Abstract
A prebiotically plausible route to enantioenriched glyceraldehyde is reported via a kinetic resolution mediated by peptides. The reaction proceeds via a selective reaction between the l-peptide and the l-sugar producing an Amadori rearrangement byproduct and leaving d-glyceraldehyde in excess. Solubility considerations in the synthesis of proline–valine (pro–val) peptides allow nearly enantiopure pro–val to be formed starting from racemic pro and nearly racemic (10%) ee val. (ee = enantiomeric excess = (|d − l|)/(d + l)) Thus enantioenrichment of glyceraldehyde is achieved in a system with minimal initial chiral bias. This work demonstrates synergy between amino acids and sugars in the emergence of biological homochirality.
A prebiotically plausible route to enantioenriched glyceraldehyde is reported via a kinetic resolution mediated by peptides.
Introduction
The origin of biological homochirality remains a fundamental question in the origin of life.1 While recent advances in prebiotic chemistry have significantly furthered our understanding of how the key building blocks of life may have emerged,2 the question of how the stereochemistry of these molecules might be controlled has been left unaddressed in many of these reports.3 We have carried out extensive studies of chemical and physical approaches to the enantioenrichment of amino acids and sugars, including glyceraldehyde 1, the “primordial” sugar.4 It may be argued that the stereochemistry of the pentose sugars of RNA and DNA might have been set in the step that controls the enantioenrichment of glyceraldehyde. Glyceraldehyde also plays several important roles in cyanosulfidic chemistry,5 serving as a substrate for nucleotide synthesis,6 pyruvate metabolism,7 and higher sugars via the formose reaction.8 Our lab has demonstrated that l-amino acids mediate the enantioenrichment of d-glyceraldehyde9 in the Powner-Sutherland RNA synthesis.6
There is a rich interplay between the enantioselective formation of sugars and amino acids/peptides. Amino acids and peptides catalyse the formation of enantioenriched tetrose and pentose sugars via the formose reaction.10,11 Conversely, pentose sugars can mediate the enantioselective synthesis of amino acids.12 The group of Clarke and coworkers has studied extensively the formation of glyceraldehyde and tetroses catalyzed by amino acid derivatives, including in hydrogel form, under prebiotically relevant conditions.13 The provenance of enantioenriched peptides has also been well studied. Orgel and Ghadiri used COS/CS2 (ref. 14) to polymerise amino acids through formation of N-carboxyanhydrides, whereas Powner et al. reported an activation–ligation cycle using aminonitrile monomers.15 Alternative approaches are wet-dry cycling,16 mineral catalysis,17 or self-assembly of aggregates or gels.18 Stochastic oligomerisation of scalemic amino acids produces mixtures of peptide stereoisomers, but we recently demonstrated how highly enantioenriched products may be formed by combining polymerisation with physical processes.19
We report here that enantioenriched glyceraldehyde may be obtained from rac-glyceraldehyde by a peptide-mediated kinetic resolution. Combining physical and chemical processes affords nearly homochiral glyceraldehyde using peptides synthesized from nearly racemic amino acid monomers. This work reaffirms the close connection between the emergence of enantioenrichment in sugars and amino acids.
Results and discussion
We recently reported the prebiotically relevant synthesis of libraries of enantioenriched di- and tripeptides that catalyze the enantioselective formation of tetrose sugars.19 In the current work, we turn our attention to the interaction of di- and tripeptide library candidates with rac-glyceraldehyde (Scheme 1). The kinetic resolution proceeds smoothly in phosphate buffer, and screening studies showed that enantioenrichment toward the d-sugar occurred with N-terminal proline di-peptides (Table 1, entries 1–3), as shown in Fig. 1 for the case of ll-PV. Peptides without proline as the N-terminus afforded less enantioenrichment. Carbonate buffer gave similar results, while borate buffer afforded little enantioselectivity (see ESI†). Fig. 1 shows the evolution of ee as a function of conversion for the PV system.
Scheme 1. Kinetic resolution of racemic glyceraldehyde rac-1 mediated by enantioenriched peptides.
