Abstract
The third-generation of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), represented by osimertinib, has achieved remarkable clinical outcomes in the treatment of non-small-cell lung cancer (NSCLC) with EGFR mutation. However, resistance eventually emerges in most patients and the underlying molecular mechanisms remain to be fully understood. In this study, we generated an osimertinib-acquired resistant lung cancer model from a NSCLC cell line H1975 harboring EGFR L858R and T790M mutations. We found that the capacity of DNA damage repair was compromised in the osimertinib resistant cells, evidenced by increased levels of γH2AX and higher intensity of the comet tail after withdrawal from cisplatin. Pharmacological inhibiting the activity or genetic knockdown the expression of DNA-PK, a key kinase in DNA damage response (DDR), sensitized the resistant cells to osimertinib. Combination of osimertinib with the DNA-PK inhibitor, PI-103, or NU7441, synergistically suppressed the proliferation of the resistant cells. Mechanistically, we revealed that DNA-PK inhibitor in combination with osimertinib resulted in prolonged DNA damage and cell cycle arrest. These findings shed new light on the mechanisms of osimertinib resistance in the aspect of DNA repair, and provide a rationale for targeting DNA-PK as a therapeutic strategy to overcome osimertinib-acquired resistance in NSCLC.
Keywords: NSCLC, EGFR-TKI resistance, osimertinib, DNA damage repair, DNA-PK
Introduction
Epidermal growth factor receptor (EGFR) is one of the most important driver oncogenes in non-small-cell lung cancer (NSCLC). Over the past decades, EGFR tyrosine kinase inhibitors (EGFR-TKIs), including gefitinib [1–3], erlotinib [4–6], and afatinib [7, 8], have been used as the standard first-line therapies for patients with EGFR mutation (especially exon 19 deletion or exon 21 L858R). Although these first-and second-generation EGFR-TKIs show better efficacy than standard platinum-based chemotherapy, unfortunately, acquired resistance eventually occurs in most patients after treatment for 9–13 months. One of the most dominant resistance mechanisms involves a secondary mutation in EGFR, T790M, which is found in ~50% of EGFR-mutant cases [9–11]. In order to overcome this resistance, the third-generation EGFR-TKI osimertinib was developed to selectively inhibit EGFR T790M as well as the sensitizing mutations. Importantly, osimertinib demonstrates better median progression-free survival and overall survival benefits than other EGFR-TKIs and exhibits remarkable activity in the central nervous system [12, 13]. However, resistance to osimertinib is still inevitable. To date, multiple mechanisms of resistance to osimertinib have been identified, such as EGFR amplification, C797S mutation, T790M loss, MET amplification, HER2 amplification, alternative kinase activation, and histological transformation. However, additional mechanisms underlying resistance remain to be fully understood, and novel therapeutic strategies are needed to overcome the resistance.
One of the hallmarks of cancer is genomic instability. DNA damage response (DDR) pathways can sense DNA lesions, initiate signaling and promote repair of damaged DNA. The key proximal DDR signaling components are ataxia-telangiectasia mutated (ATM), ataxia telangiectasia and Rad3-related protein (ATR), and DNA-dependent protein kinase (DNA-PK) [14–16]. ATM-mediated homologous recombination repair (HR) and DNA-PK-mediated nonhomologous end joining (NHEJ) are the two dominant repair pathways for DNA double-strand breaks (DSBs), one of the most deleterious forms of DNA lesions [17]. HR results in high-fidelity repair, while NHEJ is an intrinsically error-prone pathway. In NHEJ, DSBs are recognized by the Ku protein, which binds and activates DNA-PK, leading to recruitment and activation of DNA repair proteins. As a member of the phosphoinositide 3 lipid kinase (PI3K)-related protein kinase (PIKK) family, DNA-PK has become a potential therapeutic target for cancer therapy. DNA-PK deficiency sensitizes cancer cells to radiotherapy, chemotherapy, and other DSB-inducing agents [18, 19]. However, whether DNA-PK inhibition will benefit molecular targeted therapy in lung cancer is unclear.
Accumulating data suggest that EGFR plays an important role in DNA repair [20–22]. EGFR has been shown to translocate to the nucleus to interact with DNA repair proteins and regulate DNA damage repair following chemotherapy, radiotherapy or gefitinib treatment [19, 20, 23]. The activity of EGFR is associated with resistance to radiotherapy and chemotherapy. In addition, blockade of EGFR with anti-EGFR antibodies can enhance the sensitivity to radiation therapy [24, 25]. However, it remains unknown whether DNA repair pathways are involved in EGFR-TKI resistance.
