Abstract
BACKGROUND:
Spinal muscular atrophy is a progressive neurodegenerative disorder that can be treated with intrathecal antisense oligonucleotide therapy (nusinersen). However, administration is often complicated by posterior spinal fusion and neuromuscular scoliosis, necessitating a transforaminal approach.
PURPOSE:
To assess the safety profile of the transforaminal approach for intrathecal access.
DATA SOURCES:
Searches of the PubMed, Web of Science, and SCOPUS databases.
STUDY SELECTION:
Thirteen articles were selected based on inclusion of transforaminal access and appropriate clinical information about the procedure.
DATA ANALYSIS:
Complications were taken from the included articles and aggregated based on Cardiovascular and Interventional Radiological Society of Europe scale adverse event grading.
DATA SYNTHESIS:
Total number of complications and grade of complications were analyzed, by year and in total.
LIMITATIONS:
Selection bias in publication, small patient population size, and variability of the procedure limits the available data.
CONCLUSIONS:
Transforaminal approach is a safe alternative for intrathecal access in patients with spinal muscular atrophy and may be applicable to a larger patient population.
Spinal muscular atrophy (SMA) is a genetic disease of spinal motor neurons characterized by progressive muscle weakness and hypotonia due to progressive degeneration of motor neurons in the spine and brain stem.1,2 It is inherited in an autosomal recessive pattern and roughly 1/10,000 live births have a mutation or deletion of the survival of motor neuron 1, telomeric (SMN1) gene, resulting in SMA.1,3
In 2016, nusinersen became the first therapeutic drug approved for the treatment of SMA. The drug must be administered as an intrathecal injection because it is unable to cross the blood-brain barrier.3 Nusinersen requires intrathecal administration with loading doses on the first, 15th, 30th, and 60th day of treatment, followed by maintenance doses every 4 months for the patient's lifetime. Traditional interlaminar or interspinous lumbar puncture is difficult in this population due to a high prevalence of concomitant neuromuscular scoliosis and spinal deformity correction surgery with long-segment instrumented posterior fusion. Cervical punctures were also initially considered in some patients; however, all were precluded by the complex spinal anatomy. The transforaminal approach to lumbar puncture has emerged as an alternative approach; however, given the small number of patients with SMA in any one center, it is difficult to accurately assess a complication rate.
To assess the safety profile for our institution, we aggregated our own center's experience and compared this with the results of our comprehensive literature review. With our analysis, we hope to establish a broader, more generalizable safety profile for the transforaminal lumbar puncture that has proved to be a relatively safe and reliable method of intrathecal access as demonstrated in the SMA population.
MATERIALS AND METHODS
This retrospective study was conducted under an institutional review board protocol for Clinical Imaging and Outcomes Research. Funding was provided by the Herman and Gwendolyn Shapiro Foundation through the University of Wisconsin School of Medicine and Public Health.
