Abstract
The guideline provides a practical step-by-step guide in order to facilitate high-quality echocardiographic studies of patients with aortic stenosis. In addition, it addresses commonly encountered yet challenging clinical scenarios and covers the use of advanced echocardiographic techniques, including TOE and Dobutamine stress echocardiography in the assessment of aortic stenosis.
Keywords: aortic stenosis, guideline
Introduction
Aortic valve stenosis is a significant health burden, particularly in older individuals, with a prevalence of up to 5% in individuals over 75 years of age (1). Aortic stenosis is the most common valve disease necessitating surgical or percutaneous intervention (2). Echocardiography is central in the diagnosis, assessment and management of individuals with aortic valve disease. The British Society of Echocardiography (BSE) has previously published a guideline document in order to facilitate high-quality echocardiography in the assessment of patients. This document is intended as an update to the previously published work.
This guide should be seen as supplementary to the BSE minimum dataset (3). The intended benefit of this supplementary document is to:
Support cardiologists, cardiac physiologists and clinical scientists to develop local protocols for the assessment of aortic valve disease.
Promote quality by defining the optimal methodology in the assessment of aortic valve disease and linking this to the current evidence-base.
Ensure that the management of patients with aortic valve disease is based around contemporary data and optimal echocardiographic assessment.
In some situations, this BSE guidance differs from the most recent European or American guidelines (4, 5, 6). In those areas, these decisions were made in order to reflect contemporaneous data or as the result of differing interpretation. This guidance is divided into a number of subsections, which are listed in Table 1.
Table 1.
Subsections of the BSE aortic valve guidance.
|
1. Anatomy - Standard anatomy and imaging planes - Variant anatomy |
2. Calcification and aetiology of AS |
3. Haemodynamic principles of AS |
4. Standard echocardiographic images |
|
5. Essential parameters in the echocardiographic assessment of AS severity - Aortic valve maximal velocity (AV Vmax) - Mean aortic valve gradient (mean AVG) - Aortic valve area (AVA) - Potential sources of error and troubleshooting |
6. Approach to the patient |
|
7. Grading of severity - Aortic sclerosis - Mild, moderate and severe AS - Very severe AS |
|
8. Additional parameters to define severity - Indexed aortic valve area (AVAi) - Dimensionless index - Planimetry - Energy loss index (ELI) |
|
9. Other considerations - Atrial fibrillation - Blood pressure |
|
10. Additional prognostic markers - Left ventricular ejection fraction - Indexed left ventricular mass - Global longitudinal strain - Pulmonary hypertension |
|
11. Additional echocardiographic imaging modalities - Trans-oesophageal imaging - Exercise stress echocardiography |
|
12. Special circumstances - Low-gradient AS with LVEF ≥50% - Low-gradient AS with impaired LVEF - High gradient high valve area |
|
13. Combined valve disease - Aortic stenosis and aortic regurgitation - Aortic stenosis and mitral regurgitation - Aortic stenosis and mitral stenosis |
14. Aortic stenosis and amyloid |
Anatomy
The aortic valve usually consists of three cusps, suspended within the aortic root, which together form a gate between the left ventricular outflow tract (LVOT) and the aorta. Each cusp is usually associated with a specific outpouching or ‘sinus’ of the aorta: the left and right coronary cusps (LCC; RCC) are associated with the left and right coronary sinuses respectively, which are the usual point of origin of the left and right coronary arteries. The third or ‘non-coronary’ cusp (NCC) is associated with a sinus from which no arteries arise. Two-thirds of the circumference of the aortic root are attached to the muscular interventricular septum. One-third of the aortic root, which corresponds with the majority of the non-coronary cusp and a portion of the left coronary cusp, forms a fibrous continuity with the adjacent mitral valve (called the aorto-mitral continuity) (7). Using echocardiography, normal anatomy of the aortic valve and aortic root is depicted in Fig. 1.
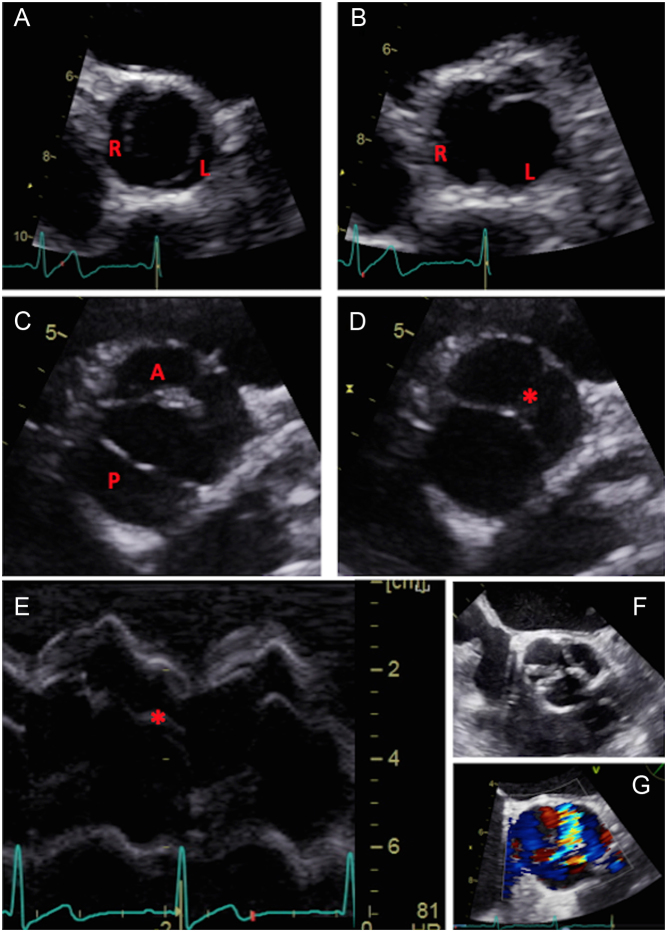
Figure 1.
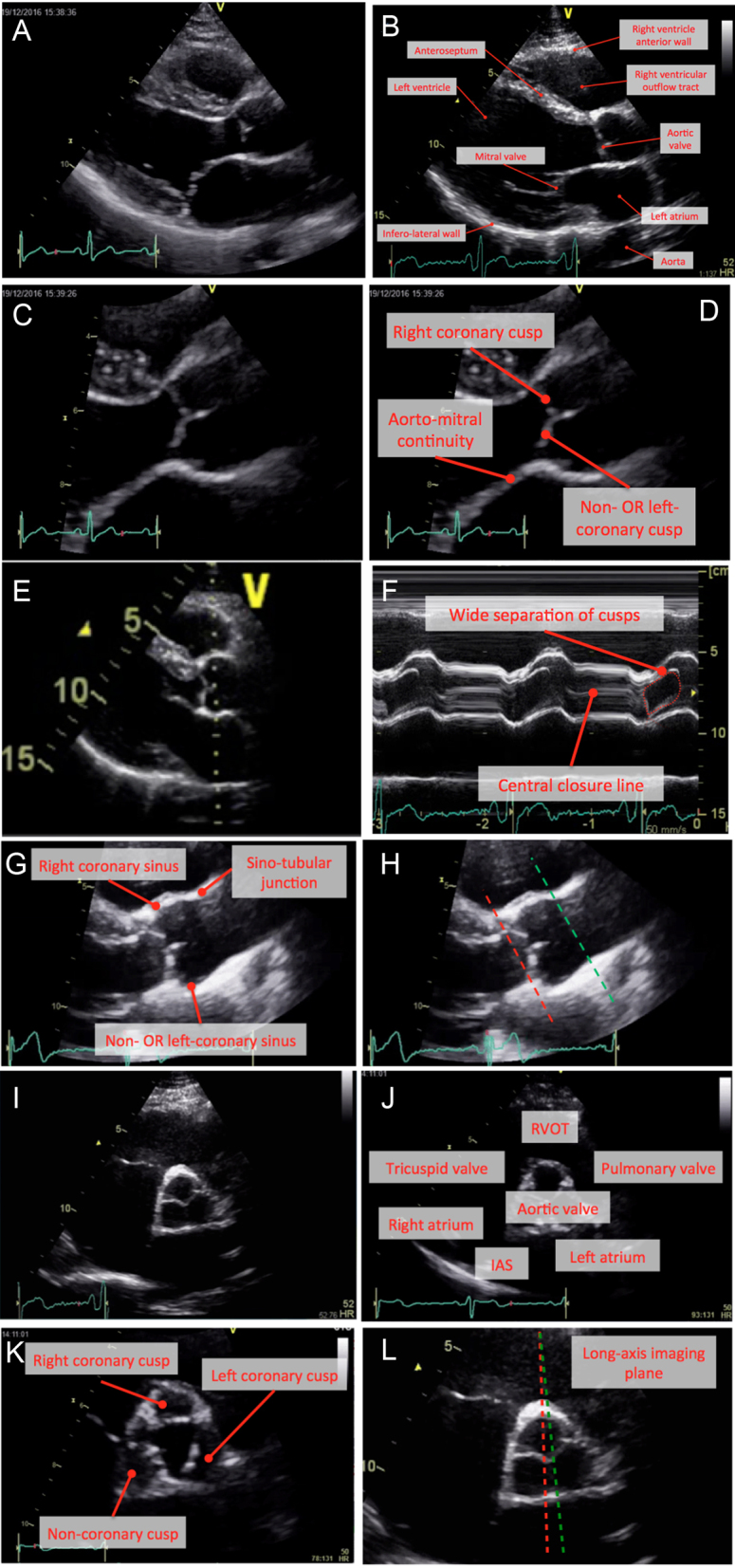
Normal anatomy. Parasternal long-axis window (A and B), with zoom images (C and D). M-mode recording of the aortic valve should be obtained with the cursor perpendicular to the axis of the aorta (E). Normal M-mode trace (F): note central closure line (labelled). Anatomy of the aorta (G). Image (H) marks the insertion of the cusps (red dotted line) and sino-tubular junction (green dotted line). Parasternal short-axis window at the level of the aortic cusps (images I and J). Image (K) represents a zoomed image of the aortic valve during systole with the three cusps labelled. In image (L), the lines represent potential parasternal long-axis imaging planes. Whilst both transect the right coronary cusp, it is evident that a subtle change in angle of the transducer will lead to the inclusion of either the non-coronary cusp (red dotted line) or left coronary cusps (green dotted line).
Variant anatomy
Bicuspid valve disease
Key points.
- The BSE recommend that bicuspid valves (BAV) be described as either ‘antero-posterior (AP)’ or ‘right-left (RL)’ orientation, with an additional comment on the presence or absence of a raphe (see Fig. 2).
Figure 2.
Bicuspid aortic valve. In the top panes there are images of a bicuspid valve with right-left (RL) configuration (annotated; systole (A) and (B) diastole). Images (C) and (D) depict a bicuspid valve with anterior-posterior configuration (AP) with a raphe in the anterior cusps (marked with asterisk). M-mode recording of a bicuspid valve with AP configuration (E): note eccentric closure line (marked with asterisk). Image (F) depicts a calcified aortic valve obtained using TOE, which appears tricuspid. Colour Doppler is applied in image (G) and demonstrates a ‘crescent-shaped’ opening indicating this is a bicuspid valve (AP configuration with raphe). All patients with BAV should undergo a comprehensive assessment of the aorta to assess for dilatation and coarctation.
All patients with BAV should be offered echocardiographic surveillance.
Echocardiographic screening should be offered to first degree relatives of patients with BAV.
BAV has a prevalence of between 0.5 and 1% (8, 9, 10). Identification of BAV is important as they are disproportionately responsible for more advanced valve dysfunction and are associated with aortic dilatation (11). The appearance and function of the valve at diagnosis are useful tools to inform discussions with the patient regarding prognosis and decisions concerning the frequency of follow-up. Patients in whom the valve displays no thickening or calcification, and functions normally at baseline, have an excellent prognosis with fewer than 20% requiring aortic valve surgery over 20 years follow-up. Such individuals only require infrequent echocardiographic surveillance. Conversely, around 75% of patients with thickening, calcification or valve dysfunction will need surgery over a similar timeframe and therefore should be monitored more closely (12, 13).
Differing classifications of BAV have been advocated in the literature, which means that comparisons and nomenclature are not standardized (14, 15, 16, 17). Importantly, there is no consensus as to associations between the sub-type of BAV and the pattern of valve dysfunction or aortic dilatation (14, 15, 16).
Where there is uncertainty about the potential diagnosis of BAV, this should prompt review of any past echocardiographic images and consideration of advanced imaging techniques (i.e. TOE) to resolve the uncertainty, given the importance of the diagnosis for long-term prognosis.
Unicuspid and quadricuspid aortic valves
Key points.
Unicuspid valves (UAV) may display advanced aortic stenosis in the absence of heavy calcification.
Patients with UAV and severe AS should be intervened upon according to standard indications in international guidance.
Less common anatomical variants are also recognized, which include quadricuspid or unicuspid aortic valves (QAV; UAV). Quadricuspid aortic valves are rare, with an estimated prevalence of <0.01% (18). There is an association between QAV and aortic dilatation, and the predominant valve lesion is that of regurgitation, with stenosis a less frequent presentation (18, 19).
The estimated prevalence of UAV is around 0.02% (20). Transthoracic (TTE) and trans-oesophageal echocardiography (TOE) are both highly specific for the identification of UAV, but are poorly sensitive, with unicuspid valves often mistakenly identified as bicuspid particularly in the presence of marked calcification (21). Unicuspid valves are a disproportionate contributor to the overall burden of severe aortic stenosis, particularly in young individuals (11, 22). An example of UAV is in Fig. 3.
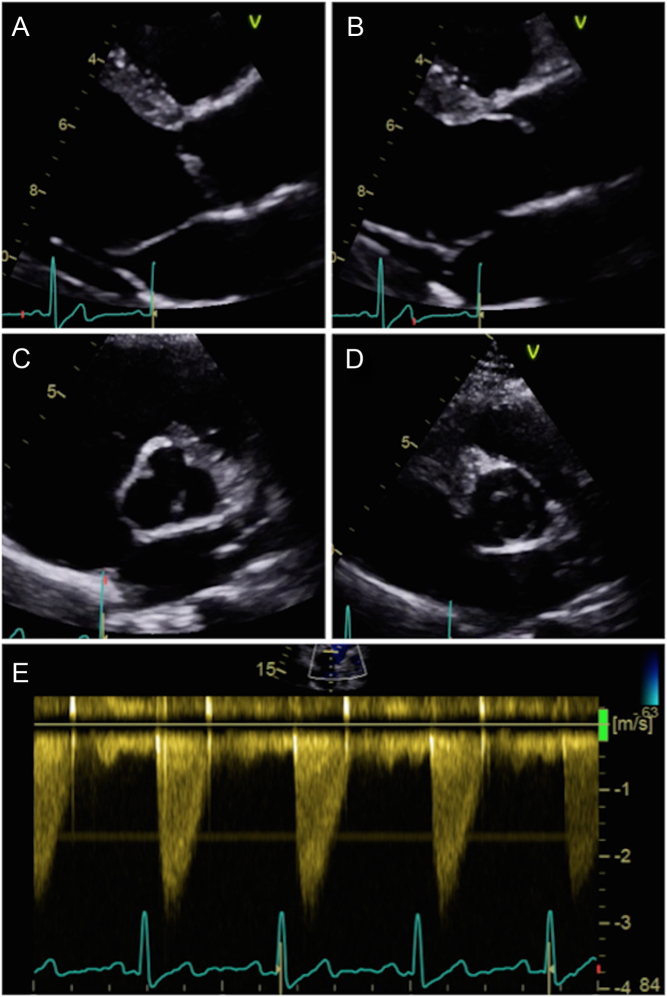
Figure 3.
Parasternal long-axis window of a unicuspid aortic valve during diastole (A) and systole (B). Note the marked ‘doming appearance’ during systole. Parasternal short-axis window during diastole (C) and systole (D): note how the orifice is eccentric. This valve has minimal calcification and retains mobility. Image (E) represents CW Doppler through the unicuspid valve. There is an obstruction to flow despite the lack of calcification.
Calcification and aetiology of AS
Key points.
Calcification is central to the development of aortic stenosis.
Echo studies should describe the pattern and burden of calcification.
Echo studies should identify thickening of cusps and restriction of motion.
In the absence of significant calcification, important aortic stenosis should not usually be considered.
There are three main aetiological factors contributing towards the development of AS within the UK. BAV is described above. Rheumatic heart valve disease is the most frequently seen worldwide but is rare in the UK. Rheumatic AS is characterized by calcification affecting the margins of the cusps and commissures, with relative sparing of the body of the cusps. Rheumatic aortic valve disease is almost never seen in the absence of mitral stenosis (MS) (4).
The commonest cause of AS in the UK is age-related calcific degeneration of the valve, which is characterized by progressive thickening, fibrosis and calcification of the aortic cusps. Such patients often display an abundance of calcification at the base of the cusps, whereas the commissures tend to be spared. Risk factors for calcific degenerative AS include hypertension, dyslipidaemia, smoking, diabetes and impaired renal function (23, 24).
Haemodynamic principles of aortic stenosis
The normal aortic valve is compliant and opens fully, and presents almost no obstruction to blood flow out of the heart during systole. With worsening aortic stenosis, the valve becomes progressively more restricted, and consequently aortic valve maximal velocity (Vmax) and mean gradient (AVG) are correspondingly higher. Importantly, as blood approaches a fixed obstruction, it accelerates prior to the point of maximal obstruction, which has implications when estimating flow within the LV outflow tract (Fig. 4) (25, 26, 27, 28). As blood accelerates, the jet additionally ‘contracts’ in order to fit through the narrowed aortic orifice (29). It may be assumed that once the jet has passed the valve it will immediately expand to fill the aorta, but in fact, the jet continues to contract for a short distance, forming a vena contracta. This vena contracta represents the ‘effective orifice area’ which is always smaller than the ‘anatomic orifice area’. This principle is important, as, whilst it is the anatomical valve area that is responsible for the flow obstruction, the effective orifice area is the key determinant of survival and long-term outcomes in patients with aortic stenosis (Fig. 4) (29). Maximal AV velocity, mean gradient and the estimated aortic valve area (AVA) using the continuity equation all assess the haemodynamic impact of the effective orifice area and as such are useful for the determination of prognosis (30, 31).
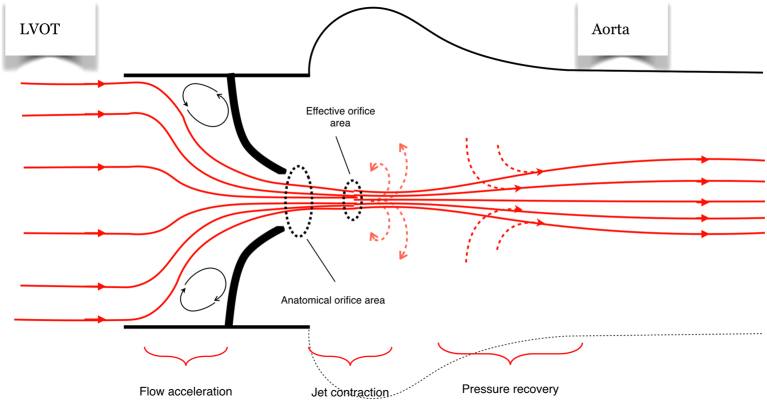
Figure 4.
Haemodynamic considerations of AS. In a patient with severe AS, blood flow accelerates prior to the valve (flow acceleration region marked). The jet contracts in order to fit through the anatomical orifice and continues to contract for a short period, forming the effective orifice. Doppler indices of AS measure the severity of obstruction at the level of the effective orifice. In the ascending aorta, there is a degree of ‘pressure recovery’.
Standard echocardiographic images
In all patients with aortic valve disease, it is imperative that they undergo a complete and careful echocardiographic evaluation, according to the principles of the minimum dataset (3). The specific windows and images required for the echocardiographic assessment of AS are described in Table 2 followed by a suggested reporting template in Table 3.
Table 2.
