7h). Correspondingly, the impaired cardiac function was also attenuated by matrine, as evidenced by the ameliorated FS and ±dp/dt (Fig. 7i). Together, these data indicated that matrine treatment was capable of blocking the induction and progression of cardiac fibrosis, thereby improving cardiac dysfunction; therefore, matrine might be a promising therapeutic agent for treating pathological cardiac fibrosis.
Fig. 7. Matrine is a promising therapeutic agent for treating pathological cardiac fibrosis.
a–d Protein levels of α-SMA and relative mRNA levels of Col1 or Col3 in human cardiac fibroblasts stimulated with TGF-β or Ang II (n = 6). e The average collagen volume in preexisting fibrotic hearts in the presence or absence of matrine (n = 6). f Representative images of Western blots and statistical results of α-SMA in mice (n = 6). g The relative mRNA levels of Col1 and Col3 in murine hearts (n = 6). h Statistical result of HW/TL (n = 8). i Echocardiography and hemodynamic analysis of FS and ±dp/dt, respectively (n = 8). Values represent the means ± SEM. *P < 0.05 versus the matched group.
Discussion
In the present study, we found that matrine treatment prevented pathological cardiac fibrosis in mice and suppressed the proliferation, migration, and transdifferentiation of neonatal rat cardiac fibroblasts in vitro. Mechanistically, we demonstrated that matrine could suppress p38 phosphorylation by upregulating RPS5 expression, and genetic activation of p38 or deletion of RPS5 abrogated the matrine-mediated beneficial effects on fibrotic remodeling and cardiac dysfunction. In addition, we also observed that matrine treatment suppressed the α-SMA protein level and the mRNA levels of Col1 and Col3 in human cardiac fibroblasts and was capable of blocking preexisting cardiac fibrosis in mice.
Beyond the cardiomyocyte-centric view of heart failure, it has been established that alterations of the ECM also play crucial roles in the development of structural remodeling and determine the evolution of heart failure [3, 4]. Extensive studies have indicated that myocardial collagen turnover is primarily regulated by cardiac fibroblasts, and disturbing the homeostasis between the synthesis and degradation of matrix proteins results in fibrotic remodeling and cardiac dysfunction [5]. Therefore, exploring pharmacological agents that target cardiac fibroblasts may be of great therapeutic interest for treating cardiac fibrosis. We previously found that matrine treatment evidently prevented doxorubicin-induced cardiomyocyte apoptosis, whereas in this study, we found that matrine attenuated the proliferation, migration, and transdifferentiation of cardiac fibroblasts in vitro and protected against fibrotic remodeling and cardiac dysfunction in the experimental mice. In accordance with our data, previous studies indicated that matrine and its derivatives strongly inhibited the proliferation, migration, and collagen synthesis of hepatic stellate cells, thereby preventing their activation and alleviating experimental fibrosis in the liver [10, 19, 41]. Ma et al. recently found that matrine treatment can suppress atrial fibrosis and has the potential to reduce susceptibility to atrial fibrillation in mice, which is different from our paper that focusing on ventricular fibrosis, a more clinically relevant situation [29]. In addition, the results from Zhang et al. and Liu et al. both verified the antifibrotic effect of matrine in diabetic hearts [42, 43]. However, emerging evidence indicates that the occurrence of fibrotic remodeling in diabetes might involve distinct fibrogenic pathways that depend on high glucose and metabolic disorders [44, 45]. Furthermore, numerous studies have revealed that the same intervention can cause diametrically opposite outcomes in different disease models [46, 47]. Accordingly, in this study, we found that matrine treatment cannot alter Smad3 phosphorylation but causes p38 inhibition (differences between our data and the results from Zhang et al. [42]). More importantly, we confirmed that matrine incubation notably decreases collagen synthesis in human cardiac fibroblasts and that matrine treatment can even reverse preexisting cardiac fibrosis in the experimental mice, which is a more clinically relevant situation (Fig. 7). In addition, matrine has been clinically approved for the treatment of viral hepatitis and hepatic tumors and induces few adverse effects, indicating that matrine might be an effective and safe therapeutic agent to use against fibrotic remodeling and cardiac dysfunction [48].
