Abstract
Bacteriophages (hence termed phages) are viruses that target bacteria and have long been considered as potential future treatments against antibiotic-resistant bacterial infection. However, the molecular nature of phage interactions with bacteria and the human host has remained elusive for decades, limiting their therapeutic application. While many phages and their functional repertoires remain unknown, the advent of next-generation sequencing has increasingly enabled researchers to decode new lytic and lysogenic mechanisms by which they attack and destroy bacteria. Furthermore, the last decade has witnessed a renewed interest in the utilization of phages as therapeutic vectors and as a means of targeting pathogenic or commensal bacteria or inducing immunomodulation. Importantly, the narrow host range, immense antibacterial repertoire, and ease of manipulating phages may potentially allow for their use as targeted modulators of pathogenic, commensal and pathobiont members of the microbiome, thereby impacting mammalian physiology and immunity along mucosal surfaces in health and in microbiome-associated diseases. In this review, we aim to highlight recent advances in phage biology and how a mechanistic understanding of phage–bacteria–host interactions may facilitate the development of novel phage-based therapeutics. We provide an overview of the challenges of the therapeutic use of phages and how these could be addressed for future use of phages as specific modulators of the human microbiome in a variety of infectious and noncommunicable human diseases.
Keywords: Microbiota, Microbiome, Phages
Subject terms: Mucosal immunology, Viral infection
Introduction
Bacteriophages (referred to hereafter as phages) are viruses infecting prokaryotes, which collectively encompass the most genetically variable reservoir on Earth. Phages have been detected wherever a bacterial host exists, including in water sediments, soil, and along mucosal surfaces of the human body.1–3 Phages are ‘adsorbed’ on the membrane of the host bacterium, interacting with bacterial receptors through external structures (e.g., tail fibers) to inject their viral DNA through the bacterial cell wall by enzymes present in the tail. During this interaction, the phage capsid remains outside the cell, while the viral genetic material may localize in the cytoplasm, where it can exist in two major states. The first state, characteristic of lytic phages, includes phage exploitation of the replicative machinery of the host to produce more virions, which are then released by lysis of the host cell.4,5 In the second state, characteristic of temperate phages, the phage genome may be integrated into the bacterial chromosome, where it remains in a dormant state, replicating with the bacterial genome. Importantly, microbial stress signals may induce a transformation between the lysogenic and lytic states in a highly regulated manner (Fig. 1a).
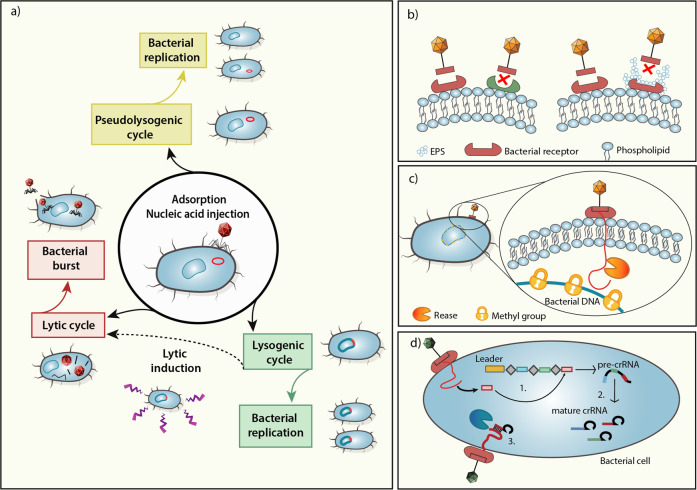
Fig. 1.
Mechanisms of phage–bacterial interactions. a Following adsorption and injection of a phage genome into a bacterial host, phages can undergo a lytic cycle, leading to viral replication and bacterial cell lysis. Alternatively, in the lysogenic cycle, the phage genome is integrated as a prophage into the bacterial genome, until lytic induction triggered by a stress signal. Pseudolysogeny is the stable persistence of viral DNA in the host when growth conditions become unfavorable, and the phage is not replicating; thus, it will be inherited only by a single progeny cell. Innate bacterial phage evasion mechanisms include (b) the generation of mutated phage receptors or receptor masking by extracellular polymeric substances (EPSs), and (c) cleavage of the phage genome following its injection into the bacterial cell, while methylated bacterial DNA remains protected. The adaptive bacterial antiphage machinery is exemplified by (d) the CRISPR-Cas system, which has several phases: 1. Acquisition: a short viral DNA portion (red rectangle) is inserted between palindromic sequences (gray rhombuses) to form a CRISPR array. 2. Expression: The CRISPR array is transcribed into a pre-crRNA that is then cleaved into mature crRNA ready for recognition. 3. Interference: the endonuclease recognizes and cleaves the complex crRNA-phage nucleic acid
Maintenance of lysogeny requires continuous transcription of repressor proteins to suppress lytic genes (such as CI protein in λ phage). A drop in repressor protein levels, or stress signals such as DNA damage, is interpreted by the integrated phage as a threat to its own genomic stability, inducing excision from the bacterial chromosome and the start of the lytic cycle4 (Fig. 1a). For example, temperate phages release a pro-lysogenic hexapeptide, named ‘arbitrium’, that transfers information guiding the next phage generation toward either the lytic or lysogenic cycle. According to this model, aimR, an intracellular phage protein, binds to the phage DNA, thereby increasing transcription of the aimX gene, which in turn promotes the lytic cycle through currently unknown mechanisms. Accumulation of arbitrium after several generations of phage production suppresses expression of aimR, which leads to prevention of DNA-binding, suppressed activation of aimX and lytic induction, thereby promoting lysogeny.6 As such, at the beginning of a phage infection, few viral particles would increase their ecological success by initiating the lytic cycle, but when infection is spread over an entire bacterial colony, lysogeny could represent a more efficient choice in preserving the phage and its bacterial host. Such a mechanism may help avoid extinction of both bacteria and associated phages7 or, alternatively, provide phages with information pertaining to the number of available hosts or of lysogens (i.e., bacteria already infected by a prophage), thereby enabling the phage to maximize infection efficiency.8 Importantly, in this model, the choice between lysis and lysogeny happens independently during infection for each phage, and phages coordinate an unanimous decision to enter a lytic versus a lysogenic state only in cases of multiple phages infecting the same host.9
Many prophages confer immunity to the lysogen they infect by different mechanisms, collectively termed prophage-mediated superinfection exclusion.10 For example, phages harboring CRISPR-Cas sequences directed against other phages confer resistance to their lysogenic bacteria upon integration into the chromosome.11 Interestingly in Vibrio’s phage VP882, the lysis-lysogeny decision depends on quorum sensing molecules produced by its bacterial host. The cytoplasmic receptor-transcription factor Vibrio quorum modulator A represses virulence- and biofilm-associated bacterial genes. Strikingly, the VP882 phage encodes a homolog of the host’s protein that the phage uses as an antirepressor of lytic genes, thereby initiating a lytic cycle, while acquiring direct control over its host’s quorum sensing and virulence.12 Bacteria, in turn, can influence phage fitness by generating metabolic cues, such as resisting phage infection by dampening metabolic functions along the GI tract13–15 or by inducing prophage production via release of short chain fatty acids.16,17 Phages may react to these bacterial-induced metabolic signals by altering metabolite composition via targeting of susceptible commensals.18 Collectively, such an extensive ‘arms race’ between bacteria and their phages underlines the constant crosstalk between the counterparts at the molecular, cellular, and organ levels.
Given these phage–bacterial interactions, harnessing phageome members for therapeutic purposes was studied in the past century as a promising tool in redirecting bacterial communities toward homeostatic configurations. The first therapeutic use of phages was documented by d’Herelle.19 Shortly thereafter, phages gained great interest as antimicrobial agents, initially for treating staphylococcal skin infections, and then as therapy against life-threatening diseases such as cholera and bubonic plague, and were marketed by companies such as L’Oréal and by Eli Lilly.20 However, the efficacy of phage preparations remained controversial,21 and the concomitant discovery of antibiotics in 1928 led to a decrease in the popularity of phage treatment.22 Nonetheless, phages were still extensively used as antimicrobial therapy in the former Soviet Union, where antibiotics were not easily accessible. This decades-long experience and the knowledge gained in clinical phage exploitation have remained elusive, as scientific reports from Eastern Europe were mainly written in Russian and were thus inaccessible to the global scientific community. In addition, these reports often lacked adequate randomization and controls,20 but provided, at least to some extent, proof of the safety of phages as potential therapeutic agents. Currently, the threat of multi-drug-resistant and carbapenem-resistant pathogenic bacteria has led to a renewed interest in antibacterial agents, among them phage therapy.
In this review, we illuminate how phage therapy could potentially reemerge as an effective antimicrobial treatment, including the potential to modulate the human microbiome. Furthermore, we aim to analyze the biological limitations of phage therapy, including regulatory obstacles that limit its wider application, and the means of addressing these challenges in moving phage therapy toward clinical use.
Bacterial antiphage defenses
Phages represent a major threat to bacterial species. Reactive to their phage predators, bacteria have developed resistance mechanisms; as a result, phages find strategies to overcome such defenses, resulting in a constantly evolving ‘arms race’.23 The first line of bacterial immunity, defined as innate immunity, akin to more evolved immune systems, includes several nonspecific mechanisms.24 Following phage infection, bacteria can mutate, downregulate expression or lose receptors exploited by the phage to penetrate the cell.25 Alternatively, bacteria can mask phage receptors by a layer of extracellular polymers (EPS) or capsular material. Phages can reactively develop the capacity to recognize a novel receptor26 or to quickly adapt to a mutated receptor.27 Moreover, tailed phages contain an endosialidase domain in their tail structure, which allows them to digest the capsule or EPS covering masked receptors28 (Fig. 1b).
After entering the bacterial cell, phages can encounter restriction–modification (R–E) systems that employ a restriction endonuclease (REase) to cleave foreign nucleic acids, while the bacterial DNA is protected by methylation. Phages can resist this defense system in several ways, including naturally integrating modified bases that will alter the restriction site29 or actively self-methylate their own DNA, as was shown for the L. lactis phage φ5030 (Fig. 1c). Abortive infection systems (Abi) trigger a cascade reaction to induce an ‘altruistic cell suicide’ of phage-infected bacteria, preventing further infection of the bacterial community.31 A widespread example of Abi are toxin-antitoxin systems, involving a toxin directed against vital cellular processes, and a protective antitoxin that is constantly expressed, avoiding bacterial extinction.32 In the presence of stress signals (such as phage infection), the antitoxin, being structurally more labile than the toxin, is degraded, leading to a dormant state or cell death.31 Phages may overcome this strategic defense by expressing small, repetitive RNA molecules resembling the antitoxin transcript that neutralizes the toxin.33,34
The adaptive bacterial antiphage system consists of several more sophisticated systems, such as CRISPR-Cas (clustered regularly interspaced short palindromic repeats; CRISPR-associated), comprising a series of short fragments of foreign DNA (spacers) inserted into repeated palindromic sequences, named CRISPR loci. Small RNAs are then transcribed and associated with Cas proteins, enabling recognition and cleavage of phage genomes matching the RNA sequence35 (Fig. 1d). This mechanism is characterized by a rapid evolution because of the high intraspecies phage diversity; as a result, point mutations are no longer sufficient to overcome it.36 However, if directed against a temperate phage inserted in the host genome, CRISPR systems could also cause “autoimmunity” and become deleterious to the host bacterium.37 In addition to its antiphage properties, CRISPR biology is also recognized as an efficient tool for providing insights into phage-bacteria ecology. Moreover, conservation of ancient spacers in the CRISPR loci allows the identification of unknown phages targeting bacteria residing in the gut.38 In order to survive, and as part of the bacteria-phage arms race, phages developed anti-CRISPR proteins (Acrs), which can block CRISPR immunity by inhibiting components of its protein complex.39 Phages can cooperate by producing Acrs to overcome CRISPRs and successfully infect resistant bacteria.40,41 These counter-regulatory mechanisms were shown to evolve based on the diversity of the surrounding bacterial community.42
Besides CRISPR, newly identified antiphage defense systems include, for example, an abortive system inducing cyclic GMP–AMP signaling, leading to membrane damage and bacterial death upon phage infection, even before the phage is able to replicate. Therefore, the death of the single bacterial cell prevents the spreading of the virus and protects the remaining bacterial colony from further infection. Another recently discovered bacterial defense mechanism is termed the defense island system associated with restriction–modification, and is based on a DNA restriction system providing a broad defense against multiple phage types.43,44
From these observations, it is clear that phages can drive microbial diversity at a genotypic, phenotypic, and community level, affecting competition between bacteria,45 and preserving bacterial diversity.46 Further unraveling the mechanisms by which bacteria develop phage resistance could provide valuable insights into overcoming these mechanisms in effectively targeting bacteria without inducing the onset of phage resistance.