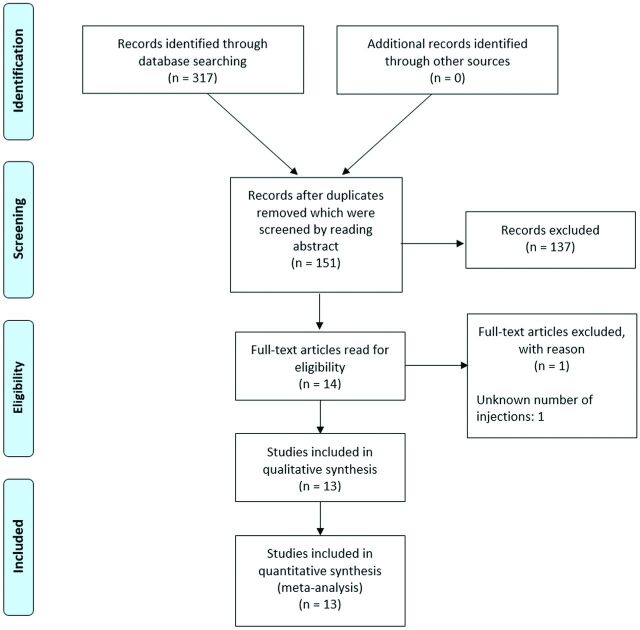
Following consultation with a health sciences librarian because no review protocol exists, a literature search was performed in PubMed, Web of Science, and SCOPUS by using the keywords “transforaminal,” “foraminal,” “spinal puncture,” “lumbar puncture,” and “intrathecal” on June 24, 2020 (Table 1). Articles were compiled, duplicates were removed, and article abstracts were reviewed for inclusion in the study. Animal research or articles that did not contain transforaminal access to the intrathecal space were excluded (Figure). Eligible articles were reviewed, and data were extracted as feasible on patient demographics, the number of transforaminal injections, procedure technique, adverse events, and conclusions of the studies. Adverse events were graded based on the Cardiovascular and Interventional Radiological Society of Europe (CIRSE) scale.3 Grade I complications are those that did not require any additional therapy or change from normal postprocedural course. Grade II complications are defined as those requiring a prolonged hospital stay but no additional therapy. Grade III complications require either additional therapy or a prolonged hospital stay (> 2 days), but without long-term sequelae. Given the small number of patients with SMA currently being treated via the transforaminal approach, all studies were included, despite the high risk of selection bias, to increase the overall power of the study.
Table 1:
Search queries as entered for the specific databases
| Data Base | PubMed | Web of Science | Scopus |
|---|---|---|---|
| Search query | (“transforaminal*"[All Fields] OR “foraminal*"[All Fields]) AND (“spinal puncture"[MeSH Terms] OR (“spinal"[All Fields] AND “puncture*"[All Fields]) OR (“lumbar"[All Fields] AND “puncture*"[All Fields]) OR “intrathecal*"[All Fields]) | TS=(transforaminal* OR foraminal*) AND TS=((spinal AND puncture*) OR (lumbar AND puncture*) OR intrathecal*) | TITLE-ABS-KEY ((transforaminal* OR foraminal*)) AND (TITLE-ABS-KEY ((spinal AND puncture*) OR (lumbar AND puncture*) OR intrathecal*)) AND (LIMIT-TO (LANGUAGE, “English”)) |
FIGURE.
Flowchart demonstrating the identification of eligible articles.
The SMA data base at our institution was queried for all patients undergoing transforaminal lumbar punctures for nusinersen administration. All patients underwent a standard procedure planning CT scan at the start of their therapy, followed by limited localization scanning at the time of procedure to minimize patient radiation exposure. All procedures were performed by using 22-ga Quincke tip spinal needles. Needle placement was performed under intermittent visualization with CT fluoroscopy aiming for the Kambin triangle in the inferior-posterior foramen. All procedures were performed in conjunction with the anesthesiology department, which administered appropriate sedation for patient comfort. Routine patient follow-up was performed before patient discharge and at regular intervals after the procedure. The number of patients, number of injections, and adverse events were collated; adverse events were also graded on the basis of the scale.3 Adverse events were collated for each year, as well as an aggregate total based on the available years' data.
RESULTS
A total of 317 articles were identified by searching the 3 data bases, in which there were 151 unique articles after duplicates were reviewed and removed. In addition, 137 records were excluded after reviewing the article abstracts, because they were either animal research studies (n = 9), did not include a transforaminal approach for intrathecal access (n = 125), or were not research articles (n = 1) and articles not available in English (n = 2). Fourteen full-text articles were then reviewed and 1 additional article was excluded because it did not include the number of injections performed (Figure). Most interesting, despite not including “SMA” in the search terms, no additional articles were identified outside of this patient population. Only 12 articles advocated for the transforaminal approach and 1 article (with the highest complication rate) argued against the safety of this approach, thus further demonstrating the selection bias present in these articles. The included articles are summarized below and in Table 2.
