On January 11, the vaccination center in Lausanne—a city of 140 000 inhabitants in western Switzerland—started the coronavirus disease 2019 (COVID-19) vaccination with mRNA-based vaccines. As of February 9, 13 296 vaccine doses were administered with 12 349 patients receiving the first dose (10501 Pfizer/BioNTech, 1848 Moderna) and 947 receiving a second dose (945 Pfizer/BioNTech, 2 Moderna). The center offers vital signs monitoring for any patient reporting symptoms compatible with a serious adverse event such as suspicion of anaphylactic reaction, malaise, shortness of breath, or pain (eg, headache and chest pain). Any adverse event is medically monitored and those which are serious or unexpected are reported to Swissmedic—the Swiss Agency for Therapeutic Products.
We report a case series of 9 patients with stage III hypertension documented within minutes of vaccination during the first 30 days, of which 8 were symptomatic. Inclusion criteria for monitoring are detailed in Table. Vital signs were measured with an oscillometric manometer (Omron Healthcare Europe; a HEM 907-E7) with at least 3 sets of separate values at 5-minute intervals. Median age was 73 (IQR, 22) years and sex distribution was 7 women for 2 men. Eight of 9 patients had a history of arterial hypertension with most patients on antihypertensive therapy. All but one patient received the Pfizer/BioNTech (BNT162b2) vaccine. Of note, the Moderna (mRNA-1273) vaccine was only introduced in late January in Switzerland. One of the patients (n=3) reported a cerebral aneurysm that was coiled within the last year, with a targeted SBP <140 mm Hg.1 Due to developing headache, the patient underwent imaging with no sign of intracranial hemorrhage. Patient No. 4 did not have associated ECG changes or an increase in hs-troponins. Importantly, all patients recovered but required at most several hours of monitoring at our tertiary center’s emergency department.
Table.
Patient characteristics.
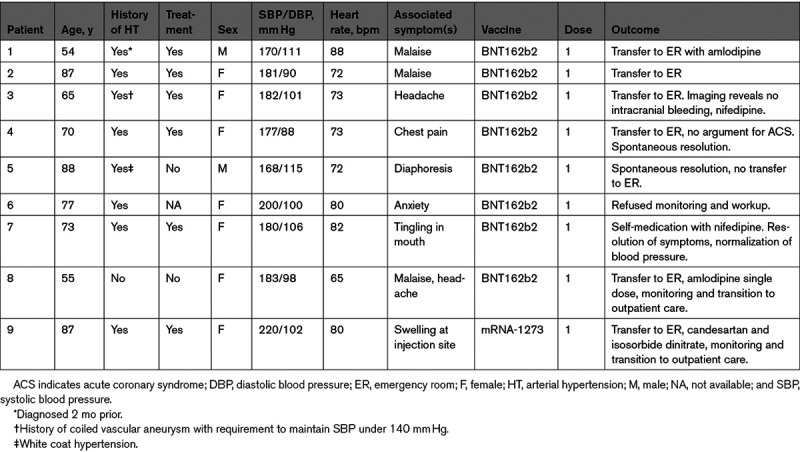
Due to the patient high throughput in our vaccination center, we do not have prevaccination BP values. However, 8 of 9 patients reported otherwise well controlled hypertension. Our case series suggests that a fraction of hypertensive patients may react with symptomatically significant increases in both systolic and diastolic blood pressure. A stress response is likely in view of the public debate, in addition to pain response and white coat effect—the latter being associated with age and female sex.2 However, the relatively low heart rate (median, 73 bpm) may soften this hypothesis. Alternative mechanisms could theoretically include hypertension to components of the vaccines such as polyethylenglycol, although this seems unlikely due to the presumably low dosage and as patients reacted within minutes of the injection. As tromethamine is only contained in the MRNA-1273 vaccine, its causative role is ruled out. An interaction between the S-protein and angiotensin converting enzyme 2 also seems highly unlikely as patients reacted within minutes of the injection, not leaving time for mRNA cellular uptake, translation, and S-protein presentation at the membrane of macrophages and dendritic cells.
The rollout of vaccines in many parts of the world focuses on the vulnerable populations (>75 years of age, risk factor groups including hypertension). The mRNA vaccines have received intense scrutiny for immediate hypersensitivity reactions in the wake of an initial report signaling 21 cases of anaphylaxis.3 Hypertension, on the contrary, has not been mentioned explicitly as an adverse event in both safety/immunogenicity trials. However, both phase I/II and III clinical trials for the mRNA vaccines included predominantly younger populations with a mean and median age of 31 and 52 years for the BNT162b2 vaccine4 and 31 and 51 for the mRNA-1273 vaccine.5 Although more data are needed to understand the extent and the mechanism of hypertension after mRNA-based vaccination, our data indicate that in elderly patients with a history of hypertension or significant prior cardiovascular comorbidities, prevaccination control of blood pressure and post-vaccination monitoring, including symptom screening, may be warranted.
Acknowledgments
We thank all collaborators of the coronavirus disease 2019 (COVID-19) vaccination center in Lausanne. Special thanks to Thierry Calandra for insightful comments.
Sources of Funding
None.
Disclosures
None.
Footnotes
For Sources of Funding and Disclosures, see page e57.
Contributor Information
Françoise Livio, Email: Francoise.Livio@chuv.ch.
Maryline Foerster, Email: Maryline.Foerster-Pidoux@chuv.ch.
Patrick James Genoud, Email: Patrick.Genoud@chuv.ch.
François Marguet, Email: Francois.Marguet@chuv.ch.
Gregoire Wuerzner, Email: gregoire.wuerzner@chuv.ch.
References
- 1.Thompson BG, Brown RD, Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES, Jr, Duckwiler GR, Harris CC, Howard VJ, Johnston SC, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, and Council on Epidemiology and Prevention; American Heart Association; American Stroke Association. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2368–2400. doi: 10.1161/STR.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 2.Thomas O, Shipman KE, Day K, Thomas M, Martin U, Dasgupta I. Prevalence and determinants of white coat effect in a large UK hypertension clinic population. J Hum Hypertens. 2016;30:386–391. doi: 10.1038/jhh.2015.95 [DOI] [PubMed] [Google Scholar]
- 3.CDC COVID-19 Response Team; Food and Drug Administration. Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine — United States, December 14–23, 2020. Morbidity Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]