The TGF-β axis is the best known fibrogenic signaling pathway that directly mediates myofibroblast activation, ECM synthesis and fibrotic remodeling [49]. Although an exact picture of all downstream pathways remains unclear, activation of the Smad-dependent pathway plays crucial roles in accelerating the fibrotic process [1]. Upon TGF-β stimulation, TGF-β type I receptor and type II receptor form a heterodimer, which mediates Smad3 phosphorylation and nuclear translocation and subsequently transduces stimulus-induced fibrotic signals to the nucleus [49]. We previously found that the inhibition of the Smad pathway with either genetic or pharmacological methods markedly restrained the progression of cardiac fibrosis [21, 50]. However, previous studies found that myofibroblast transdifferentiation continued in Smad3-deficient mice despite a reduction in ECM secretion [51]. Furthermore, the overexpression of inhibitory Smads failed to block TGF-β-dependent α-SMA expression in isolated cardiac fibroblasts [52]. All these data suggest that additional pathways might be required for myofibroblast activation and fibrogenesis, except for Smad-dependent pathways. Recent studies have identified that Smad-independent pathways, especially AKT and MAPK pathways, are also essential for fibrotic remodeling [6, 8]. Previous studies indicated that matrine or its synthesized derivatives can inhibit AKT activation and exert beneficial effects on various diseases, including hepatic fibrosis [10, 16]. Unexpectedly, the findings in our study showed that the protective effect of matrine on cardiac fibrosis was not attributable to AKT inhibition. Recent studies proved that p38 is a nodal signaling effector within cardiac fibroblasts, and inductive mechanical and paracrine signals converge on p38 to initiate programmed fibroblast-to-myofibroblast transdifferentiation and fibrogenesis. Moreover, Molkentin et al. confirmed that fibroblast-specific deletion of p38 reduced the fibrotic response to ischemic injury and to chronic neurohumoral stimulation, whereas the fibroblast-specific activation of p38 signaling promoted the expansion of cardiac fibroblasts and their conversion to myofibroblasts [8]. In line with these data, matrine significantly suppressed p38 activation, and the protective effects of matrine on myofibroblast transdifferentiation and fibrotic remodeling were abolished when p38 was overexpressed both in vivo and in vitro. With findings consistent with the present study, Zhou et al. recently verified that oxidized low-density lipoprotein-induced p38 activation in macrophages was prevented by matrine treatment [53]. In addition, Ren et al. and Wu et al. found that matrine treatment obviously reduced p38 phosphorylation and suppressed the proliferation and migration of cancer cells [38, 39].
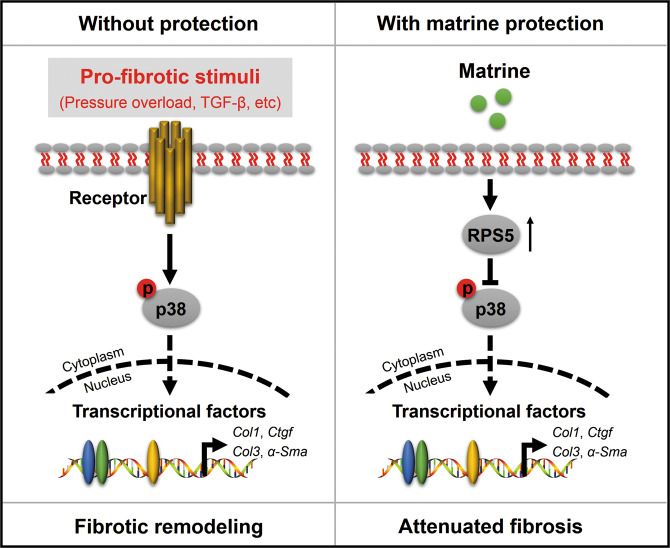
RPS5 is an important component of ribosomes that plays an essential role in ribosome assembly and protein translation and was recently identified as a direct molecular target for matrine and its derivatives [10, 13]. Increasing evidence suggests that RPS5 extends well beyond its ribosome-dependent functions, and its extraribosomal functions have also been increasingly appreciated [10, 13, 54]. Xu et al. found that RPS5 deficiency was associated with an insufficient dephosphorylation capacity in hepatic stellate cells, thereby resulting in increased phosphorylation of AKT/GSK3β [10]. More recently, RPS5 was reported to regulate AKT, MAPKs and nuclear factor κB (NF-κB) phosphorylation, serving as a potential candidate to inhibit osteoclastogenesis and osteoporosis [15]. Herein, we reported that matrine treatment restrained p38 activation by upregulating RPS5 expression and that RPS5 knockdown abrogated the inhibitory effects of matrine on p38 phosphorylation and fibrotic response in vivo and in vitro. With these findings in mind, we speculate that RPS5 is downregulated upon fibrotic stimulation, which triggers a loss of dephosphorylation in cardiac fibroblasts. In the presence of matrine protection, RPS5 expression is maintained, and p38 phosphorylation is decreased, thereby preventing the development of fibroblast-to-myofibroblast transdifferentiation and cardiac fibrosis (Fig. 8).