Table 2:
Number of injections performed by year in the literature and our data with complications, number, and ratea
| Year | Transforaminal Injections | Patients | CIRSE Grade 1 | CIRSE Grade 2 | CIRSE Grade 3 | Overall Adverse Event Rate |
|---|---|---|---|---|---|---|
| 2018 | 36 | 16 | 2 | 0 | 0 | 5.6% |
| 2019 | 69 | 16 | 1 | 0 | 0 | 1.4% |
| 2020 | 451 | 74 | 31 | 2 | 3 | 6.9% |
| Total | 556 | 106 | 34 | 2 | 3 | 7% |
| Adverse event rate | 5.8% | 0.3% | 0.5% | |||
| Institutional experience | 42 | 8 | 1 | 0 | 0 | 2.4% |
Data include the total adverse event rate categorized by CIRSE grade as well as by year.
In 2018, Mousa et al4 published a review of 26 children, undergoing a total of 104 intrathecal injections. There were 44 injections performed in 11 patients with complex spines, all of which were preceded by a noncontrast lumbar spine CT. There were 19 injections performed via an interspinous approach, 3 via cervical puncture, and 7 patients underwent a total of 22 transforaminal injections. Three of the transforaminal injections were performed by using conebeam CT (CBCT) and 19 by using a biplane fluoroscopy machine. The spinal needles were 22-ga with the length dependent on patient body habitus, but the type of needle was not noted. They report a single adverse event of meningismus and back pain at the injection site, but it is uncertain if this was a patient undergoing a transforaminal approach. For the purposes of this analysis, this was included as a CIRSE grade 1 adverse event. In addition, they note that a transforaminal approach was preferable to a cervical puncture when available.
The same year, Nascene et al5 published a review of 26 transforaminal injections in 9 patients during a 21-month period. Two patients did not have SMA (both were complicated postoperative patients requiring myelography) and 1 injection was performed with CT guidance and the other under fluoroscopy. All 7 patients with SMA underwent preprocedural imaging and CT was used for procedural guidance. The review noted that 22-ga Quincke (cutting) needles were used and 24 total injections were performed. Four patients developed self-limited headaches and 1 reported injection site pain, for a total of 5 CIRSE grade 1 adverse events. These data are not included in the 2018 data because they overlap with an ensuing article from the same group published in 2020.
Finally, the last article from 2018 was published in Muscle and Nerve by Geraci et al,6 who had a total of 14 injections in 5 patients. This article was slightly different in that patients did not undergo preprocedural imaging and instead had a full diagnostic-quality CT of the lumbar spine at the time of intrathecal injection. CT was also used to guide needle placement and a 22-ga Chiba needle was used for each procedure. A single patient developed postprocedural headache, CIRSE grade 1.
For 2018, there were a total of 36 transforaminal injections published with 2 adverse events reported, both CIRSE grade 1 (adverse event rate 5.6%).
The following year, 2019, there were 2 published studies. Bortolani et al7 performed 27 transforaminal injections in 7 patients, all of whom had preprocedural imaging. All procedures were performed under CT guidance, by using a 22-ga spinal needle in 4 patients and a 20-ga Chiba needle in 3 patients. They reported that 1 patient developed a mild headache, CIRSE grade 1. Towbin et al8 had a cohort of 9 patients who underwent 42 transforaminal injections. Patients underwent either radiography or CT before the procedure and the procedure was performed under fluoroscopic or CBCT guidance. They used 22-ga Quincke needles for most of the procedures; however, Whitacre needles were used if the patient had a history of prior spinal headache, and a coaxial approach was used with an 18-ga Chiba as a guide if necessitated by body habitus, while still using a 22-ga needle for dural puncture. No complications were reported.
