Abstract
Objective
To assist women and their physicians in making decisions regarding the prevention of breast cancer with tamoxifen and raloxifene.
Evidence
Systematic review of English-language literature published from 1966 to August 2000 retrieved from MEDLINE, HealthSTAR, Current Contents and Cochrane Library.
Values
The strength of evidence was evaluated using the methods of the Canadian Task Force on Preventive Health Care and the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer.
Recommendations
· Women at low or normal risk of breast cancer (Gail risk assessment index < 1.66% at 5 years): There is fair evidence to recommend against the use of tamoxifen to reduce the risk of breast cancer in women at low or normal risk of the disease (grade D recommendation). · Women at higher risk of breast cancer (Gail index ≥ 1.66% at 5 years): Evidence supports counselling women at high risk on the potential benefits and harms of breast cancer prevention with tamoxifen (grade B recommendation). The cutoff for defining high risk is arbitrary, but the National Surgical Adjuvant Breast and Bowel Project P-1 Study included women with a 5-year projected risk of at least 1.66% according to the Gail index, and the average risk of patients entered in the trial was 3.2%. Examples of high-risk clinical situations are 2 first-degree relatives with breast cancer, a history of lobular carcinoma in situ or a history of atypical hyperplasia. As the risk of breast cancer increases above 5% and the benefits outweigh the harms, a woman may choose to take tamoxifen. The duration of tamoxifen use in such situations is 5 years based on the results from trials of tamoxifen involving women with early breast cancer. If a woman raises concerns or has already been evaluated and is calculated to be at high risk, then individuals experienced and skilled in counselling may discuss the potential benefits and harms of tamoxifen use.
Important additional issues
· Prevention of breast cancer with raloxifene: Current evidence does not support recommending chemoprevention of breast cancer with raloxifene outside of a clinical trial setting. · Screening using the Gail risk assessment index: This index was the main eligibility criterion for enrolling women in the one study that showed potential benefit from chemoprevention. However, it has not been evaluated for use as a routine screening or case-finding instrument; validation of the index is required. Overall, current evidence does not support a shift to its routine use in physicians' offices for screening or case finding. However, when a woman or her physician is concerned about the woman's increased risk of breast cancer, the index can be a useful tool in deciding whether to pursue an in-depth discussion of the potential benefits and harms of chemoprevention. Hence, the approach to identifying women at higher risk who warrant counselling and shared decision-making will vary across practices. (The risk assessment index is available online at http://bcra.nci.nih.gov/brc/). [A patient version of these guidelines appears in Appendix 2.]
Validation
The authors' original text was revised by both the Canadian Task Force on Preventive Health Care and the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. The final document reflects a consensus of these contributors.
Sponsor
Health Canada.
Completion date
February 2001.
Evidence from epidemiological and experimental animal studies suggests that both endogenous and exogenous estrogens play a significant role in breast cancer development. Many of the established risk factors relate to the timing of exposures and the cumulative exposure of breast tissue to these hormones.1,2,3,4 This suggests that a pharmacological approach to modulating risk could be effective.3,5
Tamoxifen is a selective estrogen-receptor modulator that is active against metastatic breast cancer and is used as adjuvant therapy for early breast cancer. Today, primary chemoprophylaxis for breast cancer has evolved from the use of tamoxifen to treat established breast cancer. In various adjuvant trials, tamoxifen therapy not only reduced the risk of ductal carcinoma in situ (DCIS)6 and recurrent invasive breast cancer,7 but it also significantly reduced the risk of contralateral breast cancer.7,8 It was this latter observation that made it particularly attractive to study as a chemoprophylactic agent. Raloxifene, another selective estrogen-receptor modulator, acts as both an agonist and an antagonist to estrogen. It is used for the prevention and treatment of osteoporosis in postmenopausal women. In animal models, raloxifene has been found to prevent the development of new mammary cancers and to inhibit growth of existing cancers.9
Methods
This guideline is a joint project of the Canadian Task Force on Preventive Health Care and the Canadian Breast Cancer Initiative's Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer.