In this study, we established an osimertinib-acquired resistant lung cancer model that harbors EGFR L858R and T790M mutations. Interestingly, we found that DNA repair was compromised in osimertinib-resistant cells. Inhibition of the activity or expression of DNA-PK, a key kinase mediating NHEJ repair, enhanced the sensitivity of the cells to osimertinib. These findings reveal a novel molecular mechanism of osimertinib resistance in the aspect of DNA repair and provide a rationale for combination of DNA-PK inhibitors as a therapeutic strategy to overcome acquired osimertinib resistance in NSCLC.
Materials and methods
Cell lines, compounds, and antibodies
The NCI-H1975 human lung adenocarcinoma cell line was obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The osimertinib-resistant H1975 cell line (denoted as H1975-OR) was established by our laboratory. The cells were cultured in RPMI-1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) at 37 °C in a humidified atmosphere with 5% CO2. Osimertinib, NU7441, PI-103, VE-822, KU60019, and cisplatin were obtained from Selleck (Houston, TX, USA). LipofectamineTM 3000 was obtained from Life Technologies (Carlsbad, CA, USA). The PE-Annexin V Apoptosis Detection Kit I was obtained from BD Biosciences (San Jose, CA, USA). The comet assay reagent kit (Catalog # 4250–050-K) was obtained from Trevigen (Gaithersburg, MD, USA). EGFR, p-EGFR (Tyr1068), γH2AX, DNA-PKcs, β-actin, and p-DNA-PKcs (S2056) antibodies were purchased from Cell Signaling Technology (Cambridge, MA, USA).
Generation of cells with acquired osimertinib resistance
The H1975 cell line harboring EGFR L858R and T790M mutations was treated with increasing doses of osimertinib ranging from 30 nM to 1.5 μM over a period of 22 weeks and then maintained with 1.5 μM osimertinib over 3 months. The H1975 osimertinib-resistant cell line is denoted as H1975-OR, and the parental H1975 cell line, which was sensitive to osimertinib, is denoted as H1975-S.
Cell proliferation assay
Cell viability was determined by Cell Counting Kit-8 (CCK8) assay (Bimake.cn, Shanghai, China). Cells (4 × 103 cells/100 μL/well) were seeded in 96-well plates and then subjected to drug treatments for 72 h. After drug exposure, 10 µL of CCK8 reagent was added to each well, and the cells were cultivated at 37 °C for 2 h with 5% CO2. The absorbance at 450 nm was measured with a microplate reader (Beckman Coulter, Brea, CA, USA). The half-maximal inhibitory concentration (IC50) values were calculated using GraphPad Prism. The combination index (CI) for drug interaction was analyzed with Calcusyn software.
Crystal violet staining
Cells were seeded in a 24-well plate and incubated for 18–24 h to enable adhesion to wells. The cells were then subjected to drug treatment for 48 h. After that, the cells were fixed with cold methanol (500 μL/well) at −20 °C for 0.5 h, stained with 0.5% crystal violet staining solution (500 μL/well) at room temperature for 30 min, and then washed three times with deionized water.
Flow cytometry analysis of apoptosis and cell cycle progression
A PE-Annexin V/7-AAD Apoptosis Detection Kit was used to evaluate apoptosis and the cell cycle. Briefly, for apoptosis analysis, cells were treated with drugs for 48 h and then collected and stained with PE-Annexin V and 7-AAD for 15 min in the dark. The cells were analyzed by flow cytometry (BD Biosciences) within 1 h. The data were analyzed with FlowJo software. For cell cycle analysis, cells were fixed in 75% cold ethanol in the dark for 24 h, resuspended in stain buffer and stained with 7-AAD at room temperature for 15 min in the dark. Analysis was performed on a flow cytometer within 1 h. The data were analyzed with ModFit software.
Generation of DNA-PK-knockdown cells by lentiviral infection
The DNA-PK shRNA sequences were as follows: shDNA-PK#1, ccGGTAAAGATCCTAATTCTA; shDNA-PK#2, gcAGCCTTATTACAAAGACAT; and shDNA-PK#3, ccAGTGAAAGTCTGAATCATT. To generate stable DNA-PK-knockdown cells, 293T cells were cotransfected with DNA-PK shRNA-expressing constructs (4 μg), pCMV-dR8.2 (3 μg), and pCMV-VSVG (1 μg) helper constructs using Lipofectamine 3000 reagent (Life Technologies). Viral stocks were harvested from the culture medium after 2 days and then filtered to remove nonadherent 293T cells. To select cells that stably expressed DNA-PK shRNA, cells were plated at subconfluent densities and infected with a cocktail of 1 mL of virus-containing medium, 1 mL of regular medium, and 8 μg/mL polybrene and then selected in 1 μg/mL puromycin (Invitrogen, San Diego, CA, USA) 48 h after lentivirus infection.
