Abstract
BACKGROUND:
Surveillance of patients with colorectal adenomas has limited long-term evidence to support current practice.
OBJECTIVE:
To compare the lifetime benefits and costs of high-intensity vs. low-intensity surveillance.
DESIGN:
Microsimulation model.
DATASOURCES:
U.S. cancer registry, cost data, and published literature.
TARGET POPULATION:
U.S. patients aged 50, 60 or 70 years old with low-risk adenomas (LRA; 1–2 small adenomas) or high-risk adenomas (HRA; 3–10 small or ≥1 advanced adenomas) removed after colonoscopy or FIT screening.
TIME HORIZON:
Lifetime.
PERSPECTIVE:
Societal.
INTERVENTIONS:
No further screening or surveillance, routine screening after 10 years, low-intensity surveillance 10 years after LRA and 5 years after HRA removal, and high-intensity surveillance after 5 and 3 years, respectively.
OUTCOME MEASURES:
Colorectal cancer (CRC) incidence; incremental cost-effectiveness.
BASE-CASE RESULTS:
Without surveillance or screening, lifetime CRC incidence for 50-year-olds was 10.9% after LRA and 17.2% after HRA removal at screening colonoscopy. Subsequent colonoscopy screening, low-intensity or high-intensity surveillance decreased incidence by 39%, 46–48% and 55–56%, respectively. CRC risk and surveillance benefits were higher for adenomas detected at FIT screening and lower for older patients. High-intensity vs. low-intensity surveillance cost <$30,000/QALY gained.
SENSITIVITY ANALYSIS:
High-intensity surveillance cost <$100,000/QALY gained in most alternative scenarios for adenoma recurrence, CRC risk, longevity, quality of life, screening ages, surveillance ages, test performance, disutilities, and cost.
LIMITATIONS:
Few surveillance outcome data exist.
CONCLUSIONS:
Our model suggests high-intensity surveillance, as recommended in the U.S., provides modest but clinically-relevant benefits over low-intensity surveillance at acceptable cost.
PRIMARY FUNDING SOURCE:
National Cancer Institute within the U.S. National Institutes of Health.
INTRODUCTION
Colorectal cancer (CRC) is a leading cause of cancer mortality with 50,260 deaths in the U.S. in 2017.(1) Robust evidence suggests that screening substantially reduces the risk of CRC death through removal of precancerous adenomas and early detection of CRC.(2–4) In contrast, surprisingly few CRC outcome data exist to inform the appropriate management of patients in whom adenomas have been removed. The landmark National Polyp Study (NPS) demonstrated that colonoscopy surveillance may be highly effective for reducing CRC risk (5) and that surveillance could occur at 3 years instead of 1 year.(6) Based on trials focusing on adenoma outcomes,(7, 8) longer intervals of at least 5 years have since been recommended for lower-risk patients.(9) Current U.S. surveillance guidelines recommend surveillance after 5–10 years for patients with 1–2 small tubular adenomas or sessile serrated polyps without dysplasia, and after 3 years for patients with ≥3 small tubular adenomas or ≥1 large tubular adenoma, adenoma with villous features, adenoma with high-grade dysplasia, or dysplastic or large sessile serrated polyp.(10) With such surveillance in the U.S. requiring substantial resources,(11) concerns have arisen that surveillance may not be cost-effective if colonoscopy quality increases (12–14) to the extent that removing adenomas may provide sufficient protection to defer further testing for at least 10 years.(15) Observational studies on cancer mortality risk after adenoma removal do not address surveillance intensity.(16–18) Results from trials on the intermediate-term effectiveness of more vs. less intensive surveillance after adenoma resection are not expected for at least another decade.(19)
We performed a modeling study to inform clinicians and policymakers on the potential benefits and costs of high vs. low-intensity surveillance for persons in whom adenomas have been removed. Using an established Cancer Intervention and Surveillance Modeling Network (CISNET) model of CRC that was validated against published observational outcomes during up to 20 years of follow-up, we predicted the lifetime benefits, costs and cost-effectiveness of alternative surveillance strategies in patients who have had low-risk or high-risk adenomas removed.
METHODS
Study population
The simulated study population comprised average-risk patients aged 50, 60, and 70 years following removal of low-risk adenomas (LRA) or high-risk adenomas (HRA) at screening with colonoscopy or fecal immunochemical test (FIT). LRA patients were defined as those with baseline findings of 1–2 small adenomas (<10mm in diameter), and HRA patients as those with baseline findings of 3–10 small adenomas or ≥1 large (≥10mm) adenoma. The approximately 3% of LRA patients whose 1–2 small adenomas harbor villous histology or high-grade dysplasia were not distinguished from other LRA patients in the base case (20) but were examined in sensitivity analysis. Sessile serrated polyps were not simulated due to the lack of high-quality data on their prevalence and CRC risk.(10)
Surveillance strategies
The model evaluated high-intensity vs. low-intensity surveillance strategies, with intervals of 5 vs. 10 years for LRA patients, and 3 vs. 5 years for HRA patients, respectively. Surveillance intervals or potential return to routine screening were determined based on the adenoma findings during up to two preceding examinations (Supplementary Table 1), consistent with the U.S. Multi-Society Task Force guideline recommendations.(10) Surveillance was stopped at the earliest of age 80 years, CRC diagnosis, or death. As comparison strategies, the model also evaluated no further surveillance or screening beyond the baseline exam, and as recommended for persons without prior adenoma in the U.S. (21) and for LRA patients in Europe (22), return to routine screening after 10 years with either screening colonoscopy every 10 years or annual FIT screening.
MISCAN-Colon model
The Microsimulation Screening Analysis-CRC (MISCAN-Colon) is a stochastic, semi-Markov, microsimulation model for CRC developed within the Erasmus MC University Medical Center, Department of Public Health, Rotterdam, the Netherlands. The model simulates the relevant life histories for a population of individuals who move through the model one at a time (microsimulation), with variable continuous durations in progressive states of disease (semi-Markov structure), and random variability in outcomes (stochasticity). As each simulated individual ages, one or more adenomas may develop (Supplementary Figure 1).(23) Adenomas are either progressive or non-progressive: both types may grow from small (≤5mm or 6–9mm in diameter) to large (≥10mm) size, but only progressive adenomas develop into CRC. A cancer may progress through stages I to IV without symptoms (“preclinical”) or be diagnosed during each stage because of symptoms (“clinical”). Some patients die from CRC and lose life-years, while others die from competing causes before or after developing cancer. Screening and surveillance may avert CRC death through early detection and removal of adenomas or early diagnosis of CRC (Supplementary Figure 2a) but may also result in overdiagnosis (Supplementary Figure 2b). The model predicts the associated effects, harms and costs, and has been used to inform U.S. Preventive Services Task Force (24, 25) and American Cancer Society (26, 27) CRC screening recommendations. The model, its assumptions and underlying data sources are presented in detail in Supplementary Appendix §1–2, Supplementary Table 2-3, Supplementary Figure 1-5, and previous publications.(14, 28)
Data sources
The main data sources that informed the model’s natural history assumptions include (Table 1): the observed prevalence and number of adenomas per patient in autopsy studies;(29–38) the size distribution of adenomas detected in a colonoscopy screening trial;(39) the incidence of CRC according to patient age, stage at diagnosis,(40) and anatomic site as observed in 1975–1979 Surveillance Epidemiology and End Results program data (prior to the emergence of routine CRC screening);(41) and, the relative survival after CRC diagnosis by patient age, disease stage, and anatomic site (colon, rectum).(42)
Table 1.