The databases MEDLINE, CINAHL, HealthSTAR, Current Contents and Cochrane Library were searched for English-language articles published from 1966 to August 2000 with the use of the following medical subject headings: (a) “breast neoplasms/prevention and control” and “chemoprevention” and (b) “breast neoplasms/prevention and control” and “clinical trials” plus any one of “chemoprevention,” “tamoxifen” or “raloxifene.” Abstracts of all retrieved articles were read, and the reference lists of key articles were searched by hand for additional relevant articles. In addition, experts in the field were consulted to ensure that no important studies (up to January 2001) were missed.
The literature search identified 3 randomized controlled clinical trials designed to assess the effects of tamoxifen in women without established breast cancer. The search did not identify any trials designed specifically to assess the use of raloxifene for breast cancer prevention; however, a secondary analysis of a trial investigating the use of raloxifene for osteoporosis was identified that reported on breast cancer outcomes.
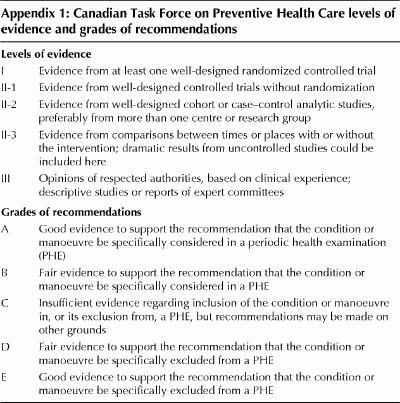
All evidence was systematically reviewed using the procedures of the task force and the steering committee. The primary authors collaborated in applying standardized evidence-based evaluation methods. Appendix 1 provides the definitions of the levels of evidence and grades of recommendations.
Effect of breast cancer chemoprevention
Tamoxifen studies
Three clinical trials evaluated tamoxifen for the prevention of breast cancer (Table 1).10,11,12
Table 1
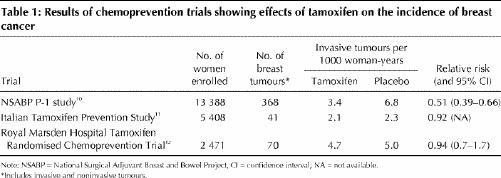
National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 Study: In the NSABP P-1 study 13 388 women were randomly assigned to receive either 5 years of tamoxifen (20 mg/d) or placebo (Table 1).10 Women were enrolled in the study if they were at increased risk for breast cancer because of age (>> 60 years) or a history of lobular carcinoma in situ (LCIS), or if they had a 5-year risk of at least 1.66% according to the modified risk assessment instrument described by Gail and associates13 (the Gail index). The Gail index was developed to predict an individual woman's risk of breast cancer. The index translates an individual's risk factors into an overall risk score by multiplying her relative risk scores in several categories (current age, age at menarche, number of breast biopsies, family history, age at first live birth and ethnic origin), and then multiplying this value by an adjusted population risk score to determine the individual's lifetime and 5-year risks. Women with a history of deep vein thrombosis or pulmonary embolism were ineligible. No hormone therapy or oral contraceptive use was permitted during the trial.
Almost all (96.4%) of the study subjects were white, and many (39.2%) were less than 50 years of age. Three quarters (76.2%) of the women had at least 1 first-degree relative with breast cancer. About 7% of the women had LCIS. The participants' average risk of breast cancer was 3.2%.14 The proportion of women who discontinued treatment early was 21.6% in the tamoxifen group and 19.7 % in the placebo group. Two thirds (67.0%) of the study subjects were followed for more than 48 months.
In total, 368 breast tumours (264 invasive and 104 noninvasive) were detected over the course of the study. Tamoxifen reduced the risk of invasive breast cancer by 49% (p < 0.00001). The risk of noninvasive breast cancer was reduced by 50% (p < 0.002). The occurrence of estrogen receptor (ER)-positive tumours was reduced by 69%. There was no evidence of a significant effect on ER-negative tumours.
