Abstract
BACKGROUND AND PURPOSE:
Embolization of the middle meningeal artery for treatment of refractory or recurrent chronic subdural hematomas has gained momentum during the past few years. Little has been reported on the use of the n-BCA liquid embolic system for middle meningeal artery embolization. We present the technical feasibility of using diluted n-BCA for middle meningeal artery embolization.
MATERIALS AND METHODS:
We sought to examine the safety and technical feasibility of the diluted n-BCA liquid embolic system for middle meningeal artery embolization. Patients with chronic refractory or recurrent subdural hematomas were prospectively enrolled from September 2019 to June 2020. The primary outcome was the safety and technical feasibility of the use of diluted n-BCA for embolization of the middle meningeal artery. The secondary end point was the efficacy in reducing hematoma volume.
RESULTS:
A total of 16 patients were prospectively enrolled. Concomitant burr-hole craniotomies were performed in 12 of the 16 patients. Two patients required an operation following middle meningeal artery embolization for persistent symptoms. The primary end point was met in 100% of cases in which there were no intra- or postprocedural complications. Distal penetration of the middle meningeal artery branches was achieved in all the enrolled cases. A 7-day post–middle meningeal artery embolization follow-up head CT demonstrated improvement (>50% reduction in subdural hematoma volume) in 9/15 (60%) patients, with 6/15 (40%) showing an unchanged or stable subdural hematoma. At day 21, available CT scans demonstrated substantial further improvement (>75% reduction in subdural hematoma volume).
CONCLUSIONS:
Embolization of the middle meningeal artery using diluted n-BCA and ethiodized oil (1:6) is safe and feasible from a technical standpoint. The use of a dextrose 5% bolus improves distal penetration of the glue.
Despite traditional treatment with surgical evacuation, chronic subdural hematomas (cSDHs) tend to have an indolent course with frequent recurrences.1 In recent years, embolization of the middle meningeal artery (MMA) for treatment of refractory or recurrent cSDH has gained momentum, with recent literature showing a significant reduction in the size of the cSDH as well as lower rates of recurrence.2 The primary endovascular techniques used to date have involved the use of polyvinyl alcohol particles (PVA) and Onyx liquid embolic (ethylene-vinyl alcohol dissolved in dimethyl-sulfoxide; Medtronic). Another commonly used liquid embolic agent in the neurointerventional area is n-BCA, which is a liquid adhesive that polymerizes rapidly on contact with ionic substances and can be injected to achieve permanent vessel occlusion. The rates of polymerization and flow and the penetration depth can be modified using varying amounts of ethiodized oil as well as concurrent infusion of dextrose 5% in water (D5W) during n-BCA (Trufill, Cordis Neurovascular) injection (D5W-push technique).3 Data on the use of n-BCA as an embolic agent in cases of cSDH are extremely limited. Herein, we sought to study the safety and technical feasibility of using diluted n-BCA for embolization of the MMA for cSDHs.
MATERIALS AND METHODS
Study Population and Patient Selection
Prospective patients with chronic refractory or recurrent subdural hematomas admitted to our quaternary care level 1 trauma center (Westchester Medical Center) were enrolled from September 2019 to June 2020. Informed consent was obtained from patients and/or their families. This study was approved by the institutional review board at our institution. Enrolled patients were older than 18 years of age and had symptomatic cSDH.
We enrolled 3 subgroups of patients in our study. Patients with a previously untreated subdural hematoma (SDH) were offered treatment with MMA embolization if they were clinically symptomatic, had failed conservative management, and did not require urgent surgical evacuation. Patients with a history of SDH (acute or chronic) who underwent surgical evacuation with radiographic evidence of recurrence were offered treatment with MMA embolization if they were clinically symptomatic, had failed conservative management, but did not require urgent surgical re-evacuation. Finally, for patients who underwent surgical evacuation for acutely symptomatic SDHs due to mass effect with evidence of herniation on cranial imaging, MMA embolization was offered in the acute postoperative period as a means of prophylaxis in patients who needed to be on anticoagulation therapy, had platelet dysfunction due to systemic conditions (such as renal failure or alcohol use), or had re-accumulation or incomplete hematoma evacuation, to potentially reduce the risk of recurrence regardless of postoperative symptomatology.