For 2019, there were a total of 69 transforaminal injections published with 1 adverse event reported, CIRSE grade 1 (adverse event rate 1.4%). Combining the articles from the 2 years gives a total of 105 injections with 3 adverse events, again all CIRSE grade 1 (adverse event rate 2.9%).
Most the articles were published in the first half of 2020. Velayudhan et al9 published a series of 17 transforaminal injections in 3 patients, all of whom underwent a preprocedural planning CT and they used CT guidance for the procedure. Either a 20-ga or 22-ga Quincke needle was used for the procedure. There were 2 headaches in patients in whom 20-ga needles were used, both of which were self-limited, CIRSE grade 1. In addition, they report 3 episodes of transient radiculopathy in 2 patients, also categorized as CIRSE grade 1.
An additional single report of 1 patient undergoing a single injection was published by Cartwright et al.10 This patient was transitioned from a fluoroscopically-guided interlaminar lumbar puncture to a CT-guided transforaminal approach secondary to discomfort during prior 7 interlaminar approach procedures. No complication was reported.
Weaver et al11 published the largest series of transforaminal injections – 200 injections in 28 patients. Preprocedural planning was performed with CT and a 22-ga Quincke needle was used in 199 procedures; a single procedure was performed with a 24-ga Sprotte Spinal needle (Teleflex Medical). There were 187 procedures performed with CBCT and 13 performed under fluoroscopic guidance. Three patients developed transient radiculopathy, 2 patients developed self-limited headache, and 2 patients developed headache that responded to gabapentin for a total of 7 CIRSE grade 1 complications. One patient developed meningitis that was attributed to the nusinersen after CSF tests were negative for bacterial infection (CIRSE grade 2) and 1 patient was admitted for observation and antibiotics after possible traversal of the large bowel during the procedure (CIRSE grade 3).
Another large group of procedures, 85 transforaminal injections in 9 patients, was reported the same year by Shokuhfar et al,12 including both adult and pediatric patients. All patients underwent preprocedural planning CT and most patients underwent a fluoroscopically-guided procedure (all pediatric patients and 29 of 39 adult patients), with the remainder of procedures performed using CBCT guidance. Whitacre needles were used for all procedures; however, the gauge was not reported. Two complications were reported, but the technique used was not included. A pediatric patient developed constipation and urinary tenesmus, which was successfully treated with polyethylene glycol 3350 (Miralax) (CIRSE grade 1). An adult patient developed bilateral radicular pain after the first injection; no epidural hematoma was visualized on imaging and the pain responded to a single dose of opioid medication (CIRSE grade 1).
A different approach was described by Jacobson et al,13 using a coaxial curved-needle technique in 59 procedures performed in 12 patients. Three of the patients had preprocedural CT with the procedures performed under fluoroscopy using a 25-ga coaxial Pakter Curved Needle (Cook). One patient developed a headache that required admission for transforaminal epidural blood patch placement (CIRSE grade 3) and 2 patients had dorsal muscular arterial branch puncture during placement of the 21-ga coaxial needle without complication (CIRSE grade 1).
Özütemiz et al14 published a series of 65 transforaminal injections in 13 patients; however, there is a 10-month overlap with the previous publication from this group (Nascene et al,5 2018). It is unclear how many of these patients were included in the original analysis. For the purposes of our analysis, we elected to include this cohort of patients in the analysis. All patients underwent preprocedural low-dose CT scanning of the lumbar spine. One patient started the procedure by using fluoroscopic guidance but was transitioned to CT guidance after concern for renal puncture (without adverse outcome – CIRSE grade 1); the remaining procedures were performed under CT guidance from the initiation. The length of the needle was determined by patient body habitus with 64 patients having the procedure performed with a 22-ga Quincke needle and 1 patient with a 25-ga Quincke needle. Eight patients had postprocedural headaches, 1 of which lasted more than a day (CIRSE grade 1). One patient developed hot flashes, 2 had soreness, and 1 developed self-limited radicular pain (CIRSE grade 1). There was a single CIRSE grade 2 complication of radiculopathy requiring emergency department admittance and use of narcotic pain management.
