Patients underwent spinal venography following catheterization of the azygous vein and then selective catheterization of the paraspinal vein followed by embolization of the vein with Onyx.
SUMMARY:
We report a consecutive case series of patients who underwent transvenous embolization of the paraspinal vein, which was draining the CSF-venous fistula, for treatment of spontaneous intracranial hypotension. These are the first-ever reported cases of this treatment for CSF-venous fistulas. All patients underwent spinal venography following catheterization of the azygous vein and then selective catheterization of the paraspinal vein followed by embolization of the vein with Onyx. All patients had improvement of clinical and radiologic findings with 4 patients having complete resolution of headaches and 1 patient having 50% reduction in headache symptoms. Pachymeningeal enhancement resolved in 4 patients and improved but did not resolve in 1 patient. Brain sag resolved in 4 patients and improved but did not resolve in 1 patient. There were no cases of permanent neurologic complications. All patients were discharged home on the day of the procedure.
CSF-venous fistulas have been increasingly recognized as a cause of spontaneous intracranial hypotension (SIH).1 The exact pathogenesis of these lesions is unclear, with potential hypotheses being that these lesions represent aberrant connections between the nerve root sleeve and a paraspinal vein or that these lesions are secondary to increased CSF drainage via spinal arachnoid granulations into adjacent radicular veins. Advanced imaging techniques such as digital subtraction myelography (DSM) and decubitus CT myelography have improved the detection of these occult lesions.2-5 A number of treatment options have been proposed for CSF-venous fistulas, including surgical ligation, nerve root skeletonization (ie, isolation of the nerve root and surrounding vasculature), and blood/fibrin patching.6 Surgical ligation has been shown to be extremely effective in treating CSF-venous fistulas, likely by means of disconnecting the venous outflow pathway of the CSF-venous fistula.
On reviewing the anatomy of the paraspinal venous system in the cervical, thoracic, and lumbar spine, it is apparent that catheter-based navigation of these veins is feasible. In fact, multiple studies dating back to the 1970s and 1980s have documented techniques for spinal phlebography, which, in those times, were used primarily to diagnose mass effect from disc herniations and spinal masses and have shown that catheter-based venography of the paraspinal venous system is safe.7-9 Given the relatively simple organization of the paraspinal venous system, we considered it feasible to navigate into these veins and close the venous outflow of the CSF-venous fistula using liquid embolic materials such as Onyx (Covidien). Herein, we report the first series of patients undergoing transvenous embolization of paraspinal veins draining CSF-venous fistulas for treatment of SIH.
MATERIALS AND METHODS
Patient Selection
Following institutional review board approval at the Mayo Clinic, we retrospectively reviewed our series of patients with CSF hypotension secondary to definite CSF-venous fistula diagnosed on DSM. All patients had imaging evidence of CSF hypovolemia on MR imaging, including brain sag and pachymeningeal enhancement and no extradural fluid collection on total spine MR imaging, and all patients underwent clinical evaluation by neurology subspecialists in headache and CSF disorders. All included patients were imaged and embolized between July 2020 and August 2020, when all CSF venous fistulas were treated endovascularly only. All images were reviewed on the PACS.
DSM Technique
Our technique for DSM has been previously described.2 Briefly, all DSMs were performed with spinal angiography set to 1 frame per 1–2 seconds. A tiltable table was used to adjust spine angling. All DSMs were performed with a single plane. In general, a 20-ga spinal needle was placed at L2–L3 or below and into the thecal sac. Following return of CSF, in general, 10 mL of saline was slowly injected into the thecal sac. Following this, 5-6 mL of iohexol (Omnipaque 300; GE Healthcare) was injected in rapid fashion while the imaging system was centered on the thoracic spine, and angiography was performed. Another 5-6 mL of contrast was then injected in the thoracolumbar region, and angiography was performed. Of note, for the first run in all patients, the first rib was clearly in the FOV to allow us to clearly identify the level of the leak. For the run focusing on the thoracolumbar spine, we clearly had the lowest rib in the FOV so that rib counting could be easily performed. In general, patients were imaged on consecutive days with the left side down being performed on day 1 and right side down performed on day 2.