The gut phageome
The last decades have seen a tremendous improvement in understanding the structure of microbial communities inhabiting the human host, collectively termed the microbiome. The microbiome has been increasingly recognized as playing a central modulatory role in the development of noncommunicable or nontransmissible diseases including metabolic syndrome, cardiovascular disease, and cancer. Development and utilization of high-throughput next-generation sequencing technologies allows for characterization of complex, heterogenous, and poorly culturable microbial ecosystems in the gut and elsewhere (see Box 1). Early studies suggested that the viral fraction in the human gut, also defined as the “phageome”, encompasses 5–6% of normalized sequencing reads in the human gut.47 However, the total amount of DNA per gram of stool in the gut derived from phages is still debated and depends on the extraction method.48–50
Viruses sequenced from fecal samples of healthy people are mostly temperate and largely composed of the tailed, double-stranded DNA phage Caudovirales. It is estimated that 82% of bacteria in the human gut harbor at least one phage sequence, a so-called “prophage”.51,52 This estimation is based on the presence of numerous sequences coding for annotated integrases, utilized by temperate phages to integrate into the bacterial genome.53,54 The human gut phageome displays a common reservoir of a few highly abundant families shared among unrelated, geographically distant subjects. These include Siphoviridae, Myoviridae, the ubiquitous crAssphage, and the single-stranded Microviridae,54,55 as well as several low abundance families that are responsible for interpersonal variability.38,54,56 In contrast to prokaryotic viruses, eukaryotic viruses are less commonly found in human stool samples, although some studies reported their constant presence and fecal shedding.57,58
Despite these observations of phage presence as part of the gut microbiome, we are still far from fully elucidating the full repertoire of the phageome composition. Out of 2500 virus-like particles (VLPs) identified in the gut,53,54 the large majority cannot be linked to a precise bacterial host,56 and over 90% of obtained sequences cannot be assigned to any of the viral families proposed by the International Committee on Taxonomy of Viruses (ICTV).59 The major difficulties of characterizing the composition of the gut virome stem from the lack of a common genetic marker that would allow recognition of phage sequences (as in the case of the 16S rRNA gene for bacteria) and the lack of annotated phage genes in confirmed databases. Early studies relied on sequence homology to known viral genes or proteins to identify new prophages. More unbiased and efficient approaches utilizing the percentage of GC compositional skews or the presence of consecutive genes on the same DNA strand may enable recognition of new phages. One example is the cross-assembly phage (later called crAssphage), which is present from early infancy in 50% of healthy adults,60 but features no encoded proteins matching previously known viral sequences.61,62 Another major obstacle to accurate phage identification is the possible contamination of prophage sequences inserted in bacterial genomes upon analysis of the “active” part of the virome (lytic phages and activated prophages). These regions are technically impossible to distinguish by sequencing because of their viral origin.63
To date, metagenomic studies have identified an increasing number of viral sequences derived from the vast unclassified genetic material, or “dark matter”, of the virome in commonly used databases.60,64–67 Nonetheless, metagenomics approaches lack the high resolution necessary to reconstruct genomes of different, but closely related, microorganisms, which would be important in future research in decoding host–phage relationships, considering the mosaic structure of the genome in viruses and their high degree of interindividual variability.53,65 Novel technologies such as single-virus genomics,68 frequently employed in marine ecosystems, and viral tagging (VT) metagenomics69 could shed new light on phage–bacteria interactions.70 Likewise, increasing the sequencing depth in the analyses of metagenomic libraries may be more informative in identifying new sequences50 (see Box 1).
Box 1.
In the last decades, the biological role of phages in modulating complex ecological environments was facilitated by recent advances in high-throughput sequencing (HTS) technologies. However, technical limitations are frequently noted in generating metagenomic libraries from viral DNA, further complicated by variable and often noncomparable protocols utilized in different studies. Establishing validated methods for in-depth study of the human virome would significantly help in understanding the role of the virome in general, specifically the phageome, in health and disease.
After DNA purification, the majority of DNA obtained from a sample is nonviral. DNA is then fragmented to an appropriate size in preparation for next-generation sequencing. Illumina Nextera XT is widely used for creation of virome libraries, although it has limitations in recovery of end sequences. Considering that a low yield of DNA is often obtained from purification, it is necessary to amplify nucleic acids in order to build a library, usually by the use of whole-genome amplification (WGA) or random amplification. Relative abundances of the viral community could be altered by this step, as previously reported for specific enzymes227 or PCR protocols.228 Careful selection could help avoid such technical biases.229
Several efforts in generating an optimized protocol utilized mock viral communities.230–232 An approach called NETOvir was developed to enrich for viral DNA compared to bacterial DNA.233 Although it is time consuming and labor intensive, this method allows high-throughput sample preparation with almost total elimination of bacterial reads.233 Another challenge in preparation of viral metagenomics libraries is the incorporation of contamination from environmental, bacterial, and human sequences, and from commercial kits.234 To address this problem, multiple negative controls must be included in each run, as a means of differentiating between background noise and true viral sequences of biological relevance. Separation of virus-like particles (VLPs) from contaminants by flow cytometry based on DNA content and size of particles may further address contamination issues. Indeed, such an approach may improve the number of assembled long contigs while reducing contamination from human ribosomal RNA or mitochondrial DNA.235 Annotation of viral sequences by publicly available databases remains poor, given their high genomic variability, resulting in virome research greatly relying on de novo assembly of short sequencing reads.236 A study assessing assembling performances of four different datasets reported large database-related variations and low accuracy coverage impacting analyses, with SPADES performing better than the other databases.237 This result highlights the importance of implementing a multi-assembler approach using several datasets at a time for virome assembly in complex microbiome analyses.
Other phageomes
To date, most of the efforts to characterize the endogenous human virome and phage populations focused on the gut, as the richest ecological niche of the human body. Other viral communities in the human holobiome are only starting to be elucidated. In early studies, phages isolated from human skin were used to distinguish different serotypes of their host.71,72 Recently, it has been discovered that the skin virome displays a core of double-stranded DNA phages, mainly Caudovirales, which are directed against the most prevalent bacteria on the skin, such as Propionibacterium, Streptococcus, and Staphylococcus. Phages are mostly segregated at a specific site of their bacterial hosts (e.g., sebaceous sites for Propionibacterium acnes). Likewise, phage-bacterium dynamics seem to be site-specific. In sebaceous sites, an observed negative host–phage correlation suggests antagonism, while in moist and dry sites, a positive correlation could imply the presence of temperate phages.73 Phage communities in the skin, and their most abundant genes, display a low variability in composition and diversity over time.16 For instance, phages targeting Propionibacterium spp. in healthy subjects were shown to be dominated by a few strains mostly belonging to the Syphoviridae family,74 which feature low genetic variability and morphology.75,76 These skin phages are mostly lytic, but in rare cases, they may undergo a pseudolysogenic cycle,77 while collectively exhibiting a broad host range toward several P. acnes clinical isolates.78 As such, these phages could be regarded as a promising putative tool for treating P. acnes-associated acne.78 However, they may also induce the generation of phage-resistant clones,71,72,79 which could limit treatment efficacy.
Comparing phages in the lungs in children affected by multiple acute respiratory tract infections to those in children suffering from a single respiratory infection revealed that phages targeting Propionibacterium spp. are more prevalent in the former compared to the latter in a clinical context. Furthermore, the abundance of phages increases with recurrent infections, while the overall diversity of phages is comparable between the two groups. Importantly, Propionibacterium spp. colonization of the respiratory tract has been previously associated with a propensity for viral infection80 and pulmonary inflammation.81 Indeed, the respiratory phageome has been suggested as a biomarker for the susceptibility of individuals to airway infections, as phages targeting Propionibacterium spp. are correlated with higher levels of cytokines, which are associated with respiratory disease.82 A metagenomic study of the lung phageome in cystic fibrosis (CF) patients revealed a specific composition and metabolic signature associated with the disease and lower species richness in CF than healthy individuals.83 In addition, the lung phageome differs between smokers and nonsmokers, with the latter featuring higher species richness. Interestingly, the smokers’ lung virome configuration was correlated with levels of IL-8, a potential marker of COPD (chronic obstructive pulmonary disease) severity. Strikingly, the differences in the phageome were not reflected by the microbiome composition.84 Collectively, these observations could provide new insights for the use of the lung phageome as a diagnostic tool in COPD, although further investigation is required to determine its potential causative impacts on human lung physiology.
Little is known about the vaginal phageome, although this ecosystem could play an important role in modulating the onset of microbiome-related diseases, such as bacterial vaginosis (BV). Early in vitro studies suggested that lytic phages could target healthy members of the vaginal microbiome, such as Lactobacillus jensenii85 or other Lactobacillus species,86 thereby contributing to dysbiosis in BV. Conversely, the Human Microbiome Project87 discovered that the ‘healthy’ vaginal microbial ecosystem is characterized by low species diversity compared to the gut. In addition, phages were found to correlate with bacterial hosts in a physiological equilibrium, while high microbial diversity was correlated with dysbiosis. Interestingly, BV is more prevalent in women who smoke,88 while in clinical isolates of Lactobacillus spp., prophage activation was induced by cigarette-derived chemicals, such as benzo(a)pyrene diol epoxide, which is also present in the vaginal secretions of smoking women; this might suggest a possible role of cigarette-derived chemicals in nonphysiological phage activation, potentially disrupting the vaginal microbiome.89 The vaginal phageome of BV-positive and BV-negative women is mainly composed of dsDNA phages, with a clear correlation noted between phage composition and presence of their specific bacterial hosts in healthy and BV conditions. As such, most of the phages targeting Lactobacillus spp. (a hallmark of a healthy vaginal microbiome) are negatively correlated with phages targeting Gardnerella vaginalis (a hallmark of the dysbiotic vaginal microbiome in BV). Differences in the composition of the vaginal virome are prominent in women suffering from BV, suggesting that the vaginal phageome could be a marker or even a predictor of this condition.90 Recently, vaginal microbiome transplantation (VMT) performed on women affected by intractable BV resulted in full long-term remission and reconstitution of a Lactobacillus-dominated microbiome configuration.91 Future studies may assess whether the transferred vaginal phageome could contribute to the observed clinical impacts noted with VMT.
Phages also constitute the majority of viruses in the oral cavity,92 yet their physiological effects remain unknown.93,94 Interestingly, the oropharynx is constantly exposed to pathobionts, whose translocation to other microhabitats may be associated with disease states.95 In accordance with this concept, a plethora of virulence factor homologs was recently found in the salivary phageome,92 and phages from the mouth were shown to encode for Streptococcus mitis virulence factors, which are responsible for platelet-attachment and associated with endocarditis.96 This link potentially suggests a dynamic network between phages and their pathogenic hosts. Interestingly, the oral cavity is characterized by a high phage diversity compared to the gut, hallmarked by the presence of jumbo phage genomes.97,98 This diversity could be enhanced by the presence of dense biofilms that were shown to be promoted, among other factors, by phages.99
Overall, information is still largely lacking as to the full spectrum and interindividual variability of the phageome in various niches of the human body. Gaining further information in this area could shed light on previously unexplained bacteria-phage networks in unexplored sites of the body and their impacts on host microbiome-related phenotypes, while boosting the potential applications of the phageome as a diagnostic or therapeutic organ- and disease-specific tool.
Modulation of gut phageome repertoires
Host intrinsic factors
The phageome is modulated by a complex interplay of endogenous and extrinsic factors, which have remained elusive to date. Longitudinal studies suggested that phageomes generally remain stable in healthy adults featuring a stable lifestyle. However, phageomes can change due to external conditions such as changes in diet,53,56 differences in mode of delivery60, and other varying environmental signals. In addition to the role of environmental signals in controlling phageome composition (discussed below), genetic factors were also found to play important roles in phage dynamics modulation. For example, young monozygotic twins shared more similar virotypes than unrelated individuals.100 Of note, these twins were also more likely to share a similar environment and diet; thus, a key role of the environment in influencing phage dynamics was not ruled out even in this setting. Specific host genotypes influencing phageome composition remain elusive to date. These include genetic factors regulating direct viral nucleic acid recognition or immune-related genes controlling phages directly or through induced alterations in microbiome configuration.
Host extrinsic factors
While host intrinsic factors regulating phageome composition remain largely unknown, key environmental factors determining phageome composition are being increasingly identified. Indeed, in a cohort of adult monozygotic twins, twins exhibiting a similar microbiome also shared more viral taxa in comparison to twins with different microbiomes.101 Moreover, a positive correlation was noted between the virome and microbiome β-diversity regardless of genetic relatedness, suggesting an association between the virome and microbiome diversity, even in genetically unrelated individuals.55,102 In a longitudinal study, phage richness was highest at birth and decreased with age.66 While the mode of acquisition of phages starting from birth has not been fully explained, a significant proportion of phages seem to be transferred to the infant through breast milk.103 However, phages could also derive from prophages integrated into the genome of gut commensal bacteria,104 which implies that the mode of delivery may represent an extrinsic factor in shaping the phageome, possibly through its effect on the microbiota.
Similarly, a change in dietary composition also significantly impacts the gut phageome. For example, individuals switching to a similar diet showed reduced interindividual differences in their virome configuration.53 However, some virome changes exhibit resilience following their modification by diet, suggesting the existence of other intrinsic or extrinsic factors determining phage composition. For example, in mice, the gut virome does not revert to its initial composition following exposure to a diet rich in refined sugars, even if animals are subjected to a period of washout.105 Different dietary regimens seem to impact the temperate fraction of phages in the gut,106 possibly contributing to the observed variability in the phageome following a dietary change.107 Similarly in mice, the fraction of prophages integrated in the genome of different gut bacterial taxa changed in a diet-specific manner.63 Ingested fructose, possibly acting as a stress signal, could induce the activation of a prophage from the lysogenic gut commensal Lactobacillus reuteri in an AckA-dependent manner.17 Such mechanisms could likely initiate a cascade reaction altering the microbiome composition, both due to further prophage excision following the spread of stress signals and potential phage-induced lysis of microbiome elements closely related to L. reuteri.106 In support of this notion, phages were suggested to modulate microbiome diversity upon increasing consumption of refined foods.108 Better mechanistic understanding of environmental and indigenous regulators of the human phageome may explain how environmental–phage interactions impact human traits in treatment and disease, while identifying altering phage repertoires as a means of impacting human disease.
Phages and host immunity
Although it remains unclear if different types of phages (i.e., lytic, temperate or pseudolysogenic) directly affect the human host,109 it has been repeatedly suggested that phages can indirectly modulate the immune system. Such an impact on a pathogenic or commensal bacterial host may alter their host infectivity and virulence. For instance, lysogeny could increase the fitness of bacteria through a mechanism called “lysogenic conversion”,110 which occurs when integrated DNA from the phage modifies bacteria. Lysogenic conversion often translates into beneficial mutualism, either protecting bacteria from infection by other phages or increasing their virulence through gene transfer (see Table 1). Another interesting example includes “active lysogeny”, in which integration of phage DNA disrupts bacterial genes that increase fitness, thereby enhancing survival chances of both partners.111 For example, excision of the integrated DNA of phage φ10403S from the Listeria monocytogenes genome during infection of mammalian cells leads to restoration of genes encoding for phagosome escape but not to virion production or bacterial lysis.112 Strikingly, Pasechnek et al. showed that translation of late genes responsible for activation of the lytic cycle in phage φ10403S are temperature-dependent and downregulated at 37 °C. Thus, when L. monocytogenes infects mammalian cells, the phage φ10403S lytic cycle is inhibited at a post-transcriptional level.113 In addition, persistence of a pseudolysogenic phage may be transmitted unevenly to daughter cells, conferring a metabolic advantage upon genomic integration.114 Interestingly, maintenance of the pesudolysogenic state in bacteria could lead to generation of phage mutants able to infect the newly generated phage-resistant bacteria, thereby generating larger colonies.115 Collectively, both lysogenic and pseudolysogenic states could have an impact on host–bacterial virulence and fitness, ultimately bearing an indirect downstream influence on bacterial–mammalian host interactions and the associated immune responses.