Tamoxifen was found to be effective in preventing breast cancer in all subgroups, including all age groups, women with a history of LCIS (56% reduction), those with a history of atypical hyperplasia (86% reduction) and those with any category of predicted 5-year risk of breast cancer. There was no statistically significant evidence of heterogeneity of relative risks across these subgroups. After 48 months of follow-up, no significant difference in survival was observed. Three women in the tamoxifen group and 6 in the placebo group died of breast cancer.
Although serious adverse events occurred more frequently in the tamoxifen group than in the placebo group, the difference was statistically significant only for endometrial cancer, pulmonary embolism and new cataracts (Table 2). Most of the vascular events occurred in women aged 50 years and older.
Table 2
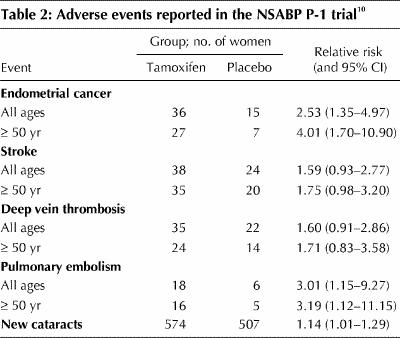
The NSABP P-1 study did not detect beneficial effects of tamoxifen on cardiac events.15 There was a trend toward fewer fractures at sites typical of osteoporosis (hip, spine and wrist) with tamoxifen, but the differences did not reach statistical significance. Hot flashes were reported as bothersome by a greater proportion of women in the tamoxifen group than in the placebo group (45.7% v. 28.7%). Similarly, the proportion of women who reported bothersome vaginal discharge was greater in the tamoxifen group than in the placebo group (29.0% v. 13.0%).
Italian Tamoxifen Prevention Study: A total of 5408 healthy women aged 35 to 70 who had undergone a hysterectomy for reasons other than cancer were recruited and randomly assigned to receive either tamoxifen or placebo for 5 years (Table 1).11 Participants were permitted to use hormone replacement therapy during the trial. No risk factor criteria were established. Women with a history of venous thromboembolism were excluded. In 1997 recruitment was halted early because of concerns about dropouts and side effects. The dropout rate as a result of voluntary withdrawal or side effects was 27.8% in the tamoxifen group and 24.7% in the placebo group. The average duration of treatment was 46 months at the time of the interim report.
The participants were generally at low risk of breast cancer. Fifteen percent of the subjects had a first-degree relative with breast cancer, about 37% were under 50 years of age, and 48% had undergone bilateral oophorectomy.
In total, 41 breast tumours were detected: 19 in the tamoxifen group and 22 in the placebo group (p = 0.64). A subgroup analysis of breast cancer incidence among women who were also taking hormone replacement therapy during the trial indicated a significant reduction in the incidence of breast cancer in the tamoxifen group: 8 cases among 390 women receiving placebo versus 1 case among 362 women receiving tamoxifen (p = 0.02). No deaths from breast cancer were reported. All-cause mortality was not significantly different between the 2 groups, with 6 deaths in the tamoxifen group and 9 in the placebo group.
There were 38 women who experienced thromboembolic events in the tamoxifen group, as compared with 18 in the placebo group (p = 0.0053). Although most events were cases of superficial phlebitis, there were 6 cases of deep vein thrombosis in the tamoxifen group, as compared with 3 in the placebo group. All 5 of the confirmed cases of stroke reported during the trial occurred in the tamoxifen group. The rate of endometrial cancer was not reported.
