Abstract
Aberrant inflammasome activation contributes to the pathogenesis of various human diseases, including atherosclerosis, gout, and metabolic disorders. Elucidation of the underlying mechanism involved in the negative regulation of the inflammasome is important for developing new therapeutic targets for these diseases. Here, we showed that Raf kinase inhibitor protein (RKIP) negatively regulates the activation of the NLRP1, NLRP3, and NLRC4 inflammasomes. RKIP deficiency enhanced caspase-1 activation and IL-1β secretion via NLRP1, NLRP3, and NLRC4 inflammasome activation in primary macrophages. The overexpression of RKIP in THP-1 cells inhibited NLRP1, NLRP3, and NLRC4 inflammasome activation. RKIP-deficient mice showed increased sensitivity to Alum-induced peritonitis and Salmonella typhimurium-induced inflammation, indicating that RKIP inhibits NLRP3 and NLRC4 inflammasome activation in vivo. Mechanistically, RKIP directly binds to apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC) and competes with NLRP1, NLRP3, or NLRC4 to interact with ASC, thus interrupting inflammasome assembly and activation. The depletion of RKIP aggravated inflammasome-related diseases such as monosodium urate (MSU)-induced gouty arthritis and high-fat diet (HFD)-induced metabolic disorders. Furthermore, the expression of RKIP was substantially downregulated in patients with gouty arthritis or type 2 diabetes (T2D) compared to healthy controls. Collectively, our findings suggest that RKIP negatively regulates NLRP1, NLRP3, and NLRC4 inflammasome activation and is a potential therapeutic target for the treatment of inflammasome-related diseases.
Keywords: Raf kinase inhibitor protein (RKIP), Inflammasome, Gouty arthritis, HFD-induced metabolic disorders
Subject terms: Inflammasome, Chronic inflammation
Introduction
Inflammasomes are intracellular protein complexes that are important for host defense against infection, as well as various inflammatory and autoimmune disorders.1 During inflammasome assembly, pro-caspase-1 and sensor proteins are linked by an adapter protein known as apoptosis-associated speck-like protein containing a caspase-recruitment domain (ASC), followed by the cleavage of pro-caspase-1 and the maturation of proinflammatory cytokines such as IL-1β or IL-18.2 The sensor proteins include nucleotide-binding oligomerization domain, leucine-rich repeat and pyrin domain-containing protein family members (NLR), such as NLRP1, NLRP3 (also known as NALP3), and NLRC4 (also known as IPAF or CARD12), or absent in melanoma 2 (AIM2).3 The NLRP3 inflammasome recognizes endogenous and exogenous danger signals, including adenosine triphosphate (ATP), monosodium urate crystals (MSU), nigericin (bacterial toxin), and Alum. The NLRP1 inflammasome is stimulated by lethal toxin and muramyl dipeptide (MDP), the AIM2 inflammasome senses double-stranded DNA (dsDNA), and the NLRC4 inflammasome is stimulated by intracellular flagellin.4 In addition, these canonical inflammasomes are complemented by a noncanonical inflammasome that activates caspases-4, -5, or -11 in response to intracellular lipopolysaccharide (LPS).5,6 Typically, activation of the inflammasome requires two signals, referred to as priming and activation signals.7
Adequate activation of inflammasomes is important for the elimination of pathogens. For instance, the AIM2 inflammasome is critical for innate immunity to Francisella tularensis.8 However, inflammasome hyperactivation is responsible for diverse inflammatory diseases.4,9 Aberrant activation of the NLRP3 inflammasome is responsible for the progression of several human diseases, including septic shock,10 atherosclerosis,11 gout,12 Alzheimer’s disease,13 and type 2 diabetes (T2D).14 A series of NLRP3 inflammasome inhibitors, such as MCC950, β-hydroxybutyrate, and NSAIDs,15–17 have been demonstrated to have beneficial effects on NLRP3 inflammasome-dependent diseases. Thus, understanding the underlying mechanism involved in the negative regulation of inflammasome activation will help develop new therapeutic targets for these diseases.
Raf kinase inhibitor protein (RKIP) is the prototypical member of the phosphatidylethanolamine-binding protein family.18,19 It was originally identified as a Raf1-binding protein that suppresses the phosphorylation and activation of MEK.20 Emerging evidence indicates that RKIP is involved in the regulation of many signaling cascades, such as Raf/MEK/ERK,20,21 G-protein-coupled receptors (GPCR),22 glycogen synthase kinase-3B (GSK3B)23, and NF-κB signal transduction cascades.24 Therefore, RKIP plays important roles in various physiological processes, including cell differentiation, migration, cell cycle, and apoptosis.25,26 Recently, we demonstrated that RKIP contributes to the progression of inflammatory bowel disease (IBD),27 innate immune responses,28,29 mast cell-mediated anaphylactic responses and allergic asthma,30 and autoimmune diseases.31 However, the regulatory role of RKIP in inflammasome activation remains unclear.
In this study, we showed that RKIP negatively regulates the activation of the NLRP1, NLRP3, and NLRC4 inflammasomes but not the AIM2 inflammasome. Mechanistically, RKIP directly binds to ASC and thereby prevents the interaction of ASC with NLRP1, NLRP3, or NLRC4 to inhibit inflammasome assembly and activation. Rkip-deficient mice developed more severe peritonitis and Salmonella typhimurium-induced inflammation than wild-type (WT) mice. Furthermore, RKIP deficiency increased the sensitivity of mice to NLRP3 inflammasome-related diseases, including gouty arthritis and T2D. Collectively, our results provide new insight into the physiological role of RKIP in inflammatory diseases.
Materials and methods
Mice
Rkip-knockout (Rkip-KO) mice were provided by Professor John Sedivy of Brown University, and Rkip floxed (Rkipf/f) mice were provided by Professor Kam C. Yeung from the University of Toledo Health Sciences Campus. Rkip-KO mice and their littermates with a C57BL/6 background were used in this study. Mice were genotyped by PCR analysis of isolated tail DNA. The following primers were used: Rkip -KO sense (5′-GAGCCCTGGCCGGTCTCCCTTGTCCCAAACTTT-3′), Rkip -WT antisense (5′-CACAAAACCAATCTTAAAGAGCCA-3′), and Rkip-KO antisense (5′-CCAAAAGGGTCTTTGAGCACCAGAGGACATCCG-3′). For generation of Rkip myeloid KO mice, Rkip f/f mice were crossed to LysM-Cre mice (B6.129P2- Lyz2tm1(cre)Ifo/J; stock no. 04781) from the Jackson Laboratory for at least five generations before starting in vivo experiments. The mice were maintained and bred in specific pathogen-free conditions. Animal care and experiments were undertaken in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals with approval from the Scientific Investigation Board of Zhejiang University, Hangzhou.
Human subjects
The peripheral blood samples of healthy controls and patients with T2D and gouty arthritis were obtained from the Second Affiliated Hospital, School of Medicine, Zhejiang University. The omental adipose tissue of OB/D patients was collected during bariatric surgery, and nonobese (NO) samples were collected during explorative laparoscopically assisted colonoscopy at the Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. We obtained written informed consent from the patients and the donors or from their families before enrollment. The study was performed with approval from the Ethics Committee of the Second Affiliated Hospital, School of Medicine, Zhejiang University and the Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University.
Reagents, antibodies, and plasmids
ATP and nigericin (Cat:tlrl-nig) were purchased from InvivoGen (San Diego, CA, USA). Imject Alum was purchased from Thermo Scientific (Rockford, IL, USA). LPS and anti-Flag mouse magnetic (M2) beads were purchased from Sigma-Aldrich. Antibodies against NLRP3, caspase-1 p20, and ASC were purchased from Adipogen International (San Diego, CA, USA). Antibodies against GFP and Flag were purchased from Abclonal. Antibodies against RKIP and GAPDH were purchased from Diagbio. Anti-CD11b-Fitc was purchased from BioLegend, and anti-Gr-1-APC and anti-Ly6C-PE were purchased from Sungene Biotech. PEI was purchased from Polyscience. Lipofectamine RNAiMAX was purchased from Invitrogen. RKIP and ASC were amplified from Jurkat cells by PCR and ligated into pcDNA3.1-Flag-His, pcDNA3.1-Myc, or pcDNA3.1-HA to construct the expression plasmids, and Flag-tagged NLRP1, AIM2, NLRP3, NLRC4, and pro-caspase-1 were a gift from Dr. Rongbin Zhou (University of Science and Technology of China).
