Abstract
Maternal HIV infection is associated with adverse pregnancy outcomes, but the mechanisms remain unknown. The course of pregnancy is regulated by immunological processes and HIV infection and antiretroviral therapy (ART) impact key immune mechanisms, which may disrupt the immune programme of pregnancy. We evaluated a broad range of systemic cytokines at each trimester of pregnancy in 56 women living with HIV (WLHIV) and 68 HIV-negative women, who were enrolled in a prospective pregnancy cohort study in Soweto, South Africa. The pro-inflammatory cytokine IP-10 was detected in each trimester in all WLHIV, which was significantly more than in HIV-negative women. The anti-viral cytokine IFNλ1 was detected more frequently in WLHIV, whereas IFNβ and IFNλ2/3 were detected more frequently in HIV-negative women. Th1 cytokines IL-12 and IL-12p70, Th2 cytokine IL-5, and Th17 cytokine IL-17A were detected more frequently in WLHIV throughout pregnancy. Il-6, IL-9, and IL-10 were more commonly detected in WLHIV in the first trimester. Trends of increased detection of Th1 (IL-2, IL-12p70), Th2 (IL-4, Il-5, Il-13) and Th17 (IL-17A, Il-17F, IL-21, IL-22) cytokines were associated with small-for-gestational-age babies. Our findings indicate that maternal HIV/ART is associated with distinct systemic cytokine profiles throughout pregnancy.
Subject terms: Cytokines, HIV infections
Introduction
HIV/AIDS currently affects an estimated 38 million people worldwide including approximately 1.3 million pregnant women, the majority of whom reside in sub-Saharan Africa1. A systematic review and meta-analysis demonstrated that WLHIV not receiving antiretroviral therapy (ART) experience higher rates of adverse pregnancy outcomes, including preterm birth, low birth weight, small-for-gestational-age (SGA), and stillbirth, compared to HIV-negative women2. Preterm birth is the leading cause of neonatal and child mortality globally, and is associated with significant short- and long-term morbidities3. Intra-uterine growth restriction is associated with stillbirth and SGA, which is also associated with neonatal morbidity and mortality4. While the administration of ART during pregnancy is effective at improving maternal outcomes and reducing mother-to-child HIV transmission, it does not reverse HIV-associated adverse pregnancy outcomes5–10.
Pregnancy is an immunologically dynamic period involving specific temporal adaptations in immune function and regulation11. This includes shifts in the prevailing cytokines which correspond to the biological processes occurring at each stage of pregnancy. For instance, in early pregnancy, implantation and placentation involve inflammatory processes as does labour, whereas the second and third trimester are predominantly anti-inflammatory periods12–14. HIV infection is characterised by a decline in CD4 T cells, CD8 T cell expansion and a shift from a Th1 to a Th2 immune response. ART aims to suppress viral replication as well as restore CD4/CD8 ratios and redress the Th1/Th2 balance15. However, ART administered during pregnancy may disrupt the regular immune programme of pregnancy16. We hypothesised that immunological changes caused by maternal HIV and ART might impact immunological changes occurring during pregnancy, which might in turn affect pregnancy outcomes. To gain insight into these immunological processes, we evaluated systemic pro-inflammatory, anti-viral, Th1, Th2, Th17, anti-inflammatory and pleiotropic cytokines and chemokines at each trimester of pregnancy in WLHIV and HIV-negative women in South Africa to investigate how HIV infection and ART impact cytokine responses during pregnancy.
Results
Patient characteristics
Fifty-six WLHIV and 68 HIV-negative women were included in this study. Maternal age was greater in WLHIV (p = 0.034) and there was a difference in the number of years of education (p = 0.038) (Table 1). However, there were no differences in either body mass index, obstetric history, smoking or alcohol intake (Table 1). CD4 counts were available for 21 WLHIV, with a median (IQR) of 498 (397–662) cells/mm3 (Table 1). Viral loads were only available in early pregnancy in 9 of the WLHIV, with median (IQR) of 97 (23–7029) copies/ml, and at delivery 15 viral loads were available, 12 of which were undetectable and the highest VL detected was 119 copies/ml. There were no cases of mother-to-child transmission of HIV.
Table 1.
Patient characteristics.
| HIV+ | HIV− | p | |
|---|---|---|---|
| Number of patients | 56 | 68 | |
| Maternal age (mean [SD]) | 32 [5.2] | 30 [5.3] | 0.034 |
| Pre-pregnancy body mass index (mean [SD]) | 27.7 [4.2] | 26.4 [3.9] | 0.091 |
| Smoking during pregnancy (n) | 5 [9%] | 2 [3%] | 0.148 |
| Alcohol intake during pregnancy (n) | 9 [16%] | 5 [7%] | 0.159 |
| Number of years of education (median [IQR]) | 12 [11–12] | 12 [12–12] | 0.038 |
| Number of previous pregnancies (mean [SD]) | 2 [1.1] | 2 [1.3] | 0.864 |
| History of adverse pregnancy outcome (n) | |||
| Yes | 30 [53.6%] | 39 [57%] | 0.241 |
| No | 24 [43%] | 22 [32%] | |
| Unknown | 2 [3.6%] | 7 [10%] | |
| Preterm birth (n) | 23 [41%] | 25 [37%] | 0.712 |
| Term birth (n) | 33 [59%] | 43 [63%] | |
| Small-for-gestational-age (n) | 19 [34%] | 11 [16%] | 0.055 |
| CD4 count (Trimester 1) (median cells/mm3 [IQR]) (n = 21) | 498 [397–662] | ||
| Antiretroviral therapy initiation (n [%]) | |||
| Preconception | 21 [38%] | N/A | |
| Post-conception | 23 [41%] | N/A | |
| Unknown | 12 [21%] | N/A | |
| Number of samples (n) | |||
| Trimester 1 | 54 | 68 | |
| Trimester 2 | 45 | 54 | |
| Trimester 3 | 31 | 37 | |
| Delivery | 11 | 0 | |
| Postnatal | 18 | 0 | |
| Weeks + days of gestation at sample collection (median [IQR]) | |||
| Trimester 1 | 11 + 6 [10 + 5 − 13 + 1] | 12 + 2 [11 + 1 − 13 + 2] | 0.311 |
| Trimester 2 | 26+0 [24 + 6 − 27 + 2] | 26+0 [23 + 3 − 27+0] | 0.869 |
| Trimester 3 | 35+0 [32 + 2 − 36+0] | 35 + 2 [33+0 − 37+0] | 0.398 |
Characteristics of pregnant women living with HIV (HIV+) and pregnant HIV-negative women (HIV−) enrolled in prospective pregnancy cohort study in Soweto, South Africa, of whom plasma samples were analysed. Statistical comparisons were made using unpaired t test, Mann–Whitney U test, Chi-square test or Fisher’s exact test, as appropriate. History of adverse pregnancy outcome: at least one occurrence of preterm birth, low birth weight, miscarriage, stillbirth or neonatal death.
