Abstract
Fas/Fas ligand (FasL)-mediated cell apoptosis involves a variety of physiological and pathological processes including chronic hepatic diseases, and hepatocytes apoptosis contributes to the development of liver fibrosis following various causes. However, the mechanism of the Fas/FasL signaling and hepatocytes apoptosis in liver fibrogenesis remains unclear. The Fas/FasL signaling and hepatocytes apoptosis in liver samples from both human sections and mouse models were investigated. NF-κBp65 wild-type mice (p65f/f), hepatocytes specific NF-κBp65 deletion mice (p65Δhepa), p53-upregulated modulator of apoptosis (PUMA) wild-type (PUMA-WT) and PUMA knockout (PUMA-KO) littermate models, and primary hepatic stellate cells (HSCs) were also used. The mechanism underlying Fas/FasL-regulated hepatocytes apoptosis to drive HSCs activation in fibrosis was further analyzed. We found Fas/FasL promoted PUMA-mediated hepatocytes apoptosis via regulating autophagy signaling and NF-κBp65 phosphorylation, while inhibition of autophagy or PUMA deficiency attenuated Fas/FasL-modulated hepatocytes apoptosis and liver fibrosis. Furthermore, NF-κBp65 in hepatocytes repressed PUMA-mediated hepatocytes apoptosis via regulating the Bcl-2 family, while NF-κBp65 deficiency in hepatocytes promoted PUMA-mediated hepatocytes apoptosis and enhanced apoptosis-linked inflammatory response, which contributed to the activation of HSCs and liver fibrogenesis. These results suggest that Fas/FasL contributes to NF-κBp65/PUMA-modulated hepatocytes apoptosis via autophagy to enhance liver fibrogenesis, and this network could be a potential therapeutic target for liver fibrosis.
Subject terms: Apoptosis, Extracellular signalling molecules
Introduction
Liver fibrosis represents one of the major consequences of morbidity and mortality worldwide1,2, and the activation of hepatic stellate cells (HSCs), which is regulated by multiple cell populations or soluble mediators, is the major source of extracellular matrix substances (ECM)3–5. Hepatocytes are the major parenchymal cells of the liver and essential for maintaining the function and organization of the liver6. It’s widely accepted that the progression of hepatic fibrosis is associated with considerable injury and loss of hepatocytes, which may present as a main inflammatory stimulus for HSCs activation7,8. The apoptotic cells could release the nucleotides ATP and UTP, which bind to purinergic receptors (especially the P2Y2 receptor) on macrophages and HSCs, leading to their activation9,10. As a classic apoptosis modulator, Fas, following Fas ligand (FasL) engagement, leads to the recruitment of Fas-associated proteins having death domains and the initiators into a death-inducing signaling complex (DISC) to cause apoptosis11–13, and the Fas/FasL has been demonstrated to participate in the pathological processes of hepatic fibrosis/cirrhosis, acute/chronic hepatitis and hepatocarcinoma14–16.
Autophagy is an evolutionarily conserved and catabolic process that targets cytosolic material, organelles and long-lived proteins to lysosomes to be degraded for survival, development, differentiation, and homeostasis17–19. Hepatocytes have been revealed to own a high level of autophagic flux because of their increased abundance of lysosomes and lysosomal enzymes, and the enhanced autophagy could modulate the progression to hepatocytes death20, and our previous study has suggested autophagy was required for liver fibrogenesis21. Nuclear factor-κB (NF-κB), as a ubiquitous and inducible transcription factor responsible for mediating the expression of a large number of genes involved in differentiation, apoptosis, and proliferation22, and NF-κBp65, as the main functional element, involves in various physiological and pathological events and influences the survival of hepatocytes and activation of HSCs23. Activation of NF-κB in non-parenchymal cells promotes inflammation, fibrosis, and hepatocarcinogenesis in the liver, whereas suppression of NF-κB in parenchymal cells enhances hepatocarcinogenesis in some cases and retards hepatocarcinogenesis in others10,24. The activation of NF-κB in HSCs appears to promote hepatic fibrosis via modulating fibrogenic effects and anti-apoptotic effects9. While decreased or absent NF-κB activity in hepatocytes might lead to subsequent fibrosis by regulating hepatocytes injury and the primary trigger of fibrogenic responses in liver23–26.
Considerable death and loss of hepatocytes induced by different etiologies are always associated with the initiation and progression of hepatic fibrosis1,27. p53-upregulated modulator of apoptosis (PUMA), is one of the most potent mediators of apoptosis induced by various stimuli28. PUMA deficiency could block cell apoptotic responses to p53 activation, DNA-damaging agents, and hypoxia in hepatocytes29. In response to multiple insults, PUMA functions through other Bcl-2 family members, including Bcl-2, Mcl-1, and Bcl-xL, to induce mitochondrial dysfunction and caspases activation30,31. PUMA-mediated apoptotic response in hepatocytes is a direct cause of compensatory proliferation and carcinogenesis in the liver32. Mice deficient in NF-κB signaling or the anti-apoptotic Bcl-2 family members developed spontaneous hepatocellular carcinoma secondary to hepatocytes death33, and PUMA has been demonstrated to be a target of NF-κBp65 and a critical mediator of TNF-α-induced hepatocytes apoptosis34. However, the role of NF-κBp65 in PUMA-regulated hepatocytes apoptosis in liver fibrogenesis remains unknown.
In the current study, we found that Fas/FasL promoted PUMA-mediated hepatocytes apoptosis via autophagy and NF-κBp65 signaling, while inhibition of autophagy or PUMA deficiency attenuated Fas/FasL-modulated hepatocytes apoptosis and liver fibrosis. Furthermore, hepatocytes deletion of NF-κBp65 promoted PUMA-mediated hepatocytes apoptosis via regulating the Bcl-2 family, and the enhanced hepatocytes apoptosis linked inflammatory action to drive HSCs activation and liver fibrogenesis. Thus, we suggest that Fas/FasL contributes to NF-κBp65/PUMA-regulated hepatocytes apoptosis via autophagy to enhance liver fibrogenesis.
Results
Fas/FasL-mediated apoptosis involved in liver fibrogenesis
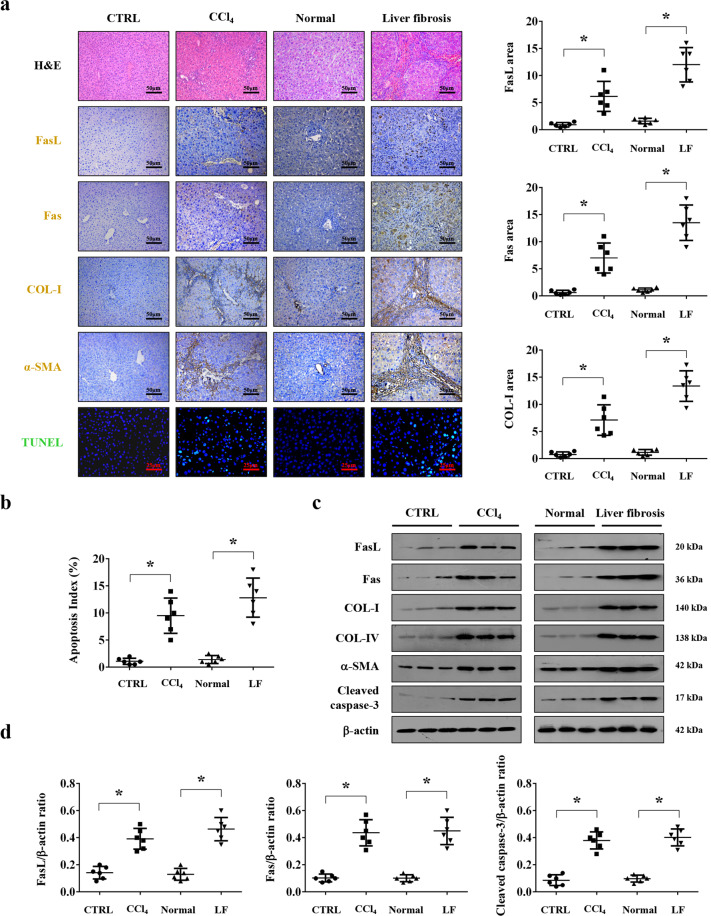
To evaluate the role of Fas/FasL in liver fibrosis, liver specimens from healthy volunteers and liver fibrosis patients were analyzed. Histological staining presented a loss of preserved architecture and excess deposition of ECM, associated with obvious hepatic apoptosis and upregulated Fas/FasL expression, were observed in the liver fibrotic tissues compared with the normal samples (Fig. 1a, b). Western blotting also confirmed a similar condition (Fig. 1c, d). By using mouse model, we found that CCl4 injection-induced prominent liver fibrosis in mice as shown by α-SMA and COL-I staining, which was accompanied with obvious apoptosis and Fas/FasL upregulation (Fig. 1a, b), and western blotting further represented that the levels of Fas, FasL, α-SMA, COL-I, COL-IV, and cleaved caspase-3 were enhanced in the fibrotic sections (Fig. 1c, d). These results indicated that Fas/FasL involved in hepatic apoptosis in liver fibrogenesis.