Patients were excluded if the hematoma had an underlying chronic cause such as a vascular lesion, brain tumor, arachnoid cyst, or spontaneous intracranial hypotension. Patients with mixed-density subdural hematomas were excluded if the acute component exceeded 50%. Patients with ophthalmic collaterals from the MMA or other signs of dangerous anastomoses identified during angiography were excluded. Patients were also excluded if they were clinically asymptomatic.
Clinical Management
Clinical management conformed to Brain Trauma Foundation and the Neurocritical Care Society guidelines.4,5
Data Collection
Prospective comprehensive data on each patient were collected, including demographics, medical history, baseline clinical status, imaging results, as well as treatment and complications during hospitalization.
Clinical and Radiologic Variables
The diagnosis of the SDH was established by admission CT based on standard radiographic characteristics. After endovascular treatment, a follow-up CT scan was obtained on day 7, day 21, and at 3 months to assess stability and/or a change in size of the cSDH. Percentage hematoma reduction based on volume was assessed. Hematoma volume was measured by applying the ABC/2 technique modified for ellipsoid cSDH to noncontrast head CT.6
MMA Embolization Procedure
All embolization procedures were performed with the patient under general anesthesia using an arterial line for blood pressure monitoring. Femoral or radial 6F access was obtained. A Benchmark 071 (2.03 mm) Intracranial Access Catheter (Penumbra) was advanced into the common carotid artery ipsilateral to the cSDH, and intracranial angiography was performed to evaluate dangerous external carotid artery–ICA collaterals via the MMA. In case of MMA–ophthalmic artery collaterals, embolization was not performed. Embolization of the frontal and parietal branches of the MMA was performed using a dilute mixture of 1:6 n-BCA and ethiodized oil, with dextrose 5% (D5) boluses from the guide catheter to improve the distal penetration of the glue. Visibility was improved using tantalum powder. Cases in which there were ophthalmic collaterals from the MMA or other signs of dangerous anastomoses were excluded. The Prowler Select Plus microcatheter (Codman), 2.8F/2.3F 0.021 inch, was used for all cases.
After we advanced the Benchmark catheter into the internal maxillary artery, a Prowler Select Plus 2.8/2.3F straight-tip microcatheter was advanced into the frontal and parietal branches of the MMA. Initially, particulate embolysate (150–300 µm) was infused into the distal frontal and parietal branches of the MMA until there was stagnation of anterograde flow. Following PVA embolization, a dilute preparation of n-BCA was prepared with ethiodized oil (14%; 1:6 mixture of n-BCA/ethiodized oil). The guide catheter was connected to two 60-mL syringes filled with D5W. As the n-BCA mixture was injected through the microcatheter into the frontal and parietal branches of the MMA, a second operator simultaneously infused D5W through the guide catheter to enhance distal penetration (D5W-push technique). Depending on the duration of the procedure, some patients were given 25–50 U/kg of heparin to prevent thromboembolic complications. An XperCT Dual Cone Beam CT of the head (Philips Healthcare) was performed on the table immediately after the endovascular procedure.
Outcome Assessment
The primary outcome measure was the safety and technical feasibility of MMA embolization using diluted n-BCA, defined as any intra- or postprocedural neurologic or non-neurologic complication. Procedural complications were defined as nontarget embolization or unintentional retention of the catheter. The secondary end point was the efficacy in reducing hematoma volume, defined as >50% reduction in SDH volume. Global outcome was assessed by virtual interview or a telephone structured interview using the 7-point version of the mRS rated from death (6) to symptom-free full recovery (0). Poor outcome was defined as death or moderate-to-severe disability (unable to walk or tend to bodily needs, mRS score of 4–6).
RESULTS
Demographics
A total of 16 patients were prospectively enrolled in our study between September 2019 and June 2020. The mean age was 72 years, with a male/female ratio of 2:1. A history of trauma was confirmed in 13 of the 16 patients. Of the 16 patients enrolled, 10 patients were on anticoagulation at the time of the SDH identification (Online Supplemental Data).