Royal Marsden Hospital Tamoxifen Randomised Chemoprevention Trial: This trial began as a pilot study in which women were randomly assigned to receive either tamoxifen (20 mg/d) for 8 years or placebo (Table 1).12 The trial was later expanded to include a total of 2471 women. Women were considered eligible if they were aged 30 to 70 and had a first-degree relative with breast cancer. Women with a history of venous thromboembolism were ineligible. Oral contraceptive use was not permitted, but postmenopausal women were allowed to continue or initiate hormone replacement therapy. The number of women who discontinued treatment early was 320 (26%) in the tamoxifen group, as compared with 176 (14%) in the placebo group (p < 0.0005). The median follow-up was 70 months.
Participants in this study were generally younger than those in the NSABP P-1 study and the Italian Tamoxifen Prevention Study: the median age was 47 years, and 62% of the subjects were less than 50 years of age. Twenty-six percent of the women were taking concurrent hormone replacement therapy during the trial.
The number of breast cancer events did not differ significantly between the tamoxifen and the placebo groups (34 and 36 respectively; relative risk 0.94) (Table 1). There were 4 deaths from breast cancer in the tamoxifen group and 1 in the placebo group.
The number of cases of endometrial cancer was 4 in the tamoxifen group and 1 in the placebo group. The corresponding number of cases of venous thromboembolism was 7 and 4. The most frequently reported side effects leading to early discontinuation of study medication were hot flashes, vaginal discharge and unspecified gynecological problems.
Raloxifene study
Multiple Outcomes of Raloxifene Evaluation (MORE) Trial: The MORE trial was a multicentre, randomized, double-blind, controlled trial designed to determine whether postmenopausal women with osteoporosis who took raloxifene for 3 years had a reduced risk of fracture.16 Development of breast cancer was a secondary outcome, and breast cancer risk was not evaluated for entry into the trial.
Participants were randomly assigned to receive 60 mg of raloxifene daily, 120 mg of raloxifene daily or placebo. A total of 7705 women (mean age 66.5 years) were recruited. Of these, 12.3% reported a family history of breast cancer. The median follow-up was 40 months.
In total, 40 breast tumours were detected: 13 in the raloxifene group and 27 in the placebo group (p < 0.001). Raloxifene was found to reduce the risk of ER-positive breast tumours by 90% (relative risk 0.10 [95% confidence interval (CI) 0.04–0.24]), but not the risk of ER-negative tumours (relative risk 0.88 [95% CI 0.26–3.0]). The reduction in risk of invasive breast cancer was similar for both doses of raloxifene.
Raloxifene increased the risk of venous thromboembolic disease (relative risk 3.1 [95% CI 1.5–6.2]). One death from pulmonary embolism occurred in the 60-mg raloxifene subgroup. Significantly more women in the entire raloxifene group than in the placebo group had influenza-like symptoms, hot flashes, leg cramps, endometrial cavity fluid and peripheral edema. Thirty-three women assigned to the raloxifene group (0.6%) and 2 assigned to the placebo group (0.1%) dropped out because of hot flashes (p < 0.001). More women in the raloxifene group than in the placebo group also reported new or worsening diabetes mellitus (p < 0.009). There was no increase in the risk of endometrial cancer (relative risk 0.8 [95% CI 0.2–2.7]). Regarding beneficial outcomes, there was a reduction in the risk of vertebral fractures, but no reduction in risk of other types of fractures.
Summary
Tamoxifen research
The largest tamoxifen trial — the NSABP P-1 study10 — demonstrated a reduction in breast cancer incidence, whereas the 2 smaller studies11,12 detected no difference in breast cancer incidence between the tamoxifen and placebo groups. All 3 trials reported an apparent excess of vascular events as a secondary outcome in the tamoxifen groups. None of the trials found a difference in the number of deaths from breast cancer between the treatment groups. The difference in the results of the NSABP study from those of the 2 European trials may have been related to differences in design, study populations and power. The populations in the studies were heterogeneous (the ages and risk profiles differed), and the duration of treatment differed. The Italian study was smaller than the NSABP study. Almost half of the women in the Italian study had undergone oophorectomy, which may have reduced the risk of breast cancer, and compliance was poor. The Royal Marsden Hospital trial involved a younger population with stronger family histories of breast cancer. Such women may have had the genetic variant of breast cancer, which could be less influenced by tamoxifen. Finally, because of the sample size the Royal Marsden Hospital trial may have been underpowered to detect a difference in treatment effect.