Screening of l-dipeptide-mediated kinetic resolution of rac-glyceraldehydea.
| Entry | Peptideb | % Conversion | % ee | s-Factorc |
|---|---|---|---|---|
| 1 | PV | 54 | 24 | 1.86 |
| 2 | PA | 35 | 12 | 1.79 |
| 3 | PF | 52 | 23 | 1.88 |
| 4 | VL | 82 | 9 | 1.11 |
| 5 | VV | 88 | 11 | 1.11 |
| 6 | LV | 84 | 2 | 1.02 |
| 7 | VD | 84 | 10 | 1.11 |
| 8 | AA | 86 | 7 | 1.08 |
Reaction conditions: 100 mM l-peptide, 100 mM rac-glyceraldehyde; ambient temperature; 0.4 M phosphate buffer, pH 9.5, 4–6 h.
Single letter amino acid abbreviations: P = proline; V = valine; A = alanine; F = phenylalanine; D = aspartic acid.
Selectivity factor refers to relative rates of the reaction of 1: s = kfast/kslow,20 see ESI for derivation.
Fig. 1. Enantiomeric excess as a function of % conversion of rac-glyceraldehyde (10 mM) mediated by PV dipeptide (100 mM) ambient temperature; 0.4 M phosphate buffer, pH 9.5 over the course of 5 h.
Monitoring the time course of the separate reactions of each hand of glyceraldehyde using PV confirmed first order kinetics and the faster decomposition of l-1 (Fig. 2a). Similar results were observed using the opposite hand of the dipeptide catalyst, which afforded a faster reaction with d-1 (see ESI†). Reaction of glyceraldehyde produces two main reaction products, as is shown in Fig. 2b. Isomerization to dihydroxyacetone (DHA) is a minor product, and the temporal conversion profile to DHA differs little between l-1 and d-1. The major product of the kinetic resolution is a species identified as the Amadori rearrangement product (ARP, Scheme 2) between the peptide and glyceraldehyde identified by both mass spectroscopy and 1H and 13C NMR spectroscopy (see ESI† for further details).
Fig. 2. Temporal profiles of the separate reactions of d- and l-1 using ll-PV dipeptide under the conditions of Table 1. (a) Consumption of 1; (b) formation of products from the reaction of 1. ARP = Amadori rearrangement product.
Scheme 2. Formation of the Amadori rearrangement product (ARP) from the reaction of 1 with ll-PV identified by 13C NMR spectroscopy (see ESI† for details).
The plots of Fig. 2 reveal that selectivity in the kinetic resolution of rac-1 is achieved via selective reaction of the ll-dipeptide catalyst with l-1, leaving d-1 behind. The plot also shows that isomerization of 1 to DHA is unselective, as expected, and this side reaction decreases the overall selectivity. The stereochemical identity of 1 is lost in formation of both DHA and ARP.
The observation of an irreversible reaction between the peptide and 1 indicates that the kinetic resolution is not a catalytic process. Higher concentrations of peptide relative to that of the sugar were studied to probe whether the background isomerization reaction could be suppressed. Ratios up to 10 : 1 dipeptide : 1 afforded only a slight increase in the selectivity factor, as shown in Fig. 3.
Fig. 3. Selectivity factor20 in the kinetic resolution of eq. (1) as a function of the ratio of dipeptide to rac-1 using ll-PV dipeptide under the conditions of Table 1.
Further studies showed that proline monomer was unreactive in the reaction of Scheme 1 while derivatives of proline showed activity under the conditions of Table 1 (Table 2, entries 1). Proline amide was only slightly selective (Table 2, entry 2), while PV-methoxy dipeptide exhibited a selectivity factor similar to ll-PV dipeptide (Table 2, entry 3). Tri and tetra peptides with P at the N-terminus and incorporating V and F (Table 2, entries 4–8) performed slightly better than the dipeptides shown in Table 1. The low solubility of the higher order peptides made it difficult to carry out comparisons of the peptide : sugar ratio as was shown for PV in Fig. 3.