Table 1.
Phage-encoded virulence factors transmitted to bacteria
| Bacterium | Phage | Acquired gene, phenotype or protein | Bibliography |
|---|---|---|---|
| C. botulinum | Phage C1 | C1; neurotoxin | 204 |
| Corynebacterium diphtheria | β-phage | tox gene; diphtheria toxin | 205 |
| E. coli K-12 | 9 cryptic prophages (CP4-6, DLP12, e14, rac, Qin, CP4-44, CPS-53, CPZ-55 and CP4-57) | kilR and dicB; resistance to quinolone and β-lactam antibiotics, improved survival to osmotic stress | 206 |
| E. coli O157:H7 | ΦFC3208 | hly2; Enterohemolysin | 207 |
| P. aeruginosa | ΦCTX | ctx; cytotoxin | 208 |
| S. aureus | Φ13 | entA, sak; Enterotoxin A, staphylokinase | 209 |
| S. flexneri | Sf6 | oac; O-antigen acetylase | 210 |
| S. pyogenes | T12 | speA, B and C; pyrogenic exotoxins (SPE) A, B and C | 211 |
| S. Typhimurium LT2 | Fels-1 | sodCII; SodCII enzyme | 212 |
| Salmonella spp. | Gifsy-2 | SodC1; periplasmic superoxide dismutase (SodCI) | 213 |
| Salmonella spp. | sopEϕ | sopE; type III effector | 214 |
| Shigella dysenteriae | Phage 7888 | stx; Shiga toxin | 215 |
| V. cholerae O139 | CTXΦ | ctxAB; cholera toxin (CT) | 216 |
In addition to indirect host influences mediated through phage-induced modification of bacterial behaviors, phages may also directly affect the mammalian host immune response.116 This is important from a therapeutic perspective, as the host immune response has been suggested to limit phage therapeutic efficacy.117 Some phages, such as phage 536_P1, directly promote an increase in the production of antiviral cytokines like interferon-γ (IFNγ) and interleukin-12, as well as chemokines, even in the absence of host bacteria.118 This is not surprising, as phages share some features with viruses targeting mammalian cells and are thus recognized by innate host receptors such as members of the Toll-like receptor family. Indeed, evidence in humans suggests that phages of Lactobacillus, Escherichia, and Bacteroides may induce IFNγ via Toll-like receptor 9 (TLR) in the gut in a microbiota-independent manner,119 which may trigger phage-specific as well as bacteria-specific immunity119 (Fig. 2). This may be of potential relevance in human disease. For example, ulcerative colitis (UC) patients responding to fecal microbiota transplantation featured reduced levels of phages compared to nonresponders.119 Phages from UC patients induced more IFNγ than phages from healthy controls, suggesting a potential immunomodulatory role of phages in chronic intestinal autoinflammation.119 However, such innate immune activation is not observed with all phages. The T4 phage, for example, does not elicit an immune response, including production of reactive oxygen species, at least in the in vitro setting.120 Another key element in the activation of host immunity by phage preparation is the potential contamination with endotoxins, especially LPS.117 Endotoxins are potent inducers of immunity and could thus potentially influence the host immune response against the phage. Indeed, phage targeting of gram-negative bacteria leads to massive release of LPS, which in turn will likely increase host immunity toward the phage as well as bacterial clearance.
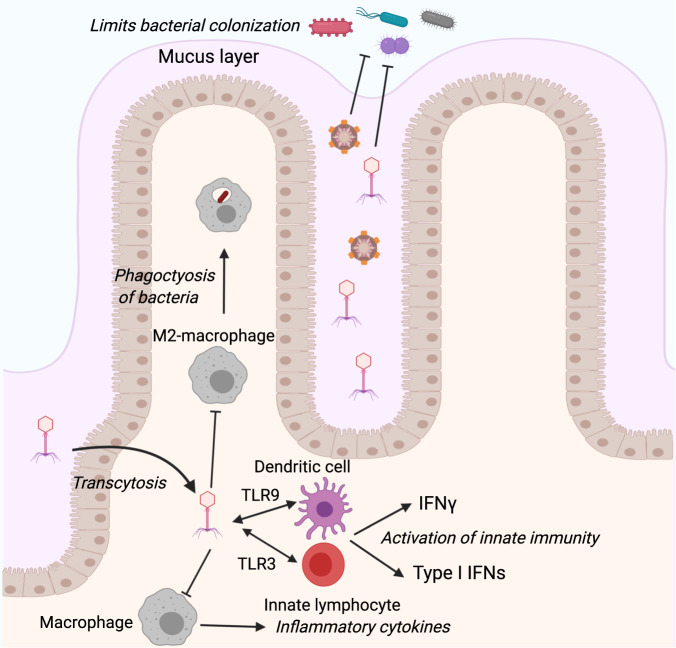
Fig. 2.
Phage–immune interactions in the gut. Phages interact with the host immune system in various ways. Depicted are some examples of such interactions occurring in the mammalian gut, including phage-induced stimulation of macrophages to inhibit phagocytosis and production of inflammatory cytokines, activation of dendritic cells and ILCs to produce IFNs, and promoting direct protection against bacterial colonization
Conversely, the general inflammatory state of the host was also shown to directly control the number of phages. LPS-induced inflammation in mice reduces phage levels in vivo in several organs, including the spleen, liver, and kidney, through induction of phage-specific antibodies.121 Interestingly, phages can exhibit some level of commensalism within the host, as high levels of phages were found in the intestinal mucus layer, as well as other mucosal barrier surfaces such as the gums.122 Phages directly bind to mucin glycoproteins via their capsids. The presence of phages close to the intestinal epithelium likely serves as a defense mechanism of the mammalian host, in preventing bacterial breach of the intestinal barrier, and may constitute an exciting avenue of future research. Conversely, phages can promote loss of intestinal barrier integrity123 through elusive mechanisms that may involve phage-induced alterations in the composition of the intestinal microbiome.
Collectively, these observations suggest that, similar to bacteria, phages could display commensal versus pathogenic behaviors. Indeed, in sponges, phage-dependent expression of Ankyrin proteins in bacteria can promote host-symbiont coexistence by suppressing eukaryote inflammatory mechanisms including phagocytosis and inflammatory cytokine secretion by macrophages.124 Conversely, phages may impact bacterial pathogens and their interactions with the host immune system during infection. In respiratory infection with Pseudomonas aeruginosa, the presence of specific phages strongly increases their in vivo infectivity, leading to higher morbidity and mortality.125 This effect is dependent on phage interaction with the host immune system through the activation of TLR3 and subsequent induction of type 1 interferons (Fig. 2). Furthermore, phages may promote inhibition of tumor necrosis factor and phagocytosis.125 Indeed, Pseudomonas phage predation was found to make strains more virulent and less prone to macrophage phagocytosis.126
In contrast to the disease-exacerbating roles of some phages mediated through their effects on bacterial pathogens, other phages were found to induce opposite effects. The filamentous phage of P. aeruginosa prevents Pseudomonas-induced lung injury, mortality, and inflammation, an activity associated with lower cytokine levels and enhanced bacterial adhesion to mucin coupled with reduced invasion of epithelial cells.127 The phage was also shown to promote M2 macrophage polarization, as well as reduced bacterial phagocytosis127 (Fig. 2). The underlying molecular mechanisms for these intriguing impacts remain elusive but are speculated to involve immune system modulation. For example, phage therapy against multi-drug-resistant P. aeruginosa depends on myeloid differentiation primary response 88 (Myd88), a critical signaling molecule downstream of pattern recognition receptors of the TLR family.128 Indeed, while neutrophils are also strictly required for efficient phage-mediated therapy of P. aeruginosa infection, lymphocytes, including innate lymphoid cells, are immune to their effects128. These findings suggest that the adaptive immune system may not play a major role in the antipathogenic effects of phages, at least in some contexts. With increased interest in using phages as a therapeutic for antibiotic-resistant bacterial infection, understanding the molecular basis of phage interactions with the immune system will become crucial for their successful in vivo implementation as an antibacterial treatment.
Gut phageome in intestinal steady state
The healthy gut microbiome features a core community of bacterial phyla that are consistently present in over 90% of healthy individuals.129 In addition, several studies have attempted to describe the composition of a “healthy gut phageome” (HGP).53,54,65 Manrique et al. used ultra-deep sequencing to study viral taxa identified in geographically distant individuals.59 Network analysis of the sequenced viral contigs identified three biological groups based on three fractions: a “core”, consisting of 9 phages shared by over 50% of healthy people, a “common” set harbored by 20–50% of individuals, and a rare set of unique or uncommonly shared phages among subjects, containing all remaining phages. The core group, for instance, included the widely spread crAssphage, defined as a benign cosmopolitan element of the gut phageome, which is correlated with different bacterial configurations and dietary habits but not with a particular disease state.104,130 The authors defined the combination of the core and the common fraction as the HGP, which is commonly present in the intestine of healthy adults, and strikingly, found it to be decreased in individuals with Crohn’s disease (CD) and UC.59
The bilateral influences of the gut phageome and bacterial microbiome on each other have been challenging to address, as over 80% of temperate phages found in the gut are absent in three commonly used databases.63 Identifiable phages integrate more frequently in the genome of Proteobacteria and Firmicutes, rather than Bacteroidetes and Actinobacteria,51,52 despite the high abundance of Bacteroidetes in the human gut. Whether this stems from an increased abundance of phages targeting these groups, or whether phages specifically targeting Bacteroidetes are less well-annotated, remains unclear. Among phage–microbiome interactions, the easiest to identify are the ones that contribute to bacterial virulence.124 Ankyphages, recently described in sponges but abundant also in humans, promote bacterial protection from macrophage-driven phagocytosis. Recently, contigs encoding similar prophage-derived proteins were also found to be expressed in oral and gut virome metadata.124 One major challenge in studying the possible effects of the gut phageome on bacterial microbiome configuration stems from the lack of a direct causal relationship between phages and their commensal bacterial hosts. As such, some of the observed dynamics in phage abundances may likely reflect the abundance of their bacterial host, as was recently observed in IBD patients.65 Moreover, available methods and databases are currently insufficiently equipped to recognize phages targeting some phyla, as is the case for crAssphage.
To address these limitations, researchers modeled the evolution of the lytic and temperate phages in the healthy gut using ecological modeling. For example, in the “kill-the-winner” model, present in several aquatic environments,131 phages kill the most abundant bacterial hosts, leading to a massive decrease in biomass and replacement by novel bacteria, which will in turn be targeted by specific phages. The resultant reduction in each targeted bacterial population also decreases their respective phage population size, leading to a dynamic cycle.132 Intriguingly, the “kill-the-winner” model may explain the gut viral kinetics noted in the healthy infant gut, in which a high diversity of phages, or predators, at birth, diminishes because of the low bacterial colonization, followed by an expansion of gut bacteria. This is consequently followed by an enrichment of phage species.66 Conversely, several studies observed mostly a temperate regime in adult individuals, in which phages are integrated within bacterial chromosomes as prophages, rather than clear predator-prey dynamics.54,56 Therefore in adults, the phageome seems to fit a stable long-term configuration54,56 rather than the rapid compositional changes characteristic in the “kill-the-winner” model. However, to date, no longitudinal cohorts have fully assessed the kinetics and ‘rules of engagement’ by which this diverse phage composition stabilizes into its adult configuration. In summary, changes in the gut microbiome and phageome are tightly linked, and dissecting cause and effect in this relationship is challenging but may lead to a more holistic understanding of the forces shaping the bacterial microbiome.
Gut phageome in inflammatory bowel disease
While numerous studies have associated the bacterial microbiome with human disease, including arteriosclerosis,133 inflammation,95 amyotrophic lateral sclerosis,134 nonalcoholic fatty liver disease135, and other diseases,136 the drivers of dysbiosis in these conditions remain largely elusive. Phages could represent one such putative dysbiosis-promoting mechanism. Indeed, severe inflammatory states of the gut such as CD and UC have been associated with dysbiosis characterized by a disproportionate outgrowth of Proteobacteria and Bacteroides phyla over Firmicutes.137 Importantly, abnormal composition of the enteric virome has also been observed in inflammatory bowel disease (IBD). IBD patients from three different geographically distant cohorts showed an increase in Caudovirales abundance and species richness and a simultaneous diminishing of Microviridae upon comparison to healthy household controls. These changes were inversely correlated with bacterial diversity in these subjects, while a disease-specific phage pattern could be identified.65 A longitudinal study in a colitis mouse model revealed phage patterns similar to those in humans with IBD, characterized by increased numbers of Caudovirales and of phages targeting Streptococcus spp. and Alistipes spp.67
These interesting observations notwithstanding, many alterations in the gut virome in IBD patients do not seem to provide a direct explanation for the changes observed in bacterial communities. For example, the unexpected increase in phages targeting a Bacteroidetes member such as Alistipes spp. seems contradictory to the decline of Bacteroidetes observed during IBD. Other alternative mechanisms may involve release of inflammatory products by the human host, enhancing excision of integrated prophages from bacterial chromosomes, thereby impacting the virome composition. Alternatively, a local lack of nutrients during active inflammation constitutes a known trigger of bacterial stress that could initiate lysogeny excision. As such, an IBD-associated, dysbiotic microbiome consisting of stressed bacteria competing for scarce nutrients would be the ideal environment to trigger the onset of an IBD-associated phage bloom.67 Further associations between phages and their host could be niche-specific. For example, while Caudovirales increased in abundance in the gut lumen of IBD patients,65,67 species diversity was decreased in the inflamed mucosa compared to biopsies from healthy gut segments of the same individuals or healthy controls.138 A functional analysis of the mucosa-associated virome in these patients revealed how genes associated with viral fitness (e.g., DNA binding and integrase) were abolished in favor of genes expressing bacterial pathogenicity or antibiotic resistance, highlighting how the mucosal virome could potentially contribute to IBD pathogenesis.138 After identifying novel prophages in the genome of Faecalibacterium prausnitzii, Cornuault et al. hypothesized that these phages could be intestinally induced by IBD-related inflammation, diminishing the presence of the beneficial bacterium and contributing to a pro-inflammatory state. Indeed, they observed a significant intestinal enrichment of F. prausnitzii-targeting phages in IBD patients when compared to healthy controls. However, these results were not corroborated in a colitis mouse model.139
Overall, a healthy gut phage community is distinct from that of the IBD-associated phage pattern, but the nature of putative phage–bacteria interactions, regulation by the host, and impact on downstream disease pathogenicity merit further study.