Standard TTE images for the assessment of AS.
| View (modality) | Measurement | Explanatory notes | Image |
|---|---|---|---|
| Parasternal long axis (PLAX); 2D | LV dimensions Calculate indexed LV mass using linear method | Visual assessment of wall motion Calcification of aortic valve (see ‘Calcification and aetiology of AS’ section) Indexed LV mass is a prognostic marker in AS (see ‘Additional prognostic markers’ section) | 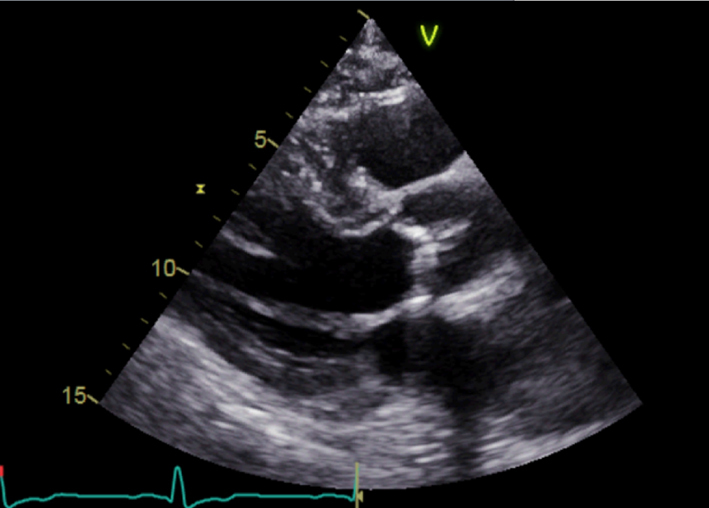 |
| Parasternal long axis; zoom 2D | Assess calcification and mobility of cusps Advanced AS unlikely without significant cusp calcification or restriction Assess for central vs eccentric closure line suggesting BAV (‘Anatomy’ section) | 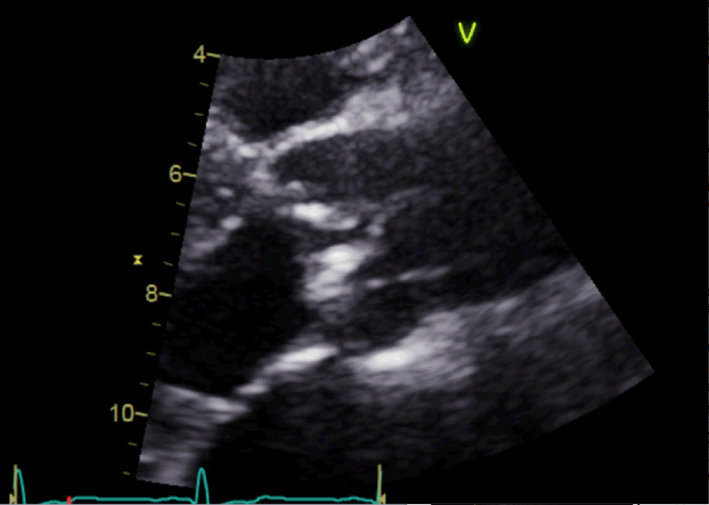 |
|
| Parasternal long axis; zoom 2D with colour Doppler | Assess for turbulence and presence of aortic regurgitation | 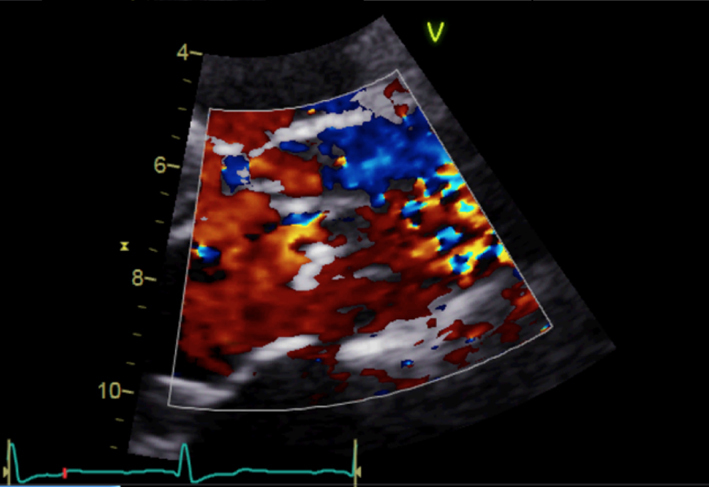 |
|
| Parasternal long axis; zoom 2D | LVOT dimension for assessment of AVA and stroke volume | Obtained at level of cusp insertion Inner-edge to inner-edge in mid-systole when LVOT is at a maximum Measurement parallel to aortic valve See ‘Essential parameters in the echocardiographic assessment of AS severity’ section | 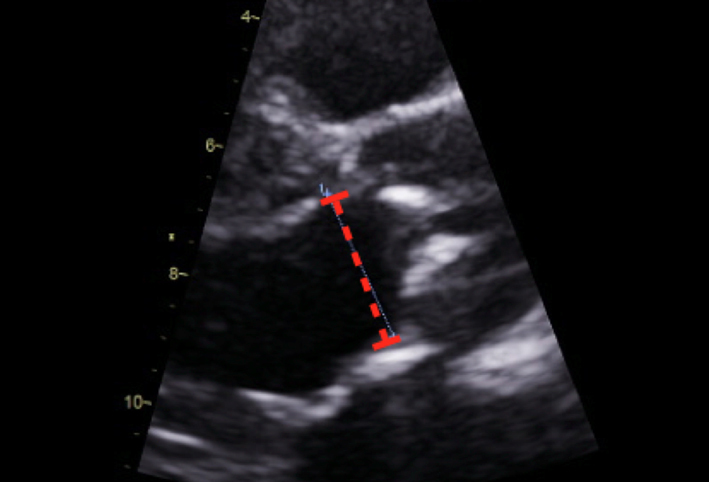 |
| Parasternal long axis; zoom 2D | Measurement of the aorta including the sino-tubular junction | Inner-edge to inner-edge method in end-diastole May be used in the assessment of the energy loss index (ELI; see ‘Additional parameters in the assessment of aortic valve stenosis’ section) | 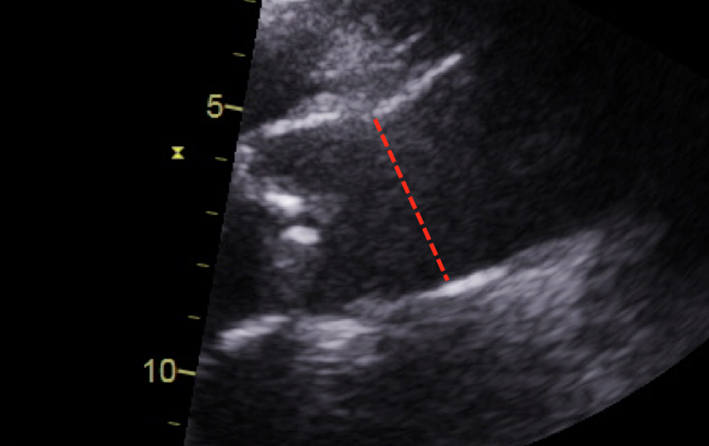 |
| Parasternal short axis (PSAX); 2D | Overview Visual appearance of aortic valve – cusp calcification and mobility | 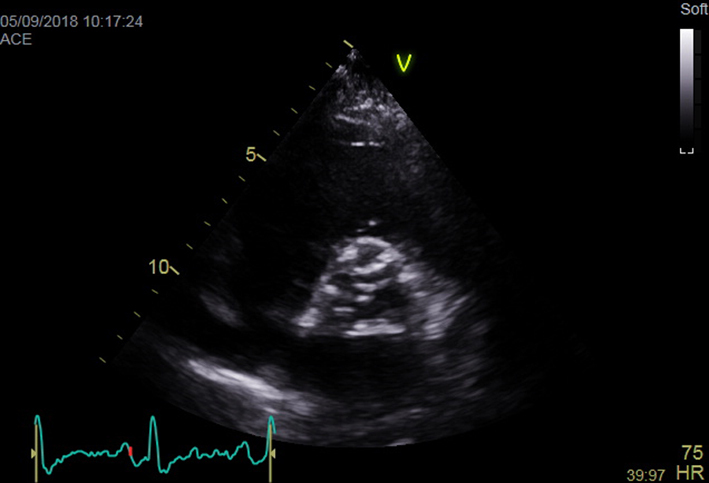 |
|
| Parasternal short axis; zoom 2D (+ colour Doppler) | Morphology of valve Visual appearance of calcification and mobility of cusps Colour Doppler to assess for presence and origin of AR | 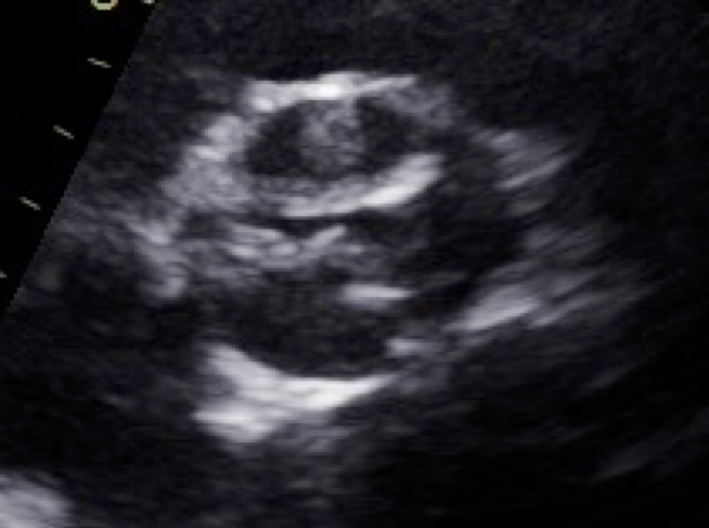 |
|
| Apical 4-chamber view; 2D imaging optimized for LV assessment | LV volumes and LVEF using quantitative methodology Consider GLS | LVEF is a prognostic marker in AS (see ‘Additional prognostic markers’ section) GLS is a potential marker of prognosis in AS (see ‘Additional prognostic markers’ section) |  |
| Apical 5-chamber view; 2D imaging (+ colour Doppler) | Overview Visual appearance of aortic valve – cusp calcification and mobility Colour Doppler assessment for AR |  |
|
| Apical 5-chamber view; 2D imaging zoom | CW Doppler tracings for AV Vmax and mean AVG | Sweep speed 50–100 mm/s Trace around dense aspect of Doppler curve Average of three tracings in sinus rhythm (SR) See ‘Essential parameters in the echocardiographic assessment of AS severity’ section for optimization and troubleshooting | 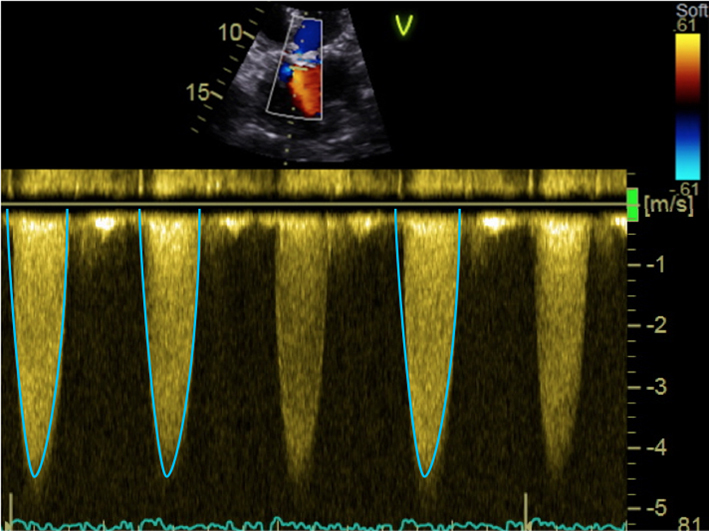 |
| Apical 5-chamber view; 2D imaging zoom | PW Doppler tracing in LVOT for calculation of stroke volume and AVA | Sweep speed 50–100 mm/s Trace around modal velocity Average three tracings in SR See ‘Essential parameters in the echocardiographic assessment of AS severity’ section for optimization and troubleshooting | 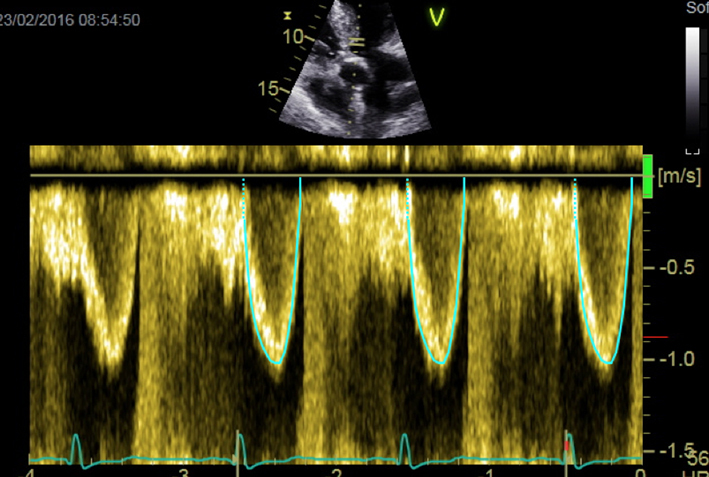 |
| Apical 2-chamber view; 2D imaging focus on LV | LV volumes and LVEF using quantitative methodology Consider assessment of GLS | LVEF is a prognostic marker in AS (see ‘Additional prognostic markers’ section) GLS is a potential marker of prognosis in AS (see ‘Additional prognostic markers’ section) | 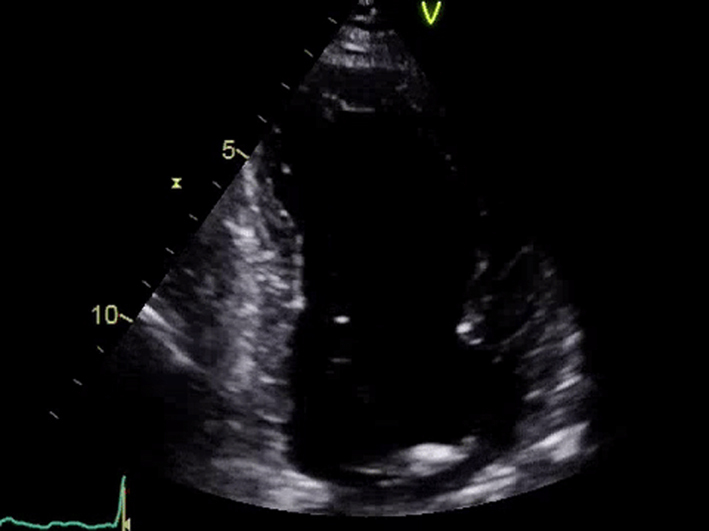 |
| Apical 3-chamber window; 2D imaging (+ colour Doppler) | Overview Calcification and mobility of aortic valve + colour Doppler for assessment of AR Consider GLS (see ‘Additional prognostic markers’ section) | 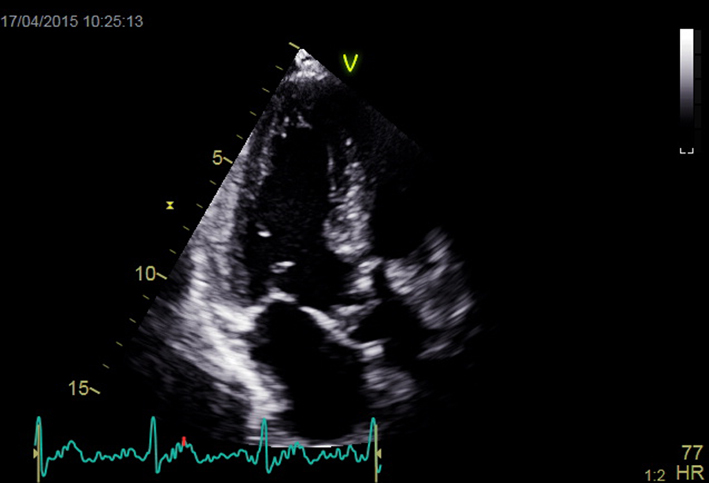 |
|
| Apical 3-chamber view; 2D imaging zoom AV + repeat CW and PW Doppler | Mobility and calcification of valve Repeat Doppler tracings for assessment of severity | 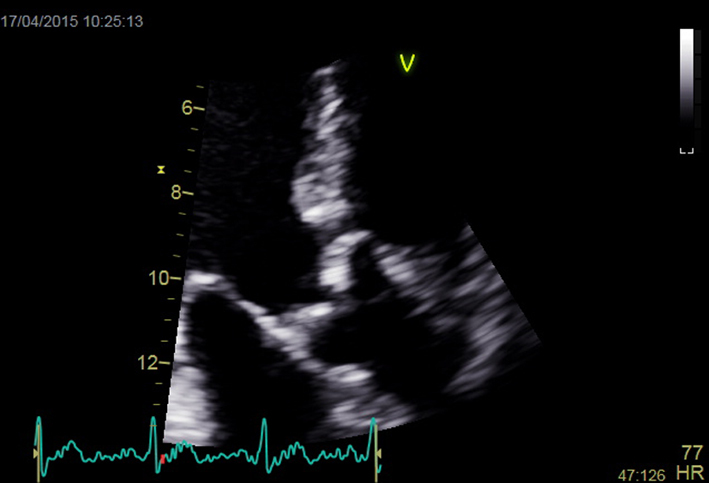 |
|
| Suprasternal notch; 2D + colour Doppler | Aortic arch | Look for turbulence and aortic pathology Repeat CW Doppler for AV Vmax and mean AVG (see ‘Essential parameters in the echocardiographic assessment of AS severity’ section) | 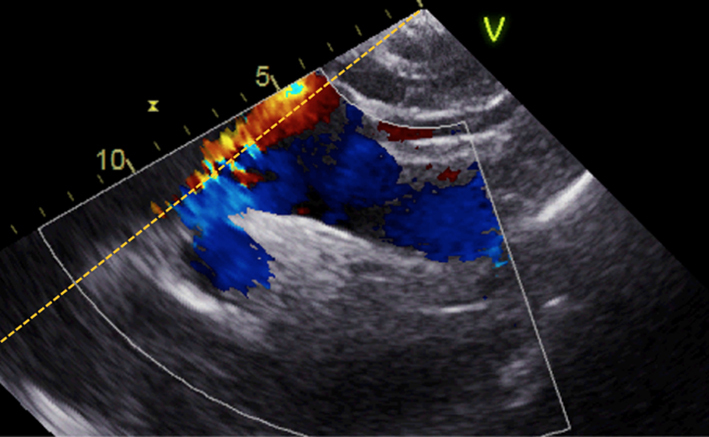 |
| Suprasternal notch; 2D + colour Doppler | Distal arch/descending aorta; look for turbulence and pathology including coarctation | 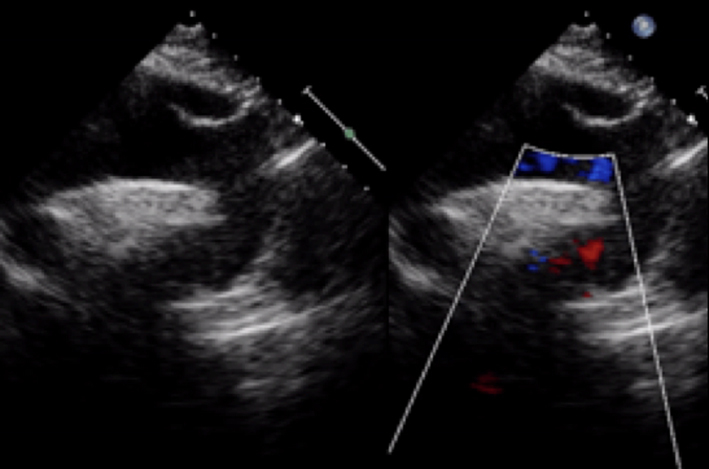 |
|
| PEDOF or standalone imaging Try all imaging windows including right parasternal (shown) | AV Vmax and mean gradient | Repeat from all imaging windows to ensure maximal values of Vmax and mean gradient are obtained | 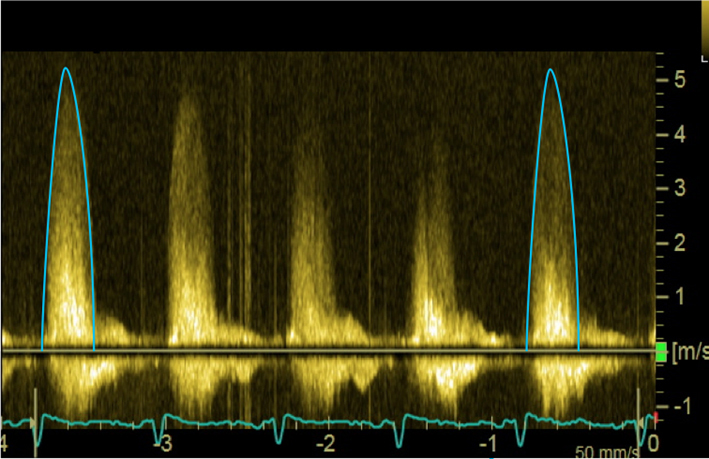 |
Table 3.
Suggested reporting template for AS.
| Demographics - Height, weight, body surface area (BSA). - Blood pressure, heart rate and rhythm. Aortic valve morphology - Tricuspid/bicuspid/unicuspid - Severity and extent of calcification LVOT - Dimensions and VTI - Report any change in the LVOTd from previous studies Aortic stenosis severity - Aortic valve Vmax; mean gradient: include window from which maximal values were obtained - Change in AV Vmax from previous echo study - Aortic valve area - Description of severity (mild/moderate/severe/very severe) Additional prognostic markers - Left ventricular ejection fraction - Global longitudinal strain - Indexed LV mass - High probability of pulmonary hypertension (see specific BSE guidance) Aortic regurgitation – note presence and severity (see specific BSE guidance) Aorta – measure size (see specific BSE guidance and reference intervals) |
Essential parameters in the echocardiographic assessment of AS severity
Aortic valve maximal velocity and mean gradient
Key points.
AV Vmax and mean gradient should be obtained in all patients undergoing the assessment of aortic valve stenosis.
The standalone or PEDOF probe should be used in all patients from multiple acoustic windows.
AV Vmax and mean gradient should be combined with the aortic valve area in order to describe AS severity.
BSE recommended methodology is demonstrated in Fig. 5.
Maximal AV Velocity and mean gradient are both obtained using continuous wave (CW) Doppler interrogation of the aortic valve (see Fig. 5) (32, 33).
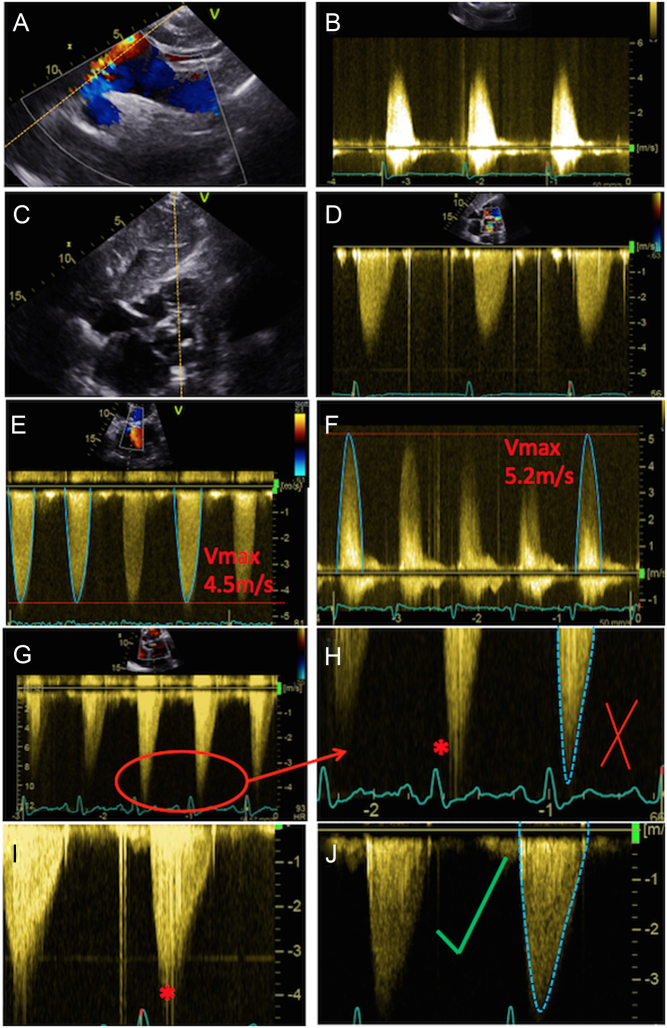
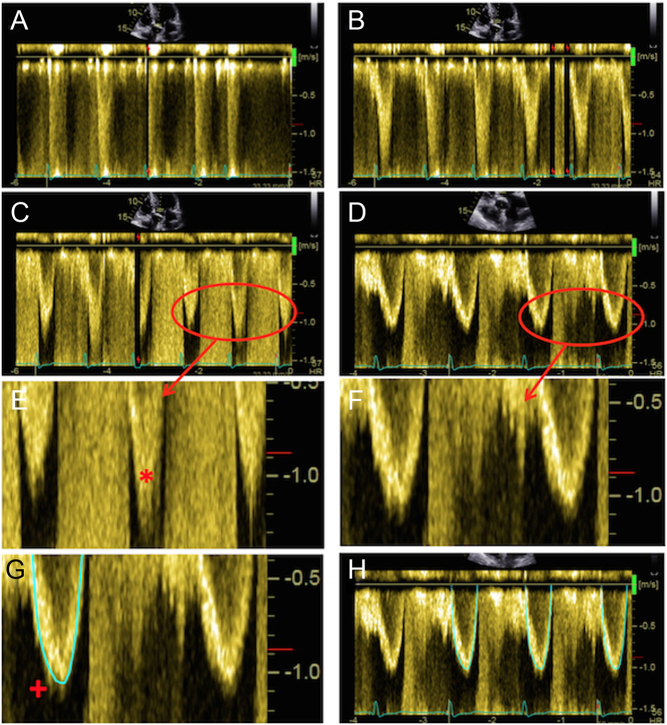
Figure 5.