Fig. 1. Fas/FasL-mediated apoptosis involved in liver fibrogenesis.
a H&E staining, Fas, FasL, collagen-I (COL-I), α-SMA immunohistochemical staining (brown), and TUNEL staining (green) in the related liver tissues were presented. FasL, Fas, and COL-I area from the histological staining were also determined. Values are presented as mean ± SEM. *P < 0.05, n = 6 per group. CTRL, the control mice (olive oil-treated mice); CCl4, 20% carbon tetrachloride-induced mouse fibrosis; Normal, healthy volunteers; LF, human liver fibrosis. b The apoptotic index from TUNEL staining was presented. n = 6 in each group, values are presented as mean ± SEM. *P < 0.05. c Western blotting represented that the levels of Fas, FasL, α-SMA, COL-I, COL-IV, and cleaved caspase-3 were enhanced in liver fibrotic sections. β-actin was used as the loading control. d The ratio of densitometry units of the normalized FasL/β-actin, Fas/β-actin, and cleaved caspase-3/β-actin was also presented (n = 6 per group), values are presented as mean ± SEM. *P < 0.05.
Autophagy participated in Fas/FasL-mediated hepatic apoptosis in liver fibrosis
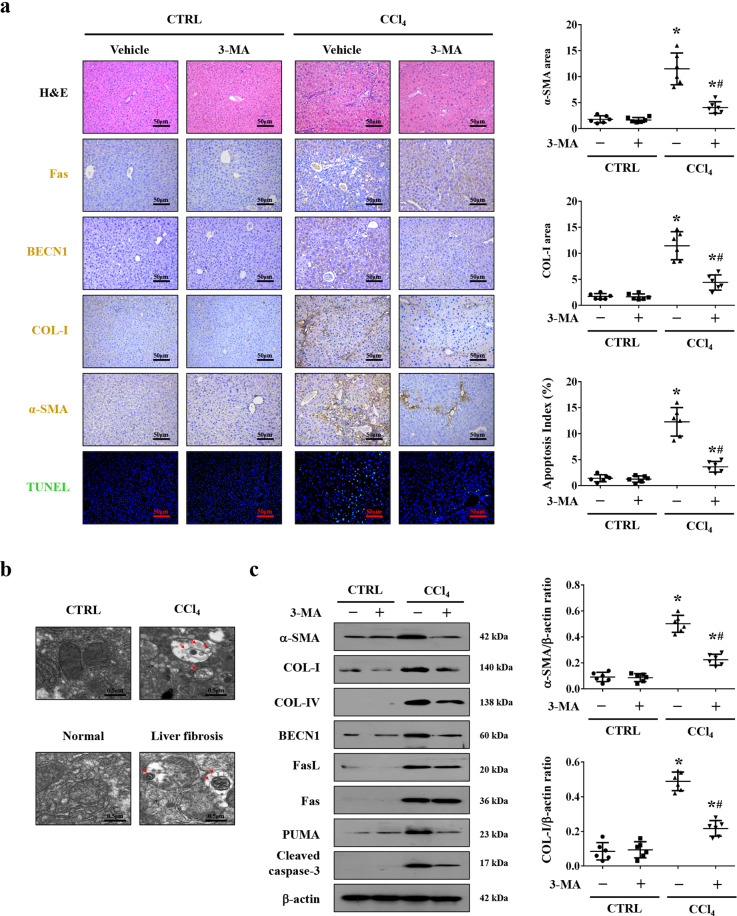
Our preceding data has testified that autophagy was required for liver fibrosis21. In the current study, we found that the autophagic element BECN1 was enhanced in liver fibrosis, which was associated with the upregulated α-SMA and COL-I expressions and apoptotic signaling (Fig. 2a), and ultrastructural analysis showed the typical autophagosomes in the hepatocytes of mouse and human liver fibrotic sections but scarce in the normal tissues (Fig. 2b). We pretreated mice with autophagic inhibitor 3-methyladenine (3-MA) and found 3-MA treatment alleviated the degree of apoptosis and liver fibrosis in mice, and did not affect the levels of Fas and FasL (Fig. 2a, c and Supplementary Fig. 1a). To sum up, these data suggested that autophagy participated in Fas/FasL-mediated hepatic apoptosis in liver fibrosis.
Fig. 2. Autophagy participated in Fas/FasL-mediated hepatic apoptosis in liver fibrosis.
a H&E staining, Fas, BECN1, COL-I, α-SMA staining (brown), and TUNEL staining (green) were presented in the indicated sections from olive oil- (as CTRL) or CCl4-treated mice, with or without 3-MA administration. α-SMA area and COL-I area from the histological staining, and the apoptotic index (TUNEL staining) were also determined. *P < 0.05 versus CTRL mice, #P < 0.05 versus CCl4-treated mice without 3-MA treatment, n = 6 per group. b Ultrastructural features in the hepatocytes of liver tissues from human tissues and mouse models were presented (red arrows indicating autophagosomes). c Western blotting depicted that inhibition of autophagy by 3-MA downregulated the expressions of BECN1, α-SMA, COL-I, COL-IV, PUMA, and cleaved caspase-3, without affecting the state of Fas and FasL, in CCl4-treated mice. The ratio of densitometry units of the normalized α-SMA/β-actin and COL-I/β-actin was presented (n = 6 per group), values are presented as mean ± SEM. *P < 0.05 versus CTRL mice, #P < 0.05 versus CCl4-treated mice without 3-MA treatment.
PUMA responded to Fas/FasL/autophagy-regulated hepatocytes apoptosis and HSCs activation during liver fibrosis
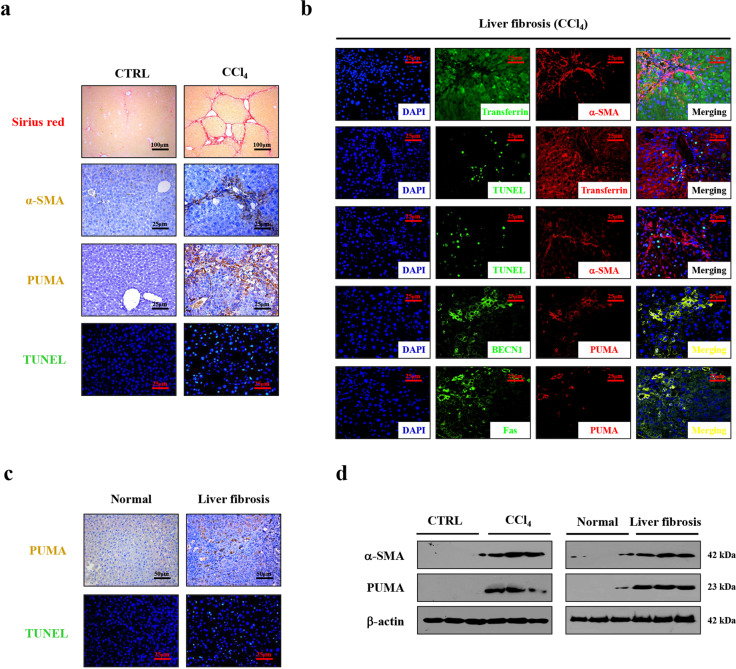
PUMA has been demonstrated to be a target of Fas/FasL signaling and a critical mediator of apoptosis in our previous study35. By analyzing the mouse models, we found that, accompanying by the loss of preserved architecture and excess production and deposition of ECM, the expressions of α-SMA, PUMA and the number of apoptotic cells (TUNEL staining) were increased in the liver fibrotic tissues (Fig. 3a). The enhanced PUMA expression and hepatic apoptosis were also observed in the human fibrotic tissues (Fig. 3c). Western blotting further revealed that the levels of α-SMA and PUMA were obviously enhanced in the mouse and human liver fibrotic tissues (Fig. 3d). Double staining by utilizing HSCs marker α-SMA and hepatocytes marker transferrin revealed the apoptotic cells were mainly localized in hepatocytes but not in HSCs (Fig. 3b). Co-staining of BECN1 and PUMA, Fas, and PUMA demonstrated they were mainly localized in similar cells (Fig. 3b). Western blotting showed that inhibition of autophagy by 3-MA repressed the upregulation of PUMA and caspase-3 cleavage in CCl4-induced mouse fibrosis (Fig. 2c and Supplementary Fig. 1a).
Fig. 3. PUMA responded to Fas/FasL/autophagy-mediated hepatocytes apoptosis during liver fibrosis.
a Sirius red staining (red), α-SMA (brown), PUMA staining (brown), and TUNEL staining (green) were presented in the indicated sections from CTRL and CCl4-treated mice (n = 6 per group). b Double IF staining by utilizing HSCs marker α-SMA (red) and hepatocytes marker transferrin (green) were presented (upper panel). Double staining of transferrin (red) and TUNEL (green), α-SMA (red), and TUNEL (green) indicated that the apoptotic cells mainly located in hepatocytes in liver fibrotic mice (middle panel). Co-staining of BECN1 (green) and PUMA (red), Fas (green), and PUMA (red) further revealed that PUMA involved in Fas and autophagy-regulated signaling (lower panel). Nuclei (blue) were counterstained with DAPI (4′6-diamidino-2-phenylindole dihydrochloride). c PUMA immunohistochemistry staining (brown) and TUNEL staining (green) in the indicated humans liver sections were presented. d PUMA and α-SMA levels were determined by western blotting.