Patient Imaging Evaluation
All patients included underwent baseline and 2- to 3-month follow-up brain MR imaging with and without IV contrast. MR imaging sequences included precontrast sagittal T1, FLAIR, T2, and gradient recalled-echo as well as postcontrast T1-weighted imaging. MRIs were analyzed according to the methods put forth by Dobrocky et al.10 For the purpose of this study, we call this the Bern SIH score. We analyzed the following imaging findings: venous sinus distension, pachymeningeal enhancement, the presence of subdural fluid collections, suprasellar cistern effacement (defined as ≤4.0 mm), effacement of the prepontine cistern of ≤5.0 mm, and mamillopontine distance of ≤6.5 mm. Measurements were performed exactly according to the criteria described by Dobrocky et al. Three imaging findings were considered major (2 points each), including pachymeningeal enhancement, venous sinus engorgement, and suprasellar cistern effacement. Three were considered minor (1 point each), including subdural fluid collection, effacement of the prepontine cistern, and mamillopontine distance of ≤6.5 mm. Patients were then classified as having a low, intermediate, or high probability of having a CSF leak with total Bern SIH scores of ≤2 points, 3–4 points, or ≥5 points, respectively, on a scale of 9 points. All imaging was reviewed by a single neurointerventional radiologist with 2.5 years of postfellowship experience who was not blinded to patient health information at the time of MR imaging.
Patient Clinical Evaluation
All patients underwent a comprehensive neurologic evaluation before and after endovascular therapy. For the evaluation, patients were queried regarding position-dependent symptoms, hearing loss, tinnitus, and cognitive disturbance (ie, brain fog).
Endovascular Technique
For embolization of the fistulas, our approach was to selectively catheterize the paraspinal vein that was identified as draining the fistula on the DSM and then embolize the vein using Onyx. For 4 patients, we obtained venous access in the right common femoral vein, and in 1 patient, we obtained venous access in the right internal jugular vein. Following this, we would advance a 5F Vertebral catheter (Merit Medical) in the superior vena cava. To enter the azygous vein, the catheter was steered to a point in a posterior direction, and with the patient taking a gentle inspiration, we would probe around the origin of the azygous vein with an 0.035-inch Glidewire (Terumo). Once the wire was able to access the azygous vein, we would advance the wire as far distally as possible, in general to the level of T12. Then, we would slowly advance the Vertebral catheter into the azygous vein. When the 0.035-inch wire did not provide enough support, we would use a stiff Glidewire. Following this, we would use a Headway Duo microcatheter (MicroVention) to selectively catheterize the paraspinal vein draining the fistula. To perform this procedure, we would point the tip of the Vertebral catheter in the direction of the paraspinal vein (right posterior for right-sided fistulas, left posterior for left-sided fistulas). Then, we would advance the Headway Duo microcatheter over a gently angled 14-inch wire into the paraspinal vein by probing with the wire. Once the wire was in the proximal paraspinal vein, we would advance the microcatheter.
Once the microcatheter was past the anatomic landmark of the pedicle (ie, in the proximal intercostal vein, roughly 1 cm from the site of fistula), a venogram was performed of the paraspinal vein. Then, we would proceed with embolization, starting with about 0.5 mL of Onyx 34 to build a plug that would not escape into the systemic venous system and then push about 1 mL of Onyx 18 into the network surrounding the nerve root sleeve. Onyx did enter the epidural venous plexus in 1 patient, but it was well tolerated with no resultant symptoms. Entry into the epidural plexus was identified by the flow of Onyx medial to the pedicle. In general, follow-up CT was performed for evaluation of the Onyx cast. For 1 lesion at T4, it was difficult to catheterize the T4 vein from a transfemoral route, given the sharp takeoff angle of the supreme intercostal vein off the apex of the azygous arch, so a transjugular approach was chosen.