Cytokines across pregnancy
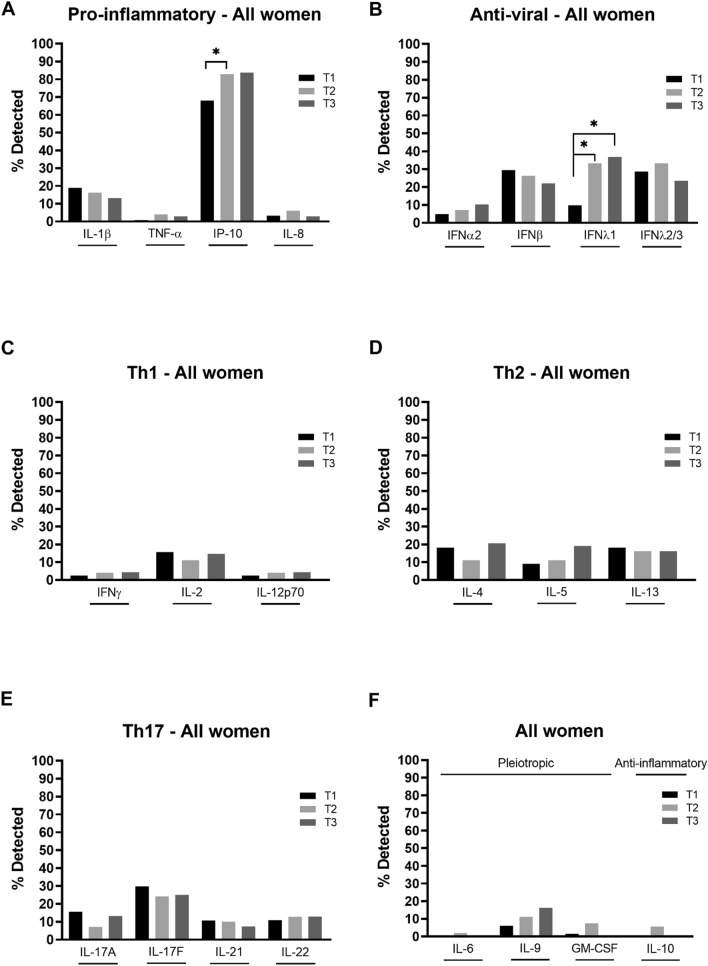
IL-1β, TNF-α, IP-10, IL-8 (pro-inflammatory), IFNα2, IFNβ, IFNλ1, IFNλ2/3 (anti-viral), IFNγ, IL-2, IL-12p70 (Th1), IL-4, IL-5, IL-13 (Th2), IL-17A, IL-17F, IL-21, IL-22 (Th17), IL-6, IL-9, GM-CSF (pleiotropic) and IL-10 (anti-inflammatory) cytokines and chemokines (hereafter referred to as cytokines) were measured in maternal plasma collected at each trimester and reported as a percentage of samples in which cytokines were detected (Fig. 1, Supp. Table 1). Across the 22 cytokines analysed among all the women of the study (i.e. both WLHIV and HIV-negative women), the rate of cytokine detection varied considerably and ranged from 1 to 84% with median detection rates of 11%, 11% and 13% across the three trimesters, respectively. IP-10 had the highest detection rates of 68%, 83% and 84% in the first, second and third trimester, respectively (Fig. 1A–F).
Figure 1.
Systemic pro-inflammatory, anti-viral, Th1, Th2, Th17, pleiotropic and anti-inflammatory cytokines across pregnancy. (A) Pro-inflammatory (IL-1β, TNF-α, IP-10, IL-8), (B) anti-viral (IFNα2, IFNβ, IFNλ1, IFNλ2/3), (C) Th1 (IFNγ, IL-2, IL-12p70), (D) Th2 (IL-4, IL-5, IL-13), (E) Th17 (IL-17A, IL-17F, IL-21, IL-22) and (F) pleiotropic and anti-inflammatory (IL-6, IL-9, GM-CSF and IL-10 respectively) cytokines were measured in maternal plasma collected during the first (T1), second (T2) and third (T3) trimester. Data are presented as the percentage of samples in which cytokines were detected by multiplex cytokine assay. *P < 0.05.
Among all the women studied, there were significant changes throughout the course of pregnancy in the detection of IP-10 and IFNλ1, with an increase in the detection of both cytokines form the first to second trimester, as well as from the first to third trimester for IFNλ1 (Fig. 1A,B). While these were the only significant changes, there were also notable trends of an increase in IFNα2, IL-5 and IL-9 and a decrease in IL-1β and IFNβ detection during pregnancy (Fig. 1A,B,D,F).
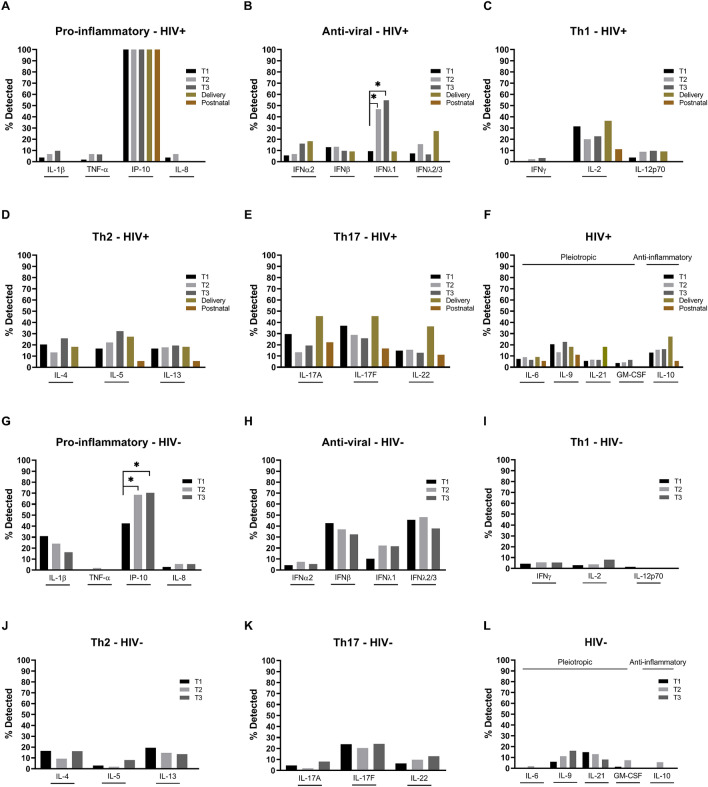
Cytokines across pregnancy in WLHIV and HIV-negative women
Cytokine detection throughout pregnancy was also analysed according to HIV status (Fig. 2, Supp. Table 1). For a number of WLHIV we also had delivery and 6 weeks postnatal samples which were included in the analysis of the WLHIV. Among the pro-inflammatory cytokines, IP-10 was detected in all WLHIV at each time-point and the detection rate of IP-10 increased throughout pregnancy in HIV-negative women (Fig. 2A,G). Therefore, IP-10 was additionally analysed according to cytokine concentration and was found to remain stable across pregnancy in WLHIV; as did IP-10 concentrations across pregnancy in all women (both WLHIV and HIV-negative women) (Supp. Figure 1A,B). There was also a trend for an increase in IL-1β detection over the course of pregnancy in WLHIV, which was in contrast to the decrease in detection seen in the analysis of HIV-negative women (Fig. 2A,G). Detection of IFNλ1, an anti-viral cytokine, increased significantly from the first to the second and third trimesters in WLHIV and there was a trend for an increase and a decrease in IFNα2 and IFNβ detection, respectively, during pregnancy (Fig. 2B). There was also a trend for an increase in the detection of the Th2 cytokine IL-5, and a decrease in the Th17 cytokine IL-17F, over the course of pregnancy in WLHIV (Fig. 2D–F). At delivery in WLHIV there was a trend for increased detection of IFNα2 and IFNλ2/3 of the anti-viral cytokines, IL-2 of the Th1 cytokines, the four Th17 cytokines measured (IL-17A, IL-17F, IL-21 and IL-22), and the anti-inflammatory cytokine IL-10, compared to during pregnancy (Fig. 2B,C,E,F). In contrast, the anti-viral cytokines IFNβ and IFNλ1, the three Th2 cytokines measured (IL-4, IL-5, IL-13), and IL-9, a pleiotropic cytokine, had a trend for decreased detection at delivery compared to during pregnancy in WLHIV (Fig. 2B,D,F). With the exception of IP-10, cytokines that were detected at delivery showed a trend for comparatively lower detection at 6 weeks postnatal in WLHIV (Fig. 2A–F). Many cytokines were not detected in any postnatal samples in WLHIV, including IL-1β, TNF-α, IL-8 (pro-inflammatory), all anti-viral cytokines, IFNγ and IL-12p70 (Th1), IL-4 (Th2), IL-21 (Th17), and GM-CSF (pleiotropic). In contrast, all the cytokines that were detected postnatally in WLHIV (IP-10 (pro-inflammatory), IL-2 (Th1), IL-5, IL-13 (Th2), IL-17A, IL-17F, IL-22 (Th17), and IL-6, IL-9, and IL-10) were also detected in all trimesters and at delivery (Fig. 2A–F).
Figure 2.
Systemic cytokines across pregnancy in women living with HIV and HIV-negative women. (A,G) Pro-inflammatory (IL-1β, TNF-α, IP-10, IL-8), (B,H) anti-viral (IFNα2, IFNβ, IFNλ1, IFNλ2/3), (C,I) Th1 (IFNγ, IL-2, IL-12p70), (D,J) Th2 (IL-4, IL-5, IL-13), (E,K) Th17 (IL-17A, IL-17F, IL-21, IL-22) and (F,L) pleiotropic and anti-inflammatory (IL-6, IL-9, GM-CSF and IL-10 respectively) cytokines were measured in the maternal plasma collected during the first (T1), second (T2) and third (T3) trimester in women living with HIV (HIV+) (A–F) and HIV-negative women (HIV−) (G–L). Data are presented as the percentage of samples in which cytokines were detected by multiplex cytokine assay. *P < 0.05.