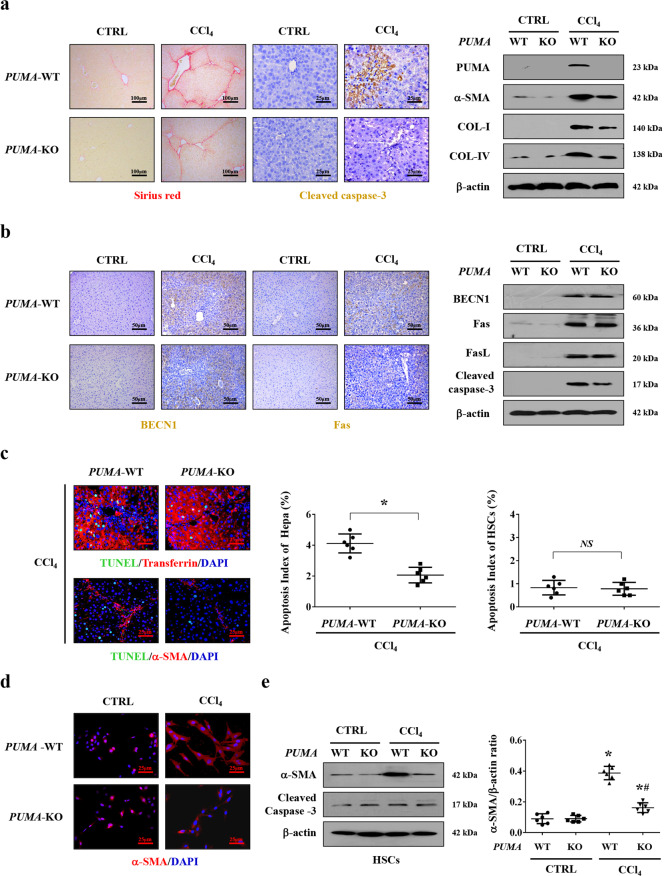
CCl4-induced PUMA-WT and PUMA-KO liver fibrotic mouse models were adopted, and we found that deletion of PUMA ameliorated the collagen deposition, the levels of COL-IV, COL-I, α-SMA, and caspase-3 cleavage in CCl4-treated mice, although there was no obvious distinction between PUMA-WT and PUMA-KO mice from the control group (Fig. 4a). By the way, PUMA was reported to be expressed lowly or barely in normal cells and tissues, but is rapidly induced in response to a wide range of stresses in human and mouse cells29,31. Due to a very low level of PUMA in normal tissues, the low concentration of total detected proteins, and the limited detection capacity, we could not detect obvious PUMA signaling in the normal PUMA-WT mouse and further make a definite distinction between normal PUMA-WT mouse and normal PUMA-KO mouse, however, the levels of PUMA were obviously enhanced in the mouse and human liver fibrotic sections (Fig. 3a, c, d and Fig. 4a). PUMA deficiency did not affect the levels of Fas, FasL, and BECN1, but downregulated cleaved caspase-3 in CCl4-induced mouse fibrosis (Fig. 4b). By utilizing co-staining analysis, we found the apoptotic cells were mainly localized in hepatocytes, and deletion of PUMA decreased the number of hepatocytes apoptosis (Fig. 4c). Lastly, the activation of primary HSCs dissociated from CCl4-treated PUMA-WT mice was enhanced compared with that from CCl4-treated PUMA-KO mice, while PUMA deletion could not interfere with the apoptosis of primary HSCs (Fig. 4d, e). These observations revealed that PUMA contributed to Fas/FasL/autophagy-regulated hepatocytes apoptosis and HSCs activation in liver fibrosis.
Fig. 4. Targeted deletion of PUMA ameliorated hepatocytes apoptosis and liver fibrosis.
a Sirius red staining (red) and cleaved caspase-3 staining (brown) presented that targeted deletion of PUMA ameliorated hepatic apoptosis and collagen deposition. Western blotting presented that targeted deletion of PUMA ameliorated the levels of collagen-IV (COL-IV), collagen-I (COL-I), and α-SMA in CCl4-treated mice (n = 6 per group). b Immunohistochemistry staining and western blotting revealed that PUMA deficiency did not affect the status of Fas and BECN1 in CCl4-treated mice. c Double immunofluorescence staining (transferrin (red) and TUNEL (green), α-SMA (red) and TUNEL (green)) and the analysis of the apoptotic index of hepatocytes or HSCs indicated that targeted deletion of PUMA mainly ameliorated hepatocytes apoptosis during liver fibrosis, n = 6 per group. Nuclei (blue) were counterstained with DAPI. *P < 0.05. NS, no significance. d α-SMA (red) staining in the primary HSCs dissociated from the indicated PUMA-WT and PUMA-KO mice was represented, nuclei (blue) were counterstained with DAPI. e The indicated proteins from primary HSCs analyzed by western blotting. The ratio of densitometry units of the normalized α-SMA/β-actin was also determined, n = 6 per group, values are presented as mean ± SEM. *P < 0.05 versus primary HSCs from CTRL mice, #P < 0.05 versus primary HSCs from CCl4-treated PUMA-WT mice.
Fas/FasL repressed the activation of NF-κBp65 in hepatocytes in liver fibrogenesis
Decreased or absent NF-κB activity in hepatocytes might lead to subsequent fibrosis by regulating hepatocytes injury and the primary trigger of fibrogenic responses in the liver, and NF-κBp65 involved in the regulation of various pathological events in chronic liver diseases9. To investigate the correlation between Fas/FasL and NF-κBp65 in liver fibrosis, liver specimens from humans and CCl4-induced mouse model were analyzed, and histological staining presented that, in contrast to the upregulation of Fas/FasL signaling in the fibrotic tissues, the activation of NF-κBp65 (phosphorylation of NF-κBp65, p-p65) was obviously repressed in the liver fibrotic tissues compared with their normal samples (Fig. 5a). Western blotting also confirmed a similar condition (Fig. 5b, c). By using primary hepatocytes isolated from the mouse models, upregulated Fas expression and downregulated p-p65 level were observed in the primary hepatocytes dissociated from CCl4-treated mice, while knockdown of Fas by siRNA significantly enhanced the activation of NF-κBp65 (p-p65) in the primary hepatocytes dissociated from CCl4-treated mice (Fig. 5d). What is more, FasL administration promoted the expression of Fas and repressed the level of p-p65 in the primary isolated hepatocytes cells, and knockdown of Fas promoted the activation of NF-κBp65 (Fig. 5e). By the way, we also found that knockdown of Fas did not obviously influence the status of NF-κBp65 and its phosphorylation in the control group, which might suggest that a very low level of Fas/FasL signaling under normal condition could not regulate NF-κBp65 pathway in hepatocytes. In summary, these findings indicated that Fas/FasL repressed the activation of NF-κBp65 in hepatocytes in liver fibrogenesis.
Fig. 5. Fas/FasL repressed the activation of NF-κBp65 in hepatocytes in liver fibrogenesis.
a Immunohistochemical staining (brown) of Fas, FasL, and p-p65 (the phosphorylation of NF-κBp65) in the indicated liver sections was presented (n = 6 per group). b Expressions of Fas, FasL, p65, p-p65, and COL-I proteins from the related humans liver sections were detected by western blotting. c Western blotting was adopted to analyze the levels of Fas, FasL, p65, p-p65, and COL-I in the liver tissues of mouse models. The ratio of densitometry units of the normalized Fas/β-actin and p-p65/β-actin from (b) and (c) was also determined, n = 6 per group, values are presented as mean ± SEM. *P < 0.05. CTRL, the control mice (olive oil-treated mice); CCl4, 20% carbon tetrachloride-induced mouse fibrosis; Normal, healthy volunteers; LF, human liver fibrosis. d Western blotting depicted that knockdown of Fas upregulated the level of p-p65 in the primary hepatocytes isolated from CCl4-treated mice. The ratio of densitometry units of the normalized p65/β-actin and p-p65/β-actin was also presented, n = 6 per group, values are presented as mean ± SEM. *P < 0.05 versus primary hepatocytes from CTRL group, #P < 0.05 versus primary hepatocytes from CCl4-treated group without siFas treatment. e FasL treatment enhanced the expression of Fas and repressed the phosphorylation of NF-κBp65 (p-p65) in the primary hepatocytes, while knockdown of Fas by siRNA upregulated the level of p-p65 in FasL-treated group. The ratio of densitometry units of the normalized p65/β-actin and p-p65/β-actin was also presented, n = 6 per group, values are presented as mean ± SEM. *P < 0.05 versus primary hepatocytes from PBS group, #P < 0.05 versus primary hepatocytes from FasL-treated group without siFas treatment.