Assessment of maximal AV velocity and mean gradient using CW Doppler. CW tracings should be obtained from multiple echocardiographic windows: suprasternal window (A), with the corresponding Doppler in (B); subcostal (C) and (D); apical 5-chamber (E); and stand-alone probe from the right parasternal window (F). Note that the AV Vmax in (E) represents a significant underestimation of maximal velocity when compared to that obtained using the standalone probe (F). Images (G) and (H) demonstrate spectral dispersion of the CW Doppler trace (marked with an asterisk). The mean gradient is obtained from tracing around the dense part of the CW Doppler curve. In images (I) and (J), the CW trace has been optimised: in (I) some spectral dispersion remains, but this has been completely eliminated in (J), resulting in an ideal CW trace.
The simplified Bernoulli equation (Box 1) is a formula by which the maximal velocity across an aortic valve can be ‘converted’ to an equivalent pressure change. There is no specific benefit of describing a peak gradient defined using echo over and above the maximal AV velocity, but an appreciation of the method is of value, as is an understanding of clinical scenarios in which CW Doppler may result in under- or over-estimation of AS severity.
The major challenge with CW Doppler is ensuring that the angle of insonation is fully aligned with the direction of the AS jet. A difference in alignment of more than 15–20 degrees between the ultrasound beam and the direction of blood flow will result in significant underestimation of Doppler indices and the severity of AS (27, 32). Sometimes this underestimation may be obvious to the sonographer, however, often traces may appear adequate only for further interrogation to demonstrate significantly higher values than first obtained (Fig. 5).
Box 1.
Simplified Bernoulli equation:
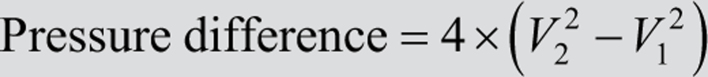
where V2 is the velocity of blood flow across the obstruction, and V1 is the velocity of blood flow prior to the level of obstruction. In most patients V1 is relatively small (≈1 m/s or lower) and is therefore negligible in the context of high values of V2. Consequently, the formula can be simplified further:
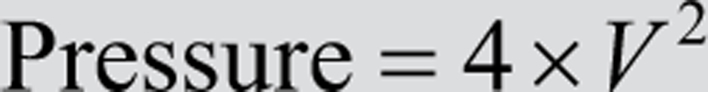
In circumstances where the sub-valve velocity is unexpectedly high (i.e. with small calibre LVOT dimensions or dynamic outflow tract obstruction), the longer equation (top) should be used.
Mean AVG is derived from an assessment of the average of the instantaneous velocities occurring during systole and is obtained from tracing around the CW Doppler waveform obtained through the aortic valve (see Fig. 5). Calculating the mean AVG is complex and is not derived from the mean velocity, but is routinely performed by measurement packages pre-installed on imaging platforms. Deriving the mean AVG utilizes the Bernoulli equation, and as such there are two important considerations when obtaining and interpreting this parameter. Firstly, owing to the squared relationship between instantaneous velocity and gradient, any under-estimation of the CW Doppler waveform will lead to an exaggerated underestimation of the mean gradient. Secondly, in the case of sub-valve obstruction and increased LVOT velocity, both the mean gradient and maximal velocity will overestimate severity of valvular stenosis. Other methods to assess AS severity will need to be employed in this scenario.
Recommended methodology
See Fig. 5:
It is essential to use multiple echocardiographic windows. Whilst in the majority of patients maximal values for AV Vmax and mean AVG are obtained from the apical window, in 20% of cases it is the suprasternal or right parasternal window that provides optimal results (26, 27).
The BSE recommends the use of the PEDOF or standalone probe in all patients.
The traces should be optimized for gain and scale. Sweep speed should be set at 50–100 mm/s.
At least three beats should be averaged for patients in sinus rhythm with a minimum of 5–10 consecutive beats for patients in AF (see ‘Other considerations’ section for details and alternative methodology).
The dense outer edge of the spectral waveform should be traced. Transit time artefact (which appears as a spectral dispersion or ‘blurring’ at the peak of the curve) should be ignored and not included within the trace (Fig. 5). The ‘reject’ function of the echo machine helps reduce transit time artefact and better delineate the modal signal.
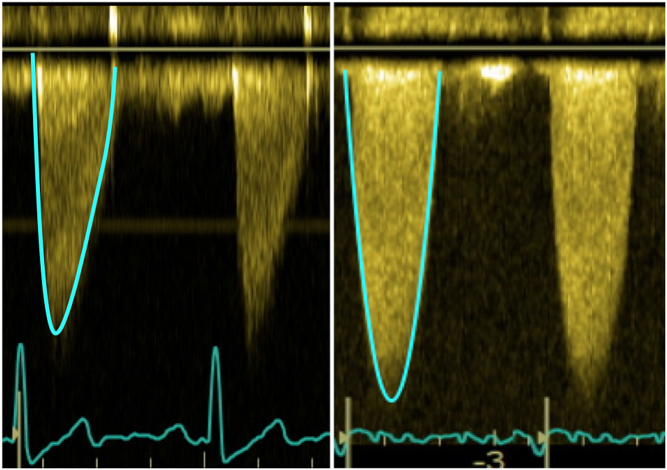
The shape of the CW waveform can provide an insight into the severity of AS. The CW curve in patients with moderate stenosis often has a rapid early peak, whereas severe AS will often display a slow acceleration with late peaking waveform (4) (Fig. 6).
The echocardiographic report should include the AV Vmax, the mean gradient, the window and transducer with which the maximal values were obtained.
Figure 6.
Comparison of the shape of the CW waveform. On the left, a patient with moderate AS: note how there is a rapid acceleration and an early peak. For comparison, the patient on the right has severe AS. There is a slower acceleration with a late peak. The shape of the CW waveform is maintained irrespective of LVEF and therefore it may be useful to help identify severe AS in difficult scenarios such as low-gradient AS.
Aortic valve area
Key points.
The effective aortic valve area, calculated using the continuity equation, should be obtained in all patients undergoing assessment of AS.
The AVA should be combined with the AV Vmax and mean gradient in order to describe AS severity.
The LVOT diameter should be measured at the insertion point of the aortic cusps and not below.
BSE recommended methodology is demonstrated in Fig. 7.
The assessment of AVA is well validated using echocardiography and is an essential aspect of the comprehensive echocardiographic assessment of AS (25, 26, 27, 28). The continuity equation is used to estimate the AVA, and is outlined in Box 2.
Box 2.
The continuity equation dictates that the volume of blood flowing through the LVOT must be the same as the volume of blood flowing through the aortic valve. We can calculate stroke volume in any conduit as the product of the velocity–time integral and the cross-sectional area (CSA) of the conduit. Using these two principles, we can derive the following formulae:
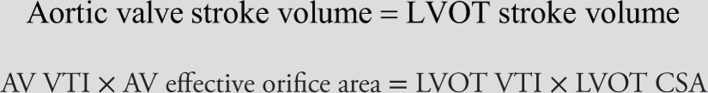
Therefore,
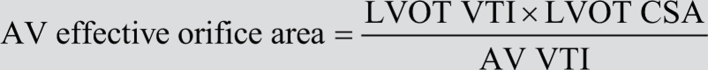
The LVOT is assumed to be circular; therefore the LVOT CSA is replaced by:
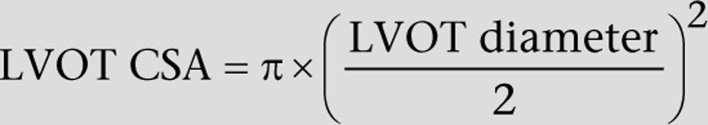
A ‘simplified’ continuity equation using velocity rather than the VTI is not recommended and is more prone to error, with a tendency to overestimate the calculated valve area (25).
Calculated AVA is useful as it is far less sensitive to alterations in transvalvular flow, and therefore will provide a more stable assessment of AS over a range of haemodynamic states (28, 34, 35). Conversely, estimated AVA requires several detailed measurements and is, therefore, more susceptible to a technical error.
In an ideal scenario, both the LVOT velocity time integral (VTI) and the LVOT cross-sectional area would be obtained at the same anatomical level. However, to all intents and purposes this is impossible. The two measurements are acquired from different echocardiographic windows, and so the sonographer can never be sure whether they are being obtained ‘at the same point’. More importantly, there is significant axial motion (up to 1 cm) of the aortic annulus during systole (25). Additionally, as mentioned previously, blood flow within the LVOT accelerates prior to the stenotic valve within the zone of acceleration, which can extend up to 1 cm from the anatomical annulus (25) (see Fig. 4). As such, even if the cross-sectional area and VTI were obtained at the same anatomical level, it would not necessarily be truly representative of stroke volume.
Recommended methodology
See Fig. 7:
Figure 7.
Optimal assessment of the LVOT Doppler. The PW Doppler sample volume should initially be placed on the aortic valve (A), which will usually result in aliasing. It should then be slowly moved apically (i.e. away from the valve; images B and C). In (C) there is a wide area of density at the apex of the trace, evident in the zoomed image (E; marked with asterisk). This represents blood flow within the zone of acceleration immediately proximal to the valve, and should not be included, as it would result in an overestimation of LV stroke volume and an underestimation of AS severity. In (D), (F), (G) and (H) the trace has been optimised further and depicts ideal assessment of LVOT Doppler. When tracing the curve, any spectral dispersion should be ignored (marked in (G) with a +). Three traces should be obtained and averaged for use in the continuity equation, with sweep speed set between 50 and100 mm/s (H).
Maximal AV velocity and mean gradient should be obtained using CW recordings as outlined in the previous section from multiple echocardiographic windows using the standalone probe. The maximal values obtained (an average of three beats) should be used in the continuity equation irrespective of which window the CW tracings were obtained from.
The LVOT diameter should be measured at the point of insertion of the aortic cusps, using an inner-edge to inner-edge methodology from the parasternal long-axis window (not the apical windows). This recommendation differs from a historical practice whereby the LVOT has often been measured up to 1 cm below the point of insertion of the cusps. This approach is no longer recommended by the BSE.
Measuring the LVOT diameter at the point of cusp insertion improves inter-observer reproducibility, better corresponds to the ‘true’ AVA derived using invasive tools, and provides a more accurate assessment of the cross-sectional area, as the LVOT is usually circular at this anatomical level (25, 26, 27).
It is essential that all sonographers within a department use the same methodology to measure the LVOT diameter.
-
In departments where the LVOTd was previously measured below cusp insertion, it is important to highlight to the referring clinician that the methodology has been updated. We would recommend that the report includes the following statement:
- The AVA is now being estimated in accordance with updated BSE guidance 2021. Changes in the estimated AVA from previous studies should therefore be interpreted with caution.
Care should be taken to exclude eccentric calcification from the measurement (see the troubleshooting section below).
The LVOT diameter should not be ‘assumed’ to be 2 cm. Careful measurement of the LVOT diameter will provide a better assessment of true AS severity (27).
For follow-up studies, it is important to note any changes in the measured LVOT diameter as this will dramatically impact on reported AVA. If there is a marked change in LVOT diameter, careful review of the previous and current study in order to establish an accurate value is required. In such cases re-reporting the earlier study is essential in order to document interval change accurately.
The PW trace should be obtained by placing the sample volume on the valve, at which point ‘aliasing’ will be noticed, reflecting rapid flow through the stenosed valve. The PW sample volume should then be slowly withdrawn apically until a suitable trace is obtained (Fig. 7).
The recordings should be optimized for gain and scale to improve accuracy. The sweep speed should be set between 50 and 100 mm/s. Three consecutive waveforms should be measured and averaged for patients in sinus rhythm, with a minimum of 5–10 consecutive waveforms measured in AF (see ‘Other considerations’ section for details and alternative methodology).
LVOT waveform should have a well-demarcated, narrow band of recorded velocities throughout systole. If any spectral dispersion or transit time artefact (‘blurring’) of the trace is noted, the PW sample volume should be moved more apically (i.e. away from the valve).
The process of obtaining PW traces should be repeated in both the apical 5- and 3-chamber windows. The maximal LVOT VTI obtained (as an average of three beats) should be used in the continuity equation.
Patients in whom accurate assessment of the LVOT dimensions cannot be obtained (for example owing to poor echocardiographic windows) the calculation of AVA using the continuity equation should be abandoned, in which case other parameters should be used to determine AS severity.
Potential sources of error and troubleshooting
Underestimation of maximal AV velocity and mean gradient owing to sub-optimal alignment of the Doppler transducer. This source of error will directly lead to an underestimation of AS severity.
-
Underestimation of LVOT cross-sectional area. If the LVOT diameter is underestimated, this will lead to an exaggerated underestimation of the cross-sectional area owing to the squared relationship between the two. Eccentric calcification of the LVOT is a major source of error in this regard. This type of error will directly result in an overestimation of AS severity.
In many circumstances, the LVOT is elliptical rather than circular. The standard method of calculating the LVOT CSA will therefore significantly underestimate the true value, resulting in an overestimation of AS severity. On such occasions direct planimetry of the LVOT cross-sectional area may improve accuracy (see ‘Special circumstances’ section). Examples of these sources of error are depicted in Figs 8 and 9.
-
Positioning of PW sample volume:
The measurement of flow using PW Doppler is highly dependent on the position of the PW sample volume. If the sample volume is positioned too close to the valve, increased velocity from the zone of acceleration will be included. This will directly lead to an underestimation of AS severity.
Conversely, if the PW sample volume is moved too far apically (i.e. away from the valve and into the LV cavity), the obtained LVOT VTI will frequently underplay ‘true’ stroke volume and lead to overestimation of AS severity.
In cases where the LVOT is short, withdrawing the PW sample from the valve to obtain a suitable LVOT Doppler tracing may result in a rapid reduction in velocities. In this scenario, 3D imaging of the LVOT closer to the anatomical level at which the PW trace was recorded may improve accuracy (Fig. 9).
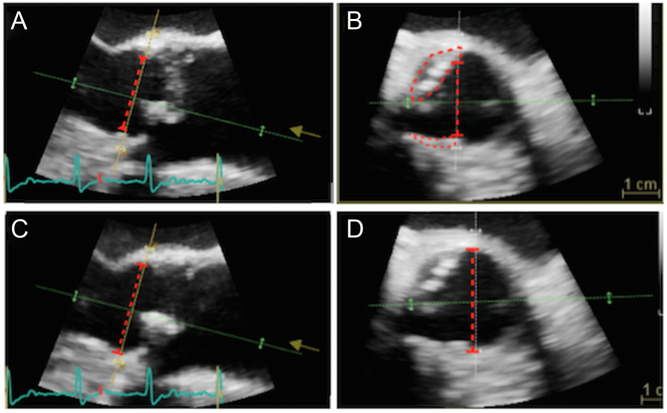
Figure 8.
Eccentric calcification can lead to important underestimation of LVOT diameter, here demonstrated with 3D imaging. (A) Depicts a long-axis view obtained during TOE with LVOT diameter marked (red dotted line). Picture (B) is an image of the LVOT taken at an orthogonal plane to image (A). Note that the LVOT diameter is an underestimate owing to two areas of eccentric calcification (marked). Adjustment of the imaging plane can remove calcification from the image (C), with the optimal LVOT diameter now shown (red dotted line in images C and D).
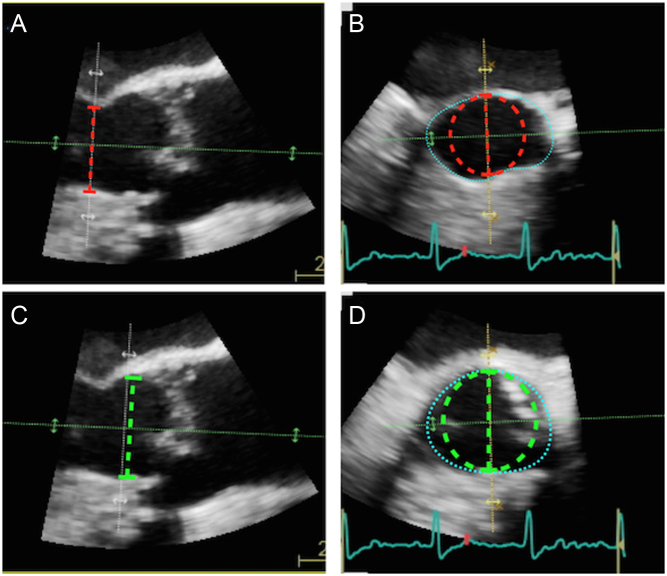
Figure 9.
Error arising from the assumption of circular LVOT. Image (A) depicts a long-axis view of the aortic valve obtained using 3D TOE. The LVOT diameter has been measured 2 cm proximal to the valve (red dotted line). Image (B) is a short axis image obtained at this anatomical level. Estimation of the LVOT cross-sectional area at this point calculates the area of the red dotted circle, and is a huge underestimate of the ‘true’ LVOT area (marked by blue dotted outline). The LVOT should be measured immediately below the insertion of the aortic cusps (c; green dotted line). Image (D) depicts the short axis view at this anatomical level: note the LVOT demonstrates a much more circular profile. Estimation of the LVOT area using π(LVOTd ÷ 2)2 would calculate the area of the green dotted circle, which is much closer to the ‘true’ LVOT area (blue dotted line). On occasion, direct planimetry of the LVOT cross-sectional area using 3D echo can be considered, particularly for difficult cases (see low-gradient AS, ‘Special circumstances’ section).
Box 3 Common errors in assessment of AS.
Failure to use PEDOF probe from multiple acoustic windows including apex and right intercostal space.
-
Inaccurate assessment of LVOT diameter and cross-sectional area:
- Calcification must be excluded.
- Measure LVOT diameter at the level of cusp insertion.
- Remember non-circular nature of LVOT.
Poor positioning of PW sample volume.
Failure to image the ascending aorta and arch.
Approach to the patient
Figure 10 describes the echocardiographic approach to classifying the severity of AS and identifying high-risk characteristics. This will allow the majority of patients to be consistently graded and ensure that important prognostic findings are highlighted to the clinician.
Figure 10.
Decision-aid to guide assessment of aortic stenosis.
The first priority in the assessment of AS is to ensure that the valve is both calcified and restricted. In the absence of either of these, significant AS is unlikely except in the (rare) circumstance of congenital AS.
Next, ensure that a robust assessment of both maximal AV velocity and mean gradient have been obtained. If either the AV Vmax is ≥4 m/s or the mean AVG is ≥40 mmHg, the patient should usually be considered as having severe AS. The rare exceptions to this are in circumstances in which there is temporary increase in flow such as tachy-arrhythmia or sepsis, in which case repeat assessment when the haemodynamic status has normalized should be considered.
Once AV Vmax is noted to be ≥4 m/s or the mean AVG is ≥40 mmHg, the focus of the study should then turn to assess for the high-risk characteristics that define prognosis and may inform clinical decision-making (see Box 4 and ‘Additional prognostic markers’ section).
If AV Vmax and mean AVG do not fulfil the criteria for severe AS, assessment of the AVA will usually confirm that the patient has non-severe AS (i.e. the AVA will be ≥1 cm2). In this scenario, the severity of AS should be defined using the AV Vmax or mean gradient (whichever is the greater; Table 4).
If, however, the Vmax and/or mean AVG suggest non-severe AS but the AVA is <1 cm2, further thought is required (Fig. 10). It is imperative to re-check all measurements, in particular ensuring that the PEDOF probe has been used from multiple echocardiographic windows. Assessment of the shape of the CW waveform may provide a clue as to the underlying severity (see Fig. 6). For individuals of small body habitus (i.e. a BSA <1.7 m2), use of the indexed AVA may confirm that the severity is moderate (see ‘Additional parameters in the assessment of aortic valve stenosis’ section for details of indexed AVA).
There is not a linear relationship between Doppler indices and the AVA. Often, the Vmax or mean gradient will fall in the range of 3.5–4 m/s or 35–40 mmHg, respectively, at which point the AVA will likely be between 0.8 and 1.0 cm2. Such patients are usually best treated as being ‘moderate AS’ but are often pragmatically reported as ‘moderate to severe AS’ to ensure that the advancing nature of the disease is highlighted to the clinician.
If a marked disagreement between the Doppler indices and AVA remains, the next stage is to refer to the ‘Special circumstances’ section. An MDT approach for challenging cases is advocated and ensures consistency within a department.
Table 4.
Grading of aortic stenosis.
| Grading of severity | Echocardiographic indices | ||
|---|---|---|---|
| AV Vmax (m/s) | Mean gradient (mmHg) | AVA (cm2) | |
| Aortic sclerosis | <2.5 | – | – |
| Mild | 2.5–2.9 | <20 | >1.5 |
| Moderate | 3–3.9 | 20–39 | 1–1.5 |
| Severe | 4–4.9 | 40–59 | <1 |
| Very severe | ≥5 | ≥60 | ≤0.6 |
Grading of severity
Aortic sclerosis
Key points.
Aortic sclerosis is defined as a thickened restricted aortic valve without significant obstruction to flow.
AV Vmax is <2.5 m/s.
Echocardiographic surveillance is not routinely recommended.
The prevalence of aortic sclerosis increases with age, and is associated with hypertension, renal disease, dyslipidaemia, and smoking (23). Approximately 2% of patients per year will progress from aortic sclerosis to aortic stenosis (36), and as such, it is important to identify these patients so they can have any risk factors addressed. In some clinical scenarios, surveillance may be considered, such as younger individuals with risk factors such as advanced renal dysfunction.
Mild, moderate and severe AS
Key points.
Maximal velocity is the preferred measure with which to define AS severity.
The echocardiographic report should document the change in maximal velocity with time.
Echocardiographic surveillance is advised (6):
- - Mild AS (Vmax <3 m/s) repeated every 3–5 years.
- - Moderate AS (Vmax 3–3.9 m/s) repeated every 1–2 years.
- - Severe AS (Vmax ≥4 m/s) repeated every 6 months.
A wealth of prospective data has demonstrated the association between AV Vmax and cardiovascular events (30, 37, 38, 39, 40). Fewer than 30% of patients with an AV Vmax <3 m/s will need aortic valve intervention within 5 years of follow-up (30, 38). If the AV Vmax is between 3 and 4 m/s, around half of patients will need surgery within 4 years. As the maximal velocity increases, so time-to-event decreases (30, 37, 39). AVA is a useful tool to assess AS severity, but there are relatively few studies in the literature demonstrating an independent association between adverse survival and AVA, with most data suggesting that the AVA cut-off for predicting poorer outcomes is 0.8 cm (41, 42).
In patients with heavily calcified valves, a change in AV Vmax of >0.3 m/s/year is associated with poor outcomes (30, 37). Accordingly, the rate of change in AV Vmax should be documented in the echo report and can be calculated by dividing the AV Vmax change by the appropriate decimal (i.e. 6 months = 0.5). Changes in AV Vmax should only be interpreted if sequential studies obtained maximal velocity from similar echocardiographic windows. It is important to review previous studies and ensure that any change in AV Vmax is not related to the measurement of artefact.
Very severe AS
Key points.
Very severe AS is defined as an AV Vmax ≥5 m/s or a mean gradient ≥60 mmHg.
Patients with very severe AS have poor event-free survival even in the absence of symptoms.