Natural history and screening performance characteristics.
| Model characteristics | All patients |
LRA patients * |
HRA patients * |
Ref. † | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 50–80 y | 50 y | 60 y | 70 y | 80 y | 50 y | 60 y | 70 y | 80 y | ||
| Adenoma prevalence, % | (29–38) | |||||||||
| Proportion with ≥1 adenoma, % | 100 | 39 | 56 | 66 | 100 | 58 | 73 | 78 | ||
| Proportion with ≥3 adenomas, % ‡ | 0 | 2 | 6 | 10 | 62 | 3 | 10 | 16 | ||
| Proportion with ≥1 large adenoma, % | 0 | 4 | 15 | 29 | 55 | 14 | 36 | 54 | (39) | |
| CRC incidence, 100,000 y−1 | (41) | |||||||||
| Stage I | 0 | 25 | 72 | 124 | 0 | 46 | 129 | 215 | ||
| Stage II | 0 | 38 | 124 | 240 | 0 | 68 | 228 | 441 | ||
| Stage III | 0 | 28 | 90 | 155 | 0 | 53 | 164 | 278 | ||
| Stage IV | 0 | 25 | 84 | 159 | 0 | 50 | 154 | 278 | ||
| Mortality, 100,000 y−1 | ||||||||||
| From CRC | 0 | 29 | 124 | 280 | 0 | 54 | 223 | 508 | (42) | |
| From other causes | 0 | 911 | 2061 | 5700 | 0 | 905 | 2079 | 5727 | (43) | |
| Colonoscopy performance, % | (44) | |||||||||
| Sensitivity for adenoma ≤5 mm | 75 | |||||||||
| Sensitivity for adenoma 6–9 mm | 85 | |||||||||
| Sensitivity for adenoma ≥10 mm | 95 | |||||||||
| Sensitivity for preclinical CRC | 95 | |||||||||
| Completion rate (average reach) | 95 | |||||||||
| Specificity | 85 | (45) | ||||||||
| Perforation rate | 0.5 | 0.9 | 1.7 | 3.1 | 0.5 | 0.9 | 1.7 | 3.1 | (28, 46) | |
| Fatality rate | <0.01 | (47) | ||||||||
| FIT performance, % | (48) | |||||||||
| Sensitivity for adenoma ≤5 mm | 11 | |||||||||
| Sensitivity for adenoma 6–9 mm | 16 | |||||||||
| Sensitivity for adenoma ≥10 mm | 63–89 | |||||||||
| Sensitivity for preclinical CRC | 63–89 | |||||||||
| Specificity | 96 | |||||||||
Abbreviations: CRC = colorectal cancer; FIT = fecal immunochemical test; HRA = high-risk adenoma (≥3 small adenomas or ≥1 large); LRA = low-risk adenoma (1–2 small adenomas); Ref = reference.
The prevalence, incidence, and mortality rates represent model-predicted rates for patients with LRA or HRA removed during screening colonoscopy at age 50 years, followed over time without further intervention. Adenoma prevalence and CRC incidence were calibrated to the data sources in the last column. LRAs were defined as 1–2 tubular adenomas <10 mm in diameter; HRAs were defined as ≥3 or more tubular adenomas <10mm in diameter, and/or ≥1 advanced adenoma (tubular adenoma ≥10 mm in diameter, tubulovillous adenoma, or adenoma with high-grade dysplasia). In the model histology was not described, and an advanced adenoma was considered a large adenoma.
The references informed model-predicted prevalence, incidence, and mortality rates for the U.S. general population. The numbers represent adenoma cohorts from this population.
The number of adenomas per patient is determined by an individual patient risk index, which governs that some patients will develop more adenomas then others.
The efficacy of screening and surveillance is determined by a test’s ability to detect and remove lesions. For colonoscopy, sensitivity for adenoma by size was based on a review of tandem colonoscopy studies (with two colonoscopies on the same day).(44) We assumed that colonoscopy is associated with a risk of complications that increases with age,(46) a fraction of which may be fatal,(47) and we based the sensitivity and specificity of FIT on a large observational study.(48)
Model validation
For this study, we externally validated the model-predicted adenoma and advanced adenoma detection rates in surveillance (i.e. adenoma “recurrence”) against data from multiple chemoprevention trials and prospective cohort studies cited in U.S. surveillance guidelines.(10) Studies generally had ≤5 years follow-up after removal of LRA or HRA (Supplementary Figure 6; Supplementary Table 4).(7, 8, 49–53)
The model’s CRC risk predictions were externally validated against the largest such study to date: a retrospective, multicenter cohort from the U.K. with observed CRC incidence during 10 year follow-up (median 7.9 years) of 11,944 patients with 3–4 small or 1–2 large HRAs removed between 1990–2010 (Supplementary Figure 7).(54)
The model’s CRC mortality predictions were validated against two of the largest and most cited studies: a retrospective, population-based study from Norway with observed CRC mortality during ≤19 year follow-up (median 7.7 years) of 40,826 patients with LRA and HRA removed between 1993–2007 (Supplementary Figure 8);(16) and the combined study arms of the NPS, a randomized clinical trial from the U.S. with observed CRC mortality during 20 year follow-up (median 15.8 years) of 2,602 patients with any adenoma removed between 1980–1990 (Supplementary Figure 9).(17)
The model reproduced observed detection rates of adenoma in surveillance, incidence in most patient subgroups, and mortality in NPS, but underestimated the detection rates of advanced adenoma in HRA patients, and CRC mortality vs. Løberg et al.(16) Because the base-case model had the best overall external validity (see Supplementary Appendix §3), we selected that for further analyses and examined alternative models in sensitivity analyses.