NF-κBp65 inhibited PUMA-mediated hepatocytes apoptosis via Bcl-2 family and attenuated liver fibrosis
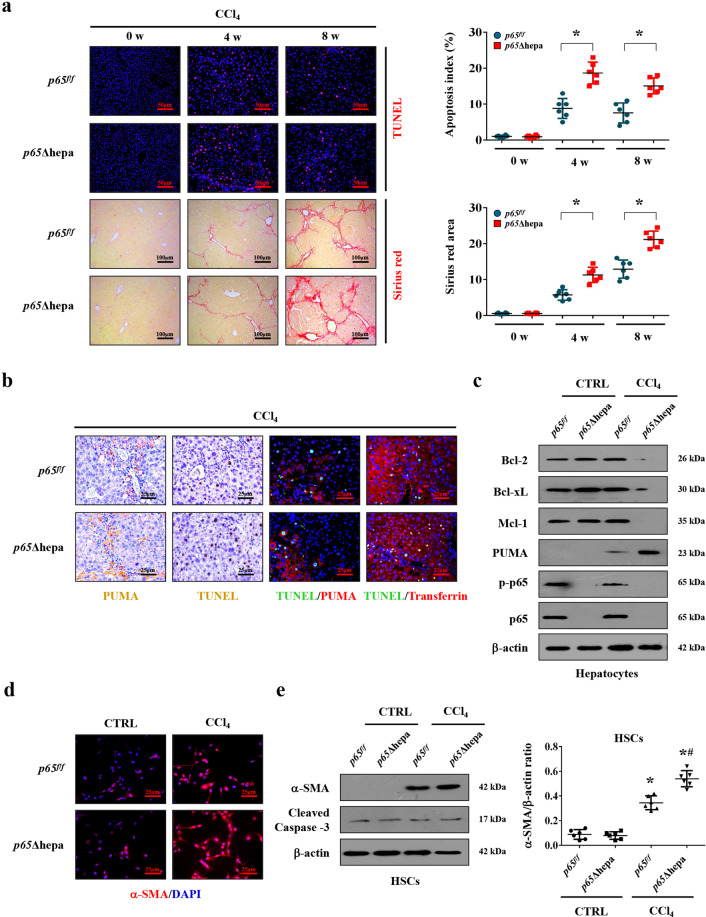
By using hepatocytes specific NF-κBp65 deletion (p65Δhepa) and NF-κBp65 wild-type (p65f/f) fibrotic mouse models, TUNEL analysis performed at the different time-points of fibrosis showed that cells apoptosis was exacerbated in CCl4-treated p65Δhepa mice, with an apoptotic index that was higher compared with p65f/f mice. What is more, sirius red staining detected at the distinct points of fibrosis development disclosed that significant enhanced accumulation of collagen was found in CCl4-treated p65Δhepa mice than CCl4-treated p65f/f mice (Fig. 6a). Moreover, the phosphorylation of NF-κBp65 (p-p65) was repressed in CCl4-treated p65f/f mice, and p-p65-positive signaling in p65Δhepa mice was only localized in the non-parenchymal cells due to hepatocytes specific NF-κBp65 deletion in p65Δhepa mice (Supplementary Fig. 2a), these data suggest that downregulated or absent NF-κBp65 activity could lead to increase hepatocytes apoptosis in the initiation of liver fibrosis. The levels of PUMA, associates with cell apoptosis, induced by CCl4 were upregulated in the liver tissues of p65Δhepa mice compared to p65f/f mice (Fig. 6b, c). Double staining of TUNEL and PUMA, TUNEL, and transferrin indicated that PUMA and TUNEL signaling were mainly located in hepatocytes of CCl4-treated p65Δhepa mice (Fig. 6b). Furthermore, we found that the activation (detected by α-SMA) of primary HSCs rather than their death (detected by cleaved caspase-3) was visibly enhanced in CCl4-treated p65Δhepa mice than that in p65f/f mice (Fig. 6d, e).
Fig. 6. NF-κBp65 inhibited PUMA-mediated hepatocytes apoptosis and attenuated liver fibrosis.
a TUENL staining (red) and sirius red staining at the indicated time points were adopted to showed that NF-κBp65 deficiency in hepatocytes promoted apoptosis and collagen deposition during fibrogenesis. The apoptotic index and sirius red area were also analyzed. *P < 0.05. p65Δhepa: hepatocytes specific NF-κBp65 deletion; p65f/f: NF-κBp65 wild-type. b Immunohistochemical staining revealed NF-κBp65 deficiency in hepatocytes induced PUMA expression and cell apoptosis (brown). Double staining of TUNEL (green) and PUMA (red) indicated that PUMA expression and TUNEL signaling were located in similar cells, and co-staining of TUNEL (green) and transferrin (red) was also presented. Cell nuclei (blue) were counterstained by DAPI. c Western blotting represented hepatocytes specific NF-κBp65 deletion promoted the upregulation of PUMA and downregulated the levels of anti-apoptotic proteins Bcl-2, Bcl-xL, and Mcl-1 in primary hepatocytes isolated from CCl4-treated mice, and the phosphorylation of NF-κBp65 (p-p65) was repressed in primary hepatocytes isolated from CCl4-treated p65f/f mice. d Primary HSCs dissociated from the indicated mice were stained by α-SMA (red). Nuclei (blue) were counterstained with DAPI. e The indicated proteins from primary isolated HSCs in the mouse models were detected by western blotting. The ratio of densitometry units of the normalized α-SMA/β-actin was also determined. *P < 0.05 versus primary HSCs from CTRL mice, #P < 0.05 versus HSCs from CCl4-treated p65f/f mice. n = 6 per group.
The pro-survival Bcl-2 family members actively sequester the pro-apoptotic BH3-only members, like PUMA, and finally inhibit the induction of apoptosis36–38. By microanalysis screening for primary hepatocytes from mouse models, we found that PUMA (BBC3) in primary hepatocytes from fibrotic tissues presented a higher expression than that from normal tissues, while the Bcl-2 family members were not affected, it seems that the unchanged Bcl-2 family could not be enough to suppress the detrimental effects of PUMA during liver fibrosis (Supplementary Fig. 2b). Western blotting revealed that the expressions of Bcl-2, Bcl-xL, and Mcl-1 were downregulated in the primary hepatocytes of p65Δhepa fibrotic mice (Fig. 6c). Adopting the Bcl-2 inhibitor ABT-199, we revealed that the expressions of PUMA and α-SMA, along with enhanced hepatocytes apoptosis and collagen deposition, were upregulated compared to that without ABT-199 administration (Supplementary Fig. 2c). By the way, the phosphorylation of NF-κBp65 (p-p65) was repressed in primary hepatocytes isolated from CCl4-treated p65f/f mice compared to those from the control group (Fig. 6c), while ABT-199 did not influence the phosphorylation of NF-κBp65 in primary hepatocytes both from the control group and CCl4-treated p65f/f mice (Supplementary Fig. 2d). Lastly, primary HSCs analysis suggested that ABT-199 administration promoted HSCs activation in liver fibrosis (Supplementary Fig. 2e), These data suggested that NF-κBp65 regulated PUMA-mediated hepatocytes apoptosis and liver fibrosis via Bcl-2 family.
NF-κBp65/PUMA-regulated hepatocytes apoptosis drove inflammatory response to promote HSCs activation and liver fibrosis
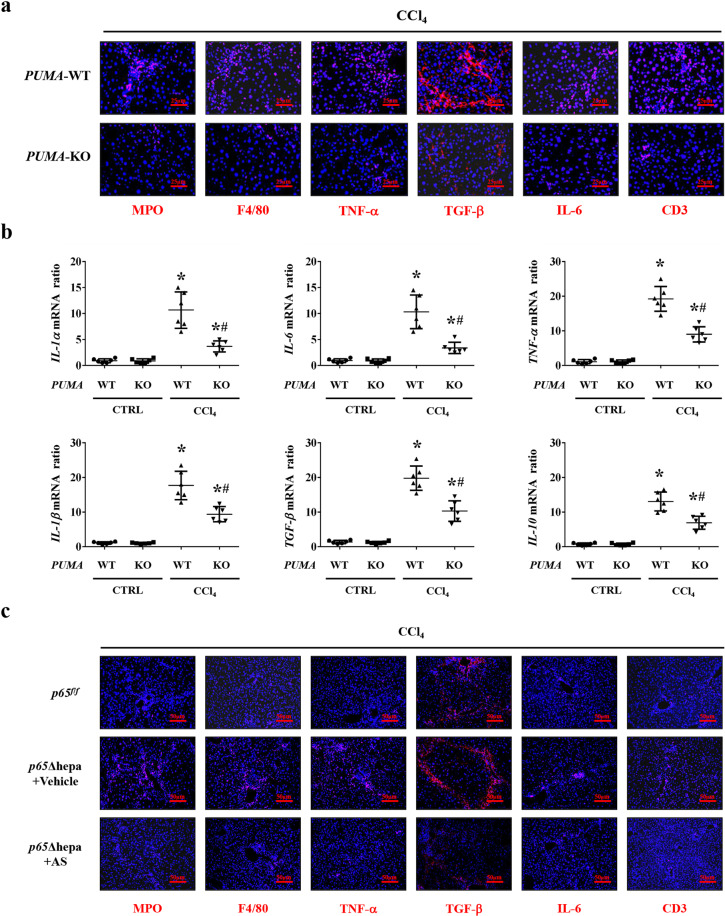
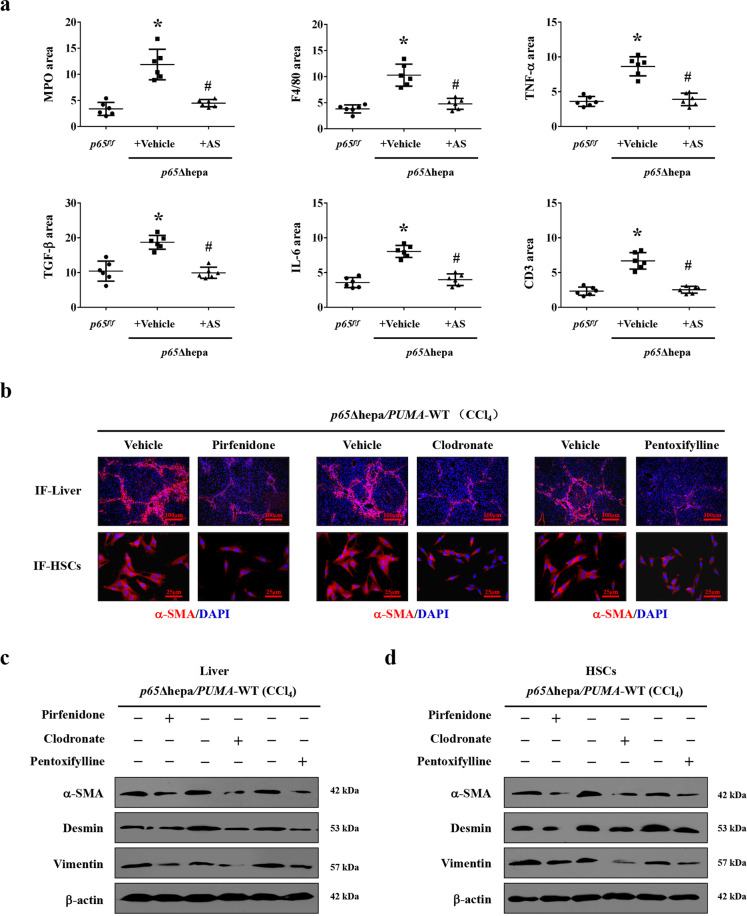
Dying hepatocytes could release alarmins and activate inflammatory cells to produce various inflammatory cytokines to contribute to the initiation and development of liver fibrosis9,10. Based on these, after CCl4 treatment, the hepatic inflammatory cells or mediators, including myeloperoxidase (MPO, neutrophil), F4/80 (macrophages), TNF-α, TGF-β, IL-6, and CD3 (lymphocytes) were upregulated in PUMA-WT mice compared to PUMA-KO mice (Fig. 7a). The inflammatory cytokines mRNA, including IL-1α, IL-6, TNF-α, IL-1β, TGF-β, and IL-10, were also enhanced in CCl4-treated PUMA-WT mice (Fig. 7b). Furthermore, MPO, F4/80, TNF-α, TGF-β, IL-6, and CD3 were upregulated in p65Δhepa mice compared to p65f/f mice, while PUMA antisense oligonucleotides (AS) treatment suppressed this inflammatory response (Figs. 7c and 8a). By adopting the TGF-β1 inhibitor pirfenidone, TNF-α inhibitor pentoxifylline, and monocytes depressor clodronate-loaded liposomes in p65Δhepa/PUMA-WT mice, we found inhibition of inflammatory action ameliorated liver fibrosis (Fig. 8b, c). α-SMA levels in primary HSCs isolated from the CCl4-treated p65Δhepa/PUMA-WT mice were visibly upregulated, while above inhibitors administration could hold back the activation of HSCs (Fig. 8b, d). These data indicated that PUMA-promoted HSCs activation depended on the inflammatory response following hepatocytes apoptosis.