Modeling host–phage interactions in animal models
Modeling host–bacterial phage interactions in small animal models may contribute to better understanding of phage impact on the bacterial microbiome and human clinical traits. For example, a striking similarity was recently reported between healthy and IBD-affected human virome composition and that of an established murine model of colitis,67 suggesting that putative causal commonalities may exist between mammalian species, with respect to phage shifts in inflammatory conditions.
Gnotobiotic mice constitute a key tool in generating mechanistic insights in the microbiome, since transferring microbial communities from disease-affected mice or humans into gnotobiotic mice enables researchers to establish causality between a clinical state and specific bacterial or viral configurations. An elegant study investigated the impact of a pool of human phages on a consortium of 15 bacterial commensals in the gut of adult germ-free (GF) mice.100 Interestingly, some unknown phages emerged and persisted in the murine gut after 7 days, without a decrease in any related bacterial species, suggesting that they have no apparent bacterial hosts. In contrast, an in vitro model of phage interactions with the same consortium did not show a parallel expansion of any of the unknown phages, highlighting the importance of factors related to the mammalian hosts in impacting these interactions. In addition, some of the phages administered to mice were isolated from stool after only 1 week. This could potentially represent a case of pseudolysogeny, in which a phage is steadily present within a prokaryotic host without undergoing duplication (Fig. 1a).100 Using a similar approach in GF mice, Hsu et al. observed that phage infection of a known bacterial consortium also impacted other nonbacterial members of the microbiome via indirect effects.18 Moreover, phages strongly impact the gut metabolite repertoire, either those associated with a single bacterial species (e.g., the neurotransmitters tryptamine and tyramine) or those more broadly associated with microbiome members (such as AAs or bile salts), suggesting that the phageome could be used as an indirect tool to manipulate the host metabolome via microbiome targeting.18,21 Taken together, these results indicate that utilizing GF mice as a microbiome-free host is a useful tool in decoding the complexity of phage–microbiome interactions and elucidating causal links in vivo. Furthermore, these results highlight the need to study phage–bacteria interactions both in vivo and in vitro, thereby enabling the study of phage-induced indirect effects on commensals and the host.
Phage therapy
In the 1930s, a full review of all existing literature on phage therapy, requested by the Council on Pharmacy and Chemistry of the American Medical Association, reported insufficient data to recommend phages as a treatment method.140 During this era, despite the use of phage therapy as treatment for numerous infectious diseases (e.g., typhoid fever, cholera, osteomyelitis, urinary infection, gonorrhea, acne), unambiguous reports demonstrating the clinical efficacy of phage therapy were lacking. The few studies on which these interventions were based lacked preclinical results in animal models and adequate control groups in human studies. In addition, phage preparation methods, administration routes, and dosage varied between groups, while phage formulations, derived from crude bacterial extracts, contained large amounts of endotoxins and other supplements such as mercury, which could have inactivated the phage.20 Moreover, many of the phage treatment indications at the time focused on self-limited diseases, while phage therapy in life-threatening disease was used as a last-resort compassionate attempt and often did not include adequate placebo controls. Furthermore, limited knowledge was available at the time about serotype identification of phage strains, host range, phage resistance development in bacteria and lysogeny, making it impossible to trace the nature and identity of phage strains utilized by different research groups and clearly establish why some resulted in more effective therapy than others.141 Consequently, several authors at the time concluded that persuasive therapeutic effects could be observed only in treating localized staphylococcic infections and cystitis.21 The development of a wide repertoire of antibiotic drugs such as penicillin in the mid-20th century led most clinicians and scientists to dismiss phage therapy as inferior and no longer suitable for use in a clinical setting.
However, major developments in molecular microbiology and genomics now make obtaining highly pure phage preparations possible, including selection of strictly lytic phages or even engineering them by deleting genes for the lysogenic cycle. This broadens their host range or prevents horizontal transfer of pathogenic and antibiotic resistance genes.20 In the last two decades, renewed interest in clinical phage utilization against antibiotic-resistant infection resulted in numerous clinical applications of phages featuring successful outcomes against pathogenic infections such as P. aeruginosa,142 Acinetobacter baumanii,143 and Vibrio parahaemolyticus144 in mice, Clostridium difficile in hamster,145 and Staphylococcus aureus in rabbits146 (Table 2). Intraperitoneal administration of a single phage strain successfully treated infections caused by B-lactamase producing Escherichia coli,147 vancomycin-resistant Enterococcus faecium,148 and imipenem-resistant P. aeruginosa149 in murine models. However, many challenges still limit the systemic use of phages as pharmaceuticals, including unknown pharmacokinetic and pharmacodynamic properties.150 Variables affecting phage pharmacokinetics include adsorption (e.g., influx into the blood stream), distribution, (e.g., influx from blood to tissues) and phage clearance by excretion and disintegration. While phage elimination mainly occurs in the liver and spleen and is mediated by serum complement or mononuclear phagocytes, long-circulating phages can be selected by repeated in vivo passages151 or through decoy peptide expression rendering them less immunogenic.152 Intravenous injection is the most effective route of administration of phages, and active phages have been observed in the blood stream within minutes of administration.153
Table 2.
Overview experimental and clinical studies of phage therapy
| Bacterium | Disease | Administration route | Comments | Model | Bibliography |
|---|---|---|---|---|---|
| E. coli | Peritonitis | i.p. | Engineered phagemids expressing antimicrobial peptides (AMP) designed to kill bacteria by a nonlytic process to avoid LPS release. | Mouse | 217 |
| E. coli | Diarrhea | oral gavage | Phage treatment shown to be safe; neither adverse events nor antiphage antibodies were observed in patients. | Humans | 156 |
| E. coli | Diarrhea | oral gavage | In a phase II randomized and placebo-controlled trial on children, the phage failed to replicate in the gut, with no improvement noted in diarrhea severity. | Humans | 218 |
| E. coli K1 | E. coli-induced septicemia | i.v. | The lytic bacteriophage ΦIK1 has successfully rescued mice from lethal E. coli K1 infection upon administration within 60 min of infection. | Mouse | 219 |
| E. faecium (VRE) | Burn wound infection | i.p.a | Phage co-injection with the infectious agent rescued 100% of animals from fatal infection. If administered within 45 min of infection, 50% of mice recovered. | Mouse | 148 |
| Imipimem-resistant P. aeruginosa | Bacteremia | i.p.a | Phage ØA392 saved 100% of animals if administered within 180 min after infection; after this time, the recovery rate dropped to 50%. | Mouse | 149 |
| K. pneumoniae | Burn wound infection | i.p.a | No therapeutic effect was observed after multiple phage treatments, with multiplicity of infection (MOI) ranging from 0.001 to 900. | Mouse | 220 |
| K. pneumoniae | Lobar pneumonia | i.p.a, intranasal | Preventive intranasal phage administration rescued mice from death, but administration after 6 h was not effective against infection. | Mouse | 153 |
| MDR A. baumannii | Wound infection | i.p., topical | A cocktail of four phages lowered the burden of infection by selecting for an unencapsulated, avirulent strain variant of the pathogen. | Mouse | 182 |
| MRSA S. aureus | Lethal lung-derived septicemia | i.p.a | Injection of phages 6 h after infection increased survival of infected mice from 10 to 67%. | Mouse | 221 |
| MRSA S. aureus | Chronic osteomyelitis | i.p. | Efficacy of phage therapy was noted when animals were treated either 4 weeks or 6 weeks after infection. | Rabbit | 222 |
| P. aeruginosa | Burn wound infection | i.m., s.c., i.p. | Mortality was decreased in animals intraperitoneally injected with phages, with an 87% survival rate noted. | Mouse | 223 |
| P. aeruginosa | Endocarditis | infusion into the superior vena cava | Phages were synergistically administered with ciprofloxacin, achieving a high therapeutic effect. Bacterial mutants resistant to the phage developed but displayed defective virulent phenotype. | Mouse | 181 |
| P. aeruginosa | Otitis | ear dropsa | A group of 24 patients suffering of chronic otitis was successfully treated by phage therapy, with reporting no adverse effects. | Humans | 224 |
| P. aeruginosa | P. aeruginosa infection in cystic fibrosis (CF) model | injection into duct of Cuvie | Phage therapy decreased lethality and improved inflammatory status. | Zebrafish | 225 |
| Shigella sonnei | Diarrhea | oral gavage | A phage cocktail was compared to ampicillin treatment, resulting in equally effective results, with no impact on gut microbiome composition noted. | Mouse | 226 |
i.v. intravenous, i.m. intramuscular, i.p. intraperitoneal
aa single dose could completely eradicate the infection
Many more variables complicate oral phage delivery. An efficient phage encounter with its target bacterium in the gut remains a daunting challenge, potentially limited by factors such as gastric acids (phages are sensitive to low pH), intestinal mucus protection and bacterial biofilm barriers. Some observations suggest that, once phages overcome the gastric barrier, they are mainly absorbed by gut lymph nodes.154 In a cohort of 120 Bangladeshi children affected by acute diarrhea, no beneficial clinical effects were observed upon oral consumption of T4-like coliphages for 4 days compared to controls. Importantly, fecal phage titers were not higher in patients infected with the bacterial target, suggesting that the phage passively transited along the gastrointestinal tract without ever meeting its target.155 No detrimental secondary effects were observed in this study, as already previously described, with healthy volunteers receiving phages for 3 weeks in drinking water.156 Interestingly, several reports noted successful intranasal delivery of phages into the lungs of mice157,158 and via intraperitoneal injection into the brain.159,160
A potential advantage of phage therapy is its self-replicating nature, coupled with a narrow host specificity,161 thereby limiting off-target effects, preserving microbiome integrity and eubiosis.20 However, narrow host specificity could also be considered a limitation, as it remains challenging to target all pathogenic strains of a given species by a single phage type. Recently, the use of cocktails of several phages, targeting different host receptors, proved to be an effective therapeutic strategy in increasing host range of phage therapy, while also delaying or preventing the onset of phage resistance.156,162,163 Alternatively, incorporation of receptor-binding proteins to broaden phage host specificity may also achieve the same task.164–166 Once they reach the targeted bacterial host, the ability of phages to actively replicate depends on both the ability of the phage to reach a sufficient burst size (i.e., number of new virions produced per infected bacterium) and on the bacterium supporting phage population growth in adequate numbers. Moreover, availability of bacterial surface receptors required for phage infection might vary in in vivo settings in a context-dependent manner and result in the emergence of bacterial phage resistance, as discussed below.
A less specific treatment approach that may involve phages is fecal microbiome transplantation (FMT), mainly reported in C. difficile infection (CDI) in humans167,168 but it has also been increasingly tested in other clinical scenarios with a variable success rate.169–172 Interestingly, patients affected by CDI display a predominance of Caudovirales. After FMT treatment, responders feature a reduced abundance of Caudovirales, coupled with an increase in species richness, and a shift in phage composition toward that of the donors.173–175 In comparison, a pure gut viral transfer (i.e., viruses purified from the bacterial fraction) from mice fed a high-fat diet (HFD) to recipients on HFD or on standard diet was sufficient to alter microbiome composition in recipient mice.176 Very recently, a high-throughput method called VT was developed to enable the pursuit of novel bacterium–phage interactions and was used to design a rational FMT preparation, in which the donated sample consisted of an in vitro mix of virome and bacterial fractions isolated from stools of different individuals. Of note, a high cross-infectivity, in which phages displayed a very broad range against several bacterial species, was observed, suggesting a potential risk of a nonspecific perturbation of the microbiome following FMT.177 Another limitation of this interesting methodology relates to its nonspecific nucleic acid staining, which does not allow for discrimination of dsDNA phages from other gut viruses.178
The most advanced experimental clinical application of phage therapy involves targeting of multi-drug resistant (MDR) bacteria. With the recent increase in MDR pathogen-mediated infection, the interest and need of the scientific and medical communities to find new ways to manipulate human bacterial ecosystems in this setting are rapidly increasing.179 Phages and antibiotics share no antimicrobial molecular mechanisms. Thus, cross-resistance is unlikely to occur between these two strategies, while synergistic use of antibiotic-phage therapy, already extensively validated in vitro and in vivo, may constitute an attractive strategy to prevent the development of phage-resistant clones180–182 (see Table 2). Indeed, phage treatment was effective against MDR Klebsiella pneumoniae biofilms,183 and it augmented the effect of antibiotics, which were ineffective when used alone.184 In 2017, a 68-year old male patient underwent phage therapy against an MDR A. baumanii-infected pancreatic pseudocyst due to necrotizing pancreatitis and associated multi-organ failure and featured a significant improvement. Interestingly, following treatment, a phage-resistant clone of A. baumanii was isolated, displaying a defective capsule configuration, which was more accessible to penetration by antibiotics.185 Of note in this case report, no control was included to confirm that the recovery was due to phage administration and not to other confounding factors. Other cases of compassionate intravenous phage therapy against human MDR infections have also been documented,185,186 including treatment for Mycobacterium abscessus infection in a 15-year-old CF patient intravenously administered a cocktail of lytic, genetically engineered phages to remove immunity repressor genes or augment infectivity. The patient showed substantial clinical improvement, with no adverse effects noted.187
Despite these promising preliminary reports, phage therapy has to undergo all phases of a clinical trial to ensure efficacy, safety, and absence of adverse effects of the novel modality.188 In 2016, the European Medicines Agency confirmed that none of the existing regulations suit the peculiar case of phage therapy and that a new regulatory framework toward phage therapy testing should be adapted.189 The exponential biological diversity of the ecosystem comprising bacteria, phages, and the human host would require a new clinical trial for every new candidate phage of a given phage cocktail, which would lead to an unrealistic and immense number of clinical trials needed for any contemplated phage treatment.190 Because of the prohibitive costs required to accomplish such standards, phage therapy enterprise has been limited so far only to academia and small biopharmaceutical companies.188 Given these issues, and the growing need and interest to move phage therapies into the clinic, a dialog is underway between scientists, clinicians, biopharma entities, and regulatory authorities to devise stringent yet viable regulatory pathways ensuring patient safety while not implementing insurmountable obstacles on phage treatment development.
