Abstract
In the last decade, approaches based on T cells and their immunomodulatory receptors have emerged as a solid improvement in treatments for various types of cancer. However, the roles of these molecules in the therapeutic context of autoimmune and cardiovascular diseases are still relatively unexplored. Here, we review the best known and most commonly used immunomodulatory T cell receptors in clinical practice (PD-1 and CTLA-4), along with the rest of the receptors with known functions in animal models, which have great potential as modulators in human pathologies in the medium term. Among these other receptors is the receptor CD69, which has recently been described to be expressed in mouse and human T cells in autoimmune and cardiovascular diseases and cancer. However, inhibition of these receptors individually or in combination by drugs or monoclonal antibodies generates a loss of immunological tolerance and can trigger multiple autoimmune disorders in different organs and immune-related adverse effects. In the coming decades, knowledge on the functions of different immunomodulatory receptors will be pivotal for the development of new and better therapies with less harmful side effects. In this review, we discuss the roles of these receptors in the control of immunity from a perspective focused on therapeutic potential in not only cancer but also autoimmune diseases, such as systemic lupus erythematosus, autoimmune diabetes and rheumatoid arthritis, and cardiovascular diseases, such as atherosclerosis, acute myocardial infarction, and myocarditis.
Keywords: Immunomodulatory receptors, Autoimmune diseases, T cells, Immunotherapy
Subject terms: Immunology, Immunosuppression
Introduction
Targeting immunomodulatory T cell receptors has emerged as a successful approach to manipulate the immune system, generating spectacular outcomes in certain diseases, such as cancer. Advances in the knowledge on the mechanisms by which tumors affect the balance between effector and regulatory T cells have revealed new therapeutic strategies for the treatment of autoimmune diseases1 and are also very promising for cardiovascular diseases.2 In this review article, we focus on receptors with the main function of triggering immunosuppressive signaling pathways in T cells, mainly programmed cell death protein (PD1), cytotoxic T-lymphocyte-associated protein-4 (CTLA-4), B- and T-lymphocyte attenuator (BTLA), Lymphocyte-activation gene 3 (LAG-3), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3), T cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif [ITIM] domain (TIGIT), 2B4 (CD244), and V-domain Ig suppressor of T cell activation (VISTA), all of which belong to the immunoglobulin (Ig) superfamily;3 CD5, a scavenger-receptor cysteine-rich (SRCR) superfamily receptor; and CD69, a C-type lectin with a role as an immunomodulatory T cell receptor that has been demonstrated in recent years.4 We summarize the main characteristics of each of the above immunomodulatory T cell receptors, their expression in different T cell subsets, the mechanism of suppression, or negative regulation of T cell activation and associated positive and negative repercussions in the contexts of human autoimmunity, cardiovascular disease, and cancer (Table 1). Moreover, we also discuss the role of the new generation of targeted immunotherapies (immune checkpoint inhibitors, ICIs) in cancer and how checkpoint inhibition is also being adapted to treat autoimmune diseases.1
Table 1.
Pathologies and treatments associated with different T cell immunoreceptors in the fields of autoimmunity, cardiovascular disease, and cancer
| Immunoreceptor | T cell type | Disease | Treatment | Ref. |
|---|---|---|---|---|
| PD-1 | ||||
| Autoimmunity | T cell | Type 1 diabetes | 81 | |
| gd T cell | Psoriasis | Nivolumab | 210 | |
| Treg | Inflammatory bowel disease | Anti-PD-L1 monoclonal antibodies: MIH2 | 230 | |
| Treg | RA | TNF blockade | 94 | |
| Treg | MS | 231 | ||
| CVD | T cell | Atherosclerosis | 123 | |
| CD8 T cell | Atherosclerosis | Anti-PD-1 monoclonal antibody (clone 29E.2A3, BioLegend) | 121 | |
| T cell | DC and myocarditis | Anti-PD1 and anti-PD1L antibodies | 152 | |
| Treg | ACS | 122 | ||
| Cancer | CD8+ T cells/Treg cells | Melanoma, lung cancer, kidney cancer, BC, head and neck cancer, urothelial carcinoma, hepatocellular carcinoma, gastric cancer, metastatic Merkel cell carcinoma and Hodgkin lymphoma | Anti-PD-1 mAbs: atezolizumab, pembrolizumab and nivolumab | 191,210 |
| CD8 T cell | AML | 232 | ||
| CTLA-4 | ||||
| Autoimmunity | CD8 T cell | RA | Fusion protein: CTLA4 Ig | 97 |
| CVD | T cell | DC and myocarditis | Anti-CTLA4 antibody | 152 |
| Chronic heart failure | 233 | |||
| Cancer | CD8 T cell | Melanoma | Ipilimumab | 178 |
| T cell | Melanoma, NSCLC, breast cancer, prostate cancer, pancreatic cancer, hepatocellular carcinoma, and mesothelioma | Anti-CTLA-4 mAbs: ipilimumab and tremelimumab | 187 | |
| LAG-3 | ||||
| Autoimmunity | MS | 234 | ||
| CVD | CD4 T cells | Cardiac dysfunction in chronic HIV | Anti-LAG-3 immunotherapy | 235 |
| CD4 T cells | CAD | 128 | ||
| Cancer | CD8 T cells | Multiple human cancers, breast cancer, ovarian cancer | Anti-LAG-3 immunotherapy | 12,206 |
| CD8 T cells | Melanoma | Anti-LAG3 mAb: relatlimab | 206 | |
| CD8 T cells | Metastatic breast cancer | LAG3-Ig fusion protein: EOC202 | 206 | |
| Tim-3 | ||||
| Autoimmunity | CD4 T cell, CD8 T cell, NKT cell | RA | 12,103 | |
| CD8 T cell | Asthma and allergy | 12 | ||
| Th1 cell | MS | 12 | ||
| Treg | Autoimmune hepatitis | 236 | ||
| CVD | CD8 | Atherosclerosis | Anti-Tim-3 mAb: F38-2E2 | 121 |
| Cancer | T cells | Hepatocellular carcinoma | 237 | |
| CD8 T cell | Prostate cancer | 238 | ||
| CD8+ T cells/Treg cells | Hepatocellular, ovarian, colon and cervical carcinomas | 237 | ||
| TIGIT | ||||
| Autoimmunity | Treg cell | Type 1 diabetes | 12 | |
| MS | 12 | |||
| RA | 12 | |||
| Cancer | CD8 T cell | NSCLC, colon cancer, melanoma, and myelogenous leukemia | 12,88 | |
| Treg cell | Melanoma | 88 | ||
| 2B4 | ||||
| Autoimmunity | CD8 T cell | RA | 18 | |
| CD8 T cell | SLE | 18 | ||
| Cancer | CD8 T cell | Multiple myeloma | 239 | |
| CD8 T cell | Melanoma, hepatocarcinoma, and AML | 239 | ||
| CD8 T cell | AML | 239 | ||
| VISTA | ||||
| Cancer | CD8 T cells, Tregs | Endometrial and ovarian cancers, solid tumors | Anti-VISTA mAb: JNJ 61610588 (clinical trial) | 207 |
| T cell, Treg | Solid tumors and lymphomas, NSCLC | CA-170 (clinical trial) | 207 | |
| T cells | Prostate cancer | 131 | ||
| CD69 | ||||
| Autoimmunity | T cells | RA | 4,111 | |
| CD4 T cells | Type 1 diabetes | 90 | ||
| T cells | Osteoarthritis | 4 | ||
| Treg cells | SLE | 4,44,68,69 | ||
| Th17 cells | Wegener’s granulomatosis | 4 | ||
| Th17 cells | MS | 4 | ||
| T cells | Optic neuromyelitis | 4 | ||
| T cells/Treg cells | Autoimmune thyroiditis | 4,74 | ||
| CVD | T cells | MI | 149 | |
| Th17/Treg cells | Atherosclerosis | 4,32,149 | ||
| CD4 T cells | Coronary heart disease | 240 | ||
| Cancer | CD4 T cells | Lung adenocarcinoma | 241 | |
| Treg cells | Hepatocellular carcinoma | 4 | ||
| CD4 T cells | NSCLC | 242 | ||
| BTLA | ||||
| Autoimmunity | CD4 and CD8 T cells | RA | 243 | |
| CD4+ T cell | Ulcerative colitis | 243 | ||
| CD4+ T cells | SLE | 243 | ||
| CD8+ T cells | MS | 243 | ||
| CD4−CD8− T cells | Vasculitis | 243 | ||
| CD4+ T cells | Vasculitis (Behçet’s disease) | 243 | ||
| CVD | Treg | Atherosclerosis | 243 | |
| Cancer | T cells | Epithelial ovarian carcinoma | 243 | |
| CD8+ T cell | Melanoma | 243 | ||
| T cells | Diffuse large B cell lymphoma | 243 | ||
| CD4+ T | Hepatocellular carcinoma | 243 | ||
| CD5 | ||||
| Autoimmunity | T cell | CTCL | Anti-CD5 mAb: T101; and radioimmunoconjugate 90Y-T101 | 244 |
| T cells | MS | 244 | ||
| Cancer | T cell | Lung carcinoma | 244 | |
| T cell | Melanoma | 244 | ||
CVD cardiovascular diseases, mAb monoclonal antibody, CD cluster of differentiation, NKT natural killer T, gd T cell gamma delta T cell, Treg regulatory T cell, TNF tumor necrosis factor, Ig immunoglobulin, Th T helper, PD1 programmed cell death, PD1L programmed cell death ligand, CTLA4 cytotoxic T-lymphocyte-associated protein-4, LAG3 Lymphocyte-activation gene 3, TIM3 T cell immunoglobulin and mucin domain-containing protein 3, TIGIT T cell immunoreceptor with Ig and ITIM domains, VISTA V-domain Ig suppressor of T cell activation, BTLA B and T lymphocyte associated, AML acute myeloid leukemia, NSCLC non-small cell lung cancer, MS multiple sclerosis, SLE systemic lupus erythematosus, CAD coronary artery disease, RA rheumatoid arthritis, DC dilated cardiomyopathy, MI myocardial infarction, ACS acute coronary syndrome
Overview of the immunomodulatory receptors expressed by T cells
PD1, CTLA-4, and BTLA belong to the CD28 Ig superfamily and share similar protein structures.5 PD-1 (CD279) is expressed by all T cells during activation, B cells, natural killer, and myeloid cells.6 PD-L1 and PD-L2 are known ligands of PD-1 expressed by T, B, and antigen-presenting cells. The binding of PD-1 to PD-L1 induces resistance to activation by positive signals from the T cell receptor (TCR) and CD28 in conventional T cells.6 CTLA-4 (CD165) is expressed on activated CD4+ T cells and competitively binds B7.1 and B7.2, acting as a coinhibitory signal to downregulate early T cell activation and proliferation.7 Constitutive CTLA-4 expression on regulatory T (Treg) cells enhances their regulatory function through suppression of antigen-presenting cells.8 BTLA (CD272) is expressed in single-positive thymocytes, mature T cells, B cells, macrophages, and dendritic cells. BTLA maintains T cell immune tolerance9, and the binding of BTLA to its ligand, herpes virus entry mediator, prevents excessive activation of T cells.10
LAG-3 is a transmembrane protein homolog to the CD4 coreceptor that binds to major histocompatibility complex (MHC) class II with high affinity.11 LAG-3 (CD223) is expressed in activated CD4+ T cells, Treg cells, Tr1 cells, activated CD8+ T cells, natural killer cells, dendritic cells, B cells, and exhausted effector T cells.12 The interaction of LAG-3 with MHC II leads to decreased proliferation and cytokine secretion by antigen-specific CD4+ T cell clones.11 T cell immunoglobulin and mucin domain 3 is a transmembrane protein belonging to the Ig superfamily that is expressed in CD4 helper 1 (Th1) cells, CD8 T cytotoxic 1 (Tc1) cells, Treg cells, dendritic cells, natural killer cells, monocytes, macrophages, and mast cells. Galectin-9, the first reported Tim-3 ligand, was shown to induce apoptosis in Th1 cells,13 and carcinoembryonic antigen cell adhesion molecule 1 forms a heterodimer with Tim-3 to mediate T cell inhibition and exhaustion.14 TIGIT is a coinhibitory transmembrane protein expressed by regulatory and memory CD4+ T cells, CD8+ T cells, and natural killer cells. The poliovirus receptor (CD155) shared with CD226, poliovirus receptor-related 2 (CD112, also known as Nectin-2), and Nectin4 are the ligands for TIGIT and deliver stimulatory signals.15,16 TIGIT also blocks T cell activation, proliferation, and maturation by targeting downstream TCR signaling pathways.17 The signaling lymphocyte activation molecule 2B4/CD244 is a CD2-related receptor expressed in antigen-experienced CD8+ αβ T cells, natural killer cells, dendritic cells, and myeloid-derived suppressor cells (MDSCs).18 The engagement of 2B4 by its receptor, CD48, contributes to CD8+ T cell exhaustion and cell function inhibition.19 VISTA is expressed in T cells, natural killer cells, and myeloid cells.20 VISTA is a member of the B7 family, and although the receptor shares homology with members of the CD28 family, the gene is located on a different chromosome. VISTA can act as both a ligand and a receptor in regulating CD4+ and CD8+ T cell proliferation and cytokine production.21
The T cell receptor-inhibiting molecule CD5, also known as Leu-1 in humans, is a transmembrane glycoprotein that belongs to the highly conserved SRCR.22 CD5 is constitutively expressed on lymphocyte precursors, mature T cells, and B1a cells and is associated with both TCR/CD3 and B cell receptor.23 The known ligands for CD5 are CD5L, CD72, and CD5 itself, but little is known about their physiological functions. CD5 clusters with the TCRζ/CD3-pMHC complex and inhibits signaling through immunological synapses in thymocytes and peripheral T cells.24,25
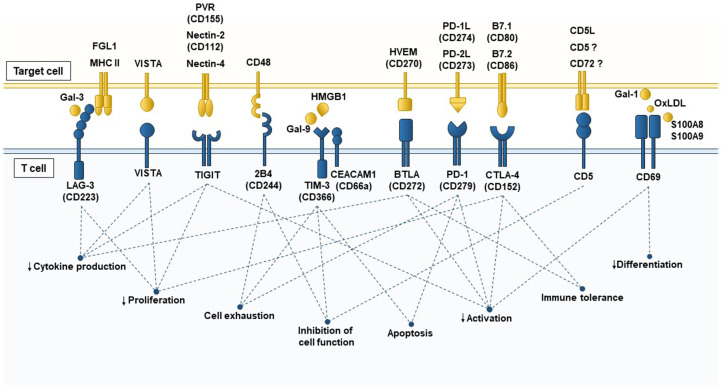
Last but not least, the early leukocyte activation antigen CD69 is a type II C-lectin membrane receptor26 that belongs to a family of immunomodulatory receptors involved in the immune response, the NK complex.4 CD69 is expressed early after cell activation in all hematopoietic cells except erythrocytes27 and has emerged as a new immunoregulatory receptor expressed by T cells in the last decade.28 Additionally, a number of ligands for CD69 with the ability to modulate T cell responses through different pathways and triggers have been identified recently. Galectin-1 (Gal-1) binds to CD69, inhibiting naive and helper T cell activation and differentiation,29,30 and interaction with the S100A8/S100A9 complex is required for the differentiation of regulatory T cells.31 Oxidized low-density lipoprotein (OxLDL) has been shown to bind specifically to CD69+ human T lymphocytes, enhancing Treg cell differentiation and inhibiting Th17 cells.32 Interestingly, the CD69 cluster with the aromatic amino acid-transporter complex LAT-1-CD98 in γδ T cells regulates l-tryptophan transport and cytokine secretion in these cells.33 The immunomodulatory T cell receptors, their ligands, and the cellular functions triggered by signaling through each receptor are summarized in Fig. 1.