Fig. 7. NF-κBp65/PUMA-regulated hepatocytes apoptosis enhanced hepatic inflammatory response.
a The indicated inflammatory cytokines in the mouse livers were analyzed via immunofluorescence staining (red), the related cytokines were significantly repressed in CCl4-treated PUMA-KO mice, nuclei (blue) were counterstained with DAPI. b Inflammatory factors mRNA expression in the livers from either CCl4-treated PUMA-WT or PUMA-KO mice were analyzed by quantitative reverse-transcription PCR. *P < 0.05 versus CTRL mice, #P < 0.05 versus CCl4-treated PUMA-WT mice. The expression of β-actin in each tissue was quantified as the internal control. n = 6 per group. c Expressions of indicated inflammatory cytokines in the livers were analyzed via immunofluorescence staining (red), the related cytokines were repressed in CCl4-treated p65Δhepa mice following PUMA antisense oligonucleotides (AS), nuclei (blue) were counterstained with DAPI.
Fig. 8. NF-κBp65/PUMA-regulated hepatocytes apoptosis-linked inflammatory response to promote HSCs activation and liver fibrosis.
a The area of the indicated inflammatory cytokines from immunofluorescence staining of Fig. 7c was analyzed. *P < 0.05 versus p65f/f mice, #P < 0.05 versus p65Δhepa mice with vehicle administration. b The administration of TGF-β1 inhibitor pirfenidone, TNF-α inhibitor pentoxifylline or monocytes inactivator clodronate-loaded liposomes, attenuated HSCs activation (α-SMA staining of primary HSCs, red) and liver fibrosis (α-SMA staining, red) in p65Δhepa/PUMA-WT mice. Nuclei (blue) were counterstained with DAPI, n = 6 per group. IF, immunofluorescence staining. c, d Western blotting presented that pirfenidone, pentoxifylline, or clodronate-loaded liposomes attenuated liver fibrosis (liver section) and the activation of HSCs (primary HSCs section) in CCl4-induced p65Δhepa/PUMA-WT mouse model, respectively. n = 6 per group.
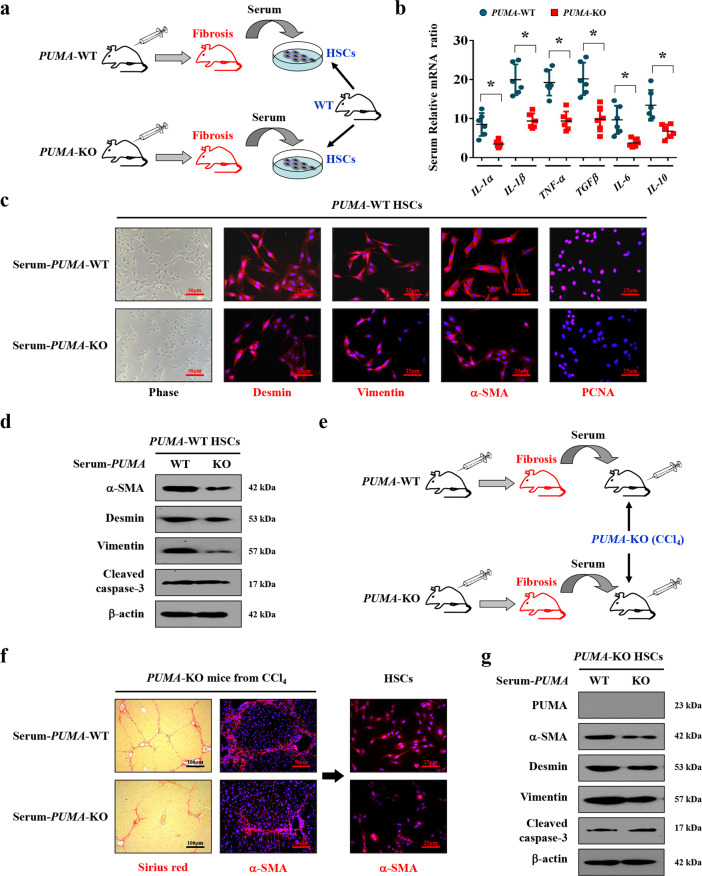
Following the induction of liver fibrosis in CCl4-induced PUMA-WT and PUMA-KO mouse models, these mice serum was extracted respectively (Fig. 9a). The inflammatory mediators mRNA levels from PUMA-WT mice, including IL-1α, IL-6, TNF-α, IL-1β, TGF-β, and IL-10, were higher than that from PUMA-KO mice (Fig. 9b). The primary HSCs (isolated from normal PUMA-WT mice) accepted the serum of PUMA-WT liver fibrotic mice showed enhanced expressions of desmin, vimentin, α-SMA, and PCNA (proliferating cell nuclear antigen), in contrast to the HSCs accepting PUMA-KO mice serum (Fig. 9c). Similar changes in the HSCs activation and growth elements were also observed by western blotting analysis (Fig. 9d). By in vivo serum test, the serum was extracted from CCl4-induced liver fibrosis models in PUMA-WT and PUMA-KO mice, respectively, and then was transfused to the different PUMA-KO littermates via tail intravenous injection (Fig. 9e). Following that, mice who accepted PUMA-WT serum appeared increased collagen deposition and enhanced HSCs activation rather than apoptosis, compared to that accepted PUMA-KO serum (Fig. 9f, g). To sum up, these data demonstrated that NF-κBp65/PUMA-regulated hepatocytes apoptosis drove inflammatory response to promote HSCs activation and liver fibrosis.
Fig. 9. NF-κBp65/PUMA-driven liver inflammation-induced HSCs activation and liver fibrosis.
a Schematic diagram of the serum test in vitro. b The genes expressions of the indicated inflammatory cytokines from CCl4-treated PUMA-WT or PUMA-KO mice were detected by quantitative reverse-transcription PCR. The expression of β-actin in each tissue was quantified as the internal control. n = 6 per group. *P < 0.05. c Representative images of the growth with activation of primary isolated HSCs following the treatment of the distinct serum extracted from CCl4-treated PUMA-WT or PUMA-KO mice. Nuclei (blue) were counterstained with DAPI. d Expressions of the related proteins of primary HSCs after serum treatment were detected by western blotting, revealing that PUMA-WT serum enhanced the activation of HSCs without affecting cell apoptosis. n = 6 per group. e Schematic diagram of the serum test in vivo. f Sirius red staining (red) and α-SMA staining (red) were examined in the liver tissues of PUMA-KO mice following the treatment of the serum extracted either from CCl4-treated PUMA-WT or PUMA-KO mice. The primary isolated HSCs were also analyzed by α-SMA staining (red). Nuclei (blue) were counterstained with DAPI. g The indicated proteins of the primary isolated HSCs from PUMA-KO mice after serum treatment were detected by western blotting, n = 6 per group.