Main analysis
Primary study outcomes were lifetime CRC incidence and mortality, quality-adjusted life-years (QALYs) gained, and cost-effectiveness ratios (incremental cost/QALY gained). Other outcomes included costs and endoscopy resources used. Costs of screening, colonoscopy complications, and CRC care were computed from a societal perspective, and associated disutilities were based on estimates of the patient time involved and literature (Supplementary Table 2-3).(55) Future QALYs and costs were discounted at 3%/year. In the U.S., interventions with incremental costs of <$50,000/QALY gained are generally considered to provide good value and those with incremental costs >$100,000/QALY gained are often considered to provide low value.(56) We treated $100,000/QALY as the threshold for cost-effectiveness but present exact ratios so readers can infer cost-effectiveness at other thresholds.
Sensitivity analysis
In sensitivity analyses, we evaluated multiple alternative scenarios. First, to assess the potential impact of the model’s underestimate vs. the observed recurrence of advanced adenoma in surveillance studies and the mortality pattern in Løberg et al.,(16) we explored an alternative model with an approximately three-fold increased recurrence of advanced adenomas and CRC risk, and another version with a three-fold increased recurrence of advanced adenomas but without increased CRC risk, to retain concordance with the Surveillance Epidemiology End Result program data used for model calibration (see Supplementary Appendix §3).(41) To assess potential misclassification of adenoma risk, we re-analyzed results by assuming that the 3% of LRA patients who harbor adenomas with villous histology or high-grade dysplasia(20) behaved as if they had HRA. To assess the potential impact of comorbidities, we evaluated scenarios with increased other-cause mortality risk for LRA patients by 11% and for HRA by 43%, as observed in the Polish screening program (personal communication, Michal Kaminski), and age-dependent quality of life of 0.72–0.82 using averages from Hanmer et al.(57) Additional scenarios included routine screening prior to the baseline colonoscopy; screening ending at age 80 instead of 75 years; surveillance up to age 100 instead of 80 years; colonoscopy sensitivities varying from 0.10 lower than the base-case assumptions to 0.98 for all lesions;(58) +/−50% higher cost of colonoscopy or CRC treatment; and, +/−25% higher utility losses for colonoscopy or treatment.
Role of the funding source
The funding source had no role in the design, conduct, analysis, or reporting of this study.
RESULTS
Patients following LRA Removal
For 50-year-old patients with LRA removed at screening colonoscopy without surveillance or further screening, the model predicted that the cumulative number of CRC cases increased non-linearly from 7 per 1,000 patients after 10 years, to 72 after 30 years, and to 109 with lifetime follow-up (Figure 2, Supplementary Table 5). The benefits of return to screening or surveillance were modest after 10 years but increased with longer follow-up (Supplementary Table 5). Return to colonoscopy screening reduced lifetime CRC risk by 39%, while low-intensity surveillance decreased risk lifetime risk by 46% and high-intensity surveillance decreased risk by 55%, with similar proportionate reductions in CRC mortality (Table 2). These risk reductions yielded progressively higher gains in QALYs per 1,000 patients compared with no screening or surveillance: 114 with return to screening, 121 with low-intensity surveillance, and 144 with high-intensity surveillance (Table 2).
Figure 2.
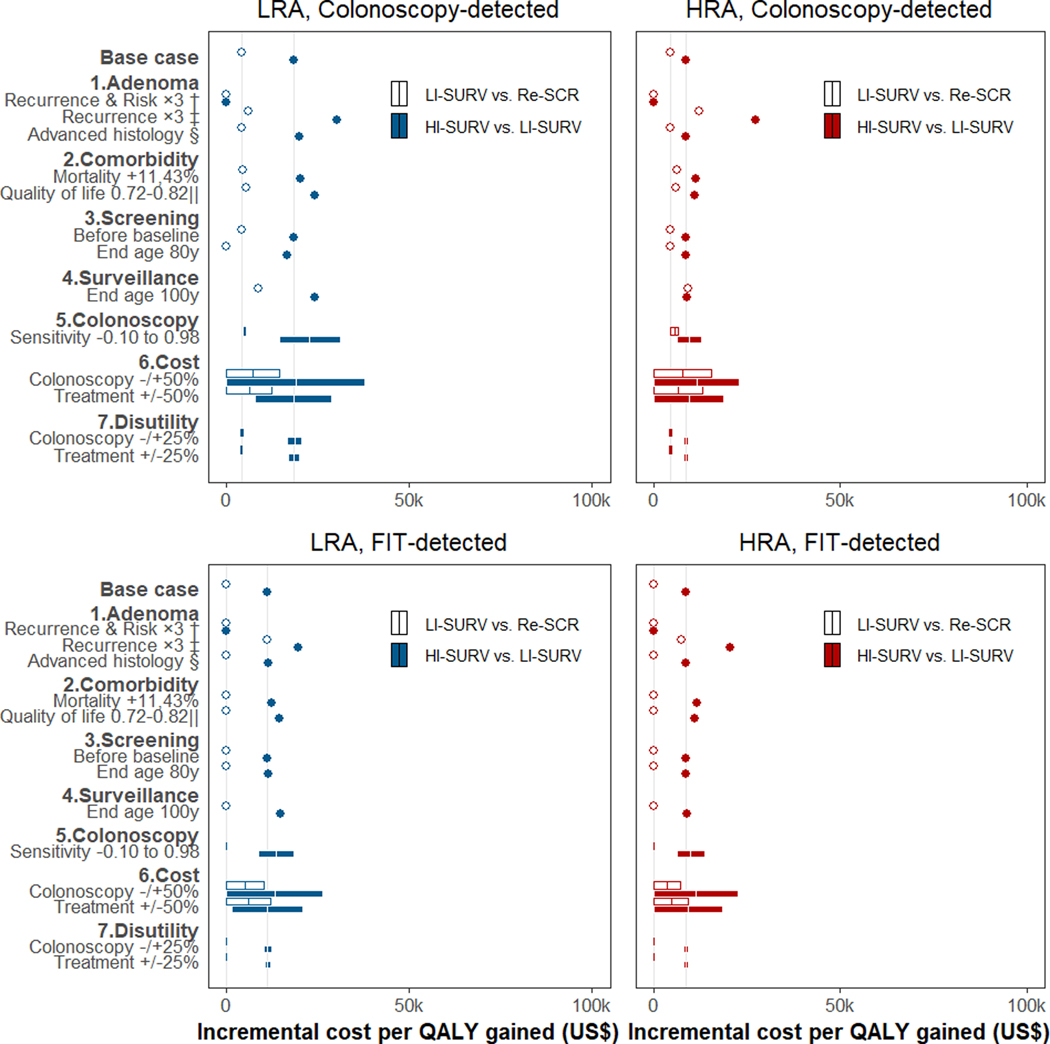
Sensitivity analysis of incremental cost-effectiveness ratios for surveillance in 50-year-old patients with adenoma detected at screening colonoscopy or FIT *
Abbreviations: HI = high-intensity; HRA = high-risk adenoma (≥3 small adenomas or ≥1 large); LI = low-intensity; LRA = low-risk adenoma (1–2 small adenomas); QALY = quality-adjusted life year; Re = return; SCR = screening; SURV = surveillance.
