Supplemental Digital Content is available in the text.
Keywords: COVID-19, ejection fraction, heart failure, prevalence, prognosis, risk factor, systole
Abstract
Presence of heart failure is associated with a poor prognosis in patients with coronavirus disease 2019 (COVID-19). The aim of the present study was to examine whether first-phase ejection fraction (EF1), the ejection fraction measured in early systole up to the time of peak aortic velocity, a sensitive measure of preclinical heart failure, is associated with survival in patients hospitalized with COVID-19. A retrospective outcome study was performed in patients hospitalized with COVID-19 who underwent echocardiography (n=380) at the West Branch of the Union Hospital, Wuhan, China and in patients admitted to King’s Health Partners in South London, United Kingdom. Association of EF1 with survival was performed using Cox proportional hazards regression. EF1 was compared in patients with COVID-19 and in historical controls with similar comorbidities (n=266) who had undergone echocardiography before the COVID-19 pandemic. In patients with COVID-19, EF1 was a strong predictor of survival in each patient group (Wuhan and London). In the combined group, EF1 was a stronger predictor of survival than other clinical, laboratory, and echocardiographic characteristics including age, comorbidities, and biochemical markers. A cutoff value of 25% for EF1 gave a hazard ratio of 5.23 ([95% CI, 2.85–9.60]; P<0.001) unadjusted and 4.83 ([95% CI, 2.35–9.95], P<0.001) when adjusted for demographics, comorbidities, hs-cTnI (high-sensitive cardiac troponin), and CRP (C-reactive protein). EF1 was similar in patients with and without COVID-19 (23.2±7.3 versus 22.0±7.6%, P=0.092, adjusted for prevalence of risk factors and comorbidities). Impaired EF1 is strongly associated with mortality in COVID-19 and probably reflects preexisting, preclinical heart failure.
Coronavirus disease 2019 (COVID-19), a highly infectious disease, first started in Wuhan, Hubei province, China and has since become a global pandemic. Risk factors for cardiovascular disease and the presence of cardiovascular disease are recognized to be factors that influence survival from COVID-19.1–5 The presence of a preinfective clinical diagnosis of heart failure, while only affecting a small proportion of cases, is associated with more severe COVID-19.6 First-phase ejection fraction (EF1), a measure of the ejection fraction up to the time of peak aortic flow velocity may be a sensitive measure of predisposition to heart failure, particularly in patients with the comorbidities of hypertension and diabetes that are highly prevalent in COVID-19.7–9 We hypothesized that predisposition to heart failure would be associated with the severity of COVID-19. We examined the association of EF1 in patients with COVID-19 to mortality in retrospective studies of patients hospitalized with COVID-19 in Wuhan, China, and in South London, United Kingdom who had undergone echocardiography. To determine whether an association of EF1 with mortality was likely to be related to preinfective, preclinical heart failure, or to acute cardiac injury arising as a result of COVID-19, we adjusted for markers of acute cardiac injury in the outcome analysis and compared values of EF1 in patients with COVID to a control group of patients without COVID-19 but with otherwise similar characteristics.
Methods
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
Patient Population
Consecutive patients diagnosed with COVID-19 according to the interim guidance of the World Health Organization10 from the West Branch of Union Hospital (UH, n=129), Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China (a designated hospital for COVID-19) were studied retrospectively. Patients from King’s Health Partners (n=251), which includes the NHS foundation trusts of Guy’s and St Thomas’ and King’s College Hospitals, London, United Kingdom between February 2020 and May 2020 who underwent echocardiography to exclude cardiovascular complications were included in a second retrospective study. Operators were protected by appropriate personal protective equipment according to China and UK national guidelines, similar to those in recommendations from the American Society of Echocardiography.11,12 The studies were approved by the Union Hospital Tongji Medical College Ethical committee (No. 20200022) and by the London SE Research Ethics Committee (reference 18/LO/248) for the use of de-identified data for research with specific work on COVID-19 approved by the King’s Electronic Records Research Interface. All patients’ clinical records were retrieved from electronic patient records, including patient demographics, medical history, treatment, and laboratory investigations. Outcome data were verified from hospital records. The primary outcome was in-hospital all-cause mortality.
In Wuhan, patients underwent echocardiography irrespective of the severity of COVID-19, and this cohort included patients who did not require intensive care. In London, echocardiography was performed mainly on patients requiring intensive care, and echocardiography requests were triaged by senior cardiologists according to local COVID-19 guidelines. In addition to hs-cTnI, (high-sensitive cardiac troponin) biochemical measures available in a subsample of the patients (Table 1) included BNP (brain natriuretic peptide), CRP (C-reactive protein, and D-dimers. Biochemical measures were taken at the time of hospital admission and in most patients were repeated at intervals during admission.