Discussion
Hepatocytes apoptosis has been considered to be in a series of biochemical steps in most forms of liver injury under a number of pathological conditions10. Regulation of hepatocytes apoptosis is crucial for liver homeostasis and increased sensitivity of hepatocytes towards apoptosis results in chronic liver injury, which is linked to trigger inflammatory and wound healing responses that in the long run promote the development of hepatic fibrosis10. So far, three classic pro-apoptotic networks, TNF-α/TNF receptor 1 (TNFR1), Fas/FasL, and TNF-related apoptosis-inducing ligand (TRAIL)/death receptor 4 (DR4 or TRAIL-R1)/death receptor 5 (DR5 or TRAIL-R2), have been widely demonstrated to mediate caspase-dependent cellular apoptosis39,40. Our previous research has revealed that autophagic flux involved in liver fibrogenesis21. Autophagy has long been recognized as a critical pathway in the regulation of cell death, and the autophagic machinery could directly interface with pro-apoptotic factors pathways to promote cell death14,21. Several studies have focused on the role of Fas/FasL signaling during autophagy, and Fas/FasL has been verified to mediate autophagy activation in cell line systems including human neuroblastoma (SH-SY5Y) cells, human lung cancer SPC-A-1 cells and HeLa cells, and Fas signaling could activate autophagic cascades in retina-retinal pigment epithelium (RPE) separation during retinal detachment via regulating the conversion of LC3-I to LC3-II and the expression of Atg5. Furthermore, inhibition of autophagy by 3-MA could obviously reverse the outcome mediated by Fas/FasL signaling41–43. They also revealed that Fas/FasL promoted the activation of autophagy via modulating Fas-activated death domain (FADD), Src, the c-Jun N-terminal kinase (JNK) family of stress kinases, BECN1, PI3K, etc41–43. Given the critical roles that Fas/FasL signaling and autophagy play in liver diseases, in this study, by analyzing the liver sections from both humans and mice, we uncovered that Fas/FasL presented distinctly higher expression in the fibrotic tissues than in normal liver sections, and Fas/FasL signaling is upstream of autophagy, while inhibition of autophagy ameliorated Fas/FasL-regulated hepatic apoptosis, α-SMA expression, and liver fibrosis, and this data suggested that Fas/FasL-mediated hepatic apoptosis by autophagy in liver fibrogenesis.
Autophagy has been reported to control the timing of mitochondrial permeabilization in apoptosis and selectively regulate PUMA level, while PUMA depletion prevents sensitization to apoptosis by autophagy inhibition44,45. PUMA transduces death signals primarily to the mitochondrial membrane to lead to caspase activation and ultimately cell death via interaction and inhibition of the anti-apoptotic Bcl-2 repertoire46. In our study, we found that, accompanying by the loss of preserved architecture and excess deposition of ECM, PUMA was induced in liver fibrosis, and deletion of PUMA attenuated HSCs activation and liver fibrosis via inhibiting hepatocytes apoptosis, without influencing the status of the Fas/FasL signaling and autophagy. Moreover, inhibition of autophagy repressed PUMA upregulation and hepatocytes apoptosis to alleviate liver fibrosis, and we suggested that PUMA contributed to the Fas/FasL/autophagy-regulated hepatocytes apoptosis and HSCs activation in liver fibrosis.
NF-κBp65 has a wide range of functions in different cellular compartments influencing the survival of hepatocytes and the activation of HSCs47. Recent study has suggested that miR-196b-5p-mediated downregulation of Fas involved in the activation of STAT3 signaling through the NF-κBp65/IL-6 axis, and Fas downregulation could activate NF-κBp65 signaling to promote lung cancer cell growth in non-small cell lung cancer (NSCLC)48, which powerfully demonstrated the interaction between Fas/FasL and NF-κBp65. In our study, we also verified the correlation between them and found that the activation of NF-κBp65 (phosphorylation of NF-κBp65, p-p65) was repressed in the liver fibrotic tissues compared with their normal samples. By using primary hepatocytes, we further demonstrated that upregulated Fas and downregulated p-p65 were observed in FasL-treated hepatocytes and in the primary hepatocytes dissociated from CCl4-treated mice, while knockdown of Fas could significantly reverse that and enhance the activation of NF-κBp65, indicating that Fas/FasL signaling repressed the activation of NF-κBp65 in hepatocytes in liver fibrogenesis. Furthermore, we revealed that deletion of NF-κBp65 in hepatocytes enhanced the expressions of collagen-IV, collagen-I, and α-SMA, along with the increased number of apoptotic hepatocytes in mice, which finally aggravated liver fibrosis. During this process, downregulated or absent NF-κBp65 activity in hepatocytes, which leads to increased hepatocytes apoptosis, plays an important role in the initiation of liver fibrosis. We then examined the Bcl-2 family, which integrates a number of inter- and intracellular cues to determine whether or not the apoptosis pathway should be activated, and found that the pro-survival Bcl-2 family members were downregulated in hepatocytes with specific deletion of NF-κBp65, while the potent pro-apoptotic element PUMA was increased following NF-κBp65 deficiency in hepatocytes, which contributed to the development of liver fibrosis. By the way, the hepatic fibrosis mouse model was established by CCl4 for 4 weeks and 8 weeks, and we found that the mouse obtained the highest apoptosis index at the 4th week, and then the apoptotic cells decreased gradually as time goes on. During this process, liver fibrosis was accelerated progressively by the accumulation of collagen and the formation of septa, with a maximum peak at 8th week. This phenomenon may be related to that hepatocyte apoptosis is a pivotal and initial step in the development of liver fibrosis, and the pathogenesis of liver fibrosis owns different pathophysiological changes (the changes of hepatocyte apoptosis, collagen deposition, etc) in the different stage6,7,21, and our further study is needed to determine the definite causes of this phenomenon.
Previous studies have recognized that dying hepatocytes release alarmins that could recruit inflammatory cells to produce inflammatory cytokines to regulate the initiation and development of liver fibrosis8. In our study, inflammatory cells and mediators were dramatically upregulated in NF-κBp65 deletion in hepatocytes in liver fibrosis, while PUMA antisense oligonucleotides or PUMA deficiency suppressed that inflammatory response. Some researches have shown that the mechanisms by which apoptosis promotes inflammation relate to the activation of the resident macrophages in the liver, and HSCs undergo a process of activation resulting from hepatocytes apoptosis and inflammation4,49,50. Adopting the TGF-β1 inhibitor pirfenidone, TNF-α inhibitor pentoxifylline, and monocytes depressor clodronate-loaded liposomes, we demonstrated that inhibition of inflammatory action ameliorated HSCs activation and liver fibrosis. Moreover, our in vivo and in vitro serum data suggested hepatocytes apoptosis contributed to inflammation-induced HSCs activation. These results indicated that HSCs activation depended on the inflammatory response following hepatocytes apoptosis. However, there is much yet to be learned about the complex interplay between the immune system and the hepatocytes status in the pathophysiological states of the liver.
In summary, our investigation provides evidence that Fas/FasL contributes to NF-κBp65/PUMA-regulated hepatocytes apoptosis via autophagy to enhance HSCs activation and liver fibrosis, and this network could be a therapeutic target for liver fibrosis.
Materials and methods
Tissue samples
Six normal liver tissues were from parahemangioma sites of hepatic hemangioma patients and the paired samples of liver fibrosis were obtained from 6 hepatitis B virus (HBV)-infected liver fibrosis patients during operations before any therapeutic intervention. Written informed consent was received from each patient and healthy volunteer prior to inclusion in the study and the acquisition of these samples was approved by the Clinical Research Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University.
Mice and treatments
All animal experiments were approved by the Institutional Animal Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University. Eight- to ten-week-old male mice (20–25 g) were used for all experiments. All mice were randomly allocated to each group and to collect and process data for analysis, and the experimenters were blinded towards the treatments or genetic background in all the experiments. LoxP NF-κBp65 (RelA) mice were generated on a C57BL/6 gene background. Hepatocytes specific NF-κBp65 deletion mice (p65Δhepa) were generated by crossing the floxed p65 mice with Alb-cre mice, which shows hepatocyte-specific expression of Cre recombinase and NF-κBp65 ablated solely in hepatocytes but not in non-parenchymal liver cells. Floxed p65 littermates (p65f/f) were used as the wild-type (WT) mice. PUMA wild type (PUMA-WT) and PUMA knockout (PUMA-KO) littermates on a C57BL/6 background were generated from PUMA heterozygote mice (Jackson Laboratory, Bar Harbor, ME, USA). p65f/f/PUMA-WT mice on a C57BL/6 background generated by crossing p65f/f and PUMA-WT littermates, p65Δhepa/PUMA-WT mice on a C57BL/6 background were generated from p65Δhepa and PUMA-WT littermates.