Cell culture
Peritoneal macrophages (PMs) and THP-1 cells were cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. We cultured 293T cells in DMEM supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin. THP-1 stable expression cells were prepared as follows: pHAGE-fEF1a-IRES-ZsGreen, pHAGE-fEF1a-IRES-ZsGreen-Flag-RKIP, and packaging plasmids were transfected into 293T cells for viral packaging using the calcium phosphate transfection method, and then, the virus was harvested to infect THP-1 cells to select stable cell lines. All cells were maintained at 37 °C in a humidified atmosphere of 5% CO2.
ASC oligomerization assay
PMs were lysed with cell lysis buffer (Cell Signaling Technology) containing protease and phosphatase inhibitors (Sigma-Aldrich) for 20 min and then centrifuged at 6000g for 10 min at 4 °C. The pellets were washed with ice-cold PBS, suspended in 300 µl of cell lysis buffer containing 2 mM disuccinimidyl suberate (Pierce) and incubated for 30 min at 37 °C. The samples were centrifuged at 6000g for 10 min, and the crosslinked pellets were analyzed by immunoblotting.
RNA isolation and real-time quantitative PCR
Total RNA from cells or peripheral blood was isolated by using TRIzol reagent (TaKaRa) according to the manufacturer’s instructions, and cDNA was synthesized using a cDNA synthesis kit (TaKaRa) according to the manufacturer’s instructions. The generated cDNA was amplified by using appropriate primers and SYBR Green mix (Yeasen).
Enzyme-linked immunosorbent assay (ELISA)
Cell culture supernatants or tissue culture supernatants were collected during the experiments, and the levels of IL-1β were measured by ELISA kits (Invitrogen) following the manufacturer’s instructions.
Lactate dehydrogenase (LDH) assay
The LDH level in the culture medium was determined by an LDH Cytotoxicity Assay Kit (Promega) according to the manufacturer’s instructions.
SiRNA-mediated interference
THP-1 cells or 293T cells were transfected with specific siRNA or nontargeting control siRNA by using Lipofectamine RNAi MAX (Invitrogen) according to the manufacturer’s instructions. The following siRNA oligonucleotide sequences were used: RKIPsiRNA (5′-TGGTCAACATGAAGGGTAA-3′).
Immunoprecipitation
The cells were harvested, washed with ice-cold PBS twice, and lysed in an immunoprecipitation buffer (50 mM Tris, pH 7.4, 150 mM NaCl, and 0.5% (vol/vol) Nonidet P-40, 1 mM EDTA) containing a protease inhibitor cocktail (Roche). Then, cell extracts were obtained after centrifugation at 13,000g for 15 min. The cell extracts were incubated with the indicated antibody together with protein G magnetic beads (Bio-Rad) or anti-Flag (M2)-agarose for 3 h at 4 °C. The immunoprecipitates were washed three times with immunoprecipitation buffer and then subjected to further immunoblotting analysis.
Immunoblotting analysis
Cell and tissue proteins were extracted with cell lysis buffer (Cell Signaling Technology) containing protease and phosphatase inhibitors (Sigma-Aldrich), quantitated using a BCA protein assay kit (Thermo Scientific) and subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad). The samples were transferred to a nitrocellulose membrane (Bio-Rad), blocked with 5% nonfat milk, incubated with appropriate primary antibodies and subsequently incubated with fluorescent secondary antibodies. The protein bands were imaged using a LiCor Odyssey scanner and analyzed by using Odyssey 3.0 analytical software (LiCor).
Immunofluorescence
Cultured cells were plated on coverslips, fixed with 4% paraformaldehyde for 10 min, permeabilized with 1% Triton X-100 for 10 min, blocked with 5% bovine serum albumin for 1 h, incubated with appropriate primary antibodies, and subsequently further incubated with appropriate secondary antibodies. The nuclei were also stained with DAPI. All immunofluorescence images were taken by using an Olympus IX81-FV1000 confocal microscope.
Flow cytometry
Single-cell suspensions of PECs were prepared, and then, the cells were stained with antibodies against surface markers. The PECs were assessed with the markers Gr-1 and CD11b for analysis of the recruitment of neutrophils and Ly6C and CD11b for monocytes. Data were acquired on a BD LSRFortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software.
Pulldown assay
The fusion protein GST-RKIP was expressed in the Escherichia coli BL21 strain, the fusion protein Flag-ASC was expressed in 293T cells, and both were purified according to standard protocols. For the GST pulldown assay, ~1 µg GST or GST-RKIP protein was mixed with 20 µl of precleared anti-Flag beads (M2 beads) in 500 µl of reaction buffer, and 1 µg Flag-ASC was subsequently added and incubated at 4 °C for 3 h with gentle mixing. The beads were then washed three times with cell lysis buffer and subjected to immunoblotting.
Histological analyses
Adipose tissues were fixed in 4% formaldehyde and embedded in paraffin. The formalin-fixed paraffin-embedded tissues were then cut into sections. The sections of adipose tissue were subjected to hematoxylin and eosin (H&E) staining to measure the pathological changes. For immunohistochemistry (IHC), the sections of adipose tissue were incubated with RKIP antibody to measure the expression level of RKIP.
In vivo peritonitis and gouty arthritis
WT and Rkip-KO mice or Rkipf/f and Rkipf/f Lysm-Cre mice were injected intraperitoneally with Alum (50 mg/kg body weight) (Thermo). The mice were sacrificed 12 h later, and the peritoneal cavities were washed with 10 ml of PBS to analyze the inflammatory cell subsets. Peritoneal exudate cells (PECs) were collected for analysis by flow cytometry and ELISA.
For induction of ankle inflammation, mice were injected with 0.5 mg MSU (dissolved in 20 μL of PBS) in the ankle to induce inflammation. Ankle swelling was measured at different time points. After 24 h, the ankles were isolated and cultured for IL-1β analysis.
Glucose tolerance tests (GTTs) and insulin tolerance tests (ITTs)
WT and Rkip-KO mice were fed a low-fat diet (LFD: D12450B, Research Diet) or a high-fat diet (HFD:D12492 Research Diet) for 24 weeks. For glucose tolerance tests (GTTs), WT and Rkip-KO mice were fasted for 16 h and then injected intraperitoneally with glucose (1.5 g/kg body weight). Blood glucose was measured with a glucometer and strips before injection (0 min) or at 30, 60, 90, or 120 min post injection. For insulin tolerance tests (ITTs), the WT and Rkip-KO mice were fasted for 4 h and then injected intraperitoneally with insulin (1 U/kg body weight) (Novolin). Blood glucose was measured before injection (0 min) or at 30, 60, 90 or 120 min post injection.
Statistical analysis
Statistical analysis was performed with Prism v5.0 (GraphPad Software). The results are expressed as the mean ± SEM. Statistical significance between two experimental groups was assessed using Student’s t test. Mouse survival curves and statistics were analyzed with the Mantel–Cox test. A value of p < 0.05 was considered statistically significant.