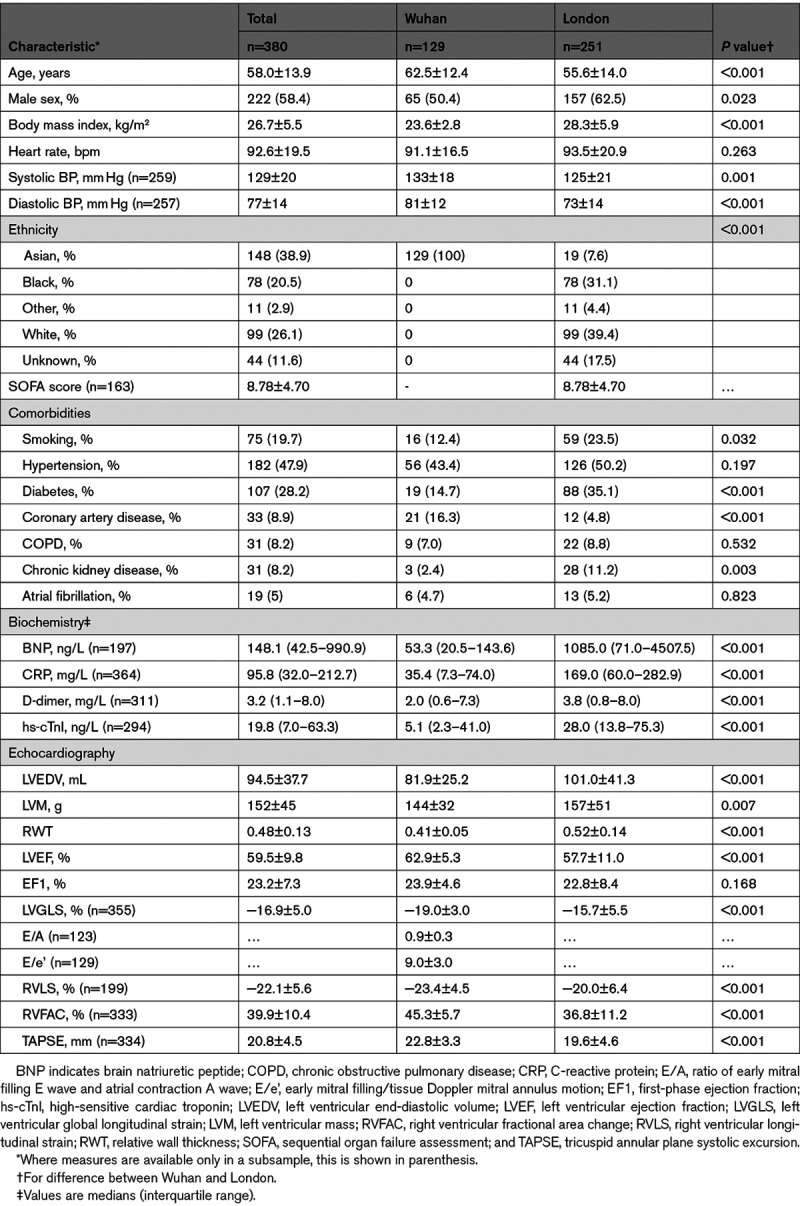
Table 1.
Clinical, Laboratory, and Echocardiographic Characteristics in the Total Cohort and Cohorts From Wuhan and London
EF1 was measured in a cohort of historical control patients (n=266) of similar age and with broadly similar comorbidities to the COVID-19 cohorts who underwent echocardiography before the COVID-19 pandemic. Measurement of EF1 was performed using the same methods as in the COVID-19 cohorts. Characteristics of these patients (some of which have previously been published8) are shown in Table S1 in the Data Supplement.
Echocardiography and EF1
Transthoracic echocardiography was performed at bedside in all patients using Philips CX50/Epic7C (Philips Medical System, Andover, MA) or GE S6 ultrasound system (GE Healthcare, Guildford, United Kingdom). Echocardiographic images were analyzed off-line by ZS for data from Wuhan and HG for data from London, who were blinded to clinical data and outcomes. The median time from admission to echocardiography was 7.5 days (interquartile range, 2–15). All conventional echocardiographic parameters were measured according to American Society of Echocardiography guidelines.13 Ejection fraction was measured using Simpson’s method. Right ventricular fractional area change (RVFAC) was measured as percentage change between RV area at the end-diastole and area at the end-systole. Tricuspid annular plane systolic excursion was measured using M-mode from an apical 4-chamber view. Left ventricular global longitudinal strain (LVGLS) and right ventricular free wall strain (right ventricular free wall strain [RVLS]) were measured with speckle tracking from apical views using the Tomtec imaging system (Tomtec, Germany). LVGLS and RVLS were measured in 355 and 199 patients in the combined cohort, respectively. EF1 was taken as the percentage volume change between end-diastole to the time of peak aortic velocity (which approximates the time of peak myocardial contraction) using the following equation: EF1=(LVEDV−V1)/LVEDV×100% where LVEDV is end-diastolic left ventricle volume, and V1 is the LV volume at the time of peak aortic velocity (Figure S1). In cases where Doppler aortic flow velocity was not recorded, the time of peak aortic velocity was taken as the time of maximal rate of change of ventricular volume obtained using wall tracking using the Tomtec imaging system. The repeatability and reproducibility of this method for measuring EF1 has been previously reported.7 In the presence of atrial fibrillation, RR intervals were measured and EF1 measurements were performed on cardiac cycles with the same R-R intervals to minimize beat-to-beat variations. LV diastolic function was measured in the Wuhan cohort. The E/A ratio was calculated as the ratio of transmitral Doppler E wave to A wave and the E/e’ ratio as the ratio of transmitral Doppler E wave velocity to the mean of basal lateral and basal septal tissue Doppler e’ wave velocity.