Indications for MMA Embolization and Surgical Evacuation
Burr-hole craniotomies were performed in 12 of the 16 patients (75%). Of the 12 patients who underwent both surgical evacuation of their subdural hematoma and middle meningeal artery embolization, 9 underwent embolization post-surgical evacuation. Postsurgical MMA embolization was performed in 8 patients within 2 weeks of the operation when there was clear re-accumulation, defined as an increase in hematoma volume of >10%, or incomplete hematoma evacuation on the 7-day follow-up head CT. One patient underwent middle meningeal embolization approximately 8 months following surgical evacuation for persistent symptoms and hematoma expansion related to cerebral hypotension. Three patients underwent MMA embolization preoperatively. Of the patients requiring burr-hole craniotomy following MMA embolization, 2 underwent an operation for persistent symptoms during their initial hospitalization, while the remaining patient underwent craniotomy after returning to the hospital following a subsequent fall with a head strike and expansion of her subdural hematoma 11 days after undergoing her MMA embolization.
Embolization Results: Technical Feasibility and Safety
Embolization of the MMA was successfully performed in all 16 patients enrolled in the study (Fig 1). A total of 20 patients were screened for the procedure. Consent could not be obtained for 2 patients. Ophthalmic collaterals from the MMA were identified in 1 patient, and the MMA was not visualized, possibly secondary to neurosurgical evacuation, in the remaining patient.
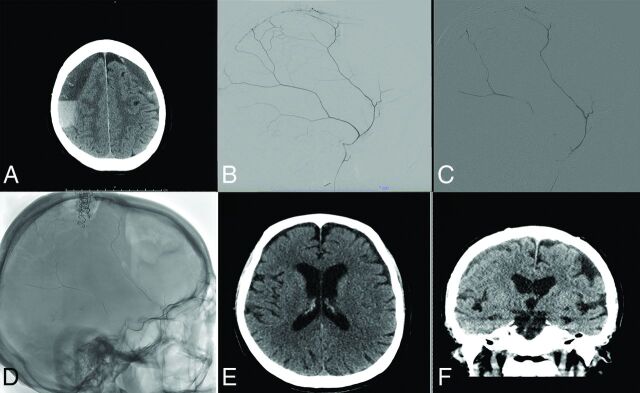
FIG 1.
Treatment course of a 77-year-old man with bilateral cSDH. Noncontrast axial head CT reveals bilateral subacute-on-chronic SDHs on admission (A). Diagnostic cerebral angiogram reveals robust filling of the MMA (B). The MMA was then embolized using n-BCA diluted with D5W (C). Postprocedural spin sequence reveals the glue cast left in the MMA (D). Noncontrast head CT at 3 months reveals significant resolution of the patient's SDHs (E and F).
The primary end point was met in 100% of cases; there were no intra- or postprocedural complications in the 16 patients enrolled. Distal penetration of the MMA branches was achieved in all cases. Average fluoroscopy time was 27.9 minutes. The fluoroscopy time attributed to glue embolization added an average of 2.5 minutes to each procedure (range, 1.5–3.5 minutes).
7-Day Follow-up CT Scan
A 7-day postembolization follow-up head CT was available for 15 of 16 patients and demonstrated improvement (>50% reduction in SDH volume) in 9/15 (60%), with 6/15 (40%) showing an unchanged or stable SDH (Fig 2). The remaining patient was discharged before the 7-day follow-up scan and was lost to follow-up
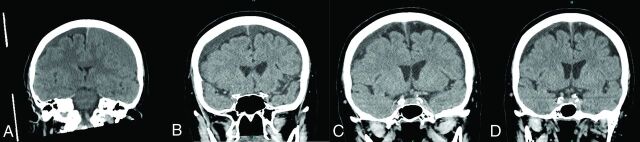
FIG 2.
Imaging course of a 75-year-old woman with bilateral cSDHs managed by unilateral burr-hole evacuation followed by bilateral MMA embolization. Noncontrast head CT images were obtained on presentation (A), postoperatively (B), 1 week postembolization (C), and 4 months postembolization (D).
21-Day and 3-Month Follow-up CT Scan
Day 21 head CTs were available for 11/16 patients, all of whom demonstrated a further >50% decrease in SDH volume (thus a total of >75% reduction in initial SDH volume). Three-month CT was available for 5/16 patients, all showing further >50% decrease in SDH volume. Four patients were lost to follow-up, and the remaining 21-day and 3-month scans are pending at the time of this writing.
