As the main antibody-producing and antigen-presenting cells, B cells play critical roles in autoimmune diseases, tumors, and transplantation. Recently, the immunoregulatory functions of B cells have attracted attention and have been extensively studied in these fields. As a specific physiological status, pregnancy shares some common immunological characteristics with these diseases. Although many immune cells, such as natural killer cells, macrophages, and T cells, have been well studied during pregnancy, research focusing on B cells and their subsets during pregnancy has just begun.
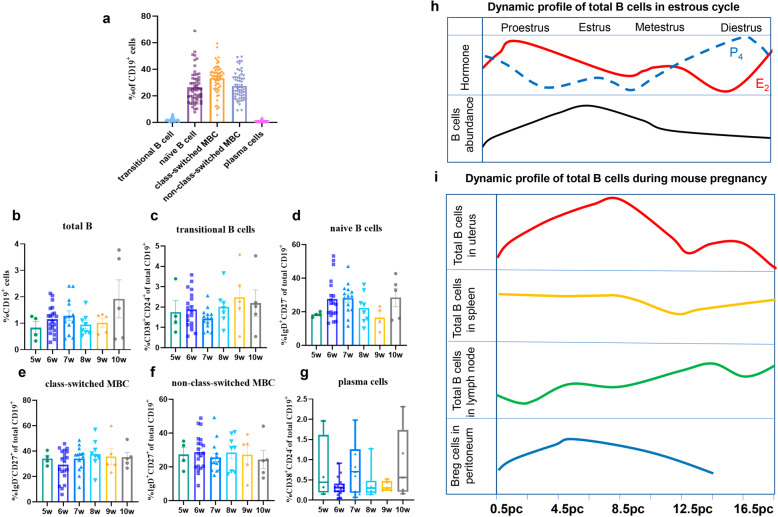
According to their developmental trajectory, B cells can be divided into five major cell subtypes in the peripheral blood: transitional B cells (TrB cells), naive B cells, class-switched memory B cells (MBCs), nonclass-switched MBCs, and plasma cells, and these subtypes account for approximately 4%, 50%, 15%, 18%, and 5% of peripheral B cells in healthy individuals, respectively.1 Although total B cells account for only 1–2% of decidual lymphocytes, we found that every stage of B-cell differentiation was present in the decidua in early pregnancy by flow cytometry (Fig. 1a, b and Table S1). Naive B cells, class-switched MBCs, and nonclass-switched MBCs represented the predominant B-cell subsets, accounting for 26.5%, 33.3%, and 27.5% of the total B-cell population, respectively (Fig. 1a and Fig. S1). In addition, total B cells and the TrB cell subset increased with increasing gestational weeks (Fig. 1b–g and Table S2). TrB cells and plasma cells comprised only a small portion of the total B-cell population (mean 2.04% and 0.73%, respectively) (Fig. 1a). The existence of different proportions of the same B-cell subsets in the peripheral blood and local decidua supported the concept that B-cell differentiation occurs in local areas beyond the peripheral blood and secondary lymphoid organs. Similarly, there are specific lymphoid aggregates found in lung cancer known as tertiary lymphoid structures, in which B-cell-rich areas contain different B-cell subsets and T cell-rich zones.2
Fig. 1.