Fig. 1.
Membrane receptors with immunomodulatory activity in T cells and their ligands. T cell responses are controlled by multiple inhibitory signals to prevent excessive inflammation that can occasionally cause more damage than repair. T cells express a battery of different receptors with immunomodulatory capacity in the membrane, such as LAG-3, VISTA, TIGIT, 2B4, TIM-3, CEACAM-1, BTLA, PD-1, CTLA-4, CD5, and CD69, which can interact with either soluble ligands or membrane proteins expressed by antigen-presenting cells (APCs), inflammatory cells, tumor cells, or damaged cells. Ligand–receptor interactions induce inhibitory signals in the T cell and sometimes also in the target cell to control inflammation, and the effects include decreasing proliferation, differentiation, activation, cytokine production and cell function and promoting apoptosis, T cell exhaustion and tolerance. LAG-3 Lymphocyte-activation Gene 3, MHC II major histocompatibility complex class II, Gal-3 Galectin-3, VISTA V-domain immunoglobulin suppressor of T cell activation, TIGIT T cell immunoreceptor with immunoglobulin and ITIM domains, PVR poliovirus receptor, Tim-3 T cell immunoglobulin and mucin domain 3, CEACAM-1 carcinoembryonic antigen cell adhesion molecule 1, Gal-9 Galectin-9, HMGB1 high-mobility group box 1, BTLA B- and T-lymphocyte attenuator, HVEM herpes virus entry mediator, PD-1 programmed cell death protein, PD-L1/PD-L2 PD-1 ligand 1/PD-1 ligand 2, CTLA-4 cytotoxic T-lymphocyte-associated protein-4, Gal-1 Galectin 1, CD5L CD5 antigen-like, OxLDL oxidized low-density lipoprotein
Opportunities related to immunomodulatory T cell receptors in human autoimmune diseases
Autoimmune diseases are the consequence of deterioration or loss of self-tolerance resulting from defects in the thymic elimination of potentially self-reactive T cells (central tolerance) or in the control mechanisms of potentially self-reactive T cells in the periphery (peripheral tolerance). The nature of autoimmune diseases is multifactorial since the mechanisms that trigger these conditions can be genetic, epigenetic, molecular, and/or cellular in origin. However, the generation of pathogenic inflammatory responses in peripheral tissues upon activation of autoantigen-specific T cells is a common denominator in all autoimmune diseases.34 T cells with a potentially autoreactive TCR escape thymic selection, and powerful mechanisms are required to control these autoreactive T cells and maintain peripheral tolerance.34 Therefore, approaches targeting immunomodulatory T cell receptors have emerged in recent years as therapeutic opportunities to restore tolerance in autoimmune diseases (Table 1).
Systemic lupus erythematosus (SLE)
The immunomodulatory receptors PD-1, CTLA-4, and BTLA play nonredundant roles in the modulation of central tolerance during the thymocyte selection process and in maintaining peripheral tolerance. We analyzed the performance of these receptors in the development of SLE since patients with this autoimmune disease display abnormal levels of these receptors in T cells with a characteristic hyperactive phenotype. PD-1 has a pivotal role in the negative regulation of positive TCR-α/β thymocyte selection by affecting the threshold of pre-TCR/CD3 complex downstream signaling.35,36 In addition, PD-1 gene ablation in mice with the additional lpr/lpr mutation also results in the development of lupus-like glomerulonephritis and destructive arthritis with age,37 which indicates the involvement of this immunomodulatory receptor in the maintenance of peripheral self-tolerance. Different animal models demonstrate that PD-1 expression can be tuned finely with anti-PD-1 monoclonal antibodies (mAbs) to preserve the number of Foxp3+ T cells or reduce the number of CD4+PD-1+ T cells and alleviate lupus-like nephritis.38,39 However, a completely opposite effect was observed with anti-PD-1 mAb therapy in experimental autoimmune encephalomyelitis and NOD diabetes animal models.40,41 The discovery that the PD-1 pathway modulates T follicular helper cell-mediated humoral immunity by negatively regulating T follicular regulatory (Tfr) cells42 indicates that PD-1 blockade may preferentially influence the Tfr cell function controlling the development of lupus, reconciling previous contradictory results. In patients with SLE, it has been reported that PD-1 expression levels in CD4+ T cells are very low43 and the frequency of PD-1+ cells is greatly decreased. It was found that patients with the PD-1.3 A/G allele have significantly lower expression of PD-1 in CD4+ T cells than other patients. Although PD-1 expression is higher in CD25+ Tregs than in effector T cells, PD-1 expression is significantly lower in SLE patients than in healthy subjects, which leads to functional and numerical reductions in Tregs in SLE patients. This was confirmed in another study in which PD-1 was found to be highly induced in CD4+CD25+ and CD4+CD69+ T cells from healthy controls but not in those from patients with SLE.44 A recent study found elevated levels of coinhibitory IgG autoantibodies against PD-1 in the serum of new-onset SLE patients that were associated with SLE Disease Activity Index (SLEDAI) scores, revealing a new pathway of PD-1 modulation.45 In contrast, a pilot study with a small number of patients showed that PD-1 expression levels in peripheral blood mononuclear cells (PBMCs) were significantly increased in SLE patients and associated with SLEDAI scores, suggesting that PD-1 inhibitors are useful tools in the treatment of SLE.46 In summary, these data indicate that both the regulation and aberrant expression of PD-1 play key roles in the regulation of this autoimmune disease. However, the clinical efficacy of manipulating this pathway requires more basic research and clinical studies. In this regard, an ongoing clinical trial (ClinicalTrials.gov Identifier: NCT03816345) is now testing the efficacy of an anti-PD1 mAb (nivolumab) in SLE and several other autoimmune diseases (Chron’s disease, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis (RA), ulcerative colitis, etc.).
CTLA-4-deficient mice develop lethal lymphoproliferative syndrome and autoimmunity,47,48 as well as a rapid and severe lupus-like autoimmune syndrome.49 These mice exhibit global T cell dysregulation promoting systemic humoral autoimmunity that triggers lupus-like autoimmunity.49 The relevance of the CTLA gene in the susceptibility to SLE is supported by evidence from different human populations with different polymorphisms.50–52 Interestingly, SLE patients display increased expression of CTLA in isolated responder T cells (Foxp3−) compared to healthy controls and patients with other autoimmune rheumatic diseases (RA or psoriatic arthritis).53 However, these T cells exhibit defective inhibition of T cell activation by CTLA-4 after CD3/CD28 costimulation. CTLA-4 receptors are displaced from membrane microdomains in SLE patients and are unable to regulate the intracellular signaling molecules triggered by T cell activation.53 Therefore, the receptor CTLA-4 in responding T cells could be a possible target to restore the function of T cells in patients with SLE, and there is in vitro evidence that soluble CTLA-4 from lupus patient PBMCs regulates effector responses.54 The administration of abatacept, a fusion protein comprising CTLA-4 linked to the Fc portion of IgG1, has been tested clinically in several autoimmune diseases. Abatacept has also been tested in different clinical trials as a therapy for SLE55 and lupus nephritis56 patients in randomized studies and, although it showed evidence of biological activity, it did not meet the endpoint criteria of achieving a complete response during treatment.56
BTLA expression in effector T cells is associated with SLEDAI scores.57,58 Lupus patients have a defect in the upregulation of BTLA expression upon activation of CD4+ T cells in comparison with healthy controls.58 BTLA is recruited to the immunological synapse and acts as an inhibitory receptor that negatively regulates the immune response; this function is defective in SLE patients but can be corrected by restoring intracellular trafficking and lipid metabolism in lupus CD4+ T cells.58 This gives BTLA characteristics similar to those of CTLA-4 and PD-1, and human anti-BTLA antibodies have been developed;59,60 however, the potential of BTLA as a possible target in autoimmune diseases has not yet been tested.
CD69-deficient mice are healthy and do not present an autoimmune phenotype,61 although the expression of this receptor in Treg cells is necessary for the development of natural Treg cells in the thymus62 and their suppressive activity in inflammatory conditions,63 indicating that the immunomodulatory receptor CD69 plays a pivotal role in the maintenance of central and peripheral tolerance.4,26 CD69 expression in Tregs negatively regulates the production of proinflammatory cytokines and is necessary for the suppressive function of these cells in mouse models of autoimmune colitis.64,65 CD4+CD69+ cell numbers are increased in lupus-prone (NZBxNZW)F1 mice and pristine-induced SLE mice, and these cells regulate cytokine production by effector T cells.66,67 SLE patients also present an increased CD69/CD3 ratio in PMBCs compared to healthy controls, which correlates with SLEDAI scores,68,69 and increased levels of CD4+CD69+TGF-β+IL-10+Foxp3− Treg cells.70–72 The CD69+ Treg cell population appears to be increased in a number of autoimmune and inflammatory disorders.73–75 However, although increased in number, CD69+TGF-β+ Foxp3− Treg cells from SLE patients are not able to inhibit the release of cytokines by autologous lymphocytes, indicating that they do not contribute to the regulation of autoimmunity in these patients.70 Moreover, SLE patients who do not respond to immunosuppressive therapy have significantly higher levels of P-glycoprotein (P-gp)+CD69+CD4+ cells in the blood and renal tissue than responsive patients. P-gp expression in lymphocytes plays a role in the active efflux of intracellular drugs, and the high proportion of P-gp+CD69+CD4+ cells in nonresponsive SLE patients is suggestive of corticosteroid resistance and renal damage.76 Therefore, CD69 plays a role in regulating the secretion of cytokines and could be a potential target to control SLE in patients refractory to immunosuppressive therapy. Clinical trials need to be conducted to underscore the real value of this molecule as a therapeutic target.
Autoimmune diabetes
The PD-1-PD-L1 interaction has central roles in regulating the initiation and progression of autoimmune diabetes in nonobese diabetic (NOD) mice. PD-1 blockade rapidly precipitates diabetes in prediabetic NOD mice,41 while overexpression of PD-L1 in pancreatic beta cells prevents diabetes in these animals.77,78 PD-1 expression is downregulated specifically in CD8+ T cells from type 2 diabetes (T2D) patients,79 suggesting that immunomodulatory receptors have a role in the development of T2D. Moreover, PD-L1 is expressed in beta cells from type 1 diabetes (T1D) mellitus patients, possibly to attenuate the autoimmune response mediated by type I and II interferons through IRF1.80,81 Along with animal evidence, these data provide the rationale for developing new therapies to target this costimulatory pathway in this disease without eliminating the protective role of the PD-1–PD-L1 pathway.
In contrast, CTLA blockade only negatively regulates autoimmune diabetes in neonatal NOD mice,40 and CTLA-4 Ig or anti-B7-2 mAb treatment was shown to reduce the incidence of diabetes in young mice, whereas treatment with any of these reagents had no effect on disease progression in older mice.82
Targeting the BTLA pathway in NOD mice with an mAb that selectively depletes pathogenic helper CD4+ T cells increases the proportion of Treg cells and protects against spontaneous disease onset in NOD mice.83 Adoptive transfer of OVA-specific CD8+ T cells isolated from BTLA-deficient mice into RIP-mOVA-recipient mice (expressing membrane-bound OVA in pancreatic beta cells) induces diabetes.9 Moreover, BTLA limits γδ T cell numbers and sustains normal γδ T cell subset frequencies by restricting interleukin-7 (IL-7) responsiveness and CD27−RORγt+ population expansion.84 γδ T cells have been implicated in the development of diabetes; however, the putative role of BTLA targeting in clinical autoimmune diabetes treatment has not been addressed. LAG-3-deficient NOD mice exhibit accelerated autoimmune diabetes development mediated by expansion of pathogenic T cell clones in the islets, which are normally restrained by LAG-3.85 However, the onset of diabetes is accelerated even more in animals doubly deficient in LAG-3 and PD-1, which also develop other autoimmune diseases, such as myocarditis.86 More recently, LAG-3 has been shown to act by limiting the function and proliferation of Tregs due to enhanced IL-2–Stat5 signaling pathway and Eos expression.87 TIGIT is a repressor of the CD226-activating pathway that functions by binding the common ligand CD155. This costimulatory axis has also been postulated to be a therapeutic target for the treatment of T1D, as targeting TIGIT would control the suppressive function of Tregs.88
Finally, the expression of CD69 has been associated with the development of T2D complicated by coronary artery disease in patients.89 Although whether there is a causal relationship is unknown, CD69 could exert its function through the regulation of the hypoxia-inducible factor short isoform I.1.90
Rheumatoid arthritis
T cells play a central role in the pathogenesis of RA; however, the first attempts to target T cells for therapeutic purposes produced a high risk of infection. Thus, the immunomodulatory receptors on T cells have been explored as better targets for the treatment of this often progressive and destructive chronic joint disease. CTLA-4 deficiency affects both central tolerance and peripheral tolerance as well as Treg-mediated suppression. Mice deficient in CTLA-4 develop severe collagen-induced arthritis,91 and patients with CTLA-4 mutations show expansion of Treg cells, which leads to subsequent inflammation and autoimmunity probably through the production of organ-specific autoantibodies.92,93 The therapeutic agent abatacept (CTLA-4-Ig) has shown efficacy in a broad spectrum of RA patients from early-stage disease to refractory disease resistant to tumor necrosis factor-α (TNF-α) blockers.94 Abatacept treatment results in significant improvement in the signs and symptoms of RA95 and in patients with juvenile idiopathic arthritis who did not respond to traditional TNF blockers.96 Treatment with belatacept, an alternative human CTLA-4 Ig, in a phase I/II clinical trial evaluating multiple doses revealed the preliminary efficacy of this drug in the treatment of RA.97
BTLA polymorphism is associated with susceptibility to RA,98 and the expression of BTLA is decreased in T cells from patients with RA.99 LAG-3+ Treg cells have been associated with the development of RA, and their frequency is lower in patients with RA than in healthy individuals.100 Moreover, LAG-3+Foxp3− Tregs are highly effective in relieving joint severity and local and systemic inflammation.101 TIM-3 is associated with RA disease activity102 and involved in the immune dysregulation mediated by TNF-α, IL-17, and interferon-γ (IFN-γ) in this disease;103 thus, TIM-3 may play an important role in the pathogenesis of RA. TIGIT overexpression in vivo improves the severity of RA by decreasing the production of IFN-γ and IL-17, increasing IL-10 cytokine levels and removing anti-collagen II antibodies.104 CD4+CD28− T cells from RA patients overexpress 2B4 together with CD226 and CRACC, suggesting that these receptors could modulate this disease.105 The roles of VISTA and CD5 in RA have been studied, and the relevant expression of these molecules in the pathology of RA relies on macrophages106 and B cells,107 respectively. Signaling by these receptors begins in the innate phase of inflammation, and their roles in T cells during RA need to be explored further. The above data suggest that these immunomodulatory receptors could have therapeutic roles in the development of RA, although at the moment, there are no data on their clinical effectiveness.