* Incremental cost-effectiveness for low-intensity surveillance scenarios was relative to the scenario of return to routine screening in 10 years; incremental cost-effectiveness for high-intensity surveillance was relative to low-intensity surveillance. Grey vertical lines represent the base-case ratios. Results for persons with adenomas detected by baseline screening at older ages are in Supplementary Figure 11, 13.
† The recurrence of advanced adenoma at surveillance and the associated CRC risk were increased approximately three-fold to better match some of the observed data (Supplementary Figure 6, Panel d; Supplementary Figure 8). The increase was accomplished by decreasing the assumed average time it takes for a new adenoma to become large and develop into cancer.
‡ The recurrence of advanced adenoma was increased three-fold to better match observed data (Supplementary Figure 6, Panel d), but CRC risk was kept consistent with the Surveillance Epidemiology and End Results program data used for model calibration. This was accomplished by decreasing the assumed time for a new polyp to become large, but increasing the subsequent time to cancer.
§ Results for LRA patients were corrected for misclassification of small adenoma with advanced histological features (villous histology or high-grade dysplasia) under the assumption that these lesions behave similar to HRA.
‖ The quality of life for the population was decreased with age to reflect overall deteriorating health with age.(57)
Table 2.
Health outcomes, costs, incremental cost-effectiveness ratios of adenoma surveillance strategies for 50-year-olds with adenomas detected at screening colonoscopy or FIT *
| Outcomes per 1000 adults |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk group † | Strategy by mode of polyp detection ‡ | CRC cases | CRC deaths | QALYs § (disc.) | QALY‖ gained (disc.) | Colonoscopies | Cost (thousand $; disc.) ¶ |
ΔCost/ΔQALY (disc.) ** | ||
| Colonoscopy | Treatment | Total | ||||||||
| LRA | Colonoscopy-detected | |||||||||
| No surveillance/No return to screening | 109 | 44 | 19,456 | - | 0 | 0 | 4,110 | 4,110 | Dom. | |
| Return to routine screening | 65 | 19 | 19,570 | 114 | 1,736 | 1,199 | 2,671 | 3,870 | Ref. | |
| Low-intensity surveillance | 59 | 16 | 19,577 | 121 | 2,013 | 1,341 | 2,557 | 3,898 | 4,000 | |
| High-intensity surveillance | 49 | 12 | 19,598 | 142 | 3,178 | 2,178 | 2,112 | 4,290 | 18,400 | |
| FIT-detected | ||||||||||
| No surveillance/No return to screening | 119 | 48 | 19,407 | - | 0 | 0 | 4,699 | 4,699 | Dom. | |
| Return to routine screening | 92 | 25 | 19,510 | 103 | 551 | 542 | 3,994 | 4,536 | Dom. | |
| Low-intensity surveillance | 74 | 20 | 19,530 | 123 | 1,591 | 1,144 | 3,310 | 4,454 | Ref. | |
| High-intensity surveillance | 55 | 14 | 19,565 | 158 | 3,207 | 2,211 | 2,630 | 4,841 | 11,100 | |
| HRA | Colonoscopy-detected | |||||||||
| No surveillance/No return to screening | 172 | 70 | 19,303 | - | 0 | 0 | 6,622 | 6,622 | Dom. | |
| Return to routine screening | 105 | 31 | 19,491 | 188 | 1,720 | 1,231 | 4,402 | 5,633 | Ref. | |
| Low-intensity surveillance | 89 | 23 | 19,525 | 222 | 2,712 | 1,960 | 3,824 | 5,784 | 4,500 | |
| High-intensity surveillance | 75 | 18 | 19,557 | 254 | 3,898 | 2,886 | 3,166 | 6,052 | 8,400 | |
| FIT-detected | ||||||||||
| No surveillance/No return to screening | 169 | 69 | 19,302 | - | 0 | 0 | 6,608 | 6,608 | Dom. | |
| Return to routine screening | 131 | 34 | 19,462 | 159 | 600 | 581 | 5,532 | 6,113 | Dom. | |
| Low-intensity surveillance | 88 | 23 | 19,520 | 218 | 2,709 | 1,955 | 3,901 | 5,856 | Ref. | |
| High-intensity surveillance | 75 | 18 | 19,553 | 251 | 3,883 | 2,872 | 3,258 | 6,131 | 8,400 | |
Abbreviations: CRC = colorectal cancer; disc. = discounted; Dom. = dominated (less effective and more costly); FIT = fecal immunochemical test; HRA = high-risk adenoma; ICER = incremental cost-effectiveness ratio; LRA = low-risk adenoma; QALY = quality-adjusted life-year; Ref. = reference scenario.
Adenomas were detected through colonoscopy screening or at colonoscopy after FIT screening.
LRAs were defined as 1–2 tubular adenomas <10 mm in diameter; HRAs were defined as ≥3 or more tubular adenomas <10mm in diameter, and/or ≥1 advanced adenoma (tubular adenoma ≥10 mm in diameter, tubulovillous adenoma, or adenoma with high-grade dysplasia). In the model histology was not described, and an advanced adenoma was considered a large adenoma.
There were four scenarios evaluated: No surveillance/No return to routine screening consisted of a baseline examination only; Return to routine screening consisted of continued colonoscopy screening after 10 years through age 70 years for colonoscopy-detected patients, and return to FIT screening through age 75 years for FIT-detected patients; Low-intensity surveillance consisted of a colonoscopy after 5 years in case of HRA detection, and colonoscopy after 10 years after detection of LRA, and 10 years or return to screening in case of no detected adenoma (Supplementary Table 1), with a stopping age of 80 years; High-intensity surveillance consisted of a colonoscopy after 3 years in case of an HRA, colonoscopy after 5 years in case of an LRA; and colonoscopy after 10 years in case of no detected adenoma in surveillance (Supplementary Table 1), with a similar stopping age of 80 years.
QALYs were discounted by 3% per annum to baseline at age 50, 60, or 70 years, respectively. See Supplementary Table 7 for a specification of quality of life adjustments.
QALY gained are presented compared to No surveillance/No return to screening.
Costs were discounted by 3% per annum to baseline at age 50, 60, or 70 years, respectively. Cost of colonoscopy complications were included. See Supplementary Table 7 for a specification of cost components.
Additional cost per QALY gained for some surveillance strategy compared to the next less effective, non-dominated strategy.
High-intensity vs. low-intensity surveillance vs. return to screening (Table 2) increased colonoscopy usage but also lowered CRC care costs (Table 2). Because the lower CRC care costs with return to screening vs. no screening or surveillance more than offset the higher colonoscopy costs, return to screening became the reference strategy for 50-year-olds. The cost-effectiveness ratios of $4,000/QALY gained for low-intensity surveillance vs. resumed screening and of $18,400/QALY gained for high-intensity vs. low-intensity surveillance fell well below the cost-effectiveness threshold (Table 2).