Fig. 8. The proposed working model of matrine on pathological cardiac fibrosis.
Matrine treatment increased RPS5 expression, which subsequently decreased profibrotic stimulation-induced p38 phosphorylation and alleviated fibrotic remodeling and cardiac dysfunction.
Taken together, these findings proved that matrine attenuated pathological cardiac fibrosis by regulating the RPS5/p38 pathway. Matrine might be a promising therapeutic agent for treating pathological cardiac fibrosis.
Supplementary information
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (nos. 81470516 and 81700254), the Key Project of the National Natural Science Foundation (no. 81530012), National Key R&D Program of China (2018YFC1311300), the Fundamental Research Funds for the Central Universities (no. 2042017kf0085 and 2042018kf1032), and Development Center for Medical Science and Technology National Health and Family Planning Commission of the People’s Republic of China (The prevention and control project of cardiovascular disease, 2016ZX-008-01).
Author contributions
XZ, CH, and QZT contributed to the conception and design of the experiment. XZ, CH, NZ, WYW, and LLL performed the study and participated in data acquisition. XZ, CH, HMW, and ZGM contributed to the data analysis and interpretation. XZ, CH, and ZGM wrote and revised the paper. QZT the guarantor of this work. All authors approved the final version of the paper. XZ and CH contributed equally to this paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Xin Zhang, Can Hu
Contributor Information
Zhen-guo Ma, Email: zhengma@whu.edu.cn.
Qi-zhu Tang, Email: qztang@whu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41401-020-0473-8) contains supplementary material, which is available to authorized users.
References
- 1.Ma ZG, Yuan YP, Wu HM, Zhang X, Tang QZ. Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci. 2018;14:1645–57.. doi: 10.7150/ijbs.28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–74. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez A, Schelbert EB, Diez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018;71:1696–706.. doi: 10.1016/j.jacc.2018.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Nagaraju CK, Robinson EL, Abdesselem M, Trenson S, Dries E, Gilbert G, et al. Myofibroblast phenotype and reversibility of fibrosis in patients with end-stage heart failure. J Am Coll Cardiol. 2019;73:2267–82.. doi: 10.1016/j.jacc.2019.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Park S, Ranjbarvaziri S, Lay FD, Zhao P, Miller MJ, Dhaliwal JS, et al. Genetic regulation of fibroblast activation and proliferation in cardiac fibrosis. Circulation. 2018;138:1224–35.. doi: 10.1161/CIRCULATIONAHA.118.035420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma ZG, Yuan YP, Zhang X, Xu SC, Wang SS, Tang QZ. Piperine attenuates pathological cardiac fibrosis via PPAR-gamma/AKT pathways. Ebiomedicine. 2017;18:179–87.. doi: 10.1016/j.ebiom.2017.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma ZG, Zhang X, Yuan YP, Jin YG, Li N, Kong CY, et al. A77 1726 (leflunomide) blocks and reverses cardiac hypertrophy and fibrosis in mice. Clin Sci. 2018;132:685–99.. doi: 10.1042/CS20180160. [DOI] [PubMed] [Google Scholar]
- 8.Molkentin JD, Bugg D, Ghearing N, Dorn LE, Kim P, Sargent MA, et al. Fibroblast-specific genetic manipulation of p38 mitogen-activated protein kinase in vivo reveals its central regulatory role in fibrosis. Circulation. 2017;136:549–61.. doi: 10.1161/CIRCULATIONAHA.116.026238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stratton MS, Bagchi RA, Felisbino MB, Hirsch RA, Smith HE, Riching AS, et al. Dynamic chromatin targeting of BRD4 stimulates cardiac fibroblast activation. Circ Res. 2019;125:662–77.. doi: 10.1161/CIRCRESAHA.119.315125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu WH, Hu HG, Tian Y, Wang SZ, Li J, Li JZ, et al. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis. Hepatology. 2014;60:648–60. doi: 10.1002/hep.27138. [DOI] [PubMed] [Google Scholar]
