Abstract
Adamantinoma-like Ewing sarcoma (ALES) is a rare tumor that demonstrates the EWSR1-FLI1 translocation characteristic of Ewing sarcoma despite overt epithelial differentiation including diffuse expression of cytokeratins and p40. Most cases of ALES described to date have occurred in the head and neck where they can mimic a wide range of small round blue cell tumors. Because distinguishing ALES from basaloid salivary gland carcinomas can be particularly difficult, we analyzed a series of 10 ALESs that occurred in the salivary glands with the aim of identifying features that allow for better recognition of this entity. The salivary ALESs included 8 parotid gland and 2 submandibular gland tumors in patients ranging from 32 to 77 years (mean 52). Nine were initially misclassified as various epithelial neoplasms. Although these tumors displayed the basaloid cytology, rosette formation, infiltrative growth, and nuclear monotony characteristic of ALES, peripheral palisading and overt keratinization were relatively rare in this site. Salivary ALESs not only displayed positivity for AE1/AE3, p40 and CD99, but also demonstrated a higher proportion of synaptophysin reactivity than has been reported for non-salivary ALESs. These morphologic and immunohistochemical findings make ALES susceptible to misclassification as various other tumors including basal cell adenocarcinoma, adenoid cystic carcinoma, squamous cell carcinoma, NUT carcinoma, large cell neuroendocrine carcinoma and myoepithelial carcinoma. Nevertheless, monotonous cytology despite highly infiltrative growth and concomitant positivity for p40 and synaptophysin can provide important clues for consideration of ALES, and identification of the defining EWSR1-FLI1 translocations can confirm the diagnosis.
Keywords: Ewing sarcoma, adamantinoma-like Ewing sarcoma, EWSR1, FLI1, salivary gland
Introduction
Adamantinoma-like Ewing sarcoma (ALES) is an unusual tumor that is currently classified as a variant of Ewing sarcoma (ES) with complex epithelial differentiation. Although these tumors harbor the EWSR1-FLI1 translocation (1) and corresponding immunohistochemical positivity for CD99 and NKX2.2 characteristic of ES (2–4), they also demonstrate overt evidence of squamous differentiation including cohesive growth, keratin pearl formation, peripheral nuclear palisading, and diffuse positivity for pancytokeratin and p40 (5). Not only does this unique morphology and immunophenotype show substantial overlap with various types of carcinoma, but a few cases that likely represent the same entity have actually been reported as “carcinoma with Ewing family tumor elements” (6–8). However, because the EWSR1-FLI1 translocation has traditionally been considered pathognomonic for ES, most tumors that demonstrate these features have been classified as the variant ALES (5, 9–17). Specific recognition of this distinctive neoplasm is essential for better understanding of its classification, prognosis, and treatment, including the role of ES-specific therapies (18–20).
While ALES was initially described in the long bones and thorax (5, 10, 12, 13), 12 of 19 cases reported to date occurred in the head and neck, including three in the sinonasal tract (9, 11), three in the parotid gland (11, 15, 16), two in the soft tissues of the neck (14, 17), two in the thyroid gland (11), and one in the orbit (11). The three similar tumors characterized as “carcinoma with Ewing family tumor elements” also arose in the thyroid (6–8). Although the diagnosis of ALES can be challenging in any location, we have observed particular diagnostic confusion when ALES unexpectedly occurs in the salivary glands due to significant histologic and immunohistochemical overlap with a wide range of more common basaloid salivary carcinomas. Here, we present the first dedicated series of 10 ALES involving the salivary glands, including 7 cases that have not been previously reported, with the aim of facilitating better recognition of this recently described entity.
Materials and Methods
With institutional review board approval, we identified 10 cases of ALES involving the major salivary glands from the surgical pathology archives of The Johns Hopkins Hospital, Brigham and Women’s Hospital, Massachusetts General Hospital, University of Texas-Southwestern Medical Center, and the University of Pittsburgh Medical Center as well as the authors’ consultation files. Three of these cases (1, 3, and 5) had been reported previously (11, 15, 16). We gathered clinical and demographic information for each case, including all available treatment and follow-up details. All available histologic sections were reviewed for each case.
We performed immunohistochemistry on all cases for CD99 (clone 12E7; Leica Microsystems, Buffalo Grove, IL; prediluted), AE1/AE3 (PCK26; Ventana Medical Systems, Tuscon, AZ; prediluted), p40 (BC28; Biocare Medical, Concord, CA; 1:100 dilution), synaptophysin (clone 27G12; Leica; prediluted), and chromogranin (clone LK2H10; Ventana; prediluted). As tissue availability permitted, we also performed immunohistochemistry for NKX2.2 (clone 74.5A5, BD Biosciences, San Jose, CA; 1:100 dilution), S100 (clone 4C4.9; Ventana; prediluted), smooth muscle actin (clone 1A4; Ventana; prediluted), desmin (clone D33, Dako, Carpinteria, CA; 1:100 dilution), and NUT1 (clone C52B1, Cell Signaling Technologies, Inc., Danvers, MA, 1:50 dilution). Immunostains were performed on a Benchmark XT autostainer (Ventana) using the Ultraview polymer detection kit (Ventana) according to manufacturer’s instructions and in the presence of appropriate controls.