Statistical Analysis
Characteristics are presented as mean±SD, except those known to be non-normally distributed which are presented as median with interquartile range. Differences between groups were tested by Student t test for continuous variables and χ2 test for categorical variables. Non-normally distributed variables were log-transformed to achieve an approximately normal distribution before statistical testing. Cox regression analysis was performed to identify potential predictors of death. Multivariate models were constructed to assess the prognostic utility of EF1, with adjustment for covariables, which were significant predictors in the univariate analysis and to calculate adjusted hazards ratios (HRs). Kaplan-Meier curves were used to examine cumulative death rate, and differences between groups were tested using a log-rank test. EF1 was dichotomized using a previously defined cut off value of 25%.7 Receiver operating characteristic analyses were performed, and the area under the curves were calculated to determine the prediction of 28-day survival by EF1 and other variables. Analyses were performed independently for the Wuhan and London cohorts and repeated in the combined cohort. Comparison of EF1 in patients with and without COVID-19 was performed by ANCOVA. All statistical analyses were performed using SPSS version 25 (SPSS, Inc, Chicago, IL).
Results
Characteristics of Patients With COVID-19
A total of 380 patients (129 from Wuhan and 251 from London) were included in the final analysis (Figure 1). Patients excluded because of poor image quality (n=95) were of similar age and sex and had similar comorbidities compared to those who were included. Clinical characteristics and echocardiographic measures of the total population stratified according to center (Wuhan or London) are shown in Table 1. Patients from the ITU population in London were younger than the unselected hospitalized population in Wuhan but had a higher prevalence of smoking, diabetes, coronary artery disease and chronic kidney disease compared with those from Wuhan. They had higher levels of D-dimers, hs-cTnI, BNP and CRP, and worse LV and RV function compared with patients from Wuhan.
Figure 1.
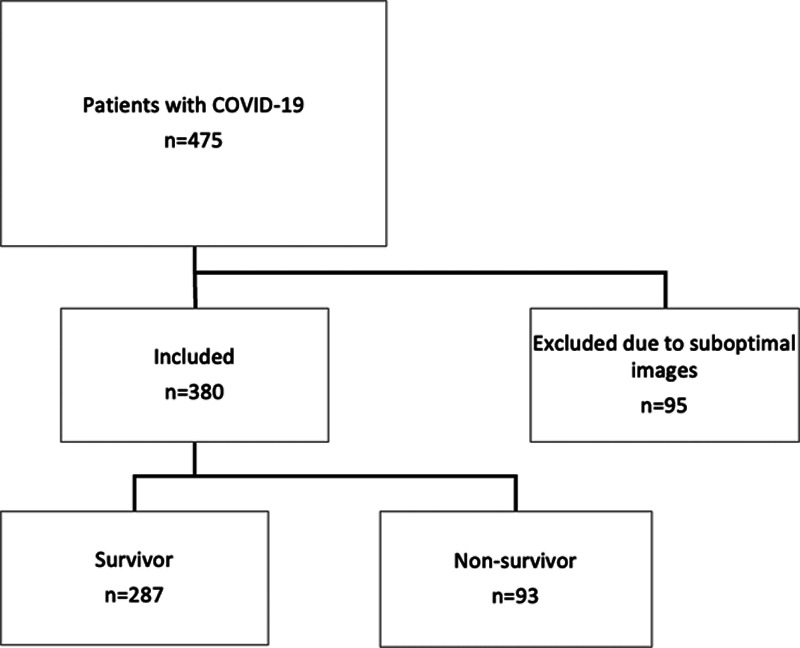
Study flow chart in the total population. COVID-19 indicates coronavirus disease 2019.