Alkaline comet assay
Analkaline comet assay was performed using a comet assay reagent kit according to the manufacturer’s instructions. Briefly, after drug treatment, cells were collected, mixed gently with LM agarose, and added onto comet slides. The slides were then placed at 4 °C in the dark for 10 min and immersed in lysis solution for 1 h at 4 °C. After that, the slides were immersed in freshly prepared alkaline unwinding solution for 20 min at room temperature and subsequently electrophoresed under alkaline conditions. The slides were then fixed with 70% ethanol, stained with SYBR Gold staining solution, and visualized with a fluorescence microscope.
Immunofluorescence microscopy
Cells were fixed in ice-cold 100% methanol for 20 min at −20 °C and blocked with 5% BSA for 1 h. The cells were then incubated with primary antibodies overnight at 4 °C, washed with PBS, and further incubated with a fluorochrome-conjugated secondary antibody for 1 h at room temperature in the dark. The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) contained in the mounting reagent. Fluorescence images were captured using an Olympus microscope.
Western blotting analysis
Cells were collected and lysed on ice in RIPA lysis buffer supplemented with a protease inhibitor cocktail (MedChemExpress, Shanghai, China). The samples were centrifuged at 15,000 r/min for 15 min at 4 °C. Protein aliquots of 25 μg were loaded for SDS-polyacrylamide gel electrophoresis, transferred to a PVDF membrane (Bio-Rad, Hercules, CA, USA), and then blocked with 5% nonfat milk at room temperature for 1 h. The membrane was then incubated with primary antibodies at 4 °C overnight and then with an HRP-conjugated species-specific secondary antibody (ZSGB-BIO, Beijing, China) at room temperature for 1 h. The immunoreactive bands were visualized using Immobilon Western HRP Substrate (Millipore, Billerica, MA, USA).
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. All data are presented as the mean ± standard deviation (SD). Unpaired two-tailed t tests were used for comparisons between two groups. P < 0.05 was considered to indicate statistical significance.
Results
Generation and characterization of a lung cancer model with acquired osimertinib resistance
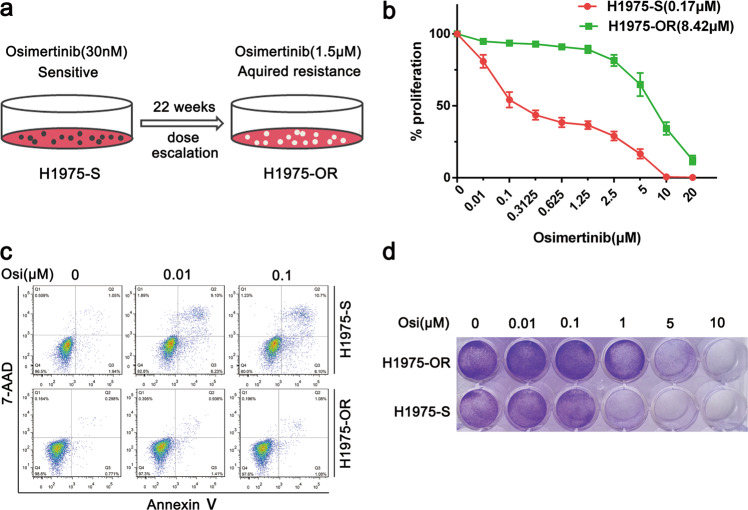
To generate a lung cancer model with acquired osimertinib resistance, we treated the NCI-H1975 NSCLC cell line, which harbors EGFR L858R and T790M mutations and is sensitive to osimertinib, with increasing doses of osimertinib for a few weeks, as indicated in Fig. 1a. Next, we determined the IC50 by CCK8 assay. As shown in Fig. 1b, the IC50 for H1975-OR cells was 8.42 μM, which was much higher than that for the parental H1975-S cells (0.17 μM). To further validate our results, we evaluated apoptosis by PE-Annexin V/7-AAD staining followed by flow cytometry. Osimertinib induced lower proportions of apoptotic cells among H1975-OR cells than among parental cells (Fig. 1c). Similarly, the crystal violet staining results demonstrated that osimertinib inhibited cell growth less significantly in H1975-OR cells than in H1975-S cells (Fig. 1d). The above results showed that the sensitivity of H1975-OR cells to osimertinib was greatly decreased, indicating that the acquired osimertinib resistance model was successfully established.