We also performed fluorescence in-situ hybridization (FISH) for EWSR1 rearrangements in all cases. In cases 2, 4–9, and 10 we performed break-apart EWSR1 FISH using commercially-available dual color probe sets for a locus on 22q12 (Abbott Molecular, Abbott Park, IL). Two independent scorers manually enumerated signals in 100 nuclei within areas of invasive tumor. Identification of greater than 10% of nuclei with a split EWSR1 signal was considered indicative of EWSR1 rearrangement. In cases 1 and 3, we performed FISH using custom probes for both EWSR1 and FLI1 rearrangements as previously described (11). Case 8 also underwent next-generation sequencing (NGS) as previously described (21, 22).
Results
Clinical and demographic findings are summarized in Table 1. The ten ALESs represented 6 men and 4 women, ranging in age from 32 to 77 years (mean 52 years). Eight tumors arose in the parotid gland and 2 in the submandibular gland. While most patients presented with a painless palpable face or neck mass, two parotid tumors were accompanied by facial pain. No patients reported previous head and neck malignancies or external beam radiation, although one patient was also found to have papillary thyroid carcinoma during workup of his parotid tumor. Nine cases were reviewed in consultation and were received with previous or preliminary diagnoses including poorly differentiated carcinoma (n=5), high-grade neuroendocrine carcinoma (n=1), Merkel cell carcinoma (n=1), basal cell adenocarcinoma (n=1), and basal cell adenoma (n=1). One tumor was diagnosed as ALES during initial workup.
Table 1:
Clinical Information and Original Diagnoses
| Case | Site | Age | Sex | Presentation | Size (cm) | Original/Submitted Diagnosis |
|---|---|---|---|---|---|---|
| 1 | Parotid | 56 | F | Painless neck mass | 2.5 | Basal cell adenocarcinoma |
| 2 | Submandibular | 58 | M | Painless neck mass | 6 | PDCA |
| 3 | Parotid | 40 | F | Painless facial mass | 2.4 | Basal cell adenoma |
| 4 | Parotid | 63 | F | Rapidly growing parotid mass | 3.8 | PDCA with basaloid features |
| 5 | Parotid | 72 | M | Painful parotid mass | 2.4 | ALES |
| 6 | Submandibular | 77 | M | Neck mass | 3.6 | PDCA with basaloid features |
| 7 | Parotid | 32 | F | Neck swelling | 3 | HGNEC |
| 8 | Parotid | 32 | M | Painless facial mass | 3.9 | PDCA with basaloid features |
| 9 | Parotid | 41 | M | Painless neck mass | 3.6 | PDCA with basaloid features |
| 10 | Parotid | 46 | M | Rapidly growing tender mass | 7.9 | Merkel cell carcinoma |
F = Female; M = Male; PDCA = poorly differentiated carcinoma; ALES = Adamantinoma-like Ewing Sarcoma; HGNEC = High-grade Neuroendocrine Carcinoma
All ALESs were centered in salivary gland parenchyma. Despite a lobulated appearance at low power (Figure 1A), tumor cells extensively permeated through surrounding acini and fibroadipose tissue (Figure 1B). The tumors also were partitioned by prominent, predominantly fibrous stroma (Figure 1C), with myxoid stromal change in 1 case (Figure 1D) and production of abundant hyalinized basement membrane-like material in 1 case (Figure 1E). All tumors demonstrated mixed architectural patterns, including prominent nests, trabeculae and lobules of cells (Figure 1F). Well-developed rosettes were at least focally present in almost all tumors (Figure 2A). Peripheral nuclear palisading was rarely seen (Figure 2B), and overt keratinization with abrupt squamous pearl formation was identified in only 1 tumor (Figure 2C). Although the tumors were highly mitotically active (Figure 2D), overt necrosis was also only present focally (Figure 2E). The tumor cells were monotonous with minimal clear to basophilic cytoplasm, round, vesicular nuclei, with vesicular chromatin and single prominent nucleoli (Figure 2F).