Results
RKIP inhibits the activation of the NLRP1, NLRP3, and NLRC4 inflammasomes
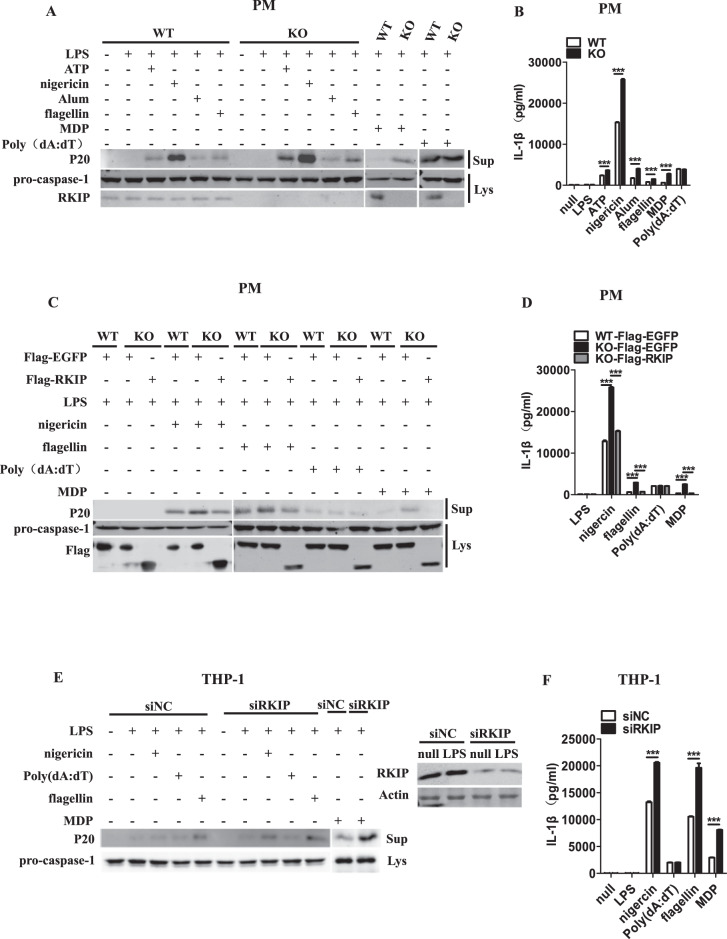
PMs from WT and Rkip-KO mice were primed with LPS and then stimulated with activators of the NLRP3 inflammasome, such as ATP, nigericin and Alum, or transfected with flagellin (an NLRC4 inflammasome activator), poly(dA:dT) (an AIM2 inflammasome activator) or MDP (an NLRP1 inflammasome activator) to activate the inflammasomes. As shown in Fig. 1a, b, PMs from the WT mice showed strong caspase-1 cleavage, as well as overproduction of IL-1β in response to the activation of the NLRP1, NLRP3, and NLRC4 inflammasomes. However, this response was substantially exaggerated in the PMs from the Rkip-KO mice, but RKIP did not alter the LPS-induced mRNA levels of pro-IL-1β (Supplementary Fig. 1A). Intriguingly, no difference was observed in AIM2 inflammasome-dependent caspase-1 cleavage and IL-1β secretion between the WT and Rkip-KO PMs. The Rkip-KO PMs were transfected with Flag-RKIP, and inflammasome activation was induced. As shown in Fig. 1c, d, compared with the WT PMs, the Rkip-KO PMs again demonstrated enhanced NLRP1, NLRP3, and NLRC4 inflammasome activation, as indicated by a notable increase in caspase-1 cleavage and IL-1β secretion, whereas the overexpression of RKIP restored the levels of these proteins to levels comparable with those of the WT PMs. No difference was observed in AIM2 inflammasome activation.
Fig. 1.
RKIP inhibits the activation of the NLRP3, NLRC4 and NLRP1 inflammasomes but not the AIM2 inflammasome. a, b A total of 1 × 106 wild-type (WT) or Rkip-knockout (KO) peritoneal macrophages (PMs) were primed with LPS (1 µg/ml) for 4 h and then treated with ATP (5 mM), nigericin (Ni) (20 µM), Alum (300 µg/ml), flagellin (1 µg/ml), poly(dA:dT) (5 µg/ml), and MDP (500 µg/ml) for the indicated times. Immunoblotting analysis of the supernatants (Sup) and cell extracts (Lys) (a) and ELISAs of the supernatants (Sup) were performed to assess IL-1β release (b). c, d WT or Rkip-KO PMs (1 × 106) were transfected with Flag-EGFP or Flag-RKIP. Forty-eight hours later, the transfectants were primed with LPS (1 µg/ml) and then treated with nigericin (Ni) (20 µM), flagellin (1 µg/ml), poly(dA:dT) (5 µg/ml) and MDP (500 µg/ml) for the indicated times. Immunoblotting analysis of the supernatants (Sup) and cell extracts (Lys) (c) and ELISAs of the supernatants (Sup) were performed to assess IL-1β release (d). e, f THP-1 cells (1 × 106) were differentiated with 100 nM PMA overnight in 12-well plates, transfected with a specific siRNA for RKIP, primed with LPS (1 µg/ml) and treated with nigericin (Ni) (20 µM), poly(dA:dT) (5 µg/ml), flagellin (1 µg/ml) and MDP (500 µg/ml) for the indicated times. Immunoblotting analysis of the supernatants (Sup) and cell extracts (Lys) (e) and ELISAs of the supernatants (Sup) were performed to assess IL-1β release (f). Data are the mean ± SEM (b, d, and f) and representative of three independent experiments. Student’s t test was used for statistical calculation. *p < 0.05; **p < 0.01; ***p < 0.001
A human monocyte-like cell line (THP-1) was used to elucidate the effect of RKIP on inflammasome activation in human cells. We knocked down RKIP in THP-1 cells via the transfection of RKIP-specific siRNA. These cells were primed with LPS and then treated with different stimuli to activate the inflammasomes. Compared with those in the control cells, the cleavage of pro-caspase-1 and secretion of IL-1β induced by NLRP1, NLRP3, and NLRC4 inflammasome activation were significantly increased in the RKIP-knockdown THP-1 cells (Fig. 1e, f). In contrast, RKIP knockdown had no effect on the activation of the AIM2 inflammasome (Fig. 1e, f). We also generated THP-1 cells stably expressing Flag-RKIP and analyzed caspase-1 cleavage and IL-1β secretion in response to NLRP1, AIM2, NLRP3, and NLRC4 inflammasome activation. RKIP overexpression significantly reduced the activation of the NLRP1, NLRP3, and NLRC4 inflammasomes but not the AIM2 inflammasome (Supplementary Fig. 1B, C). We transfected 293T cells with plasmids encoding NLRP3, ASC, pro-caspase-1, and pro-IL-1β to reconstitute the functional NLRP3 inflammasome. As expected, the overexpression of RKIP inhibited the activation of the NLRP3 inflammasome, resulting in reduced cleavage of pro-IL-1β (Supplementary Fig. 1D), whereas the knockdown of endogenous RKIP promoted NLRP3 inflammasome activation, and this activation was rescued to a level comparable with that of the control group by RKIP overexpression (Supplementary Fig. 1E). Whether RKIP regulates the activation of the noncanonical NLRP3 inflammasome was also explored. The Pam3CSK4-primed PMs were transfected with LPS, as shown by immunoblotting and ELISAs, and RKIP had no effects on noncanonical NLRP3 inflammasome activation (Supplementary Fig. 1F, G). Taken together, these results indicate that RKIP inhibits NLRP1, NLRP3, and NLRC4 inflammasome activation but not AIM2 inflammasome activation.
RKIP deficiency increases the formation of ASC specks and promotes caspase-1-dependent pyroptosis
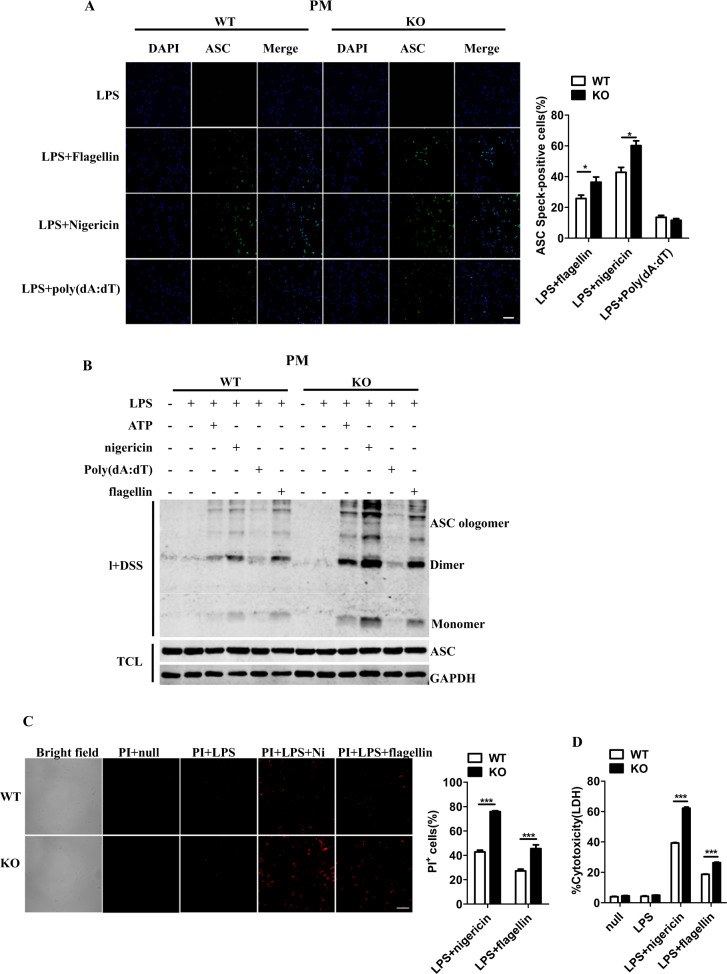
The formation of ASC “specks,” which results from the oligomerization of ASC molecules into a large protein complex, is a critical step for subsequent caspase-1 activation.3 As shown in Fig. 2a, RKIP deficiency enhanced the formation of ASC specks in PMs in response to the activation of the NLRP3 and NLRC4 inflammasomes but not the AIM2 inflammasome. We further treated the cell lysates with a nonreversible crosslinking agent and subjected the samples to ASC immunoblotting to assess oligomerization. As shown in Fig. 2b, ASC speck formation was detectable in the WT PMs after inflammasome activation and was markedly increased in the Rkip-KO PMs under NLRP3 and NLRC4 inflammasome activation conditions. In addition to causing ASC speck formation, the activation of inflammasomes results in pyroptosis.32 Significantly enhanced cell death was observed in the LPS- and nigericin- or flagellin-costimulated Rkip-KO PMs compared to the WT PMs, as shown by the positive propidium iodide (PI) staining (Fig. 2c), and the results were further confirmed by a LDH release assay (Fig. 2d). Therefore, RKIP deficiency promotes NLRP3 and NLRC4 inflammasome-dependent ASC oligomerization and pyroptosis.