Fig. 1. Generation and characterization of a lung cancer model with acquired osimertinib resistance.
a Schematic diagram of the generation of an H1975 cell line with acquired osimertinib resistance via continuous treatment with gradually increasing doses of osimertinib from 30 nM to 1.5 μM for 22 weeks. b Evaluation of cell proliferation by CCK8 assay in the H1975 parental cell line (H1975-S) and the cell line with acquired osimertinib resistance (H1975-OR) after treatment with osimertinib at the indicated dose for 72 h. The IC50 for each cell line is indicated in parentheses. All data are presented as the mean ± SD from at least three independent experiments. c Flow cytometric analysis of apoptosis in H1975-S and H1975-OR cells after treatment with osimertinib at the indicated dose for 48 h. d Cell survival was assessed by crystal violet staining in H1975-S and H1975-OR cells treated with osimertinib at the indicated dose for 48 h.
DNA damage repair is deficient in osimertinib-resistant cells
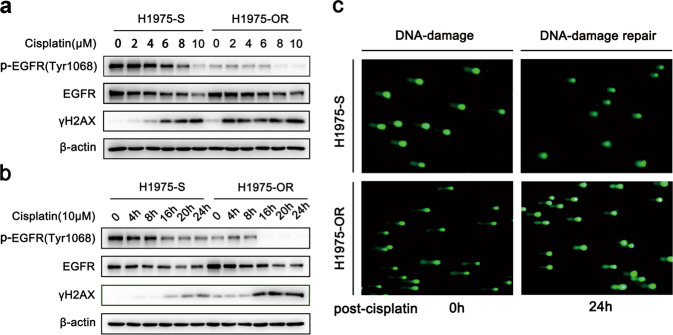
To investigate the molecular mechanisms underlying osimertinib resistance, we performed exome sequencing analysis in the resistant cell line but did not find any additional mutations in EGFR or other mutations known to be associated with resistance to EGFR-TKIs (data not shown). Given the essential role of EGFR in DNA damage repair, we therefore asked whether the DNA damage repair capacity was altered in EGFR-TKI-resistant cells, which is supposed to exhibit reduced EGFR activity upon prolonged treatment with osimertinib. To answer this question, we used cisplatin, a DNA-damaging chemotherapy drug, to treat the cells for the indicated time and at the indicated dose, and evaluated the levels of γH2AX, which is a well-established marker for DNA damage. As shown in Fig. 2a, b, compared with H1975-S cells, H1975-OR cells exhibited significantly higher levels of γH2AX in response to DNA damage induced by cisplatin, suggesting that DNA damage was enhanced in H1975-OR cells. Next, to investigate whether this phenomenon resulted from the alteration of DNA repair capacity, we evaluated the extent of DNA damage by comet assay after the cells recovered from the cisplatin treatment. As shown in Fig. 2c, after 24 h of withdrawal from cisplatin, a high comet tail intensity was still observed in H1975-OR cells but not in H1975-S cells, indicating more extensive DNA breaks and delayed DNA damage repair in H1975-OR cells compared with H1975-S cells. Thus, these results demonstrate that the DNA damage repair capacity is deficient in osimertinib-resistant cells.
Fig. 2. Osimertinib-resistant cells show deficiency in DNA damage repair.
a Western blot analysis of γH2AX and p-EGFR (Tyr1068) in H1975-S and H1975-OR cells treated with cisplatin at the indicated doses for 24 h. b Western blot analysis of γH2AX and p-EGFR (Tyr1068) in H1975-S and H1975-OR cells treated with cisplatin at the indicated time points. c Analysis of DNA comet tails by alkaline comet assay in H1975-S and H1975-OR cells treated with 5 µM cisplatin for 24 h and then released from cisplatin for 24 h to allow DNA repair.