Statistical Analysis
Descriptive statistics are reported. To compare the mean Bern SIH scores, we used a paired t test to determine whether there was improvement in imaging findings of CSF hypotension.
RESULTS
A summary of the 5 patients reported in this series is shown in the Table. Mean patient age was 60 [SD, 10] years. Four patients were men, and 1 patient was a woman. All patients had position- and Valsalva-dependent headaches. Two patients had hearing loss. Three patients had cognitive disturbances described as brain fog, and 4 patients had tinnitus.
Patient characteristics and outcomes
| Patient No. | Age (yr)/Sex | Symptoms | Fistula Level | Approach | Complications | Bern Score Pre-Tx | Bern Score Post-Tx | Improvement in Symptoms? |
|---|---|---|---|---|---|---|---|---|
| 1 | 44/M | Position- and Valsalva-dependent headache | Right T8 | Transfemoral | None | 8 | 0 | Complete resolution |
| 2 | 67/F | Position- and Valsalva-dependent headache, tinnitus, brain fog | Right T4 | Transfemoral | Pain at right T4, resolved after 1 month | 9 | 0 | Complete resolution |
| 3 | 58/M | Position- and Valsalva-dependent headache, tinnitus, brain fog, hearing loss | Right T7 | Transfemoral | None | 6 | 2 | 50% Improvement in headache and hearing loss, stable tinnitus |
| 4 | 65/M | Position- and Valsalva-dependent headache, tinnitus, brain fog | Right T4 | Transjugular | Pain at right T4, resolved after 1 month | 9 | 0 | Complete resolution |
| 5 | 68/M | Position- and Valsava-induced headache, tinnitus, hearing loss, vertigo | Bilateral T9 | Transfemoral | None | 6 | 1 | Complete resolution of headache and hearing loss, persistent tinnitus with SCC dehiscence |
Note:—SCC indicates semicircular canal; TX, treatment.
All 5 patients had bilateral DSMs. CSF-venous fistulas were located at the right T4 in 2 patients, the right T7 in 1 patient, the right T8 in 1 patient, and bilateral T9 in 1 patient. The CSF-venous fistulas are shown in Fig 1.
FIG 1.
Examples of CSF-venous fistulas from the 5 patients. A, CSF-venous fistula at the right T8 in patient 1 with contrast in the paraspinal vein (blue arrow). B, CSF-venous fistula at the right T4 in patient 2 with contrast in the paraspinal vein (blue arrows). C, CSF-venous fistula at the right T7 in patient 3 with contrast in the paraspinal vein (blue arrows). D, Large CSF-venous fistula in patient 4 with complete opacification of the right T4 paraspinal vein (blue arrow). E and F, Bilateral CSF-venous fistulas at T9 in patient 5 (blue arrows).
For the embolization procedure, the transfemoral route was used in 4 of 5 patients, and a transjugular approach, in 1 patient. In all cases, the draining paraspinal vein could be selectively catheterized and embolized with Onyx. Two patients who underwent embolization of right T4 CSF-venous fistulas reported back pain in the right T4 dermatome, which resolved after 1 month. There were no cases of permanent radiculitis or radiculopathy, no cases of new neurologic deficits after the procedure, and no access-site complications. There were no cases of embolization material entering the subarachnoid space. All patients were discharged home on the day of the procedure. No patients required narcotics for postoperative pain. No patients had rebound hypertension. An example of the steps of the embolization procedure are shown in Fig 2.
FIG 2.