CD69-deficient mice show no overt signs of autoimmunity,97 although they are more susceptible to asthma,63 contact dermatitis,108 myocarditis,109 colitis,64 and RA110 than wild-type mice due to exacerbated Th1 and Th17 responses. The role of CD69 was first described in the synovial fluid T cells of RA patients;111 these T cells fail to express CD25 or produce IL-2, and consequently, they are not able to proliferate properly. Moreover, CD69 expression by synovial T cells in RA patients correlates with disease activity.112 CD69-deficient mice show a higher incidence and severity of collagen-induced arthritis (CIA), with exacerbated T and B cell responses, than wild-type mice.110 These mice also show reductions in the levels of tumor growth factor-β1 (TGF-β1) and TGF-β2, which are protective cytokines in CIA, in inflammatory foci and parallel increases in the levels of proinflammatory cytokines, such as RANTES and IL-1β, leading to increased joint inflammation and cartilage and bone erosion. The immunomodulatory receptor CD69 expressed by T cells regulates the immune response through control of TGF-β production26 and regulates the differentiation and activation of Treg and Th17 cells through control of the Stat5/miR-155/SOCS1 (ref. 62) and Jak3–Stat5 pathways,28 respectively. CD69 is also a hallmark of tissue-resident memory T cells, together with CD103 and CD49a, with direct implications on this pathology and autoimmune diseases in general.113 However, the potential of this receptor as a possible therapeutic tool in RA has not been explored in the clinic.4 The potential roles of T cell immunoreceptors and their involvement in autoimmune pathologies are outlined in Fig. 2.
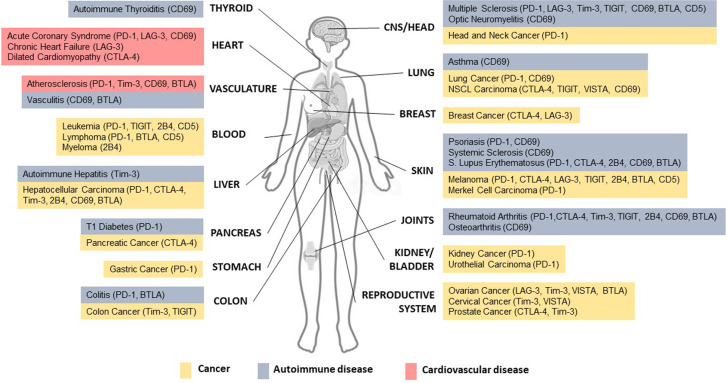
Fig. 2.
Contributions of inhibitory receptors on T cells to human autoimmune diseases (blue), cardiovascular diseases (red), and cancer (yellow) in different organs. Signaling through immunomodulatory membrane receptors is pivotal to promoting immune tolerance and avoiding excess deleterious T cell activation and reactivity. Activation or inhibition of immunomodulatory receptors on T cells may be beneficial or detrimental, depending on the disease context. In different cancer types, immunotherapy based on the blockade of these inhibitory receptors leads to increased and relatively long-lasting T cell responses against tumor cells, resulting in efficient tumor clearance. However, autoimmunity due to self-reactivity, which is related to excessive T cell activation, is the main side effect of cancer immunotherapy. Additionally, dysfunction of inhibitory receptors on T cells is linked to different autoimmune disorders, such as thyroiditis, vasculitis, hepatitis, type 1 diabetes, colitis, multiple and systemic sclerosis, optic neuromyelitis, asthma, psoriasis, systemic lupus erythematosus, rheumatoid arthritis, and osteoarthritis. Favoring signaling through these receptors has emerged as a strategy to restore tolerance in autoimmune patients. The T cell response is also an important mediator of atherosclerosis and acute and chronic myocardial disease development and progression. Thus, different immunomodulatory receptors on T cells are therapeutic candidates in cardiovascular diseases. This schematic summarizes the immunomodulatory T cell receptors (in parentheses) that have been implicated in different human diseases
The efficacy of these immunomodulatory receptors in modulating autoimmune diseases is experimentally associated with certain changes in immune markers, which can be exploited as biomarkers. Some of the receptors mentioned above may indicate the immune process that is taking place due to different treatments. In the next few years, more research will be needed to decipher whether these receptors should be exploited not only to predict the efficiency of treatments and identify autoimmune patients that could possibly benefit from the treatments but also to predict possible adverse effects of treatments.
Immunomodulatory T cell receptors in cardiovascular disease
Increasing numbers of clinical trials have been conducted in patients with acute myocardial infarction (MI) and heart failure, such as cardiomyopathy and myocarditis, using a variety of cell types, including bone marrow stem cells, mesenchymal stem cells, and cardiac resident stem cells. However, none of these preclinical trials or studies have yielded clear results in terms of regeneration of cardiac tissue. In this scenario, immunomodulation has gained interest for its use not only as an adjuvant in cellular therapies but also as cardioprotective therapy in different cardiovascular diseases. T cell responses are important in the onset, progression, and resolution of acute myocardial events and chronic heart failure.114 Here, we review the roles of immunomodulatory T cell receptors in different cardiovascular diseases, including atherosclerosis as the trigger of MI.
Immunomodulation in atherosclerosis treatment
T lymphocytes are present during all stages of development in human atherosclerotic lesions,115 although their role in the disease has been controversial for decades. Increasing evidence demonstrates the modulatory role of the immune system in experimental and clinical arteriosclerosis. The most recent and definitive evidence comes from the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study) clinical trial in which anti-inflammatory treatment was found to be associated with reduced cardiovascular risk.116 These works provide strong evidence that immunomodulatory therapies have therapeutic potential in the development and management of clinical atherosclerosis and other chronic inflammatory conditions. Even statins have been seen to regulate certain immune molecules, such as modulating MHC II through arterial cells and restraining T cell responses117 through IFN-gamma.118 T cell-mediated immunity plays a significant role in atherosclerosis development.119 The role of CD4+ T cells is quite complex; Th1 cells have a proatherogenic role, while Treg cells protect against disease development. However, the roles of Th2, Th17, and follicular helper T cells are still controversial. CD8+ T cells have pleiotropic effects on the development of atherosclerosis; they can induce an inflammatory response via inflammatory cytokines, and cytotoxic activity towards endothelial cells increases the progression of atheroma plaques, whereas the same cytotoxic activity towards macrophages and regulatory CD8+ T cell subsets can inhibit inflammatory responses and atherosclerosis progression.120 Interestingly, CD8+ T cell function is regulated by the PD-1 and Tim-3 signaling pathways in atherosclerosis.121 In humans, the CD8+PD-1+Tim3+ T cell subset, which is enriched in central memory T cells, is abundant in patients with atherosclerosis presenting with increased antiatherogenic cytokine production and decreased proatherogenic cytokine production. In patients with acute coronary syndrome, the expression of PD-1 and PD-L1 on circulating T cells is very low.122,123 Blockade of CD8+PD-1+Tim3+ T cells increases TNFα and IFNγ production. Ab-mediated inhibition of TIM-3 was shown to aggravate atherosclerosis by limiting efferocytosis and T cell responses in mice.124 In parallel, pretreatment of apolipoprotein-deficient (apoE−/−) mice with CTLA-4-IgG can reverse disease acceleration, blocking the signaling pathway in T cells and T cell activation,125 whereas CTLA-4-blocking antibodies strongly increase atherosclerotic lesion numbers,126 and the overexpression of transgenic CTLA-4 in apoE−/− mice enhances Treg-mediated suppression and prevents atherosclerosis development.127 Together, these studies demonstrate that CTLA-4 limits plaque development by inducing anti-inflammatory T cell responses, positioning CTLA-4 as a promising target in atherosclerosis.
The lipoprotein scavenger receptor BI (SCARB1) rs10846744 noncoding variant has been associated with atherosclerosis, independent of traditional cardiovascular risk factors, and with LAG3. A novel study proposed plasma LAG3 as an independent predictor of HDL-C levels and coronary heart disease risk.128
BTLA was recently described to be mostly expressed on B cells in patients with cardiovascular disease and in follicular B2 cells in Ldlr−/− mice fed a high-fat diet. However, the use of an agonistic anti-BTLA antibody was shown to inhibit atherosclerosis development in an animal model with significant increases in regulatory B and T cell numbers,129 indicating an immunomodulatory effect mediated through T cells in atherosclerosis.129 These findings suggest BTLA as a new promising target for the treatment of atherosclerosis.
Treatment with agonistic anti-TIGIT antibodies inhibits CD4+ T cell responses, although this treatment does not affect atherosclerotic lesions in LDLr−/− mice fed a high-fat diet.130 These data suggest that myeloid cells can increase their activity by crosstalk with T cells, negatively influencing disease. Similarly, VISTA is a receptor and a ligand with immunosuppressive effects on IFNγ and TNFα in both T cells and macrophages,131 which adopt an anti-inflammatory M2 phenotype that can also play a role in atherosclerosis development.132
Although CD5 is expressed in T cells from atherosclerotic lesions of apoE−/− and ldlr−/− mice,133 the role of CD5 expression in T cells during disease development is unknown. However, the role of CD69 in atherosclerosis was described recently.32 An interaction between CD69 and OxLDL was identified to be responsible for the anti-inflammatory phenotype of T cells needed to restrain plaque development. The binding of OxLDL to CD69 in mouse and human T cells induces the expression of NR4A1 and NR4A2, members of the NR4A subfamily of human nuclear receptors involved in regulatory T cell differentiation.134 CD69 depletion in the lymphoid compartment of ldlr−/− mice results in reduced expression of NR4A1 and NR4A3 in T cells in mice fed a high-fat diet. The inhibition of these signaling pathways favors the development of Th17 cells, promoting a proinflammatory environment and accelerating the development of atheroma plaques.32,135 In addition, the expression of the CD69 and NR4A1 genes in peripheral blood cells was studied in a cohort of subjects with exhaustive characterization of subclinical atherosclerosis belonging to the Progression of Early Subclinical Atherosclerosis (PESA) study,136 including in healthy and asymptomatic individuals. The data showed that CD69 and NR4A1 mRNA levels decreased with the progression of the atheroma plaque, validating the data from the in vivo model. Analysis of the CD69 receptor in the PBLs of this cohort showed that the loss of CD69 expression was very significantly associated with the development of atheroma plaques, even when this association was corrected for other cardiovascular risk parameters, such as the levels of OxLDL. Further studies on the new regulatory OxLDL/CD69 pair in human lymphoid cells during atherosclerotic disease progression will provide novel insights into targeting these pathways for the prognostic evaluation/treatment of cardiovascular diseases.
Acute myocardial infarction
MI results from occlusion of the coronary arteries, mainly due to a thrombus caused by rupture of a lipid atherosclerotic plaque, which causes ischemia in a region of the heart. Ischemia causes subsequent cardiomyocyte death and triggers inflammatory and repair mechanisms. In recent years, multiple studies have highlighted the roles of T cells not only in the development of atherosclerosis but also in the progression after MI, contributing to cardiac function and remodeling.137 Since immunomodulatory T cell receptors are also checkpoints in the inflammation underlying atherosclerosis, ICI therapy may create an increased risk for atherosclerotic cardiovascular events, such as MI, in cancer patients.138 Acute cardiovascular manifestations derived from atherosclerosis appear progressively after several years of subclinical disease. Although ICI therapy has been implemented in the clinic for the past decade, it may be too early to have a clear picture of the effect of ICIs on the incidence of acute MI. However, in recent cumulative studies, MI has been reported to be a complication in patients treated with ICIs, with an incidence ranging from 1 to 3%.139,140 Some studies indicate that MI occurs less than a year after ICI treatment, suggesting that immunomodulatory therapy can accelerate the progression and instability of pre-existing atherosclerotic lesions rather than cause de novo plaque formation.141 An analysis of coronary plaques suggested that the T cell proportion was higher in ICI-treated patients than in untreated patients,142 consistent with an exacerbated T cell response after ICI therapy in the coronary arteries, a possible trigger of acute events.142
Beyond their roles in atherosclerotic plaque instability and rupture, some immunomodulatory receptors have been proposed to be involved in regulatory mechanisms limiting the degree of inflammation after myocardial ischemic events. Variants of the PD-1-143 and CTLA-4-encoding genes144 are associated with an altered risk of MI,145 and proteomic profiling analysis identified CD5 antigen-like and others as marker proteins associated with new-onset atherosclerotic cardiovascular disease risk.145 PD-1 expression is upregulated in peripheral leukocytes when they increase in number during the first hours after MI and then decrease after reperfusion. In contrast, lower levels of PD-1 expression in T cells are associated with larger infarction lesions.146
Furthermore, analyses of the peripheral blood phenotype of MI patients revealed that circulating CD4+ T cells overexpress CD69 (ref. 147) and that regulatory T cells overexpress CTLA-4 early after MI.148 T cells show evident CD69 expression not only in the peripheral blood but also in the culprit coronary artery plaque.149 This evidence suggests that anti-inflammatory molecules are rapidly stimulated after infarction to prevent excessive inflammation and damage, although little is known about the particular mechanisms underlying these processes.