Inhibition of DNA-PK activity or expression sensitizes resistant cells to osimertinib
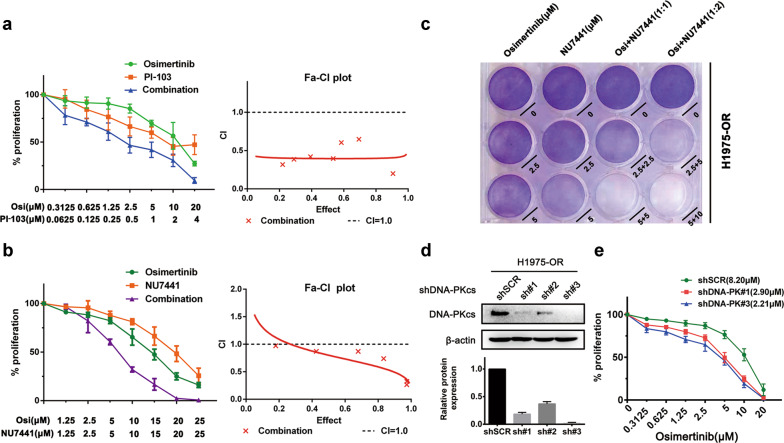
Based on the concept of “synthetic lethality”, we speculated that the vulnerability of osimertinib-resistant cells to DNA damage repair may provide potential opportunities for the use of appropriate DDR inhibitors. Next, we sought to identify which DNA repair pathways could be targeted to sensitize the resistant cells to osimertinib. We found that neither an ATM inhibitor (KU60019) nor an ATR inhibitor (VE-822) had an obvious effect on the response to osimertinib in H1975-OR cells (Supplementary Fig. 1a, b), suggesting that the ATM and ATR pathways may be dispensable for DNA repair in osimertinib-resistant cells. In contrast, we found that a combination of osimertinib and PI-103, a DNA-PK inhibitor, synergistically inhibited the proliferation of resistant cells (Fig. 2a). We further validated our findings by treating the cells with NU7441, another specific DNA-PK inhibitor tested in several clinical trials. We found that NU7441 greatly enhanced the sensitivity of the cells to osimertinib (Fig. 2b, left panel) and showed a synergistic effect with osimertinib in suppressing cell proliferation (Fig. 2b, right panel). Similar results were observed by crystal violet staining (Fig. 3c). To further investigate the role of DNA-PK in osimertinib resistance, we generated an H1975-OR cell line with stable DNA-PK knockdown (Fig. 3d) and found that DNA-PK depletion resulted in an increase in the sensitivity to osimertinib (Fig. 3e). Together, these results demonstrate that pharmacological inhibition of DNA-PK activity or genetic knockdown of DNA-PK expression sensitizes osimertinib-resistant cells to osimertinib, indicating the importance of DNA-PK for DNA repair in osimertinib-resistant cells.
Fig. 3. Inhibition of DNA-PK activity or expression sensitizes resistant cells to osimertinib.
a Evaluation of cell proliferation by CCK8 assay in H1975-OR cells treated with PI-103, osimertinib, or a combination of PI-103 and osimertinib for 72 h. The combination index (CI) and CI curve were calculated by Calcusyn software. A value <1 indicates synergy. b Evaluation of cell proliferation by CCK8 assay in H1975-OR cells treated with NU7441, osimertinib, or a combination of NU7441 and osimertinib for 72 h. The combination index (CI) and CI curve were calculated by Calcusyn software. A value <1 indicates synergy. c Crystal violet staining of H1975-OR cells after treatment with osimertinib, NU7441, or a combination of osimertinib and NU7441. d Western blot analysis of DNA-PKcs expression in H1975-OR cells with or without DNA-PK knockdown. e Evaluation of cell proliferation by CCK8 assay in osimertinib-treated H1975-OR cells with or without DNA-PK knockdown. The IC50 for each cell line is indicated in parentheses. All data are presented as the mean ± SD from at least three independent experiments.