Steps involved in CSF-venous fistula embolization (patient 5). A, The azygous vein is selectively catheterized using a 5F Vertebral catheter. The catheter is directed posteriorly to face the azygous vein. In this case, the patient had to take a shallow inspiration to open up the valve between the azygous vein and the superior vena cava. B, The catheter is then advanced into the azygous vein over a stiff Glidewire. This support is needed for the catheter to make the 180° turn. C, This patient had bilateral CSF-venous fistulas at the right T9. We advanced a Headway Duo microcatheter into the right T9 paraspinal vein, and a venogram was performed. The same procedure was performed for the left T9 paraspinal vein (D). E, Both veins are embolized with Onyx 34 to build a plug followed by Onyx 18. F, Postoperative CT shows excellent filling of the radicular veins at T9 bilaterally, essentially sealing off the CSF-venous fistulas.
On baseline imaging, all patients had MR imaging findings consistent with a high probability of CSF leak with a mean Bern SIH score of 7.6 [SD, 1.5]. At follow-up (median follow-up of 2 months; range, 1.5–3 months), the mean Bern score was 0.6 [SD, 0.9] (P = .002). Three patients had complete resolution of brain sag, subdural fluid collections, and pachy-meningeal enhancement with a final Bern score of 0. One patient had persistent effacement of the suprasellar cistern, and 1 patient had persistent-but-subtle pachymeningeal enhancement. Pre- and postimaging findings are shown in Figs 3 and 4.
FIG 3.
Pre- and posttreatment sagittal T1-weighted MRIs in patients 1–5. Patient 1 had a decreased mamillopontine distance, effacement of the prepontine and supracellar cistern, tonsillar descent, and engorgement of the pituitary gland. Following embolization, there was normalization of the suprasellar cistern and mamillopontine cistern distances as well as the prepontine cistern and resolution of tonsillar ectopia. Pituitary engorgement improved as well. Patient 2 had prepontine and suprasellar cistern effacement and decreased mamillopontine distance and tonsillar ectopia. Following embolization, these all resolved. Note the incidental pituitary cyst. Patient 3 had decreased mamilopontine distance and effacement of the suprasellar cistern and prepontine cistern, all of which normalized postembolization. Patient 4 had effacement of the suprasellar cistern and prepontine cistern and decreased mamillopontine distance, all of which resolved on follow-up MR imaging. Patient 5 had effacement of the suprasellar cistern and prepontine cistern. There was still suprasellar cistern effacement on posttreatment MR imaging, but the prepontine cistern distance normalized. Pt indicates patient; Pre, pretreatment; Post, postreatment.
FIG 4.
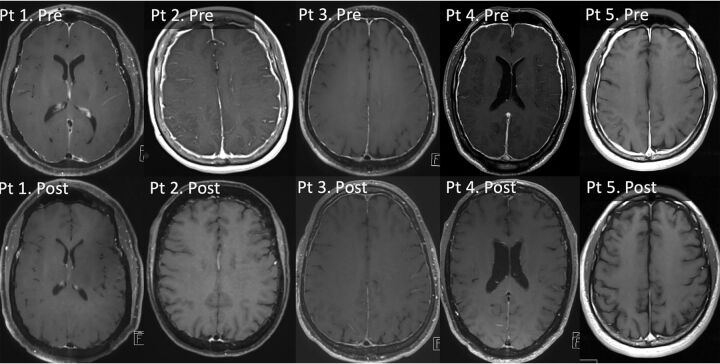
Pre- and posttreatment postcontrast T1-weighted MRIs in patients 1–5. Pretreatment MRIs show diffuse pachymeningeal enhancement in all 5 patients. Posttreatment, there is complete resolution of pachymeningeal enhancement in patients 1, 2, 4, and 5, with subtle pachymeningeal enhancement in patient 3. Pt indicates patient; Pre, pretreatment; Post, postreatment.
At last follow-up (median follow-up of 3 months; range, 2–4 months), all patients had documented clinical improvement. Three patients had complete resolution of SIH-related symptoms. One patient had complete resolution of headaches and hearing loss but had persistent tinnitus. However, this patient was found to have a semicircular canal dehiscence, which confounded the evaluation of the tinnitus. One patient, the patient with mild residual pachymeningeal enhancement, had a 50% improvement in headache and hearing loss, but persistent tinnitus.