Clinical, Laboratory, and Echocardiographic Data in COVID-19 Survivors and Nonsurvivors
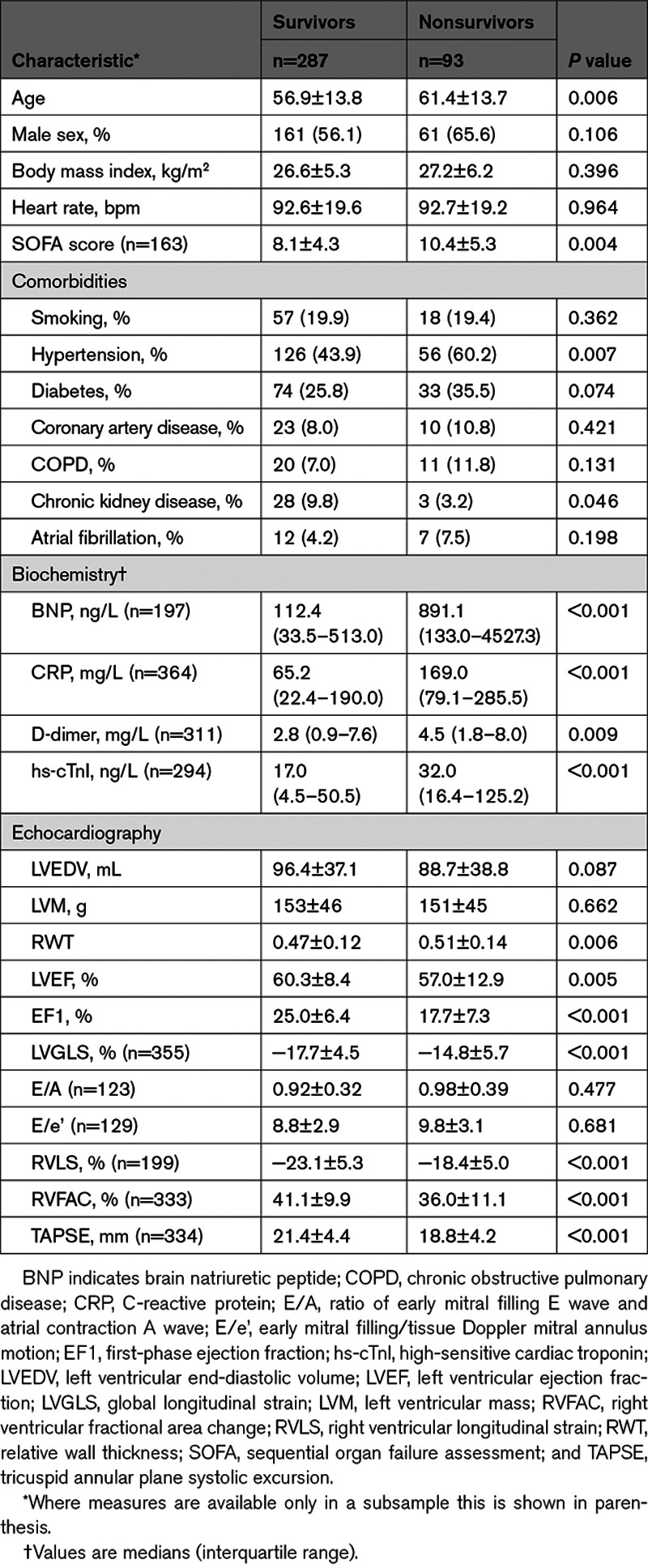
After a median of 50 (interquartile range, 27–51) days of follow-up, 93/380 (24%) patients in the total population died (16% and 29% from the Wuhan and London cohorts respectively). Cause of death was recorded as multiorgan failure (47.3%), respiratory failure (24.7%), unknown (9.7%), cardiac (8.6%), septic shock (4.3%), stroke (3.2%), and other (2.2%). Clinical, laboratory, and echocardiographic measures in survivors and nonsurvivors are shown for the 2 centers in Table S2 and for the total population in Table 2. Nonsurvivors were older and had a higher prevalence of hypertension and chronic kidney disease (and a tendency to a higher prevalence of diabetes) compared with survivors. Nonsurvivors had significantly increased D-dimer, hs-cTnl, BNP, and CRP compared with survivors. Nonsurvivors had worse LV (as measured by EF, EF1, LVGLS) and worse RV systolic function (RVLS, RVFAC, and tricuspid annular plane systolic excursion) and increased relative wall thickness.
Table 2.
Clinical, Laboratory, and Echocardiographic Characteristics in Survivors and Nonsurvivors in the Combined Cohort
Prediction of Mortality by EF1 and Other Measures
Prediction of mortality by EF1 and other measures by univariate and multivariate Cox regression analysis at each center is shown in Table S3. EF1 was the measure most consistently and strongly predictive of mortality in each center. In the combined population, other measures significantly predicting mortality on univariate analysis included the following: age, sequential organ failure assessment score, presence of hypertension, BNP, CRP, D-dimer, hs-cTnl, relative wall thickness, EF1, LVGLS, RVLS, and RVFAC (Table 3). In a multivariate model, considering age, presence of hypertension, CRP, hs-cTnl, relative wall thickness, and EF1, EF1 was the measure most strongly associated with mortality (model 1, n=316, Table 3, HR per 1% change in EF1: 0.89 [95% CI, 0.86–0.92], P<0.001). Similar HRs for EF1 were observed when all biochemical markers were included in the subsample in which these were available or when the sequential organ failure assessment score was included and did not differ substantially if peak rather than admission values were used for biochemical markers. When adjusting for LVGLS and RVFAC in addition to variables considered in model 1 (model 2, n=236, Table 3), or when replacing RVFAC with RVLS (model 3, n=150, Table 3), the HR for EF1 was similar to model 1. Receiver operating characteristic analysis showed that EF1 had the largest area under the curve (0.75) compared with age, biochemistry, and EF (Figure 2). Kaplan-Meier analysis confirmed EF1 to be a strong predictor of death (P<0.001) in each center and in the total population (Figure 3), when a previously defined threshold of 25% (sensitivity: 86.4% and specificity: 47.2%) was used.7 In the total population, when EF1 was <25%, 81/380 (35.7%) patients died compared with 12/380 (7.8%) in those with an EF1≥25%. When EF1 was considered as a categorical variable (EF1<25% versus EF1≥25%), the HR for an EF1<25% compared with ≥25% was 5.23 ([95% CI, 2.85–9.60], P<0.001) unadjusted and 4.83 ([95% CI, 2.35–9.95], P<0.001) adjusted for all variables in multivariate Cox regression model 2. Using the optimal threshold of 22.2% for EF1 had a sensitivity of 75.3% and specificity of 67.6% with similar HR for impaired versus preserved EF1 (Figure S2).