Myocarditis and dilated cardiomyopathy
Inflammation of the myocardium, or myocarditis, can be caused by infectious or noninfectious triggers. Self-reactive CD4+ T cells are a common feature of different types of myocarditis. These cells recognize cardiac antigens and increase the inflammatory response. If inflammation persists, cardiomyocyte death results in the loss of myocardial tissue and fibrosis, leading to the development of dilated chronic cardiomyopathy.150 Thymic epithelial cells present self-antigens to immature T lymphocytes to deplete the lymphocytes with self-reactive potential. Interestingly, some cardiac antigens, such as alpha myosin heavy chain (αMyHC), are poorly represented in the thymic epithelial cells (TEC) repertoire of autoantigens. As a result, heart-specific autoreactive T cells escape thymic negative selection.151 These naive T cells can recognize cardiac autoantigens presented by dendritic cells in the lymph nodes that drain the heart, but their activation may be dampened by peripheral tolerance mechanisms. Peripheral tolerance includes suppression by Treg cells and immunomodulatory receptors or immune checkpoints. Dysfunction or inhibition of immune checkpoints can cause activation and expansion of these self-reactive T cell clones. In addition, a storm of costimulatory signals occurring in the context of heart infection or tissue damage can contribute to overcoming peripheral tolerance and triggering autoimmune responses to the heart.152 The potential roles of T cell immunoreceptors and their involvement in cardiovascular diseases are outlined in Fig. 2.
Cardiovascular immune-related adverse events (irAEs) of immunomodulatory receptor therapy
Myocarditis is one of the irAEs of ICI therapy for cancer. Patients with ICI-related myocarditis have T cell infiltration of the myocardium usually accompanied by a severe clinical presentation, such as a decreased ejection fraction, cardiogenic shock, severe arrhythmia, and advanced atrioventricular block.153–155 The lack of myocarditis-specific biomarkers makes diagnosis difficult, so only severe cases are usually reported, which could explain the observed low incidence and high mortality. Approximately 0.1–1.1% of patients treated with ICI therapy develop myocarditis, although myocarditis is fulminant in up to 50% of these patients. According to different studies, the time to onset of symptoms of ICI-induced myocarditis varies between 1 and 2.5 months after the start of treatment with an ICI.154,156,157
ICI-induced myocarditis has been described in patients administered an anti-PD-1 antibody, an anti-CTLA-4 antibody or a combination of both,154 although additional studies with larger sample sizes should be performed to attribute specific myocardial adverse effects to each ICI.
Preclinical data indicate critical roles for the PD-1/PD-L1 and CTLA-4 pathways in regulating autoimmune responses to the heart. Depletion of functional PD-1 in BALB/c mice but not in BALB/c Rag2−/− mice leads to the spontaneous development of fatal autoimmune dilated cardiomyopathy,158 suggesting a critical role for PD-1 in the regulation of cardiac autoimmunity mediated by lymphoid cells. Subsequent studies have described the increased susceptibility of PD-1-deficient mice to experimental autoimmune myocarditis, which is established by immunization with an αMyHC peptide.159 Mice lacking CTLA-4 die within the first month from severe lymphoproliferative disease with involvement of the heart and development of fulminant myocarditis.160 In addition, the administration of anti-PD-1 plus anti-CTLA-4 combination therapy to nonhuman primates results in the development of multiple organ toxicities, including myocarditis, with cardiac infiltration of mononuclear cells consisting primarily of T cells and with a composition similar to that observed in patients with ICI-induced myocarditis.161 PD-L1 was first described as highly expressed in the mouse heart.162 In addition, different studies have identified PD-L1 expression in the myocardium of patients with ICI-induced myocarditis.153 Other studies have shown that the cardiac endothelium positively regulates PD-L1 in response to IFNγ as a mechanism of tissue-based tolerance and T cell depletion163 in a negative feedback loop in the heart to maintain resistance to local effector T cell-mediated responses.
The exact mechanism of ICI-induced myocarditis development remains unclear. A suggested explanation is that T cells may recognize homologous antigens in the heart and tumors by a molecular mimicry mechanism.153 Specific muscle antigens, such as troponin and desmin, were found in tumor biopsies, supporting the idea that the same T cell clones can detect antigens present in both the myocardium and tumors. Consistently, myocarditis secondary to treatment with ICIs is more common when there is also autoimmune involvement of the skeletal muscle or myositis.164 As an alternative explanation, T cell clones that recognize different antigens can undergo aberrant activation. As discussed above, some T cell clones that recognize cardiac epitopes escape negative selection in the thymus, so when peripheral tolerance mechanisms are blocked by immunotherapy, the expansion of a heart-specific T cell response can be triggered.151
Although CD69 involvement in human disease remains unexplored, preclinical studies indicate that a lack of other immunomodulatory T cell receptors increases susceptibility to myocarditis and dilated cardiomyopathy. CD69 deficiency leads to exacerbated Th17-mediated autoimmune myocarditis and subsequent cardiomyopathy after immunization with the αMyHC peptide.109 Although LAG-3 deficiency alone does not induce cardiac autoimmunity, depletion of LAG-3 in PD-1-deficient mice increases the susceptibility to spontaneous T cell-mediated fulminant myocarditis.86 Finally, administration of an anti-Tim-3 antibody exacerbates male myocardial inflammation in a mouse model of viral myocarditis.165,166 Taken together, this evidence suggests that multiple inhibitory signals in T cells prevent cardiac autoimmunity.
ICIs in cancer
In recent years, there has been a large increase in the development and implementation of cancer immunotherapies. FDA approval of the use of blocking antibodies specific for CTLA-4 (ipilimumab) or PD-1 (nivolumab) in humans and biologics such as CTLA-4-Ig (abatacept) has been key to producing significant improvements in the treatment of various types of cancer, especially melanoma. Unlike radiotherapy and chemotherapy, which are intended to directly interfere with the growth and survival of tumor cells, immunotherapies target tumors indirectly, favoring an increase in antitumor immune responses that arise spontaneously in many patients. ICI therapy could therefore be used to modulate the immune response to improve the treatment of many types of cancer (Fig. 2).
Cancer mechanisms to bypass the immune system
To understand the modes of action of ICIs, it is important to recognize the dynamic interaction between cancer and the immune system that occurs during the course of the disease. Cancer cells are genetically unstable, which contributes to their uncontrolled proliferation and expression of antigens that the immune system can recognize. These antigens include normal proteins overexpressed by cancer cells and new proteins that are generated by mutation and genetic rearrangement.167 Cytotoxic CD8+ T cells are particularly effective in mediating antitumor immune responses by recognizing tumor-specific antigens presented by MHC I. CD8+ T cells become licensed effector cells after appropriate stimulation by antigen-presenting cells, which collect antigens at the tumor site. In addition to displaying antigenic peptides presented on MHC molecules, antigen-presenting cells must provide costimulatory signals via surface receptors, such as CD28, and cytokines, such as IL-12, for effective stimulation of T cells.168
Tumor cells adopt a variety of mechanisms to prevent immune recognition and immune-mediated destruction. Established tumors arise through the selection of clones that can evade the immune system, a process known as immunoediting.169 Tumor cells can evade immune recognition directly by inhibiting molecules that make them vulnerable, such as tumor antigens or MHC class I.170 Alternatively, tumors can evade immune responses by taking advantage of mechanisms the body has developed to prevent immunopathologies. The mediators involved in these mechanisms include inhibitory cytokines, such as IL-10 and TGF-β; inhibitory cell types, such as Tregs, regulatory B cells, and MDSCs; metabolic modulators, such as indoleamine 2,3-dioxygenase; and immunomodulatory receptors, such as PD-1 and CTLA-4.171,172
Immune exhaustion also contributes to immune dysfunction in cancer. Originally described in the context of chronic viral infection, where the host cannot eliminate the pathogen, it is now evident that exhausted T cells can also promote cancer development.173,174 Under these conditions, the persistent high antigenic load leads T cells to regulate immunomodulatory receptors, whose signaling subsequently leads to a progressive loss of proliferative potential and effector functions and, in some cases, exhausted T cell elimination.175 Therefore, exhaustion is a physiological mechanism designed to limit immunopathology during persistent infection and a major obstacle to antitumor immune responses.176
Anti-CTLA4 treatment
CTLA-4 blockade has been shown to promote T cell activation and intratumoral Treg cell depletion.177 Ipilimumab (IgG1) and tremelimumab (IgG2) are the two human anti-CTLA-4 antibodies that have undergone clinical evaluation. In a 2010 phase III clinical trial, in previously treated patients, treatment with ipilimumab at a dose of 3 mg/kg with or without administration of a gp100 peptide vaccine was evaluated and compared to peptide vaccine administration alone, finding an improvement in overall survival (OS) in both groups of patients who received ipilimumab, which led to the approval of ipilimumab for patients with metastatic melanoma.178 A similar test was performed with a combination of dacarbazine with or without ipilimumab at a dose of 10 mg/kg, which improved survival but increased liver toxicity.179 In addition, a pharmacokinetic analysis showed that ipilimumab had linear pharmacokinetics in the dose range of 3–10 mg/kg.180 The efficacy of ipilimumab was validated in a randomized, double-blind, phase III trial after complete resection of high-risk stage III melanoma.181 Studies in other types of cancer have shown the efficacy of ipilimumab in some renal cell carcinoma patients, including patients who did not respond to other immunotherapies.182 In patients with B cell lymphoma, CTLA-4 blockade with ipilimumab had antitumor activity and was well tolerated at doses of 1 and 3 mg/kg;183 however, a phase III trial did not show a significant difference in terms of survival between the ipilimumab group and the placebo group in patients with castration-resistant metastatic prostate cancer.184 Tremelimumab, an alternative antibody that blocks CTLA-4, continues to be investigated in clinical trials and has also demonstrated long-lasting responses and acceptable tolerability in patients with melanoma,185 refractory metastatic colorectal cancer,186 hepatocellular carcinoma,187 or malignant mesothelioma.188
Anti-PD1 treatment
PD-1 regulates the activation of T cells in peripheral tissues and is expressed in activated T cells, Treg cells, activated B cells, and NK cells. The endogenous PD1 ligands, PD-L1 and PD-L2, are expressed in activated immune cells and nonhematopoietic cells, including tumor cells; tumor cell expression is the mechanism by which these cells circumvent the immune system189. Inhibition of these interactions with therapeutic antibodies improves the T cell response and stimulates antitumor activity.162
The first anti-PD-1 inhibitor evaluated was nivolumab (BMS-936558), a human mAb (IgG4) that blocks the immunomodulatory receptor PD1.190
A phase I trial in which different doses were tested found that nivolumab is safe and produces positive responses in 16–31% of pretreated patients across all types of solid tumors tested, with a duration of at least 1 year.191 For ipilimumab, both adverse effects and efficacy depend on the dose, but for nivolumab, these correlation are not observed, which can be explained by the high correlation between the receptor and antibody even at low doses.192
Pembrolizumab (MK-3475) is an alternative humanized high-affinity IgG4 antibody recognizing PD1. It has been evaluated in several phase I clinical trials, and three different doses have been tested in patients with metastatic melanoma who were never previously treated and in a cohort patients including patients previously treated with ipilimumab and those who were not previously treated with ipilimumab. Most of the adverse effects observed were seen with the highest dose, and no significant differences among the three treatment subgroups were, but patients with a smaller tumor burden had a greater capacity to respond to pembrolizumab.193
Pidilizumab (formerly CT-011) is a humanized anti-PD-1 IgG1 antibody. It was one of the first anti-PD-1 agents used in cancer patients. Several clinical trials at different stages, which treated patients with hematological malignancies or metastatic melanoma, have shown that treatment is generally well tolerated, with no treatment-related deaths, but in some patients, previous treatment with ipilimumab did not produce an advantage in treatment response. Pidilizumab appears to be associated with lower response rates in melanoma than nivolumab or pembrolizumab, but the 1-year OS rate is similar to that reported in studies of nivolumab.193,194
Anti-PD-L1 treatment
MPDL-3280A is an anti-human IgG1 antibody engineered to block PD-L1, with the Fc domain of IgG1 mutated to completely nullify the effects of antibody-dependent cellular cytotoxicity and complement-dependent toxicity. Furthermore, PD-L1 is particularly expressed in tumors infiltrated by immune cells, which implies that this treatment mainly benefits patients with more inflamed tumors.193 This antibody exerts its mechanism of action by blocking PD-1/PD-L1 signaling, and unlike antibodies that block PD-1, those that block PD-L1 avoid the side effects that result from blocking the interaction of PD1 with PD-L2 and that of PD-L1 with CD80. MPDL3280A has been evaluated in multiple tumor types, with safety and preliminary efficacy identified in melanoma, renal cell carcinoma, non-small cell lung carcinoma, colorectal cancer, gastric cancer, and squamous cell carcinoma of the head/neck. Other PD-L1-inhibiting antibodies are BMS-936559, which has been shown to be safe and clinically active in various types of tumors in phase I clinical trials, and MEDI-4736, which is currently in clinical development.191
Combination therapies
Blocking immune checkpoint molecules with multiple inhibitory antibodies can improve treatment efficacy. For example, for ipilimumab, the percentage of patients with a reduction in tumor size or the ORR (objective response ratio) achieved with monotherapy was 6%, and the OS time was 10 months.178 In phase I work, nivolumab had an ORR of 31%, and the OS time was 17 months. However, in a phase I trial combining ipilimumab with nivolumab, the ORR was 53% at the maximum tolerated dose, and all subjects who responded to treatment showed a ≥80% decrease in their tumor burden after 12 weeks of treatment.195 Patients treated with this combination therapy showed a more frequent rate (53%) of adverse effects. This combination, and other combinations of mAbs, should be confirmed in other phase III clinical trials, which are currently underway.191 Combination therapy with other noninhibitory immune checkpoint agents is also being evaluated. The feasibility of other combination therapy strategies is being explored in different trials, each with compelling preclinical justification. The most direct combination therapy approach would be a combination of immunotherapy and chemotherapy, as there is a multitude of preliminary data from patients who have been included in clinical trials evaluating immunoregulators after an insufficient response to conventional chemotherapy; in particular, there are treatments that combine ipilimumab with cycles of chemotherapy with carboplatin, etoposide or paclitaxel in patients with lung cancer196–198 and advanced melanoma.199
The combination of ICIs with alternative treatments that favor systemic stimulation of the immune system, such as vaccines, cytokine therapy, and adoptive cell therapy,200–202 would be an interesting approach. The combination of immunomodulatory agents with agents producing a local effect that causes tumor regression, such as radiotherapy, is based on the abscopal effect, which describes the phenomenon of tumor regression outside the irradiated field caused by the release of cytokines and antigens induced by irradiation that enhances the systemic immune response against the tumor. The combination of radiotherapy with ipilimumab has been evaluated in phase I/II trials in prostate cancer, which showed an improvement in treatment response with tolerable adverse effects.203 Similar to the combination with radiotherapy, other combinations are being investigated with different alternatives strategies for local control, such as cryoablation and electrochemotherapy. The abscopal effect is also observed after treatment with cytotoxic therapies, such as B-Raf protein or VEGF inhibitors, capable of stimulating the immune system by favoring the production of antigens.202,204 Some of these studies, such as one combining ipilimumab with GM-CSF, showed an improved survival rate with few side effects for the patients treated with the combination therapy compared to those treated only with ipilimumab. The combination of ipilimumab with bevacizumab, a VEGF-blocking antibody, improves the immune responses against tumors with tolerable toxicity. However, some combined therapies, such as treatment with vemurafenib and ipilimumab, produce high liver toxicity.205 In summary, the use of combined therapies seems to be a step forward in the search for a cure for cancer, but more studies are needed to adjust the doses very precisely and reduce adverse effects.
