Abstract
Inhibition of glycolysis process has been an attractive approach for cancer treatment due to the evidence that tumor cells are more dependent on glycolysis rather than oxidative phosphorylation pathway. Preliminary evidence shows that inhibition of phosphoglycerate kinase 1 (PGK1) kinase activity would reverse the Warburg effect and make tumor cells lose the metabolic advantage for fueling the proliferation through restoration of the pyruvate dehydrogenase (PDH) activity and subsequently promotion of pyruvic acid to enter the Krebs cycle in glioma. However, due to the lack of small molecule inhibitors of PGK1 kinase activity to treat glioma, whether PGK1 could be a therapeutic target of glioma has not been pharmacologically verified yet. In this study we developed a high-throughput screening and discovered that NG52, previously known as a yeast cell cycle-regulating kinase inhibitor, could inhibit the kinase activity of PGK1 (the IC50 = 2.5 ± 0.2 μM). We showed that NG52 dose-dependently inhibited the proliferation of glioma U87 and U251 cell lines with IC50 values of 7.8 ± 1.1 and 5.2 ± 0.2 μM, respectively, meanwhile it potently inhibited the proliferation of primary glioma cells. We further revealed that NG52 (12.5–50 μM) effectively inhibited the phosphorylation of PDHK1 at Thr338 site and the phosphorylation of PDH at Ser293 site in U87 and U251 cells, resulting in more pyruvic acid entering the Krebs cycle with increased production of ATP and ROS. Therefore, NG52 could reverse the Warburg effect by inhibiting PGK1 kinase activity, and switched cellular glucose metabolism from anaerobic mode to aerobic mode. In nude mice bearing patient-derived glioma xenograft, oral administration of NG52 (50, 100, 150 mg· kg−1·d−1, for 13 days) dose-dependently suppressed the growth of glioma xenograft. Together, our results demonstrate that targeting PGK1 kinase activity might be a potential strategy for glioma treatment.
Keywords: PGK1; NG52; kinase inhibitor; Warburg effect; glioma
Introduction
Glioma is a common and malignant brain tumor in adults [1–3]. Due to its high invasiveness and diffuse growth, surgical interference is difficult [4]. Currently, the treatment of glioma is mainly surgical resection, followed by adjuvant chemotherapy and radiotherapy [5]. Despite significant improvements in treatment, tumor recurrence is inevitable [6], and patients have an expected 5-year survival rate of only 5% [7]. Therefore, new therapeutic strategies are still urgently needed.
Most cancer cells, even when oxygen is sufficient, produce energy mainly through a high rate of glycolysis rather than pyruvate oxidation in mitochondria [8, 9], which is known as the Warburg effect. Phosphoglycerate kinase 1 (PGK1), the first ATP-producing enzyme in the glycolysis pathway, catalyzes the transfer of an energetic phosphate from 1,3-diphosphate glycerol ester to ADP, resulting in the generation of 3-phosphoglyceric acid and ATP [10, 11]. In addition to its phosphatase activity, PGK1 has been extensively studied for its kinase activity. It has been reported that PGK1 can phosphorylate important proteins and regulates important physiological processes. The expression of PGK1 is upregulated in a wide range of human diseases, including glioma [12–14], breast cancer [15, 16], and hepatocellular carcinoma [17–19]. Lu et al. found that PGK1-mediated Beclin 1 Ser30 phosphorylation is necessary for brain tumorigenesis under hypoxic conditions [12]. They also reported that PGK1 can phosphorylate pyruvate dehydrogenase kinase 1 (PDHK1) at Thr338, which inhibits the activity of the pyruvate dehydrogenase (PDH) complex. This results in an increase in lactic acid production and the occurrence of brain tumors [14]. This evidence indicates that PGK1 plays an important role in maintaining metabolic homeostasis and is an attractive molecular target for glioma therapy.
Here, we report the discovery of NG52 [20], previously known to be a yeast cell cycle-regulating kinase inhibitor, as a PGK1 kinase inhibitor via a high-throughput drug screening approach. The results show that NG52 can reverse the Warburg effect by enhancing the activity of PDH through inhibiting the activity of PGK1. Cellular and animal model studies have shown that treatment with NG52 could potently inhibit the proliferation of glioma in vitro and in vivo.
Materials and methods
Chemical reagents
NG52 (CAS #212779-48-1, purity: 99.93%) and Temoside (also known as temozolomide) (CAS #85622-93-1, purity: 99.96%) were purchased from Chemexpress Inc (Shanghai, China).
Cell lines
Human glioma cell lines U87 and U251 were purchased from Cobioer Biosciences Co, Ltd (Nanjing, China). U87 cells were cultured in MEM (Corning, Midland, NY, USA) with 10% FBS (v/v) and supplemented with 1% penicillin/streptomycin (v/v), 1% NAP (v/v), and 1% nonessential amino acids (v/v). U251 cells were cultured in DMEM (Corning, Midland, NY, USA) with 10% FBS (v/v) supplemented with 1% penicillin/streptomycin (v/v).