- 11.Zeng XY, Wang H, Bai F, Zhou X, Li SP, Ren LP, et al. Identification of matrine as a promising novel drug for hepatic steatosis and glucose intolerance with HSP72 as an upstream target. Br J Pharmacol. 2015;172:4303–18. doi: 10.1111/bph.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Zhu M, Shi R, Yang M. Radix Sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. Am J Chin Med. 2003;31:337–54. doi: 10.1142/S0192415X03001107. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Zhi X, Cao L, Weng W, Pan P, Hu H, et al. Matrine derivate MASM uncovers a novel function for ribosomal protein S5 in osteoclastogenesis and postmenopausal osteoporosis. Cell Death Dis. 2017;8:e3037. doi: 10.1038/cddis.2017.394. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chen X, Zhi X, Pan P, Cui J, Cao L, Weng W, et al. Matrine prevents bone loss in ovariectomized mice by inhibiting RANKL-induced osteoclastogenesis. FASEB J. 2017;31:4855–65.. doi: 10.1096/fj.201700316R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin Z, Jin C, Chao L, Zheng Z, Liehu C, Panpan P, et al. A matrine derivative M54 suppresses osteoclastogenesis and prevents ovariectomy-induced bone loss by targeting ribosomal protein S5. Front Pharmacol. 2018;9:22. doi: 10.3389/fphar.2018.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y, et al. Matrine derivative WM130 inhibits hepatocellular carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Cancer Lett. 2015;368:126–34.. doi: 10.1016/j.canlet.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Liu Y, Qi Y, Bai ZH, Ni CX, Ren QH, Xu WH, et al. A novel matrine derivate inhibits differentiated human hepatoma cells and hepatic cancer stem-like cells by suppressing PI3K/AKT signaling pathways. Acta Pharmacol Sin. 2017;38:120–32.. doi: 10.1038/aps.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Ma L, Wang D, Jia H, Yu M, Gu Y, et al. Design and synthesis of matrine derivatives as novel anti-pulmonary fibrotic agents via repression of the TGF-beta/Smad pathway. Molecules. 2019;24:1108. doi: 10.3390/molecules24061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feng Y, Ying HY, Qu Y, Cai XB, Xu MY, Lu LG. Novel matrine derivative MD-1 attenuates hepatic fibrosis by inhibiting EGFR activation of hepatic stellate cells. Protein Cell. 2016;7:662–72. doi: 10.1007/s13238-016-0285-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu C, Zhang X, Wei W, Zhang N, Wu H, Ma Z, et al. Matrine attenuates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via maintaining AMPKalpha/UCP2 pathway. Acta Pharm Sin B. 2019;9:690–701. doi: 10.1016/j.apsb.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Ma ZG, Yuan YP, Xu SC, Wei WY, Song P, et al. Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKalpha/Smad3 signaling. Cell Death Dis. 2018;9:102. doi: 10.1038/s41419-017-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diskin R, Askari N, Capone R, Engelberg D, Livnah O. Active mutants of the human p38alpha mitogen-activated protein kinase. J Biol Chem. 2004;279:47040–9. doi: 10.1074/jbc.M404595200. [DOI] [PubMed] [Google Scholar]