The dual blockade of LAG-3/PD-1 was recently identified to be clinically relevant and is being tested in clinical trials. A recombinant fusion protein with four extracellular domains of LAG-3 has been tested safely in combination with pembrolizumab or nivolumab in patients with melanoma with progressive disease after immunotherapy.206 Similarly, the combination of anti-VISTA molecules with anti-PD-1 therapy is under investigation. Combination of CA-170, an oral inhibitor of VISTA, with PD-1 has shown positive results in different clinical trials.207
ICI-based therapies have been able to increase the average life expectancy of cancer patients, yet mortality remains high among patients with advanced-stage disease, highlighting the need for further innovation in the field. These therapies appear to be most effective in patients with pre-existing antitumor immunity, suggesting that, in patients without such immunity, these drugs cannot mediate de novo antitumor immune responses. It would therefore be interesting to implement de novo generation of antitumor immunity with these treatments. To achieve this objective, it is essential to improve the understanding of the mechanisms of action of these treatments to identify ways to improve their use not only through specific guidance in certain types of patients who are more likely to respond but also through a correct combination with other therapies that will increase the responses of patients in whom these treatments are less efficient. Furthermore, as the understanding of the mechanisms of action of these therapies improves, it will also be easier to prevent possible adverse effects caused by these therapies.
ICIs and irAEs
Although there is no doubt that treatments targeting these immunomodulatory T cell receptors have created a new era in cancer treatment and increased patient survival, different adverse events related to the immune system have been observed. These adverse events are called irAEs and are directly related to the mechanisms of action of PD-1 and CTLA-4.139 Under physiological conditions, these molecules prevent autoimmunity and limit activation of the immune system, but inhibition of these two receptors as a cancer therapy causes a wide range of side effects that resemble autoimmune reactions. Analysis of sera and biopsies from patients with irAEs has revealed that these reactions are mediated by activated CD4+ and CD8+ T cells infiltrating tissues, as well as by increases in the levels of proinflammatory cytokines.208 IrAEs can impact multiple organs and systems, including the skin, gastrointestinal tract, liver, endocrine system, eyes, kidneys, nervous system, pancreas, and heart. Almost all patients treated with ICIs experience mild side effects, such as diarrhea, fatigue, itching, rash, nausea, and decreased appetite. Apart from myocarditis mentioned earlier in this review, serious adverse reactions include severe diarrhea, colitis, increased alanine aminotransferase levels, inflammatory pneumonitis, and interstitial nephritis.209 Patients who experience exacerbation of pre-existing autoimmune conditions, such as psoriasis,210 or who develop new autoimmune conditions, such as T1D mellitus,211 have also been reported. Particularly serious side effects may require cessation of treatment, although these patients may still respond thereafter.
Anti-CTLA-4 antibody-induced IrAEs are common but generally low grade, although severe and life-threatening cases have also been reported. The skin and gastrointestinal tract are more frequently affected, while hepatic, endocrine, and neurological events are less common.212 Rash is generally the first irAE to manifest after the first or second dose of ipilimumab.213 Colitis and diarrhea are the most common gastrointestinal IrAEs after the first few months of ipilimumab treatment, which can be very serious, particularly in rare cases where perforation of the gastrointestinal tract occurs. A phase III trial in patients with advanced melanoma demonstrated that ipilimumab adverse events were mild, with sporadic life-threatening cases.214 Anti-CTLA-4 antibodies can induce a severe and extensive form of inflammatory bowel disease, suggesting the need to avoid nonsteroidal anti-inflammatory drugs in patients treated with anti-CTLA-4 therapy.215 Hepatic irAEs are rare and may manifest as an acute hepatitis or biliary pattern, which can be reversed with corticosteroids.216 In addition, autoimmune hypophysitis has been reported in up to 17% of patients with melanoma or renal cell carcinoma treated with anti-CTLA-4 therapy, which may be related to pituitary gland enlargement and hormonal deficiencies.217 The risk of irAEs in the case of ipilimumab treatment depends on the dose, and the mean time to onset of adverse effects is 10 weeks.218
In anti-PD-1/PD-L1 treatment, 30–40% of patients experience skin toxicities.219 The most common skin toxicities are lichenoid reactions, vitiligo, pruritus, and eczema. Vitiligo is observed only in patients with metastatic melanoma. Antibodies targeting the PD-1/PD-L1 axis have shown a favorable toxicity profile in preliminary trials with rates of relatively severe adverse events of 13% in patients receiving MK-3475, 9% in patients receiving BMS-936559, and 14% in patients receiving nivolumab. A unique and life-threatening toxicity for these agents is pneumonitis, which was the cause of death in three patients (1%) in a phase I clinical trial of nivolumab despite corticosteroid treatment.219 Cardiac irAEs due to PD-1 axis blockade have attracted attention due to the high mortality rates but have an incidence of less than 1%. Endocrinopathies derived from PD1 axis blockade include hypophysitis, thyroiditis, hypothyroidism, hyperthyroidism, and T1DM. In addition to the previously described pathologies, there are some rare ICI-mediated toxicities that have neurological, ocular, or hematological manifestations.
The incidence of adverse effects is higher in patients treated with combination therapies, who are more susceptible to acute kidney injury than those treated with ipilimumab, nivolumab, or pembrolizumab monotherapy. Up to 7% of people treated with anti-PD-1 monotherapy and up to 33% of patients treated with combination anti-PD-1 and anti-CTLA-4 therapy develop hepatitis.220 Combined treatment with these ICIs causes a decrease and blockade in Treg cell function, which also produces other pathologies that are included within the irAEs, such as increases in the incidence of proatherogenic lesions or pneumonitis. Another event considered an irAE is the generation of autoantibodies, as occurs in thyroiditis, since treatment with PD1 inhibitors can promote the mobilization of pre-existing autoantibodies against the thyroid.220 The study of the pathophysiology of these adverse effects and toxicities should produce a more precise understanding of the functions of ICIs in the tumor and tissue contexts and thus favor the improvement of therapies, with avoidance of possible adverse effects.
Autoimmune diabetes mellitus is related to blockade of CTLA-4, PD-1, its ligand (PD-L1), and combination ICI therapy.
Recently, it has been demonstrated that a constitutive reduction in CTLA-4 signaling does not accelerate SLE in a susceptible NZM2328 animal model.221 Although a patient with de novo SLE nephritis after ipilimumab treatment was reported,222 SLE patients treated with ICIs do not seem to exhibit disease worsening.223 De novo cases of SLE have yet to be reported in patients treated with other ICIs.
In general, the knowledge of possible biomarkers in the field of immunotherapy is very limited in aspects related to predicting the efficiency and suitability of different treatments. The field of biomarkers for predicting possible adverse effects induced by immunotherapy is even less investigated. Therefore, the development of the field of biomarkers to cover the negative aspects of immunotherapy and potentially predictand the suitability of treatments will be very important.
Concluding remarks and future directions
T cells are key in the development and control of inflammatory diseases of autoimmune origin or cancer; very recently, it has been shown that they also play key roles in pathologies such as acute MI, atherosclerosis, and myocarditis, highlighting the relevance of T cells in cardiovascular disease.224 Different molecular tools blocking immunomodulatory receptors on T cells have emerged as some of the most promising cancer therapies in the last decade. However, the potential of targeting immunomodulatory T cell receptors in other diseases, such as autoimmunity or cardiovascular disease, which is the leading cause of death worldwide,225 has been little explored. In addition to the most well-known receptors in clinical practice, PD-1 and CTLA-4, there is a battery of immunomodulatory receptors whose immunoregulatory properties have been described more recently, but their therapeutic potential in clinical practice remains unexplored. In contrast, other T cell receptors, such as CD69, have roles in the development of autoimmunity and in cardiovascular disease in mice and humans, and blockade of this receptor has antitumor properties;226 however, its role in human cancer has never been addressed.227 In this review article, we have summarized the mechanisms of action of these immunomodulatory receptors expressed by T cells, paying special attention to the less explored receptors, including their potential development in the clinic for treatment of cancer, cardiovascular disease, or autoimmune diseases, such as diabetes, lupus, or RA.
The use of inhibitory drugs and mAbs against these immunomodulatory receptors can be equally effective in cancer and autoimmune diseases. An example is abatacept (CTLA-4-Ig), which is used mainly in melanoma, but recent clinical trials show that it has potential in the treatment of autoimmune diseases, such as RA, in patients refractory to treatment with TNF-α blockers, indicating the potential of immunomodulatory receptor-based therapies in several pathologies that have not yet been explored. In this regard, the integration of technologies to deliver and control drug or mAb release has become one of the more important challenges in the field of immunotherapy. The strategies to deliver these drugs to the target organ are almost as important as the mechanism of action. For this reason, research in nanotechnology, one of the known strategies to improve the nanodelivery of chemotherapeutic agents, is becoming of interest in the field of immunotherapy.228 In addition, the development of cellular therapies (e.g., CAR T cells) as targeted vehicles for use with regulatory molecules and/or combination with immunotherapy is a trend that we will see developed in the coming years and that, hopefully, will further improve the therapeutic potential not only in cancer but also in autoimmune and cardiovascular diseases.229 In parallel, we need to advance the knowledge on the molecular and cellular mechanisms triggered by these receptors in each tissue, especially to prevent the triggering of adverse immune effects associated with these therapies in the most vulnerable tissues.
The field of biomedicine faces a challenge in the coming years, in which the study of the immunomodulatory receptors of T cells in the context of numerous pathologies can represent a great step forward in the treatment of fatal or chronic diseases that are highly prevalent in the world and lack cures. The enormous success of targeting the immune checkpoints PD-1 and CTLA4 in cancer should be enough to encourage the scientific community to study the other immunomodulatory T cell receptors in depth in the coming years and bring therapies related to these receptors closer to clinical practice in autoimmune and cardiovascular diseases.
Acknowledgements
This study was supported by competitive grants from the Ministerio de Ciencia, Innovación y Universidades, through the Carlos III Institute of Health-Fondo de Investigación Sanitaria (PI19/00545) to P.M.; CIBER Cardiovascular (Fondo de Investigación Sanitaria del Instituto de Salud Carlos III and cofunding by Fondo Europeo de Desarrollo Regional FEDER) to P.M.; and CAM (S2017/BMD-3671-INFLAMUNE-CM) from the Comunidad de Madrid to P.M. The CNIC is supported by the Instituto de Salud Carlos III (ISCIII), the Ministerio de Ciencia e Innovación and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505). R.B.-D. is supported by the Formación de Profesorado Universitario (FPU16/02780) program of the Spanish Ministry of Education, Culture and Sports.
Competing interests
The authors declare no competing interests.