Additional challenges in phage treatment development include the need to develop effective protocols enabling optimization of phage efficacy. Considerations include optimization of phage dosage (due to their self-replicating nature) and mode of application, which should ensure contact with the bacterium to allow replication while not leading to excessive dilution of the phage. Even assessment of the host range mandates standardized protocols using efficacy of plating, which is defined as the number of plaques growing on the target bacterial cultures over the number of plaques growing on the natural bacterial cultures.191 Standardized criteria should be established to assess phage efficacy, define a minimum bactericidal level, and determine bacteriostatic or bactericidal properties over time against reference pathogens, similar to parallel approaches utilized in the development of novel antimicrobial molecules.188
To ensure safety, phages or phage-derived molecules should be produced according to good manufacturing practices (cGMP), obtaining ultrapure preparations that are LPS-free and compatible with physiological solutions to fulfill regulatory requirements.192 Safety, defined as the lack of adverse effects on healthy volunteers, should be assessed in the initial phase of a clinical trial. Although it is known that the innate and adaptive immune response can be stimulated in a phage-dependent manner as discussed above,119,193,194 numerous studies report phages to be safe, especially when orally administered.156,163 Further safety issues could arise from immune reactions to in situ released LPS or other PAMPs, generated from bacterial lysis, which could be addressed by the use of less virulent phages or by co-administration of antibiotics.
Collectively, phage cocktail development as a means of targeting pathogenic or commensal bacteria holds great promise for introducing a new class of patient-tailored precision medicine, but is limited by a number of technical and regulatory challenges. Optimistically, adaptive regulatory paths have been already proposed to address these challenges, allowing the development of in vivo precharacterized phage libraries, enabling mixing and matching of phage cocktails in targeting distinct pathogenic infections188 (Fig. 3).
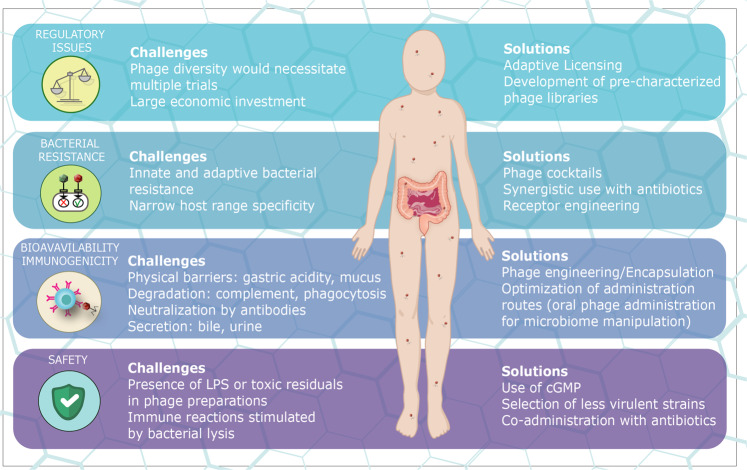
Fig. 3.
Challenges and solutions toward clinical application of phage therapy. Depicted are several of the major challenges and proposed solutions for the application of phage therapy. These include regulatory obstacles limiting phage cocktail application that may be addressed by the development of adaptive regulatory protocols; bacterial resistance can be addressed by a rational design of phage cocktails, co-administration with antibiotics, and synthetic alteration of phage structure; challenges related to bioavailability and immunogenicity could be addressed by engineering phage outer structures or phage encapsulation, (e.g., via PEGylation) making them less immunogenic, or through the development of oral routes of administration for gut microbiome modulation; safety challenges may be addressed by following cGMP regulations, pre-selecting phage strains and their co-administration with other antimicrobials
Phages as immunotherapy
Another emerging modality of phage therapy involves the exploitation of the immunomodulatory properties of phages to elicit a host immune response against a bacterial pathogen. While binding of antibodies to phages has been shown to limit phage therapy efficacy,195 immunogenic properties of phages have been exploited to induce potent immune responses. Examples include the use of bacterial strains exposed to phages, leading to their reduced fitness and growth, thereby enabling their exploitation as part of a vaccination strategy.196–198 Even structural components of phages have been used as vaccines. An example is the use of the phage protein bacterial cell wall lysin of a phage commonly targeting methicillin-resistant S. aureus (MRSA), which elicits a potent immune response and subsequently provides protection from MRSA infection.199 Similarly, a phage-derived peptide has been shown to treat Mycobacterium tuberculosis infection by promoting antibacterial immunity.200 Indeed, phages feature intrinsic immunogenic properties that can be exploited in various contexts, including VLP-mediated vaccination strategies, in which the highly repetitive nature of the page antigen arrangement favors generation of potent antibody responses.201 Such approaches have even been used to prevent fungal infection, in which recombinant phages expressing fungal antigens provided potent protection.202 Similarly, a vaccination approach targeting a phage of P. aeruginosa was shown to protect against bacterial infection by generating antiphage antibodies which, in turn, promoted opsonization and bacterial uptake.125 Moreover, specific immunological mechanisms, such as antigen presentation of specific proteins to dendritic cells through phage infection, has been used as a tool to induce potent T-cell immunity.203 These effects were also dependent on innate pattern recognition receptors such as TLR9, highlighting the role of phages not only as activators of antibody production by B cells but also as key regulators of the mononuclear phagocyte compartment. Overall, these findings highlight the broad potential use of phages or their components for immunomodulation, underlining the importance of phages in contexts outside the classical realm of phage therapy. One may speculate that these unique phage immunomodulatory features may enable future development of the immunotherapies against cancer and other noninfectious diseases.
Concluding remarks
The current popularization of research exploring phage–bacterial–host interactions and translating the resulting findings into new forms of human-relevant phage therapy is encouraging but also poses major challenges. One such challenge involves a more comprehensive characterization of phage taxonomy within the ‘dark matter’ presented in most next-generation sequencing datasets. This will be crucial in providing a holistic description of the phageome, which will, in turn, enable a better understanding of factors, which are orchestrating phage composition under different physiological contexts. With the expected maturation of phage research, an effort should be made to move from descriptive studies to those pursuing deeper mechanistic insights of phage biology and impact on complex ecologic communities such as the human microbiome. Combinations of modeling, whole-community approaches, and experimental work will be necessary in delineating whether compositional and functional alterations of the microbiome are directly, or indirectly, driven by phageome changes. The driving forces shaping phage modulation of the immune response and, conversely, immune reactivity toward phages merit further mechanistic studies. Understanding this relationship will be crucial for the design of phage therapy, while avoiding immune-mediated inhibition of phage antibacterial activity and harnessing phage-initiated immune responses toward potentiation of their antimicrobial effects. Phage–immune interactions are also likely to affect the microbiome, and how this tripartite relationship is controlled will be key in understanding the complexity of the ecosystem in health and disease.
Conversely, decoding how the phageome is influenced by disease, how it may influence interindividual microbiome differences, and how it may modulate host immune responses will constitute important areas of future research.
With respect to phage therapy, successful application will probably be different from that at the beginning of the twentieth century. It may involve personalization of phage cocktails to the individual and his/her microbiome configurations. It may require genetic engineering of phage structures to ensure safety or the use of phages in combination with other treatments or as an adjuvant for antibiotics, with the goal of maximizing efficacy. Such combinations may better overcome the rising incidence of multi-drug-resistant infections. Importantly, with the advent of data-driven phage therapy against discrete commensals in the microbiome, noncommunicable diseases impacted by dysbiosis, such as metabolic, inflammatory, neoplastic, and neurodegenerative disorders, may also benefit from phage-directed pathobiont elimination therapy. Key limitations include the need to minimize indirect nonspecific immune effects mediated by phages, while specifically targeting disease-associated commensals. This may represent a formidable task, especially when the targeted commensals are rare. In addition, exploiting different routes of phage administration may profoundly affect phage effector function and the resultant activation status of the host immune system. Exploitation of the complex relationship between phages, bacteria, and the host holds promise for future development of immunotherapies against infection and other microbiome-associated diseases.
Acknowledgements
We thank the members of the Elinav laboratory for discussions and apologize for authors whose work was not cited because of space constraints. S.P.N is funded by an EMBO Long-term Fellowship ALTF 767–2017. E.E. is the incumbent of the Sir Marc and Lady Tania Feldmann Professorial Chair, a senior fellow at the Canadian Institute of Advanced Research (CIFAR) and an international scholar at the Bill & Melinda Gates Foundation and the Howard Hughes Medical Institute (HHMI).
Author contributions
All authors performed an extensive literature research, contributed substantially to discussion of the content, and wrote and edited the manuscript.
Competing interests
E.E. is a salaried scientific consultant for DayTwo and BiomX. S.F. and S.P.N. have nothing to declare.
Footnotes
These authors contributed equally: Sara Federici, Samuel P. Nobs
References
- 1.Reyes A, et al. Going viral: next-generation sequencing applied to phage populations in the human gut. Nat. Rev. Microbiol. 2012;10:607–617. doi: 10.1038/nrmicro2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suttle CA. Viruses in the sea. Nature. 2005;437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- 3.Zablocki O, Adriaenssens EM, Cowan D. Diversity and ecology of viruses in hyperarid desert soils. Appl. Environ. Microbiol. 2015;82:770–777. doi: 10.1128/AEM.02651-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howard-Varona C, et al. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J. 2017;11:1511–1520. doi: 10.1038/ismej.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinbauer MG. Ecology of prokaryotic viruses. FEMS Microbiol. Rev. 2004;28:127–181. doi: 10.1016/j.femsre.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Erez Z, et al. Communication between viruses guides lysis–lysogeny decisions. Nature. 2017;541:488–493. doi: 10.1038/nature21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davidson AR. Phages make a group decision. Nature. 2017;541:466–467. doi: 10.1038/nature21118. [DOI] [PubMed] [Google Scholar]
- 8.Harms A, Diard M. Crowd controlled-host quorum sensing drives phage decision. Cell Host Microbe. 2019;25:179–181. doi: 10.1016/j.chom.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Zeng L, et al. Decision making at a subcellular level determines the outcome of bacteriophage infection. Cell. 2010;141:682–691. doi: 10.1016/j.cell.2010.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hynes AP, Moineau S. Phagebook: the social network. Mol. Cell. 2017;65:963–964. doi: 10.1016/j.molcel.2017.02.028. [DOI] [PubMed] [Google Scholar]
- 11.Bellas, C., Anesio, A. & Barker, G. Analysis of virus genomes from glacial environments reveals novel virus groups with unusual host interactions. Front. Microbiol. 6, 656 (2015). [DOI] [PMC free article] [PubMed]
- 12.Silpe JE, Bassler BL. A host-produced quorum-sensing autoinducer controls a phage lysis-lysogeny decision. Cell. 2019;176:268–280.e13. doi: 10.1016/j.cell.2018.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galtier M, et al. Bacteriophages to reduce gut carriage of antibiotic resistant uropathogens with low impact on microbiota composition. Environ. Microbiol. 2016;18:2237–2245. doi: 10.1111/1462-2920.13284. [DOI] [PubMed] [Google Scholar]
- 14.Galtier M, et al. Bacteriophages targeting adherent invasive Escherichia coli strains as a promising new treatment for Crohn’s Disease. J. Crohn’s Colitis. 2017;11:840–847. doi: 10.1093/ecco-jcc/jjw224. [DOI] [PubMed] [Google Scholar]
- 15.Maura D, et al. Virulent bacteriophages can target O104:H4 enteroaggregative Escherichia coli in the mouse intestine. Antimicrob. Agents Chemother. 2012;56:6235–6242. doi: 10.1128/AAC.00602-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh J, et al. Temporal stability of the human skin microbiome. Cell. 2016;165:854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh JH, et al. Dietary fructose and microbiota-derived short-chain fatty acids promote bacteriophage production in the gut symbiont Lactobacillus reuteri. Cell Host Microbe. 2019;25:273–284.e6. doi: 10.1016/j.chom.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Hsu BB, et al. Dynamic modulation of the gut microbiota and metabolome by bacteriophages in a mouse model. Cell Host Microbe. 2019;25:803–814.e5. doi: 10.1016/j.chom.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.d'Hérelle F. Sur un microbe invisible antagoniste des bacilles dysentériques. C. R. Acad. Sci. 1917;165:373–375. [Google Scholar]
- 20.Sulakvelidze A, Alavidze Z, Morris JG. Bacteriophage therapy. Antimicrobial Agents Chemother. 2001;45:649–659. doi: 10.1128/AAC.45.3.649-659.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krueger AP, Scribner E. Bacteriophage therapy. II. The bacteriophage: its nature and its therapeutic use. JAMA. 1941;19:2160–2277. [Google Scholar]
- 22.Dixon B. New dawn for phage therapy. Lancet Infect. Dis. 2004;4:186. doi: 10.1016/S1473-3099(04)00951-X. [DOI] [PubMed] [Google Scholar]
- 23.Pawluk A, Davidson AR, Maxwell KL. Anti-CRISPR: discovery, mechanism and function. Nat. Rev. Microbiol. 2018;16:12–17. doi: 10.1038/nrmicro.2017.120. [DOI] [PubMed] [Google Scholar]
- 24.Hyman P, Abedon ST. Bacteriophage host range and bacterial resistance. Adv. Appl Microbiol. 2010;70:217–248. doi: 10.1016/S0065-2164(10)70007-1. [DOI] [PubMed] [Google Scholar]