Antibodies
The Phospho-PDH (Ser293) rabbit mAb (#31866s), PDHK1 antibody rabbit mAb (#3820s), N-cadherin rabbit mAb (#D21H3s), vimentin rabbit mAb (#5741s), and GAPDH XP rabbit mAb (#D16H11) were obtained from Cell Signaling Technology (Danvers, MA, USA). The PGK1 mouse mAb (#ab113687) was obtained from Abcam (Cambridge, UK). The Phospho-PDHK1 (Tyr338) rabbit mAb (#11596-1) was obtained from SAB (College Park, MD, USA).
Primary glioma cells
Glioma samples were obtained after informed consent was obtained at Anhui Provincial Hospital (Hefei, China). Details of the patient samples are provided in Supplementary Table S1. Fragments of freshly obtained tumor tissues were dissociated using collagenase/hyaluronidase and dispase (Stem Cell Technologies, Vancouver, BC, Canada) at 37 °C for 4 h with shaking. Primary cells were collected by centrifugal filtration and plated in DMEM/F12 medium with the Gibco B-27 serum-free supplement, bFGF (40 ng/mL), EGF (20 ng/mL), and 1% insulin. The cells were cultured at 37 °C in a humidified 5% CO2 incubator, and the medium was changed every 2 days. All glioma samples were obtained through written consent under the approval of the Chinese Academy of Sciences Institutional Review Board, and all experimental protocols were approved by the Medical Ethics Committee of Hefei Institutes of Physical Science (Chinese Academy of Sciences).
Protein expression and purification
PGK1–p28a was transformed into Transetta competent cells in 4 mL of LB medium overnight. BL21 cells (4 mL) were seeded in 400 mL of LB medium, and the protein was induced by 0.5 mM IPTG at 30 °C for 4 h. The cells were lysed in extraction buffer (20 mM Tris, 500 mM NaCl, pH 8.0). The supernatant was loaded onto a nickel affinity column (GE Healthcare, Milwaukee, WI, USA) and washed with buffer containing 80 mM imidazole. The protein was eluted using 300 mM imidazole in the same buffer.
High-throughput screening for PGK1 kinase inhibitors
Recombinant PGK1 was incubated for 1 h at room temperature with substrate 3-phosphoglycerate (0.4 μg/μL) (Yuanye, Shanghai, China) and 10 μM ATP (Promega, Madison, WI) in 20 µL of reaction buffer (20 mM Tris, 100 mM NaCl, 0.1 mM MgCl2, 2 mM DTT, pH 8.6) containing compound or DMSO in 384-well plates. Then, 20 µL of ADP-Glo™ reagent (Promega) was added to each well and incubated for 40 min to stop the reaction and deplete the remaining ATP. Next, 40 µL of Kinase Detection Reagent (Promega) was added to convert the ADP to ATP, and the newly synthesized ATP was detected with a luciferase/luciferin system. Luminescence was measured with a multilabel reader (Envision, PerkinElmer, MA, USA). GI50 values were calculated using Prism 5.0 (GraphPad Software, San Diego, CA, USA).
Proliferation assay
Cells were grown in 24-well culture plates for 12 h before compounds of various concentrations were added. Cell proliferation was determined after treatment with compounds for 6 days. Cell Titer-Glo assays were performed according to the manufacturer’s instructions (Promega), and luminescence was measured with a multilabel reader (Envision, PerkinElmer). GI50 values were calculated using Prism 5.0 (GraphPad Software).
PGK1 phosphatase assay
To test the inhibitory effect of NG52 on PGK1 phosphatase activity, an assay was performed as previously reported [21]. Briefly, purified recombinant PGK1 protein (0.2 ng/μL) was mixed with DMSO or different concentrations of NG52 for 30 min. Then, a saturated amount of substrates (1.6 mM GAP, 1 mM β-NAD, 1 mM ADP and 20 ng/μL GAPDH) was added, and the reaction was conducted for 1 h in buffer (20 mM Tris, 100 mM NaCl, 0.1 mM MgSO4, 10 mM Na2HPO4, 2 mM DTT, pH 8.6). The production of ATP was detected with an ADP-Glo kinase assay (Promega).
Apoptosis analysis
The apoptosis assay was performed using the FITC Annexin V apoptosis detection kit (Becton Dickinson, Franklin Lake, NJ, USA). Assays were performed according to the manufacturer’s instructions. Data were collected with a BD FACS Calibur cytometer (Becton Dickinson) and analyzed using Flow Jo (Becton Dickinson).
Reactive oxygen species (ROS) detection
U87 and U251 cells were treated with different concentrations of NG52 for 12 h. Then, the cells were harvested and incubated with DCFH-DA (Beyotime Biotechnology, Shanghai, China) at 37 °C for 20 min. Flow cytometry was performed using a FACS Calibur (Becton Dickinson), and the results were analyzed by Flow Jo (Becton Dickinson).
Detection of ATP and lactate
The ATP level was detected using a kit from Promega (Promega). The cells were treated with DMSO or NG52 (10 μM) for 12 and 48 h, the relative total ATP level was detected with Cell Titer-Glo assays (Promega, Madison, WI, USA), and the relative ATP level for each cell was calculated using the total ATP level divided by the cell number. Lactate measurements followed a kit protocol (EnzyChrom™ lactate assay kit, BioAssay Systems). The cells were treated with NG52 for 12 h, and then lactate was measured with a detection kit.