The CCl4-induced liver fibrosis model was established via an intraperitoneal injection of 20% CCl4 (Sinopharm Chemical Reagent, Shanghai, China) dissolved in an olive oil solution (Sinopharm Chemical Reagent) at 5 ml/kg body weight, twice per week for 8 weeks. The control group was intraperitoneally injected only with olive oil at 5 ml/kg body weight, twice per week for 8 weeks. For inhibition of autophagy, the autophagic inhibitor 3-methyladenine (3-MA, Sigma, St Louis, MO, USA) was given with 3 μl of a 20 mg/ml solution prepared in saline, and mice were injected with 3-MA (10 mg/kg) at 30 min before every CCl4 injection, and the vehicle group (as Vehicle) was injected with the same volume of saline. For inhibiting inflammatory response, mice were orally administrated with TGF-β1 inhibitor pirfenidone (Sigma) at 250 mg/kg daily for 8 weeks. To inhibit TNF-α action, pentoxifylline (Sigma) at 200 mg/kg was administered by intraperitoneal injection for 8 weeks to mice at 30 min before every CCl4 injection. Clodronate-loaded liposomes (Sigma) were intraperitoneally injected in order to deplete monocytes for three consecutive days per week for 8 weeks in the process of mice models. To inhibited PUMA expression in mice, the PUMA sense oligonucleotides (5′-A*G*C*GCCATGGCCCGCGC*A*C*G-3′; *phosphorothioate bonds) and PUMA antisense oligonucleotides (PUMA-AS, 5′-C*G*T*GCGCGGGCCATGGC*G*C*T-3′) were synthesized by GenePharma (Shanghai, China). The mice were treated with PUMA sense or PUMA antisense oligo at 25 mg/kg/day via intraperitoneal injection for 8 weeks. By inhibiting the Bcl-2 activity, ABT-199 (Venetoclax, 100 mg/kg/d, Santa Cruz, Santa Cruz, CA, USA) was utilized via oral gavage. Six animals were used in each group for the above studies.
Primary cells isolation and cells culture
Primary hepatocytes and HSCs were dissociated from the indicated mice by a non-recirculating collagenase perfusion as previously described21,51. Liver was perfused through the portal vein in situ successively with Ca2+-free HBSS (Hank’s Balanced Salt solution) for 15 min, with 100 ml 0.2% pronase solution, and lastly with 0.2% collagenase Type-IV (Sigma) solution until the liver looked digested and became pale in color. This resulting cell suspension was filtered through a 100 μm pore size mesh nylon filter (Sinopharm Chemical Reagent), and then centrifuged for 10 min at 200 × g, the pellet was collected for primary hepatocytes and the supernate for primary HSCs. The primary hepatocytes were cultured in RPMI medium 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin in a humidified incubator at 37 °C with 5% CO2. For HSCs isolation, commercially available 60% Optiprep (Sigma) was added to the final concentration 11.5%. The above supernate was centrifuged at 450 × g for 15 min and then suspended with 0.5 ml HBSS, after being centrifuged at 1400 × g for 25 min, HSCs on the top of Optiprep layer were collected and also cultured in RPMI medium 1640. These cells were authenticated and tested and they were not contaminated by mycoplasma. For FasL treatment experiments in vitro, FasL (10 ng/ml, Sigma) was added for 12 h, and siRNA treatment was performed as previously described29.
Serum test
For serum test in vitro, following induction of liver fibrosis in CCl4-induced PUMA-WT and PUMA-KO mouse models, these mice were anesthetized and their serum was extracted, respectively. The above-mentioned different serum was added to the primary HSCs isolated from normal PUMA-WT mice (without any treatments), respectively, and then cultured in a humidified incubator at 37 °C with 5% CO2 for 36 h. For serum test in vivo, the serum was extracted from CCl4-induced liver fibrosis models in PUMA-WT and PUMA-KO mice, respectively. The above-mentioned distinct serum was then transfused to the different PUMA-KO littermates (by the third week of 10% CCl4 treatment, at 200 μl per mouse for three consecutive days per week until the 8th week) via tail intravenous injection. Following that, the mice were anesthetized and euthanized and the entire liver was analyzed using histopathological detection.
Histological staining
Sirius red staining was used for collagen determination. Hematoxylin-esoin (H&E) staining, immunohistochemical (IHC), immunofluorescence (IF), and double IF staining were also performed as previously described51, and the semi-quantitative analysis of the histological staining and sirius red staining was analyzed using Image-Pro Plus 6.0. IHC and IF staining were performed by using antibodies for Fas (SAB5700608), FasL (SAB4501538), NF-κBp-p65 (p-p65, SAB4504482), NF-κBp65 (p65, SAB4502615) (all from Sigma), collagen-I (COL-I, ab233080), α-SMA (ab5694), vimentin (ab92547), PUMA (ab9643), transferrin (ab278498), TGF-β (ab215715), MPO (ab208670) (all from Abcam, Cambridge, MA, USA), desmin (sc-23879), BECN1 (sc-48341), IL-6 (sc-28343), F4/80 (sc-52664) (all from Santa Cruz), cleaved caspase-3 (9661), CD3 (86603), TNF-α (11948), PCNA (13110) (all from Cell Signaling Technology, Danvers, MA, USA). TUNEL staining was performed using the In Situ Cell Death Detection Kit (Roche, Basel, Switzerland) according to the manufacturer’s instructions. The apoptotic index was determined by dividing the number of apoptotic cells by the total number of cells in the section in at least 10 randomly selected fields (×200). For transmission electron microscopy, the hepatic tissues were fixed as previously described21 and observed with a transmission electron microscope (Hitachi, H-800, Tokyo, Japan), and the images were acquired digitally from a randomly selected pool of six fields.
Western blotting
The related proteins were analyzed by western blotting using anti-α-SMA (ab5694), -vimentin (ab92547), -PUMA (ab9643), -collagen-IV (COL-IV, ab236640, ab6586), -COL-I (ab233080) (all from Abcam), -Fas (SAB5700608), -FasL (SAB4501538), -NF-κBp-p65 (p-p65, SAB4504482), -NF-κBp65 (p65, SAB4502615), -β-actin (A5441) (all from Sigma), -desmin (sc-23879), -Bcl-xL (sc-8392), -Mcl-1 (sc-74437), -Bcl-2 (sc-7382), -BECN1 (sc-48341) (all from Santa Cruz) and -cleaved caspase-3 (9661) (Cell Signaling Technology). Appropriate horseradish peroxidase conjugated secondary antibodies were used to detect the primary antibody/antigen complexes as previously described35, and then was quantified for densitometry analysis. For controlling unwanted sources of variation, the quantitative densitometry results were calculated and normalized to the loading control β-actin densitometry units.
RNA extraction and PCR assays
Total RNA was extracted using the RNAgents Total RNA Isolation System (Promega, Madison, WI, USA) according to the manufacturer’s instruction. Real-time polymerase chain reaction (PCR) was performed on a Chromo 4 Detector System (MJ Research, Sierra Point, CA, USA) using gene-specific primers and DyNAmo SYBR Green Master Mix (Finnzymes, Finland). Relative mRNA ratio was used to analyze the data, and the indicated mRNA in each tissue was normalized by β-actin gene (as the internal control) from the same tissue and expressed as fold changes relative to the matched control values.
Microarray experiment
Primary hepatocytes were dissociated from three normal liver samples with olive oil treatment and three matched pairs of fibrotic tissues with CCl4 treatment, respectively. Total RNA was isolated by using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and the total RNA was amplified, labeled, and purified by Affymetrix WT PLUS Reagent Kit (Affymetrix, Santa Clara, CA, USA) and FL-Ovation cDNA Biotin Module V2 (NuGEN, San Carlos, CA, USA) to obtain the biotin-labeled cDNA. Array hybridization and washing were performed using GeneChip Hybridization, Wash and Stain Kit (Affymetrix) in a Hybridization Oven 645 (Affymetrix) and a Fluidics Station 450, and then the arrays were scanned by Affymetrix GeneChip Scanner 3000 (Affymetrix). Command Console Software (Affymetrix) was used to control the scanner and summarize probe cell intensity data (CEL file generation) with default settings. The array data were analyzed for data summarization, normalization, and quality control using the GeneSpring software V12 (Agilent). The data were Log2 transformed and median centered by genes using the Adjust Data function of CLUSTER 3.0 software and then further analyzed with hierarchical clustering with average linkage. Finally, tree visualization was performed by using Java Treeview (Stanford University School of Medicine, Stanford, CA, USA). The microarray dataset is available in the figshare repository (10.6084/m9.figshare.14443382).
Statistical analysis
The experimenters were blinded towards the treatments or genetic background in all the experiments. To ensure adequate power to detect a pre-specified effect, the sample size was chosen using the Power Analysis and Sample Size (PASS) software. The sample size was estimated even if no statistical methods were used in animal studies. At least six mice or human sections were adopted in each group. Statistical analysis was performed only for studies where each group size was at least n = 6, unless otherwise stated. These data were normal distribution and were presented as mean ± SEM, and data statistical analysis were performed using Student’s two-tailed paired t-test or one-way ANOVA (more than two groups of data, single factor) or two-way ANOVA (more than two groups of data, two factors), followed by Bonferroni’s comparison post hoc test and post hoc tests are run only if F achieved P < 0.05. There was no significant variance inhomogeneity, and the variance was similar between the groups that were being statistically compared. Differences were considered statistically significant at the level of P < 0.05.
Supplementary information
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Author contributions
S.W.T. and X.Z.L. performed the experiments, analyzed the data, and revised the manuscript; L.J.C. and X.Q.W. collected the clinical samples and conducted the clinical study; L.T. and X.M.P. contributed the essential reagents and conducted the animals’ study; S.Y.T. and H.L.L. planned and conducted primary cells isolation; J.J. performed the cells culture study; B.W. designed the whole project, supervised the research, wrote the paper and approved of the final version.