In patients with LRA identified and removed through FIT screening, the lifetime CRC incidence and mortality risks were higher than when identified through screening colonoscopy, and surveillance yielded greater incremental benefits over return to FIT screening (Table 2). Thus, the cost-effectiveness of high-intensity vs. low-intensity surveillance was more favorable at $11,100 than in colonoscopy-identified patients, and low-intensity surveillance was cost-saving (more effective and less costly) than resuming FIT screening.
For older patients with LRA removed at ages 60 or 70 vs. 50 years, the lifetime CRC incidence and mortality risks were lower as was the benefit of return to screening or surveillance (Supplementary Tables 6, 8-9). Nevertheless, the associated gains in QALYs for high-intensity vs. low-intensity surveillance had an incremental cost-effectiveness <$30,000/QALY gained (see Supplementary Tables 7, 10).
Patients following HRA Removal
For 50-year-old patients with HRA removed at screening colonoscopy, the lifetime CRC risk was 172 per 1,000, 1.6 times higher than the risk in LRA patients (Table 2). Surveillance benefit in these patients was also modest after 10 years of follow-up (Supplementary Table 5), but return to colonoscopy screening, low-intensity surveillance, and high-intensity surveillance yielded progressively larger reductions in lifetime CRC incidence and mortality, with relative benefits comparable to those in LRA patients (Table 2). Despite more frequent surveillance colonoscopy in patients with HRA (Table 2), the incremental cost-effectiveness of high-intensity vs. low-intensity surveillance was $8,400/QALY gained (Table 2).
The overall risk and benefit of surveillance in patients with HRA identified and removed through FIT screening, vs. colonoscopy screening, were similar (Table 2). However, the relative benefit of return to screening was smaller, so low-intensity surveillance was cost-saving vs. return to screening. The incremental cost-effectiveness for high- vs. low-intensity surveillance was $8,400.
As expected, the clinical and economic outcomes benefit of return to screening and surveillance were lower for patients identified with HRA at ages 60 or 70 vs. 50 years (Supplementary Tables 5, 8). Nonetheless, the cost-effectiveness of high-intensity vs. low-intensity surveillance fell below $20,000/QALY gained (see Supplementary Tables 7, 10).
Sensitivity analyses
In sensitivity analyses, all evaluated scenarios for patients aged 50 years resulted in incremental cost-effectiveness ratios below $50,000/QALY gained for high- vs. low-intensity surveillance vs. routine screening (Figure 2). The scenario with three-fold increased recurrence and risk of advanced adenoma resulted in high-intensity vs. low-intensity surveillance being cost-saving. With increased adenoma recurrence but no change in CRC risk, cost-effectiveness ratios were higher but exceeded the cost-effectiveness threshold only for high-intensity surveillance in LRA patients detected through screening colonoscopy at age 70 years ($103,900/QALY gained; Supplementary Figure 11, 13). Scenarios with cost-effectiveness ratios exceeding $50,000/QALY gained included 50% increased colonoscopy cost ($63,000/QALY gained), surveillance up to age 100 instead of 80 ($74,200/QALY gained), and near-perfect colonoscopy quality ($455,600/QALY gained). Alternative assumptions regarding adenoma histology, other-cause mortality, quality of life, screening utilization, treatment costs, and disutilities for colonoscopy and treatment had more subtle effects irrespective of age.
DISCUSSION
Our model, which was validated against published surveillance outcome data, found that high-intensity surveillance in patients with colorectal adenomas removed at screening (after 3 years for HRA and 5 years for LRA) provided moderate additional lifetime clinical benefits compared to low-intensity surveillance (after 5 years for HRA and 10 years for LRA) at an acceptable incremental cost-effectiveness ratio for a wide variety of scenarios, settings, and patients. These findings support current U.S. guidelines that recommend surveillance colonoscopy in 3 years for patients with HRA, and suggest that a 5-year surveillance interval may be reasonable in patients with LRA.(10)
In contrast to our model’s results, previous modeling studies suggested that surveillance may not be cost-effective in LRA patients.(13, 59) A recent study primarily informed by Dutch data suggested that surveillance may not be cost-effective vs. continued FIT screening at the Dutch willingness-to-pay threshold of €36,602/life-year gained based on the Dutch gross domestic product per capita.(59) Potential explanations for the difference between model results include lower Dutch vs. U.S. CRC treatment costs(60), lower Dutch willingness-to-pay thresholds, and our model’s higher predicted lifetime CRC risk for adenoma patients based on our extensive calibration and validation.
Ideally, the predicted effectiveness of surveillance regimens would be validated against data from randomized clinical trials, but no such data currently exist. Our model’s colonoscopy performance assumptions were informed directly by data,(44) and its predictions for colonoscopy’s effect are reasonable compared to observational data (Supplementary Figures 3-5).(14) The model’s predicted adenoma recurrence rates are consistent with various prospective studies cited in U.S. guidelines (Supplementary Figure 6),(10, 61) and may even underestimate recurrence of advanced adenomas in HRA patients. Similarly, comparisons of CRC incidence and mortality predictions to observational data from settings with limited surveillance suggest we may underestimate intermediate-term adenoma recurrence and/or risk (Supplementary Figure 7-8).(16, 54) The model’s intermediate-term predictions are consistent with the following: 1) 10-year CRC risk among PLCO participants with nonadvanced adenoma,(18) 2) 10-year observed CRC incidence from a British study comparing HRA patients receiving one or multiple surveillance examinations (Supplementary Figure 7), (54) 3) high observed standardized incidence rates from Northern Ireland,(62) and 4) observed 20-year CRC mortality from NPS (Supplementary Figure 9).(17)
Intuitively, the model’s possible underestimates of adenoma recurrence and CRC risk would bias our results against high-intensity surveillance. Indeed, in sensitivity analyses, when increasing adenoma recurrence and CRC risk to levels more similar to those in Løberg et al,(16) high-intensity surveillance was cost-saving (Figure 2); when only increasing adenoma recurrence, the cost-effectiveness of high-intensity screening exceeded $100,000/QALY gained only for 70-year-olds with LRA. Some of the discrepancies between the base-case model and external validation studies may be due to selection of patients with excess risk of comorbidities and other-cause death,(16, 54) symptom-based rather than screen-based detection,(16) and initial use of flexible sigmoidoscopy for colon examination.(16) Therefore, we feel our base-case model provides a reasonably representative model of post-polypectomy CRC risk overall.