Fig. 2.
RKIP deficiency increases the formation of ASC specks and promotes caspase-1-dependent pyroptosis. a LPS (1 µg/ml)-primed WT (1 × 105) and Rkip-KO (1 × 105) PMs were treated with flagellin, nigericin (Ni) and poly(dA:dT), and immunofluorescence analysis of ASCs and then quantification of ASC clusters (5–10 images per sample) were performed. (Scale bars, 100 µm). b LPS (1 µg/ml)-primed WT (1 × 106) and Rkip-KO (1 × 106) PMs were stimulated with ATP, nigericin (Ni), poly(dA:dT) and flagellin, and an ASC oligomerization assay was then performed. c Live cell microscopy images of propidium iodide (PI) staining of WT (1 × 105) and Rkip-KO (1 × 105) PMs left untreated or primed with LPS and stimulated with nigericin (Ni) or flagellin on the left (scale bars, 100 µm). The average percentage of PI+ cells of six images (630 µm × 630 µm) is shown on the right. d Assay for LDH release in the culture supernatants of the LPS-primed WT (1 × 106) and Rkip-KO (1 × 106) PMs treated with nigericin (Ni) or flagellin. Data are the mean ± SEM (a, c, d) and representative of three independent experiments. Student’s t test was used for statistical calculation. *p < 0.05; **p < 0.01, ***p < 0.001
RKIP directly interacts with ASC to interfere with NLRP3 or NLRC4 inflammasome assembly and activation
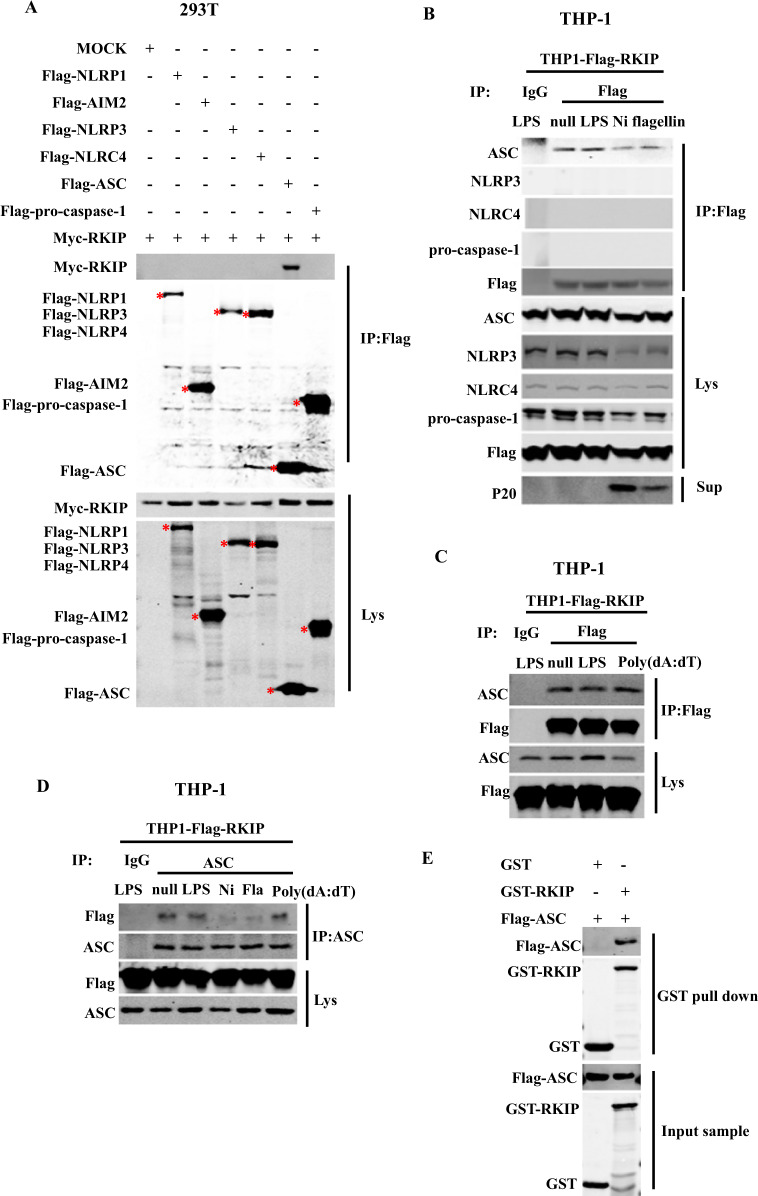
Given that our data suggested that RKIP plays a critical role in the activation of inflammasomes, we next aimed to explore which component(s) of the inflammasomes interact with RKIP. Myc-RKIP was co-overexpressed in 293T cells with Flag-NLRP1, Flag-AIM2, Flag-NLRP3, Flag-NLRC4, Flag-pro-caspase-1, or Flag-ASC, and the cell lysates were incubated with M2 beads. We found that RKIP coimmunoprecipitated (co-IP) with ASC but not NLRP1, NLRP3, NLRC4, AIM2, or pro-caspase-1 (Fig. 3a). Since the commercially available anti-RKIP antibody could not efficiently immunoprecipitate endogenous RKIP, RKIP was immunoprecipitated with M2 beads from the cell lysates of the THP-1 cells stably expressing Flag-RKIP. As shown in Fig. 3b, RKIP physically associates with ASC, and this association was dramatically reduced after NLRP3 or NLRC4 inflammasome activation but not AIM2 inflammasome activation (Fig. 3c). NLRP3 or NLRC4 inflammasome activation, but not AIM2 inflammasome activation, might induce the posttranslational modification of RKIP, which causes the disassociation of RKIP from ASC. Similar results were observed using the ASC antibody under the same experimental conditions (Fig. 3d). To determine whether RKIP interacts with ASC directly, we purified Flag-ASC protein from the ASC-overexpressing 293T cells and incubated it with recombinant GST-RKIP protein. Then, GST beads were added for an in vitro pulldown assay. As shown in Fig. 3e, RKIP directly interacts with ASC. These data demonstrate, for the first time, that RKIP binds to ASC and suggest that this association is important for the inhibitory effect of RKIP on inflammasome activation.
Fig. 3.