Dynamic profile of B-cell subsets in the decidua during early human pregnancy and in the mouse uterus. a The percentages of B-cell subsets found in the early human decidua. b–g Dynamic profile of total B cells, transitional B cells, naive B cells, class-switched MBCs, nonclass-switched MBCs, and plasma cells with gestational weeks during early human pregnancy. h B-cell abundance in the mouse estrous cycle. The upper row represents the changes in estrogen and progesterone during the estrous cycle, and the bottom row of the figure shows the dynamic changes in total B cells during the estrous cycle. i Dynamic changes in total B cells in the uterus, spleen, and lymph nodes and Breg cells in the peritoneum as mouse pregnancy progresses. The data are shown as the mean ± SEM (one-way ANOVA) or median and interquartile range (nonparametric test). Breg cells: regulatory B cells. pc post conception, MBCs memory B cells
TrB cells represent a unique subset of B cells at an intermediate stage between bone marrow immature B cells and peripheral mature B cells, and these cells are also known as human regulatory B cells (Bregs, with the immunoregulatory function of high IL-10 production).3 Although there is a low percentage of TrB cells in peripheral blood, alterations in the frequency or function of TrB cells are associated with various autoimmune diseases, such as systemic lupus erythematosus, multiple sclerosis, and some neuroimmunological diseases. However, the paradoxical roles (promoting or alleviating disease progression) played in different autoimmune diseases may depend on the different subtypes of TrB cells.3 Although there is a low percentage of TrB cells in the early decidua, this percentage increases up to 20% of the total B-cell population in the term decidua basalis.4 Compared with that of gestational age-matched controls, the reduced percentage of decidual TrB cells in women with preterm birth might suggest a protective role in late pregnancy.4
B-cell activation factor (BAFF) is a vital cytokine for the survival and differentiation of TrB cells into naive B cells and is expressed by villous cytotrophoblasts, syncytiotrophoblasts, and decidual stroma cells in early pregnancy.5 More importantly, the level of serum BAFF is significantly decreased during the second and third trimesters compared to the first trimester, which may provide a partial explanation for the increased percentage of TrB cells in the decidua at term. However, the questions of how the percentage of TrB cells among total B cells increases tenfold from the first trimester to the third trimester, which critical regulatory factors are involved, and whether the subsets and roles of TrB cells in different gestational stages are the same remain to be answered and require further investigation.
Naive B cells, as the most abundant subset in the decidua in late pregnancy,4 also account for approximately 26.5% of decidual CD19+ B cells in the first trimester (Fig. 1a). Once exposed to an antigen, naive B cells quickly develop into either MBCs or antibody-secreting plasma cells. However, whether naive B cells become tolerant or undergo transformation toward memory and plasma cells upon antigen exposure depends on the assistance of T cells.6 A high proportion of MBCs may suggest that the humoral immune responses of B cells are more intense in early pregnancy than at term. By forming a mature immunologic synapse with T cells, naive B cells induce regulatory T (Treg) cells with powerful immunosuppressive functions.7 Therefore, the interactions between naive B cells and T-cell subsets may be a key point for elucidating the mechanism of maternal-fetal tolerance.
As shown in Fig. 1a, the percentages of class-switched MBCs and nonclass-switched MBCs are also high in the decidua in early pregnancy. However, the frequencies of class-switched and nonclass-switched MBCs are decreased to 10% and 1.8%, respectively, in decidua basalis at term.4 Class-switched MBCs have undergone class switching and express IgA, IgG, or IgE. Class switching often occurs during thymus-dependent antigen-induced humoral immune responses.8 During pregnancy, asymmetric IgG antibodies cannot activate effective immune responses and may protect against paternal antigens. The serum concentration of asymmetric IgG antibodies increases with increasing gestational weeks, reaches a peak in the second trimester and then declines in the third trimester.9 The increase in MBCs in the first trimester might be related to protective antibody production.
Although less research has focused on B-cell subsets in human pregnancy, studies in mice may shed light on the functions of B cells during pregnancy. In the infertile mouse uterus, total numbers of B cells fluctuate in parallel with the estrous cycle, with the lowest abundance at proestrus and the highest abundance accounting for 1.5% of viable cells at estrus (Fig. 1h).10 Early data showed that estrogen could directly upregulate the expression of several genes involved in B-cell activation and survival and protect primary B cells from B-cell receptor-mediated apoptosis.9 Although many studies have reported the relationship between excess B-cell activation and endometriosis, the location and function of B cells in the infertile uterus are still unknown. After conception, the proportion of decidual B cells increases with the gestational day, peaking at 8.5 days post conception, declining to a nonpregnancy level in the second trimester, and then persisting until the third trimester (Fig. 1i).11 These findings indicate that B cells may play a role during early pregnancy. The percentage of total B cells in the spleen and lymph nodes also changes with the gestational week during mouse pregnancy (Fig. 1i).11 Indeed, recent studies have begun to focus on the immune regulation of several B-cell subsets, especially Breg cells and B-1a B cells, during pregnancy.