Spiliopoulos et al15 reported on 20 transforaminal injections performed in 5 patients. All patients underwent lumbar spine CT for procedure planning with limited CT used to evaluate the needle trajectory and advancement. Procedures were performed by using 23-ga Chiba needles. No complications were reported.
Finally, an article was published by Cordts et al16 detailing their experience performing 4 transforaminal injections in 3 patients. None of the patients underwent preprocedural imaging but CT was used for procedural guidance. Needle type and length were not reported, but the needle gauge was 18–22. Two patients developed self-limited radiculopathy (CIRSE grade 1). One patient developed severe head and back pain for 1 week, necessitating hospitalization; however, the treatment was not reported (CIRSE grade 3). Given the 75% adverse event rate in their patient population, the authors advocate for consideration of laminar drilling to create a posterior approach. However, the needle gauge used in this study was significantly larger than that in any of the other publications, which may have contributed to the higher complication rate.
For 2020, there were 451 transforaminal injections published with a total of 36 adverse events (6.9% adverse event rate). Of the adverse events, 30 were CIRSE grade 1, 2 were grade 2, and 3 were grade 3. In total, this gives an aggregate of 582 injections with an overall adverse event rate of 5.8% (34 total adverse events) as detailed in Table 2. The most common type of complication was headache (n = 19), followed by radiculopathy (n = 10) and back pain/soreness (n = 4). Most of the adverse events were CIRSE grade 1 (34 – 5.8% rate) with 0.3% grade 2 and 0.5% grade 3.
When available, type of needle used in the procedure was evaluated; note that this information was not included in all studies. The number of procedures performed with cutting (Quincke) needles was 216 with 17 CIRSE grade 1 adverse events. Noncutting needle types included Whitacre, Chiba, spinal, and the Pakter Curved Needle with a total of 206 procedures performed with 7 adverse events (6 CIRSE grade 1, 1 CIRSE grade 3). The Pakter Curved Needle system was the only curved system used and had 3 adverse events of which 2 were grade 1 and 1 was grade 3 (5.1% adverse event rate). The overall adverse event rate for cutting needles was 7.9% and for noncutting needles was 3.4% (Table 3).
Table 3:
Number of adverse events by needle typea
| Needle Type | Transforaminal Injections | Adverse Event Number | CIRSE Grade 1 | CIRSE Grade 2 | CIRSE Grade 3 | Adverse Event Rate |
|---|---|---|---|---|---|---|
| Quincke | 216 | 17 | 17 | 0 | 0 | |
| Cutting needles | 7.9% | |||||
| Sprotte | 1 | 0 | 0 | 0 | 0 | |
| Whitacre | 85 | 2 | 2 | 0 | 0 | |
| Chiba | 14 | 1 | 1 | 0 | 0 | |
| Spinal or Chiba | 27 | 1 | 1 | 0 | 0 | |
| Pakter | 59 | 3 | 2 | 0 | 1 | |
| Chiba | 20 | 0 | 0 | 0 | 0 | |
| Noncutting needles | 206 | 7 | 6 | 0 | 1 | 3.4% |
Note that 1 publication reported by using either a spinal or Chiba type needle without delineation of complication rate separately.
Procedural guidance varied on the basis of operator preference; granular delineation was not included in all studies. Cross-sectional guidance techniques provide the advantage of direct visualization of other structures that may be encountered along the needle path, with a theoretic decreased risk of traversing the bowel or kidneys with these far lateral needle approaches. Most procedures were performed under CT guidance, but several authors noted transitioning patients to CT or CBCT after unsuccessful fluoroscopic procedures (Table 4). The higher adverse event rate may therefore reflect the more complicated anatomy necessitating CT guidance. The only 2 groups included in the fluoroscopically-guided procedures were the patients reported by Jacobson et al13 using the curved needle technique and the pediatric cohort reported by Shokuhfar et al.12 Towbin et al8 did not report the number of each technique used, but did note that CBCT and CT were initially used for guidance before transitioning to fluoroscopy. Nearly all of the studies included preprocedural CT for planning purposes with the following exceptions: Jacobson et al13 (curved coaxial technique), Cordts et al16 (larger needle bore), and Geraci et al6 (instead performed full-dose CT for each procedure). Given the additional variables in each of these publications, the decision was made not to separately evaluate for any correlation between performing preprocedural imaging and the adverse event rate.