RKIP directly interacts with ASC to inhibit inflammasome activation. a Myc-RKIP was cotransfected with Mock, NLRP1, AIM2, NLRP3, NLRC4, ASC, or pro-caspase-1 in 293T cells (4 × 105). Cell lysates were immunoprecipitated with anti-Flag beads (M2 beads) after 48 h, and then, immunoblotting analysis of the samples was performed as indicated. The star indicates the target bands. A total of 8 × 106 THP-1 stable transfectants (which stably express Flag-RKIP) were differentiated with 100 nM PMA overnight in 6 cm dishes and then primed with LPS and treated with nigericin (Ni) and flagellin (b) or poly(dA:dT) (c). Whole cell lysates were immunoprecipitated with anti-Flag or control IgG, followed by immunoblotting analysis as indicated. d A total of 8 × 106 THP-1 stable transfectants (which stably express Flag-RKIP) were differentiated with 100 nM PMA overnight in 6 cm dishes, primed with LPS and treated with nigericin (Ni), flagellin and poly(dA:dT). Whole cell lysates were immunoprecipitated with anti-ASC antibody or control IgG, followed by immunoblotting analysis as indicated. e GST-tagged fusion proteins (GST-RKIP or GST-null) were mixed with Flag-ASC, and GST beads were added to pulldown buffer for the in vitro pulldown assay. Pulldown and input samples were immunoblotted with anti-GST or anti-Flag antibody. Data are representative of two independent experiments
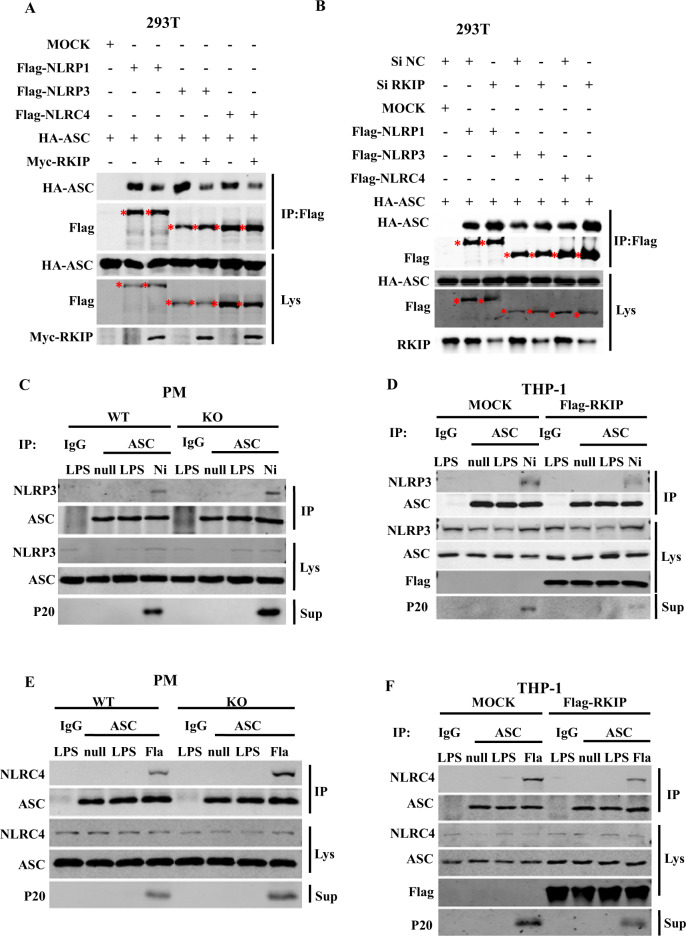
The association of ASC with NLRP1, AIM2, NLRP3, or NLRC4 was shown to be essential for the assembly and activation of inflammasomes. We then examined whether RKIP inhibits the recruitment of ASC to NLRP1, AIM2, NLRP3, or NLRC4. As shown in Fig. 4a, overexpression of RKIP inhibited the interaction between ASC and NLRP1, NLRP3, or NLRC4, whereas knockdown of RKIP enhanced these interactions in 293T cells (Fig. 4b). We further performed co-IP assays in PMs using an anti-ASC antibody. In the WT PMs, endogenous ASCs formed a complex with NLRP3 upon nigericin stimulation, and this interaction was substantially increased in the Rkip-KO PMs (Fig. 4c). In contrast, the nigericin-induced recruitment of ASC to NLRP3 was significantly attenuated in the THP-1 cells stably overexpressing Flag-RKIP compared to that in the control group (Fig. 4d). Similar results were observed after NLRC4 inflammasome activation (Fig. 4e, f); RKIP deficiency promoted the association of NLRC4 with ASC, whereas RKIP overexpression inhibited this association. Taken together, these results indicate that RKIP directly binds to ASC and interferes with the assembly and activation of the NLRP3 and NLRC4 inflammasomes in macrophages.
Fig. 4.
RKIP inhibits the interaction between ASC and NLRP3 or NLRC4 to interfere with the inflammasome. a HA-ASC was cotransfected with Mock, Flag-NLRP1, Flag-NLRP3, Flag-NLRC4 and Myc-RKIP into 293T cells (4 × 105) as indicated, and cell lysates were immunoprecipitated with anti-Flag beads (M2 beads) after 48 h, followed by immunoblotting analysis as indicated. The star indicates the target bands. b RKIP expression was knocked down in 293T cells (2 × 105) by using a specific siRNA, and then, the cells were cotransfected with HA-ASC and Mock, Flag-NLRP1, Flag-NLRP3 or Flag-NLRC4. Cell lysates were immunoprecipitated with anti-Flag beads (M2 beads) after 48 h, and the samples were analyzed by immunoblotting as indicated. The star indicates the targeted bands. c, e A total of 8 × 106 WT or Rkip-KO PMs were primed with LPS and treated with nigericin (Ni) (c) or flagellin (e). Cell lysates were immunoprecipitated (IP) with anti-ASC antibody, followed by immunoblotting with the indicated antibodies. d, f Mock or THP-1 stable transfectants (8 × 106), which stably express Flag-RKIP, were differentiated with 100 nM PMA overnight in 6 cm dishes, primed with LPS and treated with nigericin (Ni) (d) or flagellin (f). Then, the cell lysates were immunoprecipitated (IP) with anti-ASC antibody, followed by immunoblotting with the indicated antibodies. Data are representative of three independent experiments
RKIP deficiency promotes NLRP3 and NLRC4 inflammasome activation in vivo
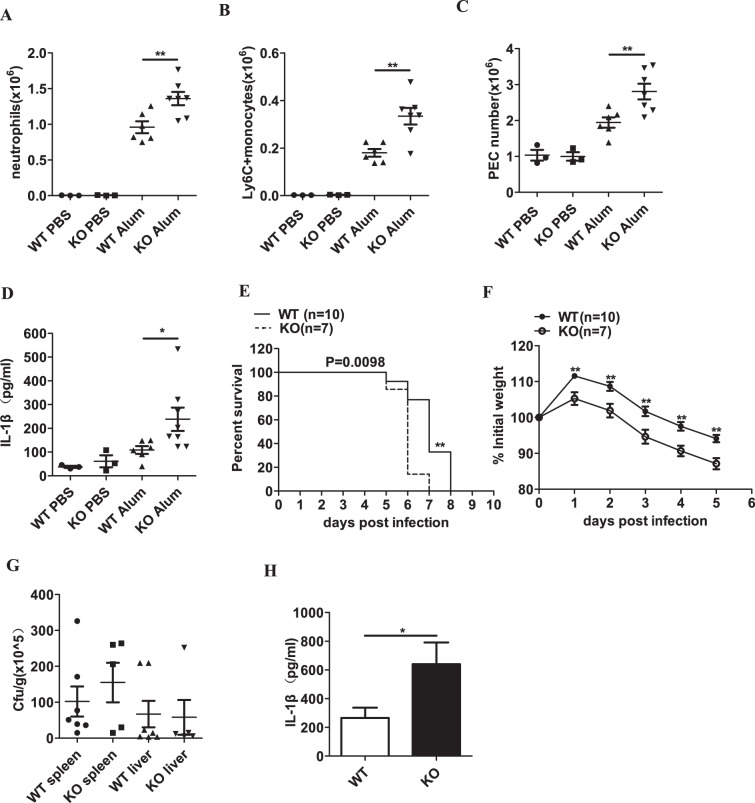
We next determined the effect of RKIP on inflammasome activation in vivo. The WT and Rkip-KO mice were challenged with Alum to induce peritonitis. Peritoneal lavage fluid (PLF) of the mice was collected 12 h after Alum injection. As shown in Fig. 5a, b, the intraperitoneal administration of Alum increased the total number of neutrophils and Ly6C+ monocytes in the peritoneal cavity. Correspondingly, the total number of PECs accumulated in the peritoneal cavity was also increased in the Rkip-KO mice compared to the WT mice (Fig. 5c). Moreover, we observed that compared with that in the PLF of the WT mice, the production of IL-1β was markedly increased in the PLF of the Rkip-KO mice (Fig. 5d). We further generated myeloid Rkip-KO mice by crossing Rkipf/f mice with Lysm-Cre transgenic mice; these mice had specifically targeted deletions in the myelomonocytic and osteoclast lineages. Compared to the Rkipf/f mice, the Rkipf/f Lysm-Cre mice showed enhanced immune cell infiltration and IL-1β secretion (Supplementary Fig. 2A–D). Caraffini et al. reported that knockdown of RKIP amplified the G-CSF-induced myelomonocytic differentiation of hematopoietic stem and progenitor cells. However, RKIP deletion was insufficient to increase the number of myelomonocytic cells,33 which is consistent with our finding that RKIP deficiency did not affect neutrophils and Ly6C+ monocytes in the peritoneal cavity. However, RKIP deletion significantly increased the Alum-induced IL-1β production and recruitment of neutrophils and Ly6C+ monocytes in the peritoneal cavity, indicating that RKIP deletion promotes Alum-induced NLRP3 inflammasome activation in vivo. To evaluate the role of RKIP in NLRC4 inflammasome activation in vivo, we infected the Rkip WT and KO mice intraperitoneally with a lethal dose of S. typhimurium. Significantly enhanced survival was observed in the WT mice compared to the Rkip-KO mice (Fig. 5e), which was further confirmed by using the myeloid Rkip-KO mice (Supplementary Fig. 2E). We then intraperitoneally infected mice with a sublethal dose of S. typhimurium and monitored the body weight changes during the course of infection. During S. typhimurium infection, RKIP deficiency exaggerated the weight loss caused by S. typhimurium (Fig. 5f). However, RKIP deficiency did not affect the bacterial loads in the spleens and livers (Fig. 5g), suggesting that the enhanced weight loss in the Rkip-KO mice was the result of excessive inflammation instead of bacterial proliferation. In addition, the PLF of the mice was collected 5 days after S. typhimurium infection, and the peritoneal production of IL-1β was significantly enhanced in the Rkip-KO mice (Fig. 5h). Collectively, these results indicate that RKIP plays a pivotal role in the activation of NLRP3 and NLRC4 inflammasomes in vivo.