Figure 1:
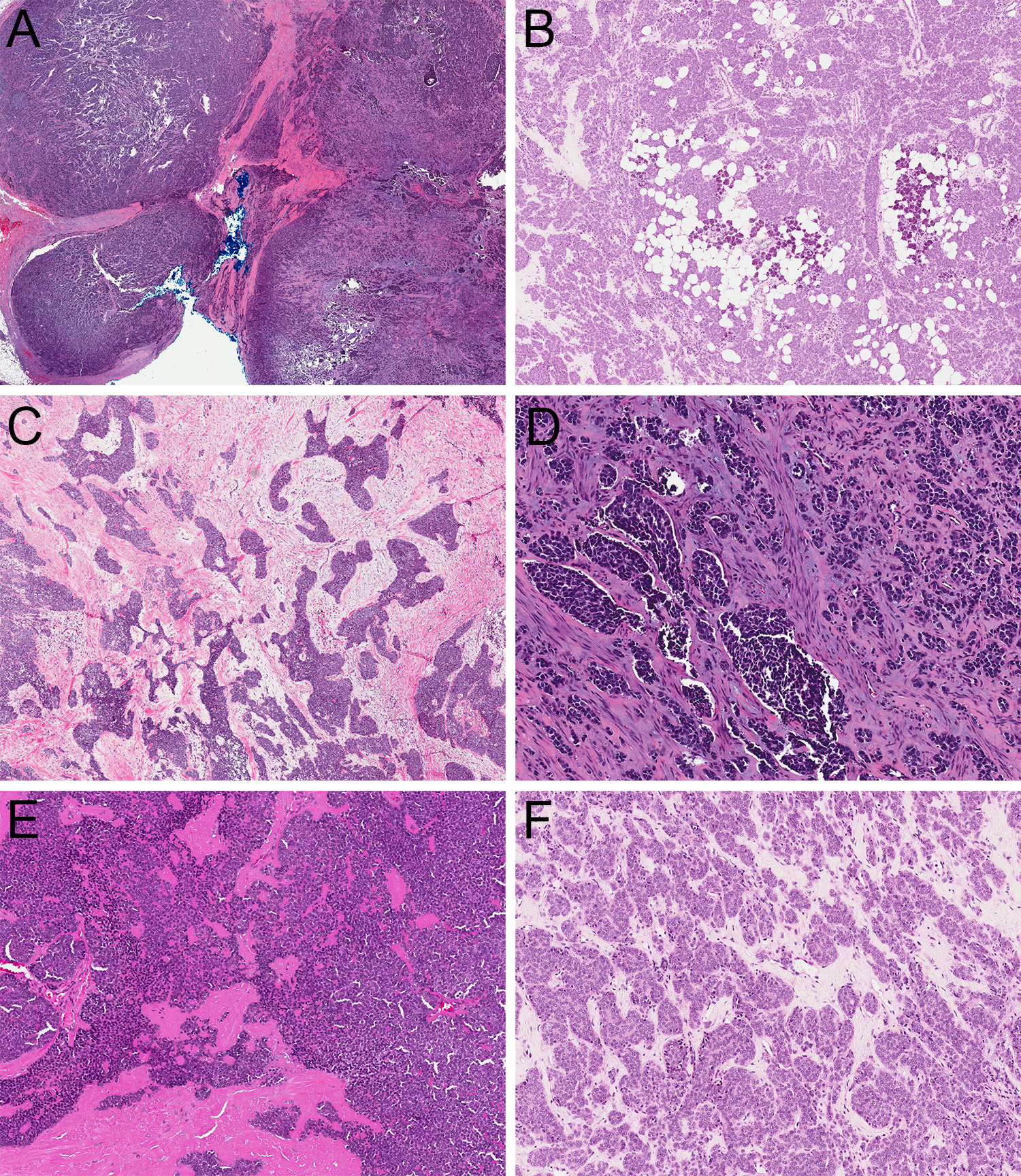
ALES demonstrated a lobulated appearance at low power (A; 2x) but were highly infiltrative into salivary parenchyma and surrounding fibroadipose tissue (B; 4x) with prominent fibrous stroma (C; 4x) that showed occasional prominent myxoid change (D; 10x) or deposition of basement-membrane like material (E; 4x) with a mix of nested, trabecular, and lobular growth (F; 10x).
Figure 2:
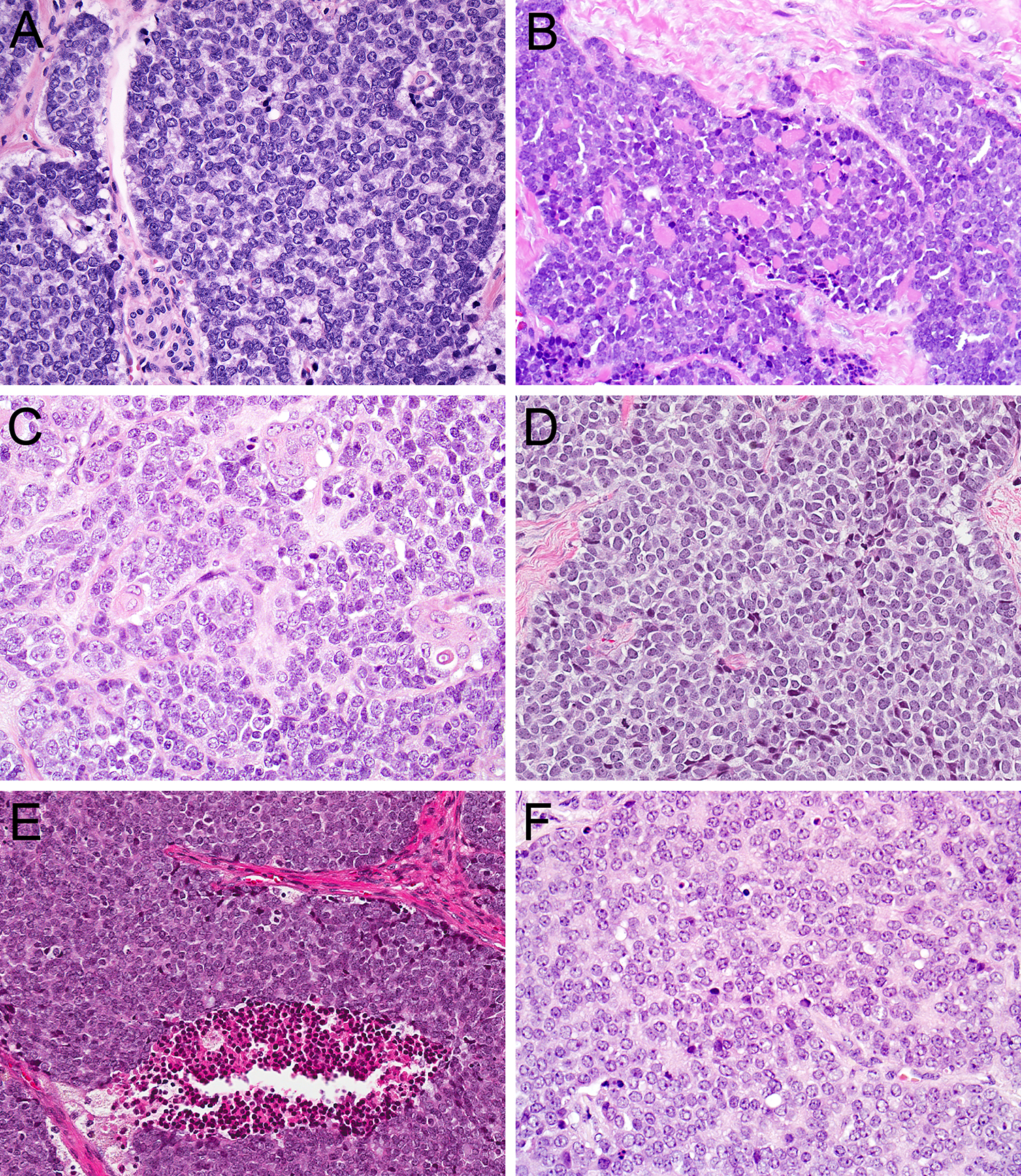
ALES showed well-developed rosettes (A; 20x) with occasional peripheral palisading (B; 20x) and only rare overt keratinization with squamous pearl formation (C; 20x). Despite high mitotic activity (D; 20x) and scattered foci of necrosis (E; 20x), tumor cells were monotonous with minimal clear to basaloid cytoplasm, round nuclei, vesicular chromatin, and single prominent nucleoli (F; 40x).
The immunohistochemical findings are summarized in Table 2. All ALESs tested were strongly and diffusely positive for cytokeratin AE1/AE3 (10 of 10) (Figure 3A), CD99 in a membranous pattern (10 of 10) (Figure 3B), NKX2.2 (8 of 8), and p40 (10 of 10) (Figure 3C). Positivity for neuroendocrine markers was also common, with staining for synaptophysin in 8 of 10 cases (80%) (Figure 3D) and chromogranin in 3 of 10 cases (30%). Only 1 of 9 cases (11%) showed focal positivity for S100, and no cases expressed smooth muscle actin (0 of 6), desmin (0 of 8) or NUT1 (0 of 9). FISH was positive for EWSR1 rearrangements in all 10 cases (Figure 3E). FLI1 rearrangements were also noted by FISH in cases 1 and 3 (Figure 3F), and a EWSR1-FLI1 fusion transcript was detected by next generation sequencing in case 8.
Table 2.
Immunohistochemical Results
| Case | CD99 | NKX2.2 | AE1/AE3 | p40 | SYN | CHR | S100 | Actin | Desmin | NUT-1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | + | + | + | + | F+ | − | − | − | − | − |
| 2 | + | ND | + | + | + | F+ | ND | ND | − | ND |