Such patients should be highlighted to the referring physician.
Very severe AS describes patients who display either very high gradients or very small aortic valve areas, associated with particularly poor outcomes. Once the AV Vmax is ≥5 m/s, survival is reduced even in the absence of symptoms (40, 43, 44). Similarly, a mean gradient ≥60 mmHg and an AVA ≤0.6 cm2 are associated with extremely high event rates and largely correspond with an AV Vmax of 5 m/s (45, 46, 47). American guidelines advocate surgery in such patients even in the absence of symptoms (Class IIa indication) (6).
The BSE discourages use of the term ‘critical AS’ as it is not clearly defined within the literature (48, 49, 50, 51).
Additional parameters in the assessment of aortic valve stenosis
These additional parameters are not usually required in every patient, but occasionally may provide additional clues as to the severity of the AS, or may be of use in certain clinical scenarios.
Indexed aortic valve area
Key points.
The indexed AVA (AVAi) is not required in all patients.
An AVAi <0.6 cm2/m2 is consistent with severe AS.
In individuals of small body habitus (i.e. a BSA <1.7 m2), the AVAi may re-classify some individuals as having moderate AS, potentially avoiding unnecessary intervention (see Fig. 10).
The AVAi should be avoided in patients who are overweight, where AVAi will overestimate AS severity.
Indexed aortic valve area was introduced in the 1960s to account for the large proportion of paediatric patients with AS (52). AVAi is not superior to absolute AVA in the assessment of individuals and identification of high risk (42, 53). Patients who are considered to have severe AS solely on the basis of an AVAi (i.e. an AVAi of <0.6 cm2/m2 but an AVA ≥1 cm2) have significantly better outcomes than individuals in whom the absolute valve area is <1 cm2 (53).
The AVAi should usually only be employed in individuals of small body habitus (i.e. a BSA <1.7 m2) in whom the AVA implies severe AS but where AV Vmax or mean AVG suggest the valve is non-severe (i.e. an AVA <1 cm2; Vmax <4 m/s and mean AVG <40 mmHg): in such patients the AVAi may re-classify AS severity as moderate, thereby avoiding unnecessary intervention (see Fig. 10) (53, 54).
Dimensionless index
Key points.
The Dimensionless Index (DI) is obtained from the ratio of LVOT: AV velocities. A value of <0.25 is consistent with severe AS.
The DI is useful when image quality prevents accurate assessment of the LVOT cross-sectional area.
If the LVOT cannot be measured accurately, DI may be used for serial studies to monitor progression of AS.
The DI removes one potential source of error (measurement of the LVOT cross-sectional area), but does not account for the true anatomy of the LVOT, and is usually less accurate than the AVA in the assessment of AS severity (25, 26, 27). If parasternal windows are challenging such that accurate assessment of the LVOT diameter cannot be obtained, the DI is useful, and may be used for surveillance. Once the DI is <0.2, outcomes are very poor, independent of the patient’s BSA (55).
Planimetry
Key points.
The BSE does not recommend routine use of planimetry.
If planimetry is to be pursued, TOE is the echocardiographic modality of choice, and 3D imaging improves accuracy.
Flow contraction means that planimetry always underestimates AS severity even if performed accurately (see Fig. 4).
Planimetry is not reliable in low-flow states as there is insufficient opening of the valve.
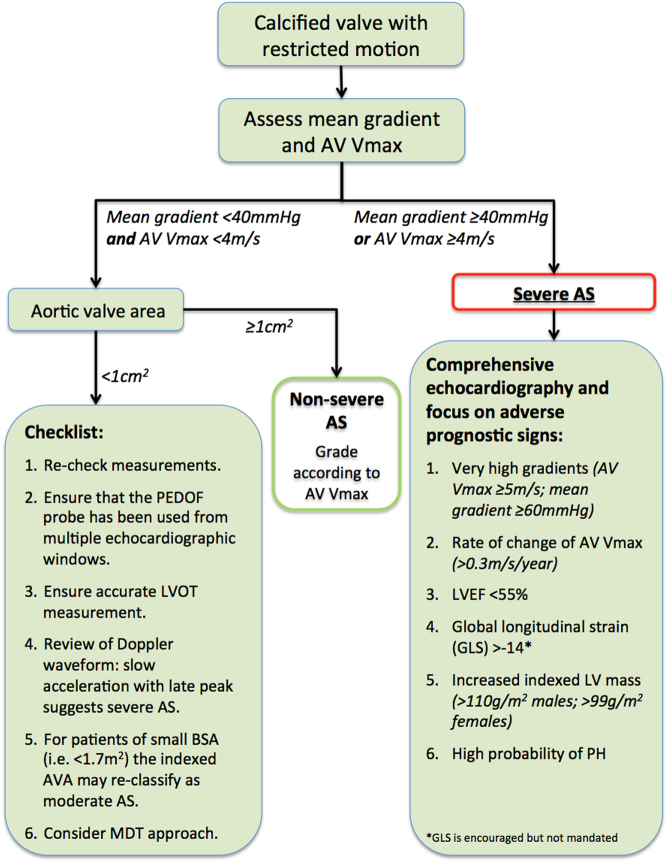
Planimetry is a technique whereby the anatomical orifice of the aortic valve is directly traced in order to provide an estimation of the AVA (Fig. 11).
Figure 11.
Planimetry using 3D TOE. A 3D volume of the aortic valve is obtained (A). The volume can be manipulated to display orthogonal planes. Image (B) depicts a long-axis view, and the dotted red line depicts the plane from which a short-axis view (C) is then shown. This plane is aligned with the point of insertion of the valve cusps. In (D), the image has been manipulated, and the ‘plane of interest’ moved further into the aorta such that it now aligns with the valve tips (dotted green line). The short axis window obtained at the level of the dotted green line is displayed in image (E). Planimetry obtained in image (C) clearly overestimates aortic valve orifice area when compared to the result obtained by planimetry in image (E).
There are multiple pitfalls to this technique. The imaging plane must be aligned with the point of maximal stenosis of the valve. Identifying the aortic orifice is challenging in heavily calcified valves in which blooming artefact will result in potential overestimation of severity. Whilst planimetry is feasible and correlates with Doppler-based estimates of AS severity, the limits of agreement are poor which restricts the value of this technique in clinical decision-making (56, 57, 58).
Energy loss index
Key points.
The energy loss index (ELI) accounts for the pressure recovery phenomenon.
The BSE does not recommend routine use of the (ELI).
An ELI <0.6 cm2/m2 is consistent with severe AS.
As blood flows past an obstructed aortic valve, the velocity of blood increases, and static energy (or pressure) is converted into kinetic energy. This manifests as a pressure drop. As blood flow continues beyond the obstructed valve into the aorta, some kinetic energy is converted back into static energy and a proportion of pressure is restored. Standard echocardiographic parameters of AS severity do not incorporate the effect of the ‘pressure-recovery’ phenomenon.
The ELI is a way to calculate a ‘corrected’ valve area that accounts for pressure-recovery, which is reported after indexing for BSA (59). The ELI will only ever re-classify a valve as being less severe than suggested by standard Doppler indices. Use of the ELI has been validated in observational studies and a randomized trial and occasionally re-classifies the severity of observed stenosis, but overall performs no better than traditional measures of AS severity at identifying individuals at risk (31, 60, 61).
Patients with a mean AVG >40 mmHg should be considered as having severe AS and not re-classified as moderate AS using the ELI. The ELI may be considered in patients with low-gradient AS (i.e. an AVA <1 cm2; Vmax <4 m/s and mean AVG <40 mmHg): in such scenarios, the ELI can identify a subset of individuals who are at lower risk and can afford to be observed (60, 61). A worked example of the ELI is in Fig. 12.
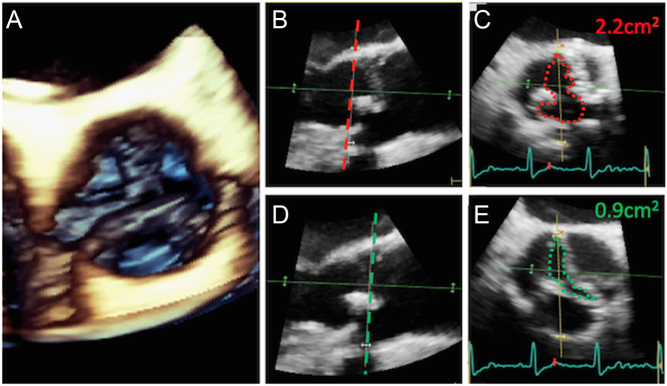
Figure 12.
Worked example of the energy-loss index (ELI): From the image (A), the LVOT cross-sectional area can be calculated: LVOT CSA = π × (LVOTd ÷ 2)2 = 3.5 cm2. The cross-sectional area of the ST junction (STJ) depicted in image (B) is: STJ CSA = π × (STJ diameter ÷ 2)2 = 6.6 cm2. Using the CW trace in image (C), AV Vmax is 3.1 m/s; mean gradient 27 mmHg; AV VTI 55 cm. Using the PW trace in image (D), LVOT VTI is 14 cm. Aortic valve area using the continuity equation is: AVA = LVOT VTI ÷ AV VTI × LVOT CSA = 14 cm ÷ 55 cm × 3.5 cm2 = 0.9 cm2. After indexing for BSA (1.7 m2 in this patient), the AVAi is 0.51 cm2/m2. This is an example of low-gradient AS whereby both the AVA and AVAi suggest severe stenosis yet the AV Vmax and mean gradient only suggest moderate stenosis. The energy-loss index is defined as: ELI = (AVA × STJ CSA) ÷ (STJ CSA – AVA). ELI = (0.9 × 6.6) ÷ (6.6 – 0.9) = 1.04 cm2. Indexed for BSA = 0.61 cm2/m2. An ELI < 0.6 cm2/m2 is consistent with severe AS, therefore this patient has been re-classified as moderate AS when accounting for the pressure-recovery phenomenon.
Other considerations
Atrial fibrillation or irregular rhythms
Key points.
For patients in AF, Doppler indices from at least 5 consecutive beats should be measured, from which the average AV maximal velocity, mean gradient and calculated AVA are derived (4). There is mixed evidence in the literature as to the number of consecutive beats required, with some studies reporting that, on average, 13 consecutive beats are needed to approximate the true cardiac output for a patient with AF, whereas other work has demonstrated that there appears to be minimal variation of the calculated AVA in patients with varied R-R intervals (62, 63).
An alternative method is to obtain Doppler recordings for AV VTI and LVOT VTI from matched cycle lengths. The method involves recording a single AV VTI measurement after a long R–R cycle, combined with a single LVOT VTI measurement obtained after a similar long R–R cycle length (where ‘long R–R’ is defined relative to the average heart rate; Fig. 13). These two single measures can then be used in the continuity equation to derive the calculated AVA. This methodology has shown to correlate highly with the traditional method of valve area calculation, with high degrees of reproducibility (64).
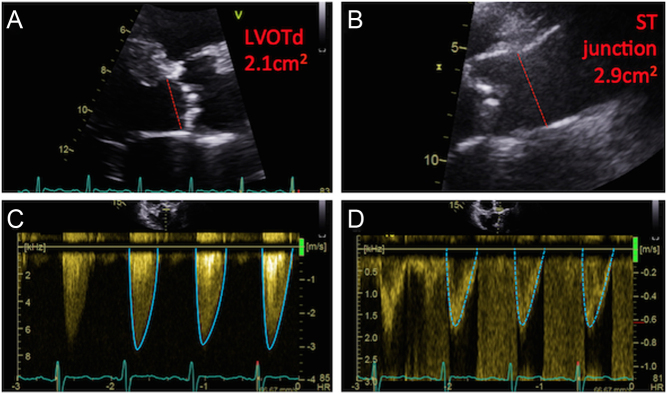
Figure 13.
Assessment of Doppler indices and AVA in AF. Doppler traces obtained from a patient in AF (A and B): note marked beat-to-beat variations of the CW and PW waveforms. Conventionally, at least 5–10 consecutive CW and PW traces are obtained for assessment of valve indices (A and B). An alternative method is the ‘matched R–R interval’ approach: in image (C) the R–R interval is seen as 600 ms, and the CW waveform is traced. In image (D) a comparable PW waveform is found, whereby the R–R interval is noted to be 607 ms, and is therefore traced. The obtained values from these comparable CW and PW traces can be used in the continuity equation. Within CW traces it is possible to appreciate a ‘phantom’ LVOT trace (E). The true LVOT trace is displayed in image (F) for comparison. This phantom trace should not be used for the continuity equation, as it will directly lead to an underestimation of AS severity.
It is often possible to see an approximation of the LVOT trace within the continuous-wave Doppler recordings through the AV (Fig. 13). These phantom tracings should not be used in the continuity equation as they systematically overestimate the calculated stroke volume as higher velocities from the flow convergence region are included within the trace, and would result in underestimation of severity of aortic stenosis using the continuity equation (63).
Blood pressure
Key points.
Hypertension may lead to either over- or under-estimation of AS severity.
In challenging clinical situations AS should be re-evaluated after adequate control of BP: target of 130–140 mmHg (systolic BP).
Assessment of AS is challenging in patients with poorly controlled hypertension, owing to the complex inter-relationship between systemic BP, afterload, transvalvular flow and indices of AS severity. In one study, systemic vascular resistance was acutely increased through the use of infused adrenaline or isometric handgrip exercise, which led to a reduction in calculated aortic valve area, although mean pressure gradient was relatively unchanged (65). Conversely, an experimental study in pigs created a hypertensive state by banding the aorta, which resulted in an acute reduction in the mean pressure gradient combined with increased calculated valve areas (66). Both these studies use experimental methods to mimic a hypertensive state and therefore are unlikely to reflect the vascular, morphological and haemodynamic alterations seen in patients with long-standing hypertensive heart disease. In fact, a study in which a circulatory model was used to examine the effect of blood pressure on Doppler indices of AS suggested that BP alterations per se did not lead to predictable changes in mean gradient or calculated valve area, but that alterations in transvalvular flow were responsible for the variations noted (67).
Poorly controlled hypertension in the context of AS is associated with worse survival (68). Historically, anti-hypertensive agents have been considered relatively contra-indicated in the presence of significant AS, but in fact most are tolerated well and appear safe (69). There is some evidence that calcium-channel blockers (CCBs) are associated with poorer outcomes and an adverse BP response to exercise (70, 71). Therefore, it is reasonable to use alternative anti-hypertensive agents where feasible (72, 73). In particular, ACE-inhibitors or other agents targeting the renin-aldosterone system are not only effective and well tolerated in AS, but improve long-term prognosis (69).
Additional prognostic markers
The following sections outline additional echocardiographic parameters that are useful in assessment of risk and clinical decision-making. These sections refer to patients in whom an AV Vmax ≥4 m/s and/or mean AVG ≥40 mmHg have been obtained. Box 4 lists the prognostic findings that should be highlighted to the referring clinician.
Box 4 Prognostic findings in severe AS (AV Vmax ≥4 m/s or mean AVG ≥40 mmHg).
Highlight to referring clinician:
Very high gradients (very severe AS): AV Vmax ≥5 m/s; mean gradient ≥60 mmHg.
Rate of change of AV Vmax: heavily calcified valve with increase of >0.3 m/s/year.
Left ventricular ejection fraction <55%.
Global longitudinal strain (GLS) >-14; (GLS is encouraged but not mandated).
Increased indexed LV mass: >110 g/m2 (males) or >99 g/m2 (females).
High likelihood of pulmonary hypertension.
Left ventricular ejection fraction
Key points.
LVEF should be assessed in all patients with aortic stenosis using quantitative methodology if possible.
Patients with severe AS (Vmax ≥4 m/s and/or mean gradient ≥40 mmHg) and an LVEF <55% should be reported as ‘impaired LVEF’ in the clinical report.
Patients with severe AS (Vmax ≥4 m/s and/or mean gradient ≥40 mmHg) with an LVEF ≤35% should be reported as ‘severely impaired LVEF with high likelihood of improvement after aortic valve intervention’.
Severe AS with a mean gradient ≥40 mmHg and an LVEF <50% is encountered infrequently in clinical practice (74, 75). Usually this scenario reflects an afterload imbalance whereby inherent contractility is largely preserved, and LVEF will typically improve after aortic valve intervention (51, 76). Such individuals are noted to have poor outcomes without surgery and accordingly it is a Class I indication for aortic valve intervention (5, 6).
Patients with an LVEF <55% have significant excess mortality compared to those in whom the LVEF is >60%, independent of whether surgery is undertaken (77). Outcomes for patients undergoing AVR with an LVEF between 55 and 60% may also be worse than those undergoing surgery with an LVEF >60% (75). Database analyses have suggested that rapid reduction in the LVEF of more that 10% per year appears to be associated with poorer survival in patients with asymptomatic severe AS (78).
Indexed LV mass
Key points.
Indexed LV mass (LVMi) should be reported for all patients with severe AS.
LVMi should be estimated using the linear method from the parasternal long-axis window and indexed to BSA (3).
- - For males an LVMi >110 g/m2 is abnormal.
- - For females an LVMi >99 g/m2 is abnormal.
Part of the adaptive mechanism of the left ventricle to an increased afterload is compensatory hypertrophy. This leads to normalization of wall stress and maintenance of cardiac output (79, 80). Whilst there are mechanistic benefits of hypertrophy in patients with aortic stenosis, it is also recognized that excessive indexed LV mass can result in increased myocardial oxygen demand, myocardial fibrosis, and increased cardiovascular mortality (81, 82).
Global longitudinal strain
Key points.
Global longitudinal strain (GLS) may identify patients who are at an increased risk of cardiovascular events.
The BSE encourages the assessment of GLS in patients with AS where image quality allows.
Significant inter-vendor variability exists for GLS, but a value more positive than −14% is very likely indicative of LV dysfunction.
Early changes in LV performance may not result in reduction in measured LVEF, and yet will confer worse prognosis on such patients. Global longitudinal strain (GLS) derived using speckle tracking is a surrogate for the burden of LV fibrosis, and may allow the identification of early LV dysfunction (83, 84).
GLS values are progressively more positive (i.e. less normal) as aortic stenosis severity worsens and are a strong predictor of cardiovascular mortality in patients with moderate or severe AS even after surgical intervention (83, 85, 86, 87). A recent participant-level meta-analysis including over 1000 patients with significant AS and an LVEF >50% demonstrated that a GLS >−14.7% predicted poor survival in the overall cohort and amongst those individuals with an LVEF >60% (88).
Pulmonary hypertension
Key points.
The echo report should include an assessment of the probability of pulmonary hypertension (PH) according to BSE guidelines (97).
A high likelihood of PH should be highlighted to the referring clinician.
There are relatively few published series in which echo-estimated pulmonary pressures have been examined in a cohort of patients with severe AS (89, 90, 91, 92, 93, 94). All are retrospective analyses, and deriving guidance is challenging, as a heterogeneous group of patients were included, many of who were already symptomatic or had other indications for intervention at the point of inclusion. Additionally, the echocardiographic methodology and threshold for defining important PH differed between reports, with some defining severe PH as an estimated PAP >50 mmHg (91, 92, 93), whereas others considered the threshold of severe PH as a PAP >60 mmHg (89, 90). The most recent study used a methodology that attempts to reflect the current approach of estimating the probability of PH rather than directly estimating PAP (94). All reports are consistent in that higher values of PAP are associated with poorer long-term survival, but the threshold that should trigger intervention remains unclear. Consequently, the BSE approach of assessing the probability of PH is appropriate for the AS cohort, and those individuals with a high probability of PH should be highlighted to the referring clinician (95).
Additional echocardiographic imaging modalities
Trans-oesophageal echocardiography
Key points.
TOE is rarely required for the assessment of AS.
If TTE is insufficient, Doppler interrogation using TOE may confirm the severity of AS.
TOE imaging windows are shown in Table 5.
Table 5.