DISCUSSION
We present the first case series of transvenous catheter–based embolization of CSF-venous fistulas causing SIH. In all cases, the fistulas were localized using DSM, and in all cases, embolization resulted in significant improvement in both imaging findings and clinical symptoms. Pain localized to the site of the embolization was a common minor complication, but in no cases did this require narcotics, and in all cases, the pain subsided in a matter of weeks. All procedures were performed on an outpatient basis, and there were no permanent neurologic deficits. Our findings are important because they suggest that CSF-venous fistulas may be amenable to obliteration via transvenous embolization and may not require surgical intervention.
Currently, surgical intervention that includes laminectomy, facetectomy, and ligation of the nerve root and associated veins has been reported to be most effective in obliterating CSF-venous fistulas.11 This intervention works by completely disconnecting the fistula, including both the CSF-filled nerve root sleeve as well as the draining vein. In a recent series of 42 patients who underwent surgical ligation of fistulas, 49% were headache-free and 27% had at least 50% im-provement at 5 months, and 80% had improvement or resolution of MR imaging findings of CSF hypotension.6 Other minimally invasive treatment options such as epidural blood patch and fibrin glue injection have been reported as potential treatment options for CSF-venous fistulas and are thought to work by compressing the fistula. However, these have been shown to be effective in a minority of patients.12
As demonstrated in this report, occlusion of the CSF draining vein with a liquid embolic agent such as Onyx leads to a reduction in shunting sufficient to result in symptomatic and radiographic cure. This effect could be due to direct occlusion of the fistula by the liquid embolic agent or by creating local venous hypertension that reverses the favorable pressure gradient for CSF to leak into the vein.13 Given that the anatomy of the nerve root sleeve has somewhat of a plexus-like organization, we have opted to use Onyx to embolize these fistulas because Onyx can permeate tinier veins and venules, especially in its more dilute formulation (Onyx 18). We build a plug with Onyx 34 because this keeps the Onyx 18 from refluxing into the azygous vein and then into the lung.
Limitations
Our case series has limitations. First, the follow-up duration ranges from 2 to 4 months in these patients. Scoring of the Bern SIH score was performed by a single neurointerventional radiologist, possibly introducing some bias. The long-term effects of this treatment have yet to be elucidated, including the possibility of delayed rebound hypertension. Given that Onyx is a permanent embolic agent and that these fistulas are slow-flow shunts with very small pressure gradients, we have no reason to believe that fistulas would recanalize. We suspect that it is more likely that patients would develop additional fistulas at other levels, which could later be embolized. Second, because patients are referred to us from multiple centers, we do not have uniform pretreatment MR imaging protocols and some MR imaging scans pretreatment were performed at 1.5T. Third, we did not collect measures such as the Headache Impact Test-6, quality of life, and other standardized questionnaires but will do so in the future. Fourth, while we do not report any technical complications in this series, there are a few complications that could potentially occur with such an embolization. One such example is that reflux of Onyx into the azygous vein could result in Onyx ending up in the lung, causing small pulmonary emboli. We suspect that these emboli would be tolerated similar to how most cement emboli following vertebroplasty are asymptomatic and well-tolerated.
CONCLUSIONS
We report the first case series of transvenous paraspinal vein embolization of a CSF-venous fistula. This novel treatment approach resulted in clinical and radiographic improvement in all patients with no permanent complications. Further studies are needed to confirm our technique and to study the durability of this treatment effect.