| 3 | + | + | + | + | F+ | − | − | − | − | − |
| 4 | + | + | + | + | − | − | F+ | − | − | − |
| 5 | + | + | + | + | + | − | − | − | − | − |
| 6 | + | + | + | + | F+ | F+ | − | − | − | − |
| 7 | + | + | + | + | + | − | − | − | − | − |
| 8 | + | + | + | + | − | − | − | ND | ND | − |
| 9 | + | ND | + | + | + | − | − | ND | − | − |
| 10 | + | + | + | + | + | F+ | − | ND | ND | − |
CHR = chromogranin; F+ = focally positive (<5% of cells), ND = not done; SYN = synaptophysin;
Figure 3:
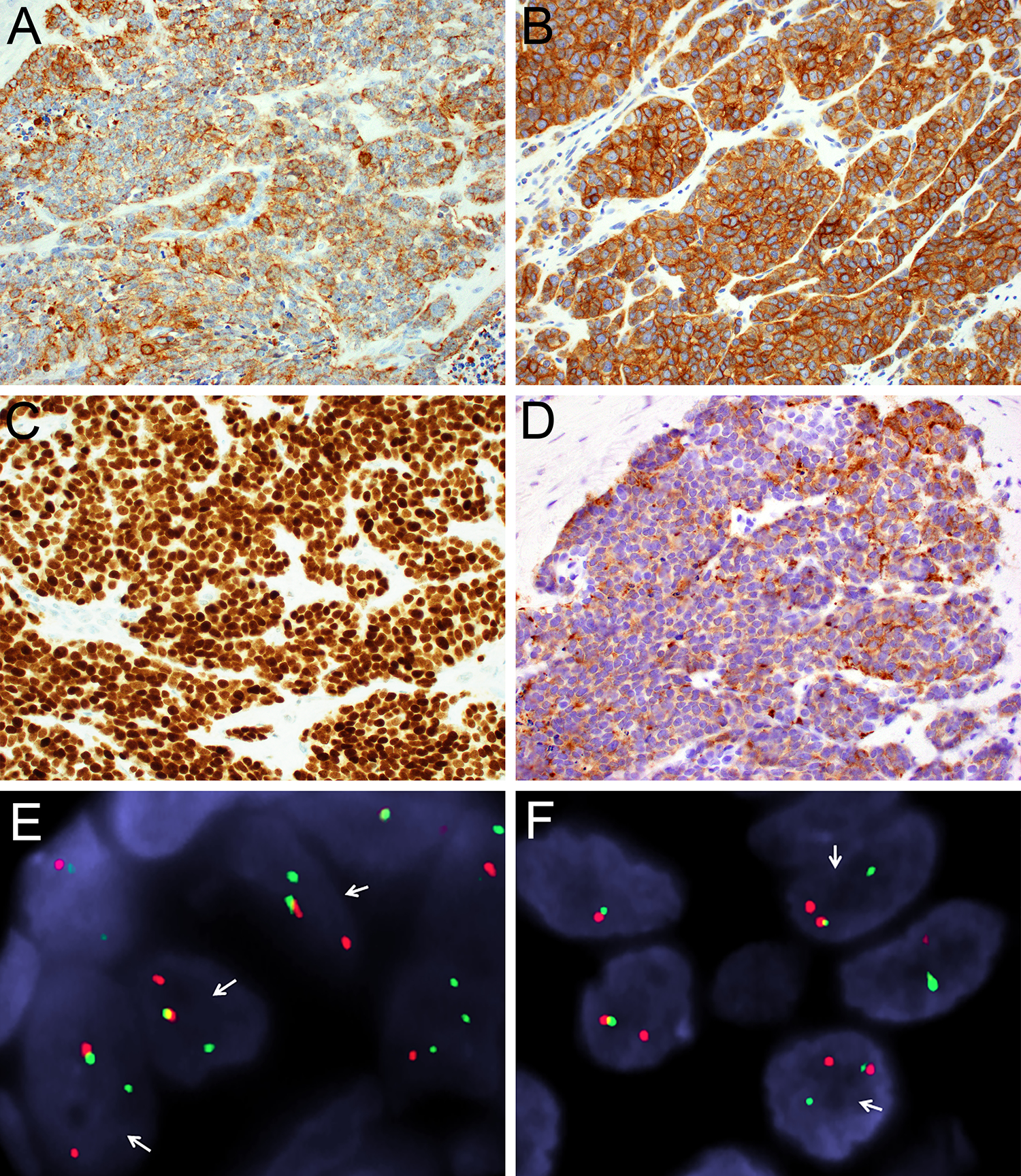
All ALES were strongly and diffusely positive for cytokeratin AE1/AE3 (A; 40x), membranous CD99 (B; 40x), and p40 (C; 40x), with frequent positivity for synaptophysin (D; 40x). The ALES also demonstrated rearrangements of EWSR1 (A) and FLI1 (B), with separated red (centromeric) and green (telomeric) signals (arrows) on break-apart fluorescent in-situ hybridization.
Treatment and follow-up information is tabulated in Table 3. Detailed treatment information was available for 6 patients. Five patients were treated with surgery followed by chemotherapy and external beam radiation; and 1 patient was treated with surgery and chemotherapy. For 2 patients, planned chemotherapy protocols were altered to an ES-specific regimen of vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide after the original diagnosis was changed to ALES. With limited follow-up of 0–27 months (mean 7 months), 7 patients were alive without disease, 1 was alive with persistent disease, and 1 died due to complications of treatment. The single patient with persistent disease underwent an alternate chemotherapy regimen of carboplatin/etoposide.
Table 3:
Treatment and Follow-Up Information
| Case | Treatment | Clinical course | Outcome | Follow-up (months) |
|---|---|---|---|---|
| 1 | Surgery + XRT + chemo (VDC/IE) | No residual disease | NED | 1 |
| 2 | Surgery, additional treatment pending | No residual disease | NED | 0 |