TOE imaging in aortic stenosis.
| View (modality) | Measurement | Explanatory notes | Image |
|---|---|---|---|
| 5-chamber view; mid-oesophageal window; 0°; zoom 2D | Observe calcification and mobility of the cusps |  |
|
| 5-chamber view; mid-oesophageal window; 0°; zoom 2D + colour Doppler | Colour Doppler imaging to assess for turbulence and valve regurgitation | 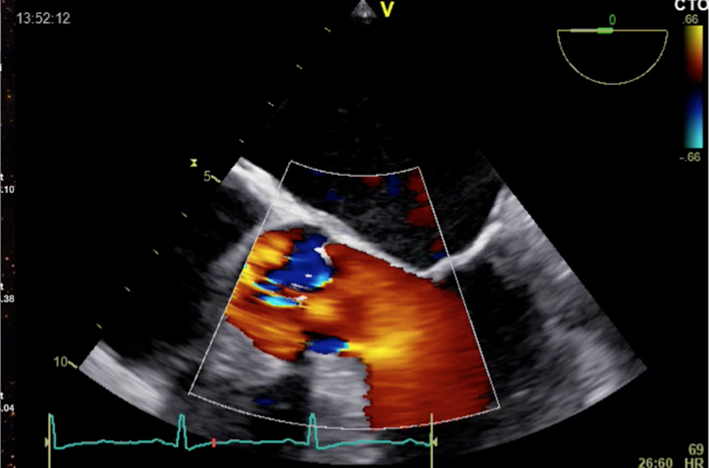 |
|
| Short-axis view; mid-oesophageal window; 40–60°; the level of the aortic cusps 2D | Assess for valve morphology, cusp mobility and calcification | 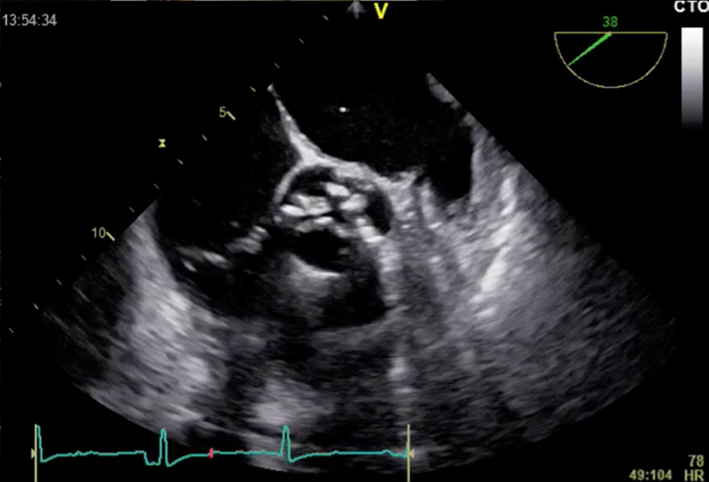 |
|
| Short-axis view; mid-oesophageal window; 40–60°; at the level of the aortic cusps 2D zoom + colour Doppler | Assess for AR Colour Doppler may help identify morphology (shown is tricuspid valve; see ‘Anatomy’ section) | 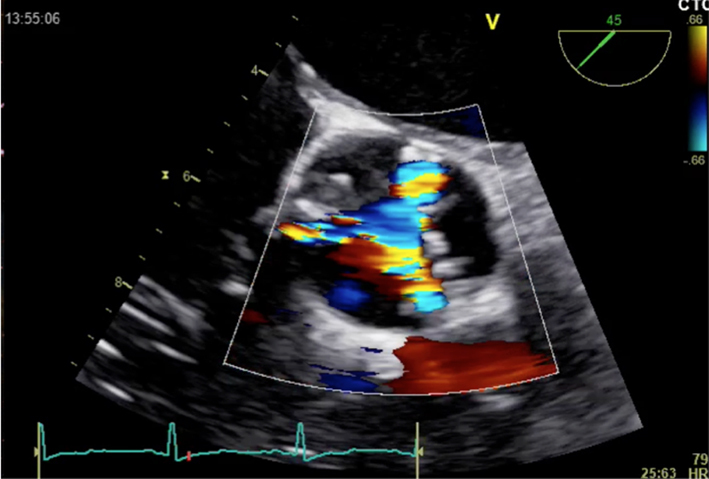 |
|
| 3D dataset | Consider planimetry | Overview and optimized images for planimetry | 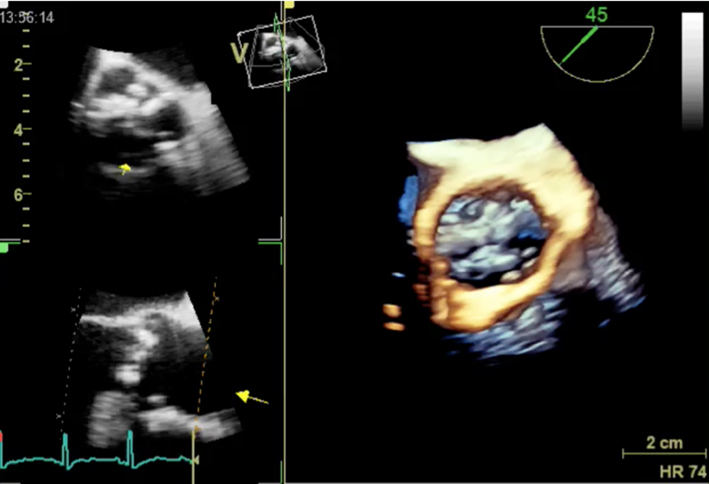 |
| Long-axis view; mid-oesophageal window; 120–140°; 2D imaging | Measurement of LV size and wall thickness Indexed LV mass calculation | Observe calcification and mobility of the cusps LV size and function Indexed LV mass is a prognostic sign | 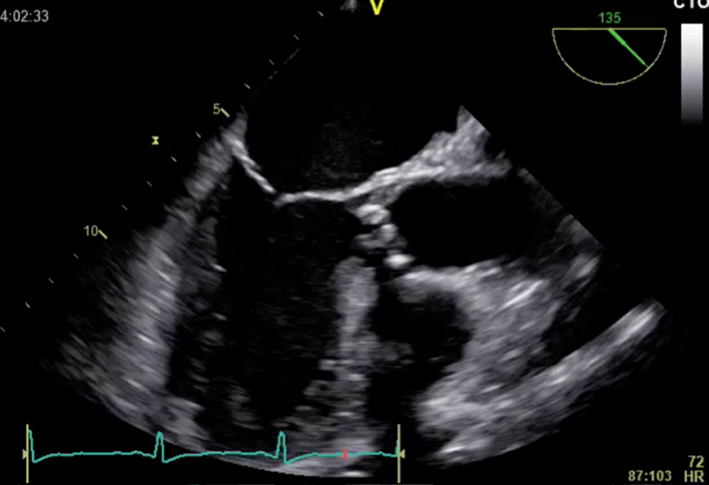 |
| Long-axis view; mid-oesophageal window; 120–140°; 2D imaging zoom | LVOT dimension (not shown) Measurement of aorta | Inner-edge to inner edge method; end-diastole Red = Sinus of Valsalva Green = ST junction Blue = Ascending | 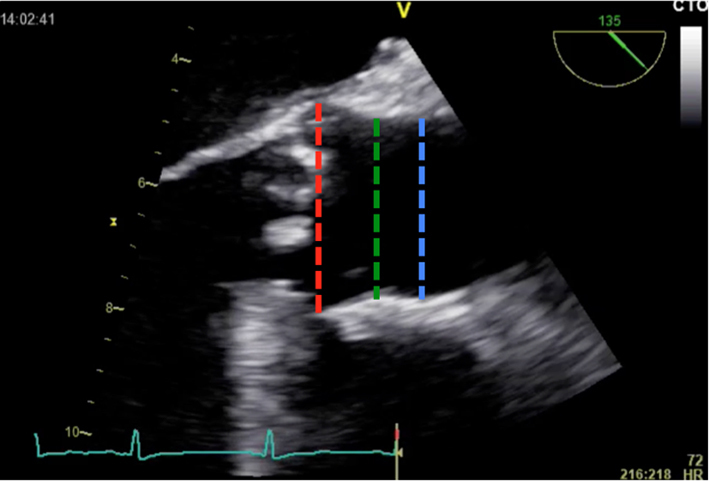 |
| Short-axis view; trans-gastric window; 0°; at the level of the aortic cusps 2D + colour Doppler | Manipulation of probe to ‘open’ aortic valve (marked with ‘AV’) Assessment of turbulence and the presence of AR | 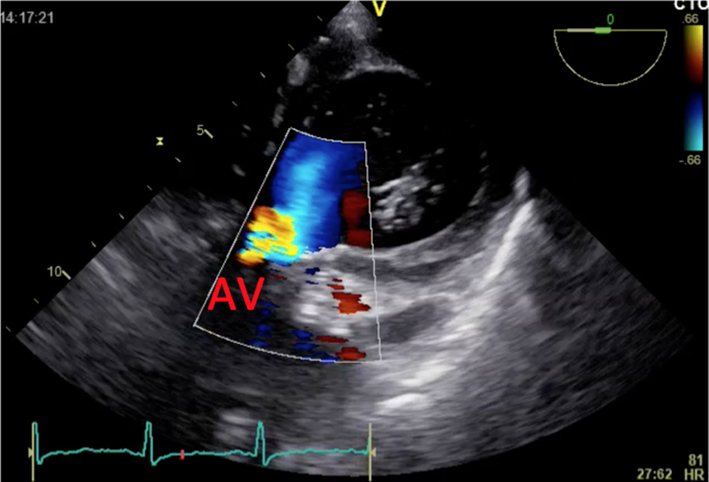 |
|
| Long-axis view; trans-gastric window; 120–140°; 2D imaging + colour Doppler | Assess for AR Colour Doppler may help identify morphology (shown is tricuspid valve; see ‘Anatomy’ section) | 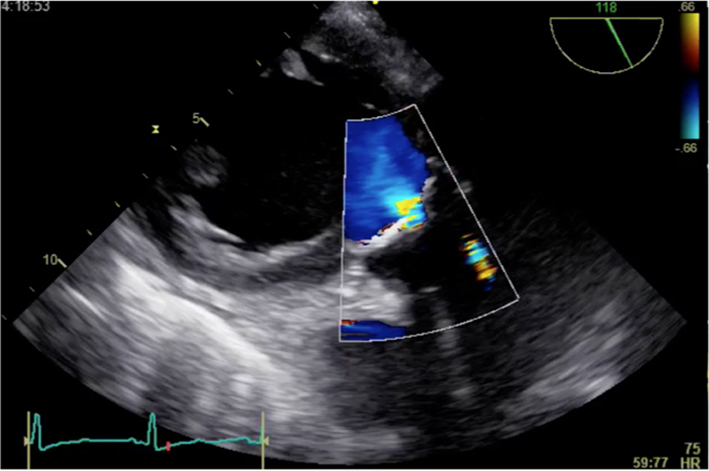 |
|
| Transgastric windows (re-attempt at all angles); CW Doppler | AV Vmax and mean gradient | Trace round modal velocity See ‘Essential parameters in the echocardiographic assessment of AS severity’ section for details | 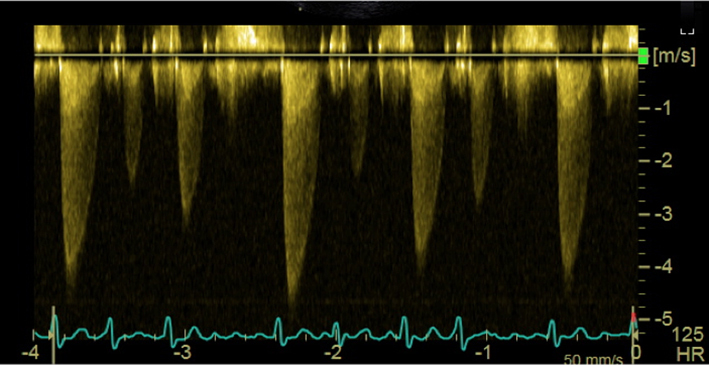 |
| Transgastric windows (re-attempt at all angles); PW Doppler | LVOT VTI for stroke volume and AVA assessment | Trace round modal velocity See ‘Essential parameters in the echocardiographic assessment of AS severity’ section for details | 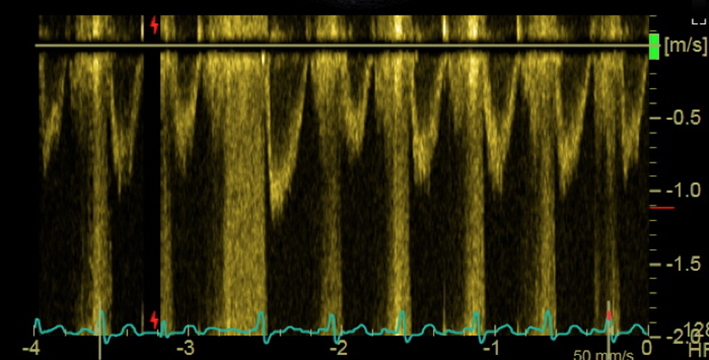 |
TOE is rarely required to assess aortic stenosis, but has use in the assessment of patients for TAVI. 3D TOE can facilitate accurate measurements of the aortic valve annulus and important characteristics such as proximity of the left main stem, combined with an assessment of calcification, and has been shown to provide equivalent information to that obtained by cardiac CT (4, 96, 97, 98, 99). 2D TOE is not adequate for TAVI assessment as it may lead to undersizing of the TAVI implant owing to the elliptical nature of the LVOT and annulus (99, 100).
Exercise stress echocardiography
Key points.
The BSE does not recommend the routine use of exercise stress echocardiography for the assessment of AS.
Exercise testing (without echocardiography) is advocated in international guidance to unmask symptoms and identify patients with severe AS who may benefit from early intervention (5). The value of exercise stress echocardiography is less clear-cut. Increases in the mean AVG and/or pulmonary pressures with exercise have both been examined in the context of severe AS, but there are contradictory reports as to whether they can identify patients at risk (101, 102, 103, 104). Consequently, the use of exercise stress echocardiography in asymptomatic severe AS is no longer recommended in international guidance (5, 6).
Special circumstances
Low-gradient AS
Most of the time, the clinical approach outlined in Fig. 10 and ‘Approach to the patient’ section will allow the sonographer to classify the severity of AS and identify and highlight any high risk features to aid decision-making.
However, in a significant minority of cases, the indices of aortic stenosis do not agree, which can present a challenge to the sonographer. There are several different reasons for this apparent disagreement (or discordance). The first is measurement error, and a priority when faced with such a scenario is to re-check all measurements and indices to ensure that accurate information has been obtained.
The second explanation is that there is not a linear relationship between AV Vmax, mean gradient and AVA (see below).
The final explanation relates to alterations in flow. Patients with reduced transvalvular flow will generate lower than expected Doppler indices for the observed severity of valve stenosis. The usual clinical example of this phenomenon is in the context of impaired LVEF, where poor systolic function means that gradients will be relatively low despite small aortic valve areas. This is low-gradient AS with impaired LVEF. In more recent times, it has been widely accepted that reduced transvalvular flow can occur in patients with relatively normal LVEF (or at least an LVEF ≥50%). This scenario is low-gradient AS with LVEF ≥50%. The echocardiographic approach to patients is very different when the LVEF is impaired compared to when it is ≥50%, and therefore it makes sense to address these patient groups separately.
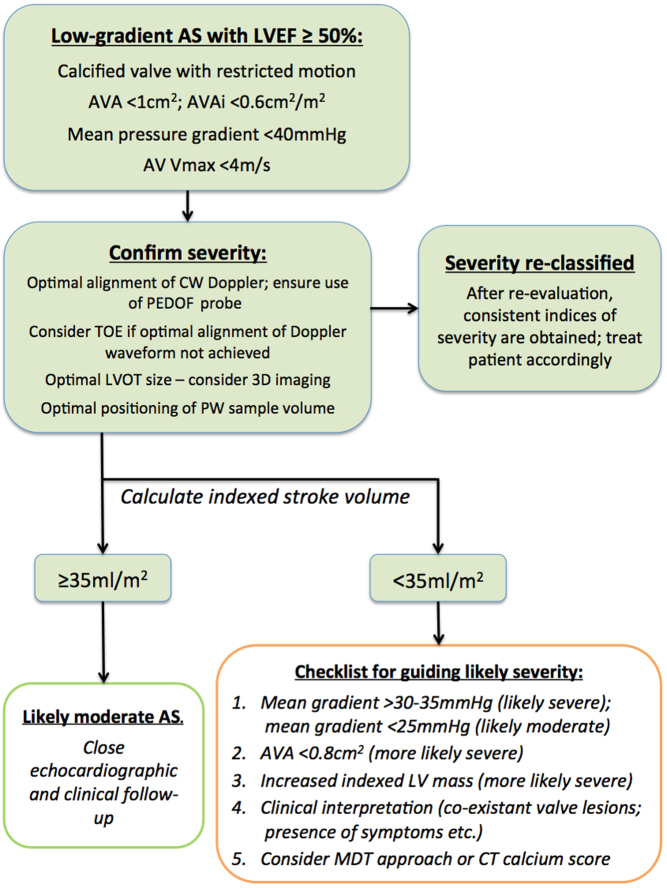
Low-gradient AS with LVEF ≥50%
Key points.
This is defined as an AVA <1 cm2; an AVAi <0.6 cm2/m2; a mean AVG <40 mmHg and an AV Vmax <4 m/s.
The BSE approach is summarized in Fig. 14.
Technical error explains a significant proportion of such findings. Optimal assessment of CW Doppler, LVOT VTI and LVOT cross-sectional area will lead to many patients being re-classified as moderate AS.
Adverse outcomes are related to the presence of higher mean gradients, lower absolute AVA, increased indexed LV mass and low stroke volume.
An MDT approach to resolve challenging cases is advocated.
Relationship between Doppler indices and AVA
There is not a linear relationship between AV Max, mean AVG and aortic valve area. The decision to make an AVA <1 cm2 the cut-off for severe AS was largely arbitrary, and has led to a significant proportion of patients in whom the indices for severe AS apparently do not agree: prospective studies and large database analyses have shown that at least 25% of patients will have an AVA <1 cm2, but a maximal AV velocity (or mean AVG) that do not fulfil the criteria for severe AS. In fact, an AVA of 0.8 cm2 corresponds much better to the Doppler threshold of severe AS (105, 106).
Given this knowledge, many ‘challenging cases’ can be resolved in a relatively straightforward manner. Very often the Vmax or mean gradient will fall in the range of 3.5–4 m/s or 35–40 mmHg, respectively, at which point the AVA will likely be between 0.8 and 1.0 cm2. In this scenario, the best study data supports such individuals as being labelled and treated as moderate AS (5, 30, 38, 41, 42, 107, 108). Pragmatically such patients are often reported as ‘moderate to severe’ AS which is reasonable as they are approaching the point at which intervention may be considered, and this terminology helps to identify the advancing nature of the disease to the clinician.
Where the apparent discrepancy is more overt: for example, if the mean AVG is <35 mmHg or AV Vmax is <3.5 m/s in the context of an AVA <1 cm2, more consideration is required.
Recommended approach
See Fig. 14:
Figure 14.
Summary of recommendations for low-gradient AS with LVEF ≥50%.
The first priority is to ensure that the valve is both heavily calcified and restricted. In the absence of these, significant AS is unlikely.
Re-evaluation of CW Doppler indices is essential. A common error is failing to insonate using the PEDOF or standalone probe from multiple acoustic windows, at the very least from both the apex and the right parasternal window. See ‘Essential parameters in the echocardiographic assessment of AS severity’ section for the optimal methodology for obtaining CW traces.
The CW waveform can sometimes provide clues as to the severity of the AS. A late peak of the CW trace with slow upstroke may indicate that the valve is truly severely stenosed (Fig. 6).
Optimal assessment of the PW trace is essential. Subtle changes in the positioning of the PW sample volume can make a dramatic difference in the assessment of the AVA (see Fig. 15).
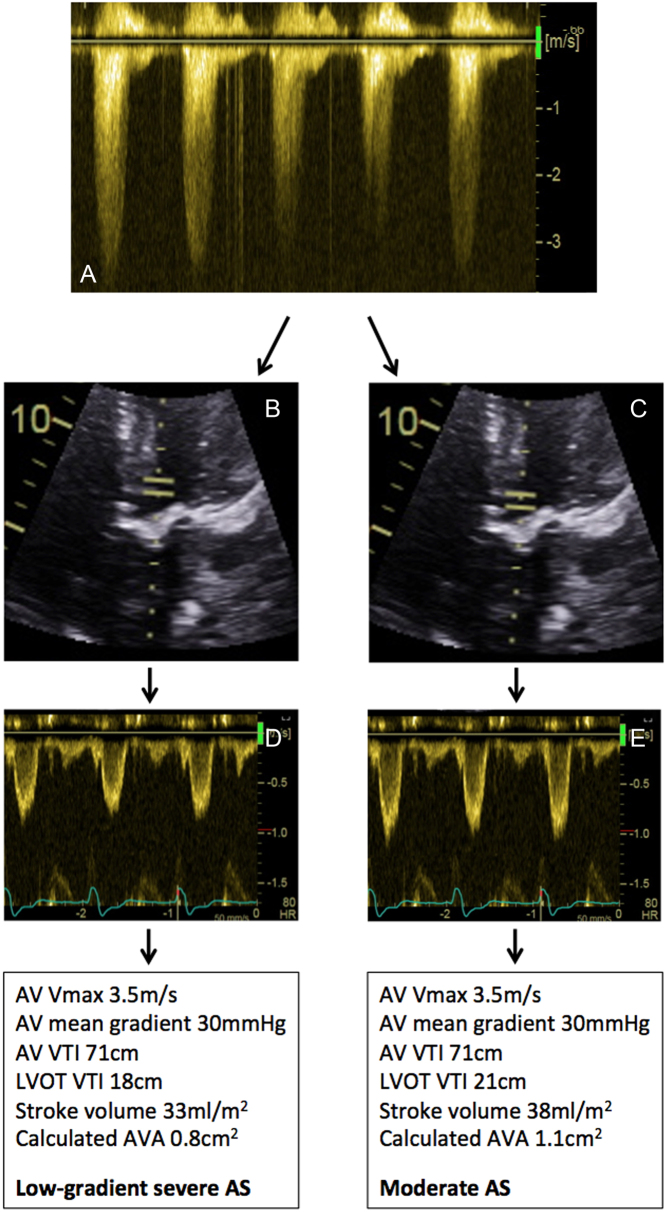
Figure 15.
Example of technical error resulting in low-gradient AS. A patient was noted to have calcified restricted aortic valve. The LVOTd is 2.0 cm (not shown). The LVOT cross-sectional area is 3.14 cm2. PEDOF values from the apical 5-chamber window demonstrate an AV Vmax 3.5 m/s; mean gradient 30 mmHg; AV VTI 71 cm (A). If the PW sample volume is placed as in image (B), corresponding Doppler traces are shown in (D). This leads to an estimated AVA of 0.8 cm2, consistent with low-gradient severe AS. In image (C), the PW sample volume has been moved closer to the valve, with corresponding Doppler trace in (E): note the amplitude of the traces is significantly larger. This leads to an estimated AVA of 1.1 cm2, consistent with moderate AS.
Consideration should be given to 3D planimetry of the LVOT cross-sectional area. The LVOT is often elliptical and the standard methodology of estimating the LVOT cross-sectional area will lead to an underestimation of stroke volume and an overestimation of AS severity using the continuity equation. Obtaining a 3D-image of the LVOT and directly tracing the LVOT cross-sectional area results in a substantial proportion of low-gradient severe AS patients being re-classified as moderate AS (109) (see Fig. 9).
If the above does not resolve the apparent discrepancy, the next stage is to evaluate the stroke volume index (SVi).
An SVi ≥35 mL/m2 is considered ‘normal’, whereas an SVi <35 mL/m2 is considered ‘low’ (110).
Patients with low-gradient AS and normal SVi (≥35 mL/m2) have a prognosis similar to conventional ‘moderate AS’ and can usually be safely observed (108). Prospective studies have shown that even in the presence of minor symptoms, such patients can have aortic valve intervention safely deferred until the mean AVG exceeds 40 mmHg (109).
-
With low SVi (<35 mL/m2), it is considerably more challenging to differentiate patients with truly-severe AS from those with non-severe AS. Additional ‘clues’ need to be obtained from the echocardiographic study in order to inform decision-making as to whether such patients are at lower or higher risk (Fig. 14).
- The presence of increased indexed LV mass makes AS more likely (115).
- An AVA <0.8 cm2 makes severe AS more likely (115).
- Clinical interpretation is important amongst this cohort. The presence of additional valve lesions, the presence of intrusive symptoms, clinical examination and co-existent coronary disease may influence decision-making.
- An MDT approach should be considered for challenging cases.
- Although there are no prospective studies using CT calcium scoring to help identify true-severe AS amongst this cohort, CT may provide complimentary data to support clinical decision-making.
Some studies have supported the value of the valvulo-vascular impedance (Zva). Higher values of Zva are associated with poor cardiovascular outcomes, although when co-morbidities are accounted for, the discriminatory value of Zva is limited and it is not useful in identifying patients who benefit from aortic valve intervention. Consequently, the BSE does not recommend Zva for routine use.
Most patients with low-gradient AS will demonstrate progressive increase in AV Vmax and mean AVG with time and therefore warrant close clinical and echocardiographic follow-up.
Flow rate assessment
There is increasing interest in the use of flow rate (FR) in aortic valve disease. Whilst FR shows promise, the current literature is insufficient to make robust guidance regarding its use in the cohort of low-gradient AS with LVEF ≥50%. Assessment of FR has theoretical benefits over the assessment of static volumes (i.e. the stroke volume index), but a lack of prospective studies means that, at the moment at least, it is not clear how patients should be managed if AS severity is defined according to the FR. It is not entirely clear what threshold of FR should be considered as normal, and given that FR is a non-indexed parameter, smaller individuals will necessarily have lower FR than larger individuals. Therefore the BSE currently does not recommend routine use of FR although acknowledges that it is an area of increasing interest.
Complimentary imaging modalities: cardiac CT
The use of CT in paradoxical low-gradient AS is reasonable, although there are no studies in which it has been used to guide intervention. It is important to appreciate that significant valve obstruction may occur even in the absence of significant calcification, particularly in bicuspid or unicuspid valve disease. Finally, there is a large ‘grey zone’ of calcium scoring in which decision-making is not clear (116).