References
- 1.Boardman DA, Levings MK. Cancer immunotherapies repurposed for use in autoimmunity. Nat. Biomed. Eng. 2019;3:259–263. doi: 10.1038/s41551-019-0359-6. [DOI] [PubMed] [Google Scholar]
- 2.Lutgens E, et al. Immunotherapy for cardiovascular disease. Eur. Heart J. 2019;40:3937–3946. doi: 10.1093/eurheartj/ehz283. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KA, et al. Immunomodulatory receptors are differentially expressed in B and T cell subsets relevant to autoimmune disease. Clin. Immunol. 2019;209:108276. doi: 10.1016/j.clim.2019.108276. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Amaro R, Cortes JR, Sanchez-Madrid F, Martin P. Is CD69 an effective brake to control inflammatory diseases? Trends Mol. Med. 2013;19:625–632. doi: 10.1016/j.molmed.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe N, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 6.Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018;18:153–167. doi: 10.1038/nri.2017.108. [DOI] [PubMed] [Google Scholar]
- 7.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, et al. Cutting edge: a critical role of B and T lymphocyte attenuator in peripheral T cell tolerance induction. J. Immunol. 2009;182:4516–4520. doi: 10.4049/jimmunol.0803161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sedy JR, et al. B and T lymphocyte attenuator regulates T cell activation through interaction with herpesvirus entry mediator. Nat. Immunol. 2005;6:90–98. doi: 10.1038/ni1144. [DOI] [PubMed] [Google Scholar]
- 11.Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4- and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. Eur. J. Immunol. 1995;25:2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- 12.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu C, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 14.Huang YH, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harjunpaa H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin. Exp. Immunol. 2020;200:108–119. doi: 10.1111/cei.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reches, A. et al. Nectin4 is a novel TIGIT ligand which combines checkpoint inhibition and tumor specificity. J. Immunother. Cancer8, 10.1136/jitc-2019-000266 (2020). [DOI] [PMC free article] [PubMed]
- 17.Yu X, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 18.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn SD, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowak EC, et al. Immunoregulatory functions of VISTA. Immunol. Rev. 2017;276:66–79. doi: 10.1111/imr.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J. Exp. Med. 2011;208:577–592. doi: 10.1084/jem.20100619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones NH, et al. Isolation of complementary DNA clones encoding the human lymphocyte glycoprotein T1/Leu-1. Nature. 1986;323:346–349. doi: 10.1038/323346a0. [DOI] [PubMed] [Google Scholar]
- 23.Huang HJ, Jones NH, Strominger JL, Herzenberg LA. Molecular cloning of Ly-1, a membrane glycoprotein of mouse T lymphocytes and a subset of B cells: molecular homology to its human counterpart Leu-1/T1 (CD5) Proc. Natl Acad. Sci. USA. 1987;84:204–208. doi: 10.1073/pnas.84.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brossard C, Semichon M, Trautmann A, Bismuth G. CD5 inhibits signaling at the immunological synapse without impairing its formation. J. Immunol. 2003;170:4623–4629. doi: 10.4049/jimmunol.170.9.4623. [DOI] [PubMed] [Google Scholar]
- 25.Tabbekh M, Mokrani-Hammani M, Bismuth G, Mami-Chouaib F. T-cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology. 2013;2:e22841. doi: 10.4161/onci.22841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sancho D, Gomez M, Sanchez-Madrid F. CD69 is an immunoregulatory molecule induced following activation. Trends Immunol. 2005;26:136–140. doi: 10.1016/j.it.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Cabrera M, et al. Molecular cloning, expression, and chromosomal localization of the human earliest lymphocyte activation antigen AIM/CD69, a new member of the C-type animal lectin superfamily of signal-transmitting receptors. J. Exp. Med. 1993;178:537–547. doi: 10.1084/jem.178.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin P, Sanchez-Madrid F. CD69: an unexpected regulator of TH17 cell-driven inflammatory responses. Sci. Signal. 2011;4:pe14. doi: 10.1126/scisignal.2001825. [DOI] [PubMed] [Google Scholar]
- 29.Martin P, et al. CD69 association with Jak3/Stat5 proteins regulates Th17 cell differentiation. Mol. Cell Biol. 2010;30:4877–4889. doi: 10.1128/MCB.00456-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Fuente H, et al. The leukocyte activation receptor CD69 controls T cell differentiation through its interaction with galectin-1. Mol. Cell Biol. 2014;34:2479–2487. doi: 10.1128/MCB.00348-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin CR, et al. Glycosylation-dependent interaction between CD69 and S100A8/S100A9 complex is required for regulatory T-cell differentiation. FASEB J. 2015;29:5006–5017. doi: 10.1096/fj.15-273987. [DOI] [PubMed] [Google Scholar]
- 32.Tsilingiri K, et al. Oxidized low-density lipoprotein receptor in lymphocytes prevents atherosclerosis and predicts subclinical disease. Circulation. 2019;139:243–255. doi: 10.1161/CIRCULATIONAHA.118.034326. [DOI] [PubMed] [Google Scholar]
- 33.Cibrian D, et al. CD69 controls the uptake of L-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 2016;17:985–996. doi: 10.1038/ni.3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosenblum MD, Remedios KA, Abbas AK. Mechanisms of human autoimmunity. J. Clin. Invest. 2015;125:2228–2233. doi: 10.1172/JCI78088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishimura H, Honjo T, Minato N. Facilitation of beta selection and modification of positive selection in the thymus of PD-1-deficient mice. J. Exp. Med. 2000;191:891–898. doi: 10.1084/jem.191.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 38.Kasagi S, et al. Anti-programmed cell death 1 antibody reduces CD4+PD-1+ T cells and relieves the lupus-like nephritis of NZB/W F1 mice. J. Immunol. 2010;184:2337–2347. doi: 10.4049/jimmunol.0901652. [DOI] [PubMed] [Google Scholar]
- 39.Wong M, La Cava A, Singh RP, Hahn BH. Blockade of programmed death-1 in young (New Zealand black x New Zealand white)F1 mice promotes the activity of suppressive CD8+ T cells that protect from lupus-like disease. J. Immunol. 2010;185:6563–6571. doi: 10.4049/jimmunol.0903401. [DOI] [PubMed] [Google Scholar]
- 40.Salama AD, et al. Critical role of the programmed death-1 (PD-1) pathway in regulation of experimental autoimmune encephalomyelitis. J. Exp. Med. 2003;198:71–78. doi: 10.1084/jem.20022119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ansari MJ, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J. Exp. Med. 2003;198:63–69. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kristjansdottir H, et al. Lower expression levels of the programmed death 1 receptor on CD4+CD25+ T cells and correlation with the PD-1.3A genotype in patients with systemic lupus erythematosus. Arthritis Rheum. 2010;62:1702–1711. doi: 10.1002/art.27417. [DOI] [PubMed] [Google Scholar]
- 44.Bertsias GK, et al. Genetic, immunologic, and immunohistochemical analysis of the programmed death 1/programmed death ligand 1 pathway in human systemic lupus erythematosus. Arthritis Rheum. 2009;60:207–218. doi: 10.1002/art.24227. [DOI] [PubMed] [Google Scholar]
- 45.Shi H, et al. Elevated serum autoantibodies against co-inhibitory PD-1 facilitate T cell proliferation and correlate with disease activity in new-onset systemic lupus erythematosus patients. Arthritis Res. Ther. 2017;19:52. doi: 10.1186/s13075-017-1258-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiao Q, et al. Upregulated PD-1 expression is associated with the development of systemic lupus erythematosus, but not the PD-1.1 allele of the PDCD1 gene. Int J. Genomics. 2014;2014:950903. doi: 10.1155/2014/950903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 48.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 49.Stohl W, et al. Global T cell dysregulation in non-autoimmune-prone mice promotes rapid development of BAFF-independent, systemic lupus erythematosus-like autoimmunity. J. Immunol. 2008;181:833–841. doi: 10.4049/jimmunol.181.1.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barreto M, et al. Evidence for CTLA4 as a susceptibility gene for systemic lupus erythematosus. Eur. J. Hum. Genet. 2004;12:620–626. doi: 10.1038/sj.ejhg.5201214. [DOI] [PubMed] [Google Scholar]
- 51.Ahmed S, et al. Association of CTLA-4 but not CD28 gene polymorphisms with systemic lupus erythematosus in the Japanese population. Rheumatology. 2001;40:662–667. doi: 10.1093/rheumatology/40.6.662. [DOI] [PubMed] [Google Scholar]
- 52.Hudson LL, Rocca K, Song YW, Pandey JP. CTLA-4 gene polymorphisms in systemic lupus erythematosus: a highly significant association with a determinant in the promoter region. Hum. Genet. 2002;111:452–455. doi: 10.1007/s00439-002-0807-2. [DOI] [PubMed] [Google Scholar]
- 53.Jury EC, et al. Abnormal CTLA-4 function in T cells from patients with systemic lupus erythematosus. Eur. J. Immunol. 2010;40:569–578. doi: 10.1002/eji.200939781. [DOI] [PubMed] [Google Scholar]
- 54.Dahal LN, et al. Immunoregulatory soluble CTLA-4 modifies effector T-cell responses in systemic lupus erythematosus. Arthritis Res. Ther. 2016;18:180. doi: 10.1186/s13075-016-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Merrill JT, et al. The efficacy and safety of abatacept in patients with non-life-threatening manifestations of systemic lupus erythematosus: results of a twelve-month, multicenter, exploratory, phase IIb, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62:3077–3087. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 56.Furie R, et al. Efficacy and safety of abatacept in lupus nephritis: a twelve-month, randomized, double-blind study. Arthritis Rheumatol. 2014;66:379–389. doi: 10.1002/art.38260. [DOI] [PubMed] [Google Scholar]
- 57.Oster, C. et al. BTLA expression on Th1, Th2 and Th17 effector T-cells of patients with systemic lupus erythematosus is associated with active disease. Int. J. Mol. Sci.20, 10.3390/ijms20184505 (2019). [DOI] [PMC free article] [PubMed]
- 58.Sawaf, M. et al. Defective BTLA functionality is rescued by restoring lipid metabolism in lupus CD4+ T cells. JCI Insight3, 10.1172/jci.insight.99711 (2018). [DOI] [PMC free article] [PubMed]
- 59.Vendel AC, et al. B and T lymphocyte attenuator regulates B cell receptor signaling by targeting Syk and BLNK. J. Immunol. 2009;182:1509–1517. doi: 10.4049/jimmunol.182.3.1509. [DOI] [PubMed] [Google Scholar]
- 60.Otsuki N, Kamimura Y, Hashiguchi M, Azuma M. Expression and function of the B and T lymphocyte attenuator (BTLA/CD272) on human T cells. Biochem. Biophys. Res. Commun. 2006;344:1121–1127. doi: 10.1016/j.bbrc.2006.03.242. [DOI] [PubMed] [Google Scholar]
- 61.Lauzurica P, et al. Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood. 2000;95:2312–2320. [PubMed] [Google Scholar]
- 62.Sanchez-Diaz, R. et al. Thymus-derived regulatory T cell development is regulated by C-type lectin-mediated BIC/MicroRNA 155 expression. Mol. Cell. Biol.37, 10.1128/MCB.00341-16 (2017). [DOI] [PMC free article] [PubMed]
- 63.Cortes JR, et al. Maintenance of immune tolerance by Foxp3+ regulatory T cells requires CD69 expression. J. Autoimmun. 2014;55:51–62. doi: 10.1016/j.jaut.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radulovic K, et al. CD69 regulates type I IFN-induced tolerogenic signals to mucosal CD4 T cells that attenuate their colitogenic potential. J. Immunol. 2012;188:2001–2013. doi: 10.4049/jimmunol.1100765. [DOI] [PubMed] [Google Scholar]
- 65.Yu L, et al. CD69 enhances immunosuppressive function of regulatory T-cells and attenuates colitis by prompting IL-10 production. Cell Death Dis. 2018;9:905. doi: 10.1038/s41419-018-0927-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ishikawa S, et al. A subset of CD4+ T cells expressing early activation antigen CD69 in murine lupus: possible abnormal regulatory role for cytokine imbalance. J. Immunol. 1998;161:1267–1273. [PubMed] [Google Scholar]
- 67.Peixoto TV, et al. CD4(+)CD69(+) T cells and CD4(+)CD25(+)FoxP3(+) Treg cells imbalance in peripheral blood, spleen and peritoneal lavage from pristane-induced systemic lupus erythematosus (SLE) mice. Adv. Rheumatol. 2019;59:30. doi: 10.1186/s42358-019-0072-x. [DOI] [PubMed] [Google Scholar]
- 68.Su CC, Shau WY, Wang CR, Chuang CY, Chen CY. CD69 to CD3 ratio of peripheral blood mononuclear cells as a marker to monitor systemic lupus erythematosus disease activity. Lupus. 1997;6:449–454. doi: 10.1177/096120339700600507. [DOI] [PubMed] [Google Scholar]
- 69.Portales-Perez D, Gonzalez-Amaro R, Abud-Mendoza C, Sanchez-Armass S. Abnormalities in CD69 expression, cytosolic pH and Ca2+ during activation of lymphocytes from patients with systemic lupus erythematosus. Lupus. 1997;6:48–56. doi: 10.1177/096120339700600107. [DOI] [PubMed] [Google Scholar]
- 70.Vitales-Noyola M, et al. Patients with systemic lupus erythematosus show increased levels and defective function of CD69(+) T regulatory cells. Mediators Inflamm. 2017;2017:2513829. doi: 10.1155/2017/2513829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Han Y, Guo Q, Zhang M, Chen Z, Cao X. CD69+ CD4+ CD25- T cells, a new subset of regulatory T cells, suppress T cell proliferation through membrane-bound TGF-beta 1. J. Immunol. 2009;182:111–120. doi: 10.4049/jimmunol.182.1.111. [DOI] [PubMed] [Google Scholar]
- 72.Crispin JC, Martinez A, de Pablo P, Velasquillo C, Alcocer-Varela J. Participation of the CD69 antigen in the T-cell activation process of patients with systemic lupus erythematosus. Scand. J. Immunol. 1998;48:196–200. doi: 10.1046/j.1365-3083.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 73.Vitales-Noyola M, et al. Quantitative and functional analysis of CD69(+) NKG2D(+) T regulatory cells in healthy subjects. Hum. Immunol. 2015;76:511–518. doi: 10.1016/j.humimm.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Rodriguez-Munoz A, et al. Levels of regulatory T cells CD69(+)NKG2D(+)IL-10(+) are increased in patients with autoimmune thyroid disorders. Endocrine. 2016;51:478–489. doi: 10.1007/s12020-015-0662-2. [DOI] [PubMed] [Google Scholar]
- 75.Vitales-Noyola M, et al. Quantitative and functional analysis of CD69(+) T regulatory lymphocytes in patients with periodontal disease. J. Oral. Pathol. Med. 2017;46:549–557. doi: 10.1111/jop.12514. [DOI] [PubMed] [Google Scholar]
- 76.Tsujimura S, Adachi T, Saito K, Tanaka Y. Role of P-glycoprotein on CD69(+)CD4(+) cells in the pathogenesis of proliferative lupus nephritis and non-responsiveness to immunosuppressive therapy. RMD Open. 2017;3:e000423. doi: 10.1136/rmdopen-2016-000423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.El Khatib MM, et al. beta-Cell-targeted blockage of PD1 and CTLA4 pathways prevents development of autoimmune diabetes and acute allogeneic islets rejection. Gene Ther. 2015;22:430–438. doi: 10.1038/gt.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li R, et al. PD-L1-driven tolerance protects neurogenin3-induced islet neogenesis to reverse established type 1 diabetes in NOD mice. Diabetes. 2015;64:529–540. doi: 10.2337/db13-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sun P, et al. Unlike PD-L1, PD-1 is downregulated on partial immune cells in type 2 diabetes. J. Diabetes Res. 2019;2019:5035261. doi: 10.1155/2019/5035261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colli ML, et al. PDL1 is expressed in the islets of people with type 1 diabetes and is up-regulated by interferons-alpha and-gamma via IRF1 induction. EBioMedicine. 2018;36:367–375. doi: 10.1016/j.ebiom.2018.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Osum KC, et al. Interferon-gamma drives programmed death-ligand 1 expression on islet beta cells to limit T cell function during autoimmune diabetes. Sci. Rep. 2018;8:8295. doi: 10.1038/s41598-018-26471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lenschow DJ, et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 1995;181:1145–1155. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Truong W, et al. BTLA targeting modulates lymphocyte phenotype, function, and numbers and attenuates disease in nonobese diabetic mice. J. Leukoc. Biol. 2009;86:41–51. doi: 10.1189/jlb.1107753. [DOI] [PubMed] [Google Scholar]