Table 3.
Univariate and Multivariate Cox Regression in the Combined Cohort
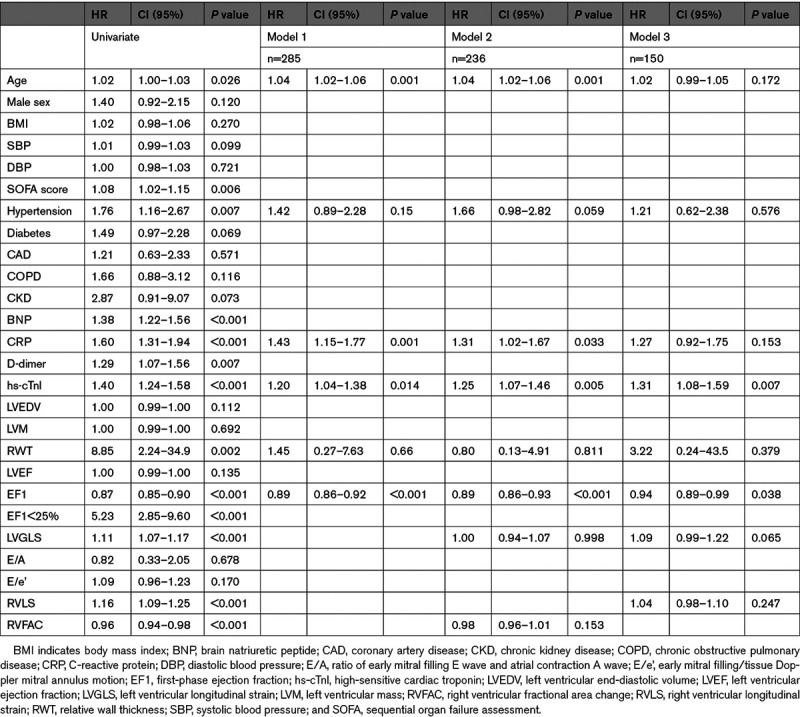
Figure 2.
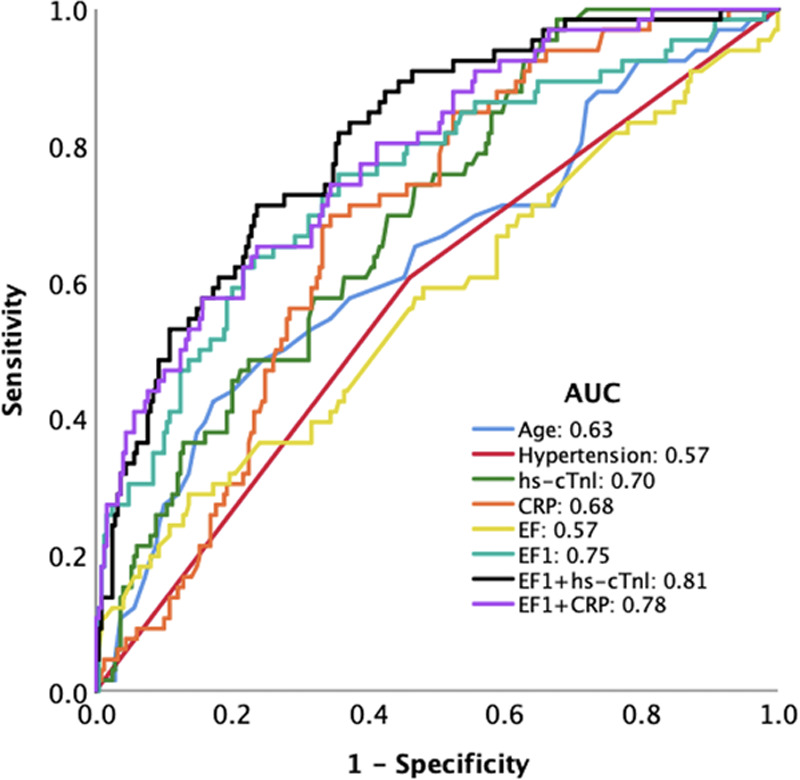
Receiver operating characteristic curve for prediction of mortality in the total population. AUC indicates area under the curve; CRP, C-reactive protein; and EF1, first-phase ejection fraction.
Figure 3.
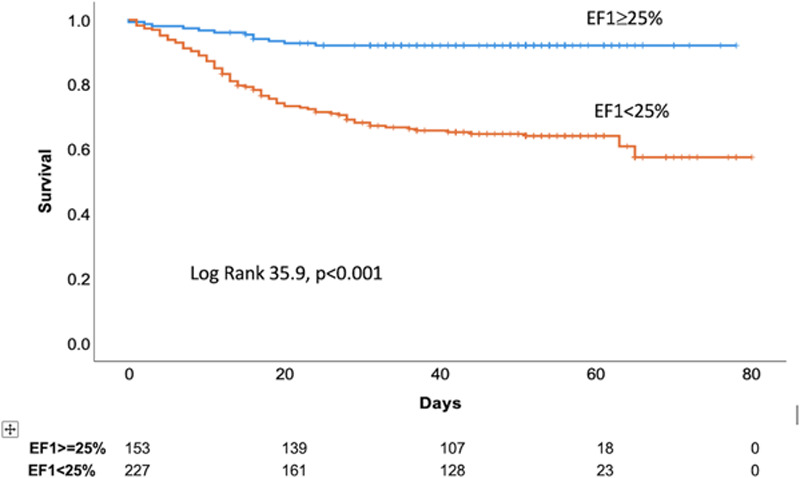
Kaplan-Meier curve of first-phase ejection fraction (EF1) stratified by a previously defined cut off value of 25% in the total population.