In HIV-negative women, similar to the analysis of all women, IP-10 detection increased significantly from 43%, to 69% and 70% in the first, second and third trimester, respectively (Fig. 2G, Supp. Table 1) and the IP-10 concentration remained stable across pregnancy (Supp. Figure 1C). The anti-viral cytokine IFNλ1, the Th17 cytokine IL-22 and the pleiotropic cytokine IL-9 also showed a trend for increasing detection over the course of pregnancy (Fig. 2H,K,L). As with the analysis of all women, IL-1β (pro-inflammatory) and IFNβ (anti-viral), showed a trend of decreasing detection over the course of pregnancy in HIV-negative women, as did the Th2 cytokine IL-13 and the Th17 cytokine IL-21 (Fig. 2G,H,J,K). Detection of the Th1 cytokines, particularly IL-2 and IL-12p70, was low in HIV-negative women (Fig. 2I).
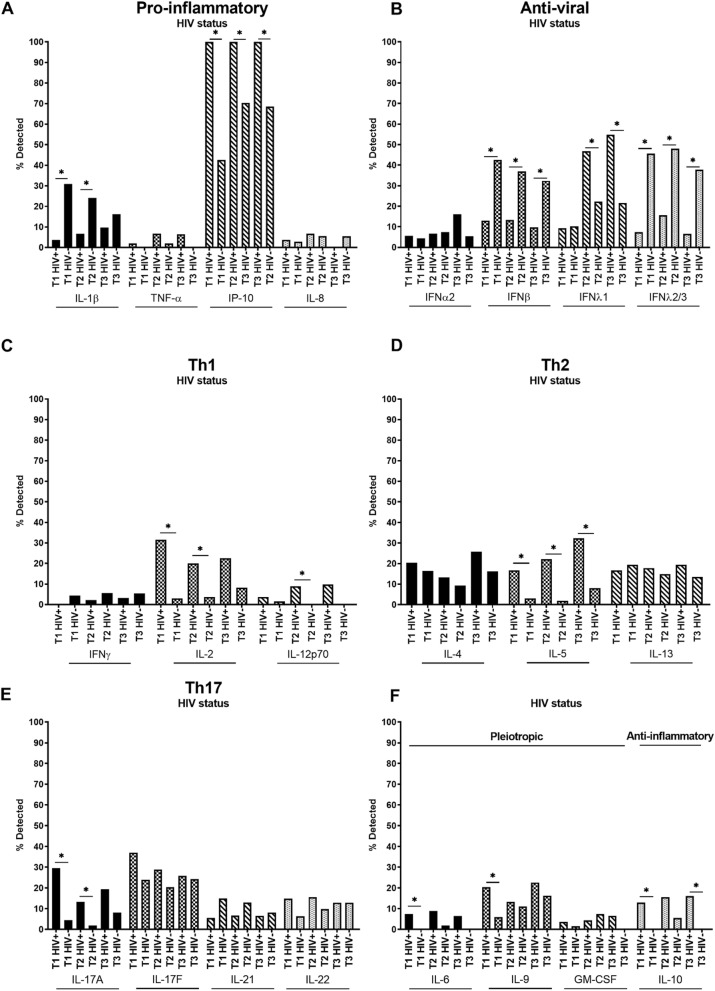
Cytokines in WLHIV versus HIV-negative women
As demonstrated in the analysis of WLHIV and HIV-negative women separately, there were differences in the patterns of cytokine detection between the two groups. Therefore, we compared cytokine detection in WLHIV and HIV-negative women over the course of pregnancy (Fig. 3, Supp. Table 2). Of the pro-inflammatory cytokines IL-1β was detected significantly less often in WLHIV compared to HIV-negative women in the first and second trimester, whereas IP-10 was detected in all WLHIV in all three trimesters, significantly more than in HIV-negative women (Fig. 3A). Additionally, IP-10 concentrations were significantly higher in WLHIV compared to HIV-negative women in the first trimester (median 48.50 vs 35.00 pg/ml) and second trimester (median 64.88 vs 26.95 pg/ml) (Supp. Figure 1D). Detection of IFNβ and IFNλ2/3 was significantly lower in WLHIV across all three trimesters, whereas detection of IFNλ1 was significantly greater in WLHIV compared to HIV-negative women in the second and third trimesters (Fig. 3B). There was also a trend for greater third trimester IFNα2 detection in WLHIV compared to HIV-negative women (Fig. 3B). Of the Th1 cytokines, detection of IL-2 was higher in WLHIV in all three trimesters with a significant difference in the first and second trimester; similarly, IL-12p70 detection was greater in WLHIV and significantly so in the second trimester, compared to HIV-negative women (Fig. 3C). In contrast, there was a trend for decreased detection of IFNγ in all three trimesters in WLHIV versus HIV-negative women (Fig. 3C). Detection of the Th2 cytokines IL-4 and IL-5 was greater in WLHIV in all three trimesters, however, the differences were only significant for IL-5 (Fig. 3D). Detection of the Th17 cytokine IL-17A was significantly greater in WLHIV in the first and second trimester with a trend for increased detection in the third trimester compared to HIV-negative women (Fig. 3E). IL-17F and IL-22 of the Th17 cytokines also showed a trend for increased detection, particularly in the first and second trimester, whereas there was a trend for lower detection of IL-21 in WLHIV versus HIV-negative women (Fig. 3E). IL-6 and IL-9, both pleiotropic cytokines, were detected at higher rates in WLHIV compared to HIV-negative women across all trimesters with a significant difference in the first trimester (Fig. 3F). Similarly, detection of the anti-inflammatory cytokine IL-10 was greater in WLHIV across all trimesters and significantly greater in the first and third trimester compared to HIV-negative women, in which IL-10 was not detected in either trimester (Fig. 3F).
Figure 3.
Systemic cytokines in women living with HIV versus HIV-negative women across pregnancy. (A) Pro-inflammatory (IL-1β, TNF-α, IP-10, IL-8), (B) anti-viral (IFNα2, IFNβ, IFNλ1, IFNλ2/3), (C) Th1 (IFNγ, IL-2, IL-12p70), (D) Th2 (IL-4, IL-5, IL-13), (E) Th17 (IL-17A, IL-17F, IL-21, IL-22) and (F) pleiotropic and anti-inflammatory (IL-6, IL-9, GM-CSF and IL-10 respectively) cytokines were measured in maternal plasma collected during the first (T1), second (T2) and third (T3) trimester in women living with HIV (HIV+) and HIV-negative women (HIV−). Data are presented as the percentage of samples in which cytokines were detected by multiplex cytokine assay. *P < 0.05.
Cytokines and adverse pregnancy outcomes
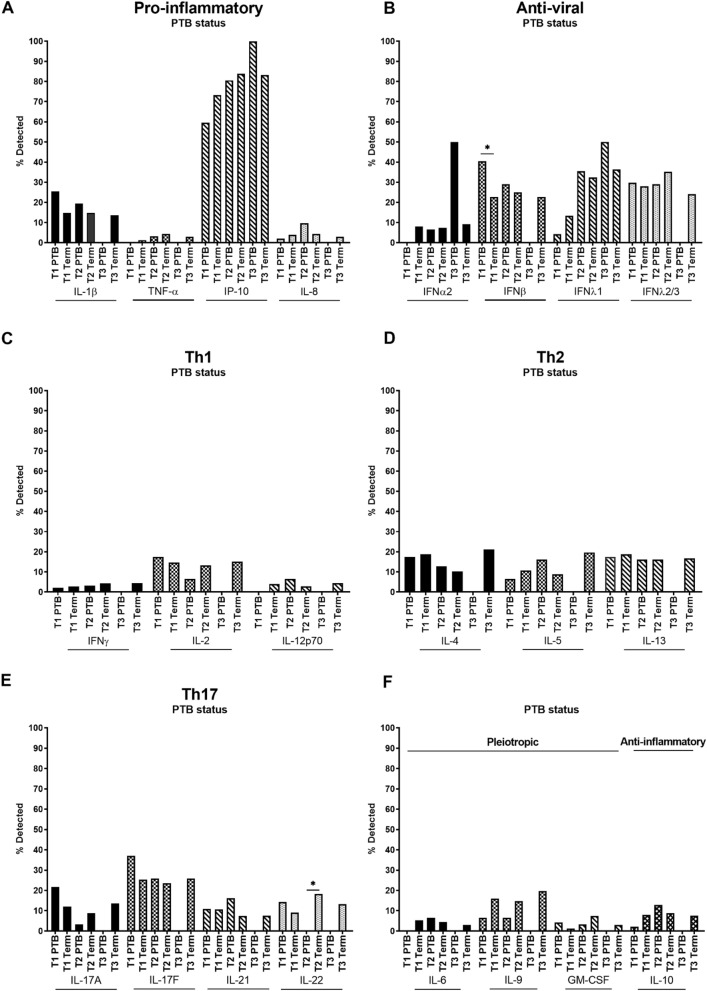
Maternal HIV infection and ART have been associated with an increased risk of adverse perinatal outcomes, including preterm birth and small-for-gestational-age infants2,9. Peripheral frequencies of a number of immune cells, including CD4 + T cells, γδ T cells, MAIT cells, and innate lymphoid cells (ILCs) have been associated with HIV infection as well as adverse perinatal outcomes, in particular preterm birth17–19. Therefore, we compared cytokine detection between women who delivered preterm versus those who delivered at term, as well as between women who delivered small-for-gestational-age (SGA) infants versus those who did not.
