Abstract
Objective: This study aims to investigate the level of peripheral blood mononuclear cells and their ratios which may point to the immunological mechanisms involved in the etiopathogenesis of ASD.
Method: The complete blood count parameters of the 45 ASD cases were compared with those of healthy controls.Childhood Autism Rating Scale (CARS) was performed to measure the disease severity.
Results: The monocytes of ASD group were significantly higher; and the lymphocyte-to-monocyte ratio (LMR) was lower than the controls’. LMR and neutrophil-to-lymphocyte ratio were found to be predictors of ASD. The decrease in LMR (B: −0.744; P=0.035; CI: −1.431 to −0.056) and the increase in age (B: 0.432; P=0.045; CI: 0.011–0.853) were related to high CARS scores in linear regression analyses.
Conclusions: The results of this study support the role of altered immune cell counts and ratios in ASD. A high monocyte level and low LMR may have diagnostic values in autism.
Keywords: ASD, autism, CBC, immunity, LMR, lymphocyte, monocyte, NLR
1. Introduction
Autism spectrum disorder (ASD) is a neuro-developmental disorder characterized by persistent impairment in reciprocal social communication and interaction and by restricted, repetitive behaviors, interests, or activities. ASD symptoms are present early childhood, and they limit or impair everyday functioning (DSM-V; American Psychiatric Association 2013). Pervasive developmental disorder (PDD) and the five PDD subtypes in the Diagnostic and Statistical Manual of Mental Disorders – IV (DSM-IV-TR; American Psychiatric Association 2000) were excluded from DSM-5 (American Psychiatric Association 2013). A new diagnostic category called ASD was created, and it is adapted to the individual’s clinical presentation. Recent reports on the incidence of ASD in the population show that its prevalence is increasing. The prevalence of ASD in the United States has been reported to be 1.46% in children eight years of age (Christensen et al. 2016). The increase in prevalence of ASD has led to an increase in efforts to understand its etiology (Meltzer and Van de Water 2016).
ASD is multifactorial and has many risk factors acting together (genetic, epigenetic, and environmental interactions) to produce the phenotype (Depino 2013; Ornoy Liza and Ergaz 2016). ASD is known to be influenced by genetic factors, but only a fraction of ASD-diagnosed individuals can be explained by genetic causes (Colvert et al. 2015). In recent years, much evidence has suggested that the symptoms of ASD may be associated with immune dysfunction (Ashwood et al. 2011; Onore, Careaga, and Ashwood 2012; López-Cacho et al. 2016).
Immunological abnormalities in individuals with ASD were first reported in 1977 (Stubbs et al. 1977). Since then, the literature on this issue has expanded rapidly. Epidemiological investigations have revealed a relationship between maternal infections and development of ASD (Chess 1971; Chess 1977; Patterson 2011). Cytokine expression in the fetal brain as a response to maternal inflammatory activation has been suggested to affect normal brain development in the fetus (Zaretsky et al. 2004; Zerbo et al. 2013). Therefore, the hypothesis that maternal immune activation leads to the development of ASD by affecting the developing fetal brain comes to the forefront (Patterson 2011). In addition, animal studies support the hypothesis that viral or bacterial prenatal fetal infections may also be involved in ASD etiology (Madore et al. 2016).
Neuro-immune interaction begins in the early stages of life and persists throughout life. Immune dysregulation, especially during early development, may be susceptible to changes in neurological functions (Goines and Ashwood 2013). Zerbo et al. (2014) conducted a population-based case – control study by measuring cytokine and chemokine concentrations from the archived neo-natal blood specimens collected for routine new-born screening. Cytokines were not detected in the vast majority of new-born samples regardless of ASD or control status. However, the chemokine monocyte chemotactic protein-1 was elevated, and the chemokine regulated upon activation normal T-cell expressed and secreted was decreased in ASD cases in comparison with GP controls. They concluded that the measurement of immune system function in the first few days of life could aid in differentiating abnormal or normal neurodevelopment (Zerbo et al. 2014). Neuroglial activation and neuroinflammation in the brain of ASD individuals have been shown to be evidence of different immunological functions in their central nervous system (CNS) (Pardo et al. 2005).
Specific immune cell types have been examined to understand post-natal immune imbalance in autism (Gregg et al. 2008; Mostafa et al. 2010; Bjørklund et al. 2016). Previous studies showed that ASD children have an altered immune cell ratio, a decreased number of T lymphocytes, and altered responses and gene expressions in peripheral blood leucocytes (Denney et al. 1996; Ashwood et al. 2006; Gregg et al. 2008; Stigler et al. 2009; Siniscalco et al. 2012; Bjørklund et al. 2016). Various sub-immunophenotypic features have been suggested to be present in the ASD population with more severe behavioral impairment (Careaga et al. 2017).
Clinical and post-mortem data show that inflammatory processes in ASD are not restricted to the peri-natal period and probably remain altered throughout ASD pathology. Chronic inflammatory diseases and the abnormal response to infection of different blood cell populations have been described in ASD children and adults (Depino 2013).
Some inflammatory markers can be examined with the conventional and inexpensive method of complete blood count (CBC). Blood cell counting can provide information on cell composition, such as red blood cells, monocytes, neutrophils, lymphocytes, platelets in blood, as well as cell ratios, such as neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio, lymphocyte–monocyte ratio (LMR), which can be used as markers for systemic inflammatory responses (Zahorec 2001; Balta et al. 2016). The current study is conducted in light of the hypothesis that the inflammatory processes in ASD etiopathogenesis remain altered throughout the lifetime (Depino 2013) and that cell counts and their ratios obtained from CBC may provide information about these different immunological processes.
Studies have been conducted on the effect of inflammation on ASD previously (Careaga et al. 2017; Meltzer and Van de Water 2016). Cell counts and their ratios obtained from CBCs are an easy, inexpensive, and accessible way to assess the effect of immune cells in ASD. Thus, in this study, CBC parameters were evaluated in ASD-diagnosed individuals and compared with the healthy control group. The study aimed to investigate whether parameters that could point to the immunological mechanisms involved in the etiopathogenesis of ASD exist.