Fig. 5.
RKIP deficiency promotes NLRP3 and NLRC4 inflammasome activation in vivo. a–d WT and Rkip-KO mice were intraperitoneally injected with Alum (50 mg/kg body weight) to induce peritonitis. After 12 h, the mice were sacrificed, and the peritoneal cavities were washed with 10 ml of ice-cold PBS. FACS analysis of neutrophils (a), Ly6C+ monocytes (b) or peritoneal exudate cells (PECs) (c) and ELISA of IL-1β (d) in the peritoneal lavage fluid (PLF) from the WT and Rkip-KO mice were performed (n = 3–7 mice per group). e Survival of the WT and Rkip-KO mice infected intraperitoneally with 5 × 102 CFU of log-phase S. typhimurium (n = 7–10 mice per group). f–h The WT and Rkip-KO mice were intraperitoneally infected with 1 × 102 CFU log-phase S. typhimurium, and weight loss was monitored until day 5 (f). At day 5 post-infection, the bacterial loads in the spleen and liver were determined (g), and PLF was collected for the detection of IL-1β (h) (n = 5–10 mice per group). Data are the mean ± SEM (a–d, g, h), and error bars represent SEM for (g) and are representative of two independent experiments. Student’s t test was used for statistical calculation. *p < 0.05, **p < 0.01, ***p < 0.001
RKIP has preventive effects in NLRP3 inflammasome-related diseases
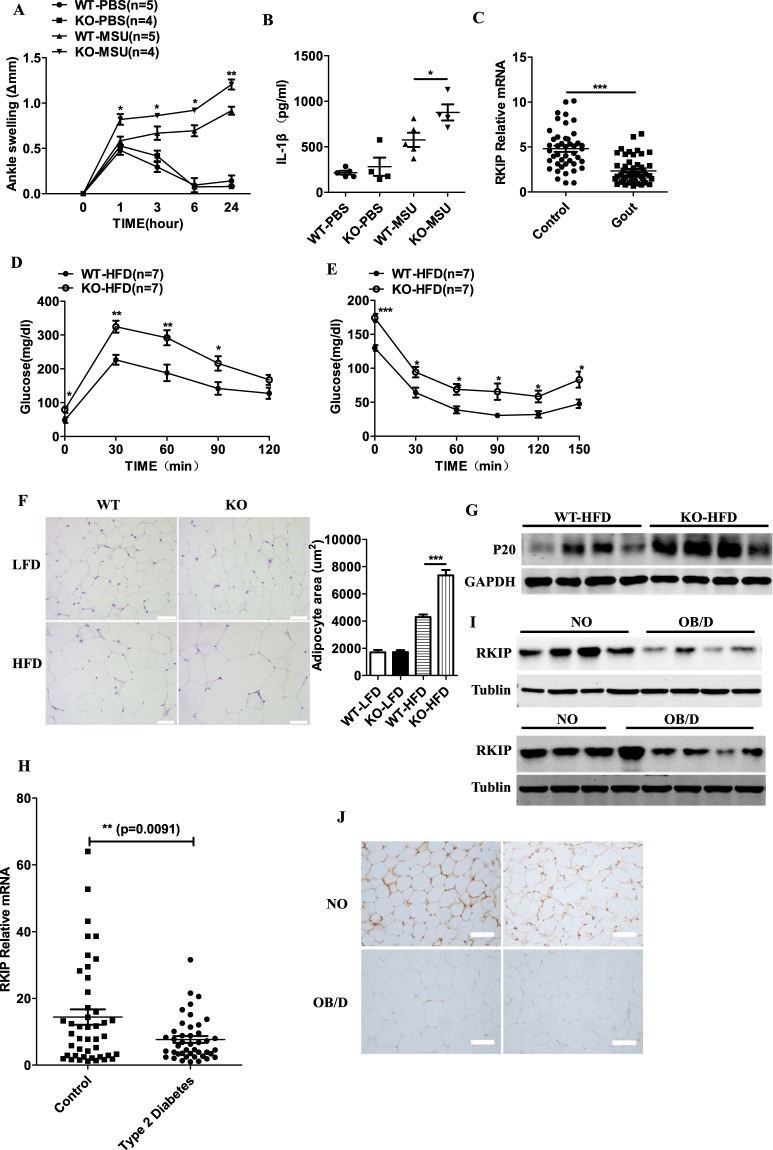
Gout is an inflammatory arthritis that is characterized by the deposition of MSU. MSU was shown to induce NLRP3-dependent caspase-1 cleavage and IL-1β production.12,34 Moreover, oridonin and tranilast, inhibitors of the NLRP3 inflammasome, attenuate the development of gouty arthritis.35,36 As shown in Supplementary Fig. 3A, B, RKIP deficiency enhanced MSU-induced caspase-1 cleavage and IL-1β secretion in macrophages. Mice were injected with MSU in the ankle, and proinflammatory cytokines in the periarticular tissue of the ankle were detected. The Rkip-deficient mice showed more severe acute ankle swelling than the WT mice (Fig. 6a), and RKIP deficiency strongly increased the secretion of IL-1β but not IL-6 in the ankle culture supernatants (Fig. 6b and Supplementary Fig. 3C). Compared to the Rkipf/f mice, the Rkipf/f Lysm-Cre mice showed enhanced MSU-induced acute ankle swelling and IL-1β but not IL-6 secretion (Supplementary Fig. 3D–F). We collected peripheral blood samples from 49 gouty arthritis patients and 44 healthy controls to detect the RKIP mRNA levels. Compared to that in the control group, the expression of RKIP was sharply decreased in the peripheral blood samples from the gouty arthritis group (Fig. 6c). Taken together, these results indicate that RKIP negatively regulates the development of inflammasome-associated gouty arthritis.
Fig. 6.