Clinical Outcomes
Overall, 6 patients showed improvement (38%), 8 were unchanged (50%), and 2 (12%) had clinically deteriorated after MMA embolization. Of the 7 patients with at least 3 months of follow-up, 6 (85.7%) had partial-to-complete resolution of clinical symptoms. Of these, 5 of 7 (71.4%) had unchanged mRS scores, while 2 had a 1-point improvement in mRS (Online Supplemental Data). Of the 9 patients who have not yet had long-term follow-up, 4 (44.4%) had improved symptoms at discharge, 3 were unchanged, and 2 patients experienced a 1-point decline in the mRS score. Because the size of the SDH had not increased, we believe that this worsening could be attributed to generalized deconditioning or poor tolerance of procedural anesthesia, given the patients' advanced age; long-term follow-up will be assessed in subsequent reports.
DISCUSSION
cSDH is a public health issue with an estimated 1-year incidence of 5–58/100,000, which is highest in elderly patients.7 cSDH presents a challenge to neurosurgeons due to high recurrence rates (2%–37%) and the numerous associated medical comorbidities. Recent results have demonstrated the effectiveness of MMA embolization as an alternative to an operation for the treatment of cSDH. Most data on MMA embolization have been achieved with PVA particle embolization. To the best of our knowledge, this study is one of the first series of patients treated with n-BCA embolization of the MMA for cSDH. In this study, 60% of patients achieved a reduction in the maximum width of the SDH of >50% at 7 days, while 31% of patients showed a reduction in volume at the longest follow-up study of 3 months. All cases were technically successful, and there were no procedure-related complications.
Development of cSDH starts with the separation of the dural border cell layer, which triggers an inflammatory response that includes dural border cell proliferation, granulation tissue formation, and macrophage deposition.7 A surrounding membrane is formed due to the inflammatory response, which develops neovascularity due to the release of angiogenic factors. The primary pathologic mechanism behind recurrent hemorrhage and growth may involve repeat microvascular hemorrhage within this surrounding membrane.8,9 The neovascularity is supplied from distal branches of the MMA; thus, embolizing the MMA could potentially halt this process and allow the collection to be resorbed.2
Surgical approaches for cSDH have traditionally included a twist-drill hole, burr-hole, and craniotomy-based evacuations. While the outcomes with surgical evacuation are favorable, after the initial evacuation, there is a tendency for cSDH to recur, oftentimes with neurologic deterioration and a requirement for an urgent or emergent re-operation.10 Conservative management leading to spontaneous resolution is rare and can be as low as 5%.11 Medical management strategies such as steroids, platelet activating factor antagonists, and statins have been explored with limited success.12 In particular, recent evidence suggests a role for atorvastatin alone and/or in combination with corticosteroids in reducing the likelihood of subdural recurrence following surgical evacuation; however, there is still a paucity of evidence to support this strategy, and several trials are currently underway.13,14
MMA embolization for cSDH aims to devascularize the surrounding subdural membranes to a sufficient extent so that the balance is shifted from the continued leakage and accumulation of blood products toward reabsorption.11 MMA embolization has been used as the sole therapy, as well as a preoperative or postoperative adjunct to surgical evacuation with the intention of reducing postoperative recurrence. A recent study evaluated 27 patients who underwent MMA embolization alone for asymptomatic cSDH and 45 patients who underwent MMA embolization in addition to surgical evacuation for symptomatic cSDH and found significantly lower recurrences in the embolization group (1.4%) compared with historical surgical controls (27.5%, P = .001).15 To date, embolization of the MMA has been most commonly performed with PVA particles. In a small randomized controlled trial with 46 patients undergoing surgical treatment, 21 patients additionally underwent MMA embolization. The addition of MMA embolization to the operation led to an increase in cSDH resorption at 3 months.15 In a systematic review and case series, the use of Onyx as a liquid embolic for MMA embolization was described with good success.16 In another review including 177 MMA embolization procedures, the most common embolic material used was PVA (91%, 160/177), occasionally supplemented with coils (4%, 7/161) and n-BCA (9%, 16/177).17
Fiorella and Arthur11 described technical considerations of using liquid embolics-versus-particulate embolysates for MMA embolization. The authors argued that particulates may only penetrate as distally as flow will allow and may be limited by the diminutive size of the meningeal arteries compared with the microcatheter used. The authors also argued that liquid embolics may be advantageous over particulate embolics due to the ability to inject under pressure from a wedged position to achieve a greater casting of the subdural membranes and retrograde reflux of adjacent meningeal branches. Furthermore, increased visualization of liquid embolics may prevent iatrogenic embolization to unintended territory. Finally, liquid agents are permanent compared with the transient nature of particulate embolysates, possibly benefitting patients who have repeat episodes of hemorrhage.