Funding
This work was supported by grants from the Science and Technology Planning Projects of Guangzhou City (201804010026), the major talent project training program of the Third Affiliated Hospital of Sun Yat-Sen University (P02089), the National Natural Science Foundation of China (81700536, 82070574), the Natural Science Foundation of Guangdong Province (2017A030310188), the Basic Research Programme of Young Teachers’ Training Project of Sun Yat-sen University (17ykpy51).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Ethics statement
Written informed consent was received from each patient and healthy volunteer prior to inclusion in the study and the research process was approved by the Clinical Research Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University. All animal experiments were approved by the Institutional Animal Ethics Committee of the Third Affiliated Hospital of Sun Yat-Sen University.
Conflict of interest
The authors declare no competing interests.
Footnotes
Edited by B. Zhivotovsky
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Siwei Tan, Xianzhi Liu
Contributor Information
Siwei Tan, Email: xiaodatou520@sina.com.
Bin Wu, Email: wubin6@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41419-021-03749-x.
References
- 1.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J. Clin. Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pradere JP, et al. Hepatic macrophages but not dendritic cells contribute to liver fibrosis by promoting the survival of activated hepatic stellate cells in mice. Hepatology. 2013;58:1461–1473. doi: 10.1002/hep.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suh YG, et al. CD11b(+) Gr1(+) bone marrow cells ameliorate liver fibrosis by producing interleukin-10 in mice. Hepatology. 2012;56:1902–1912. doi: 10.1002/hep.25817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kornek M, Popov Y, Libermann TA, Afdhal NH, Schuppan D. Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells. Hepatology. 2011;53:230–242. doi: 10.1002/hep.23999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian H, et al. An HNF1alpha-regulated feedback circuit modulates hepatic fibrogenesis via the crosstalk between hepatocytes and hepatic stellate cells. Cell Res. 2015;25:930–945. doi: 10.1038/cr.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung Y, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung SI, et al. Hepatic expression of Sonic Hedgehog induces liver fibrosis and promotes hepatocarcinogenesis in a transgenic mouse model. J. Hepatol. 2016;64:618–627. doi: 10.1016/j.jhep.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Luedde T, Schwabe RF. NF-kappaB in the liver-linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011;8:108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elliott MR, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guicciardi ME, Gores GJ. Life and death by death receptors. Faseb J. 2009;23:1625–1637. doi: 10.1096/fj.08-111005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotov DI, Kotov JA, Goldberg MF, Jenkins MK. Many Th cell subsets have Fas ligand-dependent cytotoxic potential. J. Immunol. 2018;200:2004–2012. doi: 10.4049/jimmunol.1700420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qadir AS, et al. CD95/Fas Increases stemness in cancer cells by inducing a STAT1-dependent Type I interferon response. Cell Rep. 2017;18:2373–2386. doi: 10.1016/j.celrep.2017.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14:1631–1642. doi: 10.1080/15384101.2015.1038685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, et al. Cell apoptosis and Fas gene expression in liver and renal tissues after ischemia-reperfusion injury in liver transplantation. Transpl. Proc. 2010;42:1550–1556. doi: 10.1016/j.transproceed.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 16.Reinehr R, Häussinger D. CD95 death receptor and epidermal growth factor receptor (EGFR) in liver cell apoptosis and regeneration. Arch. Biochem. Biophys. 2012;518:2–7. doi: 10.1016/j.abb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Magraoui FE, Reidick C, Meyer HE, Platta HW. Autophagy-related deubiquitinating enzymes involved in health and disease. Cells-Basel. 2015;4:596–621. doi: 10.3390/cells4040596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaffer L. Out with the bad: studying autophagy to fight infectious disease. Nat. Med. 2016;22:334–335. doi: 10.1038/nm0416-334. [DOI] [PubMed] [Google Scholar]
- 19.Sridhar S, Botbol Y, Macian F, Cuervo AM. Autophagy and disease: always two sides to a problem. J. Pathol. 2012;226:255–273. doi: 10.1002/path.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Czaja MJ, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan S, et al. beta-Arrestin1 enhances liver fibrosis through autophagy-mediated Snail signaling. Faseb J. 2019;33:2000–2016. doi: 10.1096/fj.201800828RR. [DOI] [PubMed] [Google Scholar]
- 22.Czerkies M, et al. Cell fate in antiviral response arises in the crosstalk of IRF, NF-kappaB and JAK/STAT pathways. Nat. Commun. 2018;9:493. doi: 10.1038/s41467-017-02640-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, et al. NS5ATP13 promotes liver fibrogenesis via activation of hepatic stellate cells. J. Cell Biochem. 2017;118:2463–2473. doi: 10.1002/jcb.25913. [DOI] [PubMed] [Google Scholar]
- 24.Feng GS. Conflicting roles of molecules in hepatocarcinogenesis: paradigm or paradox. Cancer Cell. 2012;21:150–154. doi: 10.1016/j.ccr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen H, et al. Thymic NF-kappaB-inducing kinase regulates CD4+ T cell-elicited liver injury and fibrosis in mice. J. Hepatol. 2017;67:100–109. doi: 10.1016/j.jhep.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koppe C, et al. IkappaB kinasealpha/beta control biliary homeostasis and hepatocarcinogenesis in mice by phosphorylating the cell-death mediator receptor-interacting protein kinase 1. Hepatology. 2016;64:1217–1231. doi: 10.1002/hep.28723. [DOI] [PubMed] [Google Scholar]
- 27.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J. Clin. Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delbridge AR, Opferman JT, Grabow S, Strasser A. Antagonism between MCL-1 and PUMA governs stem/progenitor cell survival during hematopoietic recovery from stress. Blood. 2015;125:3273–3280. doi: 10.1182/blood-2015-01-621250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan S, et al. beta-Arrestin-1 protects against endoplasmic reticulum stress/p53-upregulated modulator of apoptosis-mediated apoptosis via repressing p-p65/inducible nitric oxide synthase in portal hypertensive gastropathy. Free Radic. Biol. Med. 2015;87:69–83. doi: 10.1016/j.freeradbiomed.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Sun Q, et al. Proapoptotic PUMA targets stem-like breast cancer cells to suppress metastasis. J. Clin. Invest. 2018;128:531–544. doi: 10.1172/JCI93707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27:S71–S83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, et al. The p53-PUMA axis suppresses iPSC generation. Nat. Commun. 2013;4:2174. doi: 10.1038/ncomms3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber A, et al. Hepatocyte-specific deletion of the antiapoptotic protein myeloid cell leukemia-1 triggers proliferation and hepatocarcinogenesis in mice. Hepatology. 2010;51:1226–1236. doi: 10.1002/hep.23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192–1102. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan S, et al. IL-6-driven FasL promotes NF-kappaBp65/PUMA-mediated apoptosis in portal hypertensive gastropathy. Cell Death Dis. 2019;10:748. doi: 10.1038/s41419-019-1954-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pihán P, Carreras-Sureda A, Hetz C. BCL-2 family: integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017;24:1478–1487. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chipuk JE, Green DR. PUMA cooperates with direct activator proteins to promote mitochondrial outer membrane permeabilization and apoptosis. Cell Cycle. 2009;8:2692–2696. doi: 10.4161/cc.8.17.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren D, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu B, et al. Downregulation of cyclooxygenase-1 is involved in gastric mucosal apoptosis via death signaling in portal hypertensive rats. Cell Res. 2009;19:1269–1278. doi: 10.1038/cr.2009.97. [DOI] [PubMed] [Google Scholar]
- 40.Xiong S, Mu T, Wang G, Jiang X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014;5:737–749. doi: 10.1007/s13238-014-0089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park MA, et al. Sorafenib activates CD95 and promotes autophagy and cell death via Src family kinases in gastrointestinal tumor cells. Mol. Cancer Ther. 2010;9:2220–2231. doi: 10.1158/1535-7163.MCT-10-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Giorgio R, et al. Neurogenic chronic intestinal pseudo-obstruction: antineuronal antibody-mediated activation of autophagy via Fas. Gastroenterology. 2008;135:601–609. doi: 10.1053/j.gastro.2008.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y, et al. Fas-mediated autophagy requires JNK activation in HeLa cells. Biochem. Biophys. Res. Commun. 2008;377:1205–1210. doi: 10.1016/j.bbrc.2008.10.151. [DOI] [PubMed] [Google Scholar]
- 44.Xie W, et al. Chaperone-mediated autophagy prevents apoptosis by degrading BBC3/PUMA. Autophagy. 2015;11:1623–1635. doi: 10.1080/15548627.2015.1075688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorburn J, et al. Autophagy controls the kinetics and extent of mitochondrial apoptosis by regulating PUMA levels. Cell Rep. 2014;7:45–52. doi: 10.1016/j.celrep.2014.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan S, et al. PUMA mediates ER stress-induced apoptosis in portal hypertensive gastropathy. Cell Death Dis. 2014;5:e1128. doi: 10.1038/cddis.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang X, et al. miR-196b-5p-mediated downregulation of FAS promotes NSCLC progression by activating IL6-STAT3 signaling. Cell Death Dis. 2020;11:785. doi: 10.1038/s41419-020-02997-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bárcena C, et al. Gas6/Axl pathway is activated in chronic liver disease and its targeting reduces fibrosis via hepatic stellate cell inactivation. J. Hepatol. 2015;63:670–678. doi: 10.1016/j.jhep.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karsdal MA, et al. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015;308:G807–G830. doi: 10.1152/ajpgi.00447.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tan S, Liu H, Ke B, Jiang J, Wu B. The peripheral CB1 receptor antagonist JD5037 attenuates liver fibrosis via a CB1 receptor/beta-arrestin1/Akt pathway. Br. J. Pharm. 2020;177:2830–2847. doi: 10.1111/bph.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].