| 3 | Surgery, additional treatment pending | No residual disease | NED | 0 |
| 4 | Surgery + chemo (VDC/IE) | No residual disease | DWD | 3 |
| 5 | Surgery, additional treatment pending | No residual disease | NED | 1 |
| 6 | Surgery + XRT + chemo (doxorubicin) | No residual disease | NED | 13 |
| 7 | Surgery + XRT + chemo (carboplatin/etoposide) | Persistent disease following initial therapy | AWD | 8 |
| 8 | Surgery + XRT + chemo (VDC/IE) | No residual disease | NED | 19 |
| 9 | Surgery + XRT + chemo (initially carboplatin/paclitaxel then VDC/IE) | No residual disease | NED | 24 |
| 10 | Surgery, additional treatment pending | No residual disease | NED | 0 |
VDC/IE = alternating vincristine + doxorubicin + cyclophosphamide and ifosfamide + etoposide; XRT = external beam radiation therapy; AWD = alive with disease; chemo = systemic chemotherapy; DWD = dead with disease; NED = no evidence of disease
Discussion
ALES is a rare tumor that demonstrates the EWSR1-FLI1 translocation characteristic of ES despite complex epithelial differentiation including diffuse cytokeratin and p40 expression. Although originally described in the long bones and thorax, recent experience suggests that this tumor has a proclivity for head and neck sites; including this series, 19 of 26 reported cases have involved the head and neck. ALES presents a conspicuous diagnostic challenge when it arises in the salivary glands owing to its epithelial differentiation and morphologic overlap with a wide spectrum of basaloid salivary gland neoplasms. All 9 of our cases received in consultation were incorrectly diagnosed, including 5 as poorly differentiated carcinomas, 1 as high-grade neuroendocrine carcinoma, 1 as Merkel cell carcinoma, 1 as basal cell adenocarcinoma, and 1 as basal cell adenoma. Although very difficult, recognizing ALES and distinguishing it from a salivary gland carcinoma is critical for better understanding of tumor behavior and therapeutic options.