Breg cells producing IL-10 (B10) have been extensively studied in tumors and autoimmune diseases.2,12 The proportion of IL-10-competent Breg cells in the mouse peritoneum gradually increases during early pregnancy and then decreases during the second trimester (Fig. 1i).13 Several studies have shown that decidual B cells exhibit a regulatory effect characterized by effective suppression of the activation and proliferation of syngeneic CD4+ T cells during the preimplantation period.11 Interestingly, the adoptive transfer of regulatory B10 cells into an abortion-prone model can reverse pregnancy loss and restore pregnancy tolerance by inhibiting the maturation of dendritic cells and increasing the frequency of Treg cells.9 In addition, an elevated percentage of B10 cells was also observed in the peripheral blood of first-trimester pregnant women, and these cells play a vital peripheral regulatory role during pregnancy.9 However, the phenotype of Breg cells is variable in different studies owing to their heterogeneity, as various B-cell subsets can exhibit immune-inhibitory phenotypes in different environments.12 Therefore, research on Breg cells during pregnancy is ongoing.
B-1a B cells and B-1b B cells constitute B1 cells and are recognized as a component of innate immunity, providing protection against specific pathogens. In an abortion-prone model, B-1a B cells in the peritoneal cavity showed high CD86 expression, which induced the differentiation of initial T cells into Th1 and Th17 cells in vitro, thereby contributing to the occurrence of pregnancy complications. Surprisingly, B-1a B cells from normal pregnancies inhibited this differentiation.14 The paradox may be related to cellular heterogeneity. Based on the expression of plasma cell alloantigen 1 (PC1), B-1a B cells can be divided into two unique subsets, designated B-1a PC1hi and B-1a PC1low. PC1hi cells are more efficient in producing IL-10 and regulating Th1 cell differentiation than PC1low cells.15 Indeed, these subsets show different functions during mouse pregnancy. PC1hi cells protect the fetus from rejection after adoptive transfer into an abortion-prone model, while the transfer of PC1low cells to a normal pregnancy model increases the abortion rate.13 Huang et al.16 showed that choriodecidual B cells provide IL-10/TGF-β/IL-35-independent protection against spontaneous preterm labor (PTL), mainly via the IL-33-induced expression of progesterone-induced blocking factor 1. Increased B cells in PTL patients manifest as CD43- and CD27-positive cells, indicating the B-1a cell phenotype. These data demonstrate that the appropriate function of B-1a cells is also important for normal pregnancy.
Therefore, in addition to producing antibodies, B cells play an immunoregulatory role in the pregnancy process. However, data on the profiles of B-cell subsets during mid-gestation are still lacking, and the available research on the function of B cells in pregnancy is limited. Further investigation needs to be conducted to explain the exact functions of B-cell subsets during pregnancy.
Supplementary information
Supplemental material-B-cell subsets during pregnancy: the dynamic profile and potential function
Gating strategies and representative flow cytometry analyses of decidual B-cell subsets in early human pregnancy
Acknowledgements
This study was supported by grants from the National Key Research & Developmental Program of China (2018YFC1003900 and 2018YFC1003904).
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Liling Wang, Panpan Jiang
Contributor Information
Chaohong Liu, Email: chaohongliu@hust.edu.cn.
Aihua Liao, Email: aihua_liao@sina.com.
Supplementary information
The online version of this article (10.1038/s41423-020-00535-1) contains supplementary material.