RKIP is involved in the development of NLRP3 inflammasome-related diseases. a, b The WT and Rkip-KO mice were injected with MSU (0.5 mg) in the ankle to induce inflammation. Time course of changes in MSU-induced ankle swelling of the WT and Rkip-KO mice was performed (a), and ELISAs of IL-1β (24 h later) (b) in ankle culture from the WT and Rkip-KO mice were performed (n = 4–5 mice per group). c Quantitative PCR analysis of RKIP expression in peripheral blood samples from gout patients (n = 49) and control subjects (n = 44). Glucose tolerance tests (GTTs) (d) and insulin tolerance tests (ITTs) (e) of the WT and Rkip-KO mice after HFD feeding for 24 weeks (n = 7 mice per group). f Hematoxylin and eosin (H&E) staining of VAT from the WT and Rkip-KO mice after LFD or HFD feeding for 24 weeks on the left (scale bars, 100 µm). The size of 20 randomly selected adipocytes in 10 randomly selected images was analyzed, and the quantification of adipocyte size is shown on the right. g Immunoblotting analysis of caspase-1 (P20) levels in the VAT of the LFD- or HFD-fed WT and Rkip-KO mice. h Quantitative PCR analysis of RKIP expression in peripheral blood samples of the control subjects (n = 44) and the T2D patients (n = 43). i Immunoblotting analysis of RKIP expression in omental adipose tissue samples from the NO subjects (n = 7) and the OB/D patients (n = 9). j Representative immunohistochemical staining for RKIP in omental adipose tissue samples from the NO subjects (n = 4) and the OB/D patients (n = 4) (scale bars, 100 µm). Error bars represent SEM for (a, d, e), data are the mean ± SEM (b, c, h) and are representative of two independent experiments. Student’s t test was used for statistical calculation. *p < 0.05; **p < 0.01; ***p < 0.001
NLRP3 inflammasome activation was shown to be involved in HFD-induced metabolic disorders.37 The WT and Rkip-KO mice were fed a HFD or a LFD for 24 weeks, and then, GTT and ITT were performed. As shown in Fig. 6d, e, glucose tolerance and insulin sensitivity were impaired in the HFD-fed Rkip-KO mice compared to the WT mice, although the GTTs and ITTs showed no differences between the LFD-fed Rkip WT and KO mice (Supplementary Fig. 3G, H). The HFD-fed Rkip-KO mice showed a significant increase in adipocyte size, but RKIP deficiency had no effect on adipocyte size in the LFD-fed mice (Fig. 6f). The expression of activated caspase-1 p20 in the visceral adipose tissue (VAT) from the HFD-fed KO mice was much higher than that in the VAT from the HFD-fed WT mice (Fig. 6g). These results indicate that RKIP inhibits HFD-induced inflammasome activation and metabolic disorders. We then evaluated the expression levels of RKIP in peripheral blood samples from type 2 diabetic (T2D) patients and healthy control individuals by real-time PCR. As shown in Fig. 6h, the mRNA levels of RKIP were significantly reduced in the peripheral blood samples from the patients with T2D compared to the healthy control individuals. Lower RKIP protein levels were found in omental adipose tissue samples from obese T2D (OB/D) patients than those of NO subjects (Fig. 6i). IHC staining further confirmed that RKIP expression in crown-like structures, which consist of dead or dying adipocytes surrounded by macrophages, was obviously reduced in the omental adipose tissue samples from the OB/D patients compared to the samples from the NO controls (Fig. 6j). Taken together, these results demonstrate that RKIP inhibits HFD-induced metabolic disorders in mice and shows preventive effects in NLRP3 inflammasome-related T2D in humans.
Discussion
Inflammasomes have been demonstrated to play a central role in facilitating host defense, initiating wound healing after tissue damage and maintaining metabolic health.7 However, inappropriate inflammasome responses also cause inflammatory disease via improper and excessive cytokine release.38 Thus, a well-balanced inflammasome response is essential for the maintenance of homeostasis. Several negative regulators, such as leucine-rich repeat Fli-I-interacting protein 2, A20, small heterodimer partner, aryl hydrocarbon receptor, and lipin-2, are known to attenuate the activation of inflammasomes through different mechanisms.39–43 Here, we identified RKIP as a negative regulator of the NLRP1, NLRP3 and NLRC4 inflammasomes in vitro and in vivo. RKIP directly associates with ASC to interfere with inflammasome assembly and inhibit inflammasome activation. RKIP deficiency increases the sensitivity of mice to NLRP3 inflammasome-related diseases.
RKIP is a well-established regulator in multiple signaling pathways.20–24 We recently found that RKIP is involved in inflammation-related diseases, including IBD,27 autoimmune diseases,31 and antivirus innate immune responses,28,29 indicating that RKIP is an important regulator of the immune response. Inflammasomes have been identified as an important class of cytoplasmic protein complexes that sense endogenous or exogenous pathogen-associated molecular patterns or danger-associated molecular patterns.3 Here, we found that the loss of RKIP triggered the hyperactivation of the NLRP1, NLRP3, and NLRC4 inflammasomes but not the AIM2 inflammasome in mouse PMs. Through the use of ectopic expression in PMs, we observed that the excessive activation of the NLRP1, NLRP3, and NLRC4 inflammasomes was inhibited by the overexpression of RKIP. In human monocyte-like THP-1 cells, enhanced caspase-1 activation and IL-1β secretion were observed by RKIP depletion, whereas the overexpression of RKIP inhibited these events in response to NLRP1, NLRP3, and NLRC4 but not AIM2 inflammasome activation. We previously proposed that the lack of RKIP does not modify the LPS-induced activation of MAPK pathways.29 Here, we found that RKIP was not involved in the LPS-induced expression of pro-IL-1β, which is an essential component of inflammasome activation. In addition, the absence of RKIP did not affect the activation of noncanonical inflammasomes stimulated by LPS transfection. RKIP deficiency enhanced NLRP3 and NLRC4 inflammasome-dependent ASC speck formation as well as pyroptosis. The anti-inflammasome properties of RKIP were further confirmed in vivo. RKIP deficiency promoted Alum-induced peritonitis and S. typhimurium-mediated IL-1β secretion. The Rkip-deficient mice developed more severe peritonitis and displayed increased lethality upon S. typhimurium infection than the control mice. Inflammation is a mechanism to fight infection, and the enhancement of inflammation may be corelated to a higher survival rate. However, excessive inflammation leads to enhanced body weight loss instead of the clearance of bacteria.44 RKIP deficiency increased weight loss and IL-1β production but did not affect the bacterial load after S. typhimurium infection in the mice. These results indicated that the lower survival rate in the Rkip-deficient mice might be related to increased inflammation. In addition, the absence of RKIP might result in the activation of other processes related to inflammation, which contributes to the lower survival rate.
Co-IP assays showed that RKIP interacts with ASC but not other inflammasome components in 293T cells. RKIP interacts with endogenous ASCs in macrophages. This association was reduced upon NLRP3 or NLRC4 inflammasome activation but not AIM2 inflammasome activation, and GST pulldown experiments confirmed that RKIP directly binds to ASC. It has been demonstrated that the interaction between NLRP1 and ASC, NLRP3 and ASC, or NLRC4 and ASC is an essential step for inflammasome assembly and activation.4 We further showed that RKIP overexpression inhibits the association of ASC with NLRP1, NLRP3, or NLRC4 and that RKIP silencing promotes these associations in 293T cells. The assembly of NLRP3 or NLRC4 inflammasomes in PMs was strongly enhanced in the Rkip-KO PMs but suppressed in the THP-1 cells stably overexpressing RKIP. Thus, our results suggest that RKIP directly targets ASC to block the assembly of inflammasomes by suppressing the association of NLRP3 or NLRC4 with ASC. RKIP was first identified as a Raf1 kinase inhibitor protein by interfering with the Raf1/MEK1 association.20 Lorenz et al. reported that protein kinase C phosphorylates RKIP at Ser153, which switches RKIP from Raf1 to GPCR kinase-2, a negative regulator of GPCRs.45 We recently found that the phosphorylation of RKIP S109 by TBK1 elevates the binding affinity between RKIP and TBK1 and promotes TBK1 activation. According to the crystal structure of RKIP, the phosphorylation of S109 might induce a conformational switch in RKIP.28 The AIM2 inflammasome is a non-NLR protein that assembles an inflammasome complex in response to stimulation by a variety of pathogen components,8 such as dsDNA. The mechanism by which RKIP affects NLRP3 or NLRC4 inflammasome activation but not AIM2 inflammasome activation might involve the posttranslational modification of RKIP that leads to the dissociation of RKIP from ASC, which needs to be further studied.
Previous studies have highlighted the importance of inflammasomes in disease pathology and homeostasis. A few reagents, such as a neutralizing IL-1β antibody (canakinumab), recombinant IL-1 receptor antagonist (anakinra) and soluble decoy IL-1 receptor (rilonacept), have been applied to treat inflammasome-mediated diseases.3 However, these reagents block only the downstream events of inflammasome activation, and exploring new inhibitors that could put inhibit inflammasome-dependent diseases is important for designing future therapies. Rkip deficiency in mice exaggerated inflammasome activation and the symptoms of gouty arthritis and HFD-induced metabolic disorders, suggesting that RKIP plays a beneficial role in inflammasome-related disease. A previous study demonstrated that the expression of RKIP is low in cells belonging to the myeloid lineage but higher in lymphoid cells.33 Thus, the blood composition of the patients may lead to limitations of the results that the decreased RKIP expression in the peripheral blood from patients with gouty arthritis or T2D compared to healthy control individuals.
We recently reported that RKIP serves as a pivotal mediator of the association between IL-17R and Act1 and promotes IL-17-induced inflammation.31 RKIP positively regulated human and mouse colitis by promoting intestinal epithelial cell (IEC) apoptosis.27 We further showed that RKIP in nonhematopoietic cells promotes IL-17-mediated autoimmune diseases and DSS-induced colitis in vivo. Here, we demonstrated that RKIP inhibits NLRP3 and NLRC4 inflammasome activation in macrophages and that RKIP deficiency in myeloid cells enhances the activation of inflammasomes in vivo, indicating that RKIP plays different roles in different cells. Taken together, our data reveal a previously unrecognized protective role for RKIP in inflammasome activation, and we showed RKIP interacts with ASC to interfere with NLRP1, NLRP3, or NLRC4 inflammasome assembly and activation. Moreover, RKIP-deficient mice developed more severe symptoms of gouty arthritis and HFD-induced metabolic disorders than control mice. Considering the reduced expression of RKIP in gouty arthritis and T2D, RKIP might be a potential target for the treatment of inflammasome-related diseases.