- 25.Bertin A, de Frutos M, Letellier L. Bacteriophage-host interactions leading to genome internalization. Curr. Opin. Microbiol. 2011;14:492–496. doi: 10.1016/j.mib.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Meyer JR, et al. Repeatability and contingency in the evolution of a key innovation in phage lambda. Science. 2012;335:428–432. doi: 10.1126/science.1214449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michel A, et al. Bacteriophage PhiX174’s ecological niche and the flexibility of its Escherichia coli lipopolysaccharide receptor. Appl. Environ. Microbiol. 2010;76:7310–7313. doi: 10.1128/AEM.02721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Latka A, et al. Modeling the architecture of depolymerase-containing receptor binding proteins in Klebsiella phages. Front. Microbiol. 2019;10:2649. doi: 10.3389/fmicb.2019.02649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren RA. Modified bases in bacteriophage DNAs. Annu. Rev. Microbiol. 1980;34:137–158. doi: 10.1146/annurev.mi.34.100180.001033. [DOI] [PubMed] [Google Scholar]
- 30.Hill C, Miller LA, Klaenhammer TR. In vivo genetic exchange of a functional domain from a type II A methylase between lactococcal plasmid pTR2030 and a virulent bacteriophage. J. Bacteriol. 1991;173:4363. doi: 10.1128/jb.173.14.4363-4370.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fineran PC, et al. The phage abortive infection system, ToxIN, functions as a protein-RNA toxin-antitoxin pair. Proc. Natl Acad. Sci. USA. 2009;106:894–899. doi: 10.1073/pnas.0808832106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Short FL, et al. The bacterial Type III toxin-antitoxin system, ToxIN, is a dynamic protein-RNA complex with stability-dependent antiviral abortive infection activity. Sci. Rep. 2018;8:1013. doi: 10.1038/s41598-017-18696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blower TR, et al. Viral evasion of a bacterial suicide system by RNA-based molecular mimicry enables infectious altruism. PLoS Genet. 2012;8:e1003023. doi: 10.1371/journal.pgen.1003023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blower TR, et al. Viral molecular mimicry circumvents abortive infection and suppresses bacterial suicide to make hosts permissive for replication. Bacteriophage. 2012;2:234–238. doi: 10.4161/bact.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yanli Zheng JL, et al. Endogenous Type I CRISPR-Cas: from foreign DNA defense to prokaryotic engineering. Front. Bioeng. Biotechnol. 2020;8:62. doi: 10.3389/fbioe.2020.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Houte S, et al. The diversity-generating benefits of a prokaryotic adaptive immune system. Nature. 2016;532:385–388. doi: 10.1038/nature17436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rollie C, et al. Targeting of temperate phages drives loss of type I CRISPR–Cas systems. Nature. 2020;578:149–153. doi: 10.1038/s41586-020-1936-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stern A, et al. CRISPR targeting reveals a reservoir of common phages associated with the human gut microbiome. Genome Res. 2012;22:1985–1994. doi: 10.1101/gr.138297.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanley SY, Maxwell KL. Phage-encoded anti-CRISPR defenses. Annu. Rev. Genet. 2018;52:445–464. doi: 10.1146/annurev-genet-120417-031321. [DOI] [PubMed] [Google Scholar]
- 40.Borges AL, et al. Bacteriophage cooperation suppresses CRISPR-Cas3 and Cas9 immunity. Cell. 2018;174:917–925.e10. doi: 10.1016/j.cell.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Landsberger M, et al. Anti-CRISPR phages cooperate to overcome CRISPR-Cas immunity. Cell. 2018;174:908–916.e12. doi: 10.1016/j.cell.2018.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alseth EO, et al. Bacterial biodiversity drives the evolution of CRISPR-based phage resistance. Nature. 2019;574:549–552. doi: 10.1038/s41586-019-1662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen D, et al. Cyclic GMP–AMP signalling protects bacteria against viral infection. Nature. 2019;574:691–695. doi: 10.1038/s41586-019-1605-5. [DOI] [PubMed] [Google Scholar]
- 44.Ofir G, et al. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 2018;3:90–98. doi: 10.1038/s41564-017-0051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koskella B, et al. The costs of evolving resistance in heterogeneous parasite environments. Proc. Biol. Sci. 2012;279:1896–1903. doi: 10.1098/rspb.2011.2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rodriguez-Valera F, et al. Explaining microbial population genomics through phage predation. Nat. Rev. Microbiol. 2009;7:828–836. doi: 10.1038/nrmicro2235. [DOI] [PubMed] [Google Scholar]
- 47.Arumugam M, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoyles L, et al. Characterization of virus-like particles associated with the human faecal and caecal microbiota. Res Microbiol. 2014;165:803–812. doi: 10.1016/j.resmic.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Kim MS, et al. Diversity and abundance of single-stranded DNA viruses in human feces. Appl Environ. Microbiol. 2011;77:8062–8070. doi: 10.1128/AEM.06331-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.d’Humières C, et al. A simple, reproducible and cost-effective procedure to analyse gut phageome: from phage isolation to bioinformatic approach. Sci. Rep. 2019;9:11331. doi: 10.1038/s41598-019-47656-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kang, H. S. et al. Prophage genomics reveals patterns in phage genome organization and replication. https://www.biorxiv.org/content/10.1101/114819v1 (2017).
- 52.Kim MS, Bae JW. Spatial disturbances in altered mucosal and luminal gut viromes of diet-induced obese mice. Environ. Microbiol. 2016;18:1498–1510. doi: 10.1111/1462-2920.13182. [DOI] [PubMed] [Google Scholar]
- 53.Minot S, et al. The human gut virome: inter-individual variation and dynamic response to diet. Genome Res. 2011;21:1616–1625. doi: 10.1101/gr.122705.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Reyes A, et al. Viruses in the faecal microbiota of monozygotic twins and their mothers. Nature. 2010;466:334–338. doi: 10.1038/nature09199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreno-Gallego JL, et al. Virome diversity correlates with intestinal microbiome diversity in adult monozygotic twins. Cell Host Microbe. 2019;25:261–272.e5. doi: 10.1016/j.chom.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minot S, et al. Rapid evolution of the human gut virome. Proc. Natl Acad. Sci. USA. 2013;110:12450–12455. doi: 10.1073/pnas.1300833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kapusinszky B, Minor P, Delwart E. Nearly constant shedding of diverse enteric viruses by two healthy infants. J. Clin. Microbiol. 2012;50:3427–3434. doi: 10.1128/JCM.01589-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Witsø E, et al. High prevalence of human enterovirus a infections in natural circulation of human enteroviruses. J. Clin. Microbiol. 2006;44:4095–4100. doi: 10.1128/JCM.00653-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Manrique P, et al. Healthy human gut phageome. Proc. Natl Acad. Sci. USA. 2016;113:10400–10405. doi: 10.1073/pnas.1601060113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCann A, et al. Viromes of one year old infants reveal the impact of birth mode on microbiome diversity. PeerJ. 2018;6:e4694. doi: 10.7717/peerj.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dutilh BE, et al. A highly abundant bacteriophage discovered in the unknown sequences of human faecal metagenomes. Nat. Commun. 2014;5:4498. doi: 10.1038/ncomms5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shkoporov AN, et al. ΦCrAss001 represents the most abundant bacteriophage family in the human gut and infects Bacteroides intestinalis. Nat. Commun. 2018;9:4781. doi: 10.1038/s41467-018-07225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim MS, Bae JW. Lysogeny is prevalent and widely distributed in the murine gut microbiota. ISME J. 2018;12:1127–1141. doi: 10.1038/s41396-018-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reyes A, et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc. Natl Acad. Sci. USA. 2015;112:11941–11946. doi: 10.1073/pnas.1514285112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norman JM, et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell. 2015;160:447–460. doi: 10.1016/j.cell.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim ES, et al. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nat. Med. 2015;21:1228–1234. doi: 10.1038/nm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duerkop BA, et al. Murine colitis reveals a disease-associated bacteriophage community. Nat. Microbiol. 2018;3:1023–1031. doi: 10.1038/s41564-018-0210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez-Hernandez F, et al. Single-virus genomics reveals hidden cosmopolitan and abundant viruses. Nat. Commun. 2017;8:15892. doi: 10.1038/ncomms15892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Deng L, et al. Viral tagging reveals discrete populations in Synechococcus viral genome sequence space. Nature. 2014;513:242–245. doi: 10.1038/nature13459. [DOI] [PubMed] [Google Scholar]
- 70.Dion MB, Oechslin F, Moineau S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020;18:125–138. doi: 10.1038/s41579-019-0311-5. [DOI] [PubMed] [Google Scholar]
- 71.Jong EC, Ko HL, Pulverer G. Studies on bacteriophages of Propionibacterium acnes. Med Microbiol Immunol. 1975;161:263–271. doi: 10.1007/BF02122714. [DOI] [PubMed] [Google Scholar]
- 72.Webster GF, Cummins CS. Use of bacteriophage typing to distinguish Propionibacterium acne types I and II. J. Clin. Microbiol. 1978;7:84–90. doi: 10.1128/jcm.7.1.84-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hannigan GD, et al. The human skin double-stranded DNA virome: topographical and temporal diversity, genetic enrichment, and dynamic associations with the host microbiome. mBio. 2015;6:e01578–15. doi: 10.1128/mBio.01578-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu J, et al. The diversity and host interactions of Propionibacterium acnes bacteriophages on human skin. ISME J. 2015;9:2116. doi: 10.1038/ismej.2015.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marples RR. The microflora of the face and acne lesions. J. Investig. Dermatol. 1974;62:326–331. doi: 10.1111/1523-1747.ep12724285. [DOI] [PubMed] [Google Scholar]
- 76.Zierdt CH. Properties of Corynebacterium acnes bacteriophage and description of an interference phenomenon. J. Virol. 1974;14:1268–7. doi: 10.1128/jvi.14.5.1268-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lood R, et al. Inducible Siphoviruses in superficial and deep tissue isolates of Propionibacterium acnes. BMC Microbiol. 2008;8:139. doi: 10.1186/1471-2180-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marinelli LJ, et al. Propionibacterium acnes bacteriophages display limited genetic diversity and broad killing activity against bacterial skin isolates. mBio. 2012;3:e00279–12. doi: 10.1128/mBio.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pulverer G, Sorgo W, Ko HL. [Bacteriophages of Propionibacterium acnes (author’s transl)] Zentralbl Bakteriol. Orig. A. 1973;225:353–363. [PubMed] [Google Scholar]
- 80.Allen EK, et al. Characterization of the nasopharyngeal microbiota in health and during rhinovirus challenge. Microbiome. 2014;2:22. doi: 10.1186/2049-2618-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.McCaskill JG, et al. Pulmonary immune responses to Propionibacterium acnes in C57BL/6 and BALB/c mice. Am. J. Respir. Cell Mol. Biol. 2006;35:347–356. doi: 10.1165/rcmb.2005-0285OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li Y, et al. Altered respiratory virome and serum cytokine profile associated with recurrent respiratory tract infections in children. Nat. Commun. 2019;10:2288. doi: 10.1038/s41467-019-10294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Willner D, et al. Metagenomic analysis of respiratory tract DNA viral communities in cystic fibrosis and non-cystic fibrosis individuals. PLoS ONE. 2009;4:e7370. doi: 10.1371/journal.pone.0007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gregory AC, et al. Smoking is associated with quantifiable differences in the human lung DNA virome and metabolome. Respiratory Res. 2018;19:174. doi: 10.1186/s12931-018-0878-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Damelin LH, et al. Identification of predominant culturable vaginal Lactobacillus species and associated bacteriophages from women with and without vaginal discharge syndrome in South Africa. J. Med. Microbiol. 2011;60:180–183. doi: 10.1099/jmm.0.024463-0. [DOI] [PubMed] [Google Scholar]
- 86.Pavlova SI, et al. Phage infection in vaginal lactobacilli: an in vitro study. Infect. Dis. Obstet. Gynecol. 1997;5:36–44. doi: 10.1155/S1064744997000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huttenhower C, et al. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fethers KA, et al. Sexual risk factors and bacterial vaginosis: a systematic review and meta-analysis. Clin. Infect. Dis. 2008;47:1426–1435. doi: 10.1086/592974. [DOI] [PubMed] [Google Scholar]
- 89.Pavlova SI, Tao L. Induction of vaginal Lactobacillus phages by the cigarette smoke chemical benzo[a]pyrene diol epoxide. Mutat. Res. 2000;466:57–62. doi: 10.1016/s1383-5718(00)00003-6. [DOI] [PubMed] [Google Scholar]
- 90.Jakobsen, R. R. et al. Characterization of the vaginal DNA virome in health and dysbiosis: an opening study in patients with non-female factor infertility. https://www.biorxiv.org/content/10.1101/755710v1 (2019).
- 91.Lev-Sagie A, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019;25:1500–1504. doi: 10.1038/s41591-019-0600-6. [DOI] [PubMed] [Google Scholar]
- 92.Pride DT, et al. Evidence of a robust resident bacteriophage population revealed through analysis of the human salivary virome. ISME J. 2012;6:915–926. doi: 10.1038/ismej.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bachrach G, et al. Bacteriophage isolation from human saliva. Lett. Appl. Microbiol. 2003;36:50–53. doi: 10.1046/j.1472-765x.2003.01262.x. [DOI] [PubMed] [Google Scholar]
- 94.Hitch G, Pratten J, Taylor PW. Isolation of bacteriophages from the oral cavity. Lett. Appl. Microbiol. 2004;39:215–219. doi: 10.1111/j.1472-765X.2004.01565.x. [DOI] [PubMed] [Google Scholar]
- 95.Atarashi K, et al. Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science. 2017;358:359–365. doi: 10.1126/science.aan4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Willner D, et al. Metagenomic detection of phage-encoded platelet-binding factors in the human oral cavity. Proc. Natl Acad. Sci. USA. 2011;108:4547–4553. doi: 10.1073/pnas.1000089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang J, Gao Y, Zhao F. Phage-bacteria interaction network in human oral microbiome. Environ. Microbiol. 2016;18:2143–2158. doi: 10.1111/1462-2920.12923. [DOI] [PubMed] [Google Scholar]
- 98.Carr, V. R. et al. The human oral phageome is highly diverse and rich in jumbo phages. https://www.biorxiv.org/content/10.1101/2020.07.06.186817v1 (2020).