2. Method
The Ankara Yildirim Beyazit University Faculty of Medicine Yenimahalle Education Research Hospital Ethics Board approved the study. Necessary permissions were obtained from the hospital administration. This work was conducted in accordance with the Declaration of Helsinki as revised in 2000.
2.1. Participants
The database of our hospital in the years 2012–2016 was assessed. The cases diagnosed as F84.0 childhood autism and F84.1 atypical autism according to the International Disease Classification-10 (ICD-10) were selected. From these two diagnoses, the cases that had CBC analyses without any acute or chronic infection diseases diagnosed at the same time were included in the study. All the patients were seen by a child and adolescent psychiatrist (authors STH and MFC), diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) and ICD-10, and the Childhood Autism Rating Scale (CARS) was administered to the cases. An atypical autism diagnosis includes individuals diagnosed with pervasive developmental disorder not otherwise specified in DSM-IV. Atypical autism symptomatology is similar to autism, but the precise symptomology does not meet the criteria.
Among those in the database, 257 patients were diagnosed with F84.0 childhood autism and F84.1 atypical autism. Only 45 of these patients had CBC results that showed no infectious disease. During the diagnosis of ASD, all patients’ neurological, audiological, and medical examinations were made by necessary specialists in the first application to our clinic. Thus, CBC was a routine part of the assessment of the pediatrician. In the pediatric clinic, the first routine fasting blood tests of patients who come to the examination are taken in the morning. The CBC results obtained from these cases were compared with those of the 43 healthy controls. None of the patients had clinical findings suggestive of infection, allergic manifestations, or immunological disorders. The exclusion criteria of the study was having an infectious disease diagnosed at the time when the CBC was obtained and having a diagnosis of any chronic infections or allergic, neoplastic/leukoproliferative diseases. Patients with drug use at the time of the CBC assessment were also excluded from the study.
2.2. Data collection tool
The data were obtained from the Yildirim Beyazit University Faculty of Medicine Yenimahalle Education Research Hospital database. The CARS scores from the database were used to assess the severity of autism.
2.2.1. CARS
The CARS (Schopler et al. 1980) consists of 15 items that are used for diagnosing autism and differentiating between children with autistic disorder and those with other developmental disorders. Children with 30 points and above are considered to have autistic disorder, those with 30–36.5 points show mild–moderate autism, and those with 37–60 points show severe autism. The scale’s Turkish validity and reliability were evaluated by (İncekaş Gassaloğlu et al. 2016).
2.3. Statistical analyses
The Kolmogorov–Smirnov test was used to assess whether the data were normally distributed. The normally distributed data were analyzed by Student’s t-test and the remaining ones by the Mann–Whitney U test. Comparisons were first made between the case and the control group and then between the groups diagnosed with autism and that with atypical autism. The variables that predicted ASD diagnosis were examined by logistic regression analysis. Linear regression analysis was used to examine the variables predicting autism severity. A value of P < 0.05 was considered statistically significant.
3. Results