Raloxifene research
Although the raloxifene research is promising, there has not been a randomized controlled trial designed specifically to evaluate raloxifene as a breast cancer preventive. Questions concerning treatment duration, side effects and overall mortality are as yet unanswered. These may be answered by the Study of Tamoxifen and Raloxifene (STAR) conducted by the NSABP, which will compare the effectiveness of the 2 drugs in preventing breast cancer.
Tamoxifen use
Rockhill and associates17 recently discussed the public health implications of the widespread use of tamoxifen. One of the inclusion criteria for the NSABP P-1 study was age over 60 years. On the basis of the NSABP P-1 results, the Food and Drug Agency (FDA) recommended tamoxifen chemoprevention in women aged 35 or older with a 5-year risk of breast cancer of at least 1.66%. The FDA's indications were slightly different than the eligibility criteria for the NSABP P-1 study because it was concerned that all healthy women over 60 years could be advised to consider tamoxifen. In addition, Rockhill and associates pointed out that, in the Nurses' Health Study, only 30% of women aged 60 to 69 had a 5-year risk of 1.66% or higher.
Assessing baseline risk
The assessment of a woman's baseline risk of breast cancer presents challenges for physicians. The baseline risk is a product of a number of variables. The only instrument widely available to assess the risk is the Gail index,13 which assembles a series of risk factors for an individual woman and calculates a composite baseline risk. It estimates a woman's risk for invasive breast cancer over the next 5 years and her lifetime risk and compares her risk to that of a woman of the same age who has normal risk factors.
The Gail index was the main eligibility criterion for enrolling women in the one study that showed potential benefit from chemoprevention. However, it has not been evaluated for use as a routine screening or case-finding instrument; validation of the index is required. Overall, current evidence does not support a shift to its routine use in physicians' offices for screening or case finding. However, when a woman or her physician is concerned about the woman's increased risk of breast cancer, the index can be a useful tool in deciding whether to pursue an in-depth discussion of the benefits and harms of chemoprevention. Hence, the approach to identifying women at higher risk who warrant counselling and shared decision-making will vary across practices. (The Breast Cancer Risk Assessment Tool, which is based on the Gail index, is available online through the National Cancer Institute [http://bcra.nci.nih.gov/brc]; the program allows the entry of individual patient information and calculates the percentage risk for that person.)
Discussing benefits and risks
The decision to choose tamoxifen for chemoprevention of breast cancer will require careful balancing of benefits and risks for each woman. Individual choices will vary depending on a woman's risk of breast cancer, how she perceives that risk, and the value she attaches to the potential benefits and harms of chemoprevention. Although tamoxifen will reduce the likelihood of breast cancer for certain women at high risk, its use is associated with adverse events, some of which are severe. Data indicate that the benefits of tamoxifen therapy are more likely to outweigh the risks in younger women (35–50 years). As age increases, the risks will compete with, and at some point outweigh, potential benefits, depending on a woman's baseline risk. Because all 3 trials that evaluated tamoxifen excluded women with a history of venous thromboembolism and because of the increased risk of thrombotic events observed in the NSABP P-1 trial, it would be prudent not to consider tamoxifen therapy in women with prior thromboembolism, documented thrombophilia or a strong family history of thromboembolism.
Patients with different cultural backgrounds and education will bring different levels of understanding to a discussion of risks. In addition, the value or weight each woman places on particular adverse events will be unique. Some women may consider a tamoxifen-induced stroke equal in severity to invasive breast cancer and decide against taking the drug. Others may consider a stroke less severe and decide to take tamoxifen.