Table 4:
Number of adverse events by imaging technique
| Technique | Transforaminal Injections | Adverse Event Number | CIRSE Grade 1 | CIRSE Grade 2 | CIRSE Grade 3 | Adverse Event Rate |
|---|---|---|---|---|---|---|
| CT | 133 | 19 | 17 | 1 | 1 | 14.3% |
| Fluoroscopy | 104 | 4 | 3 | 0 | 1 | 3.8% |
| CBCT | 39 | 1 | 1 | 0 | 0 | 2.6% |
At our institution, a total of 42 transforaminal injections have been performed in 8 patients. All procedures were performed with 22-ga Quincke type spinal needles with lengths of 3.5, 5, or 7 inches depending on preprocedural images obtained for planning. A single adverse event occurred (2.4% adverse event rate) in 2018, which was deemed to be a CIRSE grade 2 because the patient required inpatient hospitalization for pain management after developing a noncompressive epidural hematoma. The epidural hematoma spontaneously resolved without surgical intervention.
DISCUSSION
The advent of an effective intrathecal treatment for SMA resulting in dramatic clinical improvement has driven the need for the development and use of alternative pathways to access the subarachnoid space.17 The presence of neuromuscular scoliosis and spinal deformity correction surgery in a large number of these patients complicates the ability to use standard posterior approaches. There are publications supporting the use of cervical puncture by using both fluoroscopic and sonographic guidance;18,19 however, this can be precluded given the nonstandard occipitocervical anatomy in this patient population.
Because the transforaminal approach is technically more challenging than a traditional posterior approach – either interlaminar or interspinous – and has a theoretically potentially higher risk, it is not advocated as a primary approach at our institution. Recently numerous patient series have been published in which transforaminal lumbar puncture was used; however, these publications were limited due to relatively small patient populations. We have aggregated the published multicenter experience with the transforaminal lumbar puncture to assess the safety profile and determine a more generalizable complication rate. Most interesting, there was no clear pattern to the adverse event percentage. It did not parallel the number of transforaminal injections performed, nor the institutional experience (as reported). One study (Cordts et al16) did report a much higher adverse event rate; however, this may be related to the large needle gauge used in their procedures, because both the needle gauge and the adverse event rate seemed to be outliers. Overall, there was a low rate of significant complication from transforaminal approach lumbar puncture in the patients with SMA after spinal fusion surgery (<1% grade 2 or 3) despite the small number of total procedures (623 procedures total).
During our literature search, we found it interesting that most transforaminal lumbar puncture literature was exclusively focused on the patient population with SMA, without a single publication outside of this patient population even though our search terms were not restricted to this population. Therefore, we must acknowledge that the generalizability of this safety profile is limited to patients with SMA, and additional work would be needed to determine the safety in the general population, despite the 2 successful patients reported by Nascene et al.5 The morphology of the foraminal anatomy with a generally exaggerated craniocaudal dimension of the neural foramen may reduce the risk of procedural complications.