- 84.Bekiaris V, Sedy JR, Macauley MG, Rhode-Kurnow A, Ware CF. The inhibitory receptor BTLA controls gammadelta T cell homeostasis and inflammatory responses. Immunity. 2013;39:1082–1094. doi: 10.1016/j.immuni.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bettini M, et al. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. J. Immunol. 2011;187:3493–3498. doi: 10.4049/jimmunol.1100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okazaki T, et al. PD-1 and LAG-3 inhibitory co-receptors act synergistically to prevent autoimmunity in mice. J. Exp. Med. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhang, Q. et al. LAG3 limits regulatory T cell proliferation and function in autoimmune diabetes. Sci. Immunol.2, 10.1126/sciimmunol.aah4569 (2017). [DOI] [PMC free article] [PubMed]
- 88.Fuhrman CA, et al. Divergent phenotypes of human regulatory T cells expressing the receptors TIGIT and CD226. J. Immunol. 2015;195:145–155. doi: 10.4049/jimmunol.1402381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diarte-Anazco, E. M. G. et al. Novel insights into the role of HDL-associated sphingosine-1-phosphate in cardiometabolic diseases. Int. J. Mol. Sci.20, 10.3390/ijms20246273 (2019). [DOI] [PMC free article] [PubMed]
- 90.Srinivasan S, et al. Sphingosine-1-phosphate reduces CD4+ T-cell activation in type 1 diabetes through regulation of hypoxia-inducible factor short isoform I.1 and CD69. Diabetes. 2008;57:484–493. doi: 10.2337/db07-0855. [DOI] [PubMed] [Google Scholar]
- 91.Klocke K, Sakaguchi S, Holmdahl R, Wing K. Induction of autoimmune disease by deletion of CTLA-4 in mice in adulthood. Proc. Natl Acad. Sci. USA. 2016;113:E2383–E2392. doi: 10.1073/pnas.1603892113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Verma N, Burns SO, Walker LSK, Sansom DM. Immune deficiency and autoimmunity in patients with CTLA-4 (CD152) mutations. Clin. Exp. Immunol. 2017;190:1–7. doi: 10.1111/cei.12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuehn HS, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345:1623–1627. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lin J, et al. TNFalpha blockade in human diseases: an overview of efficacy and safety. Clin. Immunol. 2008;126:13–30. doi: 10.1016/j.clim.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Genant HK, et al. Abatacept inhibits progression of structural damage in rheumatoid arthritis: results from the long-term extension of the AIM trial. Ann. Rheum. Dis. 2008;67:1084–1089. doi: 10.1136/ard.2007.085084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ruperto N, et al. Abatacept in children with juvenile idiopathic arthritis: a randomised, double-blind, placebo-controlled withdrawal trial. Lancet. 2008;372:383–391. doi: 10.1016/S0140-6736(08)60998-8. [DOI] [PubMed] [Google Scholar]
- 97.Larsen CP, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am. J. Transpl. 2005;5:443–453. doi: 10.1111/j.1600-6143.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 98.Oki M, et al. A functional polymorphism in B and T lymphocyte attenuator is associated with susceptibility to rheumatoid arthritis. Clin. Dev. Immunol. 2011;2011:305656. doi: 10.1155/2011/305656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang B, et al. The expression of BTLA was increased and the expression of HVEM and LIGHT were decreased in the T cells of patients with rheumatoid arthritis [corrected] PLoS ONE. 2016;11:e0155345. doi: 10.1371/journal.pone.0155345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nakachi S, et al. Interleukin-10-producing LAG3(+) regulatory T cells are associated with disease activity and abatacept treatment in rheumatoid arthritis. Arthritis Res. Ther. 2017;19:97. doi: 10.1186/s13075-017-1309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen SY, Hsu WT, Chen YL, Chien CH, Chiang BL. Lymphocyte-activation gene 3(+) (LAG3(+)) forkhead box protein 3(-) (FOXP3(-)) regulatory T cells induced by B cells alleviates joint inflammation in collagen-induced arthritis. J. Autoimmun. 2016;68:75–85. doi: 10.1016/j.jaut.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 102.Lee J, et al. Expression of human TIM-3 and its correlation with disease activity in rheumatoid arthritis. Scand. J. Rheumatol. 2011;40:334–340. doi: 10.3109/03009742.2010.547871. [DOI] [PubMed] [Google Scholar]
- 103.Koohini Z, et al. Analysis of PD-1 and Tim-3 expression on CD4(+) T cells of patients with rheumatoid arthritis; negative association with DAS28. Clin. Rheumatol. 2018;37:2063–2071. doi: 10.1007/s10067-018-4076-4. [DOI] [PubMed] [Google Scholar]
- 104.Zhao W, et al. TIGIT overexpression diminishes the function of CD4 T cells and ameliorates the severity of rheumatoid arthritis in mouse models. Exp. Cell Res. 2016;340:132–138. doi: 10.1016/j.yexcr.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 105.Fasth AE, Bjorkstrom NK, Anthoni M, Malmberg KJ, Malmstrom V. Activating NK-cell receptors co-stimulate CD4(+)CD28(−) T cells in patients with rheumatoid arthritis. Eur. J. Immunol. 2010;40:378–387. doi: 10.1002/eji.200939399. [DOI] [PubMed] [Google Scholar]
- 106.Ceeraz S, et al. VISTA deficiency attenuates antibody-induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res. Ther. 2017;19:270. doi: 10.1186/s13075-017-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verwilghen J, Corrigall V, Pope RM, Rodrigues R, Panayi GS. Expression and function of CD5 and CD28 in patients with rheumatoid arthritis. Immunology. 1993;80:96–102. [PMC free article] [PubMed] [Google Scholar]
- 108.Martin P, et al. The leukocyte activation antigen CD69 limits allergic asthma and skin contact hypersensitivity. J. Allergy Clin. Immunol. 2010;126:355–365, 365 e351–e353. doi: 10.1016/j.jaci.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 109.Cruz-Adalia A, et al. CD69 limits the severity of cardiomyopathy after autoimmune myocarditis. Circulation. 2010;122:1396–1404. doi: 10.1161/CIRCULATIONAHA.110.952820. [DOI] [PubMed] [Google Scholar]
- 110.Sancho D, et al. CD69 downregulates autoimmune reactivity through active transforming growth factor-beta production in collagen-induced arthritis. J. Clin. Invest. 2003;112:872–882. doi: 10.1172/JCI19112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernandez-Garcia C, Fernandez-Gutierrez B, Morado IC, Banares AA, Jover JA. The CD69 activation pathway in rheumatoid arthritis synovial fluid T cells. Arthritis Rheum. 1996;39:1277–1286. doi: 10.1002/art.1780390803. [DOI] [PubMed] [Google Scholar]
- 112.Iannone F, Corrigal VM, Panayi GS. CD69 on synovial T cells in rheumatoid arthritis correlates with disease activity. Br. J. Rheumatol. 1996;35:397. doi: 10.1093/rheumatology/35.4.397. [DOI] [PubMed] [Google Scholar]
- 113.Steinbach K, Vincenti I, Merkler D, Resident-Memory T. Cells in tissue-restricted immune responses: for better or worse? Front. Immunol. 2018;9:2827. doi: 10.3389/fimmu.2018.02827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blanton RM, Carrillo-Salinas FJ, Alcaide P. T-cell recruitment to the heart: friendly guests or unwelcome visitors? Am. J. Physiol. Heart Circ. Physiol. 2019;317:H124–H140. doi: 10.1152/ajpheart.00028.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Munro JM, van der Walt JD, Munro CS, Chalmers JA, Cox EL. An immunohistochemical analysis of human aortic fatty streaks. Hum. Pathol. 1987;18:375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- 116.Ridker PM, et al. Antiinflammatory therapy with Canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 117.Palinski W. Immunomodulation: a new role for statins? Nat. Med. 2000;6:1311–1312. doi: 10.1038/82107. [DOI] [PubMed] [Google Scholar]
- 118.Mach F. Statins as immunomodulatory agents. Circulation. 2004;109:II15–II17. doi: 10.1161/01.CIR.0000129502.10459.fe. [DOI] [PubMed] [Google Scholar]
- 119.Tse K, Tse H, Sidney J, Sette A, Ley K. T cells in atherosclerosis. Int Immunol. 2013;25:615–622. doi: 10.1093/intimm/dxt043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Duijn J, Kuiper J, Slutter B. The many faces of CD8+ T cells in atherosclerosis. Curr. Opin. Lipido. 2018;29:411–416. doi: 10.1097/MOL.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 121.Qiu MK, et al. PD-1 and Tim-3 pathways regulate CD8+ T cells function in atherosclerosis. PLoS ONE. 2015;10:e0128523. doi: 10.1371/journal.pone.0128523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Li SH, Chen WJ, Yan M, Shu YW, Liao YH. Expression of coinhibitory PD-L1 on CD4(+)CD25(+)FOXP3(+) regulatory T cells is elevated in patients with acute coronary syndrome. Coron. Artery Dis. 2015;26:598–603. doi: 10.1097/MCA.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 123.Lee J, et al. Contributions of PD-1/PD-L1 pathway to interactions of myeloid DCs with T cells in atherosclerosis. J. Mol. Cell Cardiol. 2009;46:169–176. doi: 10.1016/j.yjmcc.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 124.Foks AC, et al. T-cell immunoglobulin and mucin domain 3 acts as a negative regulator of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013;33:2558–2565. doi: 10.1161/ATVBAHA.113.301879. [DOI] [PubMed] [Google Scholar]
- 125.Ma K, et al. CTLA4-IgG ameliorates homocysteine-accelerated atherosclerosis by inhibiting T-cell overactivation in apoE(−/−) mice. Cardiovasc. Res. 2013;97:349–359. doi: 10.1093/cvr/cvs330. [DOI] [PubMed] [Google Scholar]
- 126.Ewing MM, et al. T-cell co-stimulation by CD28-CD80/86 and its negative regulator CTLA-4 strongly influence accelerated atherosclerosis development. Int. J. Cardiol. 2013;168:1965–1974. doi: 10.1016/j.ijcard.2012.12.085. [DOI] [PubMed] [Google Scholar]
- 127.Matsumoto T, et al. Overexpression of cytotoxic T-lymphocyte-associated antigen-4 prevents atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. 2016;36:1141–1151. doi: 10.1161/ATVBAHA.115.306848. [DOI] [PubMed] [Google Scholar]
- 128.Golden D, et al. Lymphocyte activation gene 3 and coronary artery disease. JCI Insight. 2016;1:e88628. doi: 10.1172/jci.insight.88628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Douna H, et al. B- and T-lymphocyte attenuator stimulation protects against atherosclerosis by regulating follicular B cells. Cardiovasc. Res. 2020;116:295–305. doi: 10.1093/cvr/cvz129. [DOI] [PubMed] [Google Scholar]
- 130.Foks AC, et al. Agonistic anti-TIGIT treatment inhibits T cell responses in LDLr deficient mice without affecting atherosclerotic lesion development. PLoS ONE. 2013;8:e83134. doi: 10.1371/journal.pone.0083134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao J, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat. Med. 2017;23:551–555. doi: 10.1038/nm.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Decano JL, Aikawa M. Dynamic macrophages: understanding mechanisms of activation as guide to therapy for atherosclerotic vascular disease. Front. Cardiovasc. Med. 2018;5:97. doi: 10.3389/fcvm.2018.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Roselaar SE, Kakkanathu PX, Daugherty A. Lymphocyte populations in atherosclerotic lesions of apoE −/− and LDL receptor −/− mice. Decreasing density with disease progression. Arterioscler. Thromb. Vasc. Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 134.Sekiya T, et al. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013;14:230–237. doi: 10.1038/ni.2520. [DOI] [PubMed] [Google Scholar]
- 135.Lim H, et al. Proatherogenic conditions promote autoimmune T helper 17 cell responses in vivo. Immunity. 2014;40:153–165. doi: 10.1016/j.immuni.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fernandez-Friera L, et al. Prevalence, vascular distribution, and multiterritorial extent of subclinical atherosclerosis in a middle-aged cohort: The PESA (Progression of Early Subclinical Atherosclerosis) Study. Circulation. 2015;131:2104–2113. doi: 10.1161/CIRCULATIONAHA.114.014310. [DOI] [PubMed] [Google Scholar]
- 137.Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat. Rev. Immunol. 2018;18:733–744. doi: 10.1038/s41577-018-0065-8. [DOI] [PubMed] [Google Scholar]
- 138.Lutgens, E. & Seijkens, T. T. P. Cancer patients receiving immune checkpoint inhibitor therapy are at an increased risk for atherosclerotic cardiovascular disease. J. Immunother. Cancer8, 10.1136/jitc-2019-000300 (2020). [DOI] [PMC free article] [PubMed]
- 139.Ramos-Casals M, et al. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020;6:38. doi: 10.1038/s41572-020-0160-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zaha VG, Meijers WC, Moslehi J. Cardio-immuno-oncology. Circulation. 2020;141:87–89. doi: 10.1161/CIRCULATIONAHA.119.042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bar J, et al. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur. J. Cancer. 2019;120:122–131. doi: 10.1016/j.ejca.2019.06.021. [DOI] [PubMed] [Google Scholar]
- 142.Newman JL, Stone JR. Immune checkpoint inhibition alters the inflammatory cell composition of human coronary artery atherosclerosis. Cardiovasc. Pathol. 2019;43:107148. doi: 10.1016/j.carpath.2019.107148. [DOI] [PubMed] [Google Scholar]
- 143.Bennet AM, Alarcon-Riquelme M, Wiman B, de Faire U, Prokunina-Olsson L. Decreased risk for myocardial infarction and lower tumor necrosis factor-alpha levels in carriers of variants of the PDCD1 gene. Hum. Immunol. 2006;67:700–705. doi: 10.1016/j.humimm.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 144.Yip HK, et al. Cytotoxic T lymphocyte antigen 4 gene polymorphism associated with ST-segment elevation acute myocardial infarction. Circ. J. 2007;71:1213–1218. doi: 10.1253/circj.71.1213. [DOI] [PubMed] [Google Scholar]
- 145.Yin X, et al. Protein biomarkers of new-onset cardiovascular disease: prospective study from the systems approach to biomarker research in cardiovascular disease initiative. Arterioscler. Thromb. Vasc. Biol. 2014;34:939–945. doi: 10.1161/ATVBAHA.113.302918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Forteza MJ, et al. Programmed death-1 (PD-1): a novel mechanism for understanding the acute immune deregulation in ST-segment elevation myocardial infarction. Int J. Cardiol. 2014;177:8–10. doi: 10.1016/j.ijcard.2014.09.114. [DOI] [PubMed] [Google Scholar]
- 147.Pasqui AL, et al. T cell activation and enhanced apoptosis in non-ST elevation myocardial infarction. Clin. Exp. Med. 2003;3:37–44. doi: 10.1007/s102380300014. [DOI] [PubMed] [Google Scholar]
- 148.Carvalheiro T, et al. Phenotypic and functional alterations on inflammatory peripheral blood cells after acute myocardial infarction. J. Cardiovasc. Transl. Res. 2012;5:309–320. doi: 10.1007/s12265-012-9365-8. [DOI] [PubMed] [Google Scholar]
- 149.Hosono M, et al. Increased expression of T cell activation markers (CD25, CD26, CD40L and CD69) in atherectomy specimens of patients with unstable angina and acute myocardial infarction. Atherosclerosis. 2003;168:73–80. doi: 10.1016/s0021-9150(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 150.Bruestle K, Hackner K, Kreye G, Heidecker B. Autoimmunity in acute myocarditis: how immunopathogenesis steers new directions for diagnosis and treatment. Curr. Cardiol. Rep. 2020;22:28. doi: 10.1007/s11886-020-01278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Lv H, et al. Impaired thymic tolerance to alpha-myosin directs autoimmunity to the heart in mice and humans. J. Clin. Invest. 2011;121:1561–1573. doi: 10.1172/JCI44583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Grabie N, Lichtman AH, Padera R. T cell checkpoint regulators in the heart. Cardiovasc. Res. 2019;115:869–877. doi: 10.1093/cvr/cvz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Johnson DB, et al. Fulminant myocarditis with Combination Immune Checkpoint Blockade. N. Engl. J. Med. 2016;375:1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mahmood SS, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J. Am. Coll. Cardiol. 2018;71:1755–1764. doi: 10.1016/j.jacc.2018.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shrestha KP, Carrera AE. Hair trace elements and mental retardation among children. Arch. Environ. Health. 1988;43:396–398. doi: 10.1080/00039896.1988.9935857. [DOI] [PubMed] [Google Scholar]