Screening of l-proline monomer and peptide-mediated kinetic resolution of rac-glyceraldehyde 1a.
| Entry | Peptide | % Conversion | % ee | s-Factor |
|---|---|---|---|---|
| 1 | P | n.r. | — | — |
| 2 | P-NH2 | 86 | 12.2 | 1.14 |
| 3 | PV-OMe | 56 | 26.7 | 1.95 |
| 4 | PVV | 46 | 22.0 | 2.16 |
| 5 | PFV | 56 | 28.2 | 2.00 |
| 6 | PVVV | 47 | 21.0 | 1.96 |
| 7 | PVFV | 51 | 27.7 | 2.20 |
| 8 | PFVV | 55 | 30.1 | 2.15 |
Reaction conditions: 1 : 1 peptide:1; entries 1–3: 100 mM; entry 4 : 20 mM; entries 5–8: 10 mM. Ambient temperature, phosphate buffer, pH 9.5, 6 h.
All of the peptides shown in Tables 1 and 2 were synthesized using l-amino acids. However, because the provenance of peptide homochirality is as important a question as that of the sugar under study, we probed mixtures of l and d peptides in the reaction of Scheme 1. Table 3 shows that the position next to the N-terminal proline has a strong influence, eroding the selectivity factor in glyceraldehyde kinetic resolution (Table 3, entries 3, 6, and 7), while the third position plays a less important role (Table 3, entry 5).
Influence of amino acid stereochemistry on the outcome of peptide-mediated kinetic resolution of rac-glyceraldehyde 1a.
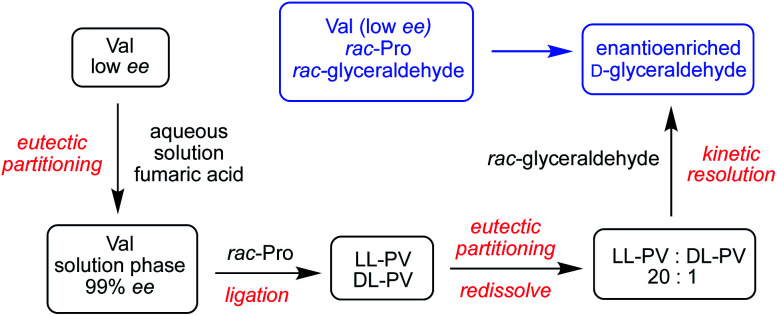
The results of Table 3 suggest that sugar enantioenrichment could proceed with peptides even in cases where homochirality has not fully emerged for the monomeric amino acids used to construct the peptides. A key feature is enantioenrichment in the first two amino acids in the sequence. We called upon our past observations of the physical phase behavior of amino acids21 to propose a sequence of steps that could lead to enantioenriched glyceraldehyde starting from rac-1 and amino acids at low ee values. We consider how the PV and PVV systems of Table 3 might be prepared from low ee P and V monomers. Aqueous valine solutions at >99% ee can be prepared from nearly racemic valine by mixing partially dissolved low ee valine with fumaric acid, a prebiotically relevant dicarboxylic acid, in a process we term eutectic partitioning.21 In our past work, we showed that a solution of nearly enantiopure proline emerges when the amino acid is partially dissolved in chloroform; however, high concentrations of this solvent may not be plausible under prebiotic conditions. In aqueous solution, proline is too highly soluble to exploit the eutectic approach discussed above. Our previous work in COS-mediated dipeptide synthesis showed that a system of rac-proline and enantiopure valine would produce equal concentrations of ll and dl PV dipeptides.21 Because selectivity is found to depend primarily on the chirality of the N-terminal residue, employing this mixture in the kinetic resolution of Scheme 1 would likely give low selectivity to d-1. However, we found that the ll and dl PV dipeptides exhibit a more than two-fold difference in solubility. By partial evaporation of a 1 : 1 mixture of the two peptides, ll-PV precipitated in solution in an almost pure form (20 : 1).
The scenario described above recruits a sequence of physical and chemical processes and prebiotically plausible wet-dry cycles to convert racemic glyceraldehyde to enantioenriched form starting from low % ee valine as the only chiral bias in the system. This scenario is illustrated in Scheme 3. We have shown previously how glyceraldehyde at ee values as low as 20 % ee can ultimately lead to enantiopure RNA monomers. The current work provides a further pathway to symmetry breaking and enantioenrichment and highlights the synergistic relationship between the two key chiral building blocks of life, sugars and amino acids.
Scheme 3. Sequence of chemical and physical processes leading to the enantioenrichment of glyceraldehyde from rac-1, rac-proline and low ee valine.