Supplementary information
The inhibitor effect of RKIP on inflammasome activation and inflammasome-dependent diseases
Acknowledgements
We thank Professor John Sedivy for the Rkip-knockout mice, Professor Kam C. Yeung for the Rkipf/f mice and Dr Rongbin Zhou for the plasmids. This work was supported by the National Natural Science Foundation of China (81972733) and the Natural Science Foundation of Zhejiang Province (LY19H160048).
Author contributions
X.W. and H.Y. designed the research; Q.Q., H.L., J.S., and Y.J. performed the research; X.W. and Q.Q. analyzed the data and wrote the paper.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Qiang Qin, Huan Liu
Contributor Information
Hong Yu, Email: yuhong-srrsh@hotmail.com.
Xiaojian Wang, Email: wangxiaojian@cad.zju.edu.cn.
Supplementary information
The online version of this article (10.1038/s41423-020-00525-3) contains supplementary material.
References
- 1.Gross O, Thomas CJ, Guarda G, Tschopp J. The inflammasome: an integrated view. Immunol. Rev. 2011;243:136–151. doi: 10.1111/j.1600-065X.2011.01046.x. [DOI] [PubMed] [Google Scholar]
- 2.Misawa T, et al. Microtubule-driven spatial arrangement of mitochondria promotes activation of the NLRP3 inflammasome. Nat. Immunol. 2013;14:454. doi: 10.1038/ni.2550. [DOI] [PubMed] [Google Scholar]
- 3.Guo HT, Callaway JB, Ting JPY. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis BK, Wen HT, Ting JPY. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–U146. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 6.Kayagaki N, et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 7.Khare S, Luc N, Dorfleutner A, Stehlik C. Inflammasomes and their activation. Crit. Rev. Immunol. 2010;30:463–487. doi: 10.1615/CritRevImmunol.v30.i5.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandes-Alnemri T, et al. The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 2010;11:385–394. doi: 10.1038/ni.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011;32:373–379. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan YQ, et al. Dopamine controls systemic inflammation through inhibition of NLRP3 inflammasome. Cell. 2015;160:62–73. doi: 10.1016/j.cell.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 11.Duewell P, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–U1357. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 13.Heneka MT, et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masters SL, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1 beta in type 2 diabetes. Nat. Immunol. 2010;11:897–U1501. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coll RC, et al. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat. Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youm YH, et al. The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat. Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniels MJD, et al. Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat. Commun. 2016;7:12504. doi: 10.1038/ncomms12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernier I, Jolles P. Purification and characterization of a Basic 23 Kda cytosolic protein from bovine brain. Biochim. Biophys. Acta. 1984;790:174–181. doi: 10.1016/0167-4838(84)90221-8. [DOI] [PubMed] [Google Scholar]
- 19.Serre L, et al. Crystal structures of YBHB and YBCL from Escherichia coli, two bacterial homologues to a Raf kinase inhibitor protein. J. Mol. Biol. 2001;310:617–634. doi: 10.1006/jmbi.2001.4784. [DOI] [PubMed] [Google Scholar]
- 20.Yeung K, et al. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature. 1999;401:173–177. doi: 10.1038/43686. [DOI] [PubMed] [Google Scholar]
- 21.Trakul N, Menard RE, Schade GR, Qian ZH, Rosner MR. Raf kinase inhibitory protein regulates Raf-1 but not B-Raf kinase activation. J. Biol. Chem. 2005;280:24931–24940. doi: 10.1074/jbc.M413929200. [DOI] [PubMed] [Google Scholar]
- 22.Lorenz K, Lohse MJ, Quitterer U. Protein kinase C switches the Raf kinase inhibitor from Raf-1 to GRK-2. Nature. 2003;426:574–579. doi: 10.1038/nature02158. [DOI] [PubMed] [Google Scholar]
- 23.Al-Mulla F, et al. Raf kinase inhibitor protein RKIP enhances signaling by glycogen synthase kinase-3 beta. Cancer Res. 2011;71:1334–1343. doi: 10.1158/0008-5472.CAN-10-3102. [DOI] [PubMed] [Google Scholar]
- 24.Yeung KC, et al. Raf kinase inhibitor protein interacts with NF-kappa B-inducing kinase and TAK1 and inhibits NF-kappa 13 activation. Mol. Cell. Biol. 2001;21:7207–7217. doi: 10.1128/Mcb.21.21.7207-7217.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeng LC, Imamoto A, Rosner MR. Raf kinase inhibitory protein (RKIP): a physiological regulator and future therapeutic target. Expert Opin. Ther. Target. 2008;12:1275–1287. doi: 10.1517/14728222.12.10.1275. [DOI] [PubMed] [Google Scholar]
- 26.Al-Mulla F, Bitar MS, Taqi Z, Yeung KC. RKIP: much more than Raf kinase inhibitory protein. J. Cell Physiol. 2013;228:1688–1702. doi: 10.1002/jcp.24335. [DOI] [PubMed] [Google Scholar]
- 27.Lin WL, et al. Raf kinase inhibitor protein mediates intestinal epithelial cell apoptosis and promotes IBDs in humans and mice. Gut. 2017;66:597–610. doi: 10.1136/gutjnl-2015-310096. [DOI] [PubMed] [Google Scholar]
- 28.Gu M, et al. RKIP and TBK1 form a positive feedback loop to promote type I interferon production in innate immunity. EMBO J. 2016;35:2553–2565. doi: 10.15252/embj.201694060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai R, et al. Raf kinase inhibitor protein preferentially promotes TLR3-triggered signaling and inflammation. J. Immunol. 2017;198:4086–4095. doi: 10.4049/jimmunol.1601672. [DOI] [PubMed] [Google Scholar]
- 30.Lin WL, et al. Raf kinase inhibitor protein negatively regulates FceRI-mediated mast cell activation and allergic response. Proc. Natl Acad. Sci. USA. 2018;115:E9859–E9868. doi: 10.1073/pnas.1805474115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin WL, et al. RKIP mediates autoimmune inflammation by positively regulating IL-17R signaling. EMBO Rep. 2018;19:e44951. doi: 10.15252/embr.201744951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caraffini V, et al. Loss of RAF kinase inhibitor protein is involved in myelomonocytic differentiation an aggravates RAS-driven myeloid leukemogenesis. Haematologica. 2020;105:375–386. doi: 10.3324/haematol.2018.209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reber LL, et al. Contribution of mast cell-derived interleukin-1 beta to uric acid crystal-induced acute arthritis in mice. Arthritis Rheumatol. 2014;66:2881–2891. doi: 10.1002/art.38747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.He HB, et al. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat. Commun. 2018;9:2550. doi: 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Rong R. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. Eur. J. Immunol. 2019;49:1892–1892. doi: 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vandanmagsar B, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17:179–U214. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. doi: 10.1038/nature10759. [DOI] [PubMed] [Google Scholar]
- 39.Jin, J. et al. LRRFIP2 negatively regulates NLRP3 inflammasome activation in macrophages by promoting Flightless-I-mediated caspase-1 inhibition. Nat. Commun.4, 2075. 10.1038/Ncomms3075 (2013). [DOI] [PMC free article] [PubMed]
- 40.Vande Walle L, et al. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512:69–73. doi: 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang, C. S. et al. Small heterodimer partner interacts with NLRP3 and negatively regulates activation of the NLRP3 inflammasome. Nature Commun.6, 6115. 10.1038/Ncomms7115 (2015). [DOI] [PMC free article] [PubMed]
- 42.Huai, W. W. et al. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nature Commun.5, 4738. 10.1038/Ncomms5738 (2014). [DOI] [PubMed]
- 43.Lorden G, et al. Lipin-2 regulates NLRP3 inflammasome by affecting P2X(7) receptor activation. J. Exp. Med. 2017;214:511–528. doi: 10.1084/jem.20161452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao KR, et al. beta-arrestin1 is critical for the full activation of NLRP3 and NLRC4 inflammasomes. J. Immunol. 2015;194:1867–1873. doi: 10.4049/jimmunol.1401989. [DOI] [PubMed] [Google Scholar]
- 45.Andersen JS, et al. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–574. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The inhibitor effect of RKIP on inflammasome activation and inflammasome-dependent diseases