- 23.Xu X, Wang Q, Long Y, Zhang R, Wei X, Xing M, et al. Stress-mediated p38 activation promotes somatic cell reprogramming. Cell Res. 2013;23:131–41. doi: 10.1038/cr.2012.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma ZG, Kong CY, Song P, Zhang X, Yuan YP, Tang QZ. Geniposide protects against obesity-related cardiac injury through AMPKalpha- and Sirt1-dependent mechanisms. Oxid Med Cell Longev. 2018;2018:6053727. doi: 10.1155/2018/6053727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma ZG, Yuan YP, Xu SC, Wei WY, Xu CR, Zhang X, et al. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 2017;60:1126–37.. doi: 10.1007/s00125-017-4232-4. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Zhu JX, Ma ZG, Wu HM, Xu SC, Song P, et al. Rosmarinic acid alleviates cardiomyocyte apoptosis via cardiac fibroblast in doxorubicin-induced cardiotoxicity. Int J Biol Sci. 2019;15:556–67.. doi: 10.7150/ijbs.29907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yuan YP, Ma ZG, Zhang X, Xu SC, Zeng XF, Yang Z, et al. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol. 2018;114:38–47. doi: 10.1016/j.yjmcc.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Bi X, Zhang G, Deng Y, Luo X, Xu L, et al. Glucose-regulated protein 78 is essential for cardiac myocyte survival. Cell Death Differ. 2018;25:2181–94.. doi: 10.1038/s41418-018-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Ma S, Yin C, Wu H. Matrine reduces susceptibility to postinfarct atrial fibrillation in rats due to antifibrotic properties. J Cardiovasc Electrophysiol. 2018;29:616–27.. doi: 10.1111/jce.13448. [DOI] [PubMed] [Google Scholar]
- 30.Guo S, Gao C, Xiao W, Zhang J, Qu Y, Li J, et al. Matrine protects cardiomyocytes from ischemia/reperfusion injury by regulating HSP70 expression via activation of the JAK2/STAT3 pathway. Shock. 2018;50:664–70.. doi: 10.1097/SHK.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 31.Avalle L, Incarnato D, Savino A, Gai M, Marino F, Pensa S, et al. MicroRNAs-143 and -145 induce epithelial to mesenchymal transition and modulate the expression of junction proteins. Cell Death Differ. 2017;24:1750–60.. doi: 10.1038/cdd.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beermann J, Kirste D, Iwanov K, Lu D, Kleemiss F, Kumarswamy R, et al. A large shRNA library approach identifies lncRNA Ntep as an essential regulator of cell proliferation. Cell Death Differ. 2018;25:307–18.. doi: 10.1038/cdd.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qu F, Holloway JL, Esterhai JL, Burdick JA, Mauck RL. Programmed biomolecule delivery to enable and direct cell migration for connective tissue repair. Nat Commun. 2017;8:1780. doi: 10.1038/s41467-017-01955-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou H, Zhu P, Wang J, Zhu H, Ren J, Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25:1080–93.. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin W, Cui B, Li P, Hua F, Lv X, Zhou J, et al. 1,25-Dihydroxyvitamin D3 protects obese rats from metabolic syndrome via promoting regulatory T cell-mediated resolution of inflammation. Acta Pharm Sin B. 2018;8:178–87.. doi: 10.1016/j.apsb.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang X, Hu C, Kong CY, Song P, Wu HM, Xu SC, et al. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020;27:540–55.. doi: 10.1038/s41418-019-0372-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lopez B, Ravassa S, Gonzalez A, Zubillaga E, Bonavila C, Berges M, et al. Myocardial collagen cross-linking is associated with heart failure hospitalization in patients with hypertensive heart failure. J Am Coll Cardiol. 2016;67:251–60. doi: 10.1016/j.jacc.2015.10.063. [DOI] [PubMed] [Google Scholar]