Given the same considerations, we describe a novel approach of a combined technique using PVA particle embolization, followed by diluted n-BCA with simultaneous D5 injection through the guide catheter. The use of a D5-push technique improves distal penetration of the n-BCA by delaying polymerization within the target vessel. This, in combination with the initial embolization with PVA, capitalizes on the advantages of each embolic material compared with their use in isolation. The initial PVA embolization markedly penetrates the microscopic neovascularity and immature capillaries of the surrounding membrane, while the n-BCA embolization provides an easily visualized, long-term solution to a chronic problem. The diluted mixture of n-BCA in combination with the D5-push may compensate for the decreased distal penetration of liquid embolics compared with smaller particulate embolysates. Further advantages of this technique include low cost, because both PVA and n-BCA are significantly more cost-effective than Onyx.
We report the efficacy of this technique in our small series because we were able to demonstrate substantial improvement in hematoma volume in >60% of patients at the latest follow-up. No procedural complications were encountered after MMA embolization. Seventy-five percent of patients underwent concurrent surgical evacuation of their subdural hematoma; however, most of these patients received MMA embolization following an operation as a means of prophylaxis against recurrent hemorrhage. Three patients required surgical evacuation of their hematoma following MMA embolization. In other words, 13 of the 16 (81.3%) included patients have been able to avoid an additional operation for recurrent subdural hematoma. A recent systematic review suggested that surgically evacuated chronic subdural hematomas have a recurrence rate approaching 35% requiring re-evacuation.18 Of the 3 patients who required an operation following MMA embolization, 2 underwent craniotomy for persistent altered mental status shortly after embolization. Most interesting, both patients had been taking antiplatelet medications before the discovery of their subdural hematoma. There was no increase in subdural hematoma volume following MMA embolization in either patient.
Limitations of our study include its small size and being a single-center study with certain patient data still pending follow-up. Given the small sample size and unique niche for this procedure, there may have been selection bias as well. Additionally, not all postoperative imaging was performed using 3D reconstructed thin-cut CT, limiting our ability to optimally calculate hematoma volume; however, we believe that we were still able to accurately compare changes in hematoma volume despite these differences in imaging techniques. However, despite these limitations, we were able to demonstrate the safety and technical feasibility of MMA embolization using diluted n-BCA and, to a lesser extent, efficacy (secondary end point). These pilot data will be instrumental in the design of any upcoming multicenter trial on the use of dilute n-BCA for MMA embolization and any comparison studies that may follow.
CONCLUSIONS
Embolization of the MMA using diluted n-BCA is safe and effective for patients with cSDH, with a low risk of recurrence, and may be considered an effective therapeutic intervention to arrest SDH enlargement and promote resolution. The use of a D5-push technique improves distal penetration of the embolic material.
ABBREVIATIONS:
- cSDH
chronic subdural hematoma
- D5
dextrose 5%
- D5W
dextrose 5% in water
- MMA
middle meningeal artery
- PVA
polyvinyl alcohol
- SDH
subdural hematoma
Footnotes
Disclosures: Stephan Mayer—UNRELATED: Consultancy: Biogen, Idorsia, BrainCool; Expert Testimony: occasional work all unrelated to this article; Stock/Stock Options: NeurOptics.