ADP-Glo biochemical assay
The ADP-Glo kinase assay (Promega) was used to test NG52 for its PGK1 kinase inhibition effects. Recombinant PGK1 was incubated for 1 h at room temperature with the substrate 3-phosphoglycerate (0.4 μg/μL) (Yuanye, Shanghai, China) and 10 μM ATP (Promega) in 10 µL of reaction buffer containing compound or DMSO. Detection was performed according to the manufacturer’s instructions.
Colony formation
U87 and U251 cells were plated into 6-well plates at 5000 cells per well, treated with NG52 or DMSO and cultured for 6 days. The colonies were fixed with 10% (v/v) methanol for 15 min and stained with 5% crystal violet staining solution for 15 min for colony visualization.
Cell culture under hypoxic conditions
Cells were cultured in a hypoxia incubator chamber (Heracell VIOS 160i incubator with variable oxygen control, Thermo Fisher Scientific, Lenexa, KS, USA) with an oxygen concentration of 2%. Culture medium and cell handling protocols were the same as those for routine cell culture under normoxic conditions.
Glioma orthotopic transplantation tumor model
Five-week-old female nu/nu mice were purchased from the Nanjing Biomedical Research Institute of Nanjing University (Nanjing, China). All animals were housed in a specific pathogen-free facility and used according to the animal care regulations of Hefei Institutes of Physical Science, Chinese Academy of Sciences (Hefei, China). Cells were harvested and resuspended in sterile PBS, and one hundred thousand primary glioma cells were injected into the brains of nu/nu mice. Animals were then randomized into treatment groups for efficacy studies. Daily treatment with the compound was initiated after 2 weeks. A range of doses of NG52 or vehicle was administered daily by oral gavage. Body weights and survival rates were measured daily after NG52 treatment.
Molecular modeling
All calculations were performed using the Schrödinger Suites (Schrödinger Release 2018-3: Schrödinger, LLC, New York, NY, USA, 2018). The human PGK1 complex (PDB ID: 5NP8) was used for docking studies. The crystal structure was prepared using the Protein Preparation Wizard, and the ligand was built in Mastro and prepared with LigPrep docked with Glide using XP mode.
Data and statistical analysis
The results are presented as the means ± SEM. Student’s t test was used for comparisons among the different groups. IC50 and GI50 values were calculated using Prism 5 (Graph Pad Software, San Diego, CA, USA) using the normalized dose response curve for inhibition (variable slope).
Results
Identification of NG52 as a PGK1 kinase inhibitor
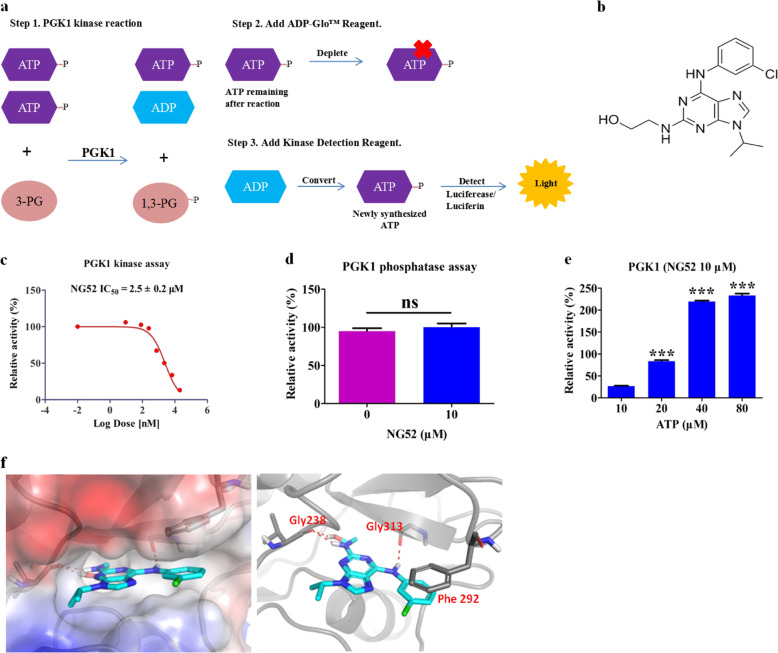
To identify PGK1 inhibitors, we performed a biochemical assay-based screening with a pool of over 5000 bioactive small molecules. Briefly, recombinant PGK1 was incubated for 1 h at room temperature with the substrate 3-phosphoglycerate (0.4 μg/μL) (Yuanye, Shanghai, China) and 10 μM ATP (Promega, Madison, WI, USA) in 20 μL of reaction buffer (20 mM Tris, 100 mM NaCl, 0.1 mM MgCl2, 2 mM DTT, pH 8.6) containing compound or DMSO in 384-well plates. Then, 20 μL of ADP-Glo™ reagent was added to each well and incubated for 40 min to stop the reaction and deplete the remaining ATP. Next, 40 μL of kinase detection reagent was added to convert the ADP to ATP, and the newly synthesized ATP was detected with a luciferase/luciferin system (Fig. 1a). As a result, we found that compound NG52 [20], a known yeast cell cycle-regulating kinase inhibitor, potently inhibits the kinase activity of PGK1 (Fig. 1b, c) without affecting its phosphatase activity [21] (Fig. 1d). To investigate the inhibition mode of NG52, ATP competition experiments were performed. Consistent with the competitive inhibition mode, the inhibition of PGK1 by NG52 decreased with increasing concentrations of ATP (Fig. 1e), which suggests that NG52 inhibits PGK1 in an ATP-competitive manner. Furthermore, molecular modeling of the hPGK1-NG52 structure (PDB ID: 5NP8) showed the possible formation of three hydrogen bonds between NG52 and PGK1, including the aromatic side chains of Gly238 and Gly313 (Fig. 1f), which indicates the direct binding of NG52 with the PGK1 protein.