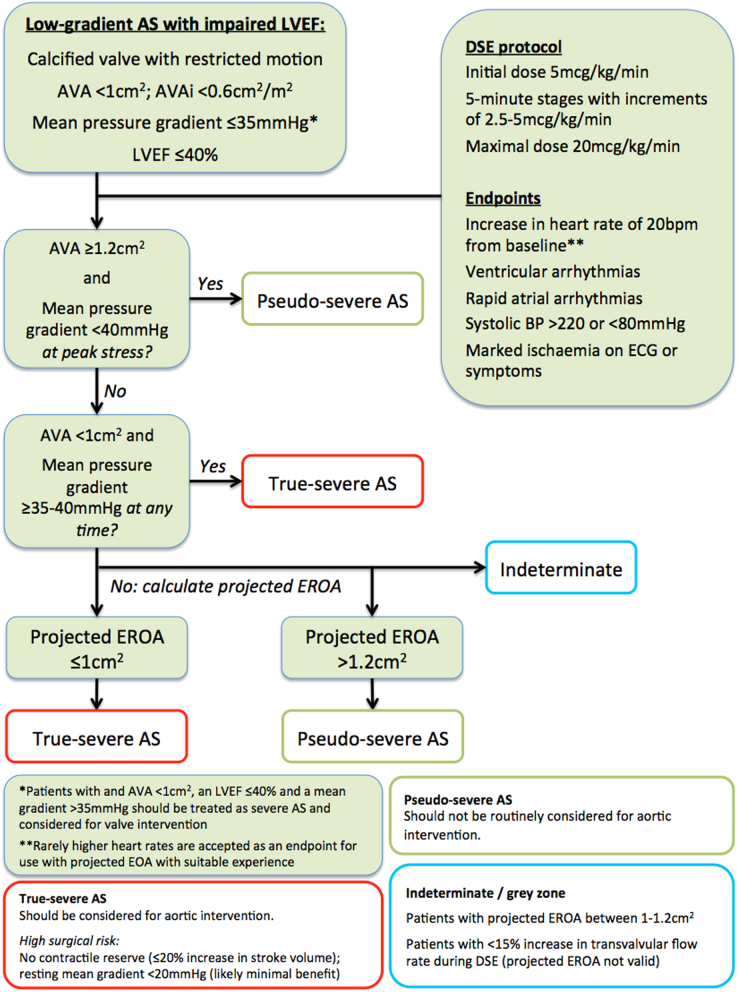
Low-gradient AS with impaired LVEF
Key points.
This is defined as: an AVA <1.0 cm2; an AVAi <0.6 cm2/m2; a mean gradient <35 mmHg; and an LVEF ≤40%.
- The BSE approach is summarized in Fig. 16.
Figure 16.
Summary of recommendations for DSE in low-gradient AS with impaired LVEF. After exclusion of technical error, such patients should be considered for dobutamine stress echocardiography (DSE).
Conventional DSE and projected EOA should be combined in order to identify patients who may benefit from intervention.
CT calcium scoring can be considered if DSE provides an indeterminate result.
An MDT approach to resolve challenging cases is advocated.
Background
In patients with a low cardiac output, recorded values for Vmax and mean gradient are lower than expected for the observed severity of AS, which could lead to individuals with severe AS being missed (27, 28, 34, 35). As the continuity equation is less flow-dependent than measured Vmax and mean gradient, it may be expected that an absolute AVA <1.0 cm2 would reliably identify patients with severe AS (28, 34). Unfortunately, this is not the case. Whilst flow has minimal effect on the calculated valve area of severely stenotic valves, if a valve is only moderately stenotic, it retains a considerable degree of compliance. A moderately stenotic valve will open relatively little in the presence of low stroke volume (and therefore have a small calculated valve area), but as cardiac output increases, the leaflets will open further, resulting in aortic orifice area that can be 30% larger (34, 35, 117). Therefore an AVA <1.0 cm2 is very sensitive but not sufficiently specific in differentiating truly severe aortic stenosis from a valve that is only moderately stenotic but appears severe owing to poor cardiac output (or pseudo-severe).
DSE has been widely used and validated in this scenario to identify patients who may benefit from aortic valve intervention (50, 117, 118, 119, 120, 121). It is important to note exercise echocardiography does not elicit adequate augmentation of cardiac output and therefore should not be used in low-gradient AS with impaired LVEF (122).
Patient selection
DSE should usually be considered in patients with an AVA <1 cm2; an AVAi <0.6 cm2/m2; a mean gradient <35 mmHg and an LVEF ≤40%. Patients with an LVEF ≤40% but in the presence of high gradients (i.e. a mean AVG ≥35 mmHg) should be considered for intervention without recourse to stress echocardiography.
The BSE has diverged from international guidance with regards patient selection for DSE. Both American and European guidance advocate the use of DSE in patients when LVEF is up to 50%, although ESC guidance includes a caveat regarding stroke volume (4, 6). The BSE has chosen not to advocate this approach on the basis that in the published literature essentially all patients undergoing DSE for low-gradient AS were observed to have an LVEF ≤40% (and very often ≤35%).
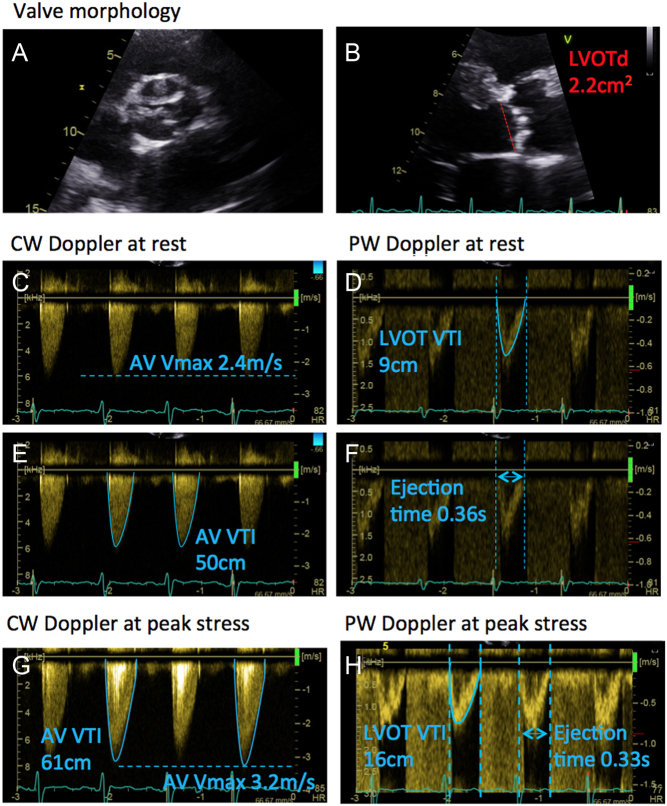
Patients with an LVEF 41–49% but who do not fulfil all the criteria for severe AS therefore represent a challenging subset in which there is almost no data in the literature to provide robust guidance. The first priority is to re-evaluate the study to identify any technical error and confirm that the LVEF is not ≤40% (in which case DSE would usually be considered). Clearly a degree of clinical pragmatism is often employed in these scenarios: if a patient has an LVEF slightly higher that 40%, with an AVA <1 cm2 but low mean gradient, it may be considered reasonable to undertake DSE. Conversely, a patient with an LVEF closer to 50%, with mean gradients of nearly 40 mmHg would usually be considered as having severe AS and DSE would not be warranted. Other clues such as the shape of the CW waveform may help to identify when a patient has severe vs non-severe AS (Fig. 6). There is some limited data in the literature regarding the use of flow rate (FR) in patients with an LVEF between 41 and 49%. FR is obtained by dividing the stroke volume by the ejection time (see Fig. 17 for an example of how to calculate FR). If FR is ≥200 mL/s, the AVA appears to be an accurate assessment of AS severity and therefore such patients may be considered as having severe AS (123).
Figure 17.
Worked example of the projected EOA methodology: A patient with severe LV impairment was noted to have calcified restricted aortic valve (A). The LVOTd is 2.2 cm (B). The LVOT cross-sectional area is 3.8 cm2. Optimal CW and PW recordings were obtained from the 5-chamber window (C and D). AV Vmax 2.4 m/s; mean gradient 16 mmHg; AV VTI 50 cm (E); LVOT VTI 9 cm. AVA at rest calculated using the continuity equation: AVArest = LVOT VTI ÷ AV VTI x LVOT CSA = 9 cm ÷ 50 cm × 3.8 cm2 = 0.68 cm2. Flow at rest (Qrest) is calculated from the stroke volume and the ejection time (F): Qrest = stroke volume ÷ ejection time = 9 cm × 3.8 cm2 ÷ 0.36 s = 95 mL/s. At maximal stress, CW Doppler was obtained (G): AV Vmax 3.2 m/s; mean gradient 28 mmHg; AV VTI 61 cm. PW Doppler at peak stress (H): LVOT VTI 16 cm; ejection time was 0.33 ms. The LVOTd is assumed to remain unchanged with stress. AVApeak = LVOT VTI ÷ AV VTI × LVOT CSA = 16 cm ÷ 61 cm × 3.8 cm2 = 1.0 cm2. Qpeak = stroke volume ÷ ejection time = 16 cm x 3.8 cm2 ÷ 0.33 s = 184 mL/s. Note that the stroke volume has increased by >20% and therefore the patient has contractile reserve. The mean gradient has only increased to 28 mmHg, and therefore this patient does not fulfil the criteria for ‘true-severe AS’. Equally, the patient has not fulfilled the usual criteria for ‘pseudo-severe’ AS, which mandates an AVA ≥1.2 cm2 at peak stress. The projected-EOA calculates the AVA at a ‘normal’ transvalvular flow rate of 250 mL/s: Projected EOA = AVArest + ((AVApeak – AVArest) ÷ (Qpeak – Qrest) × (250 – Qrest)). Projected EOA = 0.68 + ((1.0 – 0.68) ÷ (184 – 95) × (250 – 95)) > 1.2 cm2. An EOA-Proj > 1.2 cm2 is consistent with ‘pseudo-severe AS’. This patient should therefore be treated medically.
Finally, the clinical scenario may influence decision-making: for example if the patient was already being worked up for coronary revascularization, a heavily calcified aortic valve with at least moderate stenosis would usually warrant intervention thereby obviating the need for further detailed assessment of the valve. This re-iterates the central role of the clinical MDT in decision-making for challenging or borderline cases.
Conventional dobutamine stress echocardiography
Contractile reserve
Most individuals demonstrate an improvement of ≥20% in stroke volume with DSE, in which case they are considered to have ‘contractile reserve’. In these patients, repeating the assessment of the aortic valve at peak stress may demonstrate one of the following responses:
True-severe AS (TS-AS): A mean gradient that increases to ≥40 mmHg, combined with an aortic valve area that remains <1 cm2. Such patients have improved survival with AVR, whereas medical therapy results in dismal outcomes (50, 118).
Pseudo-severe AS (PS-AS): The largest series (still only 29 patients) defined PS-AS as patients with an AVA at peak stress of ≥1.2 cm2 combined with a mean gradient <40 mmHg (121). Such individuals appear to have outcomes similar to those of matched controls with LV impairment but without valve disease (121). Despite being somewhat larger than the usual cut-off for severe AS, an AVA 1.2 cm2 as a threshold for defining individuals with good outcomes under medical therapy is consistent with results from the TOPAS series (see below) (117, 119).
No contractile reserve
In around one third of patients, the stroke volume does not increase by ≥20%, that is, there is no evidence of contractile reserve. In this circumstance, conventional DSE does not readily allow the clinician to differentiate TS-AS from PS-AS. Patients without contractile reserve demonstrate high peri-procedural mortality (118, 120, 124), but routine medical therapy in such patients results in even worse survival (120). Recent work from the TOPAS registry (see more below) indicate that individuals without contractile reserve have similar medium-term survival to those patients with contractile reserve after undergoing TAVI (125, 126). Given the increasing use of TAVI, with its associated reduction in peri-procedural risk, there is a clinical need to improve decision making within this cohort in particular.
Very low resting mean gradient
Very low resting mean gradients are strongly associated with high peri-operative mortality and apparent lack of benefit of aortic valve intervention (118, 120). Therefore ‘truly-severe’ AS should be considered very unlikely in patients with resting mean aortic valve gradients of <20 mmHg, and decisions to proceed to intervention made in an MDT environment.
Projected flow area
In addition to the challenge of interpreting echo findings in patients without contractile reserve, a major difficulty with conventional DSE is that the contractile response of individual patients varies widely. For example: one patient may demonstrate a 50% increase in stroke volume, from 40 to 60 mL, whereas a different patient may only display a 20% increase, from 40 to 48 mL. Both would be labelled as having ‘contractile reserve’, but it is obvious that the aortic Doppler indices obtained at peak stress would likely be very different, with only the first patient in the example improving stroke volume to that approaching normal.
Projected flow area (EOA-Proj) is a concept whereby the clinician can use data obtained during DSE to predict what the aortic valve area would be at a ‘normal’ transvalvular flow rate. EOA-Proj was developed to help standardize the interpretation of DSE, and improve decision-making in patients without contractile reserve (117, 119, 125, 126).
Methodology
EOA-Proj relies on the fact that an increase in transvalvular flow rate (and not just absolute stroke volume) will lead to changes in mean gradient and valve area (34). If an individual undergoing DSE does not increase the transvalvular flow rate by ≥15%, the EOA-Proj is considered inaccurate and should not be used (117, 119). See Fig. 17 for a worked example.
Results of EOA-Proj
An EOA-Proj of <1.0 cm2 is best at identifying TS-AS, and outperforms other resting and stress echo criteria (117, 119). Only individuals with an EOA-Proj of >1.2 cm2 performed well without AVR, consistent with the definition of PS-AS (above).
Indeterminate result
If conventional DSE does not elicit a contractile response, and the result of projected flow area is 1–1.2 cm2 and/or the flow-rate fails to augment by ≥15%, the DSE outcome is indeterminate. In this scenario decision-making should be made in an MDT environment and complimentary imaging should be considered (see below).
Complimentary imaging modalities: cardiac CT
There is increasing interest in the use of cardiac CT in defining severity of AS and in particular scenarios where discordant indices are obtained (116, 127, 128). The reported experience of a CT approach in patients with low-gradient AS with impaired LVEF is relatively small (fewer than 100 individuals in total), and aortic valve intervention was not guided by the results of CT findings.
The value of a ‘negative’ CT calcium score within this cohort is not clear. CT cut-offs have been defined in order to identify patients who likely have an AVA <1 cm2: However, the optimal threshold for pseudo-severe AS is 1.2 cm2, on the basis that this higher AVA threshold identifies patients who perform well under medical therapy alone (117, 121). Using the standard CT threshold for severe AS therefore would risk some individuals being refused potentially beneficial intervention. As such, the BSE advises caution in the use of CT calcium scoring in the setting of low-gradient AS with impaired LVEF, and recommends that CT be employed only if DSE provides an indeterminate result.
High gradient high valve area
Key points.
High gradient-high valve area (HGHA) is defined as patients with a mean gradient ≥40 mmHg, and an AVA ≥1 cm2.
HGHA is rarely seen in routine practice.
The HGHA cohort should be carefully assessed for measurement error, particularly regarding the LVOT cross-sectional area.
HGHA patients should be considered as having severe AS.
The clinical scenario where Vmax and mean AVG are high (implying severe stenosis) but AVA >1 cm2, consistent with only moderate AS, is seen rarely, with an estimated prevalence of 1% (105). To the BSE’s knowledge, there has been no systematic echocardiographic analysis of such patients, with current guidance based upon findings from CT studies. All HGHA patients have high CT calcium levels, and accordingly current guidelines suggest that such patients should be treated as severe AS (4, 116, 128).
Combined valve disease
There is a paucity of data in the literature regarding multiple valve disease, despite the fact that the combination of one or more valve lesion occurs relatively frequently (2, 129, 130). The over-riding principle is that there should be a comprehensive assessment of all valvular lesions, evaluated according to specific guidance.
Aortic stenosis and aortic regurgitation
Key points.
When both AS and aortic regurgitation (AR) are at least moderate in severity, the lesion should be reported as ‘mixed aortic valve disease’.
AVA is not a useful marker of prognosis in mixed aortic valve disease.
Grading of mixed aortic valve disease should be guided by the AV Vmax:
- - Moderate mixed aortic valve disease (Vmax 3–3.9 m/s).
- - Severe mixed aortic valve disease (Vmax 4–4.9 m/s).
- - Very severe mixed aortic valve disease (Vmax ≥5 m/s).
Mixed aortic valve disease is a commonly encountered clinical scenario, partly as the dominant causes of aortic stenosis are similarly important aetiological factors in aortic regurgitation (AR) (4).
In the case of mixed aortic valve disease, reporting the severity is challenging. For example: the rationale for describing a valve lesion as ‘moderately severe’ is that it provides the clinician with information as to the likely prognosis. An echocardiographic report stating ‘moderate AS and moderate AR’ may be deceptive, as the prognosis of this combination of valve lesions is more in keeping with isolated severe AS (129).
Some principles are important. Mild AR does not impact upon the assessment of aortic stenosis. However, as the severity of AR increases, the stroke volume will also become larger. AV Vmax and mean gradient are proportional to stroke volume, and therefore in the presence of moderate or worse AR, higher values of AV Vmax and mean gradient will be observed relative to the aortic valve area. Given that the assessment of AVA using the continuity equation corrects for alterations in flow, it may be assumed that AVA is a better tool in the assessment of AS in the context of co-existent AR.
In fact, the prognosis of patients with combined AS and AR is far more closely linked to the maximal AV velocity, which reflects the overall haemodynamic load on the left ventricle (129). In patients with mixed AV disease, event-free survival is the same for those individuals with a calculated AVA of <1 cm2 as it is for patients with an AVA between 1 and 1.5 cm2, whereas there is a step-wise deterioration in outcomes as AV Vmax is seen to increase (129). The average time-to-event with mixed aortic valve disease closely resembles the event curves of lone AS when patients are divided according to AV Vmax (30, 38, 39, 129). The conventional approach of intervening on the valve when the patient fulfils an accepted guideline criteria for surgery (for either AR or AS) appears appropriate and does not result in excess mortality (129).
Aortic stenosis and mitral regurgitation
Key points.
With combined AS and mitral regurgitation (MR) a detailed assessment of both valve lesions is mandatory.
In the presence of severe MR, AV Vmax and mean gradient may underestimate the severity of AS.
AVA is a more accurate assessment of AS severity in the presence of significant MR.
If co-existent MR severity is more than mild, TOE is recommended to clarify the severity and mechanism of mitral regurgitation.
The combination of AS and mitral regurgitation (MR) is seen frequently, particularly in older populations (130). In the case of severe MR, forward stroke volume will be reduced. This may lead to an underestimation of AS severity when relying on the Vmax and mean gradient, whereas AVA is more reflective of true AS severity.
In such patients a comprehensive assessment of both valve lesions should be completed. Timing of intervention is informed by international guidelines (5, 6). In particular a detailed assessment of the mechanism of mitral regurgitation should be obtained as this may provide clues as to whether the MR will improve simply by intervening on the aortic valve, or whether dual valve intervention is needed (130).
Aortic stenosis and mitral stenosis
Key points.
Co-existent severe MS may result in an underestimation of AS severity using AV Vmax and mean gradient.
AVA is a more accurate assessment of AS severity in the presence of significant MS.
Patients with severe mitral stenosis will have low stroke volume, and the presence of severe MS is a well-recognised cause of low-gradient AS. The AVA provides a better assessment of true AS severity in this scenario (131). In patients undergoing surgery for MS it is recommended that detailed assessment of the aortic valve be completed to ensure that low-gradient AS is not present, which may necessitate aortic valve replacement at the time of surgery.
Aortic stenosis and amyloid
Key points.
There is an important association between amyloid and advanced AS in older patients.
Patients with combined amyloid-severe AS still derive benefit from AV intervention.
Echocardiographic features of amyloid including high LV mass and changes in GLS are not specific for amyloid in a population of severe AS.
Recent work has demonstrated an important association between aortic stenosis and cardiac amyloid in an older population (132, 133, 134, 135). Whilst the overall prevalence of cardiac amyloid in individuals over 80 years of age is considered to be around 3%, in prospective studies of patients with severe AS referred for TAVI, the observed prevalence of amyloid was considerably higher, with estimates varying between 13 and 16% of such cases (133, 135).
The value of echocardiography in identifying patients with combined amyloid-severe AS is not clear. GLS is frequently low in such patients but the pattern of ‘apical sparing’ is not specific for amyloid in this cohort and is often seen in individuals with isolated severe AS (133, 135). Consistently, average mitral annular S’ appears to be lower, and LV mass marginally higher in patients with combined amyloid-severe AS. Non-echo findings that may hint towards the existence of amyloid include the presence of RBBB on the ECG and raised biomarkers. Owing to the prevalence of abnormal myocardial systolic and diastolic function in older patients with advancing AS, no echocardiographic or clinical feature can be considered as a reliable method to differentiate a patient with combined amyloid-severe AS from someone with isolated severe AS (133, 135).
An early retrospective analyses suggested that patients with combined amyloid-severe AS had worse survival than equivalent patients with isolated AS (132). However, recent prospective studies in which all participants referred for TAVI underwent diagnostic testing have shown that individuals with combined amyloid-severe AS do not have demonstrably poorer survival and derive as much benefit from aortic valve intervention as those individuals with isolated severe AS (133, 135).
The caveat is that these recent works have only screened patients with severe AS who were referred for consideration of intervention. The prevalence, pattern, and outcomes of patients with non-severe AS combined with amyloid is not well established.