This study has limitations. Despite extensive model validation, there remains uncertainty regarding CRC development over time across individuals. The model suggests that low-intensity surveillance provides similar benefits to high-intensity surveillance for CRC incidence reduction in the intermediate-term,(19) but that high-intensity surveillance accrues greater benefits over longer periods of time. Because no studies with follow-up longer than 20 years are available, it remains uncertain whether patients, after adenoma removal, follow a lower-risk trajectory that extends the approximately linear intermediate-term cumulative incidence pattern observed in some studies (16,18) or MISCAN-Colon’s predicted rise in long-term risk.
Our model does not distinguish adenoma histology or consider sessile serrated polyps separately. For adenomas, observational studies suggest that large size or having three or more adenomas are the main independent determinants of advanced adenoma recurrence.(7) Presence of villous histology and high-grade dysplasia are strongly correlated with large size, with occurrence in <3% of small adenomas.(20) In our sensitivity analysis, considering these lesions to have the risk of HRAs, as opposed to LRAs as in our base case, had limited impact on model conclusions. Regarding serrated polyps, uncertainties persist as to their prevalence when corrected for detection bias and screening contamination and to their CRC risk.(63) As an extreme hypothetical example, if 30% of observed CRCs are attributable to serrated polyps,(63) then our model overestimates the CRC risk attributed to adenomas by 43% (100%/70%=1.43, because our model does not consider serrated polyps). Even if the model overestimated the benefits and reduced CRC costs of surveillance by 43%, the incremental cost-effectiveness ratio for high-intensity surveillance in 50-year-old LRA patients would not exceed $50,000. However, if surveillance also reduces CRC risk in patients with serrated polyps, the model would suggest timely surveillance may provide additional benefit for patients with synchronous serrated polyps.(58, 64)
In the absence of randomized trials, we believe that our model results have important clinical implications for the appropriate intensity of surveillance. Most notably, our model counterbalances previous modeling studies advocating against any surveillance in LRA patients.(13, 59) The base-case and sensitivity analyses suggest that high-intensity surveillance is reasonable, except after high-quality baseline colonoscopies in older LRA patients that clear nearly all polyps (Supplementary Figure 11). Although high-intensity surveillance yielded incremental clinical benefits at acceptable additional costs, these incremental gains were smaller than the incremental gains of return to routine screening vs. no further surveillance or screening, and high-intensity surveillance required substantially more colonoscopies than low-intensity surveillance or return to screening. Colonoscopy carries a relatively low but non-negligible risk of serious complications,(65) and the procedure and preparation may be unpleasant. Thus, for patients with a strong aversion to colonoscopy, or in settings with limited colonoscopy resources, less intensive or intrusive strategies may be acceptable in trade-off against lifetime CRC risk.
In conclusion, this modeling study suggests that high-intensity surveillance after adenoma removal achieves modest incremental clinical benefits at acceptable cost compared to low-intensity surveillance or return to routine screening. Our results support the current U.S. recommendations for 3-year surveillance after HRA removal, and suggest that 5-year surveillance after LRA removal is reasonable. Less intensive strategies could be preferred in different healthcare settings, depending on acceptable cost levels, available medical resources, and patient preferences. Evidence from planned prospective trials will be important to validate our model’s intermediate-term effectiveness predictions, but follow-up longer than 20 years may be required to adequately assess the impact of high-intensity vs. low-intensity surveillance.
Supplementary Material
Figure 1.
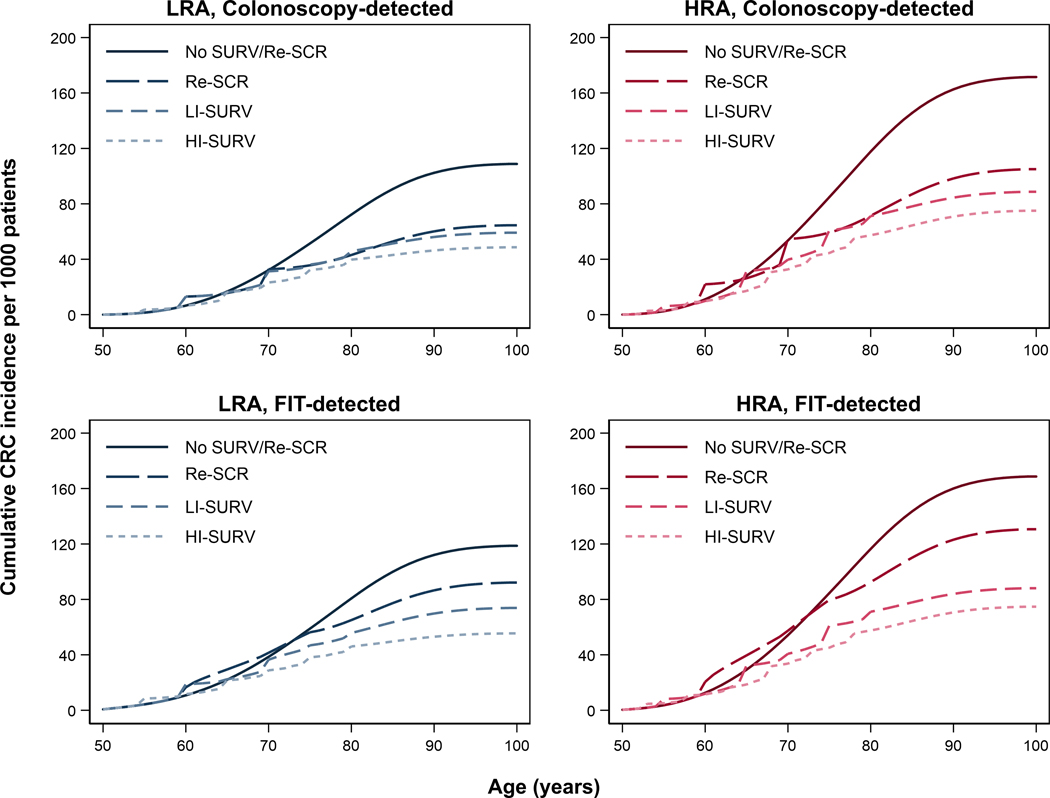
Lifetime colorectal cancer incidence in 50-year-olds with adenomas detected at screening colonoscopy or FIT * †
Abbreviations: COL = colonoscopy; Cont = continued; CRC = colorectal cancer; FIT = fecal immunochemical test; HI = High-intensity; HRA = high-risk adenoma; LI = Low-intensity; LRA = low-risk adenoma; Re = return; SCR = screening; SURV = surveillance.
* Low-risk adenomas were defined as 1–2 tubular adenoma <10 mm in diameter; high-risk adenomas were defined as ≥3 tubular adenoma <10mm in diameter, and/or ≥1 advanced adenoma (tubular adenoma ≥10 mm in diameter, tubulovillous adenoma, or adenoma with high-grade dysplasia). In the model histology was not described, and an advanced adenoma was considered a large adenoma.