Physicians may choose to engage in shared decision-making with their patients at higher risk of breast cancer, or they may also involve specialized counselling centres. Comprehensive counselling centres for familial cancer genetics are becoming more widely available in Canada. One approach to weighing the risks and benefits of tamoxifen chemoprevention and communicating this to a patient is described by Gail and associates.18 The approach involves 5 steps: (1) calculate the woman's 5-year risk for breast cancer using the Gail index; (2) calculate the reduction in risk of breast cancer associated with tamoxifen use based on the findings of the NSABP P-1 study; (3) identify the risk of adverse events associated with taking tamoxifen; (4) for a woman's baseline risk category (e.g., 2%, 4%, 6%), compare the potential benefits and risk of harms over 5 years; and (5) discuss with the woman her preferences and values associated with the benefits and harms and how they will affect her decision about tamoxifen therapy.
This approach to decision-making is elegant, but there are some important points to consider. First, this method presents the rates of events per 10 000 women, which can be misleading to the individual woman whose own net benefits are generally of the order of magnitude of 1% or 2%. Second, the Gail model appears to be accurate for women undergoing routine mammography screening, but it may overestimate the risk among young women who are not undergoing regular mammography.19 Third, the model is complex and may not be practical outside of specialized counselling centres. In Canada, family physicians rather than trained counsellors could often be the ones to assess risk and counsel women on tamoxifen therapy for breast cancer prevention. Fourth, the data used to estimate the benefits of tamoxifen therapy are based on the results of the NSABP P-1 trial10 rather than the results of the 2 European trials,11,12 which did not show the same advantages to tamoxifen use. Finally, it is difficult to advise women what happens beyond 5 years; longer follow-up in the NSABP P-1 trial is required to determine whether there will be a reduction in mortality.
Because the decision-making approach described by Gail and associates presents rates of events per 10 000 women, we suggest a minor modification when presenting the data to individuals. First, determine the 5-year risk of breast cancer and the net benefit of tamoxifen therapy. For example, for a 40-year-old woman with a 6% risk of breast cancer at 5 years, tamoxifen provides a 50% relative reduction in risk, to 3%. Because the risk of stroke and pulmonary embolism in this age group in the NSABP P-1 study was very low (0.3%), the net benefit of tamoxifen therapy at 5 years is 2.7%. Of course, it is still important to discuss the clinical significance of invasive breast cancer versus stroke and pulmonary embolism. It is also important to discuss quality-of-life issues such as tamoxifen-associated hot flashes and vaginal dryness.
Recommendations
By the Canadian Task Force on Preventive Health Care and the Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer
Recommendations for the chemoprevention of breast cancer with tamoxifen are summarized in Table 3.
Table 3
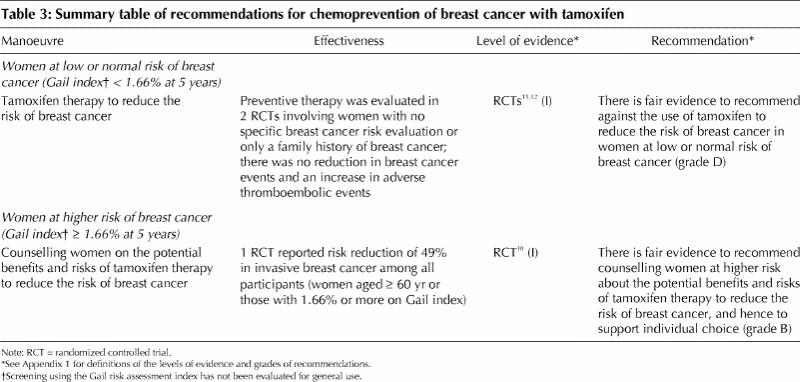
· Women at low or normal risk of breast cancer (Gail index < 1.66% at 5 years): There is fair evidence to recommend against the use of tamoxifen to reduce the risk of breast cancer in woman at low or normal risk of the disease (grade D recommendation). This recommendation is based on 3 factors: the data from the randomized controlled trials are conflicting, a reduction in breast cancer mortality has not been demonstrated, and the harms from tamoxifen reported in other similar trials outweigh any benefits in this low-risk group.