The study included 45 cases and 43 controls. The case group comprised 30 autism and 15 atypical autism cases. Female/male ratio was 9/36 (1:4) in the case group and10/33 in the control group. The mean age of the case group was 13.51 ± 4.21 and that of the control group was 11.90 ± 3.73 (Z=1.88; P=0.063). No significant differences were found between the case and the control group in terms of age and sex (Table 1).
Table 1. Comparisons of the case and control groups in terms of age and sex.
| Mean (SD) | Z | P | |||
|---|---|---|---|---|---|
| Agea | ASD | 13.51 ± 4.21 | 1.88 | 0.063 | |
| (n=45) | |||||
| Control | 11.90 ± 3.73 | ||||
| (n=43) | |||||
| Sexb | Female | Male | x2 | ||
| ASD | 9 | 36 | 0.138 | ||
| (n=45) | |||||
| Control | 10 | 33 | (P=0.711) | ||
| (n=43) | |||||
Mann–Whitney U test used
Chi-Square test used.
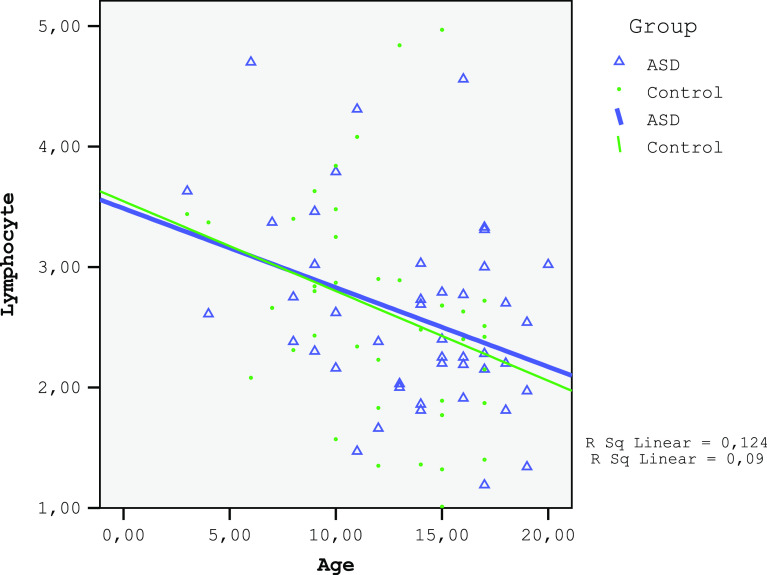
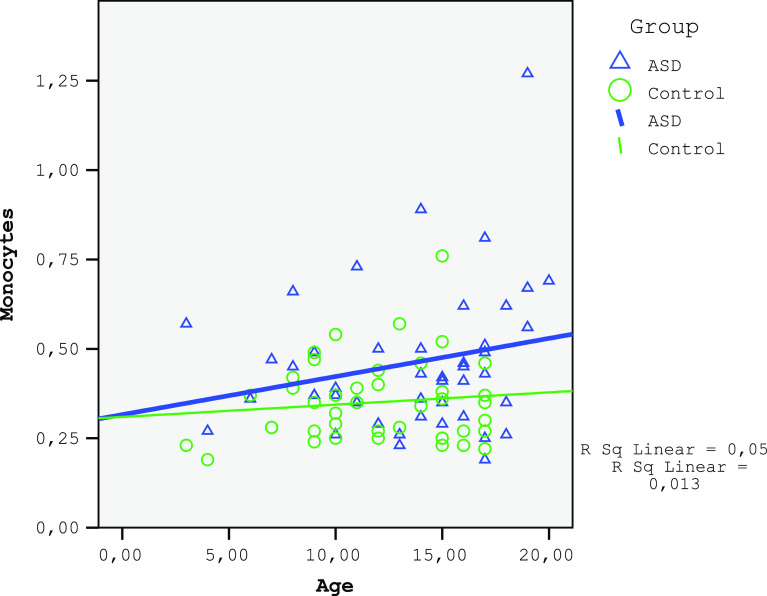
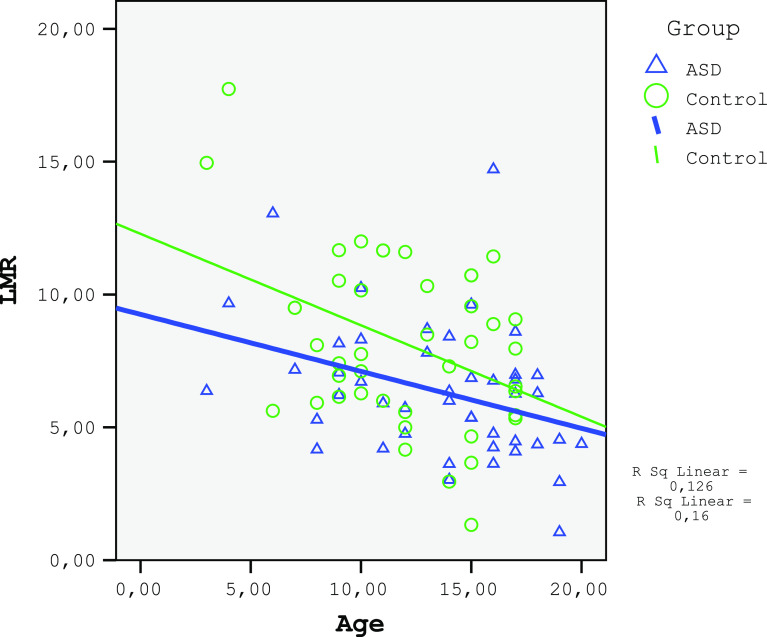
The monocytes and red cell distribution width (RDW) in the ASD group were significantly higher and the LMR was lower than those in the control (Table 2). A negative correlation was found between age and lymphocyte count for both the ASD and the control groups (ASD group: r=−0.297, P=0.047; control group: r=−0.376, P=0.013; Fig. 1). No correlation was found between age and monocyte count for both the ASD and the control group (ASD group: r=0.185, P=0.224; control group: r=−0.005, P=0.975; Fig. 2). A significant negative correlation was observed between age and LMR for the ASD group (r=−0.361, P=0.015) and an insignificant correlation for the control group (r=−0.267, P=0.084; Fig. 3). A negative relationship between the severity of the disease and the number of lymphocytes and LMR as well as a positive relationship with NLR were found (Table 3).
Table 2. Comparison of study and control groups in terms of CBC parameters.
| Study group | Control group | t or Z value | P value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| WBC (× 103/mm3) | 6.82 ± 1.67 | 6.73 ± 2.08 | 0.21 | 0.827 |
| Lymphocytes (× 103/mm3) | 2.59 ± 0.78 | 2.65 ± 0.90 | −0.33 | 0.738 |
| Monocytes (× 103/mm3) | 0.46 ± 0.20 | 0.35 ± 0.11 | 3.12 | 0.002* |
| Neutrophils (× 103/mm3) | 3.42 ± 1.37 | 3.39 ± 1.45 | −0.09 | 0.928 |
| PLT (× 103/mm3) | 297.73 ± 95.54 | 297.13 ± 77.15 | 0.03 | 0.975 |
| LMR | 6.35 ± 2.55 | 8.18 ± 3.21 | −2.96 | 0.004* |
| NLR | 1.43 ± 0.69 | 1.42 ± 0.72 | 0.08 | 0.935 |
| PLR | 123.68 ± 54.31 | 123.87 ± 52.51 | −0.01 | 0.987 |
| RBC(× 106/mm3) | 4.94 ± 0.42 | 4.93 ± 0.37 | 0.18 | 0.852 |
| RDW(%) | 13.96 ± 1.41 | 13.08 ± 1.32 | −2.44 | 0.015* |
| MPV(fL) | 8.15 ± 1.82 | 7.93 ± 4.64 | −1.94 | 0.052 |
| PDW(fL) | 38.60 ± 17.10 | 46.28 ± 6.44 | −1.02 | 0.304 |
Notes: Student t-test was used for parametric data (t values are given) and Mann–Whitney U test was used (Z values are given) to compare the non-parametric data. CBC: Complete Blood Count, WBC: White Blood Cells, PLT: Platelet (Thrombocyte), LMR: Lymphocyte-to-Monocyte Ratio, NLR: Neutrophil-to-Lymphocyte Ratio, PLR: Platelet (Thrombocyte)-to-Lymphocyte Ratio, RBC: Red Blood Cells, RDW: Red cell Distribution Width, MPV: Mean Platelet Volume, PDW: Platelet Distribution Width. *statisticaly significant (p<0.05)
Figure 1.
The corelation of lymphocytes of ASD and control groups.
Figure 2.
The corelation of monocytes of ASD and control groups.
Figure 3.
The corelation of LMR of ASD and control groups.
Table 3. Examination of the correlation between CARS score and complete blood count parameters.
| CARS score |
||
|---|---|---|
| r | P | |
| Lymphocytes (103/mm3) | −0.31 | 0.039 |
| Monocytes (103/mm3) | 0.27 | 0.074 |
| LMR | −0.42 | 0.005 |
| NLR | 0.36 | 0.016 |
Notes: Pearson correlation analyzes conducted. CARS: Childhood Autism Rating Scale, LMR: Lymphocyte-to-Monocyte Ratio, NLR: Neutrophil-to-Lymphocyte Ratio.