Therefore, the approach advocated throughout this guideline should be followed in all patients. The primary focus should be towards optimization of the echocardiographic assessment and classifying the severity of AS, before identifying any high-risk characteristics. All patients should undergo a comprehensive echocardiographic assessment according to the principles of the minimum dataset (3). Whilst the classical echocardiographic features of amyloid may not be specific for the disease in patients with severe AS, when aortic stenosis is mild or even moderate, the usual echocardiographic approach for identifying infiltrative disease is appropriate.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this guidance.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
References
- 1.Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M.Burden of valvular heart diseases: a population-based study. Lancet 2006. 368 1005–1011. ( 10.1016/S0140-6736(0669208-8) [DOI] [PubMed] [Google Scholar]
- 2.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E.A prospective survey of patients with valvular heart disease in Europe: the euro Heart Survey on valvular Heart Disease. European Heart Journal 2003. 24 1231–1243. ( 10.1016/s0195-668x(0300201-x) [DOI] [PubMed] [Google Scholar]
- 3.Robinson S, Rana B, Oxborough D, Steeds R, Harkness A, Paton M.A practical guideline for performing a comprehensive transthoracic echocardiogram in adults: the British Society of Echocardiography minimum dataset. Echo Research and Practice 2020. 7 G59–G93. ( 10.1530/ERP-20-0026) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumgartner H, Hung J, Bermejo J, Chambers JB, Edvardsen T, Goldstein S, Lancellotti P, LeFevre M, Miller Jr F, Otto CM.Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. European Heart Journal: Cardiovascular Imaging 2016. 18 254–275. [DOI] [PubMed] [Google Scholar]
- 5.Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Muñoz DR.et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. European Heart Journal 2017. 38 2739–2786.28886619 [Google Scholar]
- 6.Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin III JP, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C.ACC/AHA guideline for the management of patients with valvular heart disease. Journal of the American College of Cardiology 2020. 2020 1–173. [DOI] [PubMed] [Google Scholar]
- 7.Piazza N, De Jaegere P, Schultz C, Becker AE, Serruys PW, Anderson RH.Anatomy of the aortic valvar complex and its implications for transcatheter implantation of the aortic valve. Circulation: Cardiovascular Interventions 2008. 1 74–81. ( 10.1161/CIRCINTERVENTIONS.108.780858) [DOI] [PubMed] [Google Scholar]
- 8.Steinberger J, Moller JH, Berry JM, Sinaiko AR.Echocardiographic diagnosis of heart disease in apparently healthy adolescents. Pediatrics 2000. 105 815–818. ( 10.1542/peds.105.4.815) [DOI] [PubMed] [Google Scholar]
- 9.Tutar E, Ekici F, Atalay S, Nacar N.The prevalence of bicuspid aortic valve in newborns by echocardiographic screening. American Heart Journal 2005. 150 513–515. ( 10.1016/j.ahj.2004.10.036) [DOI] [PubMed] [Google Scholar]
- 10.Movahed MR, Hepner AD, Ahmadi-Kashani M.Echocardiographic prevalence of bicuspid aortic valve in the population. Heart, Lung and Circulation 2006. 15 297–299. ( 10.1016/j.hlc.2006.06.001) [DOI] [PubMed] [Google Scholar]
- 11.Roberts WC, Ko JM.Frequency by decades of unicuspid, bicuspid, and tricuspid aortic valves in adults having isolated aortic valve replacement for aortic stenosis, With or Without associated aortic regurgitation. Circulation 2005. 111 920–925. ( 10.1161/01.CIR.0000155623.48408.C5) [DOI] [PubMed] [Google Scholar]
- 12.Michelena HI, Desjardins VA, Avierinos JF, Russo A, Nkomo VT, Sundt TM, Pellikka PA, Tajik AJ, Enriquez-Sarano M.Natural history of asymptomatic patients with normally functioning or minimally dysfunctional bicuspid aortic valve in the community. Circulation 2008. 117 2776–2784. ( 10.1161/CIRCULATIONAHA.107.740878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tzemos N, Therrien J, Yip J, Thanassoulis G, Tremblay S, Jamorski MT, Webb GD, Siu SC.Outcomes in adults with bicuspid aortic valves. JAMA 2008. 300 1317–1325. ( 10.1001/jama.300.11.1317) [DOI] [PubMed] [Google Scholar]
- 14.Schaefer BM, Lewin MB, Stout KK, Gill E, Prueitt A, Byers PH, Otto CM.The bicuspid aortic valve: an integrated phenotypic classification of leaflet morphology and aortic root shape. Heart 2008. 94 1634–1638. ( 10.1136/hrt.2007.132092) [DOI] [PubMed] [Google Scholar]
- 15.Kang JW, Song HG, Yang DH, Baek S, Kim DH, Song JM, Kang DH, Lim TH, Song JK.Association between bicuspid aortic valve phenotype and patterns of valvular dysfunction and bicuspid aortopathy: comprehensive evaluation using MDCT and echocardiography. JACC: Cardiovascular Imaging 2013. 6 150–161. ( 10.1016/j.jcmg.2012.11.007) [DOI] [PubMed] [Google Scholar]
- 16.Sievers HH, Stierle U, Hachmann RMS, Charitos EI.New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. European Journal of Cardio-Thoracic Surgery 2016. 49 439–446. ( 10.1093/ejcts/ezv087) [DOI] [PubMed] [Google Scholar]
- 17.Kim D, Shim CY, Kim YJ, Nam K, Hong GR, Lee SH, Lee S, Ha JW.Differences in flow-gradient patterns between severe bicuspid aortic stenosis and severe tricuspid aortic stenosis – mechanistic insight from multimodal imaging. Circulation Journal 2019. 84 119–126. ( 10.1253/circj.CJ-19-0702) [DOI] [PubMed] [Google Scholar]
- 18.Tsang MYC, Abudiab MM, Ammash NM, Naqvi TZ, Edwards WD, Nkomo VT, Pellikka PA.Quadricuspid aortic valve: characteristics, associated structural cardiovascular abnormalities, and clinical outcomes. Circulation 2016. 133 312–319. ( 10.1161/CIRCULATIONAHA.115.017743) [DOI] [PubMed] [Google Scholar]
- 19.Olson LJ, Subramanian R, Edwards WD.Surgical pathology of pure aortic insufficiency: a study of 225 cases. Mayo Clinic Proceedings 1984. 59 835–841. ( 10.1016/s0025-6196(1265618-3) [DOI] [PubMed] [Google Scholar]
- 20.Novaro GM, Mishra M, Griffin BP.Incidence and echocardiographic features of congenital unicuspid aortic valve in an adult population. Journal of Heart Valve Disease 2003. 12 674–678. [PubMed] [Google Scholar]
- 21.Slostad BD, Witt CM, O’Leary PW, Maleszewski JJ, Scott CG, Dearani JA, Pellikka PA.Diagnostic accuracy of echocardiography and intraoperative surgical inspection of the unicuspid aortic valve. American Journal of Cardiology 2019. 123 967–971. ( 10.1016/j.amjcard.2018.12.010) [DOI] [PubMed] [Google Scholar]
- 22.Subramanian R, Olson LJ, Edwards WD.Surgical pathology of pure aortic stenosis: a study of 374 cases. Mayo Clinic Proceedings 1984. 59 683–690. ( 10.1016/s0025-6196(1262057-6) [DOI] [PubMed] [Google Scholar]
- 23.Stewart BF, Siscovick D, Lind BK, Gardin JM, Gottdiener JS, Smith VE, Kitzman DW, Otto CM.Clinical factors associated with calcific aortic valve disease. Cardiovascular Health Study. Journal of the American College of Cardiology 1997. 29 630–634. ( 10.1016/s0735-1097(9600563-3) [DOI] [PubMed] [Google Scholar]
- 24.Palta S, Pai AM, Gill KS, Pai RG.New insights into the progression of aortic stenosis: implications for secondary prevention. Circulation 2000. 101 2497–2502. ( 10.1161/01.cir.101.21.2497) [DOI] [PubMed] [Google Scholar]
- 25.Skjaerpe T, Hegrenaes L, Hatle L.Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two-dimensional echocardiography. Circulation 1985. 72 810–818. ( 10.1161/01.cir.72.4.810) [DOI] [PubMed] [Google Scholar]
- 26.Otto CM, Pearlman AS, Comess KA, Reamer RP, Janko CL, Huntsman LL.Determination of the stenotic aortic valve area in adults using Doppler echocardiography. Journal of the American College of Cardiology 1986. 7 509–517. ( 10.1016/s0735-1097(8680460-0) [DOI] [PubMed] [Google Scholar]
- 27.Zoghbi WA, Farmer KL, Soto JG, Nelson JG, Quinones MA.Accurate noninvasive quantification of stenotic aortic valve area by Doppler echocardiography. Circulation 1986. 73 452–459. ( 10.1161/01.cir.73.3.452) [DOI] [PubMed] [Google Scholar]
- 28.Oh JK, Taliercio CP, Holmes DR, Reeder GS, Bailey KR, Seward JB, Tajik AJ.Prediction of the severity of aortic stenosis by Doppler aortic valve area determination: prospective Doppler-catheterization correlation in 100 patients. Journal of the American College of Cardiology 1988. 11 1227–1234. ( 10.1016/0735-1097(8890286-0) [DOI] [PubMed] [Google Scholar]
- 29.Gilon D, Cape EG, Handschumacher MD, Song JK, Solheim J, VanAuker M, King MEE, Levine RA.Effect of three-dimensional valve shape on the hemodynamics ofaortic stenosis: three-dimensional echocardiographic stereolithography and patient studies. Journal of the American College of Cardiology 2002. 40 1479–1486. ( 10.1016/s0735-1097(0202269-6) [DOI] [PubMed] [Google Scholar]
- 30.Otto CM, Burwash IG, Legget ME, Munt BI, Fujioka M, Healy NL, Kraft CD, Miyake-Hull CY, Schwaegler RG.Prospective study of asymptomatic valvular aortic stenosis. Clinical, echocardiographic, and exercise predictors of outcome. Circulation 1997. 95 2262–2270. ( 10.1161/01.cir.95.9.2262) [DOI] [PubMed] [Google Scholar]
- 31.Bahlmann E, Gerdts E, Cramariuc D, Gohlke-Baerwolf C, Nienaber CA, Wachtell K, Seifert R, Chambers JB, Kuck KH, Ray S.Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 2013. 127 1149–1156. ( 10.1161/CIRCULATIONAHA.112.078857) [DOI] [PubMed] [Google Scholar]
- 32.Hatle L, Angelsen BA, Tromsdal A.Non-invasive assessment of aortic stenosis by Doppler ultrasound. British Heart Journal 1980. 43 284–292. ( 10.1136/hrt.43.3.284) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Currie PJ, Seward JB, Reeder GS, Vlietstra RE, Bresnahan DR, Bresnahan JF, Smith HC, Hagler DJ, Tajik AJ.Continuous-wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous Doppler-catheter correlative study in 100 adult patients. Circulation 1985. 71 1162–1169. ( 10.1161/01.cir.71.6.1162) [DOI] [PubMed] [Google Scholar]
- 34.Burwash IG, Thomas DD, Sadahiro M, Pearlman AS, Verrier ED, Thomas R, Kraft CD, Otto CM.Dependence of Gorlin formula and continuity equation valve areas on transvalvular volume flow rate in valvular aortic stenosis. Circulation 1994. 89 827–835. ( 10.1161/01.cir.89.2.827) [DOI] [PubMed] [Google Scholar]
- 35.Voelker W, Reul H, Nienhaus G, Stelzer T, Schmitz B, Steegers A, Karsch KR.Comparison of valvular resistance, stroke work loss, and Gorlin valve area for quantification of aortic stenosis. An in vitro study in a pulsatile aortic flow model. Circulation 1995. 91 1196–1204. ( 10.1161/01.cir.91.4.1196) [DOI] [PubMed] [Google Scholar]
- 36.Coffey S, Cox B, Williams MJ.The prevalence, incidence, progression, and risks of aortic valve sclerosis: a systematic review and meta-analysis. Journal of the American College of Cardiology 2014. 63 2852–2861. ( 10.1016/j.jacc.2014.04.018) [DOI] [PubMed] [Google Scholar]
- 37.Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H.Predictors of outcome in severe, asymptomatic aortic stenosis. New England Journal of Medicine 2000. 343 611–617. ( 10.1056/NEJM200008313430903) [DOI] [PubMed] [Google Scholar]
- 38.Rosenhek R, Klaar U, Schemper M, Scholten C, Heger M, Gabriel H, Binder T, Maurer G, Baumgartner H.Mild and moderate aortic stenosis natural history and risk stratification by echocardiography. European Heart Journal 2004. 25 199–205. ( 10.1016/j.ehj.2003.12.002) [DOI] [PubMed] [Google Scholar]
- 39.Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ.Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005. 111 3290–3295. ( 10.1161/CIRCULATIONAHA.104.495903) [DOI] [PubMed] [Google Scholar]
- 40.Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler-Klein J, Grimm M, Gabriel H, Maurer G.Natural history of very severe aortic stenosis. Circulation 2010. 121 151–156. ( 10.1161/CIRCULATIONAHA.109.894170) [DOI] [PubMed] [Google Scholar]
- 41.Pai RG, Kapoor N, Bansal RC, Varadarajan P.Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Annals of Thoracic Surgery 2006. 82 2116–2122. ( 10.1016/j.athoracsur.2006.07.043) [DOI] [PubMed] [Google Scholar]
- 42.Malouf J, Le Tourneau T, Pellikka P, Sundt TM, Scott C, Schaff HV, Enriquez-Sarano M.Aortic valve stenosis in community medical practice: determinants of outcome and implications for aortic valve replacement. Journal of Thoracic and Cardiovascular Surgery 2012. 144 1421–1427. ( 10.1016/j.jtcvs.2011.09.075) [DOI] [PubMed] [Google Scholar]
- 43.Bohbot Y, Rusinaru D, Delpierre Q, Maréchaux S, Tribouilloy C.Risk stratification of severe aortic stenosis with preserved left ventricular ejection fraction using peak aortic jet velocity: an outcome study. Circulation: Cardiovascular Imaging 2017. 10 e006760. ( 10.1161/CIRCIMAGING.117.006760) [DOI] [PubMed] [Google Scholar]
- 44.Kang DH, Park SJ, Rim JH, Yun SC, Kim DH, Song JM, Choo SJ, Park SW, Song JK, Lee JW.et al. Early surgery versus conventional treatment in asymptomatic very severe aortic stenosis. Circulation 2010. 121 1502–1509. ( 10.1161/CIRCULATIONAHA.109.909903) [DOI] [PubMed] [Google Scholar]
- 45.Maréchaux S, Ringle A, Rusinaru D, Debry N, Bohbot Y, Tribouilloy C.Prognostic value of aortic valve area by Doppler echocardiography in patients with severe asymptomatic aortic stenosis. Journal of the American Heart Association 2016. 5 II11. ( 10.1161/JAHA.115.003146) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohbot Y, Kowalski C, Rusinaru D, Ringle A, Maréchaux S, Tribouilloy C.Impact of mean transaortic pressure gradient on long‐term outcome in patients With severe aortic stenosis and preserved left ventricular ejection fraction. Journal of the American Heart Association 2017. 6 672. ( 10.1161/JAHA.117.005850) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kanamori N, Taniguchi T, Morimoto T, Watanabe H, Shiomi H, Ando K, Murata K, Kitai T, Kawase Y, Izumi C.et al. prognostic impact of aortic valve area in conservatively managed patients with asymptomatic severe aortic stenosis with preserved ejection fraction. Journal of the American Heart Association 2019. 8 1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ross J, Braunwald E.Aortic stenosis. Circulation 1968. 38 (Supplement) 61–67. ( 10.1161/01.cir.38.1s5.v-61) [DOI] [PubMed] [Google Scholar]
- 49.Carabello BA, Green LH, Grossman W, Cohn LH, Koster JK, Collins JJ.Hemodynamic determinants of prognosis of aortic valve replacement in critical aortic stenosis and advanced congestive heart failure. Circulation 1980. 62 42–48. ( 10.1161/01.cir.62.1.42) [DOI] [PubMed] [Google Scholar]
- 50.deFilippi CR, Willett DL, Brickner ME, Appleton CP, Yancy CW, Eichhorn EJ, Grayburn PA.Usefulness of dobutamine echocardiography in distinguishing severe from nonsevere valvular aortic stenosis in patients with depressed left ventricular function and low transvalvular gradients. American Journal of Cardiology 1995. 75 191–194. ( 10.1016/s0002-9149(0080078-8) [DOI] [PubMed] [Google Scholar]
- 51.Connolly HM, Oh JK, Orszulak TA, Osborn SL, Roger VL, Hodge DO, Bailey KR, Seward JB, Tajik AJ.Aortic valve replacement for aortic stenosis With severe left ventricular dysfunction. Prognostic indicators. Circulation 1997. 95 2395–2400. ( 10.1161/01.cir.95.10.2395) [DOI] [PubMed] [Google Scholar]
- 52.Morrow AG, Roberts WC, Ross J, Fisher RD, Behrendt DM, Mason DT, Braunwald E.Obstruction to left ventricular outflow. Current concepts of management and operative treatment. Annals of Internal Medicine 1968. 69 1255–1286. ( 10.7326/0003-4819-69-6-1255) [DOI] [PubMed] [Google Scholar]
- 53.Jander N, Gohlke-Bärwolf C, Bahlmann E, Gerdts E, Boman K, Chambers JB, Egstrup K, Nienaber CA, Pedersen TR, Ray S.et al. Indexing aortic valve area by body surface area increases the prevalence of severe aortic stenosis. Heart 2014. 100 28–33. ( 10.1136/heartjnl-2013-304443) [DOI] [PubMed] [Google Scholar]
- 54.Saito T, Muro T, Takeda H, Hyodo E, Ehara S, Nakamura Y, Hanatani A, Shimada K, Yoshiyama M.Prognostic value of aortic valve area index in asymptomatic patients with severe aortic stenosis. American Journal of Cardiology 2012. 110 93–97. ( 10.1016/j.amjcard.2012.02.056) [DOI] [PubMed] [Google Scholar]
- 55.Rusinaru D, Malaquin D, Maréchaux S, Debry N, Tribouilloy C.Relation of dimensionless index to long-term outcome in aortic stenosis with preserved LVEF. JACC: Cardiovascular Imaging 2015. 8 766–775. ( 10.1016/j.jcmg.2015.01.023) [DOI] [PubMed] [Google Scholar]
- 56.Stoddard MF, Arce J, Liddell NE, Peters G, Dillon S, Kupersmith J.Two-dimensional transesophageal echocardiographic determination of aortic valve area in adults with aortic stenosis. American Heart Journal 1991. 122 1415–1422. ( 10.1016/0002-8703(9190585-6) [DOI] [PubMed] [Google Scholar]
- 57.Cormier B, Iung B, Porte JM, Barbant S, Vahanian A.Value of multiplane transesophageal echocardiography in determining aortic valve area in aortic stenosis. American Journal of Cardiology 1996. 77 882–885. ( 10.1016/S0002-9149(9789190-4) [DOI] [PubMed] [Google Scholar]
- 58.Bernard Y, Meneveau N, Vuillemenot A, Magnin D, Anguenot T, Schiele F, Bassand JP.Planimetry of aortic valve area using multiplane transoesophageal echocardiography is not a reliable method for assessing severity of aortic stenosis. Heart 1997. 78 68–73. ( 10.1136/hrt.78.1.68) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia D, Dumesnil JG, Durand LG, Kadem L, Pibarot P.Discrepancies between catheter and Doppler estimates of valve effective orifice area can be. Journal of the American College of Cardiology 2003. 41 435–442. ( 10.1016/s0735-1097(0202764-x) [DOI] [PubMed] [Google Scholar]
- 60.Bahlmann E, Cramariuc D, Gerdts E, Gohlke-Baerwolf C, Nienaber CA, Eriksen E, Wachtell K, Chambers J, Kuck KH, Ray S.Impact of pressure recovery on echocardiographic assessment of asymptomatic aortic stenosis: a SEAS substudy. JACC: Cardiovascular Imaging 2010. 3 555–562. ( 10.1016/j.jcmg.2009.11.019) [DOI] [PubMed] [Google Scholar]
- 61.Yoshida H, Seo Y, Ishizu T, Izumo M, Akashi YJ, Yamashita E, Otsuji Y, Negishi K, Takeuchi M.Prognostic value of energy loss coefficient for predicting asymptomatic aortic stenosis outcomes: direct comparison with aortic valve area. Journal of the American Society of Echocardiography 2019. 32 351.e3–358.e3. ( 10.1016/j.echo.2018.10.016) [DOI] [PubMed] [Google Scholar]
- 62.Dubrey SW, Falk RH.Optimal number of beats for the Doppler measurement of cardiac output in atrial fibrillation. Journal of the American Society of Echocardiography 1997. 10 67–71. ( 10.1016/s0894-7317(9780034-x) [DOI] [PubMed] [Google Scholar]
- 63.Blackshear JL, Kapples EJ, Lane GE, Safford RE.Beat-by-beat aortic valve area measurements indicate constant orifice area in aortic stenosis: analysis of Doppler data with varying RR intervals. Journal of the American Society of Echocardiography 1992. 5 414–420. ( 10.1016/s0894-7317(1480274-5) [DOI] [PubMed] [Google Scholar]
- 64.Esquitin KA, Khalique OK, Liu Q, Kodali SK, Marcoff L, Nazif TM, George I, Vahl TP, Leon MB, Hahn RT.Accuracy of the single cycle length method for calculation of aortic effective orifice area in irregular heart rhythms. Journal of the American Society of Echocardiography 2019. 32 344–350. ( 10.1016/j.echo.2018.11.018) [DOI] [PubMed] [Google Scholar]
- 65.Little SH, Chan KL, Burwash IG.Impact of blood pressure on the Doppler echocardiographic assessment of severity of aortic stenosis. Heart 2007. 93 848–855. ( 10.1136/hrt.2006.098392) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P.Impact of systemic hypertension on the assessment of aortic stenosis. Heart 2005. 91 354–361. ( 10.1136/hrt.2003.030601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mascherbauer J, Fuchs C, Stoiber M, Schima H, Pernicka E, Maurer G, Baumgartner H.Systemic pressure does not directly affect pressure gradient and valve area estimates in aortic stenosis in vitro. European Heart Journal 2008. 29 2049–2057. ( 10.1093/eurheartj/ehn209) [DOI] [PubMed] [Google Scholar]
- 68.Nielsen OW, Sajadieh A, Sabbah M, Greve AM, Olsen MH, Boman K, Nienaber CA, Kesäniemi YA, Pedersen TR, Willenheimer R.et al. Assessing optimal blood pressure in patients with asymptomatic aortic valve stenosis: the Simvastatin Ezetimibe in Aortic Stenosis Study (SEAS). Circulation 2016. 134 455–468. ( 10.1161/CIRCULATIONAHA.115.021213) [DOI] [PubMed] [Google Scholar]
- 69.Saeed S, Scalise F, Chambers JB, Mancia G.Hypertension in aortic stenosis: a focused review and recommendations for clinical practice. Journal of Hypertension 2020. 38 1211–1219. ( 10.1097/HJH.0000000000002426) [DOI] [PubMed] [Google Scholar]
- 70.Saeed S, Mancia G, Rajani R, Parkin D, Chambers JB.Antihypertensive treatment with calcium channel blockers in patients with moderate or severe aortic stenosis: relationship with all-cause mortality. International Journal of Cardiology 2020. 298 122–125. ( 10.1016/j.ijcard.2019.09.007) [DOI] [PubMed] [Google Scholar]
- 71.Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA.Flow-gradient patterns in severe aortic stenosis With preserved ejection fraction: clinical characteristics and predictors of survival. Circulation 2013. 128 1781–1789. ( 10.1161/CIRCULATIONAHA.113.003695) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whelton PK, Carey RM, Aronow WS, Casey DE, Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW.et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines. Circulation 2018. 138 248. [DOI] [PubMed] [Google Scholar]