· Women at higher risk of breast cancer (Gail index ≥ 1.66% at 5 years): Evidence supports counselling women at high risk on the potential benefits and harms of breast cancer prevention with tamoxifen (grade B recommendation). The cutoff for defining high risk is arbitrary, but the NSABP P-1 study included women with a 5-year projected risk of at least 1.66% according to the Gail index, and the average risk of patients entered in the trial was 3.2%. Examples of high-risk clinical situations are 2 first-degree relatives with breast cancer, a history of lobular carcinoma in situ or a history of atypical hyperplasia. As the risk of breast cancer increases above 5% and the benefits outweigh the harms, a woman may choose to take tamoxifen. The duration of tamoxifen in such situations is 5 years based on the results from trials of tamoxifen involving women with early breast cancer. If a woman raises concerns or has already been evaluated and is calculated to be at high risk, then individuals experienced and skilled in counselling may discuss the potential benefits and harms of tamoxifen use.
Important additional issues
· Prevention of breast cancer with raloxifene: Current evidence does not support recommending chemoprevention of breast cancer with raloxifene outside of a clinical trial setting. Although the early evidence is promising, the use of raloxifene to prevent breast cancer remains investigational. The NSABP's STAR trial will compare the effectiveness of raloxifene and tamoxifen in reducing the incidence of breast cancer and compare the drugs' side effects.
· Screening using the Gail risk assessment index: This index was the main eligibility criterion for enrolling women in the one study that demonstrated potential benefit from chemoprevention. However, it has not been evaluated for use as a routine screening or case-finding instrument; validation of the index is required. Overall, current evidence does not support a shift to its routine use in physicians' offices for screening or case finding. However, when a woman or her physician is concerned about the woman's increased risk of breast cancer, the index can be a useful tool in deciding whether to pursue an in-depth discussion of the potential benefits and harms of chemoprevention. Hence, the approach to identifying women at higher risk who warrant counselling and shared decision-making will vary across practices. (The risk assessment index is available online at http://bcra.nci.nih.gov/brc/)
By other organizations
The American Society of Clinical Oncology has published a technology assessment of strategies to reduce the risk of breast cancer.14,20 It concluded that a woman with a defined 5-year projected risk of at least 1.66% may be offered tamoxifen (20 mg/d for up to 5 years) to reduce her risk. A woman's decision regarding tamoxifen use will depend on the importance and weight she attributes to the information provided to her. The society stated that it was premature to recommend chemoprevention of breast cancer with raloxifene outside of a clinical trial setting and that its use should currently be reserved for its approved indication of preventing postmenopausal bone loss. The results of placebo-controlled trials currently underway should be monitored and reviewed.
The American College of Obstetricians and Gynecologists issued a committee opinion statement on the use of tamoxifen as a preventive agent.21 The college recommended that the obstetrician-gynecologist take a thorough history to assess breast cancer risk in order to determine whether the benefits of tamoxifen outweigh the harms for an individual patient.
Acknowledgments
The Canadian Task Force on Preventive Health Care is funded through a partnership between the Provincial and Territorial Ministries of Health and Health Canada. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer is part of Health Canada's Canadian Breast Cancer Initiative.
Appendix 2.

Appendix 2. Continued.
Appendix 1.
Footnotes
Lists of the members of the task force and the steering committee appear at the end of the article.
A patient version of these guidelines appears in Appendix 2.
This article has been peer reviewed.
Competing interests: None declared.