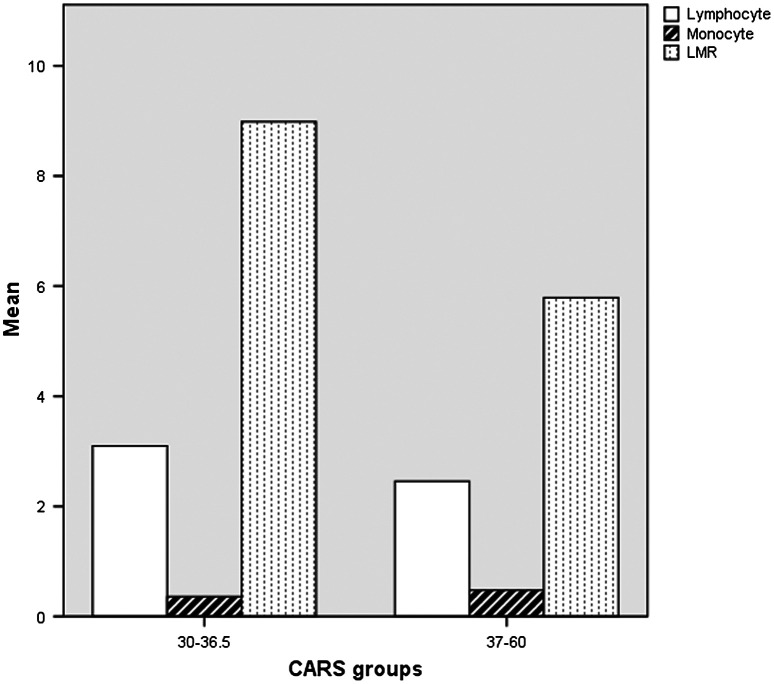
Autism (F84.0) and atypical autism (F84.1) were compared in terms of CBC parameters separately. The LMR of autism-diagnosed cases was significantly low (Table 4). Figure 4 shows the lymphocyte and monocyte counts, as well as the LMR of the ASD cases according to the CARS cut-off points (Fig. 4).
Table 4. Comparison of the complete blood count parameters between autism and atypical autism cases.
| Autism (F84.0) | Atypical autism (F84.1) | t or Z value | P value | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| WBC (103/mm3) | 6.80 ± 1.89 | 6.86 ± 1.16 | −0.11 | 0.91 |
| Lymphocytes (103/mm3) | 2.45 ± 0.65 | 2.89 ± 0.96 | −1.81 | 0.07 |
| Monocytes (103/mm3) | 0.48 ± 0.21 | 0.40 ± 0.14 | 1.28 | 0.20 |
| Neutrophils (103/mm3) | 3.54 ± 1.53 | 3.16 ± 0.99 | 0.88 | 0.38 |
| PLT (103/mm3) | 294.77 ± 100.58 | 303.67 ± 87.59 | −0.29 | 0.77 |
| LMR | 5.67 ± 1.99 | 7.70 ± 3.05 | −2.67 | 0.01 |
| NLR | 1.53 ± 0.73 | 1.23 ± 0.59 | 1.37 | 0.17 |
| PLR | 128.92 ± 59.32 | 113.21 ± 42.50 | 0.91 | 0.36 |
| RBC (106/mm3) | 4.94 ± 0.46 | 4.94 ± 0.33 | 0.002 | 0.99 |
| RDW (%) | 13.89 ± 1.55 | 14.07 ± 1.20 | −1.59 | 0.51 |
| MPV (fL) | 8.48 ± 2.06 | 7.50 ± 0.96 | −1.20 | 0.22 |
| PDW (fL) | 36.63 ± 18.63 | 43.86 ± 11.35 | −0.30 | 0.76 |
Notes: Student t-test was used for parametric data (t values are given) and Mann–Whitney U test was used (Z values are given) to compare the non parametric data. WBC: White Blood Cells, PLT: Platelet (Thrombocyte), LMR: Lymphocyte-to-Monocyte Ratio, NLR: Neutrophil–to- Lymphocyte Ratio, PLR: Platelet (Thrombocyte)-to-Lymphocyte Ratio, RBC: Red Blood Cells, RDW: Red cell Distribution Width, MPV: Mean Platelet Volume, PDW: Platelet Distribution Width.
Figure 4.
The lymphocyte, monocyte counts and LMR of the autism spectrum disorder cases in terms of CARS cut-off points.
Logistic regression analysis was applied to examine the factors affecting being in the study group (Table 5). LMR and NLR were found to be predictors of being in the ASD group (Nagelkerke R2=0.209). A multiple linear regression was applied to predict autism severity based on age, sex, and LMR. A significant equation was found (F (3.39)=4.591, P=0.008) with an R2 of 0.261. The CARS score increased 1.43-fold for each age increase and decreased 1.34-fold for each unit increase of LMR. The decrease in LMR and the increase in age were related to high CARS scores (Table 6).
Table 5. Logistic regression analyze of the factor that predict being in study group.
| B | Odds ratio | 95% CI | P | |
|---|---|---|---|---|
| Age | 0.093 | 1.098 | 0.959–1.257 | 0.174 |
| Gender | 0.170 | 1.186 | 0.381–3.689 | 0.769 |
| LMR | −0.337 | 0.713 | 0.571–0.891 | 0.003 |
| NLR | −0.965 | 0.381 | 0.155–0.933 | 0.035 |
Note: LMR: Lymphocyte–to- Monocyte Ratio, NLR: Neutrophil-to-Lymphocyte Ratio; B: non-standardized regression coefficient; CI: confidence interval.
Table 6. Linear regression analyze of the factors which predict the CARS scores.
| B | SE | 95% CI | P | |
|---|---|---|---|---|
| Age | 0.432 | 0.208 | 0.011–0.853 | 0.045 |
| Gender | −0.673 | 2.013 | −4.744–3.399 | 0.740 |
| LMR | −0.744 | 0.340 | −1.431–−0.056 | 0.035 |
Notes: CARS: Childhood Autism Rating Scale, LMR: Lymphocyte–to- Monocyte Ratio, B: non-standardized regression coefficient; S.E.: standard error; CI: confidence interval.
4. Discussion
This study points to an immunological dimension of autism. In this study, we aimed to investigate whether associations exist among autism, atypical autism, and changes in complete blood cell count parameters. Studies have been conducted on the effect of inflammation on ASD pathophysiology (Meltzer and Van de Water 2016). Recent studies have shown that complete blood cell parameters can be used as an indicator of inflammation (Balta et al. 2016). Thus, in this study; we investigated the changes in monocytes, LMR, NLR, and RDW from the complete blood cell parameters in autistic patients by comparing them with those of the healthy control group.