The combination of the DNA-PK inhibitor with osimertinib induces prolonged DNA damage and cell cycle arrest
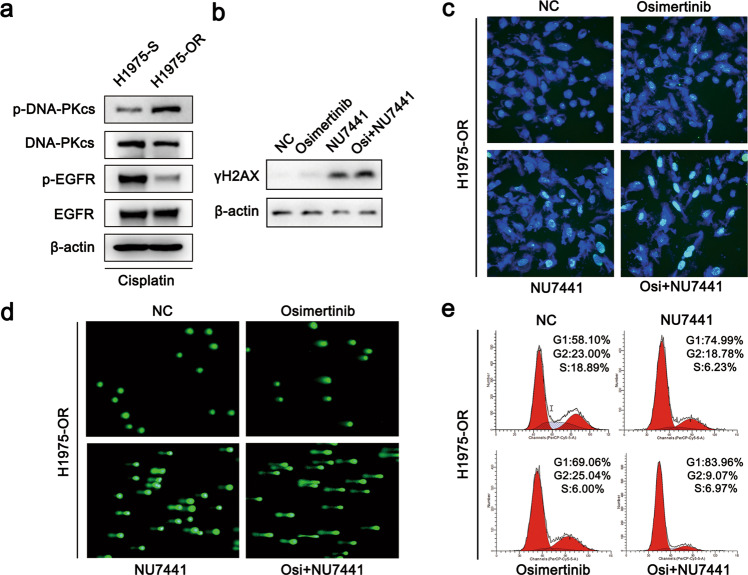
To investigate the mechanism underlying the efficacy of targeting DNA-PK in osimertinib-resistant cells, we first evaluated the activation of DNA-PK in H1975-S and H1975-OR cells in response to DNA damage induced by cisplatin. As shown in Fig. 4a, we found that the levels of p-DNA-PKcs (S2056) were markedly elevated in H1975-OR cells compared with that H1975-S cells, suggesting that H1975-OR cells may be highly reliant on DNA-PK activity for DNA repair. Combined treatment with the DNA-PK inhibitor NU7441 and osimertinib enhanced DNA damage in H1975-OR cells, as indicated by increases in the levels of rH2AX (Fig. 4b) and the numbers of rH2AX foci (Fig. 4c). Similar results were found by comet assay (Fig. 4d). Moreover, the combination treatment resulted in an induction of cell cycle arrest in H1975-OR cells (Fig. 4e). These results demonstrate that the combination of the DNA-PK inhibitor with osimertinib enhances DNA damage and induces cell cycle arrest.
Fig. 4. Combination of the DNA-PK inhibitor with osimertinib induces prolonged DNA damage and cell cycle arrest.
a Western blot analysis of p-DNA-PK (S2056) in H1975-S and H1975-OR cells treated with cisplatin for 24 h. b Western blot analysis of rH2AX in H1975-OR cells treated with NU7441 (4 μM), osimertinib (4 μM), or NU7441 (4 μM) combined with osimertinib (4 μM) for 24 h. c Immunofluorescence analysis of rH2AX in H1975-OR cells treated with NU7441 (4 μM), osimertinib (2 μM), or NU7441 (4 μM) combined with osimertinib (2 μM) for 24 h. d Evaluation of DNA damage by alkaline comet assay in H1975-OR cells treated with NU7441 (4 μM), osimertinib (4 μM), or NU7441 (4 μM) combined with osimertinib (4 μM) for 24 h. e Flow cytometric analysis of the cell cycle in H1975-OR cells treated with NU7441 (4 μM), osimertinib (4 μM), or NU7441 (4 μM) combined with osimertinib (4 μM).
Discussion
Although osimertinib has achieved impressive clinical outcomes in NSCLC patients with EGFR mutation, its efficacy is limited by the challenge of emerging resistance. It will be important to better understand the underlying molecular mechanisms and develop novel therapeutic strategies to overcome resistance to osimertinib in NSCLC. In this study, we generated a lung cancer model with acquired osimertinib resistance and identified DNA-PK as a potential target to reverse osimertinib resistance. Our study sheds new light on the molecular mechanism of osimertinib resistance in the aspect of DNA repair and provides a rationale for combined treatment with the DNA-PK inhibitor as a novel therapeutic strategy to overcome acquired osimertinib resistance in lung cancer. Given that several DNA-PK inhibitors are currently being tested in clinical trials for cancer, the in vivo antitumor synergy of those DNA-PK inhibitors in combination with osimertinib warrants further investigation in the future. Importantly, DNA-PK has also been reported to play a role in innate immunity [26, 27]; thus, it will be necessary to consider the side effects of long-term treatment with DNA-PK inhibitors.
We initiated our study by showing that the DNA repair capacity in osimertinib-resistant H1975-OR cells is decreased. This interesting phenomenon suggests that DNA damage may accumulate in the cells in response to long-term treatment with EGFR-TKIs. Based on this, we speculate that cancer cells may turn on DNA error-prone DNA repair pathways, such as DNA-PK-mediated NHEJ, to adapt to drug treatment, which would result in the generation of mutations that facilitate the emergence of drug resistance. The survival strategy used by cancer cells to develop resistance may provide new possible therapeutic strategies to overcome resistance. Consistent with our findings, a recent study [28] reported that cancer cells from patients who had received targeted therapies, including EGFR-targeted therapy, showed much higher levels of DNA damage than pretreatment samples, and combining conventional targeted cancer therapies with drugs that target DNA repair will lead to more effective therapeutic strategies.