References
- 1. Yang W, Huang J. Chronic subdural hematoma: epidemiology and natural history. Neurosurg Clin N Am 2017;28:205–10 10.1016/j.nec.2016.11.002 [DOI] [PubMed] [Google Scholar]
- 2. Haldrup M, Ketharanathan B, Debrabant B, et al. Embolization of the middle meningeal artery in patients with chronic subdural hematoma-a systematic review and meta-analysis. Acta Neurochir (Wien) 2020;162:777–84 10.1007/s00701-020-04266-0 [DOI] [PubMed] [Google Scholar]
- 3. Ashour R, Aziz-Sultan A. Preoperative tumor embolization. Neurosurg Clin N Am 2014;25:607–17 10.1016/j.nec.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 4. Carney N, Totten AM, O'Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017;80:6–15 10.1227/NEU.0000000000001432 [DOI] [PubMed] [Google Scholar]
- 5. Cook AM, Morgan Jones G, Hawryluk GW, et al. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit Care 2020;32:647–66 10.1007/s12028-020-00959-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke 1996;27:1304–05 10.1161/01.str.27.8.1304 [DOI] [PubMed] [Google Scholar]
- 7. Santarius T, Kirkpatrick PJ, Kolias AG, et al. Working toward rational and evidence-based treatment of chronic subdural hematoma. Clin Neurosurg 2010;57:112–22 [PubMed] [Google Scholar]
- 8. Asghar M, Adhiyaman V, Greenway MW, et al. Chronic subdural haematoma in the elderly: a North Wales experience. J R Soc Med 2002;95:290–92 10.1258/jrsm.95.6.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang P, Li Y, Huang J, et al. Chronic subdural haematoma in antithrombotic cohorts: characteristics, surgical outcomes, and recurrence. Br J Neurosurg 2020;34:408–15 10.1080/02688697.2020.1749987.2020/04/23 [DOI] [PubMed] [Google Scholar]
- 10. Link TW, Boddu S, Paine SM, et al. Middle meningeal artery embolization for chronic subdural hematoma: a series of 60 cases. Neurosurgery 2019;85:801–07 10.1093/neuros/nyy521 [DOI] [PubMed] [Google Scholar]
- 11. Fiorella D, Arthur AS. Middle meningeal artery embolization for the management of chronic subdural hematoma. J Neurointerv Surg 2019;11:912–15 10.1136/neurintsurg-2019-014730 [DOI] [PubMed] [Google Scholar]
- 12. Gjerris F, Schmidt K. Chronic subdural hematoma: surgery or mannitol treatment. J Neurosurg 1974;40:639–42 10.3171/jns.1974.40.5.0639 [DOI] [PubMed] [Google Scholar]
- 13. He CX, Xu J, Chen L, et al. Evaluation of the efficacy of atorvastatin in the treatment for chronic subdural hematoma: a meta-analysis. Neurosurgical Review 2020. Jan 17. [Epub ahead of print] 10.1007/s10143-019-01218-w [DOI] [PubMed] [Google Scholar]
- 14. Wang DG, Gao C, Xu X, et al. Treatment of chronic subdural hematoma with atorvastatin combined with low-dose dexamethasone: phase II randomized proof-of-concept clinical trial. J Neurosurg 2020. Jan 31. [Epub ahead of print] 10.3171/2019.11.JNS192020 [DOI] [PubMed] [Google Scholar]
- 15. Ng S, Derraz I, Boetto J, et al. Middle meningeal artery embolization as an adjuvant treatment to surgery for symptomatic chronic subdural hematoma: a pilot study assessing hematoma volume resorption. J Neurointerv Surg 2020;12:695–99 10.1136/neurintsurg-2019-015421] [DOI] [PubMed] [Google Scholar]
- 16. Waqas M, Vakhari K, Weimer PV, et al. Safety and effectiveness of embolization for chronic subdural hematoma: systematic review and case series. World Neurosurg 2019;126:228–36 10.1016/j.wneu.2019.02.208 [DOI] [PubMed] [Google Scholar]
- 17. Jumah F, Osama M, Islim AI, et al. Efficacy and safety of middle meningeal artery embolization in the management of refractory or chronic subdural hematomas: a systematic review and meta-analysis. Acta Neurochir (Wien) 2020;162:499–507 10.1007/s00701-019-04161-3 [DOI] [PubMed] [Google Scholar]
- 18. Ivamoto HS, Lemos HP, Atallah AN. Surgical treatments for chronic subdural hematomas: a comprehensive systematic review. World Neurosurg 2016;86:399–418 10.1016/j.wneu.2015.10.025 [DOI] [PubMed] [Google Scholar]