Comparison of EF1 in Patients With and Without COVID-19
Patients without COVID-19 (studied before the pandemic) were of similar age but were of higher BMI and had a higher prevalence of comorbidities to those with COVID-19 (Table S1). However, EF1 was similar in the 2 groups whether adjusted or unadjusted for risk factors and comorbidities (EF1 23.2±7.3 versus 22.1±7.6% for COVID-19 versus non-COVID-19, unadjusted, P=0.073; EF1 23.2±7.3 versus 22.0±7.6% for COVID-19 versus non-COVID-19, adjusted for BMI and presence of hypertension, diabetes, and coronary artery disease, P=0.092, Table S1).
Discussion
The main finding of the present study was a strong association between EF1, a measure of early systolic function and survival in patients with COVID-19. This was seen in Wuhan and London and in the combined cohort. The finding was robust being seen irrespective of adjustment for clinical and laboratory characteristics, comorbidities, and other echocardiographic measures of cardiac function and geometry, which made little difference to the ≈5-fold HR associated with EF1<25% compared with ≥25%. While the characteristics of the Wuhan and London cohorts differed, with the former being representative of unselected hospitalized patients and the latter of patients requiring ITU admission, the finding of a strong association of EF1 with survival in both cohorts suggests that association is likely to be generalizable to most hospitalized patients.
Previous studies of hospitalized patients who have undergone echocardiography to investigate suspected cardiovascular complications of COVID-19 have demonstrated little or no association between overall left ventricular EF and survival.14,15 Results of the present study were consistent with these observations; overall EF was not independently associated with mortality. A differential association of early as opposed to overall systolic function with mortality is not unexpected since overall EF is preserved in the majority of patients with COVID-19 and will not detect patients predisposed to heart failure with preserved EF. EF1 is, by contrast, impaired in patients with hypertension who have preclinical evidence of heart failure.7 This may be because early systolic function is more sensitive to compromise in cardiac contractility caused by acute or chronic injury with sarcomeric mechanotransduction maintaining overall contraction (and overall EF) at the expense of slower but sustained contraction during early systole.
An impairment of EF1 as a sensitive marker of early systolic dysfunction is thus likely to be a prelude to LV decompensation and to heart failure, particularly heart failure with preserved ejection fraction, and it is notable that diastolic dysfunction is common in patients with COVID-19.14,16 The slow sustained contraction of the LV required to maintain overall EF leads to an increase in late systolic myocardial wall stress, which may delay diastolic filling8,17 and result in increased LV filling pressures8 to which patients with severe lung disease may be particularly vulnerable. The association of EF1 with poor outcomes is therefore consistent with previous observations of a high prevalence of clinical signs of heart failure and/or elevation of BNP in nonsurvivors compared with survivors of COVID-19.1,18,19
An impairment of early systolic function could arise through acute or preexisting chronic cardiac injury or a combination of the 2. Some degree of acute cardiac injury as evidenced by a rise in hs-cTnl is relatively common in hospitalized patients with COVID-192,19–22 and, as in the present study, has been associated with poorer outcomes.1,2,19,23,24 However, in the present study, the association of EF1 with mortality was little influenced by adjustment for markers of acute cardiac injury such hs-cTnl and BNP. Furthermore, values of EF1 in patients with COVID-19 were similar to those in a control group of patients with broadly similar characteristics who were studied before the COVID-19 pandemic. Taken together, these observations suggest that the association of EF1 with mortality reflects preexisting preclinical injury to the LV and may, in part, explain the association of severity of COVID-19 with conditions such as hypertension, diabetes, and CKD1–4 that are also risk factors for LV dysfunction. Impaired EF1 is associated with increased late systolic wall stress that may adversely impact on LV remodeling, and our finding of increased relative wall thickness in nonsurvivors is consistent with preexisting impairment of EF1 and increased late systolic wall stress.
EF1 is a new measure of early systolic function and its dependence on loading conditions has not been fully elucidated. Preliminary studies suggest that it has modest dependence on both preload and afterload. In the present study, we did not find an association of systolic blood pressure with mortality, but patients with impaired EF1 may be sensitive to cardiac loading conditions and to drugs that may influence cardiac contractility; this would need to be tested prospectively. The much stronger association of EF1 than of individual risk factors or comorbidities with morbidity from COVID-19, if confirmed in larger series, may be useful in identifying patients who are at high risk of COVID-19, particularly in patients who have undergone cardiac imaging.