Conclusions
The kinetic resolution of glyceraldehyde mediated by peptides with N-terminal proline residues. The combination of chemical and physical processes affords enantioenriched glyceraldehyde from low ee amino acids as the only chiral bias in the system. This work demonstrates synergy and interdependence of amino acids and sugars in the emergence of biological homochirality.
Author contributions
The work was conceived by AXJ and DGB. Experiments were carried out by JY in consultation with AXJ and LL. All authors contributed to writing the manuscript.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
D. G. B acknowledges funding from the Simons Foundation through the Simons Collaboration on the Origins of Life (SCOL 287625). L. L. acknowledges the DFG for a research fellowship (project No.: 422044447). JY acknowledges funding for a research internship from Nankai University at Scripps Research.
Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc01250a
References
- Blackmond D. G. The Origin of Biological Homochirality. Cold Spring Harbor Perspect. Biol. 2019;11:a032540. doi: 10.1101/cshperspect.a032540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. D. The Origin of Life—Out of the Blue. Angew. Chem., Int. Ed. 2016;55(1):104–121. doi: 10.1002/anie.201506585. [DOI] [PubMed] [Google Scholar]
- Benner S. A. Kim H.-J. Biondi E. Prebiotic Chemistry that Could Not Not Have Happened. Life. 2019;9:84. doi: 10.3390/life9040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein J. E. Blackmond D. G. On the Origin of Single Chirality of Amino Acids and Sugars in Biogenesis. Acc. Chem. Res. 2012;45(12):2045–2054. doi: 10.1021/ar200316n. [DOI] [PubMed] [Google Scholar]
- Patel B. H. Percivalle C. Ritson D. J. Duffy C. D. Sutherland J. D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015;7(4):301–307. doi: 10.1038/nchem.2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powner M. W. Gerland B. Sutherland J. D. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459(7244):239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- Coggins A. J. Powner M. W. Prebiotic synthesis of phosphoenol pyruvate by α-phosphorylation-controlled triose glycolysis. Nat. Chem. 2017;9(4):310–317. doi: 10.1038/nchem.2624. [DOI] [PubMed] [Google Scholar]
- (a) Breslow R. On the mechanism of the formose reaction. Tetrahedron Lett. 1959;1(21):22–26. [Google Scholar]; (b) Kim H.-J. Ricardo A. Illangkoon H. I. Kim M. J. Carrigan M. A. Frye F. Benner S. A. Synthesis of C arbohydrates in Mineral-Guided Prebiotic Cycles. J. Am. Chem. Soc. 2011;133(24):9457–9468. doi: 10.1021/ja201769f. [DOI] [PubMed] [Google Scholar]
- Hein J. E. Tse E. Blackmond D. G. A Route to Enantiopure RNA from Nearly Racemic Precursors. Nat. Chem. 2011;3:704–706. doi: 10.1038/nchem.1108. [DOI] [PubMed] [Google Scholar]
- (a) Breslow R. Cheng Z.-L. L-amino acids catalyze the formation of an excess of D-glyceraldehyde, and thus of other D sugars, under credible prebiotic conditions. Proc. Natl. Acad. Sci. U. S. A. 2010;107(13):5723–5725. doi: 10.1073/pnas.1001639107. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Breslow R. Ramalingam V. Appayee C. Catalysis of Glyceraldehyde Synthesis by Primary or Secondary Amino Acids Under Prebiotic Conditions as a Function of pH. Origins Life Evol. Biospheres. 2013;43(4):323–329. doi: 10.1007/s11084-013-9347-0. [DOI] [PubMed] [Google Scholar]