In general, cases of ALES that occur in the salivary glands show substantial morphologic and immunohistochemical similarities to those in other anatomic sites. Salivary ALESs generally demonstrate highly infiltrative growth with prominent associated fibrous stroma, basaloid tumor cells with at least focal rosette formation, and monotonous cytology despite other high-grade features (e.g. necrosis, high mitotic rate). They also display consistent immunohistochemical expression of pancytokeratin, p40, CD99 and NKX 2.2. Nevertheless, some features of salivary gland ALESs depart from those in non-salivary gland sites. Salivary gland ALESs are less likely to display morphologic evidence of squamous differentiation, with characteristic keratin pearl formations noted in only a single case of our series. Furthermore, salivary gland ALESs are more consistently positive for neuroendocrine markers than previously reported, with 80% of cases positive for synaptophysin and/or chromogranin in contrast to just 20% in other head and neck sites (11). Finally, salivary gland ALESs occur in an older population than reported elsewhere, with a mean age of 52 years in patients in this series compared to 31 years in the head and neck overall (11). Notably, no salivary gland ALESs occurred in the pediatric population, where a diagnosis of ES is most common.
To compound these differences, morphologic and immunohistochemical features that facilitate recognition of ALES in other anatomic sites may not be very helpful in the salivary glands. One of the most powerful clues to a diagnosis of ALES is cytologic uniformity despite widely infiltrative growth, an elevated mitotic rate, and prominent necrosis - a finding that is highly suggestive of a translocation-associated tumor (23). However, cellular monotony is more the rule than the exception in the salivary glands since many primary salivary carcinomas also harbor recurrent gene fusions. As such, while a uniform morphologic appearance should certainly trigger consideration of ALES, its discriminatory utility is somewhat limited in this anatomic site. Another unique feature that suggests a diagnosis of ALES is concomitant immunohistochemical positivity for cytokeratin, p40, synaptophysin, and CD99. However, unlike the sinonasal tract where these markers are part of a standard first-line panel for evaluating small round blue cell tumors (24), pathologists do not routinely perform all of these markers in salivary tumors. Consequently, ALES may be prematurely assigned to an incorrect lineage if only a subset of relevant immunostains is performed. We recommend performing both p40 and synaptophysin in all high-grade basaloid salivary tumors as an initial screen for ALES.
Accordingly, pathologists should actively rule out ALES in the differential diagnosis of a wide range of basaloid salivary tumors. The basaloid cytology and occasional basement membrane deposition in ALES may initially raise consideration of basal cell adenocarcinoma or solid adenoid cystic carcinoma. However, basal cell adenocarcinoma is generally a low-grade tumor that lacks the widely infiltrative growth and high mitotic rate of ALES, and even solid adenoid cystic carcinoma usually has some areas with true cribriform architecture. Moreover, both tumors have biphasic ductal and myoepithelial/basal cell populations with abluminal rather than diffuse p40 reactivity (25, 26). The consistent p40 positivity in ALES, especially if paired with overt keratin pearl formation, can also mimic tumors with squamous differentiation, including squamous cell carcinoma and NUT carcinoma. Yet basaloid squamous cell carcinomas generally display more cytologic pleomorphism than ALES, and NUT carcinomas should demonstrate immunohistochemical expression of NUT1 (27), which was negative in this series. Similarly, synaptophysin positivity, rosette formation and peripheral nuclear palisading might be suggestive of a large cell neuroendocrine carcinoma. Nonetheless, large cell neuroendocrine carcinomas are extremely rare in the salivary glands (28), and they should lack significant p40 expression while demonstrating more notable cellular atypia than ALES. Finally, ALES may overlap with myoepithelial carcinomas, which not only demonstrate basaloid to clear cell features and diffuse cytokeratin and p40 positivity but also harbor EWSR1 rearrangements in approximately 35% of cases (29–31). Fortunately, ALES generally lack the spindled to plasmacytoid cells and significant nuclear pleomorphism that are frequently seen in salivary myoepithelial carcinomas and should be largely negative for other myoepithelial markers. Furthermore, although EWSR1 fusion partners have not yet been elucidated in most salivary myoepithelial carcinomas, identification of a EWSR1-FLI1 fusion can specifically confirm the diagnosis of ALES.