Fig. 1. Characterization of a novel PGK1 small molecule inhibitor.
a Flow chart for the high-throughput screening of PGK1 kinase inhibitors. b Chemical structure of NG52. c IC50 determination of NG52 with purified PGK1 using the ADP-Glo assay, n = 3. d The effect of NG52 on purified PGK1 phosphatase activity. The NG52 dosage is indicated, n = 3. e The mode of inhibition of NG52 with PGK1 was examined using the ADP-Glo assay with the indicated concentration of ATP, n = 3. f Molecular modeling of PGK1 and NG52 binding. The results are the mean ± SD of three independent experiments; ns not statistically significant; ***P < 0.001.
Effects of NG52 on the proliferation of glioma cells in vitro
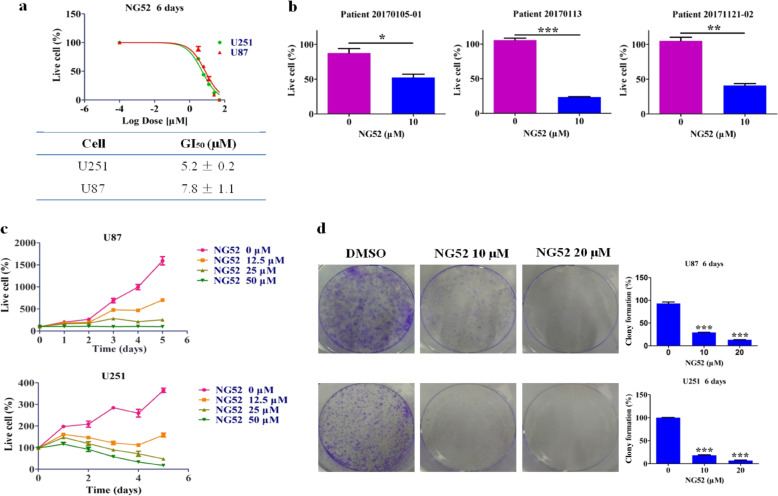
Since PGK1 is frequently overexpressed in glioma, we wanted to determine whether NG52 treatment can inhibit glioma cell viability. We examined the in vitro antiproliferative effects of NG52 in various glioma cell lines and primary glioma patient samples. The results showed that NG52 achieved considerable dose-dependent inhibition against the tumor cell lines tested with GI50 values <8 μM (Fig. 2a) and potently inhibited the proliferation of primary glioma cells (Fig. 2b). We also examined the antiproliferative effects of NG52 on normal cells and found that the inhibition of NG52 on normal cells was weaker than on glioma cells (Supplementary Table S2). In addition, a time-course antiproliferation assay showed that NG52 was able to inhibit U87 cell proliferation after 1 day of treatment, which indicates the high potency of this compound (Fig. 2c). Then, we investigated the effects of NG52 on the clonogenic formation activities of the glioma cell lines. We found that in both U87 and U251 cell lines, treatment with NG52 significantly reduced the number of the clones formed, providing more evidence for the antiproliferative effects of NG52 in glioma cells (Fig. 2d).
Fig. 2. Impact of NG52 on the proliferation of glioma cells in vitro.
a Dose-dependent inhibition against the tumor cell lines with NG52, n = 3. b The antiproliferative activity of NG52 against primary glioma cells, n = 3. c The time course of the NG52-induced effects on glioma cells, n = 3. d Effects of NG52 on glioma cell colony formation after incubation for 6 days. Quantification is shown in the right panel. Data are the mean ± SD (n = 3). The results are the mean ± SD of three independent experiments; ns not statistically significant; *P < 0.05, **P < 0.01, and ***P < 0.001.