- 73.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A.et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. European Heart Journal 2018. 39 3021–3104. [DOI] [PubMed] [Google Scholar]
- 74.Henkel DM, Malouf JF, Connolly HM, Michelena HI, Sarano ME, Schaff HV, Scott CG, Pellikka PA.Asymptomatic left ventricular systolic dysfunction in patients with severe aortic stenosis: characteristics and outcomes. Journal of the American College of Cardiology 2012. 60 2325–2329. ( 10.1016/j.jacc.2012.08.988) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dahl JS, Eleid MF, Michelena HI, Scott CG, Suri RM, Schaff HV, Pellikka PA.Effect of left ventricular ejection fraction on postoperative outcome in patients with severe aortic stenosis undergoing aortic valve replacement. Circulation: Cardiovascular Imaging 2015. 8 e002917. ( 10.1161/CIRCIMAGING.114.002917) [DOI] [PubMed] [Google Scholar]
- 76.Gunther S, Grossman W.Determinants of ventricular function in pressure-overload hypertrophy in man. Circulation 1979. 59 679–688. ( 10.1161/01.cir.59.4.679) [DOI] [PubMed] [Google Scholar]
- 77.Bohbot Y, de Meester de Ravenstein C, Chadha G, Rusinaru D, Belkhir K, Trouillet C, Pasquet A, Maréchaux S, Vanoverschelde JL, Tribouilloy C.Relationship between left ventricular ejection fraction and mortality in asymptomatic and minimally symptomatic patients With severe aortic stenosis. JACC: Cardiovascular Imaging 2019. 12 38–48. ( 10.1016/j.jcmg.2018.07.029) [DOI] [PubMed] [Google Scholar]
- 78.Minamino-Muta E, Kato T, Morimoto T, Taniguchi T, Izumi C, Nakatsuma K, Inoko M, Shirai S, Kanamori N, Murata K.et al. Decline in left ventricular ejection fraction during follow-up in patients with severe aortic stenosis. JACC: Cardiovascular Interventions 2019. 12 2499–2511. ( 10.1016/j.jcin.2019.09.015) [DOI] [PubMed] [Google Scholar]
- 79.Grossman W, Jones D, McLaurin LP.Wall stress and patterns of hypertrophy in the human left ventricle. Journal of Clinical Investigation 1975. 56 56–64. ( 10.1172/JCI108079) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aurigemma GP, Silver KH, McLaughlin M, Mauser J, Gaasch WH.Impact of chamber geometry and gender on left ventricular systolic function in patients >60 years of age with aortic stenosis. American Journal of Cardiology 1994. 74 794–798. ( 10.1016/0002-9149(9490437-5) [DOI] [PubMed] [Google Scholar]
- 81.Cioffi G, Faggiano P, Vizzardi E, Tarantini L, Cramariuc D, Gerdts E, de Simone G.Prognostic effect of inappropriately high left ventricular mass in asymptomatic severe aortic stenosis. Heart 2011. 97 301–307. ( 10.1136/hrt.2010.192997) [DOI] [PubMed] [Google Scholar]
- 82.Gerdts E, Rossebø AB, Pedersen TR, Cioffi G, Lønnebakken MT, Cramariuc D, Rogge BP, Devereux RB.Relation of left ventricular mass to prognosis in initially asymptomatic mild to moderate aortic valve stenosis. Circulation: Cardiovascular Imaging 2015. 8 e003644; discussion e003644. ( 10.1161/CIRCIMAGING.115.003644) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ng ACT, Delgado V, Bertini M, Antoni ML, van Bommel RJ, van Rijnsoever EPM, van der Kley F, Ewe SH, Witkowski T, Auger D.et al. Alterations in multidirectional myocardial functions in patients with aortic stenosis and preserved ejection fraction: a two-dimensional speckle tracking analysis. European Heart Journal 2011. 32 1542–1550. ( 10.1093/eurheartj/ehr084) [DOI] [PubMed] [Google Scholar]
- 84.Slimani A, Melchior J, de Meester C, Piérard S, Roy C, Amzulescu M, Bouzin C, Maes F, Pasquet A, Pouleur A-C.et al. Relative contribution of afterload and interstitial fibrosis to myocardial function in severe aortic stenosis. Journal of the American College of Cardiology: Imaging 2020. 13 589–600. [DOI] [PubMed] [Google Scholar]
- 85.Kearney LG, Lu K, Ord M, Patel SK, Profitis K, Matalanis G, Burrell LM, Srivastava PM.Global longitudinal strain is a strong independent predictor of all-cause mortality in patients with aortic stenosis. European Heart Journal: Cardiovascular Imaging 2012. 13 827–833. ( 10.1093/ehjci/jes115) [DOI] [PubMed] [Google Scholar]
- 86.Fries B, Liu D, Gaudron P, Hu K, Nordbeck P, Ertl G, Weidemann F, Herrmann S.Role of global longitudinal strain in the prediction of outcome in patients with severe aortic valve stenosis. American Journal of Cardiology 2017. 120 640–647. ( 10.1016/j.amjcard.2017.05.032) [DOI] [PubMed] [Google Scholar]
- 87.Zhu D, Ito S, Miranda WR, Nkomo VT, Pislaru SV, Villarraga HR, Pellikka PA, Crusan DJ, Oh JK.Left ventricular global longitudinal strain is associated with long-term outcomes in moderate aortic stenosis. Circulation: Cardiovascular Imaging 2020. 13 e009958. ( 10.1161/CIRCIMAGING.119.009958) [DOI] [PubMed] [Google Scholar]
- 88.Magne J, Cosyns B, Popescu BA, Carstensen HG, Dahl J, Desai MY, Kearney L, Lancellotti P, Marwick TH, Sato K.et al. Distribution and prognostic significance of left ventricular global longitudinal strain in asymptomatic significant aortic stenosis: an individual participant data meta-analysis. JACC: Cardiovascular Imaging 2019. 12 84–92. ( 10.1016/j.jcmg.2018.11.005) [DOI] [PubMed] [Google Scholar]
- 89.Pai RG, Varadarajan P, Kapoor N, Bansal RC.Aortic valve replacement improves survival in severe aortic stenosis associated with severe pulmonary hypertension. Annals of Thoracic Surgery 2007. 84 80–85. ( 10.1016/j.athoracsur.2007.02.094) [DOI] [PubMed] [Google Scholar]
- 90.Zuern CS, Eick C, Rizas K, Stoleriu C, Woernle B, Wildhirt S, Herdeg C, Stock U, Gawaz M, Bauer A.Prognostic value of mild-to-moderate pulmonary hypertension in patients with severe aortic valve stenosis undergoing aortic valve replacement. Clinical Research in Cardiology 2012. 101 81–88. ( 10.1007/s00392-011-0367-3) [DOI] [PubMed] [Google Scholar]
- 91.Mutlak D, Aronson D, Carasso S, Lessick J, Reisner SA, Agmon Y.Frequency, determinants and outcome of pulmonary hypertension in patients with aortic valve stenosis. American Journal of the Medical Sciences 2012. 343 397–401. ( 10.1097/MAJ.0b013e3182309431) [DOI] [PubMed] [Google Scholar]
- 92.Roselli EE, Azim AA, Houghtaling PL, Jaber WA, Blackstone EH.Pulmonary hypertension is associated with worse early and late outcomes after aortic valve replacement: implications for transcatheter aortic valve replacement. Journal of Thoracic and Cardiovascular Surgery 2012. 144 1067, .e2–1074.e2. ( 10.1016/j.jtcvs.2012.08.029) [DOI] [PubMed] [Google Scholar]
- 93.Miceli A, Varone E, Gilmanov D, Murzi M, Simeoni S, Concistrè G, Marchi F, Solinas M, Glauber M.Impact of pulmonary hypertension on mortality after operation for isolated aortic valve stenosis. International Journal of Cardiology 2013. 168 3556–3559. ( 10.1016/j.ijcard.2013.05.001) [DOI] [PubMed] [Google Scholar]
- 94.Levy F, Bohbot Y, Sanhadji K, Rusinaru D, Ringle A, Delpierre Q, Smaali S, Gun M, Maréchaux S, Tribouilloy C.Impact of pulmonary hypertension on long-term outcome in patients with severe aortic stenosis. European Heart Journal Cardiovascular Imaging 2018. 19 553–561. ( 10.1093/ehjci/jex166) [DOI] [PubMed] [Google Scholar]
- 95.Augustine DX, Coates-Bradshaw LD, Willis J, Harkness A, Ring L, Grapsa J, Coghlan G, Kaye N, Oxborough D, Robinson S.et al. Echocardiographic assessment of pulmonary hypertension: a guideline protocol from the British Society of Echocardiography. Echo Research and Practice 2018. 5 G11–G24. ( 10.1530/ERP-17-0071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buzzatti N, Maisano F, Latib A, Cioni M, Taramasso M, Mussardo M, Colombo A, Alfieri O.Computed tomography-based evaluation of aortic annulus, prosthesis size and impact on early residual aortic regurgitation after transcatheter aortic valve implantation. European Journal of Cardio-Thoracic Surgery 2013. 43 43–50; discussion 50. ( 10.1093/ejcts/ezs155) [DOI] [PubMed] [Google Scholar]
- 97.Khalique OK, Kodali SK, Paradis J-M, Nazif TM, Williams MR, Einstein AJ, Pearson GD, Harjai K, Grubb K, George I.et al. Aortic annular sizing using a Novel 3-dimensional echocardiographic method. Circulation 2014. 7 155–163. ( 10.1161/CIRCIMAGING.113.001153) [DOI] [PubMed] [Google Scholar]
- 98.Stella S, Italia L, Geremia G, Rosa I, Ancona F, Marini C, Capogrosso C, Giglio M, Montorfano M, Latib A.et al. Accuracy and reproducibility of aortic annular measurements obtained from echocardiographic 3D manual and semi-automated software analyses in patients referred for transcatheter aortic valve implantation: implication for prosthesis size selection. European Heart Journal Cardiovascular Imaging 2019. 20 45–55. ( 10.1093/ehjci/jey013) [DOI] [PubMed] [Google Scholar]
- 99.Bleakley C, Eskandari M, Monaghan M.3D transoesophageal echocardiography in the TAVI sizing arena: should we do it and how do we do it? Echo Research and Practice 2017. 4 R21–R32. ( 10.1530/ERP-16-0041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mehrotra P, Flynn AW, Jansen K, Tan TC, Mak G, Julien HM, Zeng X, Picard MH, Passeri JJ, Hung J.Differential left ventricular outflow tract remodeling and dynamics in aortic stenosis. Journal of the American Society of Echocardiography 2015. 28 1259–1266. ( 10.1016/j.echo.2015.07.018) [DOI] [PubMed] [Google Scholar]
- 101.Marechaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV.et al. Usefulness of exercise-stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. European Heart Journal 2010. 31 1390–1397. ( 10.1093/eurheartj/ehq076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lancellotti P, Magne J, Donal E, O’Connor K, Dulgheru R, Rosca M, Piérard LA.Determinants and prognostic significance of exercise pulmonary hypertension in asymptomatic severe aortic stenosis. Circulation 2012. 126 851–859. ( 10.1161/CIRCULATIONAHA.111.088427) [DOI] [PubMed] [Google Scholar]
- 103.Masri A, Goodman AL, Barr T, Grimm RA, Sabik JF, Gillinov AM, Rodriguez LL, Svensson LG, Griffin BP, Desai MY.Predictors of long-term outcomes in asymptomatic patients with severe aortic stenosis and preserved left ventricular systolic function undergoing exercise echocardiography. Circulation: Cardiovascular Imaging 2016. 9 e004689. ( 10.1161/CIRCIMAGING.116.004689) [DOI] [PubMed] [Google Scholar]
- 104.Goublaire C, Melissopoulou M, Lobo D, Kubota N, Verdonk C, Cimadevilla C, Codogno I, Brochet E, Vahanian A, Messika-Zeitoun D.Prognostic value of exercise-stress echocardiography in asymptomatic patients with aortic valve stenosis. JACC: Cardiovascular Imaging 2018. 11 787–795. ( 10.1016/j.jcmg.2017.03.020) [DOI] [PubMed] [Google Scholar]
- 105.Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N.Inconsistencies of echocardiographic criteria for the grading of aortic valve stenosis. European Heart Journal 2008. 29 1043–1048. ( 10.1093/eurheartj/ehm543) [DOI] [PubMed] [Google Scholar]
- 106.Namasivayam M, He W, Churchill TW, Capoulade R, Liu S, Lee H, Danik JS, Picard MH, Pibarot P, Levine RA.et al. Transvalvular flow rate determines prognostic value of aortic valve area in aortic stenosis. Journal of the American College of Cardiology 2020. 75 1758–1769. ( 10.1016/j.jacc.2020.02.046) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kang DH, Jang JY, Park SJ, Kim DH, Yun SC, Song JM, Park SW, Chung CH, Song JK, Lee JW.Watchful observation versus early aortic valve replacement for symptomatic patients with normal flow, low-gradient severe aortic stenosis. Heart 2015. 101 1375–1381. ( 10.1136/heartjnl-2015-307528) [DOI] [PubMed] [Google Scholar]
- 108.Chadha G, Bohbot Y, Rusinaru D, Maréchaux S, Tribouilloy C.Outcome of normal‐flow low‐gradient severe aortic stenosis with preserved left ventricular ejection fraction: a propensity‐matched study. Journal of the American Heart Association 2019. 8 e012301. ( 10.1161/JAHA.119.012301) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Maes F, Piérard S, de Meester C, Boulif J, Amzulescu M, Vancraeynest D, Pouleur AC, Pasquet A, Gerber B, Vanoverschelde JL.Impact of left ventricular outflow tract ellipticity on the grading of aortic stenosis in patients with normal ejection fraction. Journal of Cardiovascular Magnetic Resonance 2017. 19 37. ( 10.1186/s12968-017-0344-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hachicha Z, Dumesnil JG, Bogaty P, Pibarot P.Paradoxical low-flow, low-gradient severe aortic stenosis despite preserved ejection fraction is associated with higher afterload and reduced survival. Circulation 2007. 115 2856–2864. ( 10.1161/CIRCULATIONAHA.106.668681) [DOI] [PubMed] [Google Scholar]
- 111.Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesäniemi YA, Malbecq W.et al. Outcome of patients with low-gradient ‘severe’ aortic stenosis and preserved ejection fraction. Circulation 2011. 123 887–895. ( 10.1161/CIRCULATIONAHA.110.983510) [DOI] [PubMed] [Google Scholar]
- 112.Tribouilloy C, Rusinaru D, Maréchaux S, Castel AL, Debry N, Maizel J, Mentaverri R, Kamel S, Slama M, Levy F.Low-gradient, low-flow severe aortic stenosis with preserved left ventricular ejection fraction: characteristics, outcome, and implications for surgery. Journal of the American College of Cardiology 2015. 65 55–66. ( 10.1016/j.jacc.2014.09.080) [DOI] [PubMed] [Google Scholar]
- 113.Berthelot-Richer M, Pibarot P, Capoulade R, Dumesnil JG, Dahou A, Thebault C, Le Ven F, Clavel MA.Discordant grading of aortic stenosis severity: echocardiographic predictors of survival benefit associated with aortic valve replacement. JACC: Cardiovascular Imaging 2016. 9 797–805. ( 10.1016/j.jcmg.2015.09.026) [DOI] [PubMed] [Google Scholar]
- 114.Lancellotti P, Magne J, Donal E, Davin L, O’Connor K, Rosca M, Szymanski C, Cosyns B, Piérard LA.Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. Journal of the American College of Cardiology 2012. 59 235–243. ( 10.1016/j.jacc.2011.08.072) [DOI] [PubMed] [Google Scholar]
- 115.Ozkan A, Hachamovitch R, Kapadia SR, Tuzcu EM, Marwick TH.Impact of aortic valve replacement on outcome of symptomatic patients with severe aortic stenosis with low gradient and preserved left ventricular ejection fraction. Circulation 2013. 128 622–631. ( 10.1161/CIRCULATIONAHA.112.001094) [DOI] [PubMed] [Google Scholar]
- 116.Pawade T, Clavel MA, Tribouilloy C, Dreyfus J, Mathieu T, Tastet L, Renard C, Gun M, Jenkins WSA, Macron L.et al. Computed tomography aortic valve calcium scoring in patients with aortic stenosis. Circulation: Cardiovascular Imaging 2018. 11 e007146. ( 10.1161/CIRCIMAGING.117.007146) [DOI] [PubMed] [Google Scholar]
- 117.Blais C, Burwash IG, Mundigler G, Dumesnil JG, Loho N, Rader F, Baumgartner H, Beanlands RS, Chayer B, Kadem L.et al. Projected valve area at normal flow rate improves the assessment of stenosis severity in patients with low-flow, low-gradient aortic stenosis: the multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) study. Circulation 2006. 113 711–721. ( 10.1161/CIRCULATIONAHA.105.557678) [DOI] [PubMed] [Google Scholar]
- 118.Monin JL, Quéré JP, Monchi M, Petit H, Baleynaud S, Chauvel C, Pop C, Ohlmann P, Lelguen C, Dehant P.et al. Low-gradient aortic stenosis: operative risk stratification and predictors for long-term outcome: a multicenter study using dobutamine stress hemodynamics. Circulation 2003. 108 319–324. ( 10.1161/01.CIR.0000079171.43055.46) [DOI] [PubMed] [Google Scholar]
- 119.Clavel MA, Fuchs C, Burwash IG, Mundigler G, Dumesnil JG, Baumgartner H, Bergler-Klein J, Beanlands RS, Mathieu P, Magne J.et al. Predictors of outcomes in low-flow, low-gradient aortic stenosis: results of the multicenter TOPAS study. Circulation 2008. 118 (Supplement) S234–S242. ( 10.1161/CIRCULATIONAHA.107.757427) [DOI] [PubMed] [Google Scholar]
- 120.Tribouilloy C, Lévy F, Rusinaru D, Guéret P, Petit-Eisenmann H, Baleynaud S, Jobic Y, Adams C, Lelong B, Pasquet A.et al. Outcome after aortic valvereplacement for low-flow/low-gradient aortic stenosis without contractile reserve on dobutamine stress echocardiography. Journal of the American College of Cardiology 2009. 53 1865–1873. ( 10.1016/j.jacc.2009.02.026) [DOI] [PubMed] [Google Scholar]
- 121.Fougeres E, Tribouilloy C, Monchi M, Petit-Eisenmann H, Baleynaud S, Pasquet A, Chauvel C, Metz D, Adams C, Rusinaru D.et al. Outcomes of pseudo-severe aortic stenosis under conservative treatment. European Heart Journal 2012. 33 2426–2433. ( 10.1093/eurheartj/ehs176) [DOI] [PubMed] [Google Scholar]
- 122.Ennezat PV, Toussaint J, Maréchaux S, Le Tourneau T, Aubert JM, Asseman P, LeJemtel TH, Deklunder G.Low-level exercise echocardiography fails to elicit left ventricular contractile reserve in patients with aortic stenosis, reduced ejection fraction, and low transvalvular gradient. Echocardiography 2007. 24 47–51. ( 10.1111/j.1540-8175.2007.00349.x) [DOI] [PubMed] [Google Scholar]
- 123.Chahal NS, Drakopoulou M, Gonzalez-Gonzalez AM, Manivarmane R, Khattar R, Senior R.Resting aortic valve area at normal transaortic flow rate reflects true valve area in suspected low-gradient severe aortic stenosis. JACC: Cardiovascular Imaging 2015. 8 1133–1139. ( 10.1016/j.jcmg.2015.04.021) [DOI] [PubMed] [Google Scholar]
- 124.Hayek S, Pibarot P, Harzand A, Cheng JW, Gay H, Chrysohoou C, Ribeiro H, Rodés-Cabau J, Babaliaros V, Lerakis S.Dobutamine stress echocardiography for risk stratification of patients with low-gradient severe aortic stenosis undergoing TAVR. JACC: Cardiovascular Imaging 2015. 8 380–382. ( 10.1016/j.jcmg.2014.09.012) [DOI] [PubMed] [Google Scholar]
- 125.Ribeiro HB, Lerakis S, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN, Nombela-Franco L.et al. Transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis: the TOPAS-TAVI Registry. Journal of the American College of Cardiology 2018. 71 1297–1308. ( 10.1016/j.jacc.2018.01.054) [DOI] [PubMed] [Google Scholar]
- 126.Maes F, Lerakis S, Barbosa Ribeiro H, Gilard M, Cavalcante JL, Makkar R, Herrmann HC, Windecker S, Enriquez-Sarano M, Cheema AN.et al. Outcomes from transcatheter aortic valve replacement in patients with low-flow, low-gradient aortic stenosis and left ventricular ejection fraction less than 30%: a substudy from the TOPAS-TAVI Registry. JAMA Cardiology 2019. 4 64–70. ( 10.1001/jamacardio.2018.4320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cueff C, Serfaty JM, Cimadevilla C, Laissy JP, Himbert D, Tubach F, Duval X, Iung B, Enriquez-Sarano M, Vahanian A.et al. Measurement of aortic valve calcification using multislice computed tomography: correlation with haemodynamic severity of aortic stenosis and clinical implication for patients with low ejection fraction. Heart 2011. 97 721–726. ( 10.1136/hrt.2010.198853) [DOI] [PubMed] [Google Scholar]
- 128.Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R.et al. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. Journal of the American College of Cardiology 2013. 62 2329–2338. ( 10.1016/j.jacc.2013.08.1621) [DOI] [PubMed] [Google Scholar]
- 129.Zilberszac R, Gabriel H, Schemper M, Zahler D, Czerny M, Maurer G, Rosenhek R.Outcome of combined Stenoticand regurgitant aortic valve disease. Journal of the American College of Cardiology 2013. 61 1489–1495. ( 10.1016/j.jacc.2012.11.070) [DOI] [PubMed] [Google Scholar]
- 130.Zilberszac R, Gleiss A, Binder T, Laufer G, Grimm M, Gabriel H, Maurer G, Rosenhek R.Prognostic relevance of mitral and tricuspid regurgitation in patients with severe aortic stenosis. European Heart Journal Cardiovascular Imaging 2018. 19 985–992. ( 10.1093/ehjci/jey027) [DOI] [PubMed] [Google Scholar]
- 131.Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M.et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Journal of the American Society of Echocardiography 2009. 22 1–23; quiz 101. ( 10.1016/j.echo.2008.11.029) [DOI] [PubMed] [Google Scholar]
- 132.Cavalcante JL, Rijal S, Abdelkarim I, Althouse AD, Sharbaugh MS, Fridman Y, Soman P, Forman DE, Schindler JT, Gleason TG.et al. Cardiac amyloidosis is prevalent in older patients with aortic stenosis and carries worse prognosis. Journal of Cardiovascular Magnetic Resonance 2017. 19 98. ( 10.1186/s12968-017-0415-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Castaño A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T.et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. European Heart Journal 2017. 38 2879–2887. ( 10.1093/eurheartj/ehx350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Scully PR, Treibel TA, Fontana M, Lloyd G, Mullen M, Pugliese F, Hartman N, Hawkins PN, Menezes LJ, Moon JC.Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. Journal of the American College of Cardiology 2018. 71 463–464. ( 10.1016/j.jacc.2017.11.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Scully PR, Patel KP, Treibel TA, Thornton GD, Hughes RK, Chadalavada S, Katsoulis M, Hartman N, Fontana M, Pugliese F.et al. Prevalence and outcome of dual aortic stenosis and cardiac amyloid pathology in patients referred for transcatheter aortic valve implantation. European Heart Journal 2020. 41 2759–2767. ( 10.1093/eurheartj/ehaa170) [DOI] [PMC free article] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a