ABBREVIATIONS:
- DSM
digital subtraction myelography
- SIH
spontaneous intracranial hypotension
Footnotes
Disclosures: Luis Savastano—UNRELATED: Employment: VerAvanti; Stock/Stock Options: Endovascular Engineering, VerAvanti. Ivan Garza—UNRELATED: Employment: Mayo Clinic, Comments: I receive a salary for my full-time work as a neurologist at the Mayo Clinic; Royalties: UpToDate, Comments: I receive royalty payments for my work as an author for UpToDate. Jeremy K. Cutsforth-Gregory—UNRELATED: Royalties: Oxford University Press, Comments: Mayo Clinic Medical Neurosciences (textbook). Waleed Brinjikji—UNRELATED: Stock Options: Marblehead Medical LLC. Consulting Fee: Cerenovus, Microvention.* *Money paid to the institution.
References
- 1. Schievink WI, Moser FG, Maya MM. CSF-venous fistula in spontaneous intracranial hypotension. Neurology 2014;83:472–73 10.1212/WNL.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 2. Kim DK, Brinjikji W, Morris PP, et al. Lateral decubitus digital subtraction myelography: tips, tricks, and pitfalls. AJNR Am J Neuroradiol 2020;41:21–28 10.3174/ajnr.A6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Farb RI, Nicholson PJ, Peng PW, et al. Spontaneous intracranial hypotension: a systematic imaging approach for CSF leak localization and management based on MRI and digital subtraction myelography. AJNR Am J Neuroradiol 2019;40:745–53 10.3174/ajnr.A6016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kranz PG, Gray L, Amrhein TJ. Decubitus CT myelography for detecting subtle CSF leaks in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2019;40:754–56 10.3174/ajnr.A5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Amrhein TJ, Gray L, Malinzak MD, et al. Respiratory phase affects the conspicuity of CSF-venous fistulas in spontaneous intracranial hypotension. AJNR Am J Neuroradiol 2020;41:1754–56 10.3174/ajnr.A6663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duvall JR, Robertson CE, Cutsforth-Gregory JK, et al. Headache due to spontaneous spinal cerebrospinal fluid leak secondary to cerebrospinal fluid-venous fistula: case series. Cephalalgia 2019;39:1847–54 10.1177/0333102419881673 [DOI] [PubMed] [Google Scholar]
- 7. Djindjian R, Faure C. Neuro-radiological investigations (arteriography and phlebography) in vascular malformations of the spinal cord [in French]. Rontgeneur Radiodiagn Clin Eur 1963;5:171–95 [PubMed] [Google Scholar]
- 8. Tarkiainen E. Intercostal vein meningorachidography: a technical, anatomic and clinical study. Acta Radiology Diagn (Stockh) 1967(Suppl 271):1+ [PubMed] [Google Scholar]
- 9. Theron J, Djindjian R. Cervicovertebral phlebography using catheterization: a preliminary report. Radiology 1973;108:325–31 10.1148/108.2.325 [DOI] [PubMed] [Google Scholar]
- 10. Dobrocky T, Grunder L, Breiding PS, et al. Assessing spinal cerebrospinal fluid leaks in spontaneous intracranial hypotension with a scoring system based on brain magnetic resonance imaging findings. JAMA Neurol 2019;76:580–87 10.1001/jamaneurol.2018.4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang TY, Karikari IO, Amrhein TJ, et al. Clinical outcomes following surgical ligation of cerebrospinal fluid-venous fistula in patients with spontaneous intracranial hypotension: a prospective case series. Oper Neurosurg (Hagerstown) 2020;18:239–45 10.1093/ons/opz134 [DOI] [PubMed] [Google Scholar]
- 12. Kranz PG, Amrhein TJ, Schievink WI, et al. The “hyperdense paraspinal vein” sign: a marker of CSF-venous fistula. AJNR Am J Neuroradiol 2016;37:1379–81 10.3174/ajnr.A4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Scapinelli R. Antireflux mechanisms in veins draining the upper territory of the vertebral column and spinal cord in man. Clin Anat 2000;13:410–15 [DOI] [PubMed] [Google Scholar]