NG52 inhibits glioma cell proliferation through on-target inhibition of PGK1
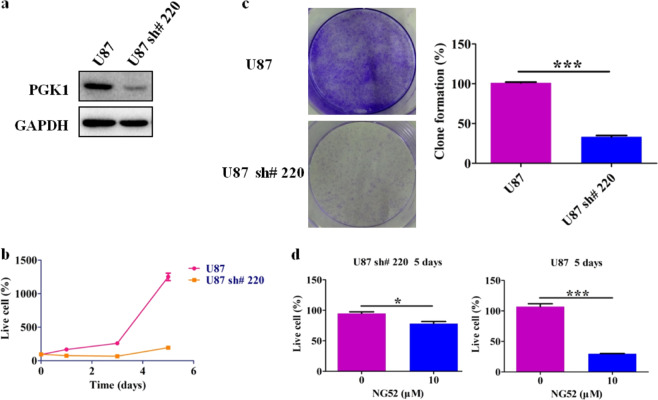
To exclude the possibility that NG52 caused cell proliferation inhibition by affecting targets other than PGK1, we further examined whether NG52 inhibits the proliferation of glioma cancer cells by directly inhibiting PGK1. First, we constructed a U87 cell line with reduced expression of PGK1 by virus-mediated RNAi knockdown. Western blot analysis showed that the expression of PGK1 in U87 sh#220 cells was significantly reduced (Fig. 3a). We then performed long-term proliferation experiments with this cell line, which showed that reduction of PGK1 significantly inhibited glioma cell proliferation (Fig. 3b). Clonogenic experiments further demonstrated that reduced expression of PGK1 led to a reduction in clony formation (Fig. 3c). These results indicate that PGK1 plays an important role in the proliferation of glioma cells and that inhibition of PGK1 can effectively impede the proliferation of glioma cells. In addition, compared with parental U87 cells, we found that the proliferation of U87 sh#220 cells with reduced PGK1 expression was less sensitive to NG52 treatment (Fig. 3d). Taken together, these results suggest that NG52 inhibits glioma cell proliferation by on-target inhibition of PGK1.
Fig. 3. NG52 inhibits the proliferation of glioma cancer cells by inhibiting PGK1.
a Western blot analysis was performed to detect the expression of PGK1 in U87 and U87 sh#220 cells. b Reduction of PGK1 inhibits the proliferation of U87 cells, n = 3. c Reduction of PGK1 inhibits clony formation of U87 cells, n = 3. d Proliferation of U87 sh#220 cells with reduced PGK1 expression was less sensitive to NG52 treatment, n = 3. The results are the mean ± SD of three independent experiments; ns not statistically significant; *P < 0.05, ***P < 0.001.
NG52 reversed the Warburg effect in glioma cells
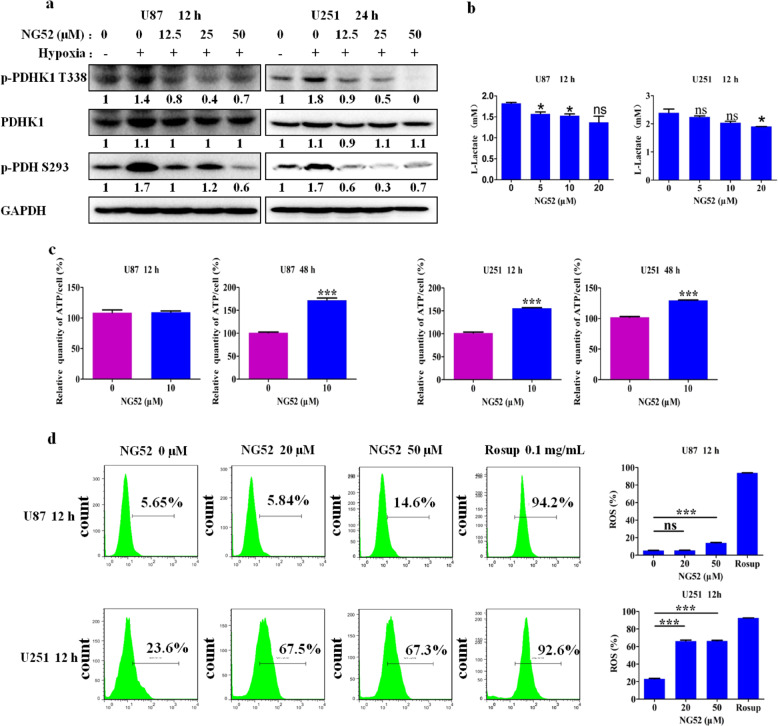
Anaerobic metabolism is a feature of cancers and plays a key role in promoting their development. In glioma, PGK1 inhibits PDH activity by phosphorylating PDHK1, which phosphorylates PDH and inhibits pyruvate from entering into the tricarboxylic acid cycle; this thereby converts cells from undergoing aerobic metabolism to undergoing anaerobic metabolism, resulting in the inhibition of mitochondrial respiration and the production ROS. We next investigated whether NG52 can reverse this Warburg effect by examining the effects of NG52 on PGK1-related proteins. Our results showed that NG52 potently inhibits the phosphorylation of PDHK1 at residue Thr338 and enhances the activity of PDH by inhibiting the phosphorylation of PDH at residue Ser293, which causes more pyruvate to enter into the tricarboxylic acid cycle (Fig. 4a). As expected, with reversal of the Warburg effect, we also found that treatment with NG52 inhibited the production of lactate (Fig. 4b), increased mitochondrial respiration to produce more ATP (Fig. 4c) and increased the production of ROS (Fig. 4d). Taken together, our results showed that NG52 can reverse the Warburg effect in glioma cells.
Fig. 4. NG52 reversed the Warburg effect in glioma cells.
a Inhibition of NG52 on the phosphorylation of PDHK1 and PDH in glioma cells was examined by Western blot after glioma cells were treated with NG52 for 12 h under hypoxic conditions. b Lactate production of U87 and U251 cells treated with NG52 was examined using an EnzyChrom™ lactate Assay Kit after treatment with NG52 for 12 h under hypoxic conditions, n = 3. c ATP levels in U87 and U251 cells were examined after glioma cells were treated with NG52 for the indicated times, n = 3. d ROS production of U87 and U251 cells treated with NG52 was examined using a FACS Calibur (Becton Dickinson), and the results were analyzed by FlowJo (Becton Dickinson), n = 3. The results are the mean ± SD of three independent experiments; ns not statistically significant; *P < 0.05, ***P < 0.001.