- 156.Moslehi JJ, Salem JE, Sosman JA, Lebrun-Vignes B, Johnson DB. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet. 2018;391:933. doi: 10.1016/S0140-6736(18)30533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Escudier M, et al. Clinical features, management, and outcomes of immune checkpoint inhibitor-related cardiotoxicity. Circulation. 2017;136:2085–2087. doi: 10.1161/CIRCULATIONAHA.117.030571. [DOI] [PubMed] [Google Scholar]
- 158.Nishimura H, et al. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- 159.Tarrio ML, Grabie N, Bu DX, Sharpe AH, Lichtman AH. PD-1 protects against inflammation and myocyte damage in T cell-mediated myocarditis. J. Immunol. 2012;188:4876–4884. doi: 10.4049/jimmunol.1200389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tivol EA, et al. CTLA4Ig prevents lymphoproliferation and fatal multiorgan tissue destruction in CTLA-4-deficient mice. J. Immunol. 1997;158:5091–5094. [PubMed] [Google Scholar]
- 161.Ji C, et al. Myocarditis in cynomolgus monkeys following treatment with immune checkpoint inhibitors. Clin. Cancer Res. 2019;25:4735–4748. doi: 10.1158/1078-0432.CCR-18-4083. [DOI] [PubMed] [Google Scholar]
- 162.Freeman GJ, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rodig N, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 164.Koelzer VH, et al. Systemic inflammation in a melanoma patient treated with immune checkpoint inhibitors-an autopsy study. J. Immunother. Cancer. 2016;4:13. doi: 10.1186/s40425-016-0117-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Frisancho-Kiss S, et al. Cutting edge: T cell Ig mucin-3 reduces inflammatory heart disease by increasing CTLA-4 during innate immunity. J. Immunol. 2006;176:6411–6415. doi: 10.4049/jimmunol.176.11.6411. [DOI] [PubMed] [Google Scholar]
- 166.Frisancho-Kiss S, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J. Immunol. 2007;178:6710–6714. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 167.Lawrence MS, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pennock ND, et al. T cell responses: naive to memory and everything in between. Adv. Physiol. Educ. 2013;37:273–283. doi: 10.1152/advan.00066.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Teng MW, Galon J, Fridman WH, Smyth MJ. From mice to humans: developments in cancer immunoediting. J. Clin. Invest. 2015;125:3338–3346. doi: 10.1172/JCI80004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Otsuka A, et al. Hedgehog pathway inhibitors promote adaptive immune responses in basal cell carcinoma. Clin. Cancer Res. 2015;21:1289–1297. doi: 10.1158/1078-0432.CCR-14-2110. [DOI] [PubMed] [Google Scholar]
- 171.Vinay DS, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015;35(Suppl):S185–S198. doi: 10.1016/j.semcancer.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 172.Sarvaria A, Madrigal JA, Saudemont A. B cell regulation in cancer and anti-tumor immunity. Cell Mol. Immunol. 2017;14:662–674. doi: 10.1038/cmi.2017.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Day CL, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 174.Baitsch L, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J. Clin. Invest. 2011;121:2350–2360. doi: 10.1172/JCI46102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wherry EJ. T cell exhaustion. Nat. Immunol. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 176.Speiser DE, et al. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat. Rev. Immunol. 2014;14:768–774. doi: 10.1038/nri3740. [DOI] [PubMed] [Google Scholar]
- 177.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Robert C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 180.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 181.Eggermont AM, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 182.Yang JC, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J. Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ansell SM, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin. Cancer Res. 2009;15:6446–6453. doi: 10.1158/1078-0432.CCR-09-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kwon ED, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15:700–712. doi: 10.1016/S1470-2045(14)70189-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ribas A, et al. Phase III randomized clinical trial comparing tremelimumab with standard-of-care chemotherapy in patients with advanced melanoma. J. Clin. Oncol. 2013;31:616–622. doi: 10.1200/JCO.2012.44.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Chung KY, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J. Clin. Oncol. 2010;28:3485–3490. doi: 10.1200/JCO.2010.28.3994. [DOI] [PubMed] [Google Scholar]
- 187.Sangro B, et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013;59:81–88. doi: 10.1016/j.jhep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 188.Calabro L, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2013;14:1104–1111. doi: 10.1016/S1470-2045(13)70381-4. [DOI] [PubMed] [Google Scholar]
- 189.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 190.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Page DB, Postow MA, Callahan MK, Allison JP, Wolchok JD. Immune modulation in cancer with antibodies. Annu. Rev. Med. 2014;65:185–202. doi: 10.1146/annurev-med-092012-112807. [DOI] [PubMed] [Google Scholar]
- 192.Lipson EJ, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin. Cancer Res. 2013;19:462–468. doi: 10.1158/1078-0432.CCR-12-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin. Ther. 2015;37:764–782. doi: 10.1016/j.clinthera.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Berger R, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008;14:3044–3051. doi: 10.1158/1078-0432.CCR-07-4079. [DOI] [PubMed] [Google Scholar]
- 195.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013;369:122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lynch TJ, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J. Clin. Oncol. 2012;30:2046–2054. doi: 10.1200/JCO.2011.38.4032. [DOI] [PubMed] [Google Scholar]
- 197.Horinouchi H, et al. Phase I study of ipilimumab in phased combination with paclitaxel and carboplatin in Japanese patients with non-small-cell lung cancer. Invest. N. Drugs. 2015;33:881–889. doi: 10.1007/s10637-015-0243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Arriola E, et al. Outcome and biomarker analysis from a multicenter phase 2 study of ipilimumab in combination with carboplatin and etoposide as first-line therapy for extensive-stage SCLC. J. Thorac. Oncol. 2016;11:1511–1521. doi: 10.1016/j.jtho.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Hersh EM, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest N. Drugs. 2011;29:489–498. doi: 10.1007/s10637-009-9376-8. [DOI] [PubMed] [Google Scholar]
- 200.Chi M, Dudek AZ. Vaccine therapy for metastatic melanoma: systematic review and meta-analysis of clinical trials. Melanoma Res. 2011;21:165–174. doi: 10.1097/CMR.0b013e328346554d. [DOI] [PubMed] [Google Scholar]
- 201.Bernatchez C, Radvanyi LG, Hwu P. Advances in the treatment of metastatic melanoma: adoptive T-cell therapy. Semin. Oncol. 2012;39:215–226. doi: 10.1053/j.seminoncol.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Boni A, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res. 2010;70:5213–5219. doi: 10.1158/0008-5472.CAN-10-0118. [DOI] [PubMed] [Google Scholar]
- 203.Slovin SF, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann. Oncol. 2013;24:1813–1821. doi: 10.1093/annonc/mdt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wilmott JS, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin. Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 205.Ribas A, Hodi FS, Callahan M, Konto C, Wolchok J. Hepatotoxicity with combination of vemurafenib and ipilimumab. N. Engl. J. Med. 2013;368:1365–1366. doi: 10.1056/NEJMc1302338. [DOI] [PubMed] [Google Scholar]
- 206.Puhr HC, Ilhan-Mutlu A. New emerging targets in cancer immunotherapy: the role of LAG3. ESMO Open. 2019;4:e000482. doi: 10.1136/esmoopen-2018-000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Tagliamento, M., Bironzo, P. & Novello, S. New emerging targets in cancer immunotherapy: the role of VISTA. ESMO Open 4, 10.1136/esmoopen-2020-000683 (2020). [DOI] [PMC free article] [PubMed]
- 208.Johnston RL, Lutzky J, Chodhry A, Barkin JS. Cytotoxic T-lymphocyte-associated antigen 4 antibody-induced colitis and its management with infliximab. Dig. Dis. Sci. 2009;54:2538–2540. doi: 10.1007/s10620-008-0641-z. [DOI] [PubMed] [Google Scholar]
- 209.Abdel-Rahman O, Fouad M. A network meta-analysis of the risk of immune-related renal toxicity in cancer patients treated with immune checkpoint inhibitors. Immunotherapy. 2016;8:665–674. doi: 10.2217/imt-2015-0020. [DOI] [PubMed] [Google Scholar]
- 210.Nonomura Y, et al. ADAMTSL5 is upregulated in melanoma tissues in patients with idiopathic psoriasis vulgaris induced by nivolumab. J. Eur. Acad. Dermatol. Venereol. 2017;31:e100–e101. doi: 10.1111/jdv.13818. [DOI] [PubMed] [Google Scholar]
- 211.Chae YK, et al. A case of pembrolizumab-induced type-1 diabetes mellitus and discussion of immune checkpoint inhibitor-induced type 1 diabetes. Cancer Immunol. Immunother. 2017;66:25–32. doi: 10.1007/s00262-016-1913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Tarhini A. Immune-mediated adverse events associated with ipilimumab ctla-4 blockade therapy: the underlying mechanisms and clinical management. Science (Cairo) 2013;2013:857519. doi: 10.1155/2013/857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fecher LA, Agarwala SS, Hodi FS, Weber JS. Ipilimumab and its toxicities: a multidisciplinary approach. Oncologist. 2013;18:733–743. doi: 10.1634/theoncologist.2012-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Weber JS, et al. Patterns of onset and resolution of immune-related adverse events of special interest with ipilimumab: detailed safety analysis from a phase 3 trial in patients with advanced melanoma. Cancer. 2013;119:1675–1682. doi: 10.1002/cncr.27969. [DOI] [PubMed] [Google Scholar]
- 215.Marthey L, et al. Cancer Immunotherapy with anti-CTLA-4 monoclonal antibodies induces an inflammatory bowel disease. J. Crohns Colitis. 2016;10:395–401. doi: 10.1093/ecco-jcc/jjv227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Kim KW, et al. Ipilimumab associated hepatitis: imaging and clinicopathologic findings. Invest N. Drugs. 2013;31:1071–1077. doi: 10.1007/s10637-013-9939-6. [DOI] [PubMed] [Google Scholar]
- 217.Dillard T, Yedinak CG, Alumkal J, Fleseriu M. Anti-CTLA-4 antibody therapy associated autoimmune hypophysitis: serious immune related adverse events across a spectrum of cancer subtypes. Pituitary. 2010;13:29–38. doi: 10.1007/s11102-009-0193-z. [DOI] [PubMed] [Google Scholar]
- 218.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Naidoo J, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Qin W, et al. The diverse gunction of PD-1/PD-L pathway beyond vancer. Front. Immunol. 2019;10:2298. doi: 10.3389/fimmu.2019.02298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stohl W, Yu N, Chalmers SA, Putterman C, Jacob CO. Constitutive reduction in the checkpoint inhibitor, CTLA-4, does not accelerate SLE in NZM 2328 mice. Lupus Sci. Med. 2019;6:e000313. doi: 10.1136/lupus-2018-000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N. Engl. J. Med. 2009;361:211–212. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 223.Richter MD, et al. Brief Report: Cancer immunotherapy in patients with preexisting rheumatic disease: The Mayo Clinic experience. Arthritis Rheumatol. 2018;70:356–360. doi: 10.1002/art.40397. [DOI] [PubMed] [Google Scholar]
- 224.Simons KH, et al. T cell co-stimulation and co-inhibition in cardiovascular disease: a double-edged sword. Nat. Rev. Cardiol. 2019;16:325–343. doi: 10.1038/s41569-019-0164-7. [DOI] [PubMed] [Google Scholar]
- 225.Mensah GA, Roth GA, Fuster V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J. Am. Coll. Cardiol. 2019;74:2529–2532. doi: 10.1016/j.jacc.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 226.Esplugues E, et al. Enhanced antitumor immunity in mice deficient in CD69. J. Exp. Med. 2003;197:1093–1106. doi: 10.1084/jem.20021337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Cibrian D, Sanchez-Madrid F. CD69: from activation marker to metabolic gatekeeper. Eur. J. Immunol. 2017;47:946–953. doi: 10.1002/eji.201646837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Song W, Das M, Chen X. Nanotherapeutics for immuno-oncology: a crossroad for new paradigms. Trends Cancer. 2020;6:288–298. doi: 10.1016/j.trecan.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Grosser R, Cherkassky L, Chintala N, Adusumilli PS. Combination immunotherapy with CAR T cells and checkpoint blockade for the treatment of solid tumors. Cancer Cell. 2019;36:471–482. doi: 10.1016/j.ccell.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gianchecchi E, Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in Treg development and their involvement in autoimmunity onset and cancer progression. Front. Immunol. 2018;9:2374. doi: 10.3389/fimmu.2018.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Michel L, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J. Clin. Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zelle-Rieser C, et al. T cells in multiple myeloma display features of exhaustion and senescence at the tumor site. J. Hematol. Oncol. 2016;9:116. doi: 10.1186/s13045-016-0345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Heinzerling L, et al. Cardiotoxicity associated with CTLA4 and PD1 blocking immunotherapy. J. Immunother. Cancer. 2016;4:50. doi: 10.1186/s40425-016-0152-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zhang Z, et al. Two genes encoding immune-regulatory molecules (LAG3 and IL7R) confer susceptibility to multiple sclerosis. Genes Immun. 2005;6:145–152. doi: 10.1038/sj.gene.6364171. [DOI] [PubMed] [Google Scholar]
- 235.Pallikkuth S, et al. Cardiac morbidity in HIV infection is associated with checkpoint inhibitor LAG-3 on CD4 T cells. PLoS One. 2018;13:e0206256. doi: 10.1371/journal.pone.0206256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liberal R, et al. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology. 2012;56:677–686. doi: 10.1002/hep.25682. [DOI] [PubMed] [Google Scholar]
- 237.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol. Rev. 2017;276:97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Japp AS, et al. Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression. Cancer Immunol. Immunother. 2015;64:1487–1494. doi: 10.1007/s00262-015-1752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Agresta L, Hoebe KHN, Janssen EM. The emerging role of CD244 signaling in immune cells of the tumor microenvironment. Front. Immunol. 2018;9:2809. doi: 10.3389/fimmu.2018.02809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Peng J, Xiang Y. Value analysis of CD69 combined with EGR1 in the diagnosis of coronary heart disease. Exp. Ther. Med. 2019;17:2047–2052. doi: 10.3892/etm.2019.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wald O, et al. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J. Immunol. 2006;177:6983–6990. doi: 10.4049/jimmunol.177.10.6983. [DOI] [PubMed] [Google Scholar]
- 242.Oja AE, et al. Functional heterogeneity of CD4(+) tumor-infiltrating lymphocytes with a resident memory phenotype in NSCLC. Front. Immunol. 2018;9:2654. doi: 10.3389/fimmu.2018.02654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yu X, Zheng Y, Mao R, Su Z, Zhang J. BTLA/HVEM signaling: milestones in research and role in chronic hepatitis B virus infection. Front. Immunol. 2019;10:617. doi: 10.3389/fimmu.2019.00617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Burgueno-Bucio E, Mier-Aguilar CA, Soldevila G. The multiple faces of CD5. J. Leukoc. Biol. 2019;105:891–904. doi: 10.1002/JLB.MR0618-226R. [DOI] [PubMed] [Google Scholar]