DNA repair pathways have become promising targets in cancer treatment. The best example involves the impressive antitumor activity of PARP inhibitors in HR-deficient (HRD) tumors with BRCA mutation, which is based on the concept of “synthetic lethality” [29–31]. Here, our findings indicate that this concept of “synthetic lethality” could also be exploited to overcome EGFR-TKI resistance in NSCLC cells. We observed a synthetic lethal effect by using an EGFR-TKI in combination with a DNA-PK kinase inhibitor. EGFR has been reported to directly or indirectly regulate DDR pathways by phosphorylating DNA repair proteins [32] or driving the expression of DNA repair-related genes. Thus, we speculate that targeting EGFR together with DDR pathway components has the potential to pharmacologically induce a phenotype of DNA repair deficiency and lead to synthetic lethality. While much attention has been given to genetic alterations of key DDR drivers, our findings suggest that the contribution of such a chemical-induced DNA repair deficiency is also worth extensively exploring. In addition, this concept may provide a rationale for diverse strategies using DDR inhibitors in combination with EGFR inhibitors. Consistent with our findings, a clinical trial (NCT 01513174) on the use of olaparib, a PARP inhibitor, with gefitinib demonstrated promising antitumor activity results.
ATM-mediated HR and DNA-PK-mediated NHEJ are the two dominant repair pathways for DSBs. Our study shows that inhibiting DNA-PK, but not ATM, leads to cell cycle arrest, induces prolonged DNA damage, and increases the sensitivity of resistant cells to osimertinib. These findings indicate that the resistant cells may be heavily reliant on the DNA-PK-mediated NHEJ repair pathway, whereas other pathways, such as ATM-mediated HR, appear to be dispensable. Given the importance of EGFR activity for ATM function [32] and constant suppression of EGFR activity by long-term treatment with osimertinib, it is very likely that ATM is dysfunctional and HR is deficient in osimertinib-resistant cells. This HRD may render the cells more dependent on DNA-PK-mediated NHEJ for DNA repair. It will be of great interest to test this hypothesis by detecting HR deficiency via staining of RAD51 foci at DNA damage sites in the future. Consistent with our findings, ATM-mutant cancer cells have been reported to be sensitive to a DNA-PK inhibitor [33]. In addition, CC-115, a dual inhibitor of DNA-PK and TOR kinase, shows enhanced antitumor activity in patients with CLL harboring biallelic ATM loss [34]. Thus, ATM dysfunction and HRD may predict sensitivity to DNA-PK inhibitors, and the use of functional biomarker assays for cancer biopsies may be important to clinically determine which patients will benefit from DNA-PK inhibitors.
In summary, in this study, we demonstrated that DNA repair is compromised in osimertinib-resistant cells. Inhibition of DNA-PK, a key kinase mediating NHEJ repair, enhances DNA damage and increases the sensitivity of the resistant cells to osimertinib. Our findings provide novel molecular insights into the mechanism of resistance to osimertinib and offer a rationale for targeting DNA-PK as a therapeutic strategy to overcome acquired osimertinib resistance in lung cancer.
Supplementary information
Acknowledgements
This work was funded by the following: National Natural Science Foundation of China (81972186 and 31301160 to LLS, 81572268 to DSZ, 81702678 to LPK); Natural Science Foundation of Tianjin (20JCYBJC00360 and 16JCYBJC24400 to LLS and 17JCYBJC25500 to DSZ).
Author contributions
LLS, DSZ, and XML designed the experiments, analyzed data, and wrote the paper; XML, QQ, BNL, XQL, and LLZ performed the experiments; JW and LPK provided the reagents; and LLS and DSZ supervised the project.
Competing interests
The authors declare no competing interests.
Contributor Information
Dian-sheng Zhong, Email: dzhong@tmu.edu.cn.
Lin-lin Sun, Email: lsun1@tmu.edu.cn.
Supplementary information
The online version of this article (10.1038/s41401-020-00577-1) contains supplementary material, which is available to authorized users.