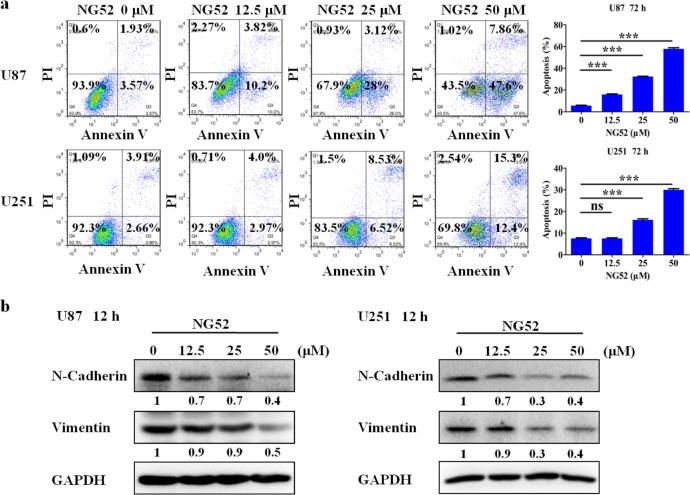
NG52 induces apoptosis and inhibits epithelial–mesenchymal transition (EMT) in glioma cells
We next studied the effects of NG52 on apoptosis. Exposure of U87 and U251 cells to NG52 for 3 days significantly increased the Annexin V-positive cell populations, indicating that NG52 can potently induce apoptosis in glioma (Fig. 5a). An increasing number of studies have confirmed that the Warburg effect also promotes tumor EMT [22]. Hence, reversing the Warburg effect may inhibit tumor EMT [23]. We therefore examined the expression of N-cadherin and vimentin, two main biomarkers of EMT [24]. Western blot analysis indicated that NG52 could downregulate N-cadherin and vimentin in both U87 and U251 cells (Fig. 5b), indicating that NG52 reversed the EMT phenotype in U87 and U251 cells.
Fig. 5. NG52 induces apoptosis and inhibits EMT in glioma cells.
a FCM analysis of cells stained with Annexin V-FITC/PI after treatment with various concentrations of NG52 for 3 days. Quantified values of apoptosis are shown, n = 3. b After treatment with or without NG52 for 12 h, Western blot analysis was performed to detect the expression of N-cadherin and vimentin in glioma cells. The NG52 group was compared with the DMSO group, and the significance of the difference is indicated; ns not statistically significant; ***P < 0.001.
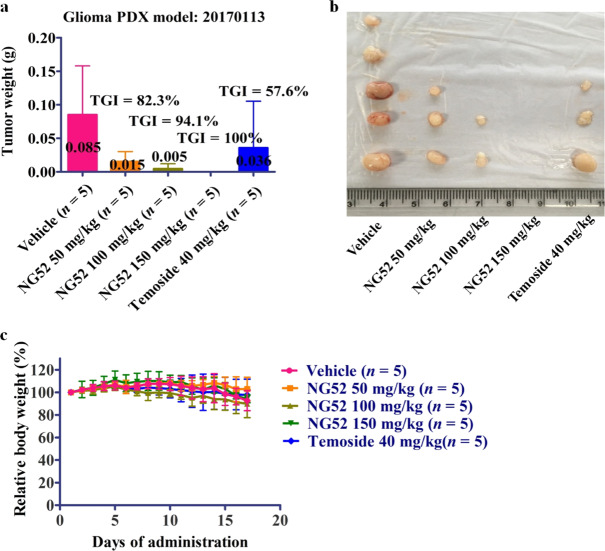
NG52 potently inhibits glioma tumor growth in vivo
Next, we evaluated the antitumor activity of NG52 in vivo using a glioma PDX model. NG52 potently inhibits the growth of glioma PDX in a dose-dependent manner. Tumor growth inhibition reached 100% at 150 mg/kg in the PDX model. These results indicate that NG52 potently inhibits the proliferation of glioma in vivo (Fig. 6a, b). We also observed that the effects of NG52 on the body weights of the mice were very small, indicating that NG52 has high safety (Fig. 6c). Overall, these results showed that NG52 potently inhibits the growth of glioma in vivo.
Fig. 6. NG52 potently inhibits glioma in vivo.
a Comparison of the final tumor weight in each group after the 17-day treatment period with NG52, n = 5. b Mice engrafted with PDXs received vehicle or NG52 for 17 days. Representative photographs of the tumors in each group are shown, n = 5. c Body weight measurements from the mice after 17 days of NG52 administration. The initial body weight was set as 100%, n = 5.
Discussion
The Warburg effect is the process by which glucose metabolism is converted from aerobic metabolism to anaerobic metabolism, even under normal oxygen concentrations [25]. Therefore, unlike normal cells, tumor cells convert most of their glucose into lactic acid rather than undergoing oxidative phosphorylation [8, 26], which serves as an adaptive response that allows tumor cells to produce ATP in the absence of oxygen [27–29], thus providing a biosynthetic advantage for tumor cells.