Beyond these specific differential diagnoses, the presence of a EWSR1-FLI1 fusion in a cytokeratin-positive salivary gland neoplasm also raises larger questions about whether ALES is really a variant of ES or if it represents a unique type of carcinoma. In the salivary glands, not only have EWSR1 rearrangements been previously reported in myoepithelial carcinomas, but also in partnership with ATF1 in clear cell carcinomas (32). Nevertheless, EWSR1-FLI1 fusions have thus far been considered pathognomonic for ES across anatomic sites, with genotype traditionally trumping phenotype to support this classification (5). However, as understanding of the phenotypic heterogeneity of tumors with identical gene rearrangements expands (23), it is conceivable that a carcinoma could also carry this translocation. Moreover, the diffuse cytokeratin positivity, p40 expression, focal overt squamous differentiation, and increasing recognition in epithelial sites raise the possibility that ALES actually represents a carcinoma with EWSR1-FLI1 fusion. Indeed, another group classified similar tumors occurring in the thyroid as “carcinoma of the thyroid with Ewing family tumor elements” (6–8). We chose to employ the ALES terminology in this series since the vast majority of tumors with this phenotype have been reported under that name, but we realize there is significant room for debate about this classification. Regardless of nomenclature, there is little question that this tumor is a distinct entity with unique morphologic and immunohistochemical features.
The prognosis of ALES is not entirely clear because of limited follow up data, but there appears to be a trend toward good outcomes in the salivary glands if treated aggressively. Although initial reports speculated that ALES in general would have a poor prognosis due to a lack of necrosis in post-chemotherapy resection specimens (5), most cases reported to date in the head and neck have actually followed an indolent course. In this series, most patients with salivary ALES achieved early disease control following surgery, external beam radiation therapy, and systemic chemotherapy using an ES-specific regimen of alternating vincristine/doxorubicin/cyclophosphamide and ifosfamide/etoposide. Indeed, the only patient with persistent disease in our cohort was treated with an alternate chemotherapy regimen. These findings suggest that, at least initially, ALES that occurs in the salivary gland does not follow the aggressive course characteristic of conventional ES. Furthermore, although insufficient follow-up information and controversial tumor classification make it difficult to recommend the most appropriate chemotherapy in this unique tumor type, the good responses to ES-specific regimens in salivary ALES suggest that it is worthwhile to consider these options in treatment discussions.
In summary, cases of ALES that occur in the salivary glands are characterized by basaloid tumor cells, highly infiltrative growth, prominent fibrous stroma, and at least focal rosette formation. However, frequent positivity for neuroendocrine markers, minimal squamous differentiation, and tendency to occur in older patient population may make the diagnosis of ALES less apparent in this anatomic site, leading to misclassification as various salivary carcinomas. Cytologic uniformity despite high-grade features and immunohistochemical positivity for both p40 and synaptophysin are useful clues that should trigger consideration of ALES, and molecular testing for EWSR1-FLI1 rearrangements can confirm the diagnosis. Pathologists should rule out ALES in the differential diagnosis of a wide range of basaloid salivary gland tumors to allow for appropriate classification, prognostication, and full consideration of treatment options.
Acknowledgments
Financial Support: No specific funding.
Footnotes
Conflicts of Interest: None declared.
References
- 1.de Alava E, Lessnick SL, Sorenson PH. Ewing Sarcoma. In: Fletcher CD, Bridge JA, Hogendoorn PC, et al. , eds. WHO Classification of Tumours of Soft Tissue and Bone. Lyon, France: International Agency for Research on Cancer; 2013:306–309. [Google Scholar]
- 2.Fadul J, Bell R, Hoffman LM, et al. EWS/FLI utilizes NKX2–2 to repress mesenchymal features of Ewing sarcoma. Genes Cancer. 2015;6:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shibuya R, Matsuyama A, Nakamoto M, et al. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465:599–605. [DOI] [PubMed] [Google Scholar]
- 4.Smith R, Owen LA, Trem DJ, et al. Expression profiling of EWS/FLI identifies NKX2.2 as a critical target gene in Ewing’s sarcoma. Cancer Cell. 2006;9:405–416. [DOI] [PubMed] [Google Scholar]
- 5.Bridge JA, Fidler ME, Neff JR, et al. Adamantinoma-like Ewing’s sarcoma: genomic confirmation, phenotypic drift. Am J Surg Pathol. 1999;23:159–165. [DOI] [PubMed] [Google Scholar]
- 6.Cruz J, Eloy C, Aragues JM, et al. Small-cell (basaloid) thyroid carcinoma: a neoplasm with a solid cell nest histogenesis? Int J Surg Pathol. 2011;19:620–626. [DOI] [PubMed] [Google Scholar]
- 7.Oliveira G, Polonia A, Cameselle-Teijeiro JM, et al. EWSR1 rearrangement is a frequent event in papillary thyroid carcinoma and in carcinoma of the thyroid with Ewing family tumor elements (CEFTE). Virchows Arch. 2017;470:517–525. [DOI] [PubMed] [Google Scholar]