This study is subject to several important limitations. The sample size was relatively small, patients were studied at different stages in the evolution of COVID-19, and were on a variety of different treatments. While the Wuhan cohorts were unselected, selection bias could have influenced findings in the London cohort. However, this would not be expected to affect the relative strengths of associations of EF1 and other cardiac biomarkers with outcomes, and it is notable that we observed similar findings in both cohorts. EF1 may be influenced by arterial stiffness, which associates with mortality in COVID-19.5 Although we adjusted for systolic blood pressure, this may be a poor surrogate of arterial stiffness especially in acute COVID-19; direct measurement of EF1 and arterial stiffness will be required to assess their relative associations with COVID-19 mortality. Image quality was insufficient to allow measurement of EF1 in a relatively high proportion of cases. However, measurement of EF1 was not mandated at the time of study. If the clinical utility of EF1 is accepted, then it is likely that the addition of appropriate quality control would allow EF1 to be measured in most patients. The cross-sectional nature of the study limits conclusions with regard to causality.
Perspectives
EF1 is a sensitive measure of early left ventricular systolic function and can be measured from standard bedside echocardiography in most patients with COVID-19. EF1 is strongly predictive of survival from COVID-19 and implicates early left ventricular dysfunction, probably arising as a chronic condition before infection, as a determinant of survival. EF1 may be useful in stratifying preventive or therapeutic treatments and as a therapeutic target.
In conclusion, in hospitalized patients with COVID-19, EF1, a measure of early systolic function, is strongly predictive of survival.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (grant 81922033 to L. Zhang; grant 81727805 to M. Xie; grant 81701716 to Z. Sun) and by a British Heart Foundation, UK project grant (PG/19/23/34259). H. Gu is supported by National Institute for Health Research, UK ICA Lectureship (ICA-CL-2018-04-ST2-012). We acknowledge financial support from the Department of Health via the National Institute for Health Research comprehensive Biomedical Research Centre and Clinical Research Facilities awards to Guy’s and St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Disclosures
H. Gu and P. Chowienczyk are named on a patent application for first-phase ejection fraction. The other authors report no conflicts.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- BNP
- brain natriuretic peptide
- COVID-19
- coronavirus disease 2019
- CRP
- C-reactive protein
- EF1
- first-phase ejection fraction
- HR
- hazard ratio
- hs-cTnI
- high-sensitive cardiac troponin
- RVFAC
- right ventricular fractional area change
H. Gu, C. Cirillo, A.A. Nabeebaccus, Z. Sun, L. Fang, and Y. Xie contributed equally as co-first authors.
L. Zhang, G. Carr-White, A.M. Shah, M. Xie, and P. Chowienczyk contributed equally as co-senior authors.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.121.17099.
For Sources of Funding and Disclosures, see page 2021.
Contributor Information
Haotian Gu, Email: haotian.gu@kcl.ac.uk.
Chiara Cirillo, Email: chiara.cirillo@ouh.nhs.uk.
Adam A. Nabeebaccus, Email: adam.a.nabeebaccus@kcl.ac.uk.
Zhenxing Sun, Email: sunzhenxing05@163.com.
Lingyun Fang, Email: 876190603@qq.com.
Yuji Xie, Email: xiemx@hust.edu.cn.
Ozan Demir, Email: ozanmdemir@gmail.com.
Nishita Desai, Email: Nishita.Desai@gstt.nhs.uk.
Lin He, Email: helinwh@163.com.
Qing Lü, Email: unionlq2003@aliyun.com.
Eleni Nakou, Email: eleni.nakou@nhs.net.
Kevin O’Gallagher, Email: kevin.o'gallagher@kcl.ac.uk.
Christos Tountas, Email: tountasxristos@yahoo.gr.
Apostolia Marvaki, Email: apostolia.marvaki@nhs.net.
Mark Monaghan, Email: mark.monaghan@nhs.net.
Divaka Perera, Email: divaka.perera@kcl.ac.uk.
Ana Pericao, Email: anapericao@gmail.com.
Matthew Ryan, Email: matthew.ryan@kcl.ac.uk.
Hannah Sinclair, Email: Hannah.Sinclair@gstt.nhs.uk.
Vasileios Stylianidis, Email: Vasileios.Stylianidis@gstt.nhs.uk.
Kelly Victor, Email: Kelly.Victor@gstt.nhs.uk.
Bin Wang, Email: ruiwang9604@qq.com.
Jing Wang, Email: ruiwang9604@qq.com.
Rui Wang, Email: ruiwang9604@qq.com.
Chun Wu, Email: wlky707@126.com.
Yali Yang, Email: chhmao@aliyun.com.
Hongliang Yuan, Email: 825162756@qq.com.
Danqing Zhang, Email: 18907131488@189.cn.
Yongxing Zhang, Email: 18907131488@189.cn.
Luca Faconti, Email: luca.faconti@kcl.ac.uk.
Alexandros Papachristidis, Email: alexandros.papachristidis@nhs.net.
Li Zhang, Email: 18907131488@189.cn.
Gerald Carr-White, Email: gerry.carr-white@gstt.nhs.uk.
Ajay M. Shah, Email: ajay.shah@kcl.ac.uk.
Novelty and Significance
What Is New?
First-phase ejection fraction (EF1), a measure of the ejection fraction up to the time of peak aortic flow velocity, is a sensitive measure of predisposition to heart failure, particularly in patients with the comorbidities of hypertension and diabetes that are highly prevalent in coronavirus disease 2019 (COVID-19).