References
- 1.Zirakzadeh AA, Marits P, Sherif A, Winqvist O. Multiplex B cell characterization in blood, lymph nodes, and tumors from patients with malignancies. J. Immunol. 2013;190:5847–5855. doi: 10.4049/jimmunol.1203279. [DOI] [PubMed] [Google Scholar]
- 2.Wang SS, et al. Tumor-infiltrating B cells: their role and application in anti-tumor immunity in lung cancer. Cell Mol. Immunol. 2019;16:6–18. doi: 10.1038/s41423-018-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, et al. Transitional B cells involved in autoimmunity and their impact on neuroimmunological diseases. J. Transl. Med. 2020;18:131. doi: 10.1186/s12967-020-02289-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leng Y, et al. Are B cells altered in the decidua of women with preterm or term labor? Am. J. Reprod. Immunol. 2019;81:e13102. doi: 10.1111/aji.13102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundell AC, et al. IFN type I and II induce BAFF secretion from human decidual stromal cells. Sci. Rep. 2017;7:39904. doi: 10.1038/srep39904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner JS, Marthi M, Benet ZL, Grigorova I. Transiently antigen-primed B cells return to naive-like state in absence of T-cell help. Nat. Commun. 2017;8:15072. doi: 10.1038/ncomms15072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reichardt P, et al. Naive B cells generate regulatory T cells in the presence of a mature immunologic synapse. Blood. 2007;110:1519–1529. doi: 10.1182/blood-2006-10-053793. [DOI] [PubMed] [Google Scholar]
- 8.Weisel F, Shlomchik M. Memory B cells of mice and humans. Annu. Rev. Immunol. 2017;35:255–284. doi: 10.1146/annurev-immunol-041015-055531. [DOI] [PubMed] [Google Scholar]
- 9.Dutta S, Sengupta P, Haque N. Reproductive immunomodulatory functions of B cells in pregnancy. Int. Rev. Immunol. 2020;39:53–66. doi: 10.1080/08830185.2019.1674299. [DOI] [PubMed] [Google Scholar]
- 10.Diener KR, Robertson SA, Hayball JD, Lousberg EL. Multi-parameter flow cytometric analysis of uterine immune cell fluctuations over the murine estrous cycle. J. Reprod. Immunol. 2016;113:61–67. doi: 10.1016/j.jri.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Guzman-Genuino RM, Eldi P, Garcia-Valtanen P, Hayball JD, Diener KR. Uterine B cells exhibit regulatory properties during the peri-implantation stage of murine pregnancy. Front. Immunol. 2019;10:2899. doi: 10.3389/fimmu.2019.02899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin X, et al. IL-10-producing regulatory B cells restrain the T follicular helper cell response in primary Sjogren’s syndrome. Cell. Mol. Immunol. 2019;16:921–931. doi: 10.1038/s41423-019-0227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schumacher A, et al. Plasma cell alloantigen 1 and IL-10 secretion define two distinct peritoneal B1a B Cell subsets with opposite functions, PC1(high) cells being protective and PC1(low) cells harmful for the growing fetus. Front. Immunol. 2018;9:1045. doi: 10.3389/fimmu.2018.01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muzzio DO, et al. B-1a B cells regulate T cell differentiation associated with pregnancy disturbances. Front. Immunol. 2014;5:6. doi: 10.3389/fimmu.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H, et al. Expression of plasma cell alloantigen 1 defines layered development of B-1a B-cell subsets with distinct innate-like functions. Proc. Natl Acad. Sci. USA. 2012;109:20077–20082. doi: 10.1073/pnas.1212428109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang B, et al. Interleukin-33-induced expression of PIBF1 by decidual B cells protects against preterm labor. Nat. Med. 2017;23:128–135. doi: 10.1038/nm.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material-B-cell subsets during pregnancy: the dynamic profile and potential function
Gating strategies and representative flow cytometry analyses of decidual B-cell subsets in early human pregnancy