- 8.Eloy C, Oliveira M, Vieira J, et al. Carcinoma of the thyroid with ewing family tumor elements and favorable prognosis: report of a second case. Int J Surg Pathol. 2014;22:260–265. [DOI] [PubMed] [Google Scholar]
- 9.Alexiev BA, Tumer Y, Bishop JA. Sinonasal adamantinoma-like Ewing sarcoma: A case report. Pathol Res Pract. 2017;213:422–426. [DOI] [PubMed] [Google Scholar]
- 10.Barroca H, Souto Moura C, Lopes JM, et al. PNET with neuroendocrine differentiation of the lung: Report of an unusual entity. Int J Surg Pathol. 2014;22:427–433. [DOI] [PubMed] [Google Scholar]
- 11.Bishop JA, Alaggio R, Zhang L, et al. Adamantinoma-like Ewing family tumors of the head and neck: a pitfall in the differential diagnosis of basaloid and myoepithelial carcinomas. Am J Surg Pathol. 2015;39:1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folpe AL, Goldblum JR, Rubin BP, et al. Morphologic and immunophenotypic diversity in Ewing family tumors: a study of 66 genetically confirmed cases. Am J Surg Pathol. 2005;29:1025–1033. [PubMed] [Google Scholar]
- 13.Fujii H, Honoki K, Enomoto Y, et al. Adamantinoma-like Ewing’s sarcoma with EWS-FLI1 fusion gene: a case report. Virchows Arch. 2006;449:579–584. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi Y, Kishimoto T, Ota S, et al. Adamantinoma-like Ewing family tumor of soft tissue associated with the vagus nerve: a case report and review of the literature. Am J Surg Pathol. 2013;37:772–779. [DOI] [PubMed] [Google Scholar]
- 15.Lezcano C, Clarke MR, Zhang L, et al. Adamantinoma-like Ewing sarcoma mimicking basal cell adenocarcinoma of the parotid gland: a case report and review of the literature. Head Neck Pathol. 2015;9:280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lilo MT, Bishop JA, Olson MT, et al. Adamantinoma-like Ewing sarcoma of the parotid gland: Cytopathologic findings and differential diagnosis. Diagn Cytopathol. 2018;46:263–266. [DOI] [PubMed] [Google Scholar]
- 17.Weinreb I, Goldstein D, Perez-Ordonez B. Primary extraskeletal Ewing family tumor with complex epithelial differentiation: a unique case arising in the lateral neck presenting with Horner syndrome. Am J Surg Pathol. 2008;32:1742–1748. [DOI] [PubMed] [Google Scholar]
- 18.Grier HE, Krailo MD, Tarbell NJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing’s sarcoma and primitive neuroectodermal tumor of bone. N Engl J Med. 2003;348:694–701. [DOI] [PubMed] [Google Scholar]
- 19.Huang M, Lucas K. Current therapeutic approaches in metastatic and recurrent ewing sarcoma. Sarcoma. 2011;2011:863210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Galindo C, Spunt SL, Pappo AS. Treatment of Ewing sarcoma family of tumors: current status and outlook for the future. Med Pediatr Oncol. 2003;40:276–287. [DOI] [PubMed] [Google Scholar]
- 21.Garcia EP, Minkovsky A, Jia Y, et al. Validation of OncoPanel: A Targeted Next-Generation Sequencing Assay for the Detection of Somatic Variants in Cancer. Arch Pathol Lab Med. 2017;141:751–758. [DOI] [PubMed] [Google Scholar]
- 22.Sholl LM, Do K, Shivdasani P, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight. 2016;1:e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonescu CR, Dal Cin P. Promiscuous genes involved in recurrent chromosomal translocations in soft tissue tumours. Pathology. 2014;46:105–112. [DOI] [PubMed] [Google Scholar]
- 24.Rooper LM, Bishop JA. Sinonasal Small Round Blue Cell Tumors: An Immunohistochemical Approach. Surg Pathol Clin. 2017;10:103–123. [DOI] [PubMed] [Google Scholar]
- 25.Owosho AA, Aguilar CE, Seethala RR. Comparison of p63 and p40 (DeltaNp63) as Basal, Squamoid, and Myoepithelial Markers in Salivary Gland Tumors. Appl Immunohistochem Mol Morphol. 2016;24:501–508. [DOI] [PubMed] [Google Scholar]
- 26.Rooper L, Sharma R, Bishop JA. Polymorphous low grade adenocarcinoma has a consistent p63+/p40- immunophenotype that helps distinguish it from adenoid cystic carcinoma and cellular pleomorphic adenoma. Head Neck Pathol. 2015;9:79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agaimy A, Fonseca I, Martins C, et al. NUT Carcinoma of the Salivary Glands: Clinicopathologic and Molecular Analysis of 3 Cases and a Survey of NUT Expression in Salivary Gland Carcinomas. Am J Surg Pathol. 2018;42:877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chernock RD, Duncavage EJ. Proceedings of the NASHNP Companion Meeting, March 18th, 2018, Vancouver, BC, Canada: Salivary Neuroendocrine Carcinoma-An Overview of a Rare Disease with an Emphasis on Determining Tumor Origin. Head Neck Pathol. 2018;12:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalin MG, Katabi N, Persson M, et al. Multi-dimensional genomic analysis of myoepithelial carcinoma identifies prevalent oncogenic gene fusions. Nat Commun. 2017;8:1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ni H, Zhao PY, Wang XT, et al. EWSR1 rearrangement is present in a subset of myoepithelial tumors of salivary glands with variable morphology and does not correlate with clinical behavior. Ann Diagn Pathol. 2017;28:19–23. [DOI] [PubMed] [Google Scholar]
- 31.Skalova A, Weinreb I, Hyrcza M, et al. Clear cell myoepithelial carcinoma of salivary glands showing EWSR1 rearrangement: molecular analysis of 94 salivary gland carcinomas with prominent clear cell component. Am J Surg Pathol. 2015;39:338–348. [DOI] [PubMed] [Google Scholar]
- 32.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. [DOI] [PubMed] [Google Scholar]