† There were four scenarios evaluated: No surveillance/No return to routine screening consisted of a baseline examination only; Return to routine screening consisted of continued colonoscopy screening after 10 years through age 70 years for colonoscopy-detected patients, and return to FIT screening through age 75 years for FIT-detected patients; Low-intensity surveillance consisted of a colonoscopy after 5 years in case of HRA detection, and colonoscopy after 10 years after detection of LRA, and 10 years or return to screening in case of no detected adenoma (Supplementary Table 1), with a stopping age of 80 years; High-intensity surveillance consisted of a colonoscopy after 3 years in case of an HRA, colonoscopy after 5 years in case of an LRA, and colonoscopy after 10 years in case of no detected adenoma in surveillance (Supplementary Table 1), with a similar stopping age of 80 years. The numbers associated with each of the scenarios are provided in Supplementary Tables 5 and 8.
ACKNOWLEDGMENTS
OTHER ACKNOWLEDGMENTS: We thank Michal F. Kaminski MD PhD and Magnus Løberg MD for their contributions to the model analysis (MFK; by sharing data from the Polish colorectal cancer screening program on other-cause mortality in adenoma patients) and validation (ML; for interpretation of the post-polypectomy cancer mortality data from the Norwegian cancer registry). Neither of the above received any compensation for this. We also acknowledge the late Prof. Wendy S. Atkin OBE for her important contributions to the field of colorectal cancer prevention. Two of her studies were used in this paper for model validation.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U01 CA 199335 (RM, ILV, AZ, AK) and P30 CA 008748 (SW, AZ). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
AUTHOR DISCLOSURES: None of the authors report any conflict of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–93. [DOI] [PubMed] [Google Scholar]
- 2.Holme O, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev. 2013;9:CD009259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ. 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.U. S. Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr., et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–75. [DOI] [PubMed] [Google Scholar]
- 5.Winawer SJ, Zauber AG, Ho MN, O’Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329(27):1977–81. [DOI] [PubMed] [Google Scholar]
- 6.Winawer SJ, Zauber AG, O’Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med. 1993;328(13):901–6. [DOI] [PubMed] [Google Scholar]
- 7.Martinez ME, Baron JA, Lieberman DA, Schatzkin A, Lanza E, Winawer SJ, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136(3):832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martinez ME, Thompson P, Messer K, Ashbeck EL, Lieberman DA, Baron JA, et al. One-year risk for advanced colorectal neoplasia: U.S. versus U.K. risk-stratification guidelines. Ann Intern Med. 2012;157(12):856–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladabaum U, Schoen RE. Post-Polypectomy Surveillance That Would Please Goldilocks-Not Too Much, Not Too Little, but Just Right. Gastroenterology. 2016;150(4):791–6. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 11.Lieberman DA, Holub J, Eisen G, Kraemer D, Morris CD. Utilization of colonoscopy in the United States: results from a national consortium. Gastrointest Endosc. 2005;62(6):875–83. [DOI] [PubMed] [Google Scholar]
- 12.Austin GL, Fennimore B, Ahnen DJ. Can colonoscopy remain cost-effective for colorectal cancer screening? The impact of practice patterns and the Will Rogers phenomenon on costs. Am J Gastroenterol. 2013;108(3):296–301. [DOI] [PubMed] [Google Scholar]
- 13.Saini SD, Schoenfeld P, Vijan S. Surveillance colonoscopy is cost-effective for patients with adenomas who are at high risk of colorectal cancer. Gastroenterology. 2010;138(7):2292–9, 9 e1. [DOI] [PubMed] [Google Scholar]
- 14.Meester RG, Doubeni CA, Lansdorp-Vogelaar I, Jensen CD, van der Meulen MP, Levin TR, et al. Variation in Adenoma Detection Rate and the Lifetime Benefits and Cost of Colorectal Cancer Screening: A Microsimulation Model. JAMA. 2015;313(23):2349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loberg M, Kalager M, Holme O, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371(9):799–807. [DOI] [PubMed] [Google Scholar]
- 17.Zauber A, Winawer SJ, Waye JD et al. Colonoscopic Polypectomy and Long-Term Prevention of Colorectal-Cancer Deaths. New England Journal of Medicine. 2012;366(8):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. JAMA. 2018;319(19):2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jover R, Bretthauer M, Dekker E, Holme O, Kaminski MF, Loberg M, et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy. 2016. June;48(6):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner KO, Genta RM, Sonnenberg A. Lesions of All Types Exist in Colon Polyps of All Sizes. Am J Gastroenterol. 2018;113(2):303–6. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, Kaltenbach T, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients From the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2017;153(1):307–23. [DOI] [PubMed] [Google Scholar]
- 22.Atkin WS, Valori R, Kuipers EJ, Hoff G, Senore C, Segnan N, et al. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Colonoscopic surveillance following adenoma removal. Endoscopy. 2012;44 Suppl 3:SE151–63. [DOI] [PubMed] [Google Scholar]
- 23.Muto T, Bussey HJ, Morson BC. The evolution of cancer of the colon and rectum. Cancer. 1975;36(6):2251–70. [DOI] [PubMed] [Google Scholar]
- 24.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149(9):659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knudsen AB, Zauber AG, Rutter CM, Naber SK, Doria-Rose VP, Pabiniak C, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. JAMA. 2016. June 21;315(23):2595–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterse EFP, Meester RGS, Siegel RL, Chen JC, Dwyer A, Ahnen DJ, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124(14):2964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meester RGS, Peterse EFP, Knudsen AB, de Weerdt AC, Chen JC, Lietz AP, et al. Optimizing colorectal cancer screening by race and sex: Microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer. 2018;124(14):2974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Hees F, Habbema JD, Meester RG, Lansdorp-Vogelaar I, van Ballegooijen M, Zauber AG. Should colorectal cancer screening be considered in elderly persons without previous screening?: a cost-effectiveness analysis. Ann Intern Med. 2014;160(11):750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arminski TC, McLean DW. Incidence and Distribution of Adenomatous Polyps of the Colon and Rectum Based on 1,000 Autopsy Examinations. Dis Colon Rectum. 1964;7:249–61. [DOI] [PubMed] [Google Scholar]
- 30.Blatt LJ. Polyps of the colon and rectum: incidence and distribution. Dis Colon Rectum. 1961;4:277–82. [Google Scholar]
- 31.Bombi JA. Polyps of the colon in Barcelona, Spain. An autopsy study. Cancer. 1988;61(7):1472–6. [DOI] [PubMed] [Google Scholar]
- 32.Chapman I Adenomatous polypi of large intestine: incidence and distribution. Ann Surg. 1963;157:223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark JC, Collan Y, Eide TJ, Esteve J, Ewen S, Gibbs NM, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int J Cancer. 1985;36(2):179–86. [DOI] [PubMed] [Google Scholar]
- 34.Jass JR, Young PJ, Robinson EM. Predictors of presence, multiplicity, size and dysplasia of colorectal adenomas. A necropsy study in New Zealand. Gut. 1992;33(11):1508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand J Gastroenterol. 1989;24(7):799–806. [DOI] [PubMed] [Google Scholar]
- 36.Rickert RR, Auerbach O, Garfinkel L, Hammond EC, Frasca JM. Adenomatous lesions of the large bowel: an autopsy survey. Cancer. 1979;43(5):1847–57. [DOI] [PubMed] [Google Scholar]
- 37.Vatn MH, Stalsberg H. The prevalence of polyps of the large intestine in Oslo: an autopsy study. Cancer. 1982;49(4):819–25. [DOI] [PubMed] [Google Scholar]
- 38.Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut. 1982;23(10):835–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoop EM, de Haan MC, de Wijkerslooth TR, Bossuyt PM, van Ballegooijen M, Nio CY, et al. Participation and yield of colonoscopy versus non-cathartic CT colonography in population-based screening for colorectal cancer: a randomised controlled trial. Lancet Oncol. 2012;13(1):55–64. [DOI] [PubMed] [Google Scholar]
- 40.American Joint Committee on Cancer. AJCC Cancer Staging Manual. 5th ed. Philadelphia: Lippincott - Raven Publishers; 1997. [Google Scholar]
- 41.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2013 Sub (1973-2011) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2012 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2014, based on the November 2013 submission.