- 99.Hansen MF, et al. Big impact of the tiny: bacteriophage-bacteria interactions in biofilms. Trends Microbiol. 2019;27:739–752. doi: 10.1016/j.tim.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 100.Reyes A, et al. Gnotobiotic mouse model of phage-bacterial host dynamics in the human gut. Proc. Natl Acad. Sci. USA. 2013;110:20236–20241. doi: 10.1073/pnas.1319470110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goodrich JK, et al. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rothschild D, et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- 103.Pannaraj PS, et al. Shared and distinct features of human milk and infant stool viromes. Front. Microbiol. 2018;9:1162. doi: 10.3389/fmicb.2018.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garmaeva S, et al. Studying the gut virome in the metagenomic era: challenges and perspectives. BMC Biol. 2019;17:84. doi: 10.1186/s12915-019-0704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Howe A, et al. Divergent responses of viral and bacterial communities in the gut microbiome to dietary disturbances in mice. ISME J. 2016;10:1217–1227. doi: 10.1038/ismej.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Duerkop BA, et al. A composite bacteriophage alters colonization by an intestinal commensal bacterium. Proc. Natl Acad. Sci. USA. 2012;109:17621–17626. doi: 10.1073/pnas.1206136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chatterjee A, Duerkop BA. Sugar and fatty acids Ack-celerate prophage induction. Cell Host Microbe. 2019;25:175–176. doi: 10.1016/j.chom.2019.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deehan EC, Walter J. The fiber gap and the disappearing gut microbiome: implications for human nutrition. Trends Endocrinol. Metab. 2016;27:239–242. doi: 10.1016/j.tem.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 109.Van Belleghem, J. D. et al. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses11, 10 (2018). [DOI] [PMC free article] [PubMed]
- 110.Frobisher M, B. J. Transmissible toxicogenicity of Streptococci. Bull. Johns. Hopkins Hosp. 1927;41:167–173. [Google Scholar]
- 111.Feiner R, et al. A new perspective on lysogeny: prophages as active regulatory switches of bacteria. Nat. Rev. Microbiol. 2015;13:641–650. doi: 10.1038/nrmicro3527. [DOI] [PubMed] [Google Scholar]
- 112.Rabinovich L, et al. Prophage excision activates Listeria competence genes that promote phagosomal escape and virulence. Cell. 2012;150:792–802. doi: 10.1016/j.cell.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 113.Pasechnek A, et al. Active lysogeny in Listeria monocytogenes is a bacteria-phage adaptive response in the mammalian environment. Cell Rep. 2020;32:107956. doi: 10.1016/j.celrep.2020.107956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cenens W, et al. Phage-host interactions during pseudolysogeny: lessons from the Pid/dgo interaction. Bacteriophage. 2013;3:e25029. doi: 10.4161/bact.25029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Latino L, et al. Investigation of Pseudomonas aeruginosa strain PcyII-10 variants resisting infection by N4-like phage Ab09 in search for genes involved in phage adsorption. PLoS ONE. 2019;14:e0215456. doi: 10.1371/journal.pone.0215456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen, S. et al. Bacteriophage transcytosis provides a mechanism to cross epithelial cell layers. mBio8, e01874-17 (2017). [DOI] [PMC free article] [PubMed]
- 117.Krut O, Bekeredjian-Ding I. Contribution of the immune response to phage therapy. J. Immunol. 2018;200:3037–3044. doi: 10.4049/jimmunol.1701745. [DOI] [PubMed] [Google Scholar]
- 118.Dufour, N. et al. Phage therapy of pneumonia is not associated with an overstimulation of the inflammatory response compared to antibiotic treatment in mice. Antimicrob. Agents Chemother. 63, e00379-19 (2019). [DOI] [PMC free article] [PubMed]
- 119.Gogokhia L, et al. Expansion of bacteriophages is linked to aggravated intestinal inflammation and colitis. Cell Host Microbe. 2019;25:285–299 e8. doi: 10.1016/j.chom.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Miernikiewicz P, et al. T4 phage and its head surface proteins do not stimulate inflammatory mediator production. PLoS ONE. 2013;8:e71036. doi: 10.1371/journal.pone.0071036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hodyra-Stefaniak K, et al. Mammalian host-versus-phage immune response determines phage fate in vivo. Sci. Rep. 2015;5:14802. doi: 10.1038/srep14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barr JJ, et al. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc. Natl Acad. Sci. USA. 2013;110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tetz G, Tetz V. Bacteriophage infections of microbiota can lead to leaky gut in an experimental rodent model. Gut Pathog. 2016;8:33. doi: 10.1186/s13099-016-0109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jahn MT, et al. A phage protein aids bacterial symbionts in eukaryote immune evasion. Cell Host Microbe. 2019;26:542–550.e5. doi: 10.1016/j.chom.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 125.Sweere, J. M. et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science363, eaat9691 (2019). [DOI] [PMC free article] [PubMed]
- 126.Hosseinidoust Z, van de Ven TG, Tufenkji N. Evolution of Pseudomonas aeruginosa virulence as a result of phage predation. Appl. Environ. Microbiol. 2013;79:6110–6116. doi: 10.1128/AEM.01421-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Secor, P. R. et al. Filamentous bacteriophage produced by Pseudomonas aeruginosa alters the inflammatory response and promotes noninvasive infection in vivo. Infect. Immun.85, e00648-16 (2017). [DOI] [PMC free article] [PubMed]
- 128.Roach DR, et al. Synergy between the host immune system and bacteriophage is essential for successful phage therapy against an acute respiratory pathogen. Cell Host Microbe. 2017;22:38–47.e4. doi: 10.1016/j.chom.2017.06.018. [DOI] [PubMed] [Google Scholar]
- 129.Qin J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Edwards RA, et al. Global phylogeography and ancient evolution of the widespread human gut virus crAssphage. Nat. Microbiol. 2019;4:1727–1736. doi: 10.1038/s41564-019-0494-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rodriguez-Brito B, et al. Viral and microbial community dynamics in four aquatic environments. ISME J. 2010;4:739–751. doi: 10.1038/ismej.2010.1. [DOI] [PubMed] [Google Scholar]
- 132.Sutton TDS, et al. Choice of assembly software has a critical impact on virome characterisation. Microbiome. 2019;7:12. doi: 10.1186/s40168-019-0626-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Z, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blacher E, et al. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–480. doi: 10.1038/s41586-019-1443-5. [DOI] [PubMed] [Google Scholar]
- 135.Le Roy T, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62:1787–1794. doi: 10.1136/gutjnl-2012-303816. [DOI] [PubMed] [Google Scholar]
- 136.Kåhrström CT, Pariente N, Weiss U. Intestinal microbiota in health and disease. Nature. 2016;535:47–47. doi: 10.1038/535047a. [DOI] [PubMed] [Google Scholar]
- 137.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–1499. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zuo T, et al. Gut mucosal virome alterations in ulcerative colitis. Gut. 2019;68:1169–1179. doi: 10.1136/gutjnl-2018-318131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cornuault JK, et al. Phages infecting Faecalibacterium prausnitzii belong to novel viral genera that help to decipher intestinal viromes. Microbiome. 2018;6:65. doi: 10.1186/s40168-018-0452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eaton MD, B.-J. S. Bacteriophage therapy. Review of the principles and results of the use of bacteriophage in the treatment of infections. JAMA. 1934;23:1769–1776. [Google Scholar]
- 141.Debattista J. Phage therapy: where East meets West. Expert Rev. Anti Infect. Ther. 2004;2:815–819. doi: 10.1586/14789072.2.6.815. [DOI] [PubMed] [Google Scholar]
- 142.Watanabe R, et al. Efficacy of bacteriophage therapy against gut-derived sepsis caused by Pseudomonas aeruginosa in mice. Antimicrob. Agents Chemother. 2007;51:446–452. doi: 10.1128/AAC.00635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lood R, et al. Novel phage lysin capable of killing the multidrug-resistant Gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrobial Agents Chemother. 2015;59:1983–1991. doi: 10.1128/AAC.04641-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Jun JW, et al. Bacteriophage therapy of a Vibrio parahaemolyticus infection caused by a multiple-antibiotic-resistant O3:K6 pandemic clinical strain. J. Infect. Dis. 2014;210:72–78. doi: 10.1093/infdis/jiu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nale JY, et al. Bacteriophage combinations significantly reduce clostridium difficile growth in vitro and proliferation in vivo. Antimicrob. Agents Chemother. 2016;60:968–981. doi: 10.1128/AAC.01774-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wills QF, Kerrigan C, Soothill JS. Experimental bacteriophage protection against Staphylococcus aureus abscesses in a rabbit model. Antimicrob. Agents Chemother. 2005;49:1220–1221. doi: 10.1128/AAC.49.3.1220-1221.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang J, et al. Therapeutic effectiveness of bacteriophages in the rescue of mice with extended spectrum beta-lactamase-producing Escherichia coli bacteremia. Int. J. Mol. Med. 2006;17:347–355. [PubMed] [Google Scholar]
- 148.Biswas B, et al. Bacteriophage therapy rescues mice bacteremic from a clinical isolate of vancomycin-resistant Enterococcus faecium. Infect. Immun. 2002;70:204–210. doi: 10.1128/IAI.70.1.204-210.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang J, et al. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int J. Mol. Med. 2006;17:309–317. [PubMed] [Google Scholar]
- 150.Dąbrowska K, Abedon ST. Pharmacologically aware phage therapy: pharmacodynamic and pharmacokinetic obstacles to phage antibacterial action in animal and human bodies. Microbiol. Mol. Biol. Rev. 2019;83:e00012–e00019. doi: 10.1128/MMBR.00012-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Capparelli R, et al. Selection of an Escherichia coli O157:H7 bacteriophage for persistence in the circulatory system of mice infected experimentally. Clin. Microbiol. Infect. 2006;12:248–253. doi: 10.1111/j.1469-0691.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 152.Hodyra-Stefaniak K, et al. Bacteriophages engineered to display foreign peptides may become short-circulating phages. Microb. Biotechnol. 2019;12:730–741. doi: 10.1111/1751-7915.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chhibber S, Kaur S, Kumari S. Therapeutic potential of bacteriophage in treating Klebsiella pneumoniae B5055-mediated lobar pneumonia in mice. J. Med. Microbiol. 2008;57:1508–1513. doi: 10.1099/jmm.0.2008/002873-0. [DOI] [PubMed] [Google Scholar]
- 154.Wolochow H, Hildebrand GJ, Lamanna C. Translocation of microorganisms across the intestinal wall of the rat: effect of microbial size and concentration. J. Infect. Dis. 1966;116:523–528. doi: 10.1093/infdis/116.4.523. [DOI] [PubMed] [Google Scholar]
- 155.Sarker SA, et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine. 2016;4:124–137. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bruttin A, Brüssow H. Human volunteers receiving Escherichia coli phage T4 orally: a safety test of phage therapy. Antimicrob. Agents Chemother. 2005;49:2874–2878. doi: 10.1128/AAC.49.7.2874-2878.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cao F, et al. Evaluation of the efficacy of a bacteriophage in the treatment of pneumonia induced by multidrug resistance Klebsiella pneumoniae in mice. BioMed. Res. Int. 2015;2015:752930. doi: 10.1155/2015/752930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Debarbieux L, et al. Bacteriophages can treat and prevent Pseudomonas aeruginosa lung infections. J. Infect. Dis. 2010;201:1096–1104. doi: 10.1086/651135. [DOI] [PubMed] [Google Scholar]
- 159.Liu KY, et al. Inhalation study of mycobacteriophage D29 aerosol for mice by endotracheal route and nose-only exposure. J. Aerosol Med. Pulm. Drug Deliv. 2016;29:393–405. doi: 10.1089/jamp.2015.1233. [DOI] [PubMed] [Google Scholar]
- 160.Nishikawa H, et al. T-even-related bacteriophages as candidates for treatment of Escherichia coli urinary tract infections. Arch. Virol. 2008;153:507–515. doi: 10.1007/s00705-007-0031-4. [DOI] [PubMed] [Google Scholar]
- 161.Nobrega FL, et al. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018;16:760–773. doi: 10.1038/s41579-018-0070-8. [DOI] [PubMed] [Google Scholar]
- 162.Rhoads DD, et al. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J. Wound Care. 2009;18:237–238. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 163.Sarker SA, et al. Oral T4-like phage cocktail application to healthy adult volunteers from Bangladesh. Virology. 2012;434:222–232. doi: 10.1016/j.virol.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 164.Ando, H. et al. Engineering modular viral scaffolds for targeted bacterial population editing. https://www.biorxiv.org/content/10.1101/020891v1.full (2015). [DOI] [PMC free article] [PubMed]
- 165.Mahichi F, et al. Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol. Lett. 2009;295:211–217. doi: 10.1111/j.1574-6968.2009.01588.x. [DOI] [PubMed] [Google Scholar]
- 166.Yoichi M, et al. Alteration of tail fiber protein gp38 enables T2 phage to infect Escherichia coli O157:H7. J. Biotechnol. 2005;115:101–107. doi: 10.1016/j.jbiotec.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 167.Kelly CR, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am. J. Gastroenterol. 2014;109:1065–1071. doi: 10.1038/ajg.2014.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.van Nood E, et al. Duodenal infusion of donor feces for recurrent clostridium difficile. N. Engl. J. Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 169.Allegretti JR, et al. Fecal microbiota transplantation in patients with primary sclerosing cholangitis: a pilot clinical trial. Am. J. Gastroenterol. 2019;114:1071–1079. doi: 10.14309/ajg.0000000000000115. [DOI] [PubMed] [Google Scholar]
- 170.Aroniadis OC, et al. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2019;4:675–685. doi: 10.1016/S2468-1253(19)30198-0. [DOI] [PubMed] [Google Scholar]