Although there were no significant differences among pro-inflammatory cytokines, IL-1β showed a trend of increased detection in the first and second trimester in women who would go on to deliver preterm, whereas detection of TNF-α and IP-10 tended to be lower in women who went on to deliver preterm versus term (Fig. 4A). First trimester detection of IFNβ, an anti-viral cytokine, was significantly greater in women who delivered preterm versus those who delivered at term (Fig. 4B). The Th1 cytokine IL-2 and the Th17 cytokines IL-17A, IL-17F and IL-22 tended to decrease from the first to second trimester in women who went on to deliver preterm but tended to remain stable in women who delivered at term (Fig. 4C,E). In contrast, the Th2 cytokine IL-5, tended to increase from the first to second trimester in women who went on to deliver preterm compared to women who delivered at term (Fig. 4D). The Th17 cytokine IL-22 was not detected in the second trimester in any women who went on to deliver preterm, a significant reduction compared to women who delivered at term (Fig. 4E). It should be noted that there were few third trimester PTB samples due to delivery before planned sample date (Fig. 4A–F).
Figure 4.
Systemic cytokines across pregnancy in women who went on to deliver preterm (PTB) versus women who delivered at term. (A) Pro-inflammatory (IL-1β, TNF-α, IP-10, IL-8), (B) anti-viral (IFNα2, IFNβ, IFNλ1, IFNλ2/3), (C) Th1 (IFNγ, IL-2, IL-12p70), (D) Th2 (IL-4, IL-5, IL-13), (E) Th17 (IL-17A, IL-17F, IL-21, IL-22) and (F) pleiotropic and anti-inflammatory (IL-6, IL-9, GM-CSF and IL-10 respectively) cytokines were measured in maternal plasma collected during the first (T1), second (T2) and third (T3) trimester. Data are presented as the percentage of samples in which cytokines were detected by multiplex cytokine assay. *P < 0.05.
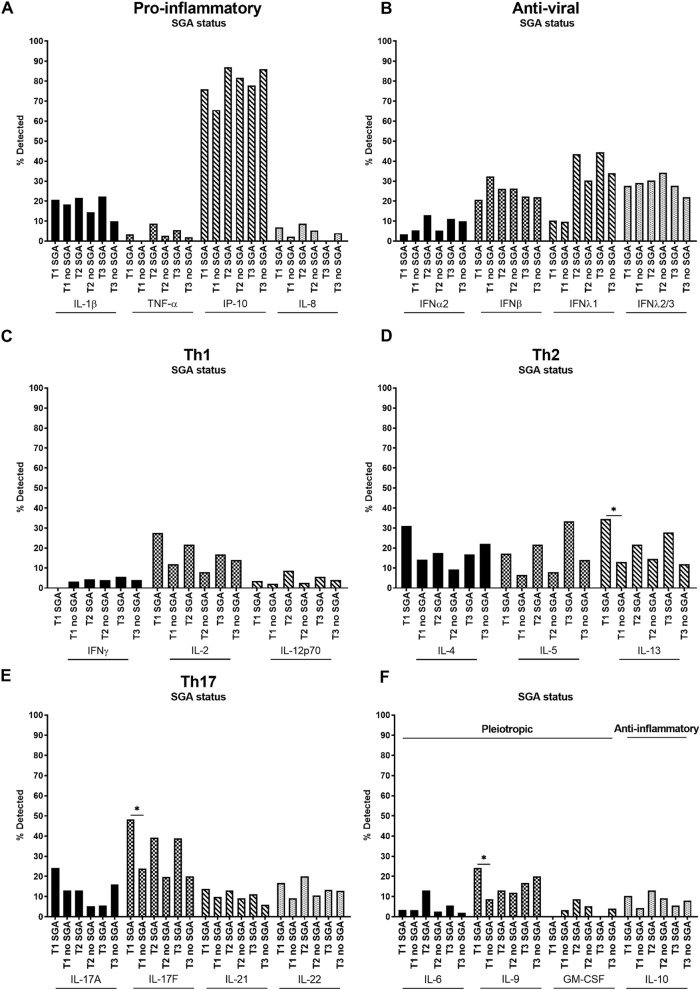
In the analysis of women with and without SGA babies, among pro-inflammatory cytokines there was a trend towards higher detection of all cytokines in SGA compared to non-SGA pregnancies, in particular in the first and second trimesters (Fig. 5A). Among anti-viral cytokines, IFNα2 and IFNλ1 also showed higher detection in SGA cases compared to non-SGA cases (Fig. 5B). Among Th1 cytokines, IL-2 and IL-12p70 were higher in SGA compared to non-SGA cases in all trimesters, although these trends were not statistically significant (Fig. 5C). All Th2 cytokines, IL-4, IL-5, and IL-13, showed trends of increased detection across pregnancy in women who delivered SGA infants (Fig. 5D). IL-13 detection was significantly greater in the first trimester in women who went on to deliver SGA infants (Fig. 5D). Additionally, all Th17 cytokines, IL-17A, IL-17F, IL-21 and IL-22, showed trends of increased detection in women who went on to deliver SGA infants versus those who did not, across pregnancy (Fig. 5E). Detection of the IL-17F was significantly greater in the first trimester in women who delivered SGA infants (Fig. 5E). Increased detection of the pleiotropic cytokine IL-9 in the first trimester was significant in women who went on to deliver SGA infants compared those who did not (Fig. 5F).
Figure 5.
Systemic cytokines across pregnancy in women who went on to deliver small-for-gestational-age (SGA) infants versus women who delivered non-SGA infants. (A) Pro-inflammatory (IL-1β, TNF-α, IP-10, IL-8), (B) anti-viral (IFNα2, IFNβ, IFNλ1, IFNλ2/3), (C) Th1 (IFNγ, IL-2, IL-12p70), (D) Th2 (IL-4, IL-5, IL-13), (E) Th17 (IL-17A, IL-17F, IL-21, IL-22) and (F) pleiotropic and anti-inflammatory (IL-6, IL-9, GM-CSF and IL-10 respectively) cytokines were measured in maternal plasma collected during the first (T1), second (T2) and third (T3) trimester. Data are presented as the percentage of samples in which cytokines were detected by multiplex cytokine assay. *P < 0.05.