The finding of a strong association of EF1 with survival in COVID-19 in 2 independent cohorts from China and United Kingdom suggests that association is likely to be generalizable to most hospitalized patients.
The association of EF1 with mortality probably reflects preexisting chronic injury to the left ventricle and may, in part, explain the association of severity of COVID-19 with conditions predisposing to left ventricle dysfunction.
What Is Relevant?
An impairment of early systolic function as evidenced by reduced EF1 could be causally implicated in worse outcomes from COVID-19 through an elevation of left ventricle filling pressure.
The much stronger association of EF1 than of individual risk factors or comorbidities with morbidity from COVID-19, if confirmed in larger series, may be useful in identifying patients who are at high risk of COVID-19, in patients who have undergone cardiac imaging.
Summary
EF1 is strongly predictive of survival from COVID-19 and implicates early left ventricular dysfunction, probably arising as a chronic condition before infection, as a determinant of survival. EF1 may be useful in stratifying preventive or therapeutic treatments and as a therapeutic target.
References
- 1.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albitar O, Ballouze R, Ooi JP, Sheikh Ghadzi SM. Risk factors for mortality among COVID-19 patients. Diabetes Res Clin Pract. 2020;166:108293. doi: 10.1016/j.diabres.2020.108293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodilla E, López-Carmona MD, Cortes X, Cobos-Palacios L, Canales S, Sáez MC, Campos Escudero S, Rubio-Rivas M, Díez Manglano J, Freire Castro SJ, et al. ; SEMI-COVID-19 Network. Impact of arterial stiffness on all-cause mortality in patients hospitalized with COVID-19 in Spain. Hypertension. 2021;77:856–867. doi: 10.1161/HYPERTENSIONAHA.120.16563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez-Garcia J, Lee S, Gupta A, Cagliostro M, Joshi AA, Rivas-Lasarte M, Contreras J, Mitter SS, LaRocca G, Tlachi P, et al. Prognostic impact of prior heart failure in patients hospitalized with COVID-19. J Am Coll Cardiol. 2020;76:2334–2348. doi: 10.1016/j.jacc.2020.09.549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu H, Saeed S, Boguslavskyi A, Carr-White G, Chambers JB, Chowienczyk P. First-phase ejection fraction is a powerful predictor of adverse events in asymptomatic patients with aortic stenosis and preserved total ejection fraction. JACC Cardiovasc Imaging. 2019;12:52–63. doi: 10.1016/j.jcmg.2018.08.037 [DOI] [PubMed] [Google Scholar]
- 8.Gu H, Li Y, Fok H, Simpson J, Kentish JC, Shah AM, Chowienczyk PJ. Reduced first-phase ejection fraction and sustained myocardial wall stress in hypertensive patients with diastolic dysfunction: a manifestation of impaired shortening deactivation that links systolic to diastolic dysfunction and preserves systolic ejection fraction. Hypertension. 2017;69:633–640. doi: 10.1161/HYPERTENSIONAHA.116.08545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bing R, Gu H, Chin C, Fang L, White A, Everett RJ, Spath NB, Park E, Jenkins WS, Shah AS, et al. Determinants and prognostic value of echocardiographic first-phase ejection fraction in aortic stenosis. Heart. 2020;106:1236–1243. doi: 10.1136/heartjnl-2020-316684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, 28 January 2020. 2020. Accessed January 30, 2020. https://apps.who.int/iris/handle/10665/330893
- 11.Kirkpatrick JN, Mitchell C, Taub C, Kort S, Hung J, Swaminathan M. ASE statement on protection of patients and echocardiography service providers during the 2019 novel coronavirus outbreak: endorsed by the American College of Cardiology. J Am Coll Cardiol. 2020;75:3078–3084. doi: 10.1016/j.jacc.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E, Petersen S, Gimelli A, Haugaa KH, Muraru D, et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging. 2020;21:592–598. doi: 10.1093/ehjci/jeaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. doi: 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 14.Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Rothschild E, Baruch G, Peri Y, et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation. 2020;142:342–353. doi: 10.1161/CIRCULATIONAHA.120.047971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li Y, Li H, Zhu S, Xie Y, Wang B, He L, Zhang D, Zhang Y, Yuan H, Wu C, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging. 2020;13:2287–2299. doi: 10.1016/j.jcmg.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freaney PM, Shah SJ, Khan SS. COVID-19 and heart failure with preserved ejection fraction. JAMA. 2020;324:1499–1500. doi: 10.1001/jama.2020.17445 [DOI] [PubMed] [Google Scholar]
- 17.Chirinos JA, Segers P, Gupta AK, Swillens A, Rietzschel ER, De Buyzere ML, Kirkpatrick JN, Gillebert TC, Wang Y, Keane MG, et al. Time-varying myocardial stress and systolic pressure-stress relationship: role in myocardial-arterial coupling in hypertension. Circulation. 2009;119:2798–2807. doi: 10.1161/CIRCULATIONAHA.108.829366 [DOI] [PubMed] [Google Scholar]
- 18.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020;41:1861–1862. doi: 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID-19 and cardiovascular disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 22.Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, Pipavath S, Wurfel MM, Evans L, et al. Covid-19 in critically ill patients in the Seattle Region - case series. N Engl J Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippi G, Lavie CJ, Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