In our study, significant differences were found between the case and the control group in terms of complete blood cell parameters. The first finding of our study is that the monocytes are significantly higher in the study group than in the control group. This increase in monocytes may play a role in the etiology of the disorder as an indicator of chronic inflammation. Monocytes are a type of white blood cell, or leucocyte, originating from the bone marrow and circulating in the blood. Some of these cells enter tissues and mature into macrophages (Cline 1978; Abbas et al. 2007). Monocytes have an important role in both innate and adaptive immunity. Their effector functions in innate immunity are to phagocytose microbes as mononuclear phagocytes and to produce cytokines that activate other inflammatory cells. They also have an antigen-presenting function in adaptive immunity (Abbas et al. 2007). Denny et al. (1996) initially found an increase in monocytes in autistic patients but suggested overlooking this increase because of the lack of previous reports. However, Sweeten et al. (2003) examined 31 autistic children and also found an increase in monocytes. Similarly, López-Cacho et al. (2016) found an increased number of circulating monocytes and an increased percentage of monocyte apoptosis in their study that evaluated the adaptive immune status of adult ASD cases. Monocytes and macrophages produce neopterin when stimulated by IFNγ, and increased levels of neopterin can serve as an indicator of monocyte/macrophage activation (Zhao et al. 2015). Previous studies showed a high monocyte count, a significantly high percentage of monocytes, and a high neopterin level in ASD patients (Bodur et al. 2014; Zhao et al. 2015). Moreover, immunological impairment in innate immunity was reported in ASD. Recently, peripheral blood monocytes have been found to increase the release of proinflammatory cytokines, such asIL-1, IL-6, TNF-α, in autism (Manzardo et al. 2012; Depino 2013; Jyonouchi et al. 2014). Increased rates of IL-6 and IL-1β were associated with increased impaired social behaviors in ASD cases (Enstrom et al. 2009). Children with autism also have an increased prevalence of specific immune-related comorbidities, such as infections, allergies, asthma, unexplained skin rashes (Zerbo et al. 2015; Li and Zhou 2016). The involvement of the immune system in the neurobiological pattern of ASD is a topic of current research (Bjørklund et al. 2016). We found an increase in monocytes in our study. This finding suggests a reactive increase in monocytes due to the chronic inflammation in ASDs. Previous findings also support the innate and adaptive immunity differences in ASD patients (López-Cacho et al. 2016). Supplementary table presents some evidence from the literature indicating the altered immune cell counts.
Our second finding is the higher RDW in the ASD group than in the control group. RDW can be obtained with a basic hemogram test. Narci et al. (2013) reported that RDW increases more significantly in chronic inflammatory processes than in acute processes. RDW was found to be high in major depression (Demircan et al. 2016). The abnormal immune response in different blood cell populations was determined in ASD children and adults (Depino 2013). The elevated RDW in the patient group in our study could be a consequence of the chronic inflammatory process.
The third finding is that LMR is significantly lower in the study group than in the control, and it is a predictor of being in the study group. LMR is inversely correlated with autism severity and is a variable that predicts an increase in CARS scores. LMR was found to be higher in the atypical autism group, which has less severe symptoms, than in the autism group. LMR decreases as the severity of autism increases. The reason for this condition is that the lymphocyte level decreases and the monocyte amount increases in the study group. It was shown that LMR is a prognostic factor for many types of cancer and elevated LMR is associated with better recurrence-free survival and overall survival for some cancers; decreased LMR is associated with poor oncologic outcome. (Peng et al. 2017; Xue et al. 2017). In general, peripheral blood lymphocyte count serves as a simple surrogate marker of the host immune status (Lin et al. 2015). Previous studies showed that some T lymphocytes decrease in autism (Mostafa et al. 2010). LMR is a ratio and is affected by the lymphocyte and monocyte counts. The number of lymphocytes decreases with age in both the study and the control group. For this reason, the main factor affecting the LMR ratio is the number of monocytes. In our study, as the severity of disease increased, the more that the decreases in lymphocyte count could lead to a deficiency in the immune system. The chronic inflammatory process in autism does not only appear in the peri-natal period but also in the later stages of the lives of autistic patients (Depino 2013). The immune dysfunction seen in autism continues through adulthood (López-Cacho et al. 2016). All these results indicate that the immunity problem may be related to disease severity, as shown in the study of Careaga et al. (2017). LMR may be a biomarker for autism and for identifying the severity of the disorder.
Our fourth finding is the positive correlation of NLR with autism severity. This result may indicate a decrease in lymphocyte as disease severity increases. NLR increase has been reported in psychiatric disorders associated with inflammation, such as schizophrenia (Semiz et al. 2014), bipolar disorder (Kalelioglu et al. 2015), major depressive disorder (Demircan et al. 2016). The NLR is a simple and easily calculated systemic inflammation-based score that has been investigated in a variety of diseases, including inflammatory, cardiovascular, and neoplastic conditions (de Jager et al. 2010; Beliaev et al. 2016; Turri-Zanoni et al. 2017). The correlation between NLR and CARS scores supports the inflammation hypothesis in ASD severity.
Autism is a neurobiological disease and there are many structural changes in the CNS (Aylward et al. 2002; Courchesne et al. 2004). Immune dysregulation, especially during early development, may be susceptible to changes in neurological functions (Goines and Ashwood 2013). However, our findings are consistent with the evidence that revealed altered inflammatory processes in later ages in ASD individuals (Bjørklund et al. 2016). The findings of this study show that the immune cell numbers and their ratios which can be measured using CBC counting, are altered in ASD cases; and that their ratios are affected by autism severity. The immune cells and molecules can affect brain structure and function, such as learning, memory, neuronal plasticity (Yirmiya and Goshen 2011). For this reason, changes in immunity may contribute to the formation of neurobiological diseases by affecting the brain cell functions, including the synthesis of neurotransmitters and synaptic functions and plasticity (Ceylan et al. 2014). Further research must be conducted to understand whether neuroimmune regulation can positively contribute to ASD severity.