- Weber A. L. Pizzarello S. The peptide-catalyzed stereospecific synthesis of tetroses: A possible model for prebiotic molecular evolution. Proc. Natl. Acad. Sci. U. S. A. 2006;103(34):12713–12717. doi: 10.1073/pnas.0602320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A. J. Zubarev D. Yu. Aspuru-Guzik A. Blackmond D. G. Chiral Sugars Drive Enantioenrichment in Prebiotic Amino Acid Synthesis. ACS Cent. Sci. 2017;3:322–328. doi: 10.1021/acscentsci.7b00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Hawkins K. Patterson A. K. Clarke P. A. Smith D. K. Catalytic Gels for a Prebiotically Relevant Asymmetric Aldol Reaction in Water: From Organocatalyst Design to Hydrogel Discovery and Back Again. J. Am. Chem. Soc. 2020;142:4379–4389. doi: 10.1021/jacs.9b13156. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Burroughs L. Clarke P. A. Forintos H. Gilks J. A. R. Hayes C. J. Vale M. E. Wade W. Zbytniewski M. Asymmetric organocatalytic formation of protected and unprotected tetroses under potentially prebiotic conditions. Org. Biomol. Chem. 2012;10:1565–1570. doi: 10.1039/c1ob06798b. [DOI] [PubMed] [Google Scholar]; (c) Burroughs L. Vale M. E. Gilks J. A. R. Forintos H. Hayes C. J. Clarke P. A. Efficient asymmetric organocatalytic formation of erythrose and threose under aqueous conditions. Chem. Commun. 2010:4776–4778. doi: 10.1039/c0cc00613k. [DOI] [PubMed] [Google Scholar]
- (a) Leman L. Orgel L. Ghadiri M. R. Carbonyl Sulfide-Mediated Prebiotic Formation of Peptides. Science. 2004;306(5694):283–286. doi: 10.1126/science.1102722. [DOI] [PubMed] [Google Scholar]; (b) Leman L. J. Huang Z.-Z. Ghadiri M. R. Peptide Bond Formation in Water Mediated by Carbon Disulfide. Astrobiology. 2015;15(9):709–716. doi: 10.1089/ast.2015.1314. [DOI] [PubMed] [Google Scholar]
- Canavelli P. Islam S. Powner M. W. Peptide ligation by chemoselective aminonitrile coupling in water. Nature. 2019;571(7766):546–549. doi: 10.1038/s41586-019-1371-4. [DOI] [PubMed] [Google Scholar]
- Campbell T. D. Febrian R. McCarthy J. T. Kleinschmidt H. E. Forsythe J. G. Bracher P. J. Prebiotic condensation through wet–dry cycling regulated by deliquescence. Nat. Commun. 2019;10(1):4508. doi: 10.1038/s41467-019-11834-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Gillams R. J. Jia T. Z. Mineral Surface-Templated Self-Assembling Systems: Case Studies from Nanoscience and Surface Science towards Origins of Life Research. Life. 2018;8(2):10. doi: 10.3390/life8020010. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Doran D. Abul-Haija Y. M. Cronin L. Emergence of Function and Selection from Recursively Programmed Polymerisation Reactions in Mineral Environments. Angew. Chem., Int. Ed. 2019;58(33):11253–11256. doi: 10.1002/anie.201902287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena-Solsona M. Nanda J. Diaz-Oltra S. Chotera A. Ashkenasy G. Escuder B. Emergent Catalytic Behavior of Self-Assembled Low Molecular Weight Peptide-Based Aggregates and Hydrogels. Chem.-Eur. J. 2016;22:6687–6694. doi: 10.1002/chem.201600344. [DOI] [PubMed] [Google Scholar]
- Jones A. X. Wen L. Legnani L. Chen J. Blackmond D. G. Asymmetric Amplification in Peptide-Catalyzed Formation of Tetrose Sugars from Nearly Racemic Amino Acids. Systems Chemistry. 2020;8:63–72. [Google Scholar]
- Fiaud J. C. and Kagan H. B., Kinetic Resolution, in Topics in Stereochemistry, ed. E.L. Eliel and S.H. Wilen, John Wiley and Sons, Inc., New York, 1988, vol. 18, pp. 249–340 [Google Scholar]
- (a) Klussmann M. Iwamura H. Mathew S. P. Wells Jr D. H. Pandya U. Armstrong A. Blackmond D. G. Thermodynamic Control of Asymmetric Amplification in Amino Acid Catalysis. Nature. 2006;441:621–623. doi: 10.1038/nature04780. [DOI] [PubMed] [Google Scholar]; (b) Klussmann M. Izumi I. T. White A. J. P. Armstrong A. Blackmond D. G. Emergence of Solution Phase Homochirality via Crystal Engineering of Amino Acids. J. Am. Chem. Soc. 2007;129:7657–7660. doi: 10.1021/ja0708870. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.