Cytokine subgroups
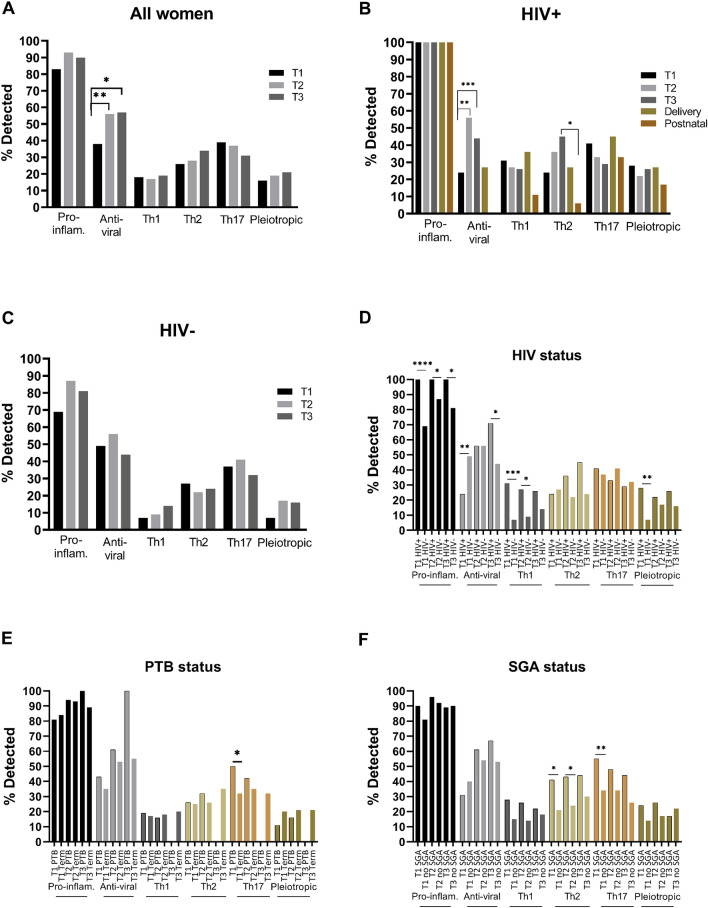
In an effort to summarise the data on the broad range of cytokines, we aggregated the data according to detection of different cytokine subgroups, i.e. pro-inflammatory, anti-viral, Th1, Th2, Th3, Th17 and pleiotropic cytokines (Fig. 6, Supp. Tables 3, 4). Among all women, we found an increase in detection of anti-viral cytokines from the first to the second trimester, as well as from the first to the third trimester (Fig. 6A). Among WLHIV, a similar increase in detection of anti-viral cytokines from the first to the second and third trimesters was seen. In addition, among WLHIV a significant drop in detection of Th2 cytokines was seen from the third trimester to 6 weeks postnatally (Fig. 6B). Among HIV-negative women no significant changes were seen during pregnancy (Fig. 6C). In the comparison of WLHIV and HIV-negative women, significant differences were seen in the detection of pro-inflammatory, antiviral, Th1 and pleiotropic cytokines (Fig. 6D). Pro-inflammatory cytokines were detected more often among WLHIV than HIV-negative women in each trimester. Anti-viral cytokines were detected more often among HIV-negative women in the first trimester, but more often among WLHIV in the third trimester. Th1 cytokines were detected more often among WLHIV in each trimester, and significantly so in the first and second trimesters. Pleiotropic cytokines were detected more often among WLHIV in the first trimester (Fig. 6D). Th17 cytokines were detected more often in the first trimester of women who would go on to deliver preterm compared to women who delivered at term (Fig. 6E). Th2 cytokines were detected more often in each trimester among women with SGA newborns, and significantly so in the first and second trimesters. In addition, Th17 cytokines were detected more often among women with SGA newborns, which reached statistical significance in the first trimester (Fig. 6F).
Figure 6.
Systemic cytokine subgroups across pregnancy. (A) All women, (B) women living with HIV (HIV+), (C) HIV-negative women (HIV-), (D) HIV+ and HIV− women, (E) preterm (PTB) and term birth, and (F) small-for-gestational-age (SGA) and non-SGA infants. Pro-inflammatory (IL-1β, TNF-α, IP-10, IL-8), anti-viral (IFNα2, IFNβ, IFNλ1, IFNλ2/3), Th1 (IFNγ, IL-2, IL-12p70), Th2 (IL-4, IL-5, IL-13), Th17 (IL-17A, IL-17F, IL-21, IL-22) and pleiotropic (IL-6, IL-9, GM-CSF and IL-10 respectively) cytokines were measured in maternal plasma collected during the first (T1), second (T2) and third (T3) trimester in women living with HIV (HIV+) and HIV-negative (HIV−) women. Data are presented as the percentage of samples in which subgroups of cytokines were detected by multiplex cytokine assay. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Discussion
In this study we evaluated a broad range of cytokines at each trimester of pregnancy in WLHIV and HIV-negative women in South Africa. We found that the pro-inflammatory cytokine IP-10 was detected in each trimester in all WLHIV, which was significantly more than in HIV-negative women. The anti-viral cytokine IFNλ1 was detected more frequently in WLHIV, whereas IFNβ and IFNλ2/3 were detected more frequently in HIV-negative women. Th1 cytokines IL-12 and IL-12p70, Th2 cytokine IL-5, and Th17 cytokine IL-17A were detected more frequently in WLHIV throughout pregnancy. Il-6, IL-9, and IL-10 were more commonly detected in WLHIV in the 1st trimester. Trends of increased detection of Th1 (IL-2, IL-12p70), Th2 (IL-4, Il-5, Il-13) and Th17 (IL-17A, Il-17F, IL-21, IL-22) cytokines were also associated with small-for-gestational-age babies. Analysis of cytokine subgroups showed that pro-inflammatory, Th1 and pleiotropic cytokines were more often detected among WLHIV, compared to HIV-negative women. Preterm birth was associated with increased detection of Th17 cytokines, and SGA was associated with increased detection of Th2 and Th17 cytokines.
IP-10 is an inflammatory chemokine involved in the migration and adhesion of immune cells including CD4 + and CD8 + T cells and NK and NKT cells during an inflammatory response20. This chemokine is secreted by a range of cell types, including monocytes, leukocytes, neutrophils and endothelial cells, predominantly in response to IFNγ20,21. The combined analysis of all women revealed significant increases in the detection of IP-10 over the course of pregnancy. This was attributable to HIV-negative women, in whom detection of IP-10 increased significantly from the first to the second and third trimester. Similar to our findings, peripheral IP-10 levels during pregnancy in HIV-negative women have been shown to increase over time22,23. However, our analysis of IP-10 concentration did not show a significant increase in IP-10 over pregnancy in the women analysed. HIV infection is associated with elevated peripheral IP-10 levels which are indicative of HIV disease progression and positively associated with HIV viral load24. While IP-10 levels in people living with HIV (PLHIV) are lowered by ART, they remain higher than controls24. This was demonstrated in our analysis, in which IP-10 was detected in all WLHIV at all three trimesters, as well as at delivery and 6 weeks postnatal, and therefore had significantly greater detection rates in WLHIV compared to HIV-negative women. Labour is associated with an infiltration of inflammatory cells and an increase in inflammatory cytokines, including IP-10, IL-1β, TNF-α and IL-8, in tissues including the cervix, myometrium and chorio-amniotic membranes13,25–28. Therefore, the observed increase in IP-10 detection during the course of pregnancy in HIV-negative women may align with an increase in inflammatory processes towards the end of pregnancy involved in labour. In the decidua IP-10 levels are correlated with decidual macrophage numbers and IP-10 is expressed by monocytes and myeloid dendritic cells in the periphery in PLHIV27,29. Elevated peripheral and decidual IP-10 levels have been associated with preeclampsia30,31 and suggests WLHIV may be at increased risk of preeclampsia32. In our analysis of women who experienced preterm versus term births and women with and without small-for-gestational-age infants, there were no significant differences in IP-10 detection. This is in accordance with a study which found no difference in peripheral IP-10 levels between women with and without small-for-gestational-age babies30, suggesting IP-10 is involved in specific rather than generalised pathologies of pregnancy.
The remaining pro-inflammatory cytokines, IL-1β, TNF-α and IL-8, did not show any statistically significant changes in detection over the course of pregnancy. However, there was a striking opposite trend in the detection of IL-1β throughout pregnancy in WLHIV and HIV-negative women, with an upward trend from the first to third trimester in WLHIV and the opposite in HIV-negative women. Additionally, this was associated with significantly lower IL-1β detection rates in WLHIV compared to HIV-negative women. Studies in HIV-negative women have shown that peripheral IL-1β levels decrease over pregnancy, including in plasma33,34, suggesting increases in IL-1β over the course of pregnancy are specific to HIV infection. However, other studies suggest IL-1β levels are not changed over pregnancy or have a positive association with gestational weeks22,23. Elevated levels of peripheral IL-1β, TNF-α and IL-8, as well as IL-6, are associated with pre-eclampsia in pregnancy and are raised during HIV infection35–37. While we did not find significant differences in the detection rates of these cytokines according to preterm birth or small-for-gestational age status, IL-1β detection tended to be greater in women who delivered preterm and small-for-gestational-age infants.