- 171.Huttner BD, et al. A 5-day course of oral antibiotics followed by faecal transplantation to eradicate carriage of multidrug-resistant Enterobacteriaceae: a randomized clinical trial. Clin. Microbiol Infect. 2019;25:830–838. doi: 10.1016/j.cmi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 172.Yu EW, et al. Fecal microbiota transplantation for the improvement of metabolism in obesity: The FMT-TRIM double-blind placebo-controlled pilot trial. PLoS Med. 2020;17:e1003051. doi: 10.1371/journal.pmed.1003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Broecker F, et al. Stable core virome despite variable microbiome after fecal transfer. Gut Microbes. 2017;8:214–220. doi: 10.1080/19490976.2016.1265196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Draper LA, et al. Long-term colonisation with donor bacteriophages following successful faecal microbial transplantation. Microbiome. 2018;6:220. doi: 10.1186/s40168-018-0598-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zuo T, et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut. 2018;67:634–643. doi: 10.1136/gutjnl-2017-313952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Lin DM, et al. Transplanting fecal virus-like particles reduces high-fat diet-induced small intestinal bacterial overgrowth in mice. Front. Cell Infect. Microbiol. 2019;9:348. doi: 10.3389/fcimb.2019.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Džunková M, et al. Defining the human gut host-phage network through single-cell viral tagging. Nat. Microbiol. 2019;4:2192–2203. doi: 10.1038/s41564-019-0526-2. [DOI] [PubMed] [Google Scholar]
- 178.Johnson CN, Duerkop BA. Dyeing to connect. Nat. Microbiol. 2019;4:2033–2034. doi: 10.1038/s41564-019-0616-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Moelling, K., Broecker, F. & Willy, C. A wake-up call: we need phage therapy now. Viruses10, 688 (2018). [DOI] [PMC free article] [PubMed]
- 180.Chaudhry WN, et al. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS ONE. 2017;12:e0168615. doi: 10.1371/journal.pone.0168615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oechslin F, et al. Synergistic interaction between phage therapy and antibiotics clears Pseudomonas aeruginosa Infection in endocarditis and reduces virulence. J. Infect. Dis. 2017;215:703–712. doi: 10.1093/infdis/jiw632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Regeimbal JM, et al. Personalized therapeutic cocktail of wild environmental phages rescues mice from Acinetobacter baumannii wound infections. Antimicrob. Agents Chemother. 2016;60:5806–5816. doi: 10.1128/AAC.02877-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Horváth M, et al. Identification of a newly isolated lytic bacteriophage against K24 capsular type, carbapenem resistant Klebsiella pneumoniae isolates. Sci. Rep. 2020;10:5891. doi: 10.1038/s41598-020-62691-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bao J, et al. Non-active antibiotic and bacteriophage synergism to successfully treat recurrent urinary tract infection caused by extensively drug-resistant Klebsiella pneumoniae. Emerg. Microbes Infect. 2020;9:771–774. doi: 10.1080/22221751.2020.1747950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schooley RT, et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrobial Agents Chemother. 2017;61:e00954–17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chan BK, et al. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol. Med. Public Health. 2018;2018:60–66. doi: 10.1093/emph/eoy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dedrick RM, et al. Engineered bacteriophages for treatment of a patient with a disseminated drug-resistant Mycobacterium abscessus. Nat. Med. 2019;25:730–733. doi: 10.1038/s41591-019-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Cooper, C. J., Khan Mirzaei, M. & Nilsson, A. S. Adapting drug approval pathways for bacteriophage-based therapeutics. Front Microbiol7, 1209 (2016). [DOI] [PMC free article] [PubMed]
- 189.Furfaro LL, Payne MS, Chang BJ. Bacteriophage therapy: clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 2018;8:376. doi: 10.3389/fcimb.2018.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pelfrene E, et al. Bacteriophage therapy: a regulatory perspective. J. Antimicrobial Chemother. 2016;71:2071–2074. doi: 10.1093/jac/dkw083. [DOI] [PubMed] [Google Scholar]
- 191.Khan Mirzaei M, Nilsson AS. Isolation of phages for phage therapy: a comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE. 2015;10:e0118557. doi: 10.1371/journal.pone.0118557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Parracho HM, et al. The role of regulated clinical trials in the development of bacteriophage therapeutics. J. Mol. Genet. Med. 2012;6:279–286. doi: 10.4172/1747-0862.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dąbrowska K, et al. Immunogenicity studies of proteins forming the T4 phage head surface. J. Virol. 2014;88:12551–12557. doi: 10.1128/JVI.02043-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Górski A, et al. Phage as a modulator of immune responses: practical implications for phage therapy. Adv. Virus Res. 2012;83:41–71. doi: 10.1016/B978-0-12-394438-2.00002-5. [DOI] [PubMed] [Google Scholar]
- 195.Beaudoin J, Pratt D. Antiserum inactivation of electrophoretically purified M13 diploid virions: model for the F-specific filamentous bacteriophages. J. Virol. 1974;13:466–469. doi: 10.1128/jvi.13.2.466-469.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Araya DV, et al. Deletion of a prophage-like element causes attenuation of Salmonella enterica serovar Enteritidis and promotes protective immunity. Vaccine. 2010;28:5458–5466. doi: 10.1016/j.vaccine.2010.05.073. [DOI] [PubMed] [Google Scholar]
- 197.Capparelli R, et al. Bacteriophage-resistant Staphylococcus aureus mutant confers broad immunity against staphylococcal infection in mice. PLoS ONE. 2010;5:e11720. doi: 10.1371/journal.pone.0011720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Won G, et al. A Salmonella Typhi ghost induced by the E gene of phage phiX174 stimulates dendritic cells and efficiently activates the adaptive immune response. J. Vet. Sci. 2018;19:536–542. doi: 10.4142/jvs.2018.19.4.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yang, H. et al. Staphylococcus aureus virulence attenuation and immune clearance mediated by a phage lysin-derived protein. EMBO J. 37, e98045 (2018) [DOI] [PMC free article] [PubMed]
- 200.Yang Y, et al. A small mycobacteriophage-derived peptide and its improved isomer restrict mycobacterial infection via dual mycobactericidal-immunoregulatory activities. J. Biol. Chem. 2019;294:7615–7631. doi: 10.1074/jbc.RA118.006968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tian M, et al. B cell-intrinsic MyD88 signaling promotes initial cell proliferation and differentiation to enhance the germinal center response to a virus-like particle. J. Immunol. 2018;200:937–948. doi: 10.4049/jimmunol.1701067. [DOI] [PubMed] [Google Scholar]
- 202.Chen F, et al. Recombinant phage elicits protective immune response against systemic S. globosa infection in mouse model. Sci. Rep. 2017;7:42024. doi: 10.1038/srep42024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sartorius R, et al. Antigen delivery by filamentous bacteriophage fd displaying an anti-DEC-205 single-chain variable fragment confers adjuvanticity by triggering a TLR9-mediated immune response. EMBO Mol. Med. 2015;7:973–988. doi: 10.15252/emmm.201404525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Barksdale L, Arden SB. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu. Rev. Microbiol. 1974;28:265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- 205.Freeman VJ. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J. Bacteriol. 1951;61:675–688. doi: 10.1128/jb.61.6.675-688.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang X, et al. Cryptic prophages help bacteria cope with adverse environments. Nat. Commun. 2010;1:147. doi: 10.1038/ncomms1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Beutin L, Stroeher UH, Manning PA. Isolation of enterohemolysin (Ehly2)-associated sequences encoded on temperate phages of Escherichia coli. Gene. 1993;132:95–99. doi: 10.1016/0378-1119(93)90519-9. [DOI] [PubMed] [Google Scholar]
- 208.Hayashi T, et al. Molecular analysis of a cytotoxin-converting phage, phi CTX, of Pseudomonas aeruginosa: structure of the attP-cos-ctx region and integration into the serine tRNA gene. Mol. Microbiol. 1993;7:657–667. doi: 10.1111/j.1365-2958.1993.tb01157.x. [DOI] [PubMed] [Google Scholar]
- 209.Coleman DC, et al. Staphylococcus aureus bacteriophages mediating the simultaneous lysogenic conversion of beta-lysin, staphylokinase and enterotoxin A: molecular mechanism of triple conversion. J. Gen. Microbiol. 1989;135:1679–1697. doi: 10.1099/00221287-135-6-1679. [DOI] [PubMed] [Google Scholar]
- 210.Clark CA, Beltrame J, Manning PA. The oac gene encoding a lipopolysaccharide O-antigen acetylase maps adjacent to the integrase-encoding gene on the genome of Shigella flexneri bacteriophage Sf6. Gene. 1991;107:43–52. doi: 10.1016/0378-1119(91)90295-m. [DOI] [PubMed] [Google Scholar]
- 211.McShan WM, Ferretti JJ. Genetic diversity in temperate bacteriophages of Streptococcus pyogenes: identification of a second attachment site for phages carrying the erythrogenic toxin A gene. J. Bacteriol. 1997;179:6509–6511. doi: 10.1128/jb.179.20.6509-6511.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Figueroa-Bossi N, et al. Variable assortment of prophages provides a transferable repertoire of pathogenic determinants in Salmonella. Mol. Microbiol. 2001;39:260–271. doi: 10.1046/j.1365-2958.2001.02234.x. [DOI] [PubMed] [Google Scholar]
- 213.Figueroa-Bossi N, Ammendola S, Bossi L. Differences in gene expression levels and in enzymatic qualities account for the uneven contribution of superoxide dismutases SodCI and SodCII to pathogenicity in Salmonella enterica. Microbes Infect. 2006;8:1569–1578. doi: 10.1016/j.micinf.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 214.Pelludat C, Mirold S, Hardt WD. The SopEPhi phage integrates into the ssrA gene of Salmonella enterica serovar Typhimurium A36 and is closely related to the Fels-2 prophage. J. Bacteriol. 2003;185:5182–5191. doi: 10.1128/JB.185.17.5182-5191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Strauch E, Lurz R, Beutin L. Characterization of a Shiga toxin-encoding temperate bacteriophage of Shigella sonnei. Infect. Immun. 2001;69:7588–7595. doi: 10.1128/IAI.69.12.7588-7595.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Das B, Bischerour J, Barre FX. Molecular mechanism of acquisition of the cholera toxin genes. Indian J. Med. Res. 2011;133:195–200. [PMC free article] [PubMed] [Google Scholar]
- 217.Krom RJ, et al. Engineered phagemids for nonlytic, targeted antibacterial therapies. Nano Lett. 2015;15:4808–4813. doi: 10.1021/acs.nanolett.5b01943. [DOI] [PubMed] [Google Scholar]
- 218.Sarker SA, Brüssow H. From bench to bed and back again: phage therapy of childhood Escherichia coli diarrhea. Ann. N. Y. Acad. Sci. 2016;1372:42–52. doi: 10.1111/nyas.13087. [DOI] [PubMed] [Google Scholar]
- 219.Schneider G, et al. Kinetics of targeted phage rescue in a mouse model of systemic Escherichia coli K1. BioMed. Res. Int. 2018;2018:7569645. doi: 10.1155/2018/7569645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kumari S, Harjai K, Chhibber S. Efficacy of bacteriophage treatment in murine burn wound infection induced by Klebsiella pneumoniae. J. Microbiol Biotechnol. 2009;19:622–628. doi: 10.4014/jmb.0808.493. [DOI] [PubMed] [Google Scholar]
- 221.Takemura-Uchiyama I, et al. Experimental phage therapy against lethal lung-derived septicemia caused by Staphylococcus aureus in mice. Microbes Infect. 2014;16:512–517. doi: 10.1016/j.micinf.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 222.Kishor C, et al. Phage therapy of staphylococcal chronic osteomyelitis in experimental animal model. Indian J. Med. Res. 2016;143:87–94. doi: 10.4103/0971-5916.178615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.McVay CS, Velásquez M, Fralick JA. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrobial agents Chemother. 2007;51:1934–1938. doi: 10.1128/AAC.01028-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wright A, et al. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 2009;34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 225.Cafora M, et al. Phage therapy against Pseudomonas aeruginosa infections in a cystic fibrosis zebrafish model. Sci. Rep. 2019;9:1527. doi: 10.1038/s41598-018-37636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mai V, et al. Bacteriophage administration significantly reduces Shigella colonization and shedding by Shigella-challenged mice without deleterious side effects and distortions in the gut microbiota. Bacteriophage. 2015;5:e1088124. doi: 10.1080/21597081.2015.1088124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hurwitz BL, et al. Evaluation of methods to concentrate and purify ocean virus communities through comparative, replicated metagenomics. Environ. Microbiol. 2013;15:1428–1440. doi: 10.1111/j.1462-2920.2012.02836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Parras-Moltó M, et al. Evaluation of bias induced by viral enrichment and random amplification protocols in metagenomic surveys of saliva DNA viruses. Microbiome. 2018;6:119. doi: 10.1186/s40168-018-0507-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Solonenko SA, Sullivan MB. Preparation of metagenomic libraries from naturally occurring marine viruses. Methods Enzymol. 2013;531:143–165. doi: 10.1016/B978-0-12-407863-5.00008-3. [DOI] [PubMed] [Google Scholar]
- 230.Kleiner M, Hooper LV, Duerkop BA. Evaluation of methods to purify virus-like particles for metagenomic sequencing of intestinal viromes. BMC Genom. 2015;16:7. doi: 10.1186/s12864-014-1207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Rosseel T, et al. Evaluation of convenient pretreatment protocols for RNA virus metagenomics in serum and tissue samples. J. Virol. Methods. 2015;222:72–80. doi: 10.1016/j.jviromet.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 232.Sachsenröder J, et al. Simultaneous identification of DNA and RNA viruses present in pig faeces using process-controlled deep sequencing. PLoS ONE. 2012;7:e34631. doi: 10.1371/journal.pone.0034631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Conceição-Neto N, et al. Modular approach to customise sample preparation procedures for viral metagenomics: a reproducible protocol for virome analysis. Sci. Rep. 2015;5:16532. doi: 10.1038/srep16532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Stinson LF, Keelan JA, Payne MS. Identification and removal of contaminating microbial DNA from PCR reagents: impact on low-biomass microbiome analyses. Lett. Appl. Microbiol. 2019;68:2–8. doi: 10.1111/lam.13091. [DOI] [PubMed] [Google Scholar]
- 235.Džunková, M., D’Auria, G. & Moya, A. Direct sequencing of human gut virome fractions obtained by flow cytometry. Front. Microbiol.6, 955 (2015). [DOI] [PMC free article] [PubMed]
- 236.Hurwitz, B. L., U’Ren, J. M. & Youens-Clark K. Computational prospecting the great viral unknown. FEMS Microbiol. Lett.363, fnw077 (2016). [DOI] [PubMed]
- 237.Sutton TDS, Hill C. Gut bacteriophage: current understanding and challenges. Front. Endocrinol. 2019;10:784. doi: 10.3389/fendo.2019.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]