Reprint requests to: Dr. Mark Levine, c/o Ms. Humaira Khan, Faculty of Health Sciences, McMaster University Health Sciences Centre, Rm. 2C6, 1200 Main St. W, Hamilton ON L8N 3Z5, fax 905 577-0017; or the Canadian Task Force on Preventive Health Care, Parkwood Hospital, 801 Commissioners Rd. E, London ON N6C 5J1, ctf@ctfphc.org
References
- 1.Henderson BE, Ross R, Bernstein L. Estrogen as a cause of human cancer. Cancer Res 1988;48:246-53. [PubMed]
- 2.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health 1996;17:47-67. [DOI] [PubMed]
- 3.Nayfield SG, Karp JE, Ford LG, Dorr FA, Kramer BS. Potential role of tamoxifen in prevention of breast cancer. J Natl Cancer Inst 1991;83:1450-9. [DOI] [PubMed]
- 4.Dawson DA, Thompson GB. Breast cancer risk factors and screening: United States, 1987. Vital Health Stat 10 1990;(172):iii-iv, 1-60. [PubMed]
- 5.Powles TJ, Hardy JR, Ashley SE, Cosgrove D, Davey JB, Dowsett M, et al. Chemoprevention of breast cancer. Breast Cancer Res Treat 1989;14(1):23-31. [DOI] [PubMed]
- 6.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet 1999;353:1993-2000. [DOI] [PubMed]
- 7.Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998;351:1451-67. [PubMed]
- 8.Fisher B, Costantino J, Redmond C, Poisson R, Bowman D, Couture J, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med 1989;320:479-84. [DOI] [PubMed]
- 9.Jordan VC, Morrow M. Tamoxifen, raloxifene and the prevention of breast cancer. Endocr Rev 1999;20:253-78. [DOI] [PubMed]
- 10.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst 1998;90:1371-88. [DOI] [PubMed]
- 11.Veronesi U, Maisonneuve P, Costa A, Sacchini V, Maltoni C, Robertson C, et al. Prevention of breast cancer with tamoxifen: preliminary findings from the Italian randomised trial among hysterectomised women. Italian Tamoxifen Prevention Study. Lancet 1998;352:93-7. [DOI] [PubMed]
- 12.Powles T, Eeles R, Ashley S, Easton D, Chang J, Dowsett M, et al. Interim analysis of the incidence of breast cancer in the Royal Marsden Hospital tamoxifen randomised chemoprevention trial. Lancet 1998;352:98-101. [DOI] [PubMed]
- 13.Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst 1989;81:1879-86. [DOI] [PubMed]
- 14.Chlebowski RT, Collyar DE, Somerfield MR, Pfister DG. American Society of Clinical Oncology technology assessment on breast cancer risk reduction strategies: tamoxifen and raloxifene. J Clin Oncol 1999;17:1939-55. [DOI] [PubMed]
- 15.Reis SE, Constantino JP, Wickerham DL, Tan-Chiu E, Wang J, Kavanah M. Cardiovascular effects of tamoxifen in women with and without heart disease: breast cancer prevention trial. J Natl cancer Inst 2001;93:16-21. [DOI] [PubMed]
- 16.Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 1999;281:2189-97. [DOI] [PubMed]
- 17.Rockhill B, Colditz G, Kaye J. Re: tamoxifen prevention of breast cancer: an instance of the fingerpost. J Natl Cancer Inst 2000;92:657-9. [PubMed]
- 18.Gail MH, Costantino JP, Bryant J, Croyle R, Freedman L, Helzlsouer K, et al. Weighing the risks and benefits of tamoxifen treatment for preventing breast cancer. J Natl Cancer Inst 1999;91:1829-46. [DOI] [PubMed]
- 19.Armstrong K, Eisen A, Weber B. Assessing the risk of breast cancer. N Engl J Med 2000;342:564-71. [DOI] [PubMed]
- 20.Chlebowski RT. Reducing the risk of breast cancer. N Engl J Med 2000;343:191-8. [DOI] [PubMed]
- 21.American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee opinion: tamoxifen and the prevention of breast cancer in high risk women. ACOG Today 1999;43(9):224. [PubMed]