Th1 cytokines generally mediate cellular immune responses against intracellular bacteria and viruses, while Th2 cytokines are associated with antibody responses and protection against extracellular parasites. Pregnancy is described as a Th2 predominated condition and shifts in Th1/Th2 cytokine ratios have been associated with conditions such as preeclampsia and preterm birth16,32,38. HIV infection is also classified as a Th2 biased condition in which ART redresses the Th1/Th2 balance in PLHIV and therefore may have detrimental consequences for pregnancy16. In our data set Th1 cytokine detection was particularly low in HIV-negative women and there were significantly higher detection rates of IL-2 and IL-12p70 in WLHIV. In addition, of the Th2 cytokines, IL-4 detection tended to be greater and IL-5 detection significantly greater in WLHIV. IL-13 remained stable across pregnancy and between WLHIV and HIV-negative women, as well as between women who delivered preterm and at term. In contrast, IL-13 detection was greater in women who delivered small-for-gestational-age infants, particularly in the first trimester, and IL-4 and IL-5 detection also tended to be greater in this group, as well as IL-2 and IL-12p70 of the Th1 cytokines. Overall, this data suggests that HIV/ART modulates both Th1 and Th2 cytokine responses during pregnancy. While HIV/ART mediated Th1/Th2 cytokine shifts have been associated with pathological pregnancy conditions, the immune responses associated with both HIV/ART and pregnancy are complex, and the increases in both Th1 and Th2 cytokines associated with HIV/ART suggest that it may not involve a clear-cut shift from one inflammatory state to another.
Three Th17 cytokines, IL-17A, IL-17F and IL-22, tended to be detected more often in WLHIV, with significantly greater detection of IL-17A in the first and second trimester compared to HIV-negative women. IL-17A is a pro-inflammatory cytokine produced by CD4 + Th17 helper cells, which also secrete TNFα, IL-1, IL-2, IL-21 and IL-22. Both Th17 cells and IL-17 are found in increased proportions in the peripheral blood and decidua of women who experience preterm birth, miscarriage and preeclampsia39–41. In our data IL-17A detection tended to drop in preterm birth mothers from the first to the second trimester, potentially demonstrating increased recruitment to the decidua towards the end of pregnancy. Similar to IL-17A, IL-17F is a pro-inflammatory cytokine produced by Th17 cells and in our study tended to be detected more often in WLHIV. In addition, IL-17F was more commonly detected in women who went on to have small-for-gestational-age infants versus those who did not, particularly in the first trimester (48% versus 24%). Th17 cells are preferentially lost from the gastrointestinal tract of HIV-infected individuals, which can be reversed by HAART. Furthermore, other cells, such as Vδ1 T cells, can make IL-17 in vivo during HIV infection and may contribute to the greater detection of IL-17 in WLHIV in our study42. Th17 cells have been reported to be both depleted, unchanged and increased during HIV infection42–45 but our findings of increased peripheral IL-17 detection in pregnancy may suggest WLHIV are at increased risk of IL-17 induced pregnancy complications. Viremia is known to be associated with changes in inflammatory markers, including IL-1715,43,46. Unfortunately we had few data on the viral loads of the WLHIV in our study. However, where available the viral loads were generally low. In addition, data from the prospective pregnancy cohort as a whole showed that the vast majority of women received ART and there were no cases of HIV transmission to newborns, indicating good viral suppression in the cohort overall10. IL-22 has been shown in mouse models of LPS-induced preterm birth to be important in protection against inflammation-induced preterm birth and IL-22 expression is lower in the decidua of women who experience recurrent pregnancy loss47,48. Our finding of significantly lower detection of peripheral IL-22 in women with preterm birth, may be reflective of a decline in recruitment of IL-22 producing cells, e.g. Th17 cells, to the decidua of women with preterm birth.
Detection of the anti-inflammatory cytokines IL-10 and the pleiotropic cytokines IL-6 and IL-9 was also higher in WLHIV. IL-6 is deemed a specific indicator of infection-associated preterm labour and peripheral increases, as well as at the maternal–fetal interface and within the amniotic and cervicovaginal fluid is deemed a risk factor for preterm birth49–52. However, while we saw higher IL-6 detection in WLHIV compared to HIV-negative women, in our data there were no differences in IL-6 between women who delivered preterm and at term. This could be due to several factors such as differences in sampling time-points, sample size and the presence or absence of infection. During pregnancy peripheral IL-9 levels positively associate with pregnancy progression22 and in our data this was demonstrated in HIV-negative women in whom IL-9 detection tended to increase over the course of pregnancy. Similarly, in women who did not go on to deliver small-for-gestational-age infants, IL-9 detection tended to increase from the first to third trimester. However, IL-9 detection tended to be lowest in the second trimester in WLHIV and in women who went on to deliver small-for-gestational-age infants. In a mouse model of pregnancy uterine levels of IL-9 have been shown to increase during pregnancy and be the most abundant cytokine measured in the uterus and uterine leukocytes53. Produced by a range of cell types including Th9 cells, Th2 cells, Th17 cells, Treg cells, mast cells and NK cells, IL-9 is involved in immune tolerance as well as inflammatory disorders54 and suggests IL-9 mediates a balance between the anti- and pro-inflammatory responses required for successful uncomplicated pregnancy.
In the analysis of all women, detection of anti-viral cytokine IFNλ1 significantly increased over the course of pregnancy and was attributed to significant increases in detection of the cytokine in WLHIV. In all women, IFNβ detection tended to decrease during pregnancy and IFNλ2/3 detection tended to peak during the second trimester. Type I (IFNα/β) and type III (IFNλs) IFNs are antiviral cytokines which initiate antiviral immune responses against a number of viruses55,56. In pregnancy, type III IFNs are constitutively released from human trophoblast cells and are involved in protection of the placenta against viral infection, including against HIV; and the potential contribution of maternal peripheral IFNλ may explain the significant increases in IFNλ1 detection we see in WLHIV57,58. In contrast, detection of both IFNβ and IFNλ2/3 was significantly lower in WLHIV at all three trimesters compared to HIV-negative women. In another study investigating systemic cytokine levels during acute HIV infection, IFNβ levels were not elevated29 suggesting the lower IFN detection rates maybe a result of HIV infection that is maintained during pregnancy. For instance, HIV infection is associated with chronic immune activation which, as well as the activation of immune pathways, also involves immune exhaustion, including a diminished capacity of plasmacytoid DCs, the main type I IFN producer, to secrete type I IFN59. In mice, type I IFNs are expressed in tissues including the decidua suggesting a role in pregnancy, however, type I IFN signalling has also been suggested to prime for inflammation driven preterm birth60,61. In support of this, we find that IFNβ detection is significantly increased in preterm birth mothers.
Our study has some strengths and limitations. Strengths of our study included the prospective study design, with samples collected in each trimester of pregnancy. Pregnancy outcomes were accurately determined based on a first trimester dating ultrasound and standardised birthweight measurement within 24 h of birth. Furthermore, we assayed a broad range of cytokines in parallel to obtain a comprehensive view of systemic cytokine levels. Limitations of the study included the limited information regarding viral loads and CD4 counts, which limited interpretation of our results to some extent. The sources of the cytokines, in terms of cell types and their location, remain unknown and as a consequence it is uncertain how differences and changes in systemic cytokine detection correlate with changes in the uterus.
Successful pregnancy involves the coordination of multiple immune processes and redundancy in immune function may suggest adverse outcomes result from the dysregulation of multiple processes11,62. Indeed, cytokines exemplify this complexity by their overlapping and redundant functions. We found significant differences in detection rates of individual cytokines between WLHIV and HIV-negative women throughout pregnancy, as well as differences associated with adverse pregnancy outcomes. Our findings therefore indicate that maternal HIV/ART is associated with distinct systemic cytokine profiles throughout pregnancy and further work is needed to elucidate the mechanisms that underlie the increased risk of adverse pregnancy outcomes.