In conclusion, a high monocyte level, which is an indicator of chronic inflammation, and LMR, which is associated with ASD severity, in peripheral blood may have diagnostic value in autism. Further studies are needed to understand the role of these parameters in showing response to treatment in terms of the severity of ASD.
4.1. Implications
Our findings suggest that changes in immunity may play a role in the pathophysiology of autism. Increased monocytes and RDW as well as decreased LMR are the most obvious findings in ASD cases. As disease severity increases, the more that decreases in the lymphocyte count and LMR may be involved in the pathophysiology of an immunological disorder. In addition, monocyte and lymphocyte changes (also LMR) in peripheral blood may have therapeutic and/or diagnostic value. Our findings may pioneer further clinical biochemical studies on autism.
Further investigation with a larger and more representative sample will allow for higher-level analyses examining additional cell subtypes and the relation among subtypes, severity of autism, and different clinical features. The lifecycle of monocytes and the cytokines that are released from monocytes should be investigated to elucidate the effects of monocytes on autism. Moreover, mononuclear blood cells from birth to adulthood should be investigated longitudinally.
4.2. Limitations
Our study has various limitations. The most important one is the small sample size. This study should be repeated on a larger sample size. Moreover, the study did not use data collection tools other than CARS. Thus, clinical features other than CARS scores were not compared between the study and the control group. The female/male ratio was one-fourth in the case group, consistent with the ratio in the literature (Loomes et al. 2017). Therefore, our study is reliable in terms of gender difference. However, the low number of girls limited us in analyzing the differences between genders. Another limitation is the lack of the use of standardized structured diagnostic instruments (e.g. the Autism Diagnostic Interview-Revised and the Autism Diagnostic Observation Schedule). Moreover, this work has the limitations of a retrospectively designed study.
The stress levels of ASD-affected individuals did not examined in this study. Stress may affect individual’s immune system and it may be an issue for further studies. Besides, while the high monocyte count or the low LMR rate are the signs of chronic inflammation, the increase of the proinflammatory cytokines (IL-1, IL-6, and TNF-α) in peripheral circulation of the ASD individuals also show the chronic inflammation (Depino 2013). In future studies, it may be appropriate to examine IL-1, IL-6, and TNF-α levels in combination with monocyte counts and ratios.
Conflict of interest
The authors declare no conflict of interest in this study.
Supplementary Material
Acknowledgments
There is not any source of funding in this research. The data collected retrospectively from the database of the Education and Research Hospital.
Supplementary material
Supplemental material for this article can be accessed here. [https://doi.org/10.1080/20473869.2017.1371369]
References
- Abbas, A. K., Lichtman, A. H. and Pillai, S.. 2007. Cells and tissues of adaptive immun system. In: Cellular and molecular immunology. 6th edn, Philadelphia, Saunders Elsevier, p. 56, Ch. 3. [Google Scholar]
- American Psychiatric Association . 2000. Diagnostic and statistical manual of mental disorders. 4th ed. – text revision. Washington, DC: Author. [Google Scholar]
- American Psychiatric Association . 2013. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Ashwood, P., Wills, S. and Van de Water, J.. 2006. The immune response in autism: A new frontier for autism research. Journal of Leukocyte Biology , 80, 1–15. 10.1189/jlb.1205707 [DOI] [PubMed] [Google Scholar]
- Ashwood, P., Corbett, B. A., Kantor, A., Schulman, H., Van de Water, J. and Amaral, D. G.. 2011. In search of cellular immunophenotypes in the blood of children with autism. PLoS ONE , 6, e19299. 10.1371/journal.pone.0019299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylward, E. H., Minshew, N. J., Field, K., Sparks, B. F. and Singh, N.. 2002. Effects of age on brain volume and head circumference in autism. Neurology , 59, 175–183. 10.1212/WNL.59.2.175 [DOI] [PubMed] [Google Scholar]
- Balta, S., Demırer, Z., Aparci, M., Yildirim, A. O. and Ozturk, C.. 2016. The lymphocyte-monocyte ratio in clinical practice. Journal of Clinical Pathology , 69, 88–89. 10.1136/jclinpath-2015-203233 [DOI] [PubMed] [Google Scholar]
- Beliaev, A. M., Angelo, N., Booth, M. and Bergin, C.. 2016. Evaluation of neutrophil-to-lymphocyte ratio as a potential biomarker for acute cholecystitis. Journal of Surgical Research , 29, 93–101. [DOI] [PubMed] [Google Scholar]
- Bjørklund, G., Saad, K., Chirumbolo, S., Kern, J. K., Geier, D. A., Geier, M. R. and Urbina, M. A.. 2016. Immune dysfunction and neuroinflammation in autism spectrum disorder. Acta Neurobiologiae Experimentalis , 76, 257–268. [DOI] [PubMed] [Google Scholar]
- Bodur, S., Ceylan, M. F., Iseri, E., Sener, S. and Yucel, A. A.. 2014. Serum neopterin levels in patients with autism. International Journal of Developmental Disabilities , 60, 109–115. 10.1179/2047387713Y.0000000029 [DOI] [Google Scholar]
- Careaga, M., Rogers, S., Hansen, R. L., Amaral, D. G., Van de Water, J. and Ashwood, P.. 2017. Immune endophenotypes in children with autism spectrum disorder. Biological Psychiatry, 81, 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan, M. F., Uneri, O. S., Guney, E., Ergin, M., Alisik, M., Goker, Z., Senses Dinc, G., Karaca Kara, F. and Erel, O.. 2014. Increased levels of serum neopterin in attention deficit/hyperactivity disorder (ADHD). Journal of Neuroimmunology , 273, 111–114. 10.1016/j.jneuroim.2014.06.002 [DOI] [PubMed] [Google Scholar]