Although attempts have been made to block aerobic glycolysis in tumor cells using small molecules, these attempts have not been successful thus far [30, 31]. Several new treatments targeting different steps in the glycolysis process are currently being evaluated, including inhibition of lactate dehydrogenase [32] and pyruvate kinase M2 [9, 33–36].
Since normal cells also need glycolysis and subsequent oxidative phosphorylation to provide energy, simply inhibiting the glycolysis process will inevitably affect the physiological functions of normal cells, resulting in toxic side effects. Therefore, we proposed that reversing the Warburg effect to convert tumor cell metabolism from anaerobic metabolism to aerobic metabolism may be an effective method for the treatment of tumors. PGK1 is a key enzyme in glycolysis and has also been shown to play an important role in regulating cellular glucose metabolism to undergo the oxidative phosphorylation pathway in glioma [14]. PGK1 can inhibit PDH activity by phosphorylating PDHK1, which phosphorylates PDH, blocking the entrance of pyruvic acid into the tricarboxylic acid cycle, and thus enhancing anaerobic metabolism. Therefore, targeting PGK1 not only inhibits the glycolysis process but also restores PDH activity, prompts more pyruvate to enter into the tricarboxylic acid cycle, and allows cells to return to their normal metabolic processes. Hence, we propose that the inhibition of PGK1 kinase activity is a good strategy for the treatment of glioma.
Through a high-throughput screening approach, we discovered a novel PGK1 kinase inhibitor, NG52. Importantly, NG52 inhibits PGK1 kinase activity without affecting its phosphatase activity. Antiproliferation assays showed that NG52 exhibited good antiproliferative effects in both glioma cell lines and primary glioma cells. A mechanistic study illustrated that NG52 can effectively inhibit the phosphorylation of PDHK1 at the Thr338 site and the phosphorylation of PDH at the Ser293 site, resulting in more pyruvic acid entering the tricarboxylic acid cycle with increased production of ATP and ROS. Therefore, NG52 can reverse the Warburg effect by inhibiting PGK1 kinase activity and switching cellular glucose metabolism from an anaerobic mode to an aerobic mode, thereby inhibiting the growth of tumor cells. These data indicated that inhibiting the kinase activity of PGK1 might be a potential novel therapeutic target for glioma.
Although our data showed that the small molecule NG52 can impede the proliferation of glioma cells in vitro and in xenograft models, NG52 is not an ideal PGK1 kinase inhibitor, and its inhibitory effect needs further improvement. Nevertheless, this study supports the notion that PGK1 is critical for the development of glioma, and inhibiting PGK1 kinase activity may provide a novel and effective therapeutic approach for the treatment of glioma.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 81773777, 81673469, 81872748, 81803366), the National Key Research and Development Program of China (Grant 2016YFA0400900), the Natural Science Foundation of Anhui Province (Grant 1908085QH348), the Science and Technology Major Projects of Anhui Province (Grant 17030801025), the China Postdoctoral Science Foundation (Grants 2018T110634, 2018M630720, 2019M652057), the Frontier Science Key Research Program of CAS (Grant QYZDB-SSW-SLH037), the CASHIPS Director’s Fund (Grant BJPY2019A03), and the Key Program of 13th Five-Year Plan of CASHIPS (Grant KP-2017-26). We are also grateful to the National Program for Support of Top-Notch Young Professionals for Jing Liu.
Author contributions
QSL and JL designed the research. WLW, ZRJ, ALW, and CH developed the methodology. WLW, ZRJ, ZQH, and LW performed the experiments and collected the data. WLW, WCW, and QSL wrote the manuscript. LW, CC, and CH provided the experiment materials. QSL, WCW, and JL revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Wen-liang Wang, Zong-ru Jiang
Contributor Information
Jing Liu, Email: jingliu@hmfl.ac.cn.
Wen-chao Wang, Email: wwcbox@hmfl.ac.cn.
Qing-song Liu, Email: qsliulab97@hmfl.ac.cn.
Supplementary information
The online version of this article (10.1038/s41401-020-0465-8) contains supplementary material, which is available to authorized users.