Methods
Patient population and sample collection
Between 27 November 2013 and 20 October 2015, plasma samples were obtained from women enrolled in a prospective pregnancy cohort study at Chris Hani Baragwanath Academic Hospital (CHBAH), Soweto, South Africa10. Women included in the study were black South African, living in Soweto, aged 18 years or over, with a spontaneous conception resulting in a singleton pregnancy. Women with multiple pregnancies, a body mass index > 35 kg/m2 or an intellectual or physical disability, were excluded. All women had a first trimester dating ultrasound scan and HIV testing was routinely offered to those not known to be HIV-positive at enrolment. All WLHIV received antenatal antiretroviral therapy, mainly efavirenz-based HAART10. Data on timing of ART initiation was also collected. ART initiation was defined as preconception if started before the date of the last menstrual period or post-conception if initiated after the last menstrual period date. Among women initiating ART post-conception, this occurred before the first trimester sample date in the majority of women (14 out of 23), with the remaining WLHIV initiating ART after the first trimester sample. Medical and obstetric history were collected from medical records, antenatal cards and/or interviews, and perinatal outcomes of interest were recorded at delivery, including standardised birthweight measurement within 24 h of birth, as previously reported10. Preterm birth (PTB) was defined as birth from 16+0 to 36+6 weeks gestation and infants small-for-gestational age (SGA) defined as newborns under the 10th centile of the INTERGROWTH‐21st Newborn Standard birth‐weight‐for‐gestational‐age/sex63. Trained study nurses collected peripheral blood samples from WLHIV and HIV-negative pregnant women during the first, second and third trimester, as previously described17–19. Additional samples were collected at delivery and 6 weeks postnatally from a subset of the WLHIV. Samples were separated into plasma and peripheral blood mononuclear cells by standard density gradient centrifugation. Plasma samples were initially stored at − 80 °C. Samples were shipped to Oxford on dry ice where they were stored in liquid nitrogen.
Cytokine analysis
The Biolegend LEGENDplex™ bead-based multiplex immunoassays were performed using the human anti-virus response (740390) and human Th cytokine (740759) panels to measure IL-1β, IL-6, TNF-α, IP-10, IFNλ1 (IL-29), IL-8, IL-12p70, IFNα2, IFNλ2/3 (IL-28A/B), GM-CSF, IFNβ, IL-10, IFNγ, and IL-5, IL-13, IL-2, IL-9, IL-17A, IL-17F, IL-4, IL-21 and IL-22, respectively, in plasma. The kits were used according to the manufacturer’s instructions. At the end of the protocol samples were sterilised by incubation with 4% paraformaldehyde in 1X PBS then washed and resuspended in the 1X Wash Buffer supplied with the kit. All samples were tested in duplicate. The average MFI of duplicates was used to determine the final cytokine concentration of each sample. Samples testing below the sensitivity of the assay were categorised as not detected. None of the samples tested above the sensitivity of the assay. Samples were read on a LSR II flow cytometer [Becton Dickinson] and analysed using the LEGENDplex™ Data Analysis V8.0 software.
Statistical analysis
Patient characteristics were compared using the unpaired t test or Mann–Whitney U test for continuous variables and Chi-squared test or Fisher’s exact test for categorical variables. Cytokine detection rates and concentrations ranged widely and for many cytokines the detection rates were low (i.e. few patient samples had a detectable level of the respective cytokines). Reporting cytokine concentrations of all samples would have required making assumptions about the concentrations present in those samples which were below the detection limit. Therefore, we report instead cytokine detection rates (i.e. the percentages of samples in which cytokines are detected) in order to utilise all the data collected without substituting arbitrary values and introducing bias. For those cytokines detected in the majority of samples (i.e. IP-10) we also report analysis of the concentrations. Cytokine detection was also aggregated for the different cytokine subgroups: pro-inflammatory, anti-viral, Th1, Th2, Th17 and pleiotropic cytokines. In this case, for each patient at each time point the detection of cytokines in each subgroup was assessed and if one or more of the cytokines in a subgroup were detected (e.g. IL-1β), then the subgroup (e.g. pro-inflammatory cytokines) was considered to be detected.
The association of cytokine detection with trimester, HIV status, preterm birth status or small-for-gestational age status was analysed using the Chi-Squared or Fisher's exact test as appropriate. Corrected pairwise comparisons were calculated for the association between cytokine detection and time periods, to determine specific pairwise differences between two periods (trimesters one, two, three, delivery or postnatal). The Bonferroni method was used to correct for multiple comparisons. P values < 0.05 were considered statistically significant and analyses were performed using SPSS® version 26.
Ethical approval
Written informed consent was obtained from all study participants upon enrolment. Ethical approval was obtained from the University of Oxford Tropical Research Ethics Committee (OxTREC) and the Human Research Ethics Committee (Medical) of the University of Witwatersrand, Johannesburg, South Africa. All experiments were performed in accordance with relevant guidelines and regulations.
Supplementary Information
Acknowledgements
We thank all the study participants whose blood samples and data contributed to this study. We thank the doctors, nurses and other staff at Chris Hani Baragwanath Academic Hospital and the Developmental Pathways for Health Research Unit who facilitated the study. We thank the Bill & Melinda Gates Foundation (Grant number 49038) and the Higher Education Funding Council for England (HEFCE) (reference number 0005078) for funding this study.
Author contributions
C.A. conducted the cytokine multiplex assays, data analysis, including the statistical analysis, made the tables and figures and wrote the first draft of the article. S.N. coordinated the study and sample collection at the study site. J.H. conceived, designed and coordinated the study, designed the experiments and analysis plan, interpreted the data and wrote the article. All authors read and approved the submitted article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-89551-3.
References
- 1.UNAIDS. AIDSinfo. http://aidsinfo.unaids.org/.
- 2.Wedi COO, et al. Perinatal outcomes associated with maternal HIV infection: A systematic review and meta-analysis. Lancet HIV. 2016;3:e33–e48. doi: 10.1016/S2352-3018(15)00207-6. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, et al. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388:3027–3035. doi: 10.1016/s0140-6736(16)31593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee AC, et al. Estimates of burden and consequences of infants born small for gestational age in low and middle income countries with INTERGROWTH-21(st) standard: Analysis of CHERG datasets. BMJ. 2017;358:j3677. doi: 10.1136/bmj.j3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chetty T, Thorne C, Coutsoudis A. Preterm delivery and small-for-gestation outcomes in HIV-infected pregnant women on antiretroviral therapy in rural South Africa: Results from a cohort study, 2010–2015. PLoS One. 2018;13:e0192805. doi: 10.1371/journal.pone.0192805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fowler MG, et al. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N. Engl. J. Med. 2016;375:1726–1737. doi: 10.1056/NEJMoa1511691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: A meta-analysis. AIDS. 2007;21:607–615. doi: 10.1097/QAD.0b013e32802ef2f6. [DOI] [PubMed] [Google Scholar]
- 8.Malaba TR, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int. J. Epidemiol. 2017;46:1678–1689. doi: 10.1093/ije/dyx136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mofenson LM. Antiretroviral therapy and adverse pregnancy outcome: The elephant in the room? J. Infect. Dis. 2016;213:1051–1054. doi: 10.1093/infdis/jiv390. [DOI] [PubMed] [Google Scholar]
- 10.Santosa WB, Staines-Urias E, Tshivuila-Matala COO, Norris SA, Hemelaar J. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS. 2019;33:1623–1633. doi: 10.1097/qad.0000000000002222. [DOI] [PubMed] [Google Scholar]
- 11.Aghaeepour N, et al. An immune clock of human pregnancy. Sci. Immunol. 2017;2:eaan2946. doi: 10.1126/sciimmunol.aan2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am. J. Reprod. Immunol. 2014;72:141–147. doi: 10.1111/aji.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romero R, et al. Inflammation in preterm and term labour and delivery. Semin. Fetal Neonatal. Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011;1221:80–87. doi: 10.1111/j.1749-6632.2010.05938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, et al. The effect of a year of highly active antiretroviral therapy on immune reconstruction and cytokines in HIV/AIDS patients. AIDS Res. Hum. Retroviruses. 2012;29:691–697. doi: 10.1089/aid.2012.0275. [DOI] [PubMed] [Google Scholar]