- 38.Ren H, Zhang S, Ma H, Wang Y, Liu D, Wang X, et al. Matrine reduces the proliferation and invasion of colorectal cancer cells via reducing the activity of p38 signaling pathway. Acta Biochim Biophys Sin. 2014;46:1049–55. doi: 10.1093/abbs/gmu101. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, Zhou J, Cai D, Li M. Matrine inhibits the metastatic properties of human cervical cancer cells via downregulating the p38 signaling pathway. Oncol Rep. 2017;38:1312–20.. doi: 10.3892/or.2017.5787. [DOI] [PubMed] [Google Scholar]
- 40.Qin W, Du N, Zhang L, Wu X, Hu Y, Li X, et al. Genistein alleviates pressure overload-induced cardiac dysfunction and interstitial fibrosis in mice. Br J Pharmacol. 2015;172:5559–72. doi: 10.1111/bph.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahzari A, Li S, Zhou X, Li D, Fouda S, Alhomrani M, et al. Matrine protects against MCD-induced development of NASH via upregulating HSP72 and downregulating mTOR in a manner distinctive from metformin. Front Pharmacol. 2019;10:405. doi: 10.3389/fphar.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Cui L, Guan G, Wang J, Qiu C, Yang T, et al. Matrine suppresses cardiac fibrosis by inhibiting the TGFbeta/Smad pathway in experimental diabetic cardiomyopathy. Mol Med Rep. 2018;17:1775–81.. doi: 10.3892/mmr.2017.8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu Z, Zhang Y, Tang Z, Xu J, Ma M, Pan S, et al. Matrine attenuates cardiac fibrosis by affecting ATF6 signaling pathway in diabetic cardiomyopathy. Eur J Pharmacol. 2017;804:21–30. doi: 10.1016/j.ejphar.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 44.Russo I, Frangogiannis NG. Diabetes-associated cardiac fibrosis: cellular effectors, molecular mechanisms and therapeutic opportunities. J Mol Cell Cardiol. 2016;90:84–93. doi: 10.1016/j.yjmcc.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jia G, Whaley-Connell A, Sowers JR. Diabetic cardiomyopathy: a hyperglycaemia- and insulin-resistance-induced heart disease. Diabetologia. 2018;61:21–8. doi: 10.1007/s00125-017-4390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Appari M, Breitbart A, Brandes F, Szaroszyk M, Froese N, Korf-Klingebiel M, et al. C1q-TNF-related protein-9 promotes cardiac hypertrophy and failure. Circ Res. 2017;120:66–77. doi: 10.1161/CIRCRESAHA.116.309398. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Yi W, Yuan Y, Lau WB, Yi D, Wang X, et al. C1q/tumor necrosis factor-related protein-9, a novel adipocyte-derived cytokine, attenuates adverse remodeling in the ischemic mouse heart via protein kinase A activation. Circulation. 2013;128:S113–20. doi: 10.1161/CIRCULATIONAHA.112.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guo Z. The modification of natural products for medical use. Acta Pharm Sin B. 2017;7:119–36.. doi: 10.1016/j.apsb.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 50.Yan L, Wei X, Tang QZ, Feng J, Zhang Y, Liu C, et al. Cardiac-specific mindin overexpression attenuates cardiac hypertrophy via blocking AKT/GSK3beta and TGF-beta1-Smad signalling. Cardiovasc Res. 2011;92:85–94. doi: 10.1093/cvr/cvr159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–28. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A TRPC6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–15. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou J, Ma W, Wang X, Liu H, Miao Y, Wang J, et al. Matrine suppresses reactive oxygen species (ROS)-mediated MKKs/p38-induced inflammation in oxidized low-density lipoprotein (ox-LDL)-stimulated macrophages. Med Sci Monit. 2019;25:4130–6. doi: 10.12659/MSM.917151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou X, Liao WJ, Liao JM, Liao P, Lu H. Ribosomal proteins: functions beyond the ribosome. J Mol Cell Biol. 2015;7:92–104. doi: 10.1093/jmcb/mjv014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.