- 42.Rutter CM, Johnson EA, Feuer EJ, Knudsen AB, Kuntz KM, Schrag D. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst. 2013;105(23):1806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arias E United States Life Tables, 2008. National Vital Statistics Reports 2012;Volume 61(3). [PubMed] [Google Scholar]
- 44.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101(2):343–50. [DOI] [PubMed] [Google Scholar]
- 45.Schroy PC 3rd, Coe A, Chen CA, O’Brien MJ, Heeren TC Prevalence of advanced colorectal neoplasia in white and black patients undergoing screening colonoscopy in a safety-net hospital. Ann Intern Med. 2013;159(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warren JL, Klabunde CN, Mariotto AB, Meekins A, Topor M, Brown ML, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–57, W152. [DOI] [PubMed] [Google Scholar]
- 47.Gatto NM, Frucht H, Sundararajan V, Jacobson JS, Grann VR, Neugut AI. Risk of perforation after colonoscopy and sigmoidoscopy: a population-based study. J Natl Cancer Inst. 2003;95(3):230–6. [DOI] [PubMed] [Google Scholar]
- 48.Imperiale TF, Ransohoff DF, Itzkowitz SH, Levin TR, Lavin P, Lidgard GP, et al. Multitarget stool DNA testing for colorectal-cancer screening. N Engl J Med. 2014;370(14):1287–97. [DOI] [PubMed] [Google Scholar]
- 49.Chung SJ, Kim YS, Yang SY, Song JH, Kim D, Park MJ, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut. 2011;60(11):1537–43. [DOI] [PubMed] [Google Scholar]
- 50.Gupta S, Jacobs ET, Baron JA, Lieberman DA, Murphy G, Ladabaum U, et al. Risk stratification of individuals with low-risk colorectal adenomas using clinical characteristics: a pooled analysis. Gut. 2017. March;66(3):446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laiyemo AO, Murphy G, Albert PS, Sansbury LB, Wang Z, Cross AJ, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148(6):419–26. [DOI] [PubMed] [Google Scholar]
- 52.Lieberman DA, Weiss DG, Harford WV, Ahnen DJ, Provenzale D, Sontag SJ, et al. Five-year colon surveillance after screening colonoscopy. Gastroenterology. 2007;133(4):1077–85. [DOI] [PubMed] [Google Scholar]
- 53.Pinsky PF, Schoen RE, Weissfeld JL, Church T, Yokochi LA, Doria-Rose VP, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clin Gastroenterol Hepatol. 2009;7(1):86–92. [DOI] [PubMed] [Google Scholar]
- 54.Atkin W, Wooldrage K, Brenner A, Martin J, Shah U, Perera S, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316(10):1093–103. [DOI] [PubMed] [Google Scholar]
- 56.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371(9):796–7. [DOI] [PubMed] [Google Scholar]
- 57.Hanmer J, Hays RD, Fryback DG. Mode of administration is important in US national estimates of health-related quality of life. Med Care. 2007;45(12):1171–9. [DOI] [PubMed] [Google Scholar]
- 58.Zhao S, Wang S, Pan P, Xia T, Chang X, Yang X, et al. Magnitude, Risk Factors, and Factors Associated With Adenoma Miss Rate of Tandem Colonoscopy: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156(6):1661–74 e11. [DOI] [PubMed] [Google Scholar]
- 59.Greuter MJE, de Klerk CM, Meijer GA, Dekker E, Coupe VMH. Screening for Colorectal Cancer With Fecal Immunochemical Testing With and Without Postpolypectomy Surveillance Colonoscopy: A Cost-Effectiveness Analysis. Ann Intern Med. 2017;167(8):544–54. [DOI] [PubMed] [Google Scholar]
- 60.Bekelman JE, Halpern SD, Blankart CR, Bynum JP, Cohen J, Fowler R, et al. Comparison of Site of Death, Health Care Utilization, and Hospital Expenditures for Patients Dying With Cancer in 7 Developed Countries. JAMA. 2016;315(3):272–83. [DOI] [PubMed] [Google Scholar]
- 61.Dube C, Yakubu M, McCurdy BR, Lischka A, Kone A, Walker MJ, et al. Risk of Advanced Adenoma, Colorectal Cancer, and Colorectal Cancer Mortality in People With Low-Risk Adenomas at Baseline Colonoscopy: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112(12):1790–801. [DOI] [PubMed] [Google Scholar]
- 62.Coleman HG, Loughrey MB, Murray LJ, Johnston BT, Gavin AT, Shrubsole MJ, et al. Colorectal Cancer Risk Following Adenoma Removal: A Large Prospective Population-Based Cohort Study. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107(9):1315–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anderson JC, Butterly LF, Robinson CM, Weiss JE, Amos C, Srivastava A. Risk of Metachronous High-Risk Adenomas and Large Serrated Polyps in Individuals With Serrated Polyps on Index Colonoscopy: Data From the New Hampshire Colonoscopy Registry. Gastroenterology. 2018;154(1):117–27 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang L, Mannalithara A, Singh G, Ladabaum U. Low Rates of Gastrointestinal and Non-Gastrointestinal Complications for Screening or Surveillance Colonoscopies in a Population-Based Study. Gastroenterology. 2018;154(3):540–55 e8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