References
- 1.Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129:829–48. doi: 10.1007/s00401-015-1432-1. [DOI] [PubMed] [Google Scholar]
- 2.Hess KR, Broglio KR, Bondy ML. Adult glioma incidence trends in the United States, 1977–2000. Cancer. 2004;101:2293–9. doi: 10.1002/cncr.20621. [DOI] [PubMed] [Google Scholar]
- 3.Roth P, Gramatzki D, Weller M. Management of elderly patients with glioblastoma. Curr Neurol Neurosci Rep. 2017;17:35. doi: 10.1007/s11910-017-0740-3. [DOI] [PubMed] [Google Scholar]
- 4.Molina JR, Hayashi Y, Stephens C, Georgescu M-M. Invasive glioblastoma cells acquire stemness and increased Akt activation. Neoplasia. 2010;12:453–63. doi: 10.1593/neo.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 7.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14:1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 9.Yang W, Lu Z. Regulation and function of pyruvate kinase M2 in cancer. Cancer Lett. 2013;339:153–8. doi: 10.1016/j.canlet.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein BE, Hol WGJ. Crystal structures of substrates and products bound to the phosphoglycerate kinase active site reveal the catalytic mechanism. Biochemistry. 1998;37:4429–36. doi: 10.1021/bi9724117. [DOI] [PubMed] [Google Scholar]
- 11.Li X, Zheng Y, Lu Z. PGK1 is a new member of the protein kinome. Cell Cycle. 2016;15:1803–4. doi: 10.1080/15384101.2016.1179037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qian X, Li X, Cai Q, Zhang C, Yu Q, Jiang Y, et al. Phosphoglycerate kinase 1 phosphorylates Beclin1 to induce autophagy. Mol Cell. 2017;65:917–31.e6. doi: 10.1016/j.molcel.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qian X, Li X, Lu Z. Protein kinase activity of the glycolytic enzyme PGK1 regulates autophagy to promote tumorigenesis. Autophagy. 2017;13:1246–7. doi: 10.1080/15548627.2017.1313945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Jiang Y, Meisenhelder J, Yang W, Hawke DH, Zheng Y, et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61:705–19. doi: 10.1016/j.molcel.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D, Tai LK, Wong LL, Chiu LL, Sethi SK, Koay ES. Proteomic study reveals that proteins involved in metabolic and detoxification pathways are highly expressed in HER-2/neu-positive breast cancer. Mol Cell Proteomics. 2005;4:1686–96. doi: 10.1074/mcp.M400221-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Grundker C, Wokoun U, Hellriegel M, Emons G. Inhibition of aerobic glycolysis enhances the anti-tumor efficacy of Zoptarelin doxorubicin in triple-negative breast cancer cells. J Obstet Gynaecol Res. 2019;45:1334–42. doi: 10.1111/jog.13980. [DOI] [PubMed] [Google Scholar]
- 17.Ai J, Huang H, Lv X, Tang Z, Chen M, Chen T, et al. FLNA and PGK1 are two potential markers for progression in hepatocellular carcinoma. Cell Physiol Biochem. 2011;27:207–16. doi: 10.1159/000327946. [DOI] [PubMed] [Google Scholar]
- 18.Xie H, Tong G, Zhang Y, Liang S, Tang K, Yang Q. PGK1 drives hepatocellular carcinoma metastasis by enhancing metabolic process. Int J Mol Sci. 2017;18:1630. doi: 10.3390/ijms18081630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu H, Zhu W, Qin J, Chen M, Gong L, Li L, et al. Acetylation of PGK1 promotes liver cancer cell proliferation and tumorigenesis. Hepatology. 2017;65:515–28. doi: 10.1002/hep.28887. [DOI] [PubMed] [Google Scholar]
- 20.Gray NS, Wodicka L, Thunnissen A-MWH, Norman TC, Kwon S, Espinoza FH, et al. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science. 1998;281:533–8. doi: 10.1126/science.281.5376.533. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Zhao C, Li X, Wang T, Li Y, Cao C, et al. Terazosin activates Pgk1 and Hsp90 to promote stress resistance. Nat Chem Biol. 2015;11:19–25. doi: 10.1038/nchembio.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–64. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Dong B. The correlation of hypoxic tumor microenvironment, Warburg effect and epithelial-mesenchymal transition. Chin J Biochem Mol Biol. 2018;34:949–53. [Google Scholar]
- 24.Zhang Q, Dong P, Liu X, Sakuragi N, Guo SW. Enhancer of Zeste homolog 2 (EZH2) induces epithelial-mesenchymal transition in endometriosis. Sci Rep. 2017;7:6804. doi: 10.1038/s41598-017-06920-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 26.Luengo A, Gui DY, Vander Heiden MG. Targeting metabolism for cancer therapy. Cell Chem Biol. 2017;24:1161–80. doi: 10.1016/j.chembiol.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakazawa MS, Keith B, Simon MC. Oxygen availability and metabolic adaptations. Nat Rev Cancer. 2016;16:663–73. doi: 10.1038/nrc.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–97. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, et al. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A. 2010;107:2037–42. doi: 10.1073/pnas.0914433107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–34. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Zachar Z, Marecek J, Maturo C, Gupta S, Stuart SD, Howell K, et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med. 2011;89:1137–48. doi: 10.1007/s00109-011-0785-8. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun. 2014;5:4436. doi: 10.1038/ncomms5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anastasiou D, Yu Y, Israelsen WJ, Jiang JK, Boxer MB, Hong BS, et al. Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat Chem Biol. 2012;8:839–47. doi: 10.1038/nchembio.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J, Xie J, Jiang Z, Wang B, Wang Y, Hu X. Shikonin and its analogs inhibit cancer cell glycolysis by targeting tumor pyruvate kinase-M2. Oncogene. 2011;30:4297–306. doi: 10.1038/onc.2011.137. [DOI] [PubMed] [Google Scholar]
- 36.Giannoni E, Taddei ML, Morandi A, Comito G, Calvani M, Bianchini F, et al. Targeting stromal-induced pyruvate kinase M2 nuclear translocation impairs OXPHOS and prostate cancer metastatic spread. Oncotarget. 2015;6:24061–74. doi: 10.18632/oncotarget.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.