Although this literature review addresses the overall safety of the technique, it does not address procedural specifics. The common pathway in all publications is that the neural foramen is traversed by a needle, with a favorable safety profile; however, there is a large heterogeneity in procedural technique, including imaging guidance (CT versus fluoroscopy) and needle characteristics (needle gauge, type of tip, needle curve, and the use or absence of a guide needle). While most articles focused on CT guidance, there are several institutions that preferentially use either fluoroscopy or CBCT and some that report a combination (starting the procedures with fluoroscopy and transitioning to either CT or CBCT if difficulty was encountered). The determination of the benefits of one technique over the other is beyond the scope of this article and is dependent on multiple factors, including operator preference and experience. An additional procedural component not addressed in this review is the use of general anesthesia or other methods of sedation during the procedures; this was variable between institutions and was not regularly mentioned in the available literature. It is unlikely to affect the complication rate.
A significant limitation of our review is the inability to accurately report complications, because not all complications are likely included in the publication, resulting in significant selection bias. In addition, there is little information regarding how adverse events were reported and what postprocedural follow-up was performed, further limiting the accuracy of the adverse event rate. The published rates (<1% CIRSE grade 2 or higher) are lower than our institutional experience (2.4%); however, we have a small internal patient population with a single adverse event.
There are now both oral (risdiplam) and intravenous (onasemnogene abeparvovec-xioi) treatments for SMA; however, anecdotally, most of our patient population has continued with nusinersen treatment given their previous responses to therapy. Treatment of patients with SMA requires an interdisciplinary team approach for success. Maintaining intrathecal access is of paramount importance to ensure continued delivery of therapy. The published literature supports our anecdotal experience that the transforaminal approach has a favorable safety profile in the SMA population.
CONCLUSIONS
This literature review of all available transforaminal lumbar puncture complication data in the administration of nusinersen demonstrates a favorable safety profile with a low complication rate (<1% CIRSE grades 2 and 3). This is congruent with our own institution experience. Despite a heterogeneous approach to the procedure across institutions, it appears that this is a safe alternative option for intrathecal access in patients with SMA and may have larger applicability in the general population.
ABBREVIATIONS:
- CBCT
conebeam CT
- CIRSE
Cardiovascular and Interventional Radiological Society of Europe
- SMA
spinal muscular atrophy
Footnotes
Financial support for this work was provided by the University of Wisconsin School of Medicine and Public Health, Herman and Gwendolyn Shapiro Foundation Summer Research Program.
Disclosures: Marissa Schoepp—RELATED: Grant: Shapiro Grant, Comments: summer research funding program for medical students at the University of Wisconsin. Anthony Kuner—UNRELATED: Consultancy: Avexis Inc, Comments: Research consultant.
References
- 1. Lunn MR, Wang CH. Spinal muscular atrophy. Lancet 2008;371:2120–33 10.1016/S0140-6736(08)60921-6 [DOI] [PubMed] [Google Scholar]
- 2. Stolte B, Totzeck A, Kizina K, et al. Feasibility and safety of intrathecal treatment with nusinersen in adult patients with spinal muscular atrophy. Ther Adv Neurol Disord 2018;11:1756286418803246 10.1177/1756286418803246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filippiadis DK, Binkert C, Pellerin O, et al. CIRSE quality assurance document and standards for classification of complications: the CIRSE classification system. Cardiovasc Intervent Radiol 2017;40:1141–6 10.1007/s00270-017-1703-4 [DOI] [PubMed] [Google Scholar]