References
- 1.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57.. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 2.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–74.. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46.. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 5.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802) Ann Oncol. 2015;26:1877–83.. doi: 10.1093/annonc/mdv276. [DOI] [PubMed] [Google Scholar]
- 6.Park K, Yu CJ, Kim SW, Lin MC, Sriuranpong V, Tsai CM, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION study. JAMA Oncol. 2016;2:305–12. doi: 10.1001/jamaoncol.2015.4921. [DOI] [PubMed] [Google Scholar]
- 7.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22.. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 8.Schuler M, Wu YL, Hirsh V, O’Byrne K, Yamamoto N, Mok T, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016;11:380–90.. doi: 10.1016/j.jtho.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 9.Oxnard GR, Arcila ME, Sima CS, Riely GJ, Chmielecki J, Kris MG, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17:1616–22.. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–7. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi S, Boggon TJ, Dayaram T, Janne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–92.. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 12.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25.. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 13.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 14.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukas J, Lukas C, Bartek J. More than just a focus: the chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13:1161–9. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- 16.Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khanna KK, Jackson SP. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247–54.. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- 18.Shinohara ET, Geng L, Tan J, Chen H, Shir Y, Edwards E, et al. DNA-dependent protein kinase is a molecular target for the development of noncytotoxic radiation-sensitizing drugs. Cancer Res. 2005;65:4987–92.. doi: 10.1158/0008-5472.CAN-04-4250. [DOI] [PubMed] [Google Scholar]
- 19.Sand-Dejmek J, Adelmant G, Sobhian B, Calkins AS, Marto J, Iglehart DJ, et al. Concordant and opposite roles of DNA-PK and the “facilitator of chromatin transcription” (FACT) in DNA repair, apoptosis and necrosis after cisplatin. Mol Cancer. 2011;10:74. doi: 10.1186/1476-4598-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liccardi G, Hartley JA, Hochhauser D. EGFR nuclear translocation modulates DNA repair following cisplatin and ionizing radiation treatment. Cancer Res. 2011;71:1103–14.. doi: 10.1158/0008-5472.CAN-10-2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liccardi G, Hartley JA, Hochhauser D. Importance of EGFR/ERCC1 interaction following radiation-induced DNA damage. Clin Cancer Res. 2014;20:3496–506.. doi: 10.1158/1078-0432.CCR-13-2695. [DOI] [PubMed] [Google Scholar]
- 22.Oberthur R, Seemann H, Gehrig J, Rave-Frank M, Bremmer F, Halpape R, et al. Simultaneous inhibition of IGF1R and EGFR enhances the efficacy of standard treatment for colorectal cancer by the impairment of DNA repair and the induction of cell death. Cancer Lett. 2017;407:93–105. doi: 10.1016/j.canlet.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, Tsai SL, et al. Nuclear translocation of epidermal growth factor receptor by Akt-dependent phosphorylation enhances breast cancer-resistant protein expression in gefitinib-resistant cells. J Biol Chem. 2011;286:20558–68.. doi: 10.1074/jbc.M111.240796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milas L, Mason K, Hunter N, Petersen S, Yamakawa M, Ang K, et al. In vivo enhancement of tumor radioresponse by C225 antiepidermal growth factor receptor antibody. Clin Cancer Res. 2000;6:701–8. [PubMed] [Google Scholar]
- 25.Dittmann K, Mayer C, Kehlbach R, Rodemann HP. Radiation-induced caveolin-1 associated EGFR internalization is linked with nuclear EGFR transport and activation of DNA-PK. Mol Cancer. 2008;7:69. doi: 10.1186/1476-4598-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chu W, Gong X, Li Z, Takabayashi K, Ouyang H, Chen Y, et al. DNA-PKcs is required for activation of innate immunity by immunostimulatory DNA. Cell. 2000;103:909–18.. doi: 10.1016/S0092-8674(00)00194-X. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson BJ, Mansur DS, Peters NE, Ren H, Smith GL. DNA-PK is a DNA sensor for IRF-3-dependent innate immunity. Elife. 2012;1:e00047. doi: 10.7554/eLife.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipponi A, Goode DL, Bedo J, McCabe MJ, Pajic M, Croucher DR, et al. MTOR signaling orchestrates stress-induced mutagenesis, facilitating adaptive evolution in cancer. Science. 2020;368:1127–31.. doi: 10.1126/science.aau8768. [DOI] [PubMed] [Google Scholar]
- 29.Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377:523–33.. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 30.Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495–505.. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 31.Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381:317–27.. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee HJ, Lan L, Peng G, Chang WC, Hsu MC, Wang YN, et al. Tyrosine 370 phosphorylation of ATM positively regulates DNA damage response. Cell Res. 2015;25:225–36.. doi: 10.1038/cr.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Riabinska A, Daheim M, Herter-Sprie GS, Winkler J, Fritz C, Hallek M, et al. Therapeutic targeting of a robust non-oncogene addiction to PRKDC in ATM-defective tumors. Sci Transl Med. 2013;5:189ra78. doi: 10.1126/scitranslmed.3005814. [DOI] [PubMed] [Google Scholar]
- 34.Thijssen R, Ter Burg J, Garrick B, van Bochove GG, Brown JR, Fernandes SM, et al. Dual TORK/DNA-PK inhibition blocks critical signaling pathways in chronic lymphocytic leukemia. Blood. 2016;128:574–83.. doi: 10.1182/blood-2016-02-700328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.