- 16.Fiore S, et al. Antiretroviral therapy-associated modulation of Th1 and Th2 immune responses in HIV-infected pregnant women. J. Reprod. Immunol. 2006;70:143–150. doi: 10.1016/j.jri.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Akoto C, et al. γδ T cell frequencies are altered in HIV positive pregnant South African women and are associated with preterm birth. PLoS One. 2020;15:e0235162. doi: 10.1371/journal.pone.0235162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ravi K, et al. Changes in the Vα72+ CD161++ MAIT cell compartment in early pregnancy are associated with preterm birth in HIV-positive women. Am. J. Reprod. Immunol. 2020;83:13240. doi: 10.1111/aji.13240. [DOI] [PubMed] [Google Scholar]
- 19.Akoto C, et al. Innate lymphoid cells are reduced in pregnant HIV positive women and are associated with preterm birth. Sci. Rep. 2020;10:13265. doi: 10.1038/s41598-020-69966-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groom JR, Luster AD. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011;89:207–215. doi: 10.1038/icb.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, et al. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22:121–130. doi: 10.1016/j.cytogfr.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtan SG, et al. Growth modeling of the maternal cytokine milieu throughout normal pregnancy: Macrophage-derived chemokine decreases as inflammation/counterregulation increases. J. Immunol. Res. 2015;2015:952571. doi: 10.1155/2015/952571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kraus TA, et al. Peripheral blood cytokine profiling during pregnancy and post-partum periods. Am. J. Reprod. Immunol. 2010;64:411–426. doi: 10.1111/j.1600-0897.2010.00889.x. [DOI] [PubMed] [Google Scholar]
- 24.Lei J, Yin X, Shang H, Jiang Y. IP-10 is highly involved in HIV infection. Cytokine. 2019;115:97–103. doi: 10.1016/j.cyto.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 25.Keelan JA, et al. Cytokines, prostaglandins and parturition—a review. Placenta. 2003;24:S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 26.Osman I, et al. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labour at term. Mol. Hum. Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton SA, Tower CL, Jones RL. Identification of chemokines associated with the recruitment of decidual leukocytes in human labour: Potential novel targets for preterm labour. PLoS One. 2013;8:e56946. doi: 10.1371/journal.pone.0056946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol. Hum. Reprod. 2002;8:399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 29.Simmons RP, et al. HIV-1 infection induces strong production of IP-10 through TLR7/9-dependent pathways. AIDS (London, England) 2013;27:2505–2517. doi: 10.1097/01.aids.0000432455.06476.bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gotsch F, et al. CXCL10/IP-10: A missing link between inflammation and anti-angiogenesis in preeclampsia? J. Matern. Fetal Neona. 2007;20:777–792. doi: 10.1080/14767050701483298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lockwood CJ, et al. Decidual cell regulation of natural killer cell-recruiting chemokines: Implications for the pathogenesis and prediction of preeclampsia. Am. J. Pathol. 2013;183:841–856. doi: 10.1016/j.ajpath.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maharaj NR, et al. Pro-inflammatory cytokine levels in HIV infected and uninfected pregnant women with and without preeclampsia. PLoS One. 2017;12:e0170063. doi: 10.1371/journal.pone.0170063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denney JM, et al. Longitudinal modulation of immune system cytokine profile during pregnancy. Cytokine. 2011;53:170–177. doi: 10.1016/j.cyto.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson KK, McElrath TF, Chen Y-H, Mukherjee B, Meeker JD. Longitudinal profiling of inflammatory cytokines and C-reactive protein during uncomplicated and preterm pregnancy. Am. J. Reprod. Immunol. 2014;72:326–336. doi: 10.1111/aji.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo H, Gao J, Taxman DJ, Ting JPY, Su L. HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J. Biol. Chem. 2014;289:21716–21726. doi: 10.1074/jbc.M114.566620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Osuji FN, Onyenekwe CC, Ahaneku JE, Ukibe NR. The effects of highly active antiretroviral therapy on the serum levels of pro-inflammatory and anti-inflammatory cytokines in HIV infected subjects. J. Biomed. Sci. 2018;25:88. doi: 10.1186/s12929-018-0490-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellwanger JH, et al. Increased IL-8 levels in HIV-infected individuals who initiated ART with CD4+ T cell counts <350 cells/mm3—a potential hallmark of chronic inflammation. Microbes Infect. 2020 doi: 10.1016/j.micinf.2020.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Pillay P, et al. Exosomal Th1/Th2 cytokines in preeclampsia and HIV-positive preeclamptic women on highly active anti-retroviral therapy. Cytokine. 2020;125:154795. doi: 10.1016/j.cyto.2019.154795. [DOI] [PubMed] [Google Scholar]
- 39.Darmochwal-Kolarz D, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J. Reprod. Immunol. 2012;93:75–81. doi: 10.1016/j.jri.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Ito M, et al. A role for IL-17 in induction of an inflammation at the fetomaternal interface in preterm labour. J. Reprod. Immunol. 2010;84:75–85. doi: 10.1016/j.jri.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Nakashima A, et al. Accumulation of IL-17-positive cells in decidua of inevitable abortion cases. Am. J. Reprod. Immunol. 2010;64:4–11. doi: 10.1111/j.1600-0897.2010.00812.x. [DOI] [PubMed] [Google Scholar]
- 42.Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr. Opin. HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ndhlovu LC, et al. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. AIDS. 2008;22:990–992. doi: 10.1097/QAD.0b013e3282ff884e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenchley JM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maek-A-Nantawat W, Buranapraditkun S, Klaewsongkram J, Ruxrungthum K. Increased interleukin-17 production both in helper T cell subset Th17 and CD4-negative T cells in human immunodeficiency virus infection. Viral Immunol. 2007;20:66–75. doi: 10.1089/vim.2006.0063. [DOI] [PubMed] [Google Scholar]
- 46.Stacey AR, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 2009;83:3719. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dambaeva S, et al. Interleukin 22 prevents lipopolysaccharide-induced preterm labor in mice. Biol. Reprod. 2018;98:299–308. doi: 10.1093/biolre/iox182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'Hern Perfetto C, et al. Expression of interleukin-22 in decidua of patients with early pregnancy and unexplained recurrent pregnancy loss. J. Assist. Reprod. Genet. 2015;32:977–984. doi: 10.1007/s10815-015-0481-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorokin Y, et al. Maternal serum interleukin-6, C-reactive protein, and matrix metalloproteinase-9 concentrations as risk factors for preterm birth <32 weeks and adverse neonatal outcomes. Am. J. Perinatol. 2010;27:631–640. doi: 10.1055/s-0030-1249366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lucaroni F, et al. Biomarkers for predicting spontaneous preterm birth: An umbrella systematic review. J. Matern. Fetal Neonatal Med. 2018;31:726–734. doi: 10.1080/14767058.2017.1297404. [DOI] [PubMed] [Google Scholar]
- 51.Romero R, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 52.Keelan JA, et al. Cytokine abundance in placental tissues: Evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am. J. Obstet. Gynecol. 1999;181:1530–1536. doi: 10.1016/s0002-9378(99)70400-x. [DOI] [PubMed] [Google Scholar]
- 53.Habbeddine M, Verbeke P, Karaz S, Bobé P, Kanellopoulos-Langevin C. Leukocyte population dynamics and detection of IL-9 as a major cytokine at the mouse fetal-maternal interface. PLoS One. 2014;9:e107267. doi: 10.1371/journal.pone.0107267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Noelle RJ, Nowak EC. Cellular sources and immune functions of interleukin-9. Nat. Rev. Immunol. 2010;10:683–687. doi: 10.1038/nri2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuel CE. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Durbin RK, Kotenko SV, Durbin JE. Interferon induction and function at the mucosal surface. Immunol. Rev. 2013;255:25–39. doi: 10.1111/imr.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wells AI, Coyne CB. Type III interferons in antiviral defenses at barrier surfaces. Trends Immunol. 2018;39:848–858. doi: 10.1016/j.it.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bayer A, et al. Human trophoblasts confer resistance to viruses implicated in perinatal infection. Am. J. Obstet. Gynecol. 2015;212(71):e71–71.e78. doi: 10.1016/j.ajog.2014.07.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller E, Bhardwaj N. Dendritic cell dysregulation during HIV-1 infection. Immunol. Rev. 2013;254:170–189. doi: 10.1111/imr.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yamamoto Y, Kurohmaru M, Hayashi Y. Localization of type I interferon in murine trophoblast and decidua during decidual formation. Reproduction. 1992;95:559–565. doi: 10.1530/jrf.0.0950559. [DOI] [PubMed] [Google Scholar]
- 61.Cappelletti M, et al. Type I interferons regulate susceptibility to inflammation-induced preterm birth. JCI Insight. 2017;2:e91288–e91288. doi: 10.1172/jci.insight.91288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. Am. J. Obstet. Gynecol. 2005;192:S36–46. doi: 10.1016/j.ajog.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 63.Villar J, et al. International standards for newborn weight, length, and head circumference by gestational age and sex: The Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.