- 4. Mousa MA, Aria DJ, Schaefer CM, et al. A comprehensive institutional overview of intrathecal nusinersen injections for spinal muscular atrophy. Pediatr Radiology 2018;48:1797–805 10.1007/s00247-018-4206-9 [DOI] [PubMed] [Google Scholar]
- 5. Nascene DR, Ozutemiz C, Estby H, et al. Transforaminal lumbar puncture: an alternative technique in patients with challenging access. AJNR Am J Neuroradiol 2018;39:986–91 10.3174/ajnr.A5596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Geraci AP, Black K, Jin M, et al. Transforaminal lumbar puncture for intrathecal nusinersen administration. Muscle Nerve 2018. Jan 24 [Epub ahead of print] 10.1002/mus.26082 [DOI] [PubMed] [Google Scholar]
- 7. Bortolani S, Stura G, Ventilii G, et al. Intrathecal administration of nusinersen in adult and adolescent patients with spinal muscular atrophy and scoliosis: transforaminal versus conventional approach. Neuromuscul Disord 2019;29:742–46 10.1016/j.nmd.2019.08.007 [DOI] [PubMed] [Google Scholar]
- 8. Towbin R, Schaefer C, Kaye R, et al. The complex spine in children with spinal muscular atrophy: the transforaminal approach - a transformative technique. AJNR Am J Neuroradiol 2019;40:1422–26 10.3174/ajnr.A6131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Velayudhan V, Patel S, Danziger A, et al. Transforaminal lumbar puncture for intrathecal access: case series with literature review and comparison to other techniques. J Clin Neurosci 2020;72:114–18 10.1016/j.jocn.2019.12.056 [DOI] [PubMed] [Google Scholar]
- 10. Cartwright MS, Ward ZT, White EP, et al. Intrathecal delivery of nusinersen in individuals with complicated spines. Muscle and Nerve 2020;62:114–18 10.1002/mus.26899 [DOI] [PubMed] [Google Scholar]
- 11. Weaver JJ, Hallam DK, Chick JFB, et al. Transforaminal intrathecal delivery of nusinersen for older children and adults with spinal muscular atrophy and complex spinal anatomy: an analysis of 200 consecutive injections. J Neurointerv Surg 2021;13:75–78 10.1136/neurintsurg-2020-016058 [DOI] [PubMed] [Google Scholar]
- 12. Shokuhfar T, Abdalla RN, Hurley MC, et al. Transforaminal intrathecal access for injection of nusinersen in adult and pediatric patients with spinal muscular atrophy. J Pediatr Neurol 2020;18:88–94 10.1055/s-0039-1697583 [DOI] [Google Scholar]
- 13. Jacobson JP, Cristiano BC, Hoss DR. Simple fluoroscopy-guided transforaminal lumbar puncture: safety and effectiveness of a coaxial curved-needle technique in patients with spinal muscular atrophy and complex spines. AJNR Am J Neuroradiol 2020;41:183–88 10.3174/ajnr.A6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Özütemiz C, Karachunski P, Nascene D.R. Nusinersen injections in adults and children with spinal muscular atrophy: a single-center experience. Diagn Interv Radiol 2020;26:596–602 10.5152/dir.2020.19607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spiliopoulos S, Reppas L, Zompola C, et al. Computed-tomography-guided transforaminal intrathecal nusinersen injection in adults with spinal muscular atrophy type 2 and severe spinal deformity: feasibility, safety and radiation exposure considerations. Eur J Neurol 2020;27:1343–49 10.1111/ene.14245 [DOI] [PubMed] [Google Scholar]
- 16. Cordts I, Lingor P, Friedrich B, et al. Intrathecal nusinersen administration in adult spinal muscular atrophy patients with complex spinal anatomy. Ther Adv Neurol Disord 2020;13:1756286419887616 10.1177/1756286419887616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Claborn MK, Stevens DL, Walker CK, et al. Nusinersen: a treatment for spinal muscular atrophy. Ann Pharmacother 2019;53:61–69 10.1177/1060028018789956 [DOI] [PubMed] [Google Scholar]
- 18. Veerapandiyan A, Pal R, D'Ambrosio S, et al. Cervical puncture to deliver nusinersen in patients with spinal muscular atrophy. Neurology 2018;91:e620–24 10.1212/WNL.0000000000006006 [DOI] [PubMed] [Google Scholar]
- 19. Ortiz CB, Kukreja KU, Lotze TE, et al. Ultrasound-guided cervical puncture for nusinersen administration in adolescents. Pediatr Radiol 2019;49:136–40 10.1007/s00247-018-4240-7 [DOI] [PubMed] [Google Scholar]