- Chess, S. 1971. Autism in children with congenital rubella. Journal of Autism and Childhood Schizophrenia , 1, 33–47. 10.1007/BF01537741 [DOI] [PubMed] [Google Scholar]
- Chess, S. 1977. Follow-up report on autism in congenital rubella. Journal of Autism and Childhood Schizophrenia , 7, 69–81. 10.1007/BF01531116 [DOI] [PubMed] [Google Scholar]
- Christensen, D. L., Baio, J., Braun, K. V. N., Bilder, D., Charles, J., Constantino, J. N., Daniels, J., Durkin, M. S., Fitzgerald, R. T., Kurzius-Spencer, M., Lee, L. C., Pettygrove, S., Robinson, C., Schulz, E., Wells, C., Wingate, M. S., Zahorodny, W. and Yeargin-Allsopp, M.. 2016. Prevalence and characteristics of autism spectrum disorder among children aged 8 years – autism and developmental disabilities monitoring network, 11 Sites, United States, 2012. MMWR. Surveillance Summaries , 65, 1–23. 10.15585/mmwr.ss6503a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline, M. J. 1978. Monocytes, macrophages, and their diseases in man. Journal of Investigative Dermatology , 71, 56–58. 10.1111/1523-1747.ep12543945 [DOI] [PubMed] [Google Scholar]
- Colvert, E., Tick, B., McEwen, F., Stewart, C., Curran, S. R., Woodhouse, E., Gillan, N., Hallett, V., Lietz, S., Garnett, T., Ronald, A., Plomin, R., Rijsdijk, F., Happé, F. and Bolton, P.. 2015. Heritability of autism spectrum disorder in a UK population-based twin sample. JAMA Psychiatry , 72, 415–423. 10.1001/jamapsychiatry.2014.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne, E., Redcay, E. and Kennedy, D. P.. 2004. The autistic brain: Birth through adulthood. Current Opinion in Neurology , 17, 489–496. 10.1097/01.wco.0000137542.14610.b4 [DOI] [PubMed] [Google Scholar]
- de Jager, C. P. C., van Wijk, P. T. L., Mathoera, R. B., de Jongh-Leuvenink, J., van der Poll, T. and Wever, P. C.. 2010. Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Critical Care (London, England) , 14, R192. 10.1186/cc9309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan, F., Gözel, N., Kılınç, F., Ulu, R. and Atmaca, M.. 2016. The impact of red blood cell distribution width and neutrophil/lymphocyte ratio on the diagnosis of major depressive disorder. Neurology and Therapy , 5, 27–33. 10.1007/s40120-015-0039-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denney, D. R., Frei, B. W. and Gaffney, G. R.. 1996. Lymphocyte subsets and interleukin-2 receptors in autistic children. Journal of Autism and Developmental Disorders , 26, 87–97. 10.1007/BF02276236 [DOI] [PubMed] [Google Scholar]
- Depino, A. M. 2013. Peripheral and central inflammation in autism spectrum disorders. Molecular and Cellular Neuroscience , 53, 69–76. 10.1016/j.mcn.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Enstrom A. M., Lit L., Onore C. E., Gregg J. P., Hansen R. L., Pessah I. N., Hertz-Picciotto I., Van de Water J. A., Sharp F. R. and Ashwood P.. 2009. Altered gene expression and function of peripheral blood natural killer cells in children with autism. Brain, Behavior, and Immunity , 23, 124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines, P. E. and Ashwood, P.. 2013. Cytokine dysregulation in autism spectrum disorders (ASD): Possible role of the environment. Neurotoxicology and Teratology , 36, 67–81. 10.1016/j.ntt.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg, J. P., Lit, L., Baron, C. A., Hertz-Picciotto, I., Walker, W., Davis, R. A., Croen, L. A., Ozonoff, S. , Hansen, R., Pessah, I. N. & Sharp, F. R.. 2008. Gene expression changes in children with autism. Genomics , 91, 22–29. 10.1016/j.ygeno.2007.09.003 [DOI] [PubMed] [Google Scholar]
- İncekaş Gassaloğlu, S., Baykara, B., Avcil, S. and Demiral, Y.. 2016. Validity and reliability analysis of Turkish version of childhood autism rating scale. Türk Psikiyatri Dergisi , 27, 266–274. [PubMed] [Google Scholar]
- Jyonouchi, H., Geng, L. and Davidow, A. L.. 2014. Cytokine profiles by peripheral blood monocytes are associated with changes in behavioral symptoms following immune insults in a subset of ASD subjects: An inflammatory subtype? Journal of Neuroinflammation , 11, 74. 10.1186/s12974-014-0187-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalelioglu, T., Akkus, M., Karamustafalioglu, N., Genc, A., Genc, E. S., Cansiz, A. and Emul, M.. 2015. Neutrophil-lymphocyte and platelet-lymphocyte ratios as inflammation markers for bipolar disorder. Psychiatry Research , 228, 925–927. 10.1016/j.psychres.2015.05.110 [DOI] [PubMed] [Google Scholar]
- Li, Q. and Zhou, J. M.. 2016. The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience , 324, 131–139. 10.1016/j.neuroscience.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Lin, Z. X., Ruan, D. Y., Li, Y., Wu, D. H., Ma, X. K., Chen, J., Chen, Z. H., Li, X., Wang, T. T., Lin, Q., Wen, J. Y. and Wu, X. Y.. 2015. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World Journal of Gastroenterology , 21, 10898–10906. 10.3748/wjg.v21.i38.10898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes, R., Hull, L. and Mandy, W. P. L.. 2017. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. Journal of the American Academy of Child and Adolescent Psychiatry , 56, 466–474. 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- López-Cacho, J. M., Gallardo, S., Posada, M., Aguerri, M., Calzada, D., Mayayo, T., Lahoz, C. and Cárdaba, B.. 2016. Characterization of immune cell phenotypes in adults with autism spectrum disorders. Journal of Investigative Medicine , 64, 1179–1185. 10.1136/jim-2016-000070 [DOI] [PubMed] [Google Scholar]
- Madore, C., Leyrolle, Q., Lacabanne, C., Benmamar-Badel, A., Joffre, C., Nadjar, A. and Layé, S.. 2016. Neuroinflammation in autism: Plausible role of maternal inflammation, dietary omega 3, and microbiota. Neural Plasticity , 2016, 3597209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzardo, A. M., Henkhaus, R., Dhillon, S. and Butler, M. G.. 2012. Plasma cytokine levels in children with autistic disorder and unrelated siblings. International Journal of Developmental Neuroscience , 30, 121–127. 10.1016/j.ijdevneu.2011.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer, A. and Van de Water, J.. 2016. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology , 42, 284–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa, G. A., Al Shehab, A. and Fouad, N. R.. 2010. The frequency of CD4 + CD25 high regulatory T cells in the peripheral blood of Egyptian children with autism. Journal of Child Neurology , 25, 328–335. 10.1177/0883073809339393 [DOI] [PubMed] [Google Scholar]
- Narci, H., Turk, E., Karagulle, E., Togan, T. and Karabulut, K.. 2013. The role of red cell distribution width in the diagnosis of acute appendicitis: A retrospective case-controlled study. World Journal of Emergency Surgery , 8, 46–51. 10.1186/1749-7922-8-46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornoy, A., Liza, W. F. and Ergaz, Z.. 2016. Genetic syndromes, maternal diseases and antenatal factors associated with autism spectrum disorders (ASD). Frontiers in Neuroscience , 6, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onore, C., Careaga, M. and Ashwood, P.. 2012. The role of immune dysfunction in the pathophysiology of autism. Brain, Behavior, and Immunity , 26, 383–392. 10.1016/j.bbi.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo, C. A., Vargas, D. L. and Zimmerman, A. W.. 2005. Immunity, neuroglia and neuroinflammation in autism. International Review of Psychiatry (Abingdon, England) , 17, 485–495. [DOI] [PubMed] [Google Scholar]
- Patterson, P. H. 2011. Maternal infection and immune involvement in autism. Trends in Molecular , 17, 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, J., Li, H., Ou, Q., Lin, J., Wu, X., Lu, Z., Yuan, Y., Wan, D., Fang, Y. and Pan, Z.. 2017. Preoperative lymphocyte-to-monocyte ratio represents a superior predictor compared with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for colorectal liver-only metastases survival. OncoTargets and Therapy , 10, 3789–3799. 10.2147/OTT [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopler, E., Reichler, R., DeVellis, R. and Daly, K.. 1980. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). Journal of Autism and Developmental Disorders , 10, 91–103. 10.1007/BF02408436 [DOI] [PubMed] [Google Scholar]
- Semiz, M., Yildirim, O., Canan, F., Demir, S., Hasbek, E., Tuman, T. C., Kayka, N. and Tosun, M.. 2014. Elevated neutrophil/ lymphocyte ratio in patients with schizophrenia. Psychiatr Danub , 26, 220–225. [PubMed] [Google Scholar]
- Siniscalco, D., Sapone, A., Giordano, C., Cirillo, A., De Novellis, V., De Magistris, L., Rossi, F., Fasano, A., Maione, S. and Antonucci, N.. 2012. The expression of caspases is enhanced in peripheral blood mononuclear cells of autism spectrum disorder patients. Journal of Autism and Developmental Disorders , 42, 1403–1410. 10.1007/s10803-011-1373-z [DOI] [PubMed] [Google Scholar]
- Stigler, K., Sweeten, T., Posey, D. J. and McDougle, C.. 2009. Autism and immune factors: A comprehensive review. Research in Autism Spectrum Disorders , 3, 840–860. 10.1016/j.rasd.2009.01.007 [DOI] [Google Scholar]
- Stubbs, E. G., Crawford, M. L., Burger, D. R. and Vandenbark, A. A.. 1977. Depressed lymphocyte responsiveness in autistic children. Journal of Autism and Childhood Schizophrenia , 7, 49–55. 10.1007/BF01531114 [DOI] [PubMed] [Google Scholar]
- Sweeten, T. L., Posey, D. J. and McDougle, C. J.. 2003. High blood monocyte counts and neopterin levels in children with autistic disorder. American Journal of Psychiatry , 160, 1691–1693. 10.1176/appi.ajp.160.9.1691 [DOI] [PubMed] [Google Scholar]
- Turri-Zanoni, M., Salzano, G., Lambertoni, A., Giovannardi, M., Karligkiotis, A., Battaglia, P. and Castelnuovo, P.. 2017. Prognostic value of pretreatment peripheral blood markers in paranasal sinus cancer: Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio. Head Neck , 39, 730–736. 10.1002/hed.v39.4 [DOI] [PubMed] [Google Scholar]
- Xue, P., Hang, J., Huang, W., Li, S., Li, N., Kodama, Y., Matsumoto, S., Takaori, K., Zhu, L. and Kanai, M.. 2017. Validation of lymphocyte-to-monocyte ratio as a prognostic factor in advanced pancreatic cancer: An East Asian cohort study of 2 countries. Pancreas , 46, 1011–1017. 10.1097/MPA.0000000000000891 [DOI] [PubMed] [Google Scholar]
- Yirmiya, R. and Goshen, I.. 2011. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity , 25, 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Zahorec, R. 2001. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratislavske Lekarske Listy , 102, 5–14. [PubMed] [Google Scholar]
- Zaretsky, M. V., Alexander, J. M., Byrd, W. and Bawdon, R. E.. 2004. Transfer of inflammatory cytokines across the placenta. Obstetrics and Gynecology , 103, 546–550. 10.1097/01.AOG.0000114980.40445.83 [DOI] [PubMed] [Google Scholar]
- Zerbo, O., Iosif, A. M., Walker, C., Ozonoff, S., Hansen, R. L. and Hertz-Picciotto, I.. 2013. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (Childhood Autism Risks from Genetics and Environment) study. Journal of Autism and Developmental Disorders , 43, 25–33. 10.1007/s10803-012-1540-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo, O., Yoshida, C., Grether, J. K., Van de Water, J., Ashwood, P., Delorenze, G. N., Hansen, R. L., Kharrazi, M. and Croen, L. A.. 2014. Neonatal cytokines and chemokines and risk of autism spectrum disorder: The Early Markers for Autism (EMA) study: A case-control study. Journal of neuroinflammation , 20, 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbo, O., Leong, A., Barcellos, L., Bernal, P., Fireman, B. and Croen, L. A.. 2015. Immune-mediated conditions in autism spectrum disorders. Brain, Behavior, and Immunity , 46, 232–236. 10.1016/j.bbi.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H. X., Yin, S. S. and Fan, J. G.. 2015. High plasma neopterin levels in Chinese children with autism spectrum disorders. International Journal of Developmental Neuroscience , 41, 92–97. 10.1016/j.